Background:
This study aimed to summarize the current literature regarding the prevalence of renal stones in patients with inflammatory bowel disease (IBD). Moreover, we aimed to evaluate the risk factors of urolithiasis in patients with IBD and the difference between patients with IBD and healthy controls in terms of urinary profile.
Methods:
On February 23, 2022, a computerized search was conducted on PubMed, OVID via MEDLINE, Web of Science, and Scopus using relevant keywords. Three independent reviewers performed 2-stage screening and data extraction. The National Institutes of Health tools were employed for quality assessment. Review Manager 5.4 software was used to calculate the mean difference (MD) between IBD patients and non-IBD in terms of urine profile using the Inverse-variance model and to estimate the odds ratio of reported risk factors for renal stones with the Generic Inverse-Variance model.
Results:
Thirty-two articles (n = 13,339,065 patients) were included. The overall prevalence of renal stones in patients with IBD was 6.3%, 95% Confidence interval (4.8%–8.3%). The prevalence of urolithiasis was more common in Chron’s disease vs Ulcerative colitis (7.9% vs 5.6%) and in old studies (1964–2009) than in more recent studies (2010–2022) (7.3% vs 5.2%), respectively. Compared to non-IBD patients, patients with IBD were associated with significantly lower urine volume (MD = −518.84 mL/day, P < .00001), calcium 24-hour urine (MD = −28.46 mg/day, P < .0001), citrate 24-hour urine (MD = −144.35 mg/day, P < .00001), sodium 24-hour urine (MD = −23.72 mg/day, P = .04), and magnesium 24-hour urine (MD = −33.25 mg/day, P < .00001).
Conclusion:
The overall prevalence of renal stones in patients with IBD was comparable to the general population. Patients with Chron’s disease were associated with a higher prevalence of urolithiasis compared to Ulcerative colitis. Drugs that induce renal calculi should be stopped in high-risk patients.
Keywords: chron’s disease, IBD, nephrolithiasis, renal calculi, ulcerative colitis, urolithiasis
1. Introduction
Ulcerative colitis (UC) and Chron’s disease (CD) are the most common forms of inflammatory bowel disease (IBD), which affect more than 1.4 million people in the United States.[1] Several extraintestinal manifestations of IBD have been reported, including the formation of renal calculi, either urolithiasis or nephrolithiasis, which occurs in up to 25% to 30% of patients with IBD.[2,3] IBD is linked with malabsorption secondary to bowel resection, primary malabsorption, chronic dehydration, and metabolic disorders, all of which contribute to the development of urinary calculi.[4,5] A lack of treatment might result in an increased risk of recurrent stone formation and impaired renal function.
Urolithiasis occurs when mineral crystals accumulate in the urinary tract, ureters, and urinary bladder.[6] The prevalence of urolithiasis in the general population varies depending on the geographic region and the population studied. In general, the global prevalence of urolithiasis is estimated to be around 1% to 20%, with higher prevalence rates in industrialized countries.[7] In the United States, for example, the prevalence of kidney stones has been reported to be approximately 8.8%.[8] IBD and urolithiasis have long been known to be linked; historical studies revealed that IBD patients had 2- to 3-folded greater rates of symptomatic stone development than the general population.[9] Surgery is the primary treatment option for patients with IBD who are unable to respond to pharmacological therapy (antibiotics and biologics, immunomodulators, and anti-inflammatory drugs).[10,11] Even after surgery for IBD, the risk of urolithiasis remains to be higher. Studies associating IBD with an increased incidence of urolithiasis or nephrolithiasis tend to be outdated or based on a small number of patients.[12–16] A previous meta-analysis showed that up to 22% of IBD patients had urinary complications. Moreover, they demonstrated that patients with IBD had an increased risk ratio (RR) of contracting nephrolithiasis compared to those without IBD (RR = 3.85, 95% confidence interval [CI]: 3.08–4.82). However, this study did not investigate the urinary profile, stone composition, and risk factors of renal stones in patients with IBD. Therefore, this systematic review and meta-analysis aimed to summarize the current literature regarding the prevalence of renal stones in patients with IBD. Moreover, we aimed to evaluate the risk factors of urolithiasis in patients with IBD and the difference between patients with IBD and healthy controls in terms of urinary profile.
2. Methods
We have followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist and Cochrane Handbook for Systematic Reviews of Interventions in reporting this study.[17,18]
2.1. Eligibility criteria
We included the observational studies (case-control, cohort, and cross-sectional) that reported data (prevalence, risk factors, urine profile, and stone composition) regarding the development of renal stones (urolithiasis and nephrolithiasis) in patients with IBD (UC or CD). There were no restrictions regarding country, race, age, gender, or associated comorbidities. We excluded case reports, conference abstracts, and non-English studies.
2.2. Information sources and search strategy
On February 23, 2022, we searched the following databases: MEDLINE via PubMed and OVID, Scopus, and Web of Science, using the relevant keywords to identify the relevant citations. Table S1, Supplemental Digital Content, http://links.lww.com/MD/J100 shows the detailed search term for each database. These databases were searched from inception to the date of search. Moreover, the reference lists of all included citations were searched. The retrieved citations were imported to EndNote X9 software, and duplications were removed.
2.3. Selection process
Using Microsoft Excel software, a screening sheet was created. Study ID, publication year, title, abstract, keywords, digital object identifier, and URL are all included. The selection process was undertaken using a 2-step screening technique by 3 independent reviewers (M.R.A, A.Y.A, and S.F.A). Step 1 was screening the title and abstract of all studies found via the literature search to determine which studies might proceed to step 2 (Full-text screening), where reviewers would read and assess whether each research met eligibility criteria. Any disagreement between the reviewers was solved by the judgment of the study supervisor (A.A).
2.4. Data items and collection process
Four independent reviewers extracted the following data from the included studies to an offline preprepared Excel sheet: Demographic data of the included patients (age, gender, and residency), study characteristics (studies groups, study duration, total sample size, country, and main findings), outcomes (prevalence of renal stones, urine profile of IBD patients, risk factors of developing renal stones, and stone composition).
2.5. Risk of bias and quality assessment
Using the National Institutes of Health (NIH) quality assessment tool for observational cohort, case-control, and cross-sectional studies, 2 authors (S.M.A and N.I.A) independently evaluated the risk of bias and the quality of each included article. Reviewers can critically evaluate the internal validity of research using this tool. Studies were deemed “good,” “fair,” or “poor.” In the case when the authors disagreed on a rating, a third author (A.A) resolved any disagreements.
