Abstract
The intestinal barrier is a complex structure that allows the absorption of nutrients while ensuring protection against intestinal pathogens and balanced immunity. The development and maintenance of a functional intestinal barrier is a multifactorial process that is only partially understood. Here we review novel findings on the emerging role of mesenchymal cells in this process using insights gained from lineage tracing approaches, Cre-based gene deletion, and single-cell transcriptomics. The current evidence points toward a key organizer role for distinct mesenchymal lineages in intestinal development and homeostasis, regulating both epithelial and immune components of the intestinal barrier. We further discuss recent findings on functional mesenchymal heterogeneity and implications for intestinal regeneration and inflammatory intestinal pathologies.
INTRODUCTION
The intestine constitutes a surface barrier between the host and the environment, which contains an abundant microbiota, food antigens, and potential pathogens. As cells at barrier sites are constantly exposed to microbial and damaging elements that may compromise tissue function, a major challenge is to efficiently eliminate pathogens and promote repair while avoiding chronic inflammation. Dysregulation of the intestinal barrier leads to pathological responses to tissue damage and the microbiota, which is associated with several chronic diseases and systemic disorders, including inflammatory bowel diseases (IBDs), intestinal fibrosis, and cancer.
A functional intestinal barrier requires a tightly regulated crosstalk between a self-renewing epithelial layer and a subepithelial compartment containing innate and adaptive immune cells, blood vessels, and stromal mesenchymal cells (MCs). The intestinal epithelial layer continuously self-renews from intestinal epithelial stem cells (IESCs) located in the crypts1. IESCs generate proliferating transit-amplifying (TA) cells that differentiate into distinct epithelial lineages, including absorptive enterocytes, enteroendocrine cells (EECs), antimicrobial peptide-secreting Paneth cells, and mucus-producing Goblet cells, altogether providing a first line of intestinal defense2, 3. Terminally differentiated epithelial cells undergo apoptosis at the villus tip and are shed into the intestinal lumen or sampled by phagocytes in the small intestine lamina propria (LP)2, 4. Intestinal homeostasis requires WNT/β–catenin signaling and pro-stemness factors to maintain IESCs in the crypts and bone morphogenetic proteins (BMPs) along the crypt-villus axis to promote epithelial differentiation5. Paneth cells are closely adjacent to IESCs and produce the WNT ligand WNT3A, Notch ligands (DLL4, DLL1), and epidermal growth factor (EGF)6, suggesting that they constitute the niche for IESCs. However, in vivo depletion of Paneth cells or ablation of Wnt3, or Porcn, and Wls (essential for WNT secretion) in epithelial cells did not impact intestinal homeostasis7, 8, 9, 10. In the colon, which lacks Paneth cells, deep crypt secretory cells activate Notch signaling in IESCs11 but do not produce canonical WNT ligands, which are indispensable for IESCs. These findings support the existence of non-epithelial sources for WNT signals and other factors required for IESCs.
Beneath the intestinal epithelium lies the LP. The LP contains a dense network of MCs, which include subepithelial, perivascular, and interstitial mesenchymal populations, as well as fibroblastic reticular cells (FRCs) of the gut-associated lymphoid tissues (GALTs)12, 13. As their distinct localization suggests, MCs interact with various cell populations, including epithelial, immune, and vascular cells, and are essential to support their function. When dysregulated, MCs promote chronic inflammation, fibrosis, and cancer14, 15, 16. MCs lack expression of hematopoietic (CD45), endothelial (CD31), and epithelial (EpCAM) markers; however, the resulting CD45−CD31−EpCAM− population, historically called fibroblast (αSMA−) or myofibroblast (αSMAlow), is neither homogeneous nor defining one single cell type14. Additional markers, such as platelet-derived growth factor receptor alpha (PDGFRα), Podoplanin (PDPN, GP38), or Thy1 (CD90), can be useful in visualizing the extensive and diverse mesenchymal network of the LP, as they are broadly expressed by several mesenchymal populations. Due to paucity of specific markers, intestinal MCs were mostly studied in vitro until recently, either from bulk primary cells isolated from organs and selected based on their adherence and growth capacity or using cell lines. Although these studies provided important information, notably on the crosstalk with immune cells17, they were not reflecting the heterogeneity and functional diversity of the mesenchymal compartment in vivo.
The recent expansion of single-cell transcriptomics (scRNAseq) and development of new mouse models allowing for genetic lineage tracing and Cre-based gene/cell depletion of mesenchymal subsets is shedding new light on the functional diversity of intestinal MCs in vivo (Table 1). The resulting evidence suggests a complex mesenchymal microenvironment, with emerging key roles in postnatal maturation of the intestinal barrier, mucosal immunity, and tissue repair. We will further discuss these results in the context of intestinal inflammatory diseases, as increasing evidence suggests a role for MCs as information relays, responding to immune cell-derived signals, and directing immune function.
Table 1.
Mice models and genetic modifications to assess the function of stromal subsets in intestinal homeostasis and disease.
| Mouse model | Genetic modification | Deletion (days) | Model | Impact | Reference |
|---|---|---|---|---|---|
| Ccl19-cre |
Myd88flox Il15flox |
from conception | Infection (MHV/C. Rodentium) | Antiviral ILC1 and NK cell responses in PPs/mLNs Intestinal inflammation |
61 |
|
Ccl19-cre Col6a1-cre |
Ltbrflox Tnfr1flox Tnfr1cneo/− |
from conception | Steady-state Infection (M-CoV) |
PPs development/stromal organization Antiviral immune response |
77 |
|
Col6a1-cre Twist2-cre |
Tnfsf11flox | from conception | Steady-state | Intestinal immune homeostasis | 59, 60 |
| Col6a1-cre |
TnfΔARE Tnfr1flxneo |
from conception | Steady-state | Intestinal inflammation Joint inflammation |
105 |
| Col6a1-cre | Rosa26flstopiDTR | Adult (3d) | Steady-state Inflammation (DSS) |
Reduced enteroendocrine cells/homeostatic proliferation No effect on intestinal regeneration |
21 |
| Col1a2-creERT2 | Map3k2flox | Adult (10d) | Inflammation (DSS) | Intestinal inflammation | 99 |
|
Foxl1-cre Foxl1-hDTR |
Rosa26flstopiDTR | Adult (3d) | Steady-state | Reduced intestinal stem/progenitor cells | 33 |
| Foxl1-creERT2 | Porcnflox | Adult (1-3d) | Steady-state | Reduced intestinal stem/progenitor cells | 35 |
| Gli1-creERT2 | Wlsflox | Adult (15-21d) | Steady-state | Reduced intestinal stem/progenitor cells | 34 |
| Grem1-creERT2 | Rosa26flstopiDTR | Adult (2d) | Steady-state | Intestinal damage /lethal | 41 |
| Lepr-cre |
Rosa26flstopiDTR Igf1flox |
Adult (5-10d) From conception |
Steady-state Irradiation |
Reduced intestinal stem/progenitor cells Impaired intestinal regeneration |
49 |
| Lgr5-creERT2 | Rosa26flstopDTA | Adult (7d) | Steady-state | Dysregulated villus tip VEGFA signaling | 52 |
| Lgr5-GFP-DTR | Adult (1d) | Steady-state | Dysregulated villus tip enterocytes gene expression | 51 | |
|
LtbrtTA x tetO-cre (LC1) |
Pdgfraflox | from birth until 28d | Postnatal P28 Inflammation (DSS/ Indo) |
Increased intestinal stem/progenitor cells Reduced Goblet cells Dysregulated inflammation and repair responses |
85 |
| Pdgfra-cre |
Porcnflox Rspo3flox |
from conception |
Postnatal P5 Steady-state Inflammation (DSS) |
Reduced intestinal stem/progenitor cells Reduced Paneth cells Impaired intestinal regeneration |
30 |
|
Pdgfra-cre Grem1-creERT2 Col1a-creERT2 |
Il1r1flox | from conception Adult |
Infection (C. Rodentium) Inflammation (DSS) |
Intestinal inflammation/regeneration | 100 |
|
Twist2-cre Myh11-cre |
MiR-143/145flox | from conception | Inflammation (DSS) | Impaired intestinal regeneration | 92 |
DTR = diphtheria toxin receptor; DSS = Dextran sulfate sodium; Indo = Indomethacin; M-CoV = Murine coronavirus; MHV = Mouse hepatitis virus; ILC1 = Innate lymphoid cells 1; NK = Natural killer; mLNs = mesenteric lymph nodes; PPs = Peyer’s patches .
