Background:
The efficacy of laparoscopic surgery (LS) for the treatment of colonoscopic perforation is still controversial. The purpose of this meta-analysis was to evaluate the effectiveness and safety of LS versus open surgery (OS) for colonoscopic perforation.
Methods:
All clinical trials that compared laparoscopic with OS for colonoscopic perforation published in English were identified in PubMed, EMBASE, Web of Science, and Cochrane Library searches. A modified scale was used to assess the quality of the literature. We analyzed the age, sex ratio, aim of colonoscopy, history of abdominopelvic surgery, type of procedure, size of perforation, operation time, postoperative fasting time, hospital stay, postoperative complication morbidity, and postoperative mortality. Meta-analyses were performed using weighted mean differences for continuous variables, and odds ratios for dichotomous variables.
Results:
No eligible randomized trials were identified, but eleven nonrandomized trials were analyzed. In the pooled data of 192 patients who underwent LS and 131 OS, there were no significant differences in age, sex ratio, aim of colonoscopy, history of abdominopelvic surgery, perforation size, and operative time between the groups. LS group had shorter time of hospital stay and postoperative fasting time, less postoperative complication morbidity, but there were no significant difference in postoperative mortality rate between LS group and OS group.
Conclusions:
Based on the current meta-analysis, we conclude that LS is a safe and efficacious technique for colonoscopic perforation, with fewer postoperative complications, less hospital mortality, and faster recovery compared with OS.
Keywords: colonic perforation, colonoscopy, laparoscopic surgery, laparoscopy
1. Introduction
The frequency of perforations from colonoscopy is estimated to be 0.016 to 0.8% for diagnostic colonoscopy and 0.02 to 8% for therapeutic colonoscopy.[1,2] Because of the widespread application of endoscopic submucosal dissection for the past few years, the incidence of perforation after colonoscopy were increasing rapidly.[3] If not properly handled, it can lead to peritonitis, sepsis, and even death. Treatment must be tailored according to the patient’s comorbidities and clinical status as well as the specific conditions during the colonoscopy that led to the perforation.[4] The managements of colonic perforations included operative and nonoperative methods. There were several researches supported nonoperative management in patients with no evidence of peritonitis and good clinical condition.[5,6] Nevertheless, nonoperative management has the possibility of failure, and surgical procedures might be inevitable if the condition continue getting worse. Some studies have reported endoscopic clip closure for the treatment of iatrogenic colon perforations, but there is still some possibility of failure.[7,8] By far, operation is recognized as the most reliable treatment for colonic perforation.[9,10]
Previously, most surgeons chose open surgery (OS) for colonic perforation, but it had disadvantages such as greater trauma and slower recover. As the use of laparoscopic techniques in colorectal surgery has been widely accepted, it has become a better choice for the treatment of colonoscopic perforation.[11] In recent years, there were a few researches that have assessed the efficacy of laparoscopic surgery (LS) in the treatment of colonoscopic perforation. The advantages are evident, LS has the same therapeutic outcomes, as well as less pain, shorter hospital stay, and less perioperative morbidity, compared with traditional OS.[12–14] In general, these have been limited to case reports and single-center studies, the efficacy of LS for the treatment of colonoscopic perforation is still controversial. Therefore, we carried out this meta-analysis, which could be used to help surgeons in choosing a better approach for the management of colonic perforation during colonoscopy.
2. Materials and methods
2.1. Literature search
We identified studies by searches of electronic databases such as PubMed, EMBASE, Web of Science, and Cochrane Library. The keywords used for the search included “colon perforation,” “colonic perforation,” “colonoscopic perforation,” “colonoscopy,” “laparoscopic,” and “laparoscopy.” The latest search was updated on March 30, 2022. Reference lists from identified publications were also reviewed so as to search for potentially relevant studies.
2.2. Inclusion and exclusion criteria
Two reviewers (ZW and LCY) evaluated every retrieved study independently. Inclusion criteria were as follows: studies that made a comparison between LS and OS for the treatment of colonic perforation during colonoscopy; recorded the majority of the following: aim of colonoscopy, history of abdominopelvic surgery, perforation size, operative time, postoperative fasting time, hospital stay, postoperative complication, postoperative mortality, blood loss, types of antibiotics used after surgery and readmission rate; were written in English; if the studies were from the same population, the most informative and recent study were included. Exclusion criteria included: did not use OS as a control; duplicate publication or the publication provided insufficient data; abstracts, letters, case reports, and reviews.
2.3. Data extraction and quality assessment
Data from all the eligible studies were extracted and checked by 2 independent investigators (ZW and LCY). Disagreements in data extraction were resolved by discussion with other members (FCF) in our group. The following information were extracted from each study: the publication year, study period, the number of participants, mean age, sex ratio, aim of colonoscopy, history of abdominopelvic surgery, type of procedure, size of perforation, operation time, postoperative fasting time, hospital stay, postoperative complication morbidity, and postoperative mortality. If the study provided medians/ranges instead of means/standard deviations (SDs), we transformed the medians/ranges to mean/SDs using the technique described by Hozo.[15] A modified scale method was used to assess the quality of literature according to a previously established scoring system, the methodological index for nonrandomized studies (Table 1).[16]
Table 1.