2.6. Data synthesis
The prevalence of developing renal stones was calculated using the random-effects model with a 95% CI. Using the I2 statistic, we calculated the percentage of heterogeneity and inconsistency between studies, with values of 25%, 50%, and 75% deemed low, moderate, and high, respectively. The random-effect model was employed if the heterogeneity was considerable and I2 > 50%; otherwise, the fixed-effect model was utilized.[19] Comprehensive Meta-analysis was used for all statistical analyses (Comprehensive Meta-analysis; USA: version 3.3.070). To resolve heterogeneity, sensitivity analysis was performed by removing 1 study in each scenario, which is known as sequential sensitivity analysis. Furthermore, subgroup analysis was performed to minimize the risk of inconsistency. To assess the difference between IBD patients and non-IBD in terms of urine profile, we used the Review Manager 5.4 software to calculate the mean difference (MD) between both groups using the Inverse-variance model. Moreover, we applied the Generic Inverse-Variance model to estimate the odds ratio (OR) of reported risk factors for renal stones. Publication bias was assessed based on the criteria of Egger test, and a funnel plot was generated for the forest plots that included 10 studies or more.[20]
3. Results
3.1. Study selection
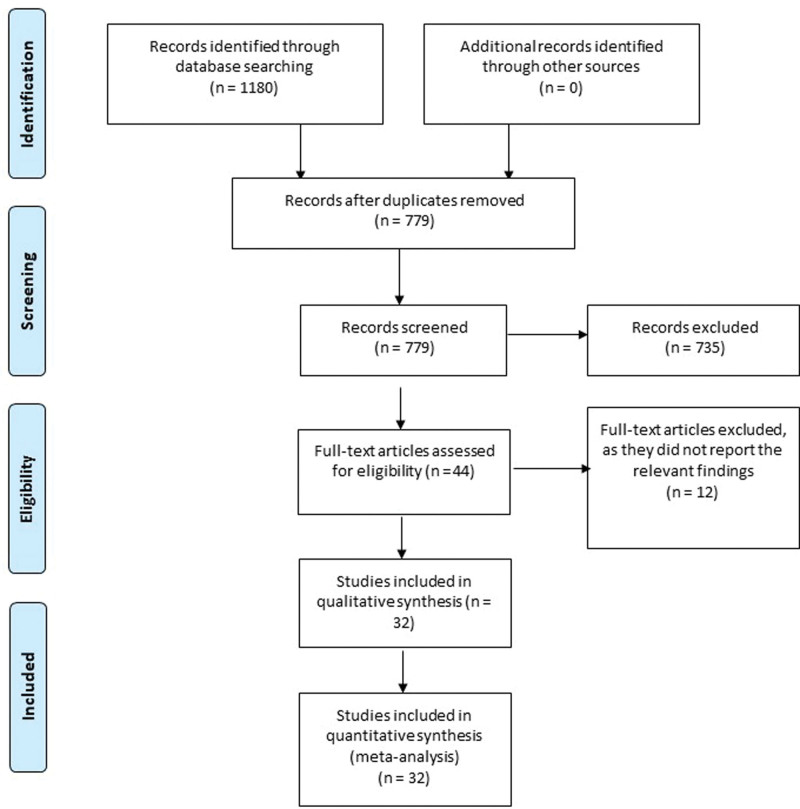
Based on our literature search, we found a total of 1180 relevant citations. After removing duplication, 779 articles underwent title/abstract screening. Then, 735 studies were deemed ineligible to our criteria. The full-text screening was performed on 44 articles, and only 12 studies were excluded. Finally, 32 articles (n = 13,339,065 patients) were included in the qualitative (systematic review) and quantitative synthesis (meta-analysis).[12–16,21–47] Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of included studies.
Figure 1.
PRISMA flow diagram. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
3.2. Characteristics of included studies and patients
Regarding the year of publication of the included studies, it ranged from 1962 to 2021. Eleven studies were conducted in the United States of America (USA), 3 in Denmark, 3 in Germany, 2 in Japan, 2 in Switzerland, and one in each of the following countries Brazil, Greece, Korea, Sweden, Australia, Tunisia, Poland, Scotland, and Spain. The majority of the included studies were cohort (25 studies), and 7 studies were case-control studies. The mean age of the included patients was 42 years (range 8–82) years. More than half of the patients (52.86%) are males across the included studies. The mean body mass index (BMI) was reported only in 6 studies, and it was found to be within the normal range. Among all patients, only 4331 patients underwent bowel surgery. Table 1 summarizes the baseline characteristics of included studies and patients.
Table 1.
Summary of included studies and patients.
