Abstract
Purpose
To compare the corneal epithelial wound healing effects of RCI001, Solcoseryl, and polydeoxyribonucleotide (PDRN) in a rat alkali burn model.
Methods
In 40 male Sprague-Dawley rats, we induced alkali burn using filter paper soaked in 0.2N sodium hydroxide. The rats were then treated with topical 0.5% RCI001, 1.0% RCI001, Solcoseryl, or PDRN twice a day for 2 weeks. Corneal epithelial integrity and epithelial healing rate were measured at day 0, 3, 5, 7, 10, and 14. Histologic and immunohistochemistry findings were also assessed.
Results
Both the 0.5% and 1.0% RCI001 groups showed significantly more epithelial healing compared to the control group at day 5, 7, 10, and 14 (each p < 0.05). No statistical difference was found between the 0.5% and 1.0% RCI001 groups. Neither the Solcoseryl nor the PDRN groups showed a significant difference from the control. RCI001 treatment resulted in significantly reduced stromal edema, and a trend towards less inflammatory cell infiltration.
Conclusions
Topical application of RCI001 showed enhanced corneal epithelial wound healing in the murine corneal alkali burn model, presumably by suppressing inflammation. Meanwhile, Solcoseryl and PDRN did not show sufficient therapeutic effects compared to RCI001.
Keywords: Corneal epithelium, Polydeoxyribonucleotides, RCI001, Solcoseryl, Wound healing
The corneal epithelium is crucial in protecting the eye against the external environment and maintaining a smooth optical surface. Disruption to the epithelium leads to blurred vision, pain, and an increased risk of infection, ulceration, and scarring. Upon injury, epithelial healing is initiated, involving migration and proliferation of epithelial cells, followed by reassembly of junctional apparatus [1]. Impaired epithelial healing prevents regeneration of the epithelial basement membrane and initiation of stromal healing, ultimately leading to stromal scarring and a decline in vision [2,3]. Therapeutic agents of corneal epithelial healing aim to stimulate the healing process or suppress inhibitors of healing.
Solcoseryl and polydeoxyribonucleotide (PDRN) are two agents used as adjunctive therapy in corneal abrasions [4,5]. Solcoseryl (Solcorin, Hanlim Pharm), the protein-free dialysate of calf blood [6,7], has been found to accelerate the re-epithelization of the cornea after mechanical injury [4,8–10]. PDRN (Re-An, PharmaResearch), a salmon sperm-derived DNA drug [11], has also been shown to facilitate corneal epithelium regeneration after photorefractive keratectomy [5]. However, their clinical efficacy in more severe corneal injury remains unclear.
RCI001 is a potential therapeutic candidate for ocular surface disorders [12]. Its main component is 8-oxo-2′-deoxyguanosine (8-oxo-dG), which is the oxidized derivative of deoxyguanosine, produced when DNA base guanine is damaged [13]. Previous experiments demonstrated excellent wound healing, antioxidant, and anti-inflammatory effects of RCI001 in ocular chemical burn and dry eye models [14–16]. In murine models of corneal ethanol injury and alkali burn, 1-week treatment with RCI001 showed marked improvement of corneal epithelial defects, with reduced inflammatory cell infiltration and proinflammatory cytokines [14,15]. Also in a murine inflammatory dry eye model, RCI001 treatment improved ocular surface staining and tear secretion, and repressed inflammatory cytokines [16].
In this study, we aimed to compare the efficacy of RCI001, Solcoseryl, and PDRN in promoting corneal epithelial wound healing using a rat corneal alkali burn model. Since Solcoseryl and PDRN have already demonstrated clinical efficacy in mechanical injuries [5,9,10], we selected the alkali burn model to achieve a definitive comparison of these agents in the severe ocular surface inflammatory model. To the best of our knowledge, no previous comparison has been made between these agents in regards to ocular surface disorders, and we hoped to provide insight into application of these agents in clinical settings of significant corneal epithelial injury.
Materials and Methods
Ethics statements
The present study’s experimental protocol was approved by the Institutional Animal Care and Use Committee of Lee Gil Ya Cancer and Diabetes Institute (No. LCDI-2018-0083). The animals were handled according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and treated following the guidelines in the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Animals and experimental design
A total of 40 male Sprague-Dawley rats at 7 weeks of age were used for the experiments (Dae Han Bio Link). The rats were kept in a pathogen-free environment with unlimited access to water and food. Anesthesia was induced with intraperitoneal injection of sodium pentobarbital (30–70 mg/kg, Entobar, Hanlim Pharm). In the right eye of each rat, alkali burn was created with a 30-second application of a 0.5 × 0.5-cm filter paper soaked in 0.2N sodium hydroxide. Then the eye was irrigated with 10 mL normal saline. To control equal corneal epithelial defect at baseline, the residual corneal epithelium was debrided with a surgical blade [14,17], and irrigated once more with 2 mL normal saline. Then each rat was treated with topical application of 10 μL eyedrops twice daily for 2 weeks according to its previously assigned group: group 1 was control (no treatment), group 2 was treated with 1.0% RCI001, group 3 with 0.5% RCI001, group 4 with Solcoseryl 120 concentrate 70.05 mg/mL, and group 5 with PDRN 0.75 mg/mL. Topical treatment was initiated 4 hours after creation of the chemical burn.