Mesenchymal-epithelial crosstalk in intestinal morphogenesis
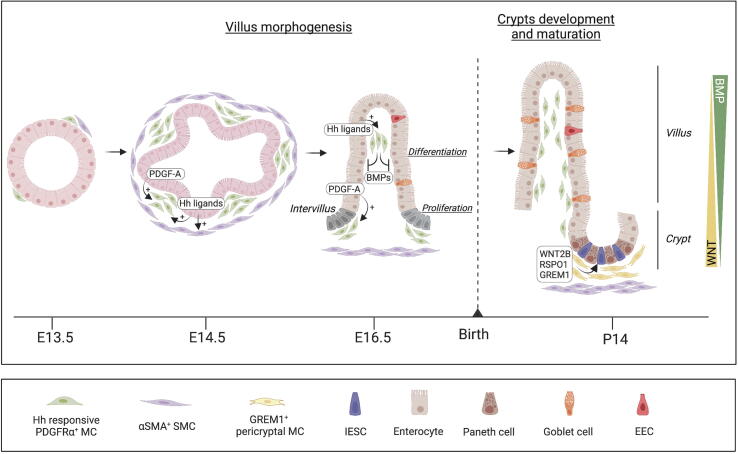
A bidirectional mesenchymal-epithelial crosstalk is required for intestinal development. Intestinal morphogenesis starts in the embryo and follows a similar order of events in most vertebrates. Hedgehog (Hh) activity directs several intestinal patterning events, such as left/right patterning, radial patterning, smooth muscle differentiation, mesenchymal expansion, and villus morphogennesis18. The Hh signaling is paracrine, as Hh ligands are produced by epithelial cells, whereas their main receptor Ptch1 is exclusively non-epithelial. Starting at E13.5 in mice (weeks 8–10 in humans), the mesenchymal-epithelial crosstalk results in the formation of villi, which represents a milestone in intestinal development18. Villus formation massively increases the intestinal epithelial surface area, which will be required for sufficient nutrient absorption. Before villus morphogenesis, the pseudostratified epithelium is uniformly proliferative, but upon villi formation, epithelial proliferation becomes restricted to intervillous areas. Initiation of villus morphogenesis starts through the formation of subepithelial mesenchymal clusters, initiated by Hh ligands secreted by the intestinal epithelium. The villus mesenchymal clusters, visualized using PDPN or COL6A1, express PDGFRα, PTCH1, and the transcription factors GLI1 and GLI2, which leads to BMP2 and BMP4 expression, blocking epithelial proliferation above each mesenchymal cluster18, 19, 20, 21, while epithelial cells located between adjacent clusters remain proliferative. Several rounds of villus morphogenesis occur as new mesenchymal clusters form adjacent to the intervillous domain (Fig. 1). The Hh pathway is critical for the initiation of MC clustering, whereas both Hh ligands and PDGF-A secreted by the epithelium are essential for subsequent mesenchymal proliferation. Genetic deletion of the Hh target genes Foxf1, Foxf2, and Foxl1 in MCs resulted in reduced villus development22, 23, and PDGF-A−/− mice have major defects in villus morphogenesis and development of pericryptal MCs and Goblet cells19. Ligands of the Hh pathway, such as SHH (Sonic hedgehog) and IHH (Indian hedgehog), produced by epithelial cells, also mediated differentiation of αSMAmid myofibroblasts and αSMAhigh smooth muscle cells (SMCs) of the intestinal muscle layer18. In the human fetal intestine, αSMA+ SMCs are localized in proximity to proliferating crypts and are a source for RSPOs (R-spondins) and WNT ligands24.
Fig. 1.
Epithelial-mesenchymal crosstalk in mouse intestinal morphogenesis. Intestinal development starts in the embryo and requires a tightly regulated crosstalk between a developing epithelial layer and underlying Hh-responsive MCs and SMCs, which orchestrate epithelial cell proliferation, migration, and differentiation. In mice, differentiation into enterocytes, Goblet cells, and EECs starts in the fetus, whereas mature crypts containing IESCs develop after birth, concomitant to development of Paneth cells and a specialized stromal niche locally promoting WNT signaling. BMPs = bone morphogenetic proteins; EECs = enteroendocrine cells; Hh = Hedgehog; IESCs = intestinal epithelial stem cells; MCs = mesenchymal cells; PDGF-A = platelet-derived growth factor A; SMCs = smooth muscle cells.
During late morphogenesis, the mesenchymal-epithelial crosstalk shapes intestinal regionalization. PDGFRαhigh MCs promoted a WNT signaling gradient, which impacted SHH epithelial expression, anterior-posterior regionalization, and villus formation25. Additional signals from subepithelial MCs, including BMPs and FGFs (Fibroblast growth factor), further drive intestinal epithelial fates and differentiation26, 27, 28. In mice, differentiation into enterocytes, Goblet cells, and EECs starts in the fetus, whereas crypts containing IESCs and rapidly proliferating TA cells develop after birth (around 10–12 days postnatal), concomitant to Paneth cells29. PDGFRα+ MCs, partially co-expressing Foxl1, PDPN, CD34, COL6A1, and GREM1, expand around the crypts in the first postnatal weeks to promote WNT signaling20, 21 (Fig. 1). In the colon, depletion of the Col6a1Cre+ mesenchymal lineage in adult mice decreased EEC differentiation21. Paneth cells were decreased when RSPO activity was reduced by adenoviral expression of decoy receptors or ablation of Rspo3 in MCs30. In contrast, the differentiation of Paneth cells and Goblet cells was increased in mice overexpressing interleukin (IL)-33, highly expressed by MCs and epithelial cells, and mice lacking Il33 were more susceptible to infection with Salmonella Typhimurium31. In fetal intestine, PDGFRαhigh cells expressed Neuregulin (NRG1), which increased the differentiation of secretory lineages in developing human intestine enteroid cultures24. As Paneth cells and Goblet cells produce notably antimicrobial peptides and mucus32, respectively, which are essential for intestinal homeostasis, dysregulation of intestinal morphogenesis might compromise the intestinal barrier.