| Study ID | Country | Groups | Sample size | Study design | Outcome | Age | Gender (male%) | BMI (mean ± SD) | Number of atient who underwent bowel surgery |
|---|---|---|---|---|---|---|---|---|---|
| Torricelli 2020 | US | IBD | 34 | Case–control | Urine parameters and stone composition | 58.4 ± 12 | 55.88 | 26.6 ± 6.6 | 34 (100) |
| Control | 34 | 58.5 ± 12.0 | 55.88 | 26.5 ± 6.5 | - | ||||
| RUDZIŃSKI 2021 | Poland | UT+ | 110 | Cohort | Association between UT and IBD and stone composition | 57 ± 16 | 53.64 | NR | 110 (100) |
| UT- | 349 | 56 ± 17 | 44.41 | 349 (100) | |||||
| Miyajima 2021 | Japan | UT+ | 34 | Case–control | Risk factors of urolithiasis and stone composition | 44.5 (22–66) | 82.35 | NR | 29 (85.3) |
| UT- | 1037 | 42.0 (11–90) | 68.56 | 586 (56.5) | |||||
| Herzog 2018 | Switzerland | CD | 481 | Cohort | Association between UT and age at disease onset of IBD | <10 to > 40 | 48.86 | NR | 159 (33.056) |
| Stark 2017 | US | CD | 19,730 | Cohort | Association between UT and IBD and risk factors of urolithiasis | 16.09 ± 0.03 | 49.94 | NR | NR |
| UC | 11,177 | 15.7 ± 0.05 | 47.07 | ||||||
| Non-IBD | 8,797,615 | 13.63 ± 0.01 | 50.22 | ||||||
| Fagagnini 2017 | Switzerland | CD | 1333 | Cohort | Association between UT and IBD and Risk factors of urolithiasis | NR | 45.46 | 23.5 (21.1-26.5) | 562 (42.2) |
| UC | 990 | 52.63 | 24.2 (21.7-26.9) | 99 (10) | |||||
| Varda 2015 | US | IBD | 14,352 | Cohort | Association between UT and IBD | <30 to > 80 | 59.70 | NR | NR |
| Non-IBD | 3,573,527 | 61.10 | |||||||
| Kima 2015 | Korea | CD | 387 | Cohort | Prevalence of UT in CD and Risk factors for urolithiasis | 35 (19-72) | 25.06 | NR | 176 (45.48) |
| Cury 2013 | Brazil | CD | 93 | Cohort | Prevalence of UT in IBD and Risk factors for urolithiasis | 41 | 48.39 | NR | 2 (2) |
| UC | 75 | 43 | 25.33 | 0 (0) | |||||
| Boussorra 2013 | Tunisia | CD | 184 | Cohort | Prevalence of UT in CD | 34.7 | 51.63 | NR | NR |
| Hueppelshaeuser 2012 | Germany | CD | 46 | Cohort | Prevalence of UT in CD and urine parameters | 6 to 62 | 63.04 | NR | 15 (32.61) |
| Ishii 2009 | Japan | UT+ | 39 | Cohort | Prevalence of CD and stone composition | NR | 76.92 | NR | 39 (100) |
| UT- | 59 | 77.97 | 59 (100) | ||||||
| PARKS 2003 | NR | IBD | 126 | Cohort | Prevalence of UT in IBD and urine parameters | 44 ± 1 | 84.92 | 96 (76.19) | |
| MCCONNELL 2002 | Scotland | CD | 25 | Case–control | Prevalence of UT in IBD and urine parameters | 39 (18-65) | 40.00 | NR | 11 (44) |
| UC | 15 | 47 (32-71) | 40.00 | 1 (6.67) | |||||
| Non-IBD | 17 | 36 (24-47) | 64.71 | Control | |||||
| Christodoulou 2002 | Greece | CD | 37 | Cohort | Prevalence of UT in IBD | 40.2 ± 11.4 | 59.46 | NR | NR |
| UC | 215 | 54.1 ± 10.1 | 57.67 | ||||||
| SOTO 2001 | Spain | CD | 42 | Case–control | Urine parameters and stone composition | 15 to 72 | 52.38 | NR | 11 (26.19) |
| Control | 18 | 25 to 65 | 44.44 | Control | |||||
| Bohles 1988 | Germany | CD | 86 | Case–control | Prevalence of UT in CD and urine parameters | 31.2 ± 10.55 | 61.63 | NR | NR |
| Control | 53 | 32.37 ± 16.67 | 71.70 | ||||||
| ANDERSSON 1987 | Sweden | CD | 107 | Cohort | Prevalence of UT in CD | NR | 51.40 | NR | 107 (100) |
| KNUDSEN 1978 | Denmark | CD | 140 | Cohort | Prevalence of UT in IBD | 34 (11-73) | 37.86 | NR | 46 (32.86) |
| UC | 88 | 42 (12-74) | 55.68 | 25 (28.41) | |||||
| Fleckenstein 2010 | Denmark | CD | 140 | Cohort | Prevalence of UT in IBD | 39 (11-79) | 37.86 | NR | NR |
| UC | 88 | 39 (10-74) | 55.68 | ||||||
| Shield 1976 | US | UC | 233 | Cohort | Prevalence of UT in IBD | 36.2 | 55.36 | NR | 148 (63.52) |
| Greenstein 1976 | US | IBD | 700 | Cohort | Prevalence of UT in UC | NR | NR | NR | NR |
| UC | 202 | ||||||||
| Farmer 1974 | US | CD | 80 | Case–control | Prevalence of UT in IBD and urine parameters | 36.5 | 52.50 | NR | 41 (51.25) |
| UC | 18 | 41.5 | 55.56 | 10 (55.56) | |||||
| Control | 27 | NR | NR | Control | |||||
| Bennett 1972 | Australia | UC | 458 | Cohort | Prevalence of UT in UC | NR | NR | NR | 333 (72.71) |
| Gelzayd 1969 | US | IBD | 885 | Cohort | Prevalence of UT in UC and stone composition | 25.23 | NR | NR | NR |
| UC | 677 | 27 | |||||||
| Grossman 1967 | US | IBD | 1100 | Case–control | Prevalence of UT in UC | NR | 2.18 | NR | 827 (75.2) |
| UC | 761 | 1.97 | 544 (71.5) | ||||||
| Dreen 1962 | US | UC | 583 | Cohort | Prevalence of UT in UC | NR | NR | NR | NR |
| Simoneaux 1996 | NR | CD | 90 | Cohort | Prevalence of UT in CD | NR | NR | NR | NR |
| Siener 2013 | Germany | UT+ | 10 | Cohort | Prevalence of UT in CD | 56.1 ± 12.6 | 60.00 | 25.5 ± 4.1 | 2 (20) |
| UT- | 41 | 48.2 ± 14.2 | 29.27 | 24.3 ± 4.5 | 10 (24.39) | ||||
| McAuliffe 2015 | US | IBD | 44,574 | Cohort | Prevalence of UT in IBD | 18 to 80 | 49.39 | NR | NR |
| Herbert 2022 | US | CD | 1778 | Cohort | Prevalence of UT in IBD and Risk factors for urolithiasis | 46.6 ± 19.7 | 40.66 | NR | NR |
| UC | 1326 | 46.9 ± 19.7 | 42.91 | ||||||
| Dimke 2020 | Denmark | IBD | 75,236 | Cohort | Prevalence of UT in IBD | NR | 45.62 | NR | NR |
| Non-IBD | 767,403 | 45.90 |
BMI = body mass index, CD = Chron’s disease, IBD = Inflammatory bowel disease, NR = Not reported, UC = Ulcerative colitis, US = United States, UT = Urolithiasis.
3.3. Quality of the included studies
Based on the NIH quality assessment tool for observational cohort studies, about 60% of the studies were deemed as “Good,” and 40% of the studies were deemed as “Fair.” In terms of case-control studies, 28.6% were deemed as “Good,” and 71.4% were deemed as “Fair.” There were no “Poor” studies. Figure S1, Supplemental Digital Content, http://links.lww.com/MD/J101 shows the detailed quality assessment based on the NIH tool.