Modified MINORS scale used for quality assessment of nonrandomized controlled trials.
| Item | Points | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Contemporary groups | Not reported | Study group compared with historical control group | Study group compared with contemporary control group |
| Prospective collection of data | Not reported | Data obtained from retrospective review of medical history | Data obtained from prospectively maintained database |
| Inclusion of consecutive patients | Not reported | Patients are not consecutive | Patients are consecutive |
| Baseline equivalence of groups | No matching analysis performed | Matching incomplete | Matching complete |
| A control group having the gold standard intervention | Not reported | Incomplete report of the standard intervention | Complete report of the standard intervention |
| Important data being presented | Lack | Less comprehensive | comprehensive |
| Sample size of LS group | <20 | 20–50 | >50 |
LS = laparoscopic surgery, MINORS = methodological index for nonrandomized studies.
2.4. Statistical analysis
Meta-analysis was performed using Review Manager Version 5.2 software (The Cochrane Collaboration, Oxford, UK). Weighted mean differences (WMD) with 95% confidence intervals (CIs) were used for analyzing continuous variables and odds ratios (ORs) for dichotomous variables. Heterogeneity was assessed by using the Cochran Q test and I2 statistics. If the P < .1 and I2 exceeded 50%, heterogeneity was considered to indicate statistical significance.[17] Random effects model was used to identify heterogeneity among the studies, otherwise, fixed-effects model was used. Potential sources of heterogeneity were explored by carrying out subgroup analyses. Sensitivity analysis was performed by eliminating each study at a time from the meta-analysis. Funnel plots were used to assess the potential publication bias.
2.5. Ethics and dissemination
Our aim is that this systematic review could be published in a peer-reviewed journal. The results will evaluate the effectiveness and safety of LS versus OS for colonoscopic perforation. Participants’ privacy not being involved, this systematic review will not require informed consent form.
3. Results
3.1. Selected studies
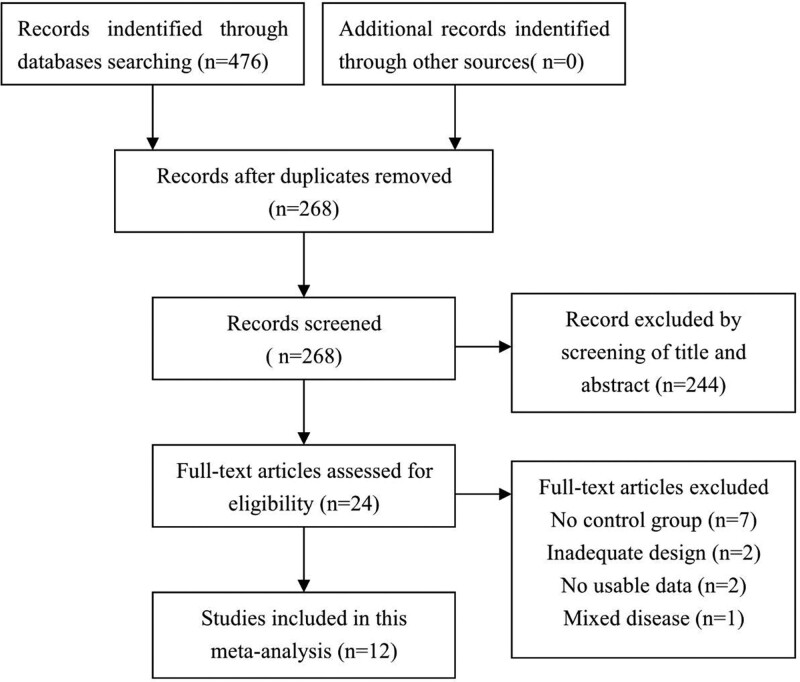
A total of 476 potential articles were retrieved according to the initial search strategy. The titles and abstracts of 268 articles were reviewed after excluding duplicated articles. Two hundred forty-four articles were excluded by this process, and the full text of 24 articles was reviewed. Of these, 12 articles were excluded for the following reasons: no control group (n = 7); inadequate design (n = 2); no usable data (n = 2); mixed disease (n = 1). Ultimately, 11 articles[12,13,18–26] (America = 2, Argentina = 1, Belgium = 1, Germany = 2, Korea = 3, China = 2) totaling 323 patients were finally eligible for the quality assessment,of whom 192 underwent LS and 131 underwent OS. A flowchart of the literature search and selection is illustrated in (Fig. 1).
Figure 1.
Literature search and selection procedures.
3.2. Study characteristics and quality assessment
These included 11 non-randomized clinical trials[12,13,18–26] (NRCTs) totaling 323 patients published between 2007 and 2020. The sample size of these studies ranged from 11 to 99. In these retrospective and nonrandomized studies, a true comparison of postoperative outcomes between open and LS cannot be performed because the 2 groups of patients are not identical. For the nature of the laparoscopy surgery trials, it was also impossible to perform blinding. The diagnosis of colon perforation are dependent on direct visualization during colonoscopy, abdominal plain radiography, or computed tomography of abdomen. The surgical management of colonoscopic perforation included primary repair or bowel resection, with or without proximal stoma diversion. The characteristics of the studies included in this meta-analysis are presented in Table 2. The quality assessment of the NRCTs is shown in Table 3.
