Abstract
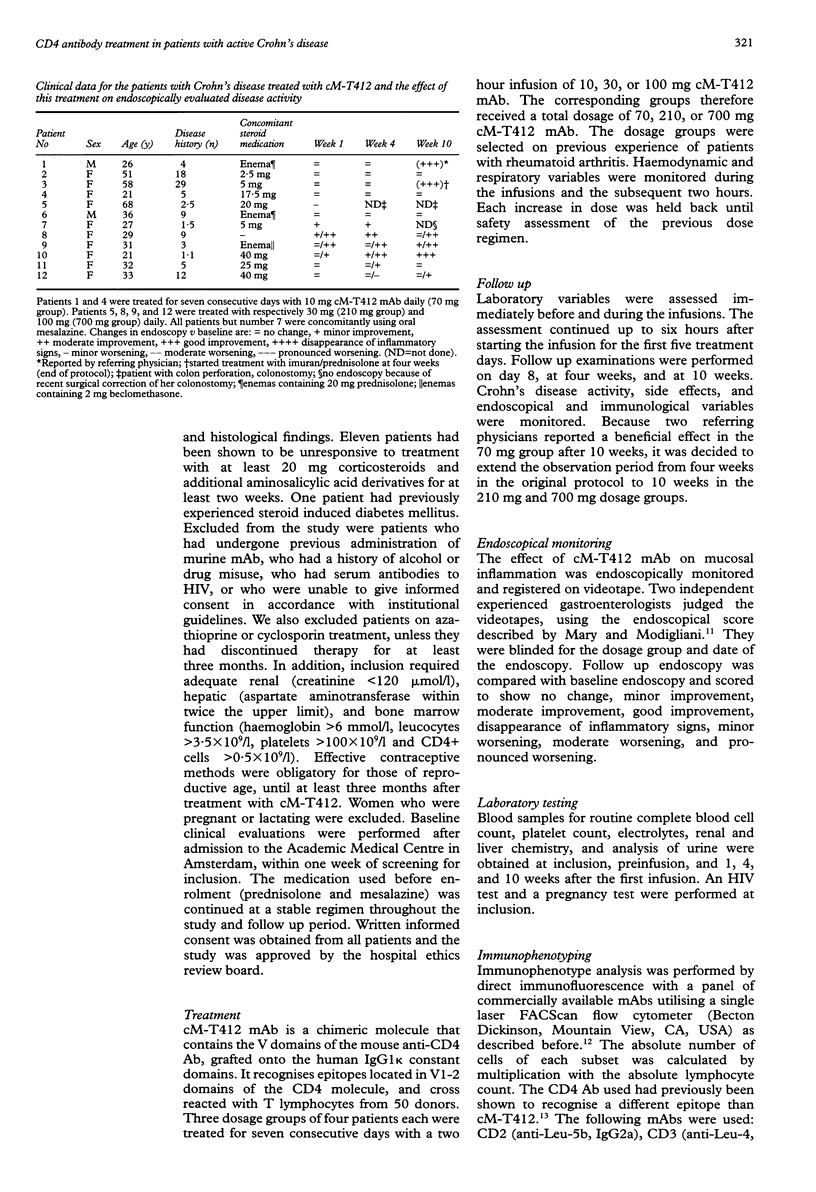
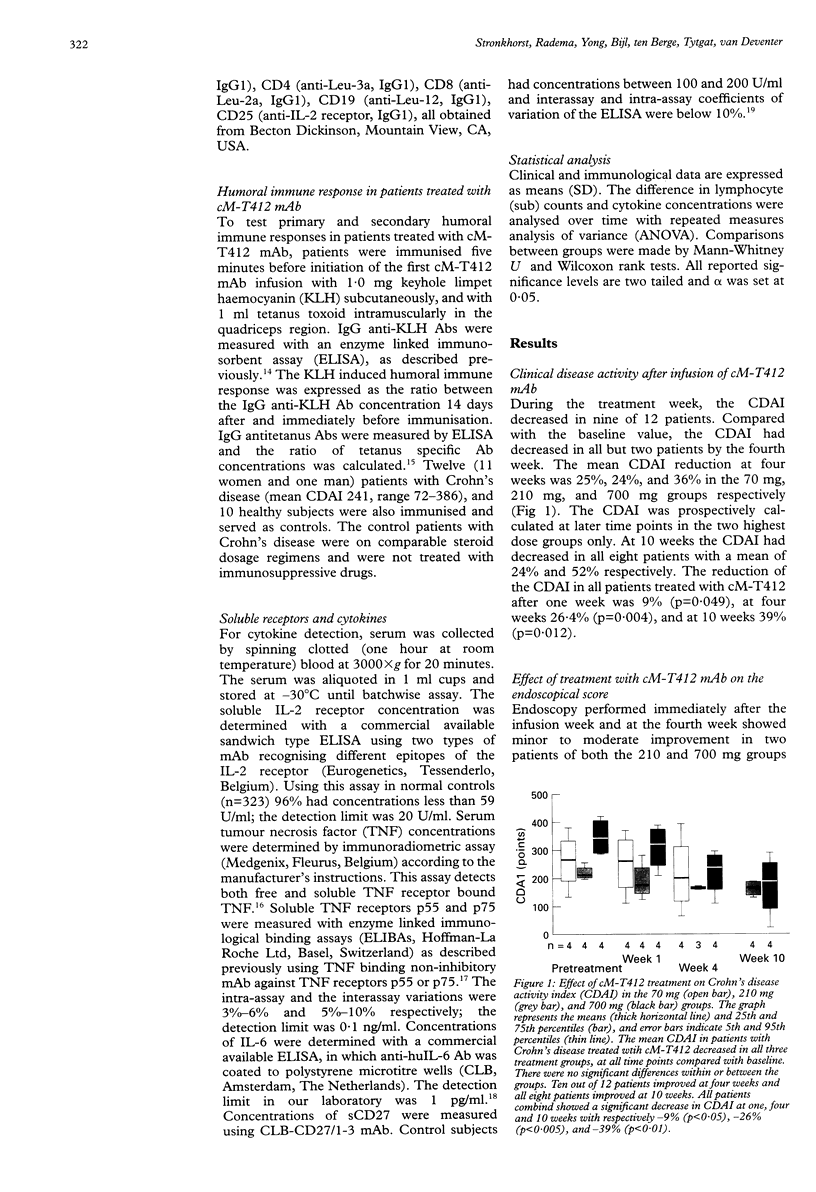
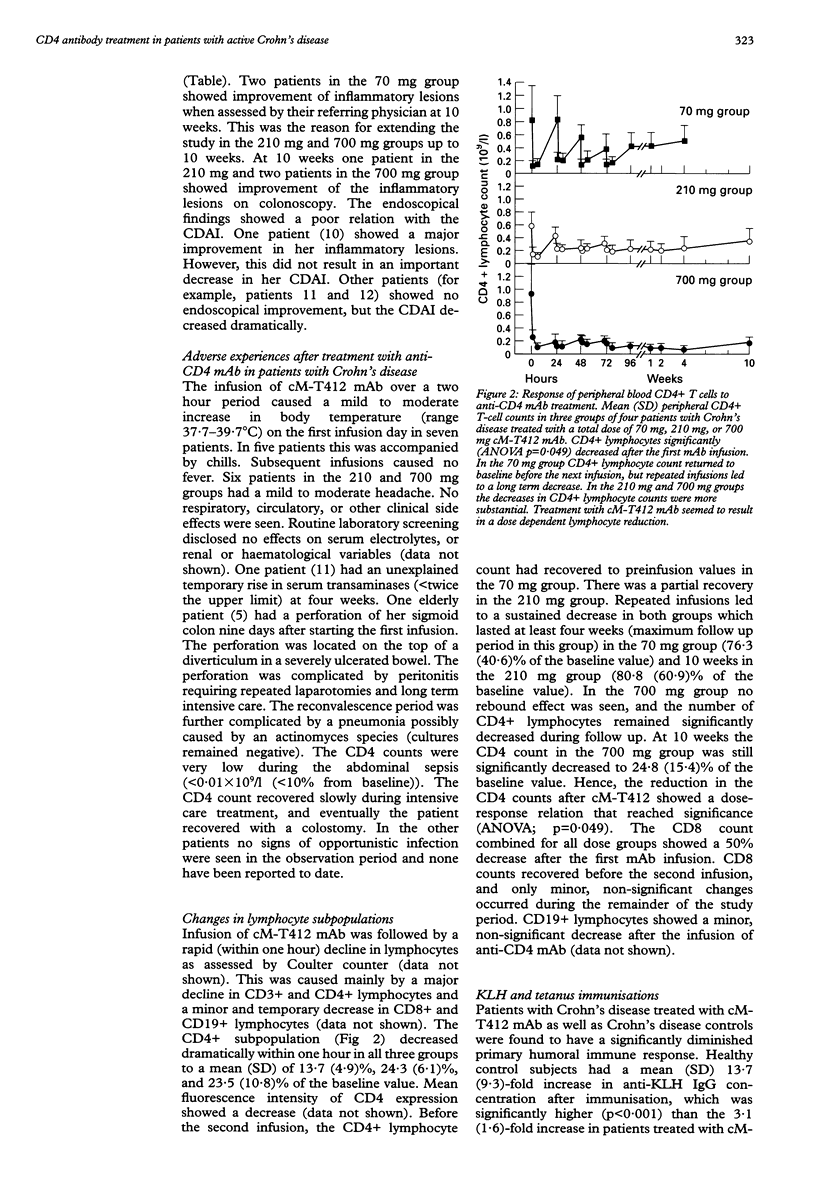
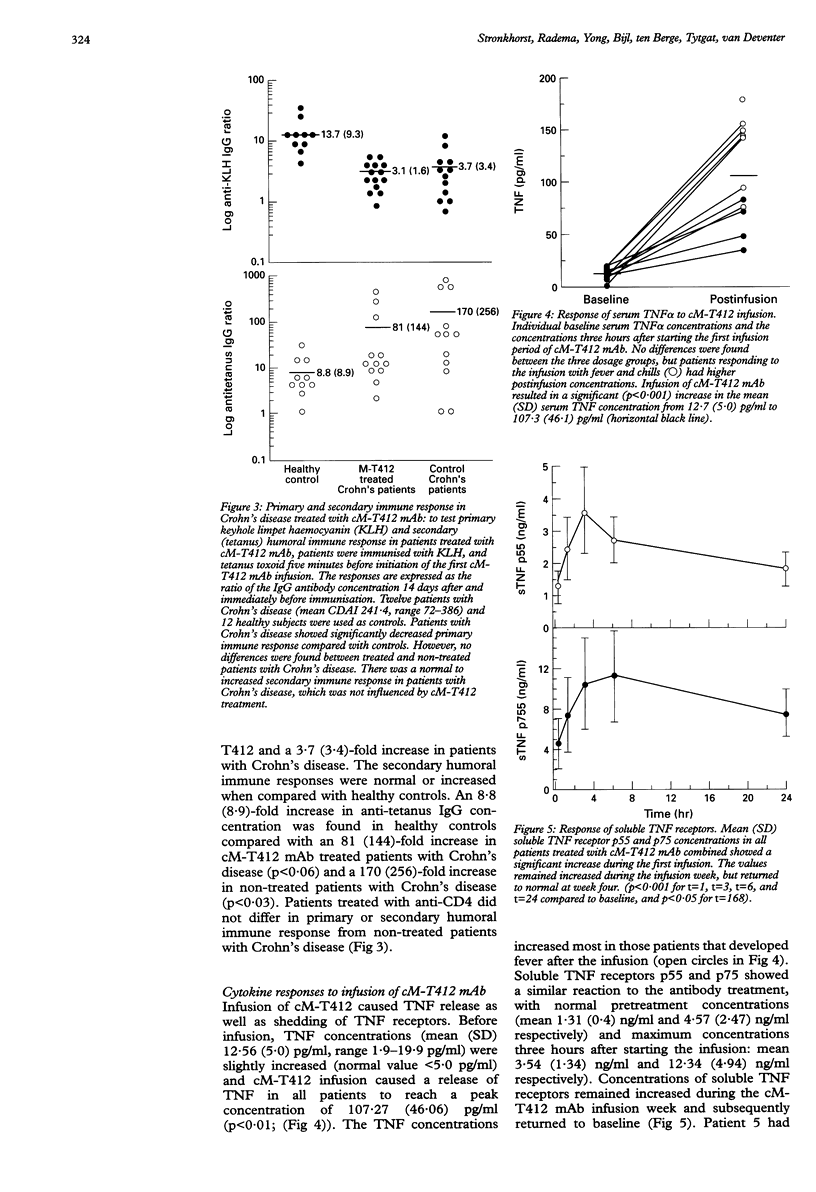
BACKGROUND: T cells play an important part in Crohn's disease. Immunomodulating therapies that target T cell activation may have clinical effects in Crohn's disease. AIM: To investigate the toxicity and potential efficacy of anti-CD4 monoclonal antibody therapy in patients with Crohn's disease. PATIENTS AND METHODS: A dose escalating pilot study was conducted in three groups of four patients with intractable Crohn's disease, refractory to steroids. They received 70, 210, or 700 mg of cM-T412, a depleting anti-CD4 monoclonal antibody (mAb). RESULTS: The mean reduction in Crohn's disease activity index (CDAI) was respectively 25%, 24%, and 36% at four weeks, and 24% and 52% at 10 weeks in the 210 mg and 700 mg groups. There was only a minor effect on endoscopically evaluated disease activity. Side effects were mild to moderate fever with chills and headache. No signs of opportunistic infection were seen. There was a sustained decrease in CD4 count which lasted at least four weeks in the 70 mg group (76.3 (SD 40.6)% of the baseline value), and 10 weeks in both the 210 mg group (80.8 (SD 60.9)%) and the 700 mg group (24.8 (SD 15.4)%). The primary and secondary humoral immune response was not influenced by anti-CD4 mAb treatment. CONCLUSION: This study shows the moderate potential efficacy of treatment of patients with Crohn's disease using a depleting chimeric monoclonal anti-CD4 antibody.