3.4. Prevalence of renal stones in IBD patients
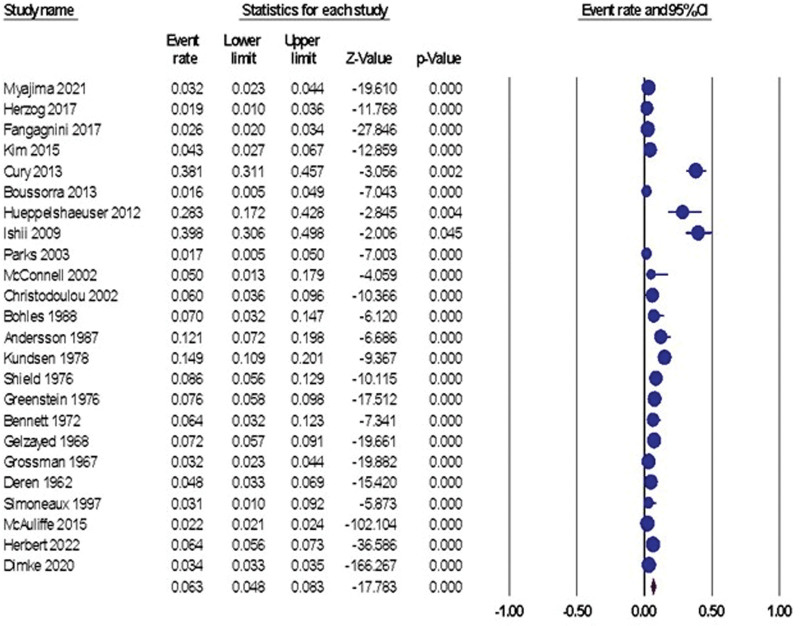
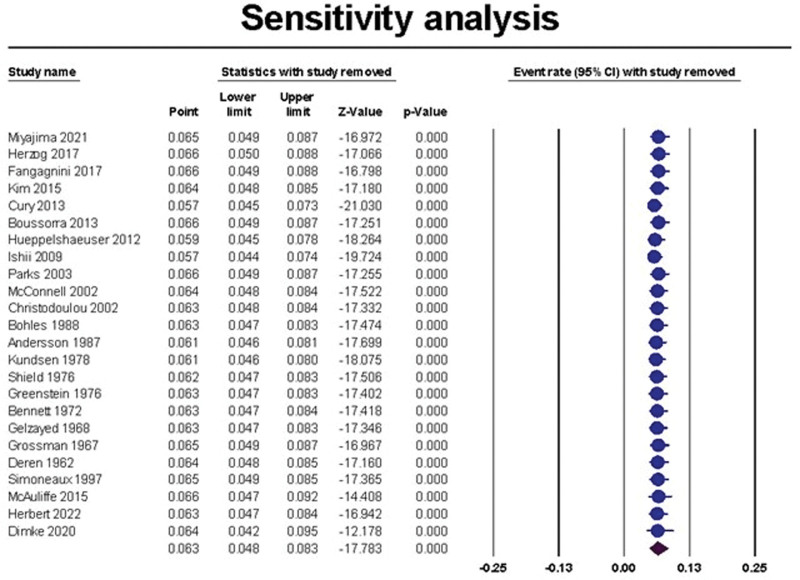
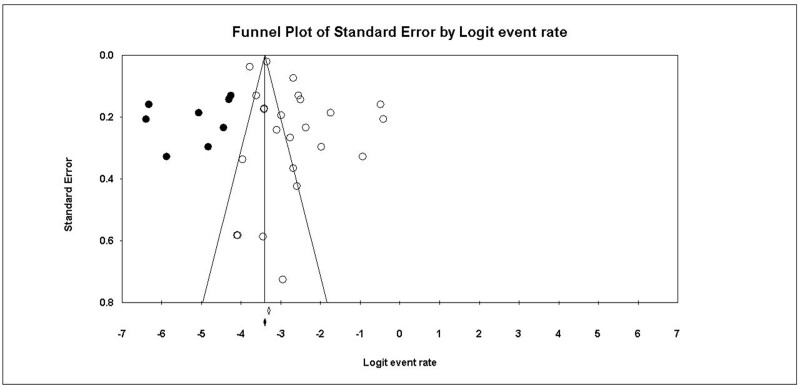
The overall prevalence of renal stones in patients with IBD was 6.3%, 95% CI (4.8% to 8.3%), Figure 2. The pooled data were heterogeneous (I2 = 97.70%, P < .001). To resolve the heterogeneity, sensitivity analysis was performed and demonstrated that Cury et al, 2013, Hueppelshaeuser et al 2012[41], and Ishii et al 2009[42] had the highest effect on the overall effect estimate[39]; by excluding them, the overall prevalence reduced to 5.7%, 5.9%, and 5.7%, respectively, Figure 3. A significant publication bias was detected based on the funnel plot (Fig. 4) and Egger test (P = .017). Trim and fill analysis showed that by trimming 5 studies, the overall prevalence would be 8.1% (5.3% to 12.1%).
Figure 2.
The random-effect forest plot of the pooled prevalence of urolithiasis in patients with IBD. IBD = inflammatory bowel disease.
Figure 3.
The sensitivity analysis of the prevalence of urolithiasis in patients with IBD. IBD = inflammatory bowel disease.
Figure 4.
shows the funnel plot of the pooled studies.
After performing the subgroup analysis to minimize the inconsistency, the random-effect estimate analysis showed that the prevalence of renal stones in CD patients was higher than patients with UC and un-specified IBD [7.9%, 95% CI (3.1%–18.7%), I2 = 95.83%, P < .001], [5.6%, 95% CI (3.9%–7.8%), I2 = 81.62%, P < .001], and [5.6%, 95% CI (3.8%–8.1%), I2 = 98.45%, P < .001], respectively. Based on the sone location, the random-effect model showed that urolithiasis, nephrolithiasis, and both in patients with IBD was [7.1%, 95% CI (3.2%–15.2%), I2 = 99.56%, P < .001], [2.1%, 95% CI (0.3%–12.8%), I2 = 99.65%, P < .001], and [11.0%, 95% CI (6.5%–18.1%), I2 = 50.52%, P = .132], respectively. A subgroup analysis based on the study design demonstrated that the overall prevalence of renal stones in case-control studies was lower than in cohort studies [4.1%, 95% CI (3.0%–5.6%), I2 = 49%, P = .097] and [6.9%, 95% CI (5.0%–9.4%), I2 = 98%, P < .001]. Regarding UC, the prevalence of urolithiasis was 6.8%, 95% CI (3.3%–13.4%), nephrolithiasis 4.2%, 95% CI (2.1%–8.1%), and both 9%, 95% CI (2.1%–31.0%). Regarding CD, the prevalence of urolithiasis was 7.1%, 95% CI (3.6%–13.6%), nephrolithiasis 4.6%, 95% CI (3.6%–5.8%), and both 11.9%, 95% CI (8.4%–16.6%). Based on the year of publications, the overall prevalence of renal stones generated from studies that were published from inception to the end of 2009 was higher than the studies published from 2010 to 2022 [7.3%, 95% CI (4.8%–11.0%), I2 = 92.23%, P < .001] vs [5.2%, 95% CI (3.5%–7.5%), I2 = 98.51%, P < .001], respectively (Table 2).