Table 2.
Characteristics of included studies comparing LS with OS for colonoscopic perforation.
| Study | Study period | Country | Number of patients | Sex of patients (M/F) | Aim of colonoscopy (diagnostic/therapeutic) | History of abdominopelvic surgery (yes/no) | Type of procedure | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LS | OS | LS | OS | LS | OS | LS | OS | ||||
| Hansen AJ[18] | 2007 | America | 7 | 4 | 3/4 | 1/3 | 6/1 | 1/3 | 7/0 | 2/2 | Primary repair, stapled repair, colostomy |
| Bleier JI[19] | 2008 | America | 11 | 7 | 2/9 | 3/4 | NA | NA | NA | NA | Primary repair |
| Rumstadt B[21] | 2008 | Germany | 10 | 3 | NA | NA | NA | NA | NA | NA | Primary repair |
| Rotholtz NA[20] | 2010 | Argentina | 14 | 6 | 5/9 | 2/4 | 8/6 | 3/3 | NA | NA | Primary repair, colonic resection, Hartmann, colostomy |
| Coimbra C[22] | 2011 | Belgium | 16 | 23 | 9/7 | 11/12 | 11/5 | 17/6 | 3/13 | 7/16 | Primary repair, colostomy |
| Schlöricke E[23] | 2013 | Germany | 24 | 12 | 14/10 | 5/7 | NA | NA | 4/20 | 4/8 | Primary repair, colonic resection |
| Kim J[24] | 2014 | Korea | 17 | 8 | 8/9 | 4/4 | NA | NA | NA | NA | Primary repair, colonic resection, Hartmann |
| Zhong W[26] | 2016 | China | 13 | 8 | 7/6 | 5/3 | NA | NA | 0/13 | 0/8 | Primary repair, |
| Shin DK[25] | 2016 | Korea | 8 | 15 | 7/1 | 5/10 | 4/4 | 7/8 | 0/8 | 3/12 | Primary repair, colonic resection, colostomy |
| Lee JS[12] | 2020 | Korea | 59 | 40 | 35/24 | 22/18 | 31/28 | 22/18 | 11/48 | 8/32 | Primary repair, colonic resection, Hartmann, colostomy |
| Li L[13] | 2020 | China | 13 | 5 | 7/6 | 2/3 | 8/5 | 5/0 | NA | NA | Primary repair, colostomy |
LS = laparoscopic surgery, OS = open surgery.
Table 3.
Modified MINORS score of all eligible nonrandomized comparative studies.
| First author | Year | Contemporary groups | Prospective data collection | Consecutive patients | Baseline equivalent of groups | Gold standard intervention | Important data being presented | LS sample size | Score |
|---|---|---|---|---|---|---|---|---|---|
| Hansen AJ | 2007 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 9 |
| Bleier JI | 2008 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 10 |
| Rumstadt B | 2008 | 2 | 1 | 2 | 2 | 2 | 0 | 0 | 9 |
| Rotholtz NA | 2010 | 2 | 1 | 2 | 2 | 2 | 0 | 0 | 9 |
| Coimbra C | 2011 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 11 |
| Schlöricke E | 2013 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 11 |
| Kim J | 2014 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 11 |
| Zhong W | 2016 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 11 |
| Shin DK | 2016 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 11 |
| Lee JS | 2020 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 13 |
| Li L | 2020 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 11 |
LS = laparoscopic surgery, MINORS = methodological index for nonrandomized studies.
4. Meta-analysis results
4.1. Age
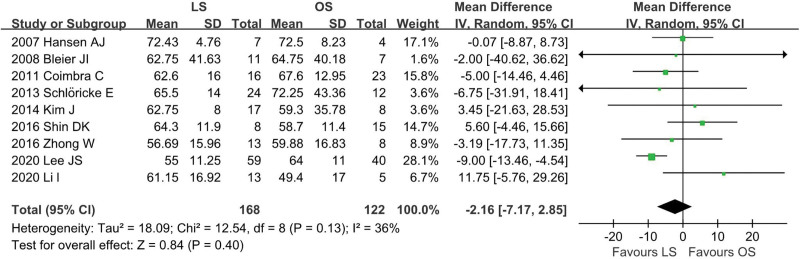
A total of 9 studies[12,13,18,19,22–26] were included (LS = 168, OS = 122) and no statistical heterogeneity was identified among these studies (I2 = 36%, P = .13). In the pooled data, there was no significant difference in the age between the groups (WMD: −2.16; 95% CI: −7.17 to 2.85; P = .40; Fig. 2).
Figure 2.
Meta-analysis of age.