Stromal regulation of intestinal homeostasis
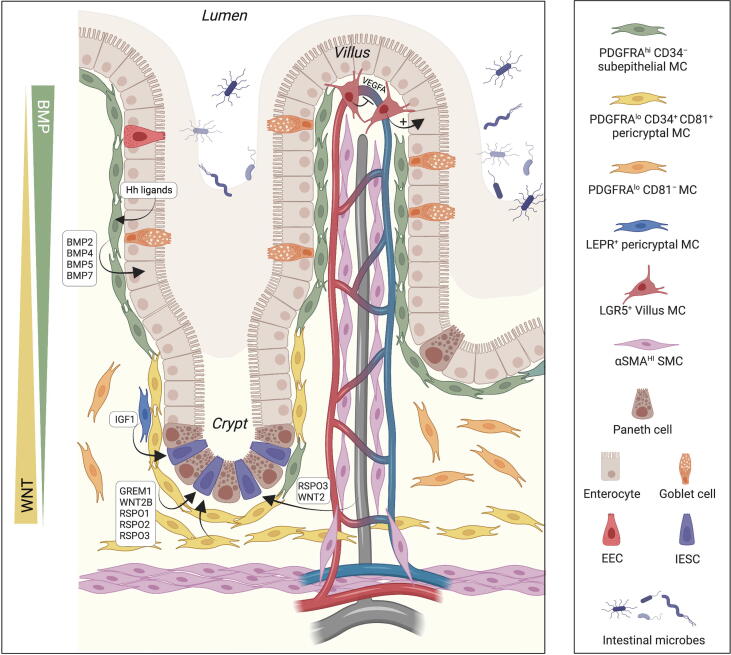
Maintenance of IESCs within crypts requires a specific environment, as the fate of Lgr5+ IESCs is to differentiate unless both RSPO and WNT ligands are present to promote self-renewal5. Several stromal populations around the crypts express BMP antagonists, WNTs, and RSPO1 and RSPO2, which potentiate canonical WNT signaling by binding to Lgr4-6 receptors on IESCs5. The identification of novel surface markers, as well as advances in genetic lineage tracing, Cre-based gene/cell depletion, intestinal organoids co-cultures with MCs, and scRNAseq, allowed to better define the niche for IESCs. Recent findings identified pericryptal MCs and trophocytes, identified by expression of PDGFRαlow, PDPN, CD34, and CD81, as major producers of canonical WNTs, the BMP antagonist GREM1, and RSPO1, RSPO2, RSPO3, which promote WNT signaling in IESCs5. They are localized around the crypts, as well as between the external muscle and the crypt bottom. PDGFRα+ cells partially (co)-express FOXL1 and GLI1, and genetic ablation of factors required for WNT secretion in PDGFRα+, FoxL1+, or GLI1+ MCs, as well as genetic depletion of FOXL1+ cells, induced loss of intestinal crypts and IESCs30, 33, 34, 35, 36, 37. In the villi, PDGFRαhigh PDPN+CD34− subepithelial MCs, which share some similarities with telocytes previously described in several organs38, 39, promoted epithelial differentiation by secreting BMPs under the control of Hh ligands, produced by epithelial cells20, 40, 41 (Fig. 2). BMPs oppose WNT signaling and suppress IESCs signature genes independently of WNT signaling42.
Fig. 2.
Stromal regulation of intestinal homeostasis. Several stromal populations around the crypts contribute to IESC maintenance by locally promoting WNT signaling, including pericryptal and subcryptal PDGFRαlow CD34+ MCs (yellow), which are the major producers of RSPOs, WNT2B, and the BMP antagonist GREM1, as well as Lyve1+ lacteals (gray). In the villi, PDGFRαhigh CD34− subepithelial MCs (green) oppose WNT signaling and promote epithelial differentiation by secreting BMPs under control of Hh ligands, produced by epithelial cells. The expression of LGR5 and LEPR identifies specific subpopulations of subepithelial or pericryptal MCs. BMPs = Bone morphogenetic proteins; EECs = Enteroendocrine cells; Hh = Hedgehog; IESCs = Intestinal epithelial stem cells; LEPR = Leptin receptor; MCs = Mesenchymal cells; PDGFR = Platelet-derived growth factor receptor; RSPOs = R-spondins; SMCs = Smooth muscle cells.
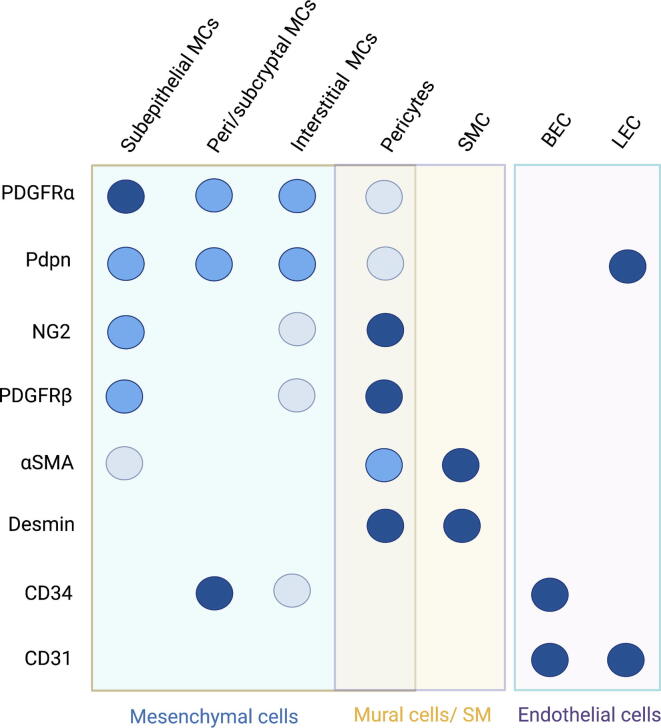
ScRNAseq of the LP identified additional stromal populations in addition to PDGFRαlow CD81+ CD34+ pericryptal cells/trophocytes and PDGFRαhigh Foxl1+Acta2low subepithelial MCs/telocytes. These include abundant PDGFRαlowCD81− interstitial MCs, Cspg4 (NG2)+Pdgfrb+ pericytes, Acta2highMyh11+Hhip+ SMCs in the villus and below the crypts, CD31+CD34+Lyve1− vascular endothelium, CD31+CD34−Lyve1+ lymphatic endothelium, S100b+ glial cells, and Acta2high Myh11+ Hhip− external smooth muscle layer34, 43, 44. Similar populations were found in the human colonic mesenchyme43, 45, although CD34 is not expressed in PDGFRαlow cells, and CD81 is expressed by most PDGFRα+ MCs. Recently, intestinal lymphatics have been shown to contribute to crypt homeostasis and repair by secreting pro-stemness factors RSPO3, WNT2, and Reelin46, 47, 48. Therefore, the mesenchymal/vascular compartment seems to have a key role in coordinating the maintenance and repair of the IESCs niche (Fig. 2). As no single marker is restricted to a single mesenchymal or vascular cell type, Cre-driven gene or cell depletion should be interpreted accordingly. Nevertheless, most studies agree on the peri/subcryptal localization of GREM1+ MCs, which can be isolated by flow cytometry using coexpression of PDGFRα and CD81, or PDPN and CD34, within the CD45−CD31− population20. A summary of the major mesenchymal and vascular intestinal populations and their markers is provided in Fig. 3.
Fig. 3.
Major mesenchymal and vascular cell types of the intestinal lamina propria. Subepithelial MCs, also known as telocytes or subepithelial myofibroblasts, express high levels of PDGFRα and are the major producers of BMPs. PDGFRαlow/mid cells are the most abundant and heterogeneous population, including pericryptal and subcryptal MCs (also known as trophocytes), which ensure a pro-stemness environment, interstitial MCs, and GALT-associated MCs. Mural cells refer to populations of the blood vessel wall, such as pericytes. It is noteworthy that, although the combination of several markers and localization allows for discrimination between these populations, no single marker identifies a unique cell type. Dark and light circles denote high and low expression. BECs = Blood endothelial cells; LECs = Lymphatic endothelial cells; MCs = Mesenchymal cells; PDGFR = Platelet-derived growth factor receptor; SMCs = Smooth muscle cells; SM = Smooth muscle.
Additional markers have been useful in characterizing the LP mesenchyme. In the crypt region, a subset of PDGFRα+ FOXL1− GLI1− MCs expressing leptin receptor (LEPR+)49, 50 has been shown to expand after irradiation. LEPR+ cells were essential for intestinal homeostasis and regeneration in a mechanism dependent on IGF149. Interestingly, the number of LEPR+ cells was modulated by diet, suggesting that alteration of niche MCs by diet (fasting, HFD) represents an additional mechanism to regulate IESC and intestinal homeostasis. In the villus tip, recent studies identified LGR5 positive PDGFRαhigh mesenchymal subsets. Subepithelial LGR5+ cells promoted enterocyte differentiation, presumably by affecting the production of BMP ligands, the non-canonical WNT ligand Wnt5a and the matrix51. Perivascular LGR5+ ADAMTS18+ cells maintained a functional endothelial cell polarization and fenestration in the villus tip by restricting fibronectin accumulation and regulating ECM-bound VEGFA52.