Table 2.
Prevalence of renal calculi in patients with IBD.
| Subgroup analysis | Studies | Prevalence (95% CI) | Heterogeneity | |
|---|---|---|---|---|
| IBD | CD | 8 | 7.9% (3.1% to 18.7%) | I2 = 95.83%, P < .001 |
| UC | 5 | 5.6% (3.9%–7.8%) | I2 = 81.62%, P < .001 | |
| Un-specified | 11 | 5.6% (3.8%–8.1%) | I2 = 98.45%, P < .001 | |
| Stone location-IBD | Urolithiasis | 5 | 7.1% (3.2–15.2) | I2 = 99.57%, P < .001 |
| Nephrolithiasis | 3 | 2.1% (0.3–12.8) | I2 = 99.65%, P < .001 | |
| Both | 3 | 11.0% (6.5–18.1) | I2 = 50.52%, P = .132 | |
| Stone location-UC | Urolithiasis | 6 | 6.8% (3.3–13.4) | I2 = 98.86%, P < .001 |
| Nephrolithiasis | 2 | 4.2% (2.1–8.1) | I2 = 77.72%, P = .034 | |
| Both | 3 | 9% (2.1–31.0) | I2 = 45.82%, P = .158 | |
| Stone location-CD | Urolithiasis | 6 | 7.1% (3.6–13.6) | I2 = 99.17%, P < .001 |
| Nephrolithiasis | 2 | 4.6% (3.6–5.8) | I2 = 0.00%, P = .812 | |
| Both | 3 | 11.9% (8.4–16.6) | I2 = 0.00%, P = .774 | |
| Study design | Cohort | 19 | 6.9% (5.0%–9.4%) | I2 = 98.19%, P < .001 |
| Case-control | 5 | 4.1% (3.0%–5.6%) | I2 = 49.02%, P = .097 | |
| Year of publication | From inception to the end of 2009 | 14 | 7.3% (4.8%–11.0%) | I2 = 92.23%, P < .001 |
| From 2010–2022 | 10 | 5.2% (3.5%–7.5%) | I2 = 98.51%, P < .001 | |
CD = Chron’s disease, CI = Confidence interval, IBD = inflammatory bowel disease, UC = Ulcerative colitis.
3.5. Urine profile of IBD patients vs non-IBD
Patients with IBD were associated with significantly lower urine volume (MD = −518.84 mL/day, 95% CI: −707.36 to −330.33, P < .00001) compared to non-IBD patients. The pooled data were homogenous (I2 = 0%, P = .77). Moreover, IBD patients were associated with significantly lower calcium 24-hour urine level (MD = −28.46 mg/day, 95% CI: −41.67 to −15.25, P < .0001), lower citrate 24-hour urine level (MD = −144.35 mg/day, 95% CI: -−198.96 to −89.75, P < .00001), lower sodium 24-hour urine level (MD = −23.72 mg/day, 95% CI: −46.24 to −1.19, P = .04), and lower magnesium 24-hour urine level (MD = −33.25 mg/day, 95% CI: −44.16 to −22.34, P < .00001), compared to non-IBD patients. On the other hand, both IBD and non-IBD patients showed comparable findings in terms of phosphate 24-hour urine level (MD = 261.88 mg/day, 95% CI: −89.94 to 613.69, P = .14) and uric acid 24-hour urine level (MD = −41.55 mg/day, 95% CI: −88.24 to 5.15, P = .08).
3.6. Risk factors of urolithiasis in patients with IBD
Pooled analysis of Inverse Generic variance showed that patients with IBD and a history of intestinal surgery were associated with a higher risk of developing urolithiasis (OR = 2.82, 95% CI: 2.122–3.525). A similar finding was observed for patients who never do physical activity (OR = 1.65, 95% CI: 1.62–1.68), male gender (OR = 1.65, 95% CI: 1.62–1.68), and history of glucocorticoid therapy (OR = 1.689, 95% CI: 1.300–2.026), ciprofloxacin (OR = 5.82, 95% CI: 2.60–9.04), and immunomodulatory (OR = 4.05, 95% CI: 2.23–5.87). On the other hand, we found a nonsignificant association between developing urolithiasis and age (OR = 1.056, 95% CI: 0.947–1.164), type of IBD (OR = 1.056, 95% CI: 0.467–1.645), and 5-aminosalicylic acid concurrent medication (OR = 0.996, 95% CI: 0.976–1.016). Individual studies[29,33,35,36,38,39] showed that there was a significant association between developing urolithiasis and the disease duration of IBD (OR = 1.03, 95% CI: 1.01–1.05), the presence of fistula, fissure, or abscess (OR = 2.01, 95% CI: 1.32–3.07), existence of stenosis (OR = 1.82, 95% CI: 1.18–2.8), NSAID intake (OR = 2.334, 95% CI: 1.415–3.851), activity index (OR = 1.032, 95% CI: 1.018–1.045), active UC (OR = 4.2, 95% CI: 1.1–15), white race (OR = 1.49, 95% CI: 1.087–2.048), number of bowel resections (OR = 1.415, 95% CI: 1.17–1.71), and CD treatment period (OR = 1.076, 95% CI: 1.04–1.113), Table 3.
Table 3.