Clinical and histological examination
Corneal epithelial integrity score and epithelial healing rate were evaluated on day 0, 3, 5, 7, 10, and 14 after the chemical burn. Ocular surface staining was done with 1% Lissamine green B (Sigma-Aldrich), and then photographed using OPMI LUMERA 300 (Carl Zeiss) and Ez-Recorder 130 (AVerMedia). Corneal epithelial integrity was graded using a scale from 0 to 4 depending on the size of the epithelial defect: 0, no defect; 1, defect less than 25%; 2, 25% to 50% epithelial defect; 3, 50% to 75% epithelial defect; and 4, more than 75% epithelial defect [18]. Epithelial healing rate was determined using ImageJ ver. 1.44 (US National Institute of Health). Area of the epithelial defect and the entire cornea were manually delineated and determined using the ImageJ software, after which the percentage of the defect area was calculated. Corneal epithelial integrity scores and epithelial healing rate were measured by an experienced researcher (YAK). After the 2 weeks of treatment, the eyes were enucleated, fixed in 10% formalin and embedded in paraffin. The tissue was cut into 4 μm sections and stained with hematoxylin-eosin (H&E). For immunohistochemistry (IHC) staining for tumor necrosis factor α (TNF-α), the sections were deparaffinized, rehydrated, and boiled in 10mM citrate buffer (pH 6) or 1mM EDTA buffer (pH 8) for antibody retrieval. Then sections were incubated with peroxidase blocking solution (PBS; Agilent) for 10 minutes, washed to remove PBS, then blocked with blocking buffer (Protein Block Serum Free, Dako) for 10 minutes. After overnight incubation with the primary antibody (TNF-α, sc-1351; Santa Cruz Biotechnology) at 4 °C, sections were incubated for 15 minutes with the secondary antibody (Horse anti-goat immunoglobulin G antibody, BA9500; Vector Laboratories), and treated with 3,3′-diaminobenzidine. Each section was counterstained with hematoxylin and mounted with Canada balsam. All slides were photographed with a digital slide scanner (Pannoramic SCAN, 3DHISTECH). The H&E-stained sections were examined for stromal thickness measurement using ImageJ. An experienced examiner (DHK) counted the number of TNF-α–positive cells at 400 high power field (HPF) magnifications in two different sections and determined the average cell count.
Statistical analysis
All data were presented as the mean ± standard error of the mean. Friedman and Kruskal-Wallis tests were used to evaluate improvement of corneal epithelial integrity, and comparison between groups was performed using Dunn test for multiple comparisons (GradPad Prism Inc). Results were considered statistically significant when p-value < 0.05.
Results
RCI001 groups showed better corneal epithelial healing effects compared to the other groups
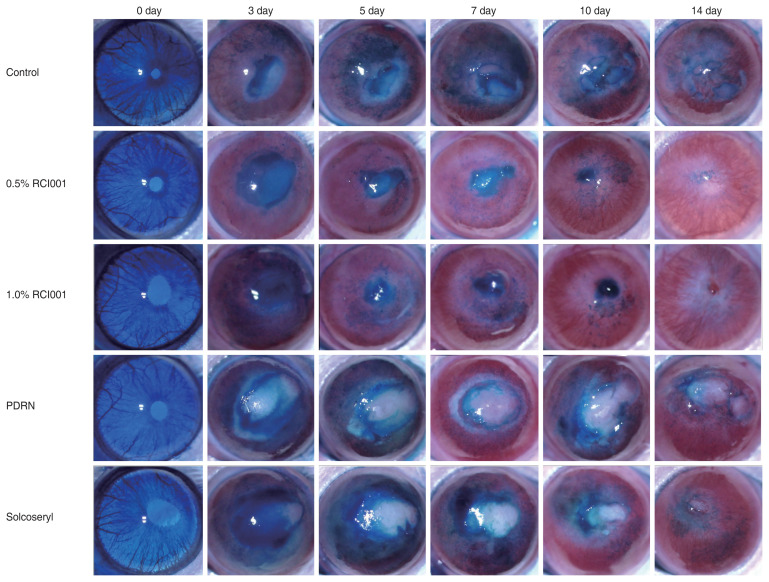
Topical application of RCI001 following alkali corneal burn accelerated healing of epithelial defects and decreased stromal opacity when compared with other two agents (Fig. 1). Starting from initial modeling, we could observe stromal opacity in all groups, which generally peaked at day 5 and 7 and diminished by day 14.