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Brynskov J., Freund L., Rasmussen S. N., Lauritsen K., de Muckadell O. S., Williams N., MacDonald A. S., Tanton R., Molina F., Campanini M. C. A placebo-controlled, double-blind, randomized trial of cyclosporine therapy in active chronic Crohn's disease. N Engl J Med. 1989 Sep 28;321(13):845–850. doi: 10.1056/NEJM198909283211301. [DOI] [PubMed] [Google Scholar]
- Choy E. H., Chikanza I. C., Kingsley G. H., Corrigall V., Panayi G. S. Treatment of rheumatoid arthritis with single dose or weekly pulses of chimaeric anti-CD4 monoclonal antibody. Scand J Immunol. 1992 Aug;36(2):291–298. doi: 10.1111/j.1365-3083.1992.tb03102.x. [DOI] [PubMed] [Google Scholar]
- Choy E. H., Kingsley G. H., Panayi G. S. Treatment with anti-CD4 monoclonal antibody and acute interstitial nephritis. Arthritis Rheum. 1993 May;36(5):723–724. doi: 10.1002/art.1780360523. [DOI] [PubMed] [Google Scholar]
- Choy M. Y., Walker-Smith J. A., Williams C. B., MacDonald T. T. Differential expression of CD25 (interleukin-2 receptor) on lamina propria T cells and macrophages in the intestinal lesions in Crohn's disease and ulcerative colitis. Gut. 1990 Dec;31(12):1365–1370. doi: 10.1136/gut.31.12.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christadoss P., Dauphinee M. J. Immunotherapy for myasthenia gravis: a murine model. J Immunol. 1986 Apr 1;136(7):2437–2440. [PubMed] [Google Scholar]
- Devey M. E., Bleasdale K., Lee S., Rath S. Determination of the functional affinity of IgG1 and IgG4 antibodies to tetanus toxoid by isotype-specific solid-phase assays. J Immunol Methods. 1988 Jan 21;106(1):119–125. doi: 10.1016/0022-1759(88)90279-7. [DOI] [PubMed] [Google Scholar]
