Abstract
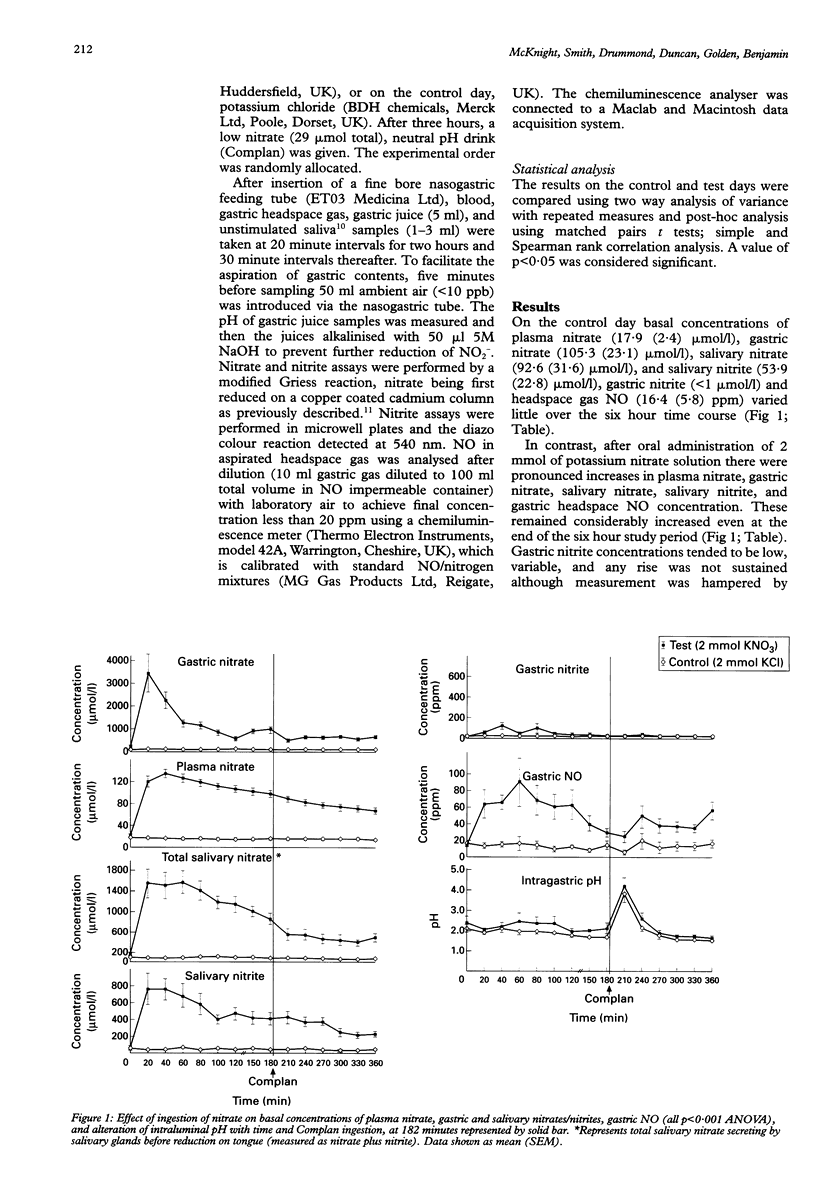
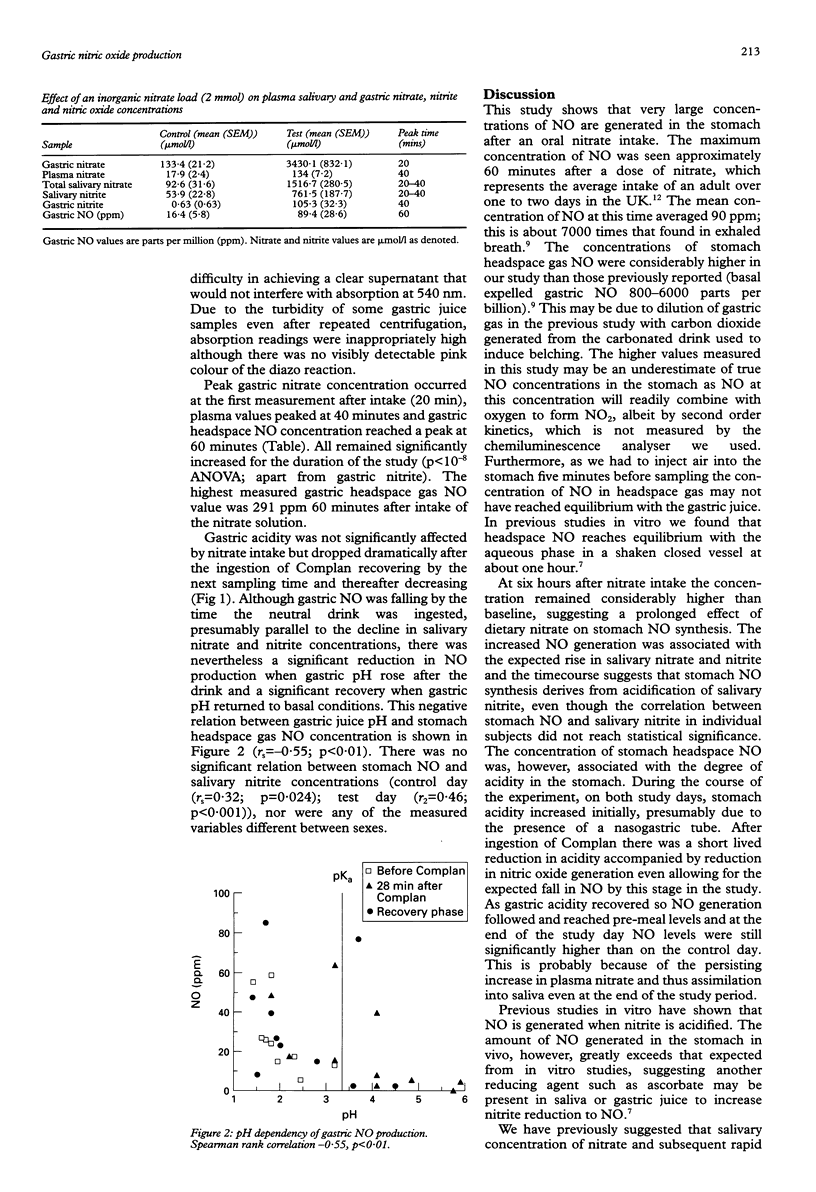
BACKGROUND/AIMS: It has been suggested that dietary nitrate, after concentration in the saliva and reduction to nitrite by tongue surface bacteria, is chemically reduced to nitric oxide (NO) in the acidic conditions of the stomach. This study aimed to quantify this in humans. METHODS: Ten healthy fasting volunteers were studied twice, after oral administration of 2 mmol of potassium nitrate or potassium chloride. Plasma, salivary and gastric nitrate, salivary and gastric nitrite, and gastric headspace NO concentrations were measured over six hours. RESULTS: On the control day the parameters measured varied little from basal values. Gastric nitrate concentration was 105.3 (13) mumol/l (mean (SEM), plasma nitrate concentration was 17.9 (2.4) mumol/l, salivary nitrate concentration 92.6 (31.6) mumol/l, and nitrite concentration 53.9 (22.8) mumol/l. Gastric nitrite concentrations were minimal (< 1 mumol/l). Gastric headspace gas NO concentration was 16.4 (5.8) parts per million (ppm). After nitrate ingestion, gastric nitrate peaked at 20 minutes at 3430 (832) mumol/l, plasma nitrate at 134 (7.2) mumol/l, salivary nitrate at 1516.7 (280.5) mumol/l, and salivary nitrite at 761.5 (187.7) mumol/l after 20-40 minutes. Gastric nitrite concentrations tended to be low, variable, and any rise was non-sustained. Gastric NO concentrations rose considerably from 14.8 (3.1) ppm to 89.4 (28.6) ppm (p < 0.0001) after 60 minutes. All parameters remained increased significantly for the duration of the study. CONCLUSIONS: A very large and sustained increase in chemically derived gastric NO concentrations after an oral nitrate load was shown, which may be important both in host defence against swallowed pathogens and in gastric physiology.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anggård E. Nitric oxide: mediator, murderer, and medicine. Lancet. 1994 May 14;343(8907):1199–1206. doi: 10.1016/s0140-6736(94)92405-8. [DOI] [PubMed] [Google Scholar]