Risk factors of urolithiasis in patients with IBD.
| Risk factors | Studies | OR (95% CI) | Heterogeneity |
|---|---|---|---|
| Age (old vs young) | 3 | 1.056 (0.947–1.164) | I2 = 99.60%, P < .001 |
| Intestinal surgery (Yes) | 2 | 2.824 (2.122–3.525) | I2 = 0.00%, P = .733 |
| Physical activity (Never) | 2 | 1.650 (1.620–1.680) | I2 = 49.53%, P = .159 |
| Gender (Male) | 3 | 1.650 (1.620–1.680) | I2 = 0.00%, P = .371 |
| Type of IBD (UC vs CD) | 3 | 1.056 (0.467–1.645) | I2 = 92.86%, P < .001 |
| History of glucocorticoid therapy (Yes) | 3 | 1.689 (1.300–2.026) | I2 = 35.37%, P = .213 |
| Concurrent medication (5-ASA) | 2 | 0.996 (0.976–1.016) | I2 = 0.73%, P = .316 |
| Ciprofloxacin | 2 | 5.821 (2.602–9.040) | I2 = 0.00%, P = .327 |
| Immunomodulatory | 2 | 4.047 (2.227–5.868) | I2 = 0.00%, P = .872 |
5-ASA = 5-aminosalicylic acid, CD = Chron’s disease, CI = Confidence interval, IBD = Inflammatory bowel disease, OR = Odds ratio, UC = Ulcerative colitis.
3.7. Stone composition
Regarding stone composition, individual studies[16,31,33,42] demonstrated that calcium oxalate and uric acid stones were more common in IBD patients at 71.4% and 21.4% compared to non-IBD 56.25% and 18.8%, respectively. Calcium phosphate, cystine, and struvite stones were more common in non-IBD patients than in IBD patients, 12.5%, 6.2%, and 6.2% versus 7.1%, 0%, and 0%, respectively.
4. Discussion
In this systematic review and meta-analysis, our findings showed that the overall prevalence of renal stones in patients with IBD was 6.3%, 95% CI (4.8%–8.3%). The prevalence of renal stones in CD patients was higher than in patients with UC and un-specified IBD. CD patients may be more susceptible to urolithiasis because their digestive systems are more severely compromised.[44] A metabolic change that has been linked to an increased risk of oxalate stones in individuals with IBD is called hyperoxaluria, and it is more common in those who have ileal dysfunction as well as those who have a CD.[5,45,46]
Patients with IBD were associated with significantly lower urine volume, calcium 24-hour urine, citrate 24-hour urine, sodium 24-hour urine, and magnesium 24-hour urine, compared to non-IBD patients. Moreover, we found that the most common stones in IBD patients were calcium oxalate and uric acid stones. One of the possible explanations for the elevated risk of calcium oxalate stone formation is that bile salt malabsorption increases urine oxalate excretion. Oxalate binds to calcium in the intestinal lumen, limiting the quantity of oxalate absorbed in the intestines under normal circumstances.[47] Patients with a compromised or resected ileum are more likely to suffer from steatorrhea because bile salts are inadequately reabsorbed in the intestines. The increased amount of absorbed oxalate in the intestines is a result of the binding between the luminal-free calcium and the unabsorbed fats in the steatorrhea.[48] Increased endogenous production of oxalate, gastrointestinal hyperabsorption, and obesity all contribute to hyperoxaluria, which may be induced by high intake or hyperabsorption.[45,46] Enteric hyperoxaluria is a complication of severe chronic bowel disease, particularly when fat absorption is impaired, and intestinal oxalate absorption is subsequently elevated.[45,49] Besides the presence of hyperoxaluria, additional variables that may contribute to the development of kidney stones in these individuals include reduced excretion in the urine of inhibitors of crystallization (citrate, magnesium), dietary factors, medications, and a low volume of urine,[42] which was observed in our study, as we found that patients with IBD were associated with significantly lower urine volume, calcium 24-hour urine, citrate 24-hour urine, sodium 24-hour urine, and magnesium 24-hour urine, compared to non-IBD patients. Urate stones have been linked to both long-term diarrheal diseases and small intestinal ostomies.[7] Small intestinal ostomies are thought to cause urate stones because of metabolic acidosis and dehydration.[50] Urate stones may occur even if the patient’s urate level is not increased because of the acidic urine that arises from bicarbonate loss and intestinal fluid.[51]
Poor socioeconomic level, gout, DM, high BMI, and male gender are all common risk factors for kidney stones in the general population [34]. IBD patients, on the other hand, had a somewhat distinct set of risk variables. A previous history of intestinal surgery, no physical activity, male gender, history of glucocorticoid therapy, ciprofloxacin, and immunomodulatory were associated with a higher risk of developing urolithiasis. Anatomical changes to the gastrointestinal system as a consequence of bowel surgery may either reduce food intake or result in nutritional malabsorption. Urolithiasis risk may be increased by bariatric surgery, even though obesity is an independent risk factor for developing stones. Stone formation is more likely to occur with restrictive operations than malabsorptive ones, which seem to provide the lowest risk.[52] Physical exercise reduces the risk of urinary stone development, but the exact mechanism by which this occurs is still a mystery. Bone resorption, hypercalciuria, and the risk of urolithiasis are all exacerbated by prolonged bed rest.[53] A recent observational study reported that among postmenopausal women, increased levels of physical activity and lower energy intake were associated with a significant reduction in the risk of kidney stones, even after taking into account animal protein, dietary sodium, calcium, intake of fluid, history of diabetes, and BMI.[54] However, a large cohort study (n = 215,133 patients) showed that there was no significant independent association between physical activity and urolithiasis [hazard ratio = 1.00, 95% CI: 0.87–1.14, P = .94].[55] A meta-analysis of 13 cohorts demonstrated that both high and low physical activity were comparable in terms of the risk of urolithiasis (RR = 0.93, 95% CI: 0.78–1.10).[56] A more recent systematic review also supports the hypothesis of no significant association between physical activity and the risk of urolithiasis.[57] The difference between our study and these studies is that they investigate the association in the general population, but in this study, we investigate it in the selected group of patients (IBD patients). Regarding the male gender, many studies have confirmed that males have a higher risk of urolithiasis than females. Wang et al showed that men contributed more calcium oxalate stones than women at age 30 to 49 years (P < .01) and more uric acid stones at 30 to 59 years (P < .05). Moreover, they reported that the prevalence peak was 50 to 59 years in men and 60 to 69 years in women, and both genders had the lowest prevalence in adolescence.[58] However, a recent systematic review highlighted that in recent years, a significant change had been observed. They claimed that the rise in prevalence of urolithiasis is higher in females compared to males, even though males are more likely to have metabolic and nutritional disorders than females. Furthermore, they showed that uric acid supersaturation in males is more common, men excrete more calcium and oxalate than women, and their urine pH is lower.[59]
Medication may also cause urinary calculi when the drugs crystallize and become the main component of the stones. In this situation, the agent’s urinary supersaturation may encourage the production of calculi. The agent’s urinary supersaturation may encourage the development of calculi in this situation. In the literature, the most common drugs that induce urolithiasis were ephedrine, indinavir, triamterene, sulfa medications, ciprofloxacin, magnesium trisilicate, and corticosteroids.[60] In this study, we could not find any significant association between urolithiasis and receiving 5-aminosalicylic acid, Azathioprine and 6-mercaptopurine, and anti-Tumor necrosis factor agents. However, a significant association between urolithiasis and corticosteroids, ciprofloxacin, and immunomodulatory was highlighted.