Fig. 1.
Representative cornea images of the control, 0.5% RCI001, 1.0% RCI001, Solcoseryl, and polydeoxyribonucleotide (PDRN) groups from days 0 to 14 following corneal alkali burn. The 0.5% and 1.0% RCI001 groups showed faster corneal epithelial healing and less stromal opacity.
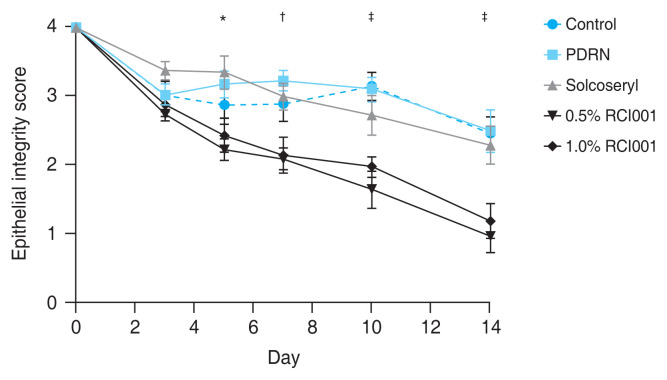
In regards to epithelial healing, the 0.5% and 1.0% RCI001 groups showed significantly better epithelial integrity scores than the control group starting from day 5 (day 5, p < 0.05; day 7, p < 0.01; day 10, p < 0.001; day 14, p < 0.001) (Fig. 2). The mean epithelial integrity scores in the control, PDRN, Solcoseryl, 0.5% RCI001, and 1.0% RCI001 groups on day 7 were 2.90 ± 0.26, 3.23 ± 0.15, 3.00 ± 0.19, 2.10 ± 0.16, and 2.15 ± 0.26, respectively. On day 14, the mean epithelial integrity scores in the control, PDRN, Solcoseryl, 0.5% RCI001, and 1.0% RCI001 groups were 2.47 ± 0.24, 2.50 ± 0.31, 2.30 ± 0.28, 0.97 ± 0.23, and 1.20 ± 0.25, respectively (Fig 2). While the Solcoseryl and PDRN groups did show a trend of improving epithelial integrity, their scores did not differ significantly from the control group. There was no significant difference between the two RCI001 groups. The area percentage analysis showed that at 1 week of treatment, 0.5% and 1.0% RCI001 groups showed significantly more epithelial healing compared to the PDRN group (p = 0.009, p = 0.013, respectively) (Table 1). On day 7, the control, PDRN, and Solcoseryl groups had 27.5%, 19.4%, and 25.0% healing of epithelial defects respectively, whereas the in 0.5% and 1.0% RCI001-treated groups exhibited 47.5% and 46.3% improvements, respectively. After 2 weeks of treatment, the two RCI001 groups each had 75.8% and 70.0% healed corneas, both significantly greater than the PDRN group (p = 0.019, p = 0.047, respectively). The control, PDRN, and Solcoseryl groups had 38.3%, 37.5%, 42.5% healing of epithelial defects, respectively (Table 1).
Fig. 2.
Comparative analysis of corneal epithelial integrity scores of the control, 0.5% RCI001, 1.0% RCI001, Solcoseryl, and polydeoxyribonucleotide (PDRN) groups from 0 to 14 days following corneal alkali burn. Starting from day 5, 0.5% and 1.0% RCI001 groups showed significant improvement of epithelial integrity compared to the control group. The Solcoseryl and PDRN groups did not significantly differ from the control group. *p < 0.05; †p < 0.01; ‡p < 0.001.
Table 1.
Comparison of corneal epithelial healing rates at 7 and 14 days after treatment
| Group | Corneal epithelial healing rate* (%) | |||
|---|---|---|---|---|
| 7 day | p-value† | 14 day | p-value† | |
| Control | 27.5 ± 7.1 | 0.999 | 38.3 ± 7.4 | 0.999 |
| PDRN | 19.4 ± 8.7 | NA | 37.5 ± 4.9 | NA |
| Solcoceryl | 25.0 ± 4.9 | 0.999 | 42.5 ± 4.1 | 0.999 |
| 0.5% RCI001 | 47.5 ± 7.1 | 0.009‡ | 75.8 ± 9.5 | 0.019‡ |
| 1.0% RCI001 | 46.3 ± 6.4 | 0.013‡ | 70.0 ± 7.2 | 0.047‡ |
| p-value‡ | 0.001 | NA | 0.001 | NA |
Values are presented as mean ± standard deviation.
PDRN = polydeoxyribonucleotide; NA, not applicable.