4.2. Sex ratio
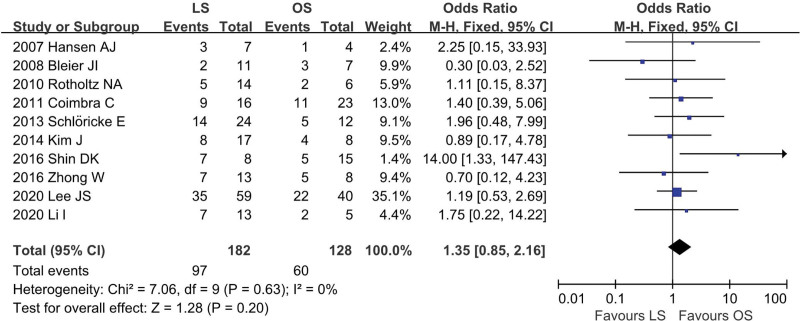
Ten studies[12,13,18–20,22–26] were included (LS = 182, OS = 128), and low heterogeneity among the studies (I2 = 0%, P = .63). In the pooled data, there was no significant difference in sex ratio between the groups (OR = 1.35; 95% CI: 0.85 to 2.16; P = .20; Fig. 3).
Figure 3.
Meta-analysis of sex ratio.
4.3. Aim of colonoscopy
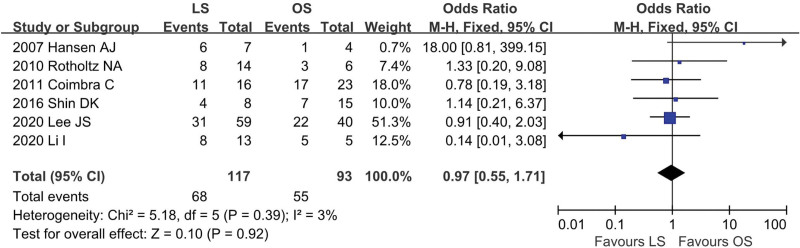
Six studies[12,13,18,20,22,25] provided data regarding the aim of colonoscopy (LS = 117, OS = 93). There was low heterogeneity among the studies (I2 = 3%, P = .39). In the pooled data, there was no significant difference in the aim of colonoscopy between the groups (OR = 0.97; 95% CI: 0.55 to 1.71; P = .92; Fig. 4).
Figure 4.
Meta-analysis of aim of colonoscopy.
4.4. History of abdominopelvic surgery
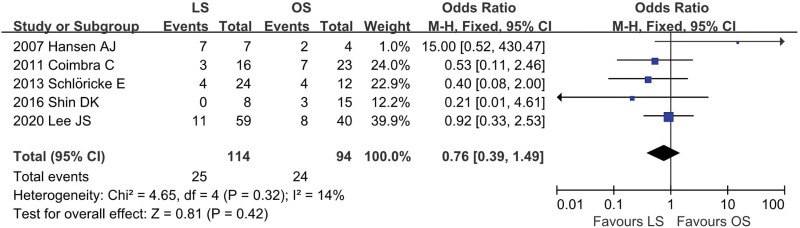
Five studies[12,18,22,23,25] provided data regarding the history of abdominopelvic surgery (LS = 114, OS = 94). There was low heterogeneity among the studies (I2 = 14%, P = .32). In the pooled data, there was no significant difference in the history of abdominopelvic surgery between the groups (OR = 0.76; 95% CI: 0.39 to 1.49; P = .42; Fig. 5).
Figure 5.
Meta-analysis of history of abdominopelvic surgery.
4.5. Perforation size
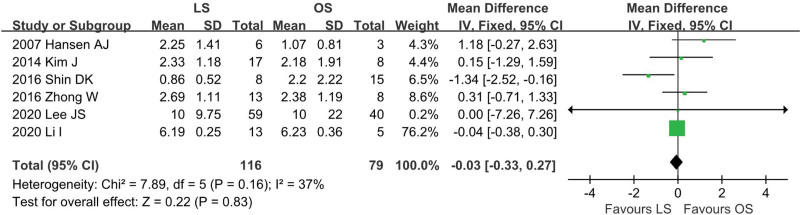
Six studies[12,13,18,24–26] provided data regarding perforation size (LS = 116, OS = 79). There was low heterogeneity among the studies (I2 = 37%, P = .16). There was no significant difference in the pooled data (WMD = −0.03; 95% CI: −0.33 to 0.27; P = .83; Fig. 6).
Figure 6.
Meta-analysis of perforation size.
4.6. Operative time
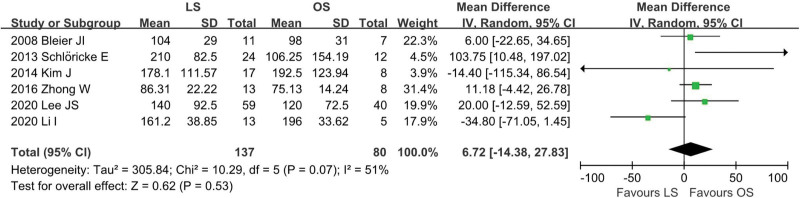
Data on operation time was available from 6 studies[12,13,19,23,24,26] (LS = 137, OS = 80). There was moderate heterogeneity among the studies (I2 = 51%, P = .07). In the pooled data, there was no significant difference in operation time between the groups (WMD = 6.72, 95% CI: −14.38 to 27.83; P = .53; Fig. 7).
Figure 7.
Meta-analysis of operative time.