This is the first systematic review and meta-analysis that investigate the prevalence and associations of urolithiasis in patients with IBD. We included a large number of studies with a huge number of patients, which may support the generalizability of our findings. We acknowledge that our study has some limitations, including the high heterogeneity observed in the prevalence analysis; however, this heterogeneity is expected in this type of analysis due to the significant variation in the year of publication and the country of population. Another limitation is the significant publication bias, which was handled by trim and fill analysis.
5. Conclusion
The overall prevalence of renal stones in patients with IBD was comparable to the general population. Patients with CD were associated with a higher prevalence of urolithiasis compared to UC. A previous history of intestinal surgery, no physical activity, male gender, history of glucocorticoid therapy, ciprofloxacin, and immunomodulatory were associated with a higher risk of developing urolithiasis. Patients with IBD were associated with significantly lower urine volume, calcium 24-hour urine, citrate 24-hour urine, sodium 24-hour urine, and magnesium 24-hour urine, compared to non-IBD patients.
Acknowledgments
We would like to thank Noha Farouk Tashkandi and her program “Research Platform” for their efforts in facilitating the process of this research.
Author contributions
Conceptualization: Abdulrhman Aldukhayel, Nurah Ibrahem Alhmed.
Data curation: Adil Alsweed, May Rashed Alotaibi, Shahad Fahad Alahmadi.
Formal analysis: Abdulrhman Aldukhayel, Adil Alsweed, May Rashed Alotaibi, Abdullah Yousef Aldakhil, Shahad Fahad Alahmadi, Saud Musallum Albishri, Nurah Ibrahem Alhmed.
Investigation: Abdulrhman Aldukhayel, Adil Alsweed.
Methodology: Abdulrhman Aldukhayel, Adil Alsweed.
Software: Abdulrhman Aldukhayel, May Rashed Alotaibi.
Supervision: Abdulrhman Aldukhayel.
Validation: Abdulrhman Aldukhayel.
Visualization: Adil Alsweed.
Writing – original draft: Abdulrhman Aldukhayel, Adil Alsweed, May Rashed Alotaibi, Abdullah Yousef Aldakhil, Shahad Fahad Alahmadi, Saud Musallum Albishri, Nurah Ibrahem Alhmed.
Writing – review & editing: Abdulrhman Aldukhayel, Adil Alsweed, May Rashed Alotaibi, Abdullah Yousef Aldakhil, Shahad Fahad Alahmadi, Saud Musallum Albishri, Nurah Ibrahem Alhmed.
Supplementary Material
Abbreviations:
- BMI
- body mass index
- CD
- Chron’s disease
- CI
- confidence interval
- IBD
- inflammatory bowel disease
- MD
- mean difference
- NIH
- National Institutes of Health
- OR
- odds ratio
- UC
- ulcerative colitis
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Abdulrhman A, Alsweed A, Alotaibi MR, Aldakhil AY, Alahmadi SF, Albishri SM, Alhmed NI. Urolithiasis in patients with inflammatory bowel disease: A systematic review and meta-analysis of 13,339,065 individuals. Medicine 2023;102:24(e33938).
Contributor Information
Adil Alsweed, Email: Adilalsweed@gmail.com.
May Rashed Alotaibi, Email: Mayrashed.21@gmail.com.
Abdullah Yousef Aldakhil, Email: Abdullahyaldakhil@gmail.com.
Shahad Fahad Alahmadi, Email: shahad.f.alahmadi@gmail.com.
Saud Musallum Albishri, Email: Abogla2018@gmail.com.
Nurah Ibrahem Alhmed, Email: IBrahemnurah@gmail.com.
References
- [1].M’Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol. 2013;6:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:235–41. [PMC free article] [PubMed] [Google Scholar]
- [3].da Silva Gaspar SR, Mendonça T, Oliveira P, et al. Urolithiasis and Crohn’s disease. Urol Ann. 2016;8:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bianchi L, Gaiani F, Bizzarri B, et al. Renal lithiasis and inflammatory bowel diseases, an update on pediatric population. Acta Biomed. 2018;89(9-S):76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gkentzis A, Kimuli M, Cartledge J, et al. Urolithiasis in inflammatory bowel disease and bariatric surgery. World J Nephrol. 2016;5:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Copelovitch L. Urolithiasis in children: medical approach. Pediatr Clin North Am. 2012;59:881–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12:e86–96. [PMC free article] [PubMed] [Google Scholar]
- [8].Litwin MS, Saigal CS, Yano EM, et al. Urologic diseases in America project: analytical methods and principal findings. J Urol. 2005;173:933–7. [DOI] [PubMed] [Google Scholar]
- [9].Abou-Elela A. Epidemiology, pathophysiology, and management of uric acid urolithiasis: a narrative review. J Adv Res. 2017;8:513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gade AK, Douthit NT, Townsley E. Medical management of Crohn’s disease. Cureus. 2020;12:e8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Farmer RG, Hossein Mir Madjlessi SH, Kiser WS. Urinary excretion of oxalate, calcium, magnesium, and uric acid in inflammatory bowel disease. Relationship to urolithiasis. Cleve Clin Q. 1974;41:109–17. [DOI] [PubMed] [Google Scholar]
- [13].Shield DE, Lytton B, Weiss RM, et al. Urologic complications of inflammatory bowel disease. J Urol. 1976;115:701–6. [DOI] [PubMed] [Google Scholar]
- [14].Bennett RC, Hughes ESR. Urinary calculi and ulcerative colitis. Br Med J. 1972;2:494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore). 1976;55:401–12. [DOI] [PubMed] [Google Scholar]
- [16].Gelzayd EA, Breuer RI, Kirsner JB. Nephrolithiasis in inflammatory bowel disease. Am J Dig Dis. 1968;13:1027–34. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Vol. Version 5., The Cochrane Collaboration; 2008. [Google Scholar]