Corneal epithelial healing rate = epithelial healing area/total corneal area;
Dunn test for multiple comparisons (comparison with PDRN group);
Kruskal-Wallis test.
RCI001 groups showed less disruption of the corneal epithelium and inflammatory cell infiltration into the stroma, with improved stromal edema
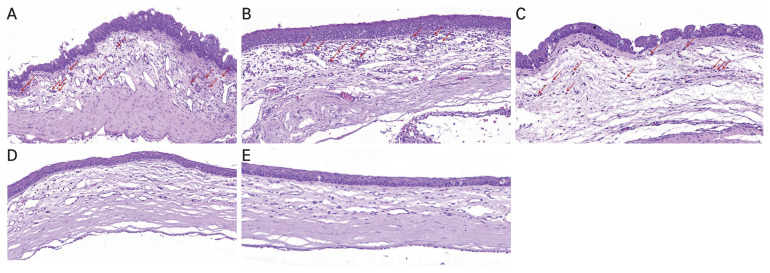
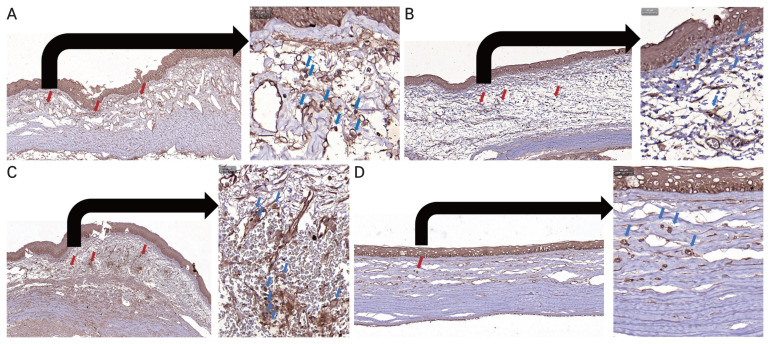
H&E staining revealed that following alkali-induced corneal burn, RCI001 treatment resulted in less stromal edema and epithelial irregularity compared to the control group (Fig. 3A–3E, 4A–4D). Although the differences in inflammatory cell and TNF-α–positive cell infiltration did not reach statistical significance due to the small sample size (n = 2), there was a trend indicating that RCI001 treated groups had fewer inflammatory cells and TNF-α–positive cells infiltrating the cornea (Fig. 3A–3E, 4A–4D). The control group showed considerable penetration of inflammatory cells, and the superficial epithelium and anterior stromal surface were irregular. The PDRN and Solcoseryl groups appeared to have smoother anterior stroma than the control, but there was still notable amount of inflammatory cell infiltration at the stroma. Notably, the PDRN group showed epithelial hyperplasia unlike other treatment groups. Immunohistochemistry results indicated that there was a trend of fewer TNF-α–positive cells in RCI001 treated eyes, whereas in the control group, PDRN, and Solcoseryl treatment groups many TNF-α–positive cells could be found (Fig. 4A–4D). The mean number of TNF-α–positive cells in the 1.0% RCI001 group was 6.5 cells/HPF, whereas for the control, Solcoseryl, and PDRN groups the mean cell count was 76.0, 57.0, and 57.5 cells/HPF, respectively.
Fig. 3.
Representative images of hematoxylin-eosin staining (×100) of the central cornea 2 weeks following corneal alkali burn. (A) Control group. (B) Polydeoxyribonucleotide group. (C) Solcoseryl group. (D) 0.5% RCI001 group. (E) 1.0% RCI001 group. RCI001-treated corneas are more organized and thinner compared to the other groups and show a trend of less inflammatory cell infiltration. Arrows indicate inflammatory cells.
Fig. 4.
Representative images of immunohistochemistry staining (×100, ×400) for tumor necrosis factor α (TNF-α) positive cells 2 weeks following corneal alkali burn. (A) Control group. (B) Polydeoxyribonucleotide group. (C) Solcoseryl group. (D) 1.0% RCI001 group. RCI001 group show a trend of less TNF-α positive cell infiltration. Red and blue arrows indicate the TNF-α positive cells.