4.7. Postoperative fasting time
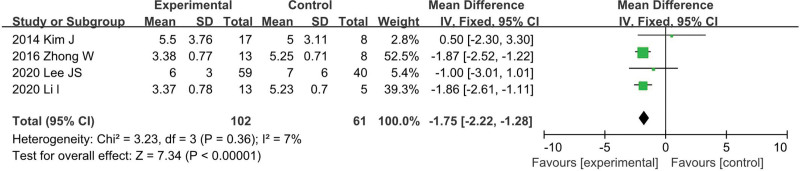
Data on operation time was available from 4 studies[12,13,24,26] (LS = 102, OS = 61). There was no heterogeneity among the studies (I2 = 7%, P = .36). In the pooled data, LS group was associated with significantly decreased postoperative fasting time among these studies (WMD = −1.75; 95% CI: −2.32 to −1.28; P < .001; Fig. 8).
Figure 8.
Meta-analysis of postoperative fasting time.
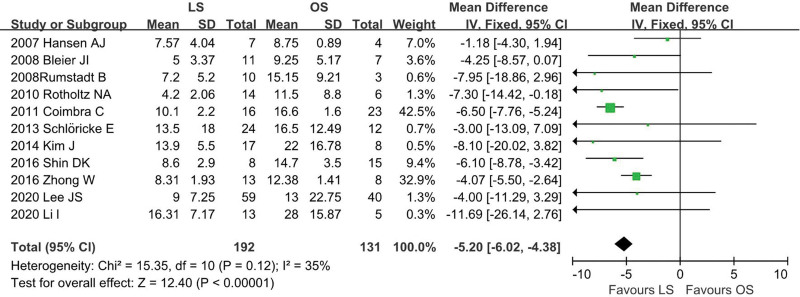
4.8. Hospital stay
Eleven studies[12,13,18–26] provided data regarding duration of postoperative hospital stay (LS = 192, OS = 131). In the pooled data, LS group was associated with significantly decreased hospital stay (WMD = −5.20; 95% CI: −6.02 to −4.38; P < .001) with low heterogeneity among these studies (I2 = 35%; P = .12; Fig. 9).
Figure 9.
Meta-analysis of hospital stay.
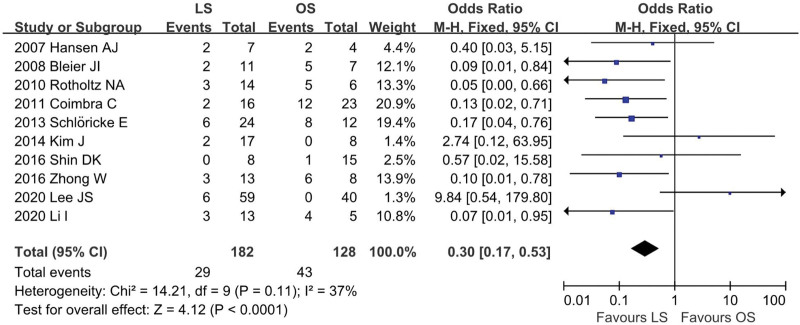
4.9. Postoperative complication morbidity
Ten researches[12,13,18–20,22–26] provided data regarding postoperative complications (LS = 182, OS = 128). The combined result indicated that the overall postoperative complication morbidity was significantly less in the LS group than in the OS group (OR = 0.30; 95% CI: 0.17 to 0.53; P < .001). The overall analysis in the studies show low heterogeneity (I2 = 37%; P = .11; Fig. 10). The complications include in this meta-analysis were ileus, wound infection, fever, abscess, pneumonia, anastomotic leak, and so on.
Figure 10.
Meta-analysis of postoperative complication morbidity.
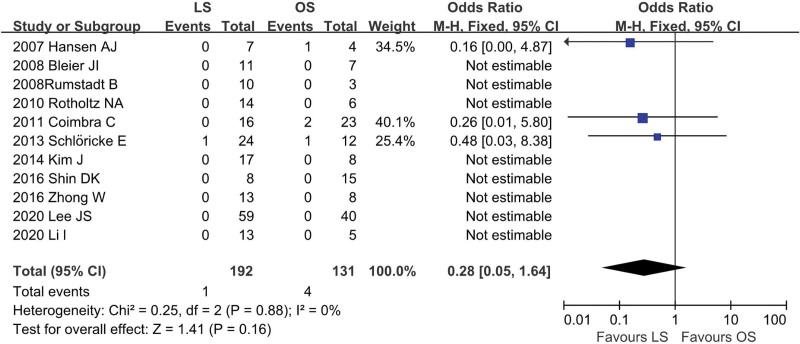
4.10. Postoperative mortality
The postoperative mortality rates were reported in 11 studies[12,13,18–26] (LS = 192, OS = 131). Meta-analysis demonstrated that no significant difference in postoperative mortality rate between LS group and OS group (OR = 0.28; 95% CI: 0.05 to 1.61; P = .16), with no significant heterogeneity among studies (I2 = 0%; P = .88; Fig. 11).
Figure 11.
Meta-analysis of postoperative mortality.