- [18].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Higgins J, Thomas J, editors. Part 2: general methods for atient reviews. In: Cochrane Handbook for Systematic Reviews of Interventions; 2022. [Google Scholar]
- [20].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Andersson H, Bosaeus I, Fasth S, et al. Cholelithiasis and urolithiasis in Crohn’s disease. Scand J Gastroenterol. 1987;22:253–6. [DOI] [PubMed] [Google Scholar]
- [22].Knudsen L, Marcussen H, Fleckenstein P, et al. Urolithiasis in chronic inflammatory bowel disease. Scand J Gastroenterol. 1978;13:433–6. [DOI] [PubMed] [Google Scholar]
- [23].Fleckenstein P, Knudsen L, Pedersen EB, et al. Obstructive uropathy in chronic inflammatory bowel disease. Scand J Gastroenterol. 1977;12:519–23. [DOI] [PubMed] [Google Scholar]
- [24].Grossman MS, Warren Nugent F. Urolithiasis as a complication of chronic diarrheal disease. Am J Dig Dis. 1967;12:491–8. [DOI] [PubMed] [Google Scholar]
- [25].Deren JJ, Porush JG, Levitt MF, et al. Nephrolithiasis as a complication of ulcerative colitis and regional enteritis. Ann Intern Med. 1962;56:843–53. [DOI] [PubMed] [Google Scholar]
- [26].Simoneaux SF, Patrick LE. Genitourinary complications of Crohn’s disease in pediatric patients. AJR Am J Roentgenol. 1997:197–9. [DOI] [PubMed] [Google Scholar]
- [27].Siener R, Petzold J, Bitterlich N, et al. Determinants of urolithiasis in patients with intestinal fat malabsorption. Urology. 2013;81:17–24. [DOI] [PubMed] [Google Scholar]
- [28].McAuliffe ME, Lanes S, Leach T, et al. Occurrence of adverse events among patients with inflammatory bowel disease in the health core integrated research database. Curr Med Res Opin. 2015;31:1655–64. [DOI] [PubMed] [Google Scholar]
- [29].Herbert J, Teeter E, Burstiner LS, et al. Urinary manifestations in African American and Caucasian inflammatory bowel disease patients: a retrospective cohort study. BMC Urol. 2022;22:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dimke H, Winther-Jensen M, Allin KH, et al. Risk of urolithiasis in patients with inflammatory bowel disease: a nationwide atien cohort study 1977–2018. Clin Gastroenterol Hepatol. 2021;19:2532–2540.e2. [DOI] [PubMed] [Google Scholar]
- [31].Torricelli FC, Reichard C, Monga M. Urolithiasis in complicated inflammatory bowel disease: a comprehensive analysis of urine profile and stone composition. Int Urol Nephrol. 2021;53:205–9. [DOI] [PubMed] [Google Scholar]
- [32].Rudziński M, Ławiński M, Gradowski L, et al. Kidney stones are common in patients with short-bowel syndrome receiving long-term parenteral nutrition: a predictive model for urolithiasis. J Parenter Enter Nutr. 2022;46:671–7. [DOI] [PubMed] [Google Scholar]
- [33].Miyajima S, Ishii T, Watanabe M, et al. Risk factors for urolithiasis in patients with Crohn’s disease. Int J Urol. 2021;28:220–4. [DOI] [PubMed] [Google Scholar]
- [34].Herzog D, Fournier N, Buehr P, et al. Age at disease onset of inflammatory bowel disease is associated with later extraintestinal manifestations and complications. Eur J Gastroenterol Hepatol. 2018;30:598–607. [DOI] [PubMed] [Google Scholar]
- [35].Stark CM, Gorman GH, Nylund CM. Association of inflammatory bowel disease and urolithiasis in hospitalized pediatric patients. Inflamm Bowel Dis. 2017;23:1777–82. [DOI] [PubMed] [Google Scholar]
- [36].Fagagnini S, Heinrich H, Rossel JB, et al. Risk factors for gallstones and kidney stones in a cohort of patients with inflammatory bowel diseases. PloS One. 2017;12:e01851931–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Varda BK, McNabb-Baltar J, Sood A, et al. Urolithiasis and urinary tract infection among patients with inflammatory bowel disease: a review of US emergency department visits between 2006 and 2009. Urology. 2015;85:764–70. [DOI] [PubMed] [Google Scholar]
- [38].Kim MJ, Woo SY, Kim ER, et al. Incidence and risk factors for urolithiasis in patients with Crohn’s disease. Urol Int. 2015;95:314–9. [DOI] [PubMed] [Google Scholar]
- [39].Bismara Cury D, Moss AC, Schor N. Nephrolithiasis in patients with inflammatory bowel disease in the community. Int J Nephrol Renovasc Dis. 2013;6:139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Boussorra H, Sallami S, Said Y, et al. Evaluation of urolithiasis in Crohn’s disease in Tunisian patients. Tunis Med. 2013;91:440–3. [PubMed] [Google Scholar]
- [41].Hueppelshaeuser R, Von Unruh GE, Habbig S, et al. Enteric hyperoxaluria, recurrent urolithiasis, and systemic oxalosis in patients with Crohn’s disease. Pediatr Nephrol. 2012;27:1103–9. [DOI] [PubMed] [Google Scholar]
- [42].Ishii G, Nakajima K, Tanaka N, et al. Clinical evaluation of urolithiasis in Crohn’s disease. Int J Urol. 2009;16:477–80. [DOI] [PubMed] [Google Scholar]
- [43].Parks JH, Worcester EM, Corey O’Connor R, et al. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int. 2003;63:255–65. [DOI] [PubMed] [Google Scholar]
- [44].McConnell N, Campbell S, Gillanders I, et al. Risk factors for developing renal stones in inflammatory bowel disease. BJU Int. 2002;89:835–41. [DOI] [PubMed] [Google Scholar]
- [45].Christodoulou DK, Katsanos KH, Kitsanou M, et al. Frequency of extraintestinal manifestations in patients with inflammatory bowel disease in Northwest Greece and review of the literature. Dig Liver Dis. 2002;34:781–6. [DOI] [PubMed] [Google Scholar]
- [46].Buño Soto A, Torres Jiménez R, Olveira A, et al. Lithogenic risk factors for renal stones in patients with Crohn’s disease. Arch Esp Urol. 2001;54:282–92. [PubMed] [Google Scholar]
- [47].Böhles H, Beifuss OJ, Brandl U, et al. Urinary factors of kidney stone formation in patients with Crohn’s disease. Klin Wochenschr. 1988;66:87–91. [DOI] [PubMed] [Google Scholar]