Mesenchymal cells of gut-associated lymphoid tissues
Effector intestinal immune cells disseminate throughout the LP and epithelial layer, whereas mostly naive cells cluster in the GALTs, such as Peyer’s patches (PPs) in the small intestine and isolated lymphoid follicles (ILFs) in the small intestinal and colonic mucosa. GALTs are local sites for the generation of intestinal effector lymphocytes, such as cytokine-producing T cells and immunoglobulin (Ig) A-secreting B cells53. In addition to immune cells, GALTs contain a fine reticulum of heterogeneous mesenchymal populations, mostly PDPN+ PDGFRαmed/low, which share similarities with FRCs of secondary lymphoid organs (SLO)54. In SLOs, FRCs are major producers of chemokines, matrix proteins, survival factors and adhesion molecules essential for DC-T cell interaction, antigen encounter, lymphocyte positioning, survival, and differentiation. FRCs of SLO and GALTs are essential players in immune homeostasis and induction/regulation of adaptive immunity55, 56, 57.
In the small intestine, ILFs mesenchymal subsets include LEPR+ PDGFRα+ telocytes at the top of follicle-associated epithelium, distinct from villus tip telocytes expressing Lgr558. Deletion of Rankl in TWIST2+ cells or COL6A1+ cells, which include several stromal and vascular populations, including MCs in the subepithelial dome (SED) of GALTs, impaired M cell-dependent antigen sampling and B cell-dendritic cell interaction in the SED, decreasing IgA production and microbial diversity59, 60. MCs of the CCL19+ lineage, which also labels FRCs of the PP, regulate the murine coronavirus (M-CoV) infection via IL15-mediated control of group 1 innate lymphoid cells (ILCs)61. Disruption of mechanosensing in CCL19+ MCs by the deletion of Piezo1 disorganized the conduit associated perivascular FRCs and high endothelial venules, impairing lymphocyte entry into PPs and initiation of mucosal antibody responses62. Lymphotoxin-driven activation of CCL19+ FRCs also played a role in the maturation of cryptopatches (CPs) into ILFs, affecting ILC homeostasis and function63. In the colon, CD34− PDPN+ MCs expressing the oxysterol 7α,25-OHC-synthesizing enzymes Ch25h and Cyp7b1 were localized to ILFs, whereas CD34+ PDPN+ MCs, which are abundant outside of ILFs, expressed higher levels of the oxysterol degrading enzyme Hsd3b7. The crosstalk between these two cell types might contribute to the localized 7α,25-OHC gradient required for migration of GPR183+ ILC3 and inflammatory tissue remodeling during colitis64.
Mesenchymal/vascular regulation of the postnatal intestinal barrier
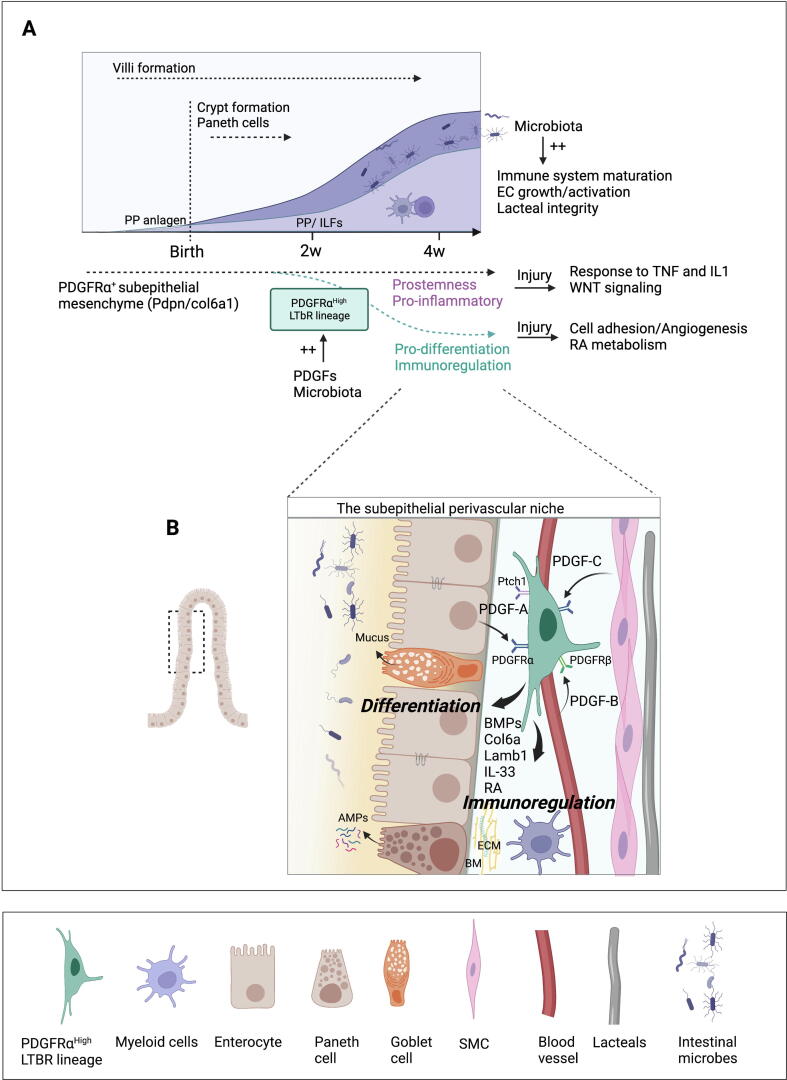
In the perinatal period, the maturation of intestinal epithelial, immune, and vascular components is required to ensure digestive and barrier functions and balanced immunity (Fig. 4). Several of these processes are regulated by the intestinal microbiota that colonize the intestine after birth65, 66, 67, 68. The human gut mucosa exhibits a fully differentiated intestinal structure at birth in contrast to mice. Although there are differences in gut maturity at birth, both humans and mice need to transition from a protected environment devoid of microbiota to a mature intestine, colonized with commensal and potentially pathogenic microorganisms. In the neonatal intestine, these structural differences, including lack of regenerative crypts/mature Paneth cells and decreased epithelial cell turnover have implications for infection. Indeed, rapid surface renewal in the adult intestine, as well as Paneth cell-derived antimicrobial peptides, represent a first line of protection from enteric pathogens69, 70 (Fig. 4A).
Fig. 4.
Stromal/vascular regulation of the postnatal intestinal barrier. (A) Microbial colonization of the intestine after birth plays a major role in maturation of immune, vascular, and stromal components. At birth, the murine neonatal intestine is mostly devoid of adaptive immune cells. Intestinal lymphoid tissue structures develop in the first few weeks after birth, whereas mesenteric lymph nodes and Peyer’s patches anlagen develop prenatally. In the first few weeks after birth, PDGFs play an essential role in the maturation of PDGFRαhigh MCs by inducing pro-differentiation and immunoregulatory genes, including BMPs, RA, and laminins (B), as well as on spatial stromal organization within the crypt-villus. Such program is essential to promote intestinal homeostasis/barrier function and prevent dysregulated repair and inflammatory responses following injury. BM = Basement membrane; BMPs = Bone morphogenetic proteins; IL = Interleukin; PP = Peyer's Patch; MCs = Mesenchymal cells; EC = Endothelial cells; PDGFR = Platelet-derived growth factor receptor; LTβR = Lymphotoxin beta receptor; RA = Retinoic acid; SMCs = Smooth muscle cells; TNF = Tumor necrosis factor; AMPs = Antimicrobial peptides; ECM = Extracellular matrix.