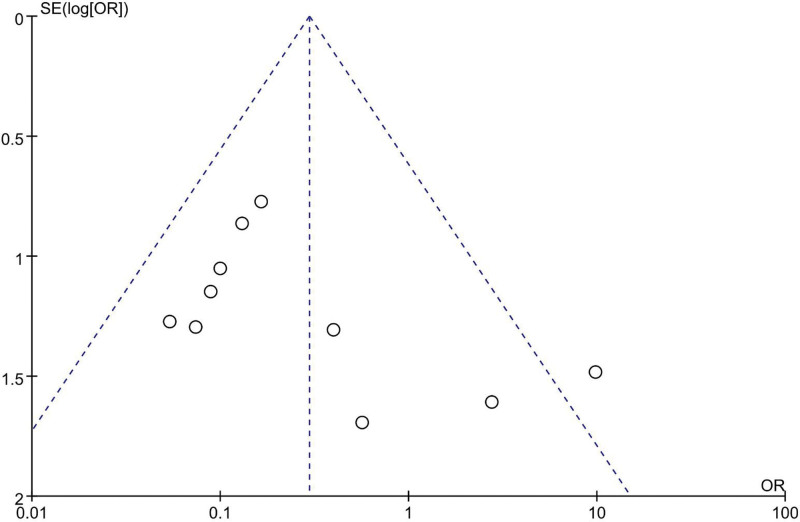
4.11. Sensitivity analysis and publication bias
Analysis of sensitivity was made by removing each study at a time from the meta-analysis, the results have not altered. Publication bias was assessed for each complication by using the funnel plot of the included studies, visual inspection of the funnel plot revealed symmetry, suggesting no severe publication bias (Fig. 12).
Figure 12.
Funnel plots of postoperative complication morbidity.
5. Discussion
LS is a technique that attracts an increasing number of surgeons, thanks to its offering important advantages (less pain, shorter hospital stay, and less perioperative morbidity) over OS for patients with colorectal diseases.[27,28] However, the efficacy of LS for the treatment of colonoscopic perforation is still controversial. Our meta-analysis included 11 nonrandomized controlled studies[12,13,18–26] that compared the short-term outcomes of LS with OS for the treatment of colonoscopic perforation. The modified methodological index for nonrandomized studies scale method was used to assess the quality of these NRCTs and the score of included studies was no less than 8. In this meta-analysis, we focused on not only the short-term outcomes but also including age, sex ratio, aim of colonoscopy, history of abdominopelvic surgery and perforation size. These subsequent 5 items were used to assess whether there had been selection bias in enrollment for the studies analyzed. Our meta-analysis revealed that there were no significant differences in age, sex ratio, aim of colonoscopy, history of abdominopelvic surgery, and perforation size between LS group and OS group. This demonstrated that the patients included in our studies were comparable between the groups.
Some researches reported that LS remains a time-consuming procedure even conducted by experienced surgeons.[29,30] Our meta-analysis revealed that there was no significant difference in operation time between the groups. There are several reasons as follows: first, a large proportion of patients included in our studies underwent laparoscopic primary repair, rather than bowel resection; second, it is well known that LS could enlarge the surgical field, which would permit better exposure and allow better identification of the site of perforation;[31] Third, during the past 20 years, equipment of laparoscopy was more advanced and the surgeons more and more skilled laparoscopic techniques. Thus, we believe that with the continued development of endoscopic equipment and laparoscopic technique, the operation time required for LS will become shorter in the future.[32,33]
Many studies have shown that LS has the advantages of less trauma and recover quickly.[33] Our meta-analysis results indicated that the time of postoperative fasting and hospital stay were shorter for the LS group than the OS group. It means that patients who underwent LS recovered more quickly than those who underwent OS. This is easy to understand, LS has a shorter incision than OS, resulting in less postoperative pain, and patients are more receptive to early ambulation, which improves gastrointestinal function and allows them to eat food earlier than the open group. Shorter postoperative fasting time can speed up the patient’s recovery process, leading to shorter hospital stays. In this meta-analysis, data from the 11 studies included showed that the length of hospital stay was shorter in the LS group than in the OS group. These strongly prove that LS is superior to OS in terms of postoperative recovery.
The incidence of surgical complications and mortality is critical for a surgical technique. Our meta-analysis revealed that postoperative complication morbidity after LS was fewer than OS. This finding might have been due to the minimal trauma of LS. LS has a smaller incision, and if the abdominal cavity is contaminated, the incision can be isolated by trocar without contamination, so the probability of infection of the incision is less than that of OS. Because patients have smaller incisions, less pain, and move out of bed earlier, complications such as pneumonia and fever were also reduced. Among the 10 studies included in the meta-analysis, only Kim’s study showed a higher incidence of postoperative complications. But interestingly, the length of hospital stay in the LS group was still shorter than that in the OS group.
One and 4 postoperative cases of death in LS group and OS group, respectively. No mortality occurred during postoperative period was reported in 8 studies.[12,13,19–21,24–26] In the pooled data, there was no significant difference in postoperative mortality rates between the groups but fewer deaths occurred in the LS group than in the OS group. This result strongly suggested that LS is an effective treatment and could achieve a comparable short-term prognosis for colonoscopic perforation compared with OS group.