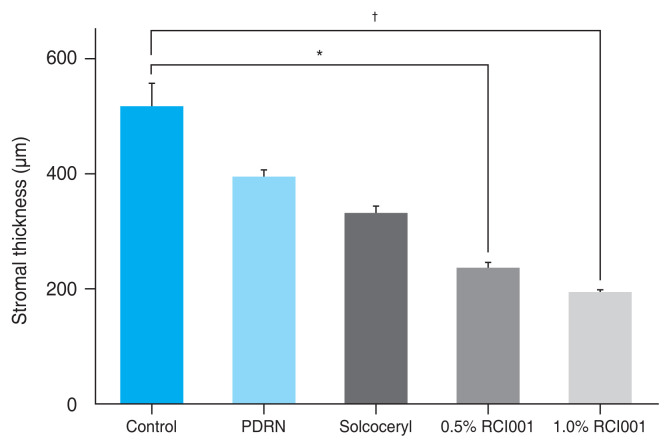
Mean corneal stromal thickness of RCI001 treated eyes were significantly thinner than the control group (Fig. 5). The mean stromal thickness of the control group was 517.8 ± 96.5 μm, while the 0.5% and 1.0% RCI001 groups measured 237.4 ± 22.2 and 195.5 ± 7.5 μm, respectively (p < 0.01 and p < 0.0001, respectively). Stromal thickness of the PDRN group (395.9 ± 26.2 μm) and Solcoseryl group (332.5 ± 28.3 μm) did not significantly differ from the control (each p > 0.05).
Fig. 5.
Comparison of central stromal thickness 2 weeks after corneal alkali burn in the control, 0.5% RCI001, 1.0% RCI001, Solcoseryl, and polydeoxyribonucleotide (PDRN) groups. The 0.5% and 1.0% RCI001 groups had significantly thinner stroma than the control group. The Solcoseryl and PDRN groups showed no statistical difference from the control group. *p < 0.01; †p < 0.0001.
Discussion
This study demonstrated that the 2-week topical application of RCI001 resulted in significant improvement of corneal epithelial healing in a murine ocular alkali burn model, unlike the two other agents, Solcoseryl and PDRN. Furthermore, RCI001 showed a significant decrease in stromal edema and a trend of less inflammatory cell infiltration.
Insults to the cornea result in oxidative stress and inflammation [19]. The damaged corneal epithelium releases reactive oxygen species (ROS) and proinflammatory cytokines which lead to recruitment of innate immune cells such as neutrophils and macrophages [20,21]. Excessive sterile inflammation has been shown to impede corneal epithelial healing [22], so early suppression of inflammation is crucial to minimize damage. Given that inflammation is controlled, the following sequence of events occurs to heal the epithelium [1]: corneal epithelial cells begin to synthesize various cytoskeletal proteins and cell surface receptors to prepare for migration [23,24]. Integrins that normally anchor the basal cells to the basement membrane dissociate and redistribute to act as adhesion molecules to the extracellular matrix (ECM) [25]. After a single layer of epithelium migrates centripetally to cover the defect area, transient amplifying cells, differentiated from limbal stem cells, proliferate to restore normal thickness [26,27]. Then the basal epithelial cells regenerate the basement membrane and reestablish junctional apparatus to attach the epithelium to the underlying stroma [26,28]. If there is excessive damage, poor epithelial proliferation due to limbal cell insufficiency, or uncontrolled inflammation, the epithelium may not heal promptly, and through the defective basement membrane, cytokines such as transforming growth factor β and platelet-derived growth factor penetrate the stroma to stimulate development of stromal keratocytes into myofibroblasts [2]. These cells then produce disordered ECM that result in stromal opacity and decreased vision [29].
The significant wound healing effects of RCI001 for alkali burns, compared to the lack of effectiveness of PDRN and Solcoseryl, may be attributed to the potent anti-inflammatory properties of RCI001, as opposed to the limited effects in the other two agents. Of note, Solcoseryl has recently stopped its production in Korea, failing to undergo clinical trials after being designated for clinical reevaluation. Also, PDRN has only recently been launched as limited over-the-counter products and is not widely evaluated as primary therapy for ocular surface damage. Solcoseryl is a deproteinized calf-blood extract containing amino acids, lipids, small peptides, and nucleotides that support wound healing in trauma, burns, and ulcers [6,30–33]. At the cornea, Solcoseryl shows accelerated epithelial healing in dry eye and foreign body injuries [4,8–10]. Its mechanism has been suggested to work by enhancing corneal epithelial cell migration and proliferation, with increased expression of mucin genes [7]. In another experiment, rat cornea treated with Solcoseryl after alkali burn showed a trend of improved epithelial healing, but its difference from the control did not reach statistical significance, which agrees with the results of our study [34]. These findings suggest that though Solcoseryl may enhance epithelial cell migration and proliferation, it may lack the anti-inflammatory properties needed in the acute phase of chemical burns; therefore, limited healing effects were observed in this study.