This is particularly important as the murine neonatal intestine has an immature immune system, mostly devoided of adaptive immune cells at birth except for a fetal wave of γδ T lymphocytes, a major source for IL1771, 72. These lymphocytes reside in the LP, as, unlike human neonates, mice do not contain epithelium-associated lymphocytes until weaning73, 74. Intestinal lymphoid tissue structures develop in the first few weeks after birth in contrast to mesenteric lymph nodes and Peyer’s patches anlagen, which develop prenatally75, 76. During the first postnatal weeks, T and B lymphocytes recruited at the site of PP anlagen induce expansion and differentiation of PP-associated FRC-like cells. The mesenchymal network of the PP develops from fetal perivascular CCL19+ and subepithelial COL6A1+ mesenchymal lineages, which colonize distinct zones of the PP in an LTβR and tumor necrosis factor (TNF)R1-dependent manner. Synergistic and partially compensatory mechanisms between both lineages ensure the IgA-dependent regulation of commensal microbiota and adaptive immune responses against intestinal viral infection77.
A prerequisite for postnatal intestinal growth is the capacity to establish a functional vasculature. The microbiota has been shown to regulate the vasculature through different mechanisms, including signaling through a bacteria-sensing epithelial cell type78, by promoting Tissue Factor (TF)–protease-activated receptor (PAR1) signaling79, through activation of Toll-like receptors (TLRs) and NOD-like receptors in endothelial cells and MCs80, as well as by inducing VEGF-C (Vascular endothelial growth factor C) in villus macrophages81. In zebrafish, which have similar intestinal organization, the microbiota promoted intestinal angiogenesis, mesenchymal metabolism, and induction of leucocytes recruiting chemokines in vascular SMCs82.
Concomitant to immune and vascular maturation, the perinatal intestinal epithelium undergoes major changes, including the formation of crypts containing stem cells and the development of Paneth cells29. PDGFRα+ MCs, partially co-expressing Foxl1, PDPN, CD34, COL6A1, and GREM1 expanded around the crypts in the first postnatal weeks20, 21. In agreement with a progenitor role of these cells, inducible lineage tracing of GREM1+ cells using the Grem1CreERT mouse model showed that early GREM1+ MCs generate most subepithelial intestinal MCs within 1 year83. PDGFRα−/− mice are embryonic lethal, which prevented investigations of the role of PDGFRα in the postnatal maturation of the intestinal mesenchyme19, 84. This question was recently investigated using a novel mouse model allowing for inducible lineage tracing and gene depletion in Lymphotoxin β receptor (LTBR)-expressing cells85. Lineage tracing of LTBR+ progenitors identified a distinct PDGFRαhigh subepithelial mesenchymal lineage developing around 2 weeks after birth in the small intestinal villi in a process dependent on the microbiota. The PDGFRαhigh LTBR lineage expressed higher levels of genes promoting immunoregulation and epithelial differentiation compared with PDGFRα+ MCs not generated from LTBR+ progenitors, which had a pro-stemness/pro-inflammatory gene signature. These distinct transcriptomic identities were maintained after injury, suggesting that the damage response of PDGFRα+ MCs is impacted by their origin or period of development85 (Fig. 4A).
PDGFRα has a major impact on the postnatal mesenchymal maturation and fate. Indeed, conditional ablation of Pdgfra in the LTBR mesenchymal lineage prevented the acquisition of factors promoting epithelial differentiation and immunoregulation, including the Hh ligands receptor Ptch1, BMPs, basement membrane (BM) proteins, laminins, retinoic acid (RA) synthesis enzymes, Il33, as well as proper spatial arrangement within the crypt-villus85. Such defect in mesenchymal maturation reduced postnatal growth and disrupted intestinal homeostasis, leading to increased IESCs and proliferative cells and decreased Goblet cells, mature enterocytes, and CD11b+CD103+ dendritic Cells (DCs), which play a key role in neonatal immunity86, 87. Overall, lack of stroma maturation dysregulated repair responses and increased susceptibility to intestinal inflammation in young individuals85 (Fig. 4B).
These data suggest that distinct PDGFRα+ lineages with pro-differentiation/immunoregulatory (PTCH1high) or pro-stemness/inflammatory (PTCH1−/low) functions are established in the first few weeks after birth and that their relative abundance determines the inflammatory status of the intestine. This is consistent with epithelial Hh ligands acting as key sensors for epithelial integrity and the observed downregulation of Hh signaling pathway in patients with IBDs88, 89. Therefore, postnatal maturation of intestinal mesenchymal/vascular elements seems to represent an essential step in the development of a functional intestinal barrier and is at least partially regulated by the microbiota (Fig. 4).
Stromal regulation of intestinal regeneration and inflammation
Tissue damage induces activation of a regenerative program to restore epithelial integrity. In the intestine, this response involves stem cells, as well as the secretory/absorptive lineages, which have the potential to revert to a stem cell state, and quiescent label-retaining cells90. The intestinal regenerative response is tightly regulated by the microenvironment, including immune cells, enteric neuronal cells, the extracellular matrix, the diet, and the microbiome 90, 91. In addition, the mesenchymal compartment is recently emerging as a critical player in intestinal regeneration and inflammation.
Several factors essential for regeneration have been identified. Knockout of the microRNAs miR-143/145 using Twist2Cre mice, which mostly label SMCs of the muscularis propria and αSMA+ myofibroblasts, resulted in disorganized regenerative responses, loss of epithelial proliferation, and exacerbated dextran sodium sulfate (DSS)-induced colitis. MiR-143 functions as a cell-autonomous tumor suppressor in colon cancer and was found to derepress Igfbp5, which impaired IGF1 signaling after injury92. IGF1 is a potent growth and repair factor in intestinal regeneration following resection, radiation injury, and DSS-induced colitis. Using a diphtheria toxin-mediated cell depletion model, ablation of LEPR+ crypt MCs and their progeny in LeprCreRosa26–iDTR mice impaired regeneration following irradiation in a mechanism dependent on IGF149. LEPR+ cell-derived WNT2B also promoted regeneration during colitis50.
An additional factor supporting colon regeneration after DSS-induced damage is prostaglandin E2 (PGE2), produced by intestinal MCs20, 76, 93. PGE2 enhanced YAP (Yes1 associated transcriptional regulator) activity, which promoted colon regeneration by increasing the expression of the PGE2 synthesis enzyme COX-2 and the prostaglandin EP4 receptor94 but also induced colon tumorigenesis through mechanisms dependent on YAP and Gs-axin-βcatenin signaling pathways94, 95, 96. Of note, Slco2a1-deficiency (which increases PGE2 concentrations) was associated with small intestinal inflammation/ulcerations in humans and increased sensitivity to colitis in mouse models97, suggesting a double-edged mechanism. During the first days after injury in mice, the non-canonical WNT5A ligand produced by wound adjacent MCs was required for transient inhibition of epithelial proliferation, an essential step in crypts regeneration98. Although the identity of the WNT5A-producing MCs remains unclear, the PDPN+CD34− cells produced higher levels of Wnt5a than pericryptal PDPN+CD34+ cells in DSS-induced colitis20.
The mesenchymal niche also maintains the stem cell compartment while regulating epithelial cell proliferation. Following DSS-induced inflammation, mesenchymal populations localized in the proximity of crypts, including PDPN+CD34+ MCs, trophocytes, GL1+ and PDPN+CD90+ MCs, overexpressed pro-stemness factors such as Grem1, Rspo1, and Rspo320, 34, 41. Several triggers activate such a protective mesenchymal response. Upregulation of Rspo1 by PDPN+CD90+ MCs after DSS was dependent on Map3k2 and ROS (Reactive oxygen species) signaling, and deletion of Map3k2 in COL1A1-expressing cells using Col1a2CreERT2Map3k2fl/fl increased the severity of DSS-induced colitis99. Additional factors promoting expression of pro-stemness factors include the pro-inflammatory cytokine IL1, consistent with the hypothesis that similar pathways are engaged in inflammation and regeneration85, 90. IL1R1 was highly expressed by pericryptal GREM1+PDGFRα+ MCs100, and IL1R1 activation induced upregulation of Rspo3 during infection to protect stem cells.