This is the first meta-analysis study to compare the short-term outcomes between OS and LS for colonoscopic perforation. We fully acknowledge that the low number of patients in our study and the included studies were all NRCTs. The findings of high-quality NRCTs might be as reliable as randomized controlled trials, particularly when pooled data are compared to evaluate the effectiveness of surgical procedures.[34] Considering the low incidence of colonic perforation during colonoscopy, we think our research is still valuable.
Nonetheless, our study actually owns some limitations: first, all the included studies are NRCTs, which may exaggerate the effect magnitude of an intervention. Second, heterogeneity was detected within several outcomes, specifically perforation size and operative time. The degree of between-study heterogeneity present may undermine the quality and legitimacy of the results obtained, even though we already used the random model.[35] Third, the number of enrolled patients in all the included studies is relatively small, which is likely to have masked the true difference in some variables. Fourth, transforming abnormal distribution (media/range) to normal distribution (mean/SD) is likely to subject to a potential bias. Fifth, all the surgical outcomes might be influenced by the surgeon’s experience. However, most studies did not explicitly state whether surgeons were proficient in the LS technique before the trial started. Finally, although an extensive literature search was done, we may miss some unpublished studies.
6. Conclusion
Based on the current meta-analysis, LS is a safe and efficacious technique for colonoscopic perforation, with fewer postoperative complications, less hospital mortality, and faster recovery compared with OS. However, this does not mean that LS is an alternative method to OS. We maintain whether LS should be implemented based on the experience of a laparoscopist, and several factors should be taken into account, such as good medical condition, history of abdominopelvic surgery, small size of perforation, and clean bowel preparation.[19] When a surgeon feels that it is difficult to complete by the laparoscopic method, conversion to an open procedure is always a consideration.
Author contributions
Conceptualization: Xueyun Guan.
Data curation: Wu Zhong.
Investigation: Wu Zhong, Chuanyuan Liu.
Methodology: Chuanfa Fang, Xianping He.
Writing – original draft: Wu Zhong, Lei Zhang, Weiquan Zhu.
Abbreviations:
- CI
- confidence interval
- LS
- laparoscopic surgery
- NRCTs
- non-randomized clinical trials
- OR
- odds ratios
- OS
- open surgery
- SD
- standard deviation
- WMD
- weighted mean differences
This article is supported by the Natural Science Foundation of Jiangxi Province (20181BAB205059).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Zhong W, Liu C, Fang C, Zhang L, He X, Zhu W, Guan X. Laparoscopic versus open surgery for colonoscopic perforation: A systematic review and meta-analysis. Medicine 2023;102:24(e34057).
Contributor Information
Wu Zhong, Email: zhongwu0016@163.com.
Chuanyuan Liu, Email: luojiangwei0016@163.com.
Chuanfa Fang, Email: fangcf123@sina.com.
Lei Zhang, Email: zhanglei5889721@sina.com.
Xianping He, Email: 1455067225@qq.com.
Weiquan Zhu, Email: 405588303@qq.com.
References
- [1].Levy I, Gralnek IM. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract Res Clin Gastroenterol. 2016;30:705–18. [DOI] [PubMed] [Google Scholar]
- [2].Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–1906, 1906.e1. [DOI] [PubMed] [Google Scholar]
- [3].Emmanuel A, Gulati S, Burt M, et al. Combining eastern and western practices for safe and effective endoscopic resection of large complex colorectal lesions. Eur J Gastroenterol Hepatol. 2018;30:506–13. [DOI] [PubMed] [Google Scholar]
- [4].Hawkins AT, Sharp KW, Ford MM, et al. Management of colonoscopic perforations: a systematic review. Am J Surg. 2018;215:712–8. [DOI] [PubMed] [Google Scholar]
- [5].Park JY, Choi PW, Jung SM, et al. The outcomes of management for colonoscopic perforation: a 12-year experience at a single institute. Ann Coloproctol. 2016;32:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Parsa H, Miroliaee A, Doagoo Z, et al. Conservative management of colonoscopic perforation: a case report. Acta Med Iran. 2017;55:477–9. [PubMed] [Google Scholar]
- [7].Yachida T, Kobara H, Nishiyama N, et al. Over-the-scope clip closure indicated as first-line therapy for iatrogenic rectal perforation. J Gastrointestin Liver Dis. 2020;29:466–7. [DOI] [PubMed] [Google Scholar]
- [8].Ryu JY, Park BK, Kim WS, et al. Endoscopic closure of iatrogenic colon perforation using dual-channel endoscope with an endoloop and clips: methods and feasibility data (with videos). Surg Endosc. 2019;33:1342–8. [DOI] [PubMed] [Google Scholar]