PDRN is a DNA drug extracted from salmon sperm cells, consisting of deoxyribonucleotides that activate adenosine A2a receptors [11]. It is known to improve wound healing by stimulating cell proliferation, improving tissue oxygenation, and anti-inflammatory effects [11]. In a rat dry eye model, PDRN reduced fluorescein staining and inflammatory cell infiltration [35] and following photorefractive keratectomy PDRN treatment stimulated corneal epithelium regeneration [5]. In a zebrafish corneal chemical burn model, PDRN accelerated corneal re-epithelialization and suppressed the increase of TNF-α and matrix metalloproteinases expression [36]. In our study, histology showed that PDRN treated corneas were more organized than the control group, and there was epithelial hyperplasia (Fig. 3A–3E). This agrees with previous studies stating PDRN enhances cell proliferation and helps tissue repair [11,36]. However epithelial healing did not significantly differ from control and the reported anti-inflammatory effect was not evident in our study; it is possible that the twice daily application is insufficient to reach the therapeutic dose, failing to control inflammation for prompt epithelial healing.
Meanwhile, RCI001 significantly enhanced epithelial healing to more than 70% at 2 weeks. This may be associated with the known robust anti-inflammatory and antioxidative effect of RCI001. In experiments of various inflammatory conditions (such as metabolic syndrome, dermatitis, gastritis, and atherosclerosis), RCI001 has demonstrated significant anti-inflammatory effect [37–40]. Though our study did not reveal significant difference of inflammatory cell infiltration due to small sample size, there was a trend of less inflammatory cell infiltration to the cornea in RCI001 groups. Moreover, previous studies of mouse ocular chemical burns showed one-week treatment with RCI001 yielded significantly less neutrophil and macrophage infiltration, with reduction of inflammatory markers interleukin 1β (IL-1β), IL-6, and TNF-α [14,15]. In addition, RCI001 was found to repress total ROS levels, with reduced Nox2 and Nox4 messenger RNA expression [15]. The anti-inflammatory and antioxidative mechanism are known to be through Rac1 inhibition in neutrophils and macrophages [40–42]. Since Rac1 pathway leads to ROS production, chemotaxis, and cytokine release [43], inhibiting Rac1 with RCI001 would exert anti-inflammatory effects and support corneal epithelial wound healing. Moreover, in an ocular chemical burn model RCI001 not only inhibited Rac1 but also the NLRP3 inflammasome [12]. Inhibition of NLRP3 inflammasome is known improve corneal epithelial healing in dry eye disease and ocular alkali burns [44–46]. These previous studies indicate that RCI001 can multimodally benefit corneal wound healing in alkali burn, in terms of anti-inflammation and anti-oxidative properties.
In conclusion, our findings revealed that in an ocular alkali burn model, 2-week treatment of RCI001 significantly improved corneal epithelial healing compared to Solcoseryl and PDRN. While further research is needed to optimize dosage and confirm efficacy in other conditions, we believe that topical RCI001 may be a promising therapeutic agent for ocular surface diseases.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Dong Hyun Kim invented a patent for the topical use of RCI001 as treatment of various ocular diseases (No. 10-1816277 [Korea], No. 10/675294 [USA]). Yong Ho Kim is the Chief Executive Officer of RudaCare, the company developing RCI001, and Young Ah Ku and Seunghoon Kim are employees at RudaCure. No other potential conflicts of interest relevant to this article were reported.
Funding: This work was supported by the Basic Science Research Program (No. NRF-2020R1C1C1007372) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT of Korea.
References
- 1.Agrawal VB, Tsai RJ. Corneal epithelial wound healing. Indian J Ophthalmol. 2003;51:5–15. [PubMed] [Google Scholar]
- 2.Wilson SE. Corneal wound healing. Exp Eye Res. 2020;197:108089. doi: 10.1016/j.exer.2020.108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krannig HM, Rohde-Germann H, Straub W. Therapy of corneal erosions and ‘dry eye’ with Solcoseryl and Vitasic eye drops. Ophthalmologica. 1989;199:100–5. doi: 10.1159/000310024. [DOI] [PubMed] [Google Scholar]
- 5.Lazzarotto M, Tomasello EM, Caporossi A. Clinical evaluation of corneal epithelialization after photorefractive keratectomy in a patient treated with Polydeoxyribonucleotide (PDRN) eye drops: a randomized, double-blind, placebo-controlled trial. Eur J Ophthalmol. 2004;14:284–9. doi: 10.5301/EJO.2008.2105. [DOI] [PubMed] [Google Scholar]