- Feagan B. G., McDonald J. W., Rochon J., Laupacis A., Fedorak R. N., Kinnear D., Saibil F., Groll A., Archambault A., Gillies R. Low-dose cyclosporine for the treatment of Crohn's disease. The Canadian Crohn's Relapse Prevention Trial Investigators. N Engl J Med. 1994 Jun 30;330(26):1846–1851. doi: 10.1056/NEJM199406303302602. [DOI] [PubMed] [Google Scholar]
- Godfried M. H., van der Poll T., Jansen J., Romijin J. A., Schattenkerk J. K., Endert E., van Deventer S. J., Sauerwein H. P. Soluble receptors for tumour necrosis factor: a putative marker of disease progression in HIV infection. AIDS. 1993 Jan;7(1):33–36. [PubMed] [Google Scholar]
- Goronzy J., Weyand C. M., Fathman C. G. Long-term humoral unresponsiveness in vivo, induced by treatment with monoclonal antibody against L3T4. J Exp Med. 1986 Sep 1;164(3):911–925. doi: 10.1084/jem.164.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle M., Boeije L., de Groot E., de Vos A., Aarden L. Sensitive ELISA for interleukin-6. Detection of IL-6 in biological fluids: synovial fluids and sera. J Immunol Methods. 1991 Apr 8;138(1):47–56. doi: 10.1016/0022-1759(91)90063-l. [DOI] [PubMed] [Google Scholar]
- Herzog C., Walker C., Müller W., Rieber P., Reiter C., Riethmüller G., Wassmer P., Stockinger H., Madic O., Pichler W. J. Anti-CD4 antibody treatment of patients with rheumatoid arthritis: I. Effect on clinical course and circulating T cells. J Autoimmun. 1989 Oct;2(5):627–642. doi: 10.1016/s0896-8411(89)80002-2. [DOI] [PubMed] [Google Scholar]
- Hintzen R. Q., de Jong R., Hack C. E., Chamuleau M., de Vries E. F., ten Berge I. J., Borst J., van Lier R. A. A soluble form of the human T cell differentiation antigen CD27 is released after triggering of the TCR/CD3 complex. J Immunol. 1991 Jul 1;147(1):29–35. [PubMed] [Google Scholar]