- [9].Thompson EV, Snyder JR. Recognition and management of colonic perforation following endoscopy. Clin Colon Rectal Surg. 2019;32:183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lim DR, Kuk JK, Kim T, et al. The analysis of outcomes of surgical management for colonoscopic perforations: a 16-years experiences at a single institution. Asian J Surg. 2020;43:577–84. [DOI] [PubMed] [Google Scholar]
- [11].Alsowaina KN, Ahmed MA, Alkhamesi NA, et al. Management of colonoscopic perforation: a systematic review and treatment algorithm. Surg Endosc. 2019;33:3889–98. [DOI] [PubMed] [Google Scholar]
- [12].Lee JS, Kim JY, Kang BM, et al. Clinical outcomes of laparoscopic versus open surgery for repairing colonoscopic perforation: a multicenter study. Surg Today. 2021;51:285–92. [DOI] [PubMed] [Google Scholar]
- [13].Li L, Xue B, Yang C, et al. Clinical characteristics of colonoscopic perforation and risk factors for complications after surgical treatment. J Laparoendosc Adv Surg Tech A. 2020;30:1153–9. [DOI] [PubMed] [Google Scholar]
- [14].Kim TW, Kim HO, Jung KU, et al. Laparoscopic repair using an endoscopic linear stapler for management of iatrogenic colonic perforation during screening colonoscopy. Wideochir Inne Tech Maloinwazyjne. 2019;14:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [18].Hansen AJ, Tessier DJ, Anderson ML, et al. Laparoscopic repair of colonoscopic perforations: indications and guidelines. J Gastrointest Surg. 2007;11:655–9. [DOI] [PubMed] [Google Scholar]
- [19].Bleier JI, Moon V, Feingold D, et al. Initial repair of iatrogenic colon perforation using laparoscopic methods. Surg Endosc. 2008;22:646–9. [DOI] [PubMed] [Google Scholar]
- [20].Rotholtz NA, Laporte M, Lencinas S, et al. Lapar,oscopic approach to colonic perforation due to colonoscopy. World J Surg. 2010;34:1949–53. [DOI] [PubMed] [Google Scholar]
- [21].Rumstadt B, Schilling D. Iatrogene kolonperforation: erfahrungen mit der umgehenden laparoskopischen therapie [Iatrogenic colon perforation: experiences with early laparoscopy]. Chirurg. 2008;79:346–50. [DOI] [PubMed] [Google Scholar]
- [22].Coimbra C, Bouffioux L, Kohnen L, et al. Laparoscopic repair of colonoscopic perforation: a new standard? Surg Endosc. 2011;25:1514–7. [DOI] [PubMed] [Google Scholar]
- [23].Schlöricke E, Bader FG, Hoffmann M, et al. Open surgical versus laparoscopic treatment of iatrogenic colon perforation-results of a 13-year experience. Zentralbl Chir. 2013;138:257–61. [DOI] [PubMed] [Google Scholar]
- [24].Kim J, Lee GJ, Baek JH, et al. Comparison of the surgical outcomes of laparoscopic versus open surgery for colon perforation during colonoscopy. Ann Surg Treat Res. 2014;87:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shin DK, Shin SY, Park CY, et al. Optimal methods for the management of iatrogenic colonoscopic perforation. Clin Endosc. 2016;49:282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhong W, Qiu C, Liu C, et al. Efficacy of laparoscopic primary repair in the treatment of colonic perforation after colonoscopy: a review of 40,127 patients. Surg Laparosc Endosc Percutan Tech. 2016;26:e105–8. [DOI] [PubMed] [Google Scholar]
- [27].Parker JM, Feldmann TF, Cologne KG. Advances in laparoscopic colorectal surgery. Surg Clin North Am. 2017;97:547–60. [DOI] [PubMed] [Google Scholar]
- [28].Harji DP, Marshall H, Gordon K, et al. Laparoscopic versus open colorectal surgery in the acute setting (LaCeS trial): a multicentre randomized feasibility trial. Br J Surg. 2020;107:1595–604. [DOI] [PubMed] [Google Scholar]
- [29].Marcelissen TA, Den Hollander PP, Tuytten TR, et al. Incidence of iatrogenic ureteral injury during open and laparoscopic colorectal surgery: a single center experience and review of the literature. Surg Laparosc Endosc Percutan Tech. 2016;26:513–5. [DOI] [PubMed] [Google Scholar]
- [30].Pascual M, Salvans S, Pera M. Laparoscopic colorectal surgery: Current status and implementation of the latest technological innovations. World J Gastroenterol. 2016;22:704–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Colás-Ruiz E, Rueda-Orgaz JA, Fernández-Cebrián JM, et al. Treatment after iatrogenic colonoscopic perforation, is the laparoscopic approach a good option? Rev Esp Enferm Dig. 2015;107:328–9. [PubMed] [Google Scholar]
- [32].Park SJ, Lee KY, Lee SH. Laparoscopic surgery for colorectal cancer in korea: nationwide data from 2013 to 2018. Cancer Res Treat. 2020;52:938–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhao JH, Sun JX, Huang XZ, et al. Meta-analysis of the laparoscopic versus open colorectal surgery within fast track surgery. Int J Colorectal Dis. 2016;31:613–22. [DOI] [PubMed] [Google Scholar]
- [34].Abraham NS, Byrne CJ, Young JM, et al. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol. 2010;63:238–45. [DOI] [PubMed] [Google Scholar]
- [35].Ng TT, McGory ML, Ko CY, et al. Meta-analysis in surgery: methods and limitations. Arch Surg. 2006;141:1125–30; discussion 1131. [DOI] [PubMed] [Google Scholar]