- 6.Wilmink JM, Stolk PW, van Weeren PR, Barneveld A. The effectiveness of the haemodialysate Solcoseryl for second-intention wound healing in horses and ponies. J Vet Med A Physiol Pathol Clin Med. 2000;47:311–20. doi: 10.1046/j.1439-0442.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 7.Nam SM, Maeng YS. Wound healing and mucin gene expression of human corneal epithelial cells treated with deproteinized extract of calf blood. Curr Eye Res. 2019;44:1181–8. doi: 10.1080/02713683.2019.1633360. [DOI] [PubMed] [Google Scholar]
- 8.Erbe W, Herrmann R, Korner WF, Rohde-Germann H, Straub W. Our experience with Solcoseryl Eye-Gel in the treatment of corneal lesions: a randomised double-blind study (with 1 color plate) Ophthalmologica. 1984;188:1–4. doi: 10.1159/000309336. [DOI] [PubMed] [Google Scholar]
- 9.Studer O. A comparative clinical study of Solcoseryl Eye-Gel and Cysteine Eye-Gel 2.4% in the treatment of foreign-body injuries of the cornea. Ophthalmic Res. 1984;16:179–84. doi: 10.1159/000265314. [DOI] [PubMed] [Google Scholar]
- 10.Egger SF, Huber-Spitzy V, Alzner E, et al. Corneal wound healing after superficial foreign body injury: vitamin A and dexpanthenol versus a calf blood extract: a randomized double-blind study. Ophthalmologica. 1999;213:246–9. doi: 10.1159/000027429. [DOI] [PubMed] [Google Scholar]
- 11.Squadrito F, Bitto A, Irrera N, et al. Pharmacological activity and clinical use of PDRN. Front Pharmacol. 2017;8:224. doi: 10.3389/fphar.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, Jang YW, Ku YA, et al. Investigating the anti-inflammatory effects of RCI001 for treating ocular surface diseases: insight into the mechanism of action. Front Immunol. 13:850287. doi: 10.3389/fimmu.2022.850287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chernikov AV, Gudkov SV, Usacheva AM, Bruskov VI. Exogenous 8-Oxo-7,8-dihydro-2′-deoxyguanosine: biomedical properties, mechanisms of action, and therapeutic potential. Biochemistry (Mosc) 2017;82:1686–701. doi: 10.1134/S0006297917130089. [DOI] [PubMed] [Google Scholar]
- 14.Im ST, Kim HY, Yoon JY, et al. Therapeutic effects of topical 8-Oxo-2′-deoxyguanosine on ethanol-induced ocular chemical injury models. Cornea. 2018;37:1311–7. doi: 10.1097/ICO.0000000000001671. [DOI] [PubMed] [Google Scholar]
- 15.Kim DH, Im ST, Yoon JY, et al. Comparison of therapeutic effects between topical 8-oxo-2′-deoxyguanosine and corticosteroid in ocular alkali burn model. Sci Rep. 2021;11:6909. doi: 10.1038/s41598-021-86440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DH, Jung Y, Moon JY, et al. Therapeutic effects of topical RCI001 on environmental and inflammation-related dry eye mouse models. Invest Ophthalmol Vis Sci. 62:1322. [Google Scholar]
- 17.Oh JY, Roddy GW, Choi H, et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A. 2010;107:16875–80. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choy EP, Cho P, Benzie IF, et al. A novel porcine dry eye model system (pDEM) with simulated lacrimation/blinking system: preliminary findings on system variability and effect of corneal drying. Curr Eye Res. 2004;28:319–25. doi: 10.1076/ceyr.28.5.319.28681. [DOI] [PubMed] [Google Scholar]
- 19.Kubota M, Shimmura S, Kubota S, et al. Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Invest Ophthalmol Vis Sci. 2011;52:427–33. doi: 10.1167/iovs.10-6167. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SE, Mohan RR, Mohan RR, et al. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–37. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 21.Gu XJ, Liu X, Chen YY, et al. Involvement of NADPH oxidases in alkali burn-induced corneal injury. Int J Mol Med. 2016;38:75–82. doi: 10.3892/ijmm.2016.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortingo N, Melnyk S, Sutton SH, et al. Innate immune system activation, inflammation and corneal wound healing. Int J Mol Sci. 2022;23:14933. doi: 10.3390/ijms232314933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zieske JD, Gipson IK. Protein synthesis during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1986;27:1–7. [PubMed] [Google Scholar]
- 24.Panjwani N, Michalopoulos G, Song J, et al. Neutral glycolipids of migrating and nonmigrating rabbit corneal epithelium in organ and cell culture. Invest Ophthalmol Vis Sci. 1990;31:689–95. [PubMed] [Google Scholar]
- 25.Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006;83:3–15. doi: 10.1016/j.exer.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Carter RT. The role of integrins in corneal wound healing. Vet Ophthalmol. 2009;12(Suppl 1):2–9. doi: 10.1111/j.1463-5224.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- 27.Zernii EY, Baksheeva VE, Yani EV, et al. Therapeutic proteins for treatment of corneal epithelial defects. Curr Med Chem. 2019;26:517–45. doi: 10.2174/0929867324666170609080920. [DOI] [PubMed] [Google Scholar]