The mesenchymal crosstalk with immune cells is tightly regulated and most likely adapted to the damage intensity. Indeed, IL1 stimulation of GREM1+ Rspo3-expressing cells was sufficient for epithelial repair in DSS-induced colitis but required IL22 production by ILCs, also expressing IL1R1, to promote epithelial proliferation and repair in intestinal infection driven by Citrobacter rodentium100. In a model of Listeria (Lm) infection, the crosstalk of several immune subsets and PDPN+ MCs, which produced IL11, was required for STAT3-dependent epithelial response and depletion of Goblet cells, overall leading to decreased Lm villus invasion but higher susceptibility to colitis101. In the colon, oxysterol production by PDPN+CD34– MCs, abundant in ILFs and CPs, played a key role in the recruitment of ILC3s expressing GPR138. Increased oxysterol production during inflammation increased susceptibility to colitis through GPR183-mediated cell recruitment64.
Mesenchymal cells in intestinal inflammatory diseases
In chronic conditions, continuous cycles of regeneration lead to dysregulated inflammatory and repair responses, as observed in IBDs, including Crohn’s disease (CD) and ulcerative colitis (UC). Recent scRNAseq studies shed new light on the heterogeneity of the mesenchymal compartment in patients with IBD, suggesting a dysregulated mesenchymal crosstalk with leucocytes, epithelial cells, and the extracellular matrix (ECM).
Single-cell RNAseq analysis of the colonic mesenchymal compartment in healthy controls and patients with UC identified several mesenchymal clusters expressing non-fibrillar collagens, matricellular proteins, and BMP/WNT factors involved in the formation of basement membrane and epithelial stemness/differentiation. One cluster overexpressing PDPN, CCL19, and IL33 strongly expanded in patients with UC43, suggesting a causal role in UC disease pathogenesis or a reaction to promote repair. A similar cluster in DSS-induced colitis overexpressed Lox and Loxl1, which catalyze covalent crosslinking of collagen and elastin, generating hydrogen peroxide as a by-product43. Blockade of Lox enzymes reduced the severity of DSS colitis most likely due to the impact on redox processes or matrix remodeling43.
Genome-wide association studies (GWASs) revealed risk alleles for UC. A cell atlas from the colon mucosa of 18 patients with UC and 12 healthy individuals identified a subset of IL11+IL13RA2+ inflammatory fibroblasts associated with resistance to anti-TNF treatment45. One of the most enriched genes in inflammatory fibroblasts is the Oncostatin M receptor (OSMR), which is also a putative risk gene for IBD102. OSM is highly expressed in myeloid cells, which also expanded during inflammation, further providing a link between inflammatory fibroblasts and OSM-mediated resistance to anti-TNF treatment103. Of note, TNF-induced response on MCs was sufficient to induce several chronic inflammatory pathologies, including in IBD104. A similar myeloid-mesenchymal interaction was recently shown in patients who carry CD-risk alleles of NOD2105. Loss of NOD2 dysregulated homeostasis of activated fibroblasts and macrophages, notably through elevated STAT3 and gp130 receptor ligands such as OSM and IL6, potentially driving fibrosis106. Therefore, the blockade of gp130 family members has been suggested to represent an alternative treatment for selected patients with CD by breaking up the circuit of fibroblast-immune cell interactions. In patients with ileal CD, scRNAseq identified pathogenic cellular modules associated with resistance to anti-TNF therapy, which included pro-inflammatory MCs and endothelial cells overexpressing IL6 and chemokines recruiting neutrophils and monocytes107. Development of mesenchymal and endothelial cells enriched in inflammatory, matrix remodeling, and Type I interferon signatures was also identified in pediatric patients with IBD108.
Increasing evidence points to a dysregulated crosstalk between activated MCs expressing cytokines such as IL6 and IL11, which respond to IL1β and OSM (mostly expressed by myeloid cells), leading to dysregulated inflammation/regeneration cycles and epithelial barrier dysfunction, key characteristics of IBD. In addition, MCs are also major producers of structural and regulatory proteins of the ECM, including collagens, proteoglycans, BM proteins, and enzymes involved in ECM degradation and remodeling. As several elements of the ECM regulate immune cell recruitment, as well as blood vessels and epithelial cell integrity, local changes in ECM composition have most likely an impact on intestinal homeostasis and inflammation109, 110. Changes in the ECM might also have systemic implications, as studies suggested that the association between skin inflammation and IBD involves recognition by colonic MC of hyaluronan (HA) fragments released in skin inflammation111. HA has also been suggested to play a role in reactive colon adipogenesis and host defense after injury112.
CONCLUSION
As key regenerative cells of the intestine, IESCs receive signals from an “expanded” niche, which includes stromal cells, paneth cells, immune cells, and the microbiota113. The type of injury, intestinal stress, age, and differences in the microbiota might further affect their niche function. Concerning the recent scRNA-sequencing experiments, it will be interesting to investigate whether and to which extent the mesenchymal clusters identified in different studies are similar, map their localization within the tissue during disease progression and define lineage relationships.
Overall, it remains unclear how to specifically target fibrotic or inflammatory MCs in pathologies. In several organs, MCs close to blood vessels have been identified as major contributors to scar tissue and fibrosis14, 114. The development of more specific Cre-based mice models might help in identifying and targeting pathological mesenchymal subsets while protecting the normal mesenchyme (in the intestine as in other organs), which is required to maintain epithelial, vascular, and immune homeostasis and for tissue repair. A “normalization” of the mesenchymal compartment, rather than targeting specific molecules, represents a promising alternative approach but requires a better understanding of the biology of MCs, which is still only partially understood.
Author contributions
All authors contributed to defining the review content and figures. LP wrote the manuscript.
Declaration of competing interests
The authors have no competing interests to declare.
Funding
The lab has received funding from the European Research Council (ERC) Consolidator grant 648428-PERIF (to LP), INSERM, and Institut Pasteur.
Acknowledgments
Figures were created in BioRender.