- Benjamin N., O'Driscoll F., Dougall H., Duncan C., Smith L., Golden M., McKenzie H. Stomach NO synthesis. Nature. 1994 Apr 7;368(6471):502–502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- Bilski J., Konturek P. C., Konturek S. J., Cieszkowski M., Czarnobilski K. Role of endogenous nitric oxide in the control of gastric acid secretion, blood flow and gastrin release in conscious dogs. Regul Pept. 1994 Oct 21;53(3):175–184. doi: 10.1016/0167-0115(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Boeing H. Epidemiological research in stomach cancer: progress over the last ten years. J Cancer Res Clin Oncol. 1991;117(2):133–143. doi: 10.1007/BF01613137. [DOI] [PubMed] [Google Scholar]
- Desai K. M., Sessa W. C., Vane J. R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991 Jun 6;351(6326):477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Duncan C., Dougall H., Johnston P., Green S., Brogan R., Leifert C., Smith L., Golden M., Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995 Jun;1(6):546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- Granli T., Dahl R., Brodin P., Bøckman O. C. Nitrate and nitrite concentrations in human saliva: variations with salivary flow-rate. Food Chem Toxicol. 1989 Oct;27(10):675–680. doi: 10.1016/0278-6915(89)90122-1. [DOI] [PubMed] [Google Scholar]
- Knight T. M., Forman D., Al-Dabbagh S. A., Doll R. Estimation of dietary intake of nitrate and nitrite in Great Britain. Food Chem Toxicol. 1987 Apr;25(4):277–285. doi: 10.1016/0278-6915(87)90123-2. [DOI] [PubMed] [Google Scholar]
- Kugler P., Drenckhahn D. Intrinsic source of stomach NO. Nature. 1994 Jul 7;370(6484):25–26. doi: 10.1038/370025a0. [DOI] [PubMed] [Google Scholar]
- Lundberg J. O., Weitzberg E., Lundberg J. M., Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994 Nov;35(11):1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Pique J. M., Whittle B. J., Esplugues J. V. The vasodilator role of endogenous nitric oxide in the rat gastric microcirculation. Eur J Pharmacol. 1989 Dec 19;174(2-3):293–296. doi: 10.1016/0014-2999(89)90324-5. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder B., Eisenbrand G., Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976 Dec;14(6):545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- Stroff T., Plate S., Respondek M., Müller K. M., Peskar B. M. Protection by gastrin in the rat stomach involves afferent neurons, calcitonin gene-related peptide, and nitric oxide. Gastroenterology. 1995 Jul;109(1):89–97. doi: 10.1016/0016-5085(95)90272-4. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S. R., Weisman M., Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet Toxicol. 1976 Dec;14(6):549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]