- 28.Wilson SE, Marino GK, Torricelli AA, Medeiros CS. Injury and defective regeneration of the epithelial basement membrane in corneal fibrosis: a paradigm for fibrosis in other organs? Matrix Biol. 2017;64:17–26. doi: 10.1016/j.matbio.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaidyanathan U, Hopping GC, Liu HY, et al. Persistent corneal epithelial defects: a review article. Med Hypothesis Discov Innov Ophthalmol. 2019;8:163–76. [PMC free article] [PubMed] [Google Scholar]
- 30.Isler H, Bauen A, Baschong W. Topical treatment of standardized burns with a protein-free haemodialysate. Burns. 1991;17:93–7. doi: 10.1016/0305-4179(91)90130-9. [DOI] [PubMed] [Google Scholar]
- 31.Isler H, Bauen A, Hubler M, Oberholzer M. Morphometric assessment of wound healing in rats treated with a protein-free haemodialysate. Burns. 1991;17:99–103. doi: 10.1016/0305-4179(91)90131-y. [DOI] [PubMed] [Google Scholar]
- 32.Konturek SJ, Brzozowski T, Dembinski A, et al. Comparison of solcoseryl and epidermal growth factors (EGF) in healing of chronic gastroduodenal ulcerations and mucosal growth in rats. Hepatogastroenterology. 1988;35:25–9. [PubMed] [Google Scholar]
- 33.Niinikoski J, Laato M, Tschannen R, Fraefel W. Effect of a hexosylceramide fraction of the hemodialysate Solcoseryl on wound-healing angiogenesis. J Surg Res. 1986;40:261–4. doi: 10.1016/0022-4804(86)90159-9. [DOI] [PubMed] [Google Scholar]
- 34.Kim H, Kim HB, Seo JH, et al. Effect of Solcoseryl in corneal alkali burn rat model. Med Lasers. 2021;10:22–30. [Google Scholar]
- 35.Choi JS, Joo CK. Polydeoxyribonucleotide (PDRN) inhibits corneal inflammation in experimental rat keratoconjunctivitis sicca model. Invest Ophthalmol Vis Sci. 2016;57:5730. [Google Scholar]
- 36.Edirisinghe SL, Nikapitiya C, Dananjaya SH, et al. Effect of polydeoxyribonucleotide (PDRN) treatment on corneal wound healing in Zebrafish (Danio rerio) Int J Mol Sci. 2022;23:13525. doi: 10.3390/ijms232113525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko SH. Lee JK, Lee HJ, et al. 8-Oxo-2′-deoxyguanosine ameliorates features of metabolic syndrome in obese mice. Biochem Biophys Res Commun. 2014;443:610–6. doi: 10.1016/j.bbrc.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Lee JK, Ko SH, Ye SK, Chung MH. 8-Oxo-2′-deoxyguanosine ameliorates UVB-induced skin damage in hairless mice by scavenging reactive oxygen species and inhibiting MMP expression. J Dermatol Sci. 2013;70:49–57. doi: 10.1016/j.jdermsci.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Ock CY, Hong KS, Choi KS, et al. A novel approach for stress-induced gastritis based on paradoxical anti-oxidative and anti-inflammatory action of exogenous 8-hydroxydeoxyguanosine. Biochem Pharmacol. 2011;81:111–22. doi: 10.1016/j.bcp.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 40.Huh JY, Son DJ, Lee Y, et al. 8-Hydroxy-2-deoxyguanosine prevents plaque formation and inhibits vascular smooth muscle cell activation through Rac1 inactivation. Free Radic Biol Med. 2012;53:109–21. doi: 10.1016/j.freeradbiomed.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HS, Ye SK, Cho IH, et al. 8-hydroxydeoxyguanosine suppresses NO production and COX-2 activity via Rac1/STATs signaling in LPS-induced brain microglia. Free Radic Biol Med. 2006;41:1392–403. doi: 10.1016/j.freeradbiomed.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Lee SH, Han ST, Choi SW, et al. Inhibition of Rac and Rac-linked functions by 8-oxo-2′-deoxyguanosine in murine macrophages. Free Radic Res. 2009;43:78–84. doi: 10.1080/10715760802609432. [DOI] [PubMed] [Google Scholar]
- 43.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–62. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 44.Bian F, Xiao Y, Zaheer M, et al. Inhibition of NLRP3 inflammasome pathway by butyrate improves corneal wound healing in corneal alkali burn. Int J Mol Sci. 18:562. doi: 10.3390/ijms18030562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Wan L, Zhang Z, et al. Topical calcitriol application promotes diabetic corneal wound healing and reinnervation through inhibiting NLRP3 inflammasome activation. Exp Eye Res. 2021;209:108668. doi: 10.1016/j.exer.2021.108668. [DOI] [PubMed] [Google Scholar]
- 46.Zheng Q, Ren Y, Reinach PS, et al. Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye. Exp Eye Res. 2014;125:1–8. doi: 10.1016/j.exer.2014.05.001. [DOI] [PubMed] [Google Scholar]