REFERENCES
- 1.Barker N., et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 2.van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 3.Bry L., et al. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc. Natl Acad. Sci. U. S. A. 1994;91:10335–10339. doi: 10.1073/pnas.91.22.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings R.J., et al. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature. 2016;539:565–569. doi: 10.1038/nature20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan K.S., et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature. 2017;545:238–242. doi: 10.1038/nature22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato T., et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durand A., et al. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc. Natl Acad. Sci. U. S. A. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farin H.F.V., Es J.H., Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529.e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Kabiri Z., et al. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 10.San Roman A.K., Jayewickreme C.D., Murtaugh L.C., Shivdasani R.A. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Rep. 2014;2:127–134. doi: 10.1016/j.stemcr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki N., et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc. Natl Acad. Sci. U. S. A. 2016;113:E5399–E5407. doi: 10.1073/pnas.1607327113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell D.W., Pinchuk I.V., Saada J.I., Chen X., Mifflin R.C. Mesenchymal cells of the intestinal lamina propria. Annu. Rev. Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Shibayama C., Gil-Cruz C., Ludewig B. Fibroblastic reticular cells at the nexus of innate and adaptive immune responses. Immunol. Rev. 2019;289:31–41. doi: 10.1111/imr.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Carlo S.E., Peduto L. The perivascular origin of pathological fibroblasts. J. Clin. Invest. 2018;128:54–63. doi: 10.1172/JCI93558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley C.D., Barone F., Nayar S., Bénézech C., Caamaño J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu. Rev. Immunol. 2015;33:715–745. doi: 10.1146/annurev-immunol-032713-120252. [DOI] [PubMed] [Google Scholar]
- 16.Davidson S., et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 2021;21:704–717. doi: 10.1038/s41577-021-00540-z. [DOI] [PubMed] [Google Scholar]
- 17.Owens B.M., Simmons A. Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol. 2013;6:224–234. doi: 10.1038/mi.2012.125. [DOI] [PubMed] [Google Scholar]
- 18.Walton K.D., Gumucio D.L. Hedgehog signaling in intestinal development and homeostasis. Annu. Rev. Physiol. 2021;83:359–380. doi: 10.1146/annurev-physiol-031620-094324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson L., Lindahl P., Heath J.K., Betsholz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–3466. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]
- 20.Stzepourginski I., et al. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc. Natl Acad. Sci. U. S. A. 2017;114:E506–E513. doi: 10.1073/pnas.1620059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melissari M.T., et al. Col6a1+/CD201+ mesenchymal cells regulate intestinal morphogenesis and homeostasis. Cell. Mol. Life Sci. 2021;79:1. doi: 10.1007/s00018-021-04071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaestner K.H., Silberg D.G., Traber P.G., Schütz G. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes Dev. 1997;11:1583–1595. doi: 10.1101/gad.11.12.1583. [DOI] [PubMed] [Google Scholar]
- 23.Ormestad M., et al. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development. 2006;133:833–843. doi: 10.1242/dev.02252. [DOI] [PubMed] [Google Scholar]
- 24.Holloway E.M., et al. Mapping development of the human intestinal niche at single-cell resolution. Cell Stem Cell. 2021;28:568–580.e4. doi: 10.1016/j.stem.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maimets M., et al. Mesenchymal-epithelial crosstalk shapes intestinal regionalisation via Wnt and Shh signalling. Nat. Commun. 2022;13:715. doi: 10.1038/s41467-022-28369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X.C., et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat. Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 27.Kim B.M., Buchner G., Miletich I., Sharpe P.T., Shivdasani R.A. The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev. Cell. 2005;8:611–622. doi: 10.1016/j.devcel.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., et al. Reciprocal epithelial-mesenchymal FGF signaling is required for cecal development. Development. 2006;133:173–180. doi: 10.1242/dev.02175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Santa Barbara P., van den Brink G.R., Roberts D.J. Development and differentiation of the intestinal epithelium. Cell. Mol. Life Sci. 2003;60:1322–1332. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greicius G., et al. PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc. Natl Acad. Sci. U. S. A. 2018;115:E3173–E3181. doi: 10.1073/pnas.1713510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahapatro M., et al. Programming of intestinal epithelial differentiation by IL-33 derived from pericryptal fibroblasts in response to systemic infection. Cell Rep. 2016;15:1743–1756. doi: 10.1016/j.celrep.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 32.Van der Sluis M., et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Aoki R., et al. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell. Mol. Gastroenterol. Hepatol. 2016;2:175–188. doi: 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degirmenci B., Valenta T., Dimitrieva S., Hausmann G., Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature. 2018;558:449–453. doi: 10.1038/s41586-018-0190-3. [DOI] [PubMed] [Google Scholar]
- 35.Shoshkes-Carmel M., et al. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature. 2018;557:242–246. doi: 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batts L.E., Polk D.B., Dubois R.N., Kulessa H. Bmp signaling is required for intestinal growth and morphogenesis. Dev. Dyn. 2006;235:1563–1570. doi: 10.1002/dvdy.20741. [DOI] [PubMed] [Google Scholar]
- 37.Kosinski C., et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc. Natl Acad. Sci. U. S. A. 2007;104:15418–15423. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popescu L.M., Faussone-Pellegrini M.S. TELOCYTES – a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J. Cell. Mol. Med. 2010;14:729–740. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kondo A., Kaestner K.H. Emerging diverse roles of telocytes. Development. 2019;146:dev175018. doi: 10.1242/dev.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosinski C., et al. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology. 2019;2010(139):893–903. doi: 10.1053/j.gastro.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarthy N., et al. Distinct mesenchymal cell populations generate the essential intestinal BMP signaling gradient. Cell Stem Cell. 2020;26:391–402.e5. doi: 10.1016/j.stem.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi Z., et al. BMP restricts stemness of intestinal Lgr5+ stem cells by directly suppressing their signature genes. Nat. Commun. 2017;8:13824. doi: 10.1038/ncomms13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinchen J., et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175:372–386.e17. doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCarthy N., et al. Delineation and birth of a layered intestinal stem cell niche. SSRN J. 2022 [Date accessed: DD Month YYYY] [Google Scholar]
- 45.Smillie C.S., et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178:714–730.e22. doi: 10.1016/j.cell.2019.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niec R.E., et al. Lymphatics act as a signaling hub to regulate intestinal stem cell activity. Cell Stem Cell. 2022;29:1067–1082.e18. doi: 10.1016/j.stem.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goto N., et al. Lymphatics and fibroblasts support intestinal stem cells in homeostasis and injury. Cell Stem Cell. 2022;29:1246–1261.e6. doi: 10.1016/j.stem.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palikuqi B., et al. Lymphangiocrine signals are required for proper intestinal repair after cytotoxic injury. Cell Stem Cell. 2022;29:1262–1272.e5. doi: 10.1016/j.stem.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng M., et al. Lepr+ mesenchymal cells sense diet to modulate intestinal stem/progenitor cells via leptin-Igf1 axis. Cell Res. 2022;32:670–686. doi: 10.1038/s41422-022-00643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumura S., et al. Stratified layer analysis reveals intrinsic leptin stimulates cryptal mesenchymal cells for controlling mucosal inflammation. Sci. Rep. 2020;10:18351. doi: 10.1038/s41598-020-75186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bahar Halpern K., et al. Lgr5+ telocytes are a signaling source at the intestinal villus tip. Nat. Commun. 2020;11:1936. doi: 10.1038/s41467-020-15714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernier-Latmani J., et al. ADAMTS18+ villus tip telocytes maintain a polarized VEGFA signaling domain and fenestrations in nutrient-absorbing intestinal blood vessels. Nat. 2022;13:3983. doi: 10.1038/s41467-022-31571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mörbe U.M., et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021;14:793–802. doi: 10.1038/s41385-021-00389-4. [DOI] [PubMed] [Google Scholar]
- 54.Rodda L.B., et al. Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity. 2018;48:1014–1028.e6. doi: 10.1016/j.immuni.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lütge M., Pikor N.B., Ludewig B. Differentiation and activation of fibroblastic reticular cells. Immunol. Rev. 2021;302:32–46. doi: 10.1111/imr.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishnamurty A.T., Turley S.J. Lymph node stromal cells: cartographers of the immune system. Nat. Immunol. 2020;21:369–380. doi: 10.1038/s41590-020-0635-3. [DOI] [PubMed] [Google Scholar]
- 57.Onder L., Cheng H.W., Ludewig B. Visualization and functional characterization of lymphoid organ fibroblasts. Immunol. Rev. 2022;306:108–122. doi: 10.1111/imr.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen N., et al. Spatial gene expression maps of the intestinal lymphoid follicle and associated epithelium identify zonated expression programs. PLoS Biol. 2021;19:e3001214. doi: 10.1371/journal.pbio.3001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagashima K., et al. Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat. Immunol. 2017;18:675–682. doi: 10.1038/ni.3732. [DOI] [PubMed] [Google Scholar]
- 60.Nagashima K., et al. Targeted deletion of RANKL in M cell inducer cells by the Col6a1-Cre driver. Biochem. Biophys. Res. Commun. 2017;493:437–443. doi: 10.1016/j.bbrc.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Gil-Cruz C., et al. Fibroblastic reticular cells regulate intestinal inflammation via IL-15-mediated control of group 1 ILCs. Nat. Immunol. 2016;17:1388–1396. doi: 10.1038/ni.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang J.E., Buechler M.B., Gressier E., Turley S.J., Carroll M.C. Mechanosensing by Peyer's patch stroma regulates lymphocyte migration and mucosal antibody responses. Nat. Immunol. 2019;20:1506–1516. doi: 10.1038/s41590-019-0505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng H.W., et al. Intestinal fibroblastic reticular cell niches control innate lymphoid cell homeostasis and function. Nat. Commun. 2022;13:2027. doi: 10.1038/s41467-022-29734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emgård J., et al. Oxysterol sensing through the receptor GPR183 promotes the lymphoid-tissue-inducing function of innate lymphoid cells and colonic inflammation. Immunity. 2018;48:120–132.e8. doi: 10.1016/j.immuni.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chin A.M., Hill D.R., Aurora M., Spence J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 2017;66:81–93. doi: 10.1016/j.semcdb.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill D.A., Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 2010;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganal-Vonarburg S.C., Hornef M.W., Macpherson A.J. Microbial-host molecular exchange and its functional consequences in early mammalian life. Science. 2020;368:604–607. doi: 10.1126/science.aba0478. [DOI] [PubMed] [Google Scholar]
- 68.Al Nabhani Z., Eberl G. Imprinting of the immune system by the microbiota early in life. Mucosal Immunol. 2020;13:183–189. doi: 10.1038/s41385-020-0257-y. [DOI] [PubMed] [Google Scholar]
- 69.Zhang K., et al. Age-dependent enterocyte invasion and microcolony formation by Salmonella. PLoS Pathog. 2014;10:e1004385. doi: 10.1371/journal.ppat.1004385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salzman N.H., et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramond C., et al. Two waves of distinct hematopoietic progenitor cells colonize the fetal thymus. Nat. Immunol. 2014;15:27–35. doi: 10.1038/ni.2782. [DOI] [PubMed] [Google Scholar]
- 72.Haas J.D., et al. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Latthe M., Terry L., MacDonald T.T. High frequency of CD8 alpha alpha homodimer-bearing T cells in human fetal intestine. Eur. J. Immunol. 1994;24:1703–1705. doi: 10.1002/eji.1830240737. [DOI] [PubMed] [Google Scholar]
- 74.Cheroutre H., Lambolez F., Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanamori Y., et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J. Exp. Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torow N., Marsland B.J., Hornef M.W., Gollwitzer E.S. Neonatal mucosal immunology. Mucosal Immunol. 2017;10:5–17. doi: 10.1038/mi.2016.81. [DOI] [PubMed] [Google Scholar]
- 77.Prados A., et al. Fibroblastic reticular cell lineage convergence in Peyer's patches governs intestinal immunity. Nat. Immunol. 2021;22:510–519. doi: 10.1038/s41590-021-00894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stappenbeck T.S., Hooper L.V., Gordon J.I. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc. Natl Acad. Sci. U. S. A. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reinhardt C., et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature. 2012;483:627–631. doi: 10.1038/nature10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schirbel A., et al. Pro-angiogenic activity of TLRs and NLRs: a novel link between gut microbiota and intestinal angiogenesis. Gastroenterology. 2013;144:613–623.e9. doi: 10.1053/j.gastro.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suh S.H., et al. Gut microbiota regulates lacteal integrity by inducing VEGF-C in intestinal villus macrophages. EMBO Rep. 2019;20:e46927. doi: 10.15252/embr.201846927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Willms R.J., Jones L.O., Hocking J.C., Foley E. A cell atlas of microbe-responsive processes in the zebrafish intestine. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110311. [DOI] [PubMed] [Google Scholar]
- 83.Worthley D.L., et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- 85.Jacob J.M., et al. PDGFRalpha-induced stromal maturation is required to restrain postnatal intestinal epithelial stemness and promote defense mechanisms. Cell Stem Cell. 2022;29:856–868.e5. doi: 10.1016/j.stem.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Lantier L., et al. Intestinal CD103+ dendritic cells are key players in the innate immune control of Cryptosporidium parvum infection in neonatal mice. PLoS Pathog. 2013;9:e1003801. doi: 10.1371/journal.ppat.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scott C.L., Aumeunier A.M., Mowat A.M. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 88.van Dop W.A., et al. Loss of Indian hedgehog activates multiple aspects of a wound healing response in the mouse intestine. Gastroenterology. 2010;139:1665–1676. doi: 10.1053/j.gastro.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 89.Lees C.W., et al. Analysis of germline GLI1 variation implicates hedgehog signalling in the regulation of intestinal inflammatory pathways. PLoS Med. 2008;5:e239. doi: 10.1371/journal.pmed.0050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hageman J.H., et al. Intestinal regeneration: regulation by the microenvironment. Dev. Cell. 2020;54:435–446. doi: 10.1016/j.devcel.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 91.Jacobson A., Yang D., Vella M., Chiu I.M. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14:555–565. doi: 10.1038/s41385-020-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chivukula R.R., et al. An essential mesenchymal function for miR-143/145 in intestinal epithelial regeneration. Cell. 2014;157:1104–1116. doi: 10.1016/j.cell.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown S.L., et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J. Clin. Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim H.B., et al. Prostaglandin E2 activates YAP and a positive-signaling loop to promote colon regeneration after colitis but also carcinogenesis in mice. Gastroenterology. 2017;152:616–630. doi: 10.1053/j.gastro.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roulis M., et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature. 2020;580:524–529. doi: 10.1038/s41586-020-2166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castellone M.D., Teramoto H., Williams B.O., Druey K.M., Gutkind J.S. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 97.Nakata R., et al. Slco2a1 deficiency exacerbates experimental colitis via inflammasome activation in macrophages: a possible mechanism of chronic enteropathy associated with SLCO2A1 gene. Sci. Rep. 2020;10:4883. doi: 10.1038/s41598-020-61775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyoshi H., Ajima R., Luo C.T., Yamaguchi T.P., Stappenbeck T.S. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu N., et al. MAP3K2-regulated intestinal stromal cells define a distinct stem cell niche. Nature. 2021;592:606–610. doi: 10.1038/s41586-021-03283-y. [DOI] [PubMed] [Google Scholar]
- 100.Cox C.B., et al. IL-1R1-dependent signaling coordinates epithelial regeneration in response to intestinal damage. Sci. Immunol. 2021;6:eabe8856. doi: 10.1126/sciimmunol.abe8856. [DOI] [PubMed] [Google Scholar]
- 101.Disson O., et al. Peyer's patch myeloid cells infection by Listeria signals through gp38+ stromal cells and locks intestinal villus invasion. J. Exp. Med. 2018;215:2936–2954. doi: 10.1084/jem.20181210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu J.Z., et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015;47:979–986. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.West N.R., et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017;23:579–589. doi: 10.1038/nm.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Armaka M., et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J. Exp. Med. 2008;205:331–337. doi: 10.1084/jem.20070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ogura Y., et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 106.Nayar S., et al. A myeloid-stromal niche and gp130 rescue in NOD2-driven Crohn's disease. Nature. 2021;593:275–281. doi: 10.1038/s41586-021-03484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin J.C., et al. Single-cell analysis of Crohn's disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell. 2019;178:1493–1508.e20. doi: 10.1016/j.cell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang B., et al. Mucosal profiling of pediatric-onset colitis and IBD reveals common pathogenics and therapeutic pathways. Cell. 2019;179:1160–1176.e24. doi: 10.1016/j.cell.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 109.Petrey A.C., de la Motte C.A. The extracellular matrix in IBD: a dynamic mediator of inflammation. Curr. Opin. Gastroenterol. 2017;33:234–238. doi: 10.1097/MOG.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimshoni E., Yablecovitch D., Baram L., Dotan I., Sagi I. ECM remodelling in IBD: innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut. 2015;64:367–372. doi: 10.1136/gutjnl-2014-308048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dokoshi T., et al. Skin inflammation activates intestinal stromal fibroblasts and promotes colitis. J. Clin. Invest. 2021;131:e147614. doi: 10.1172/JCI147614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dokoshi T., et al. Hyaluronidase inhibits reactive adipogenesis and inflammation of colon and skin. JCI Insight. 2018;3:e123072. doi: 10.1172/jci.insight.123072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Levy A., et al. Innate immune receptor NOD2 mediates LGR5+ intestinal stem cell protection against ROS cytotoxicity via mitophagy stimulation. Proc. Natl Acad. Sci. U. S. A. 2020;117:1994–2003. doi: 10.1073/pnas.1902788117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lemos D.R., Duffield J.S. Tissue-resident mesenchymal stromal cells: implications for tissue-specific antifibrotic therapies. Sci. Transl. Med. 2018;10:eaan5174. doi: 10.1126/scitranslmed.aan5174. [DOI] [PubMed] [Google Scholar]