- James S. P. Remission of Crohn's disease after human immunodeficiency virus infection. Gastroenterology. 1988 Dec;95(6):1667–1669. doi: 10.1016/s0016-5085(88)80094-5. [DOI] [PubMed] [Google Scholar]
- Julius M., Maroun C. R., Haughn L. Distinct roles for CD4 and CD8 as co-receptors in antigen receptor signalling. Immunol Today. 1993 Apr;14(4):177–183. doi: 10.1016/0167-5699(93)90282-p. [DOI] [PubMed] [Google Scholar]
- Kanof M. E., Strober W., Fiocchi C., Zeitz M., James S. P. CD4 positive Leu-8 negative helper-inducer T cells predominate in the human intestinal lamina propria. J Immunol. 1988 Nov 1;141(9):3029–3036. [PubMed] [Google Scholar]
- Mary J. Y., Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d'Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989 Jul;30(7):983–989. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel P., Nicolas J. F., Wijdenes J., Revillard J. P. Down-regulation of lymphocyte CD4 antigen expression by administration of anti-CD4 monoclonal antibody. Clin Immunol Immunopathol. 1992 Sep;64(3):248–253. doi: 10.1016/0090-1229(92)90207-5. [DOI] [PubMed] [Google Scholar]
- Moreland L. W., Bucy R. P., Tilden A., Pratt P. W., LoBuglio A. F., Khazaeli M., Everson M. P., Daddona P., Ghrayeb J., Kilgarriff C. Use of a chimeric monoclonal anti-CD4 antibody in patients with refractory rheumatoid arthritis. Arthritis Rheum. 1993 Mar;36(3):307–318. doi: 10.1002/art.1780360304. [DOI] [PubMed] [Google Scholar]
- Parlevliet K. J., ten Berge I. J., Yong S. L., Surachno J., Wilmink J. M., Schellekens P. T. In vivo effects of IgA and IgG2a anti-CD3 isotype switch variants. J Clin Invest. 1994 Jun;93(6):2519–2525. doi: 10.1172/JCI117262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Correa-Oliveira R., Mauze S., Coffman R. L. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994 Feb 1;179(2):589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasveld M. H., Bemelman F. J., Schellekens P. T., van Diepen F. N., van Dongen A., van Royen E. A., Hack C. E., ten Berge I. J. Complement activation during OKT3 treatment: a possible explanation for respiratory side effects. Kidney Int. 1993 May;43(5):1140–1149. doi: 10.1038/ki.1993.160. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Wendling D., Racadot E., Morel-Fourrier B., Wijdenes J. Treatment of rheumatoid arthritis with anti CD4 monoclonal antibody. Open study of 25 patients with the B-F5 clone. Clin Rheumatol. 1992 Dec;11(4):542–547. doi: 10.1007/BF02283116. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Mayes D. C., Woodcock J., Seaman W. E. Inhibition of humoral immunity in vivo by monoclonal antibody to L3T4: studies with soluble antigens in intact mice. J Immunol. 1985 Sep;135(3):1698–1701. [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J Immunol. 1987 May 15;138(10):3247–3253. [PubMed] [Google Scholar]
- van der Lubbe P. A., Reiter C., Miltenburg A. M., Krüger K., de Ruyter A. N., Rieber E. P., Bijl J. A., Riethmüller G., Breedveld F. C. Treatment of rheumatoid arthritis with a chimeric CD4 monoclonal antibody (cM-T412): immunopharmacological aspects and mechanisms of action. Scand J Immunol. 1994 Mar;39(3):286–294. doi: 10.1111/j.1365-3083.1994.tb03373.x. [DOI] [PubMed] [Google Scholar]
- van der Poll T., van Deventer S. J., Hack C. E., Wolbink G. J., Aarden L. A., Büller H. R., ten Cate J. W. Effects on leukocytes after injection of tumor necrosis factor into healthy humans. Blood. 1992 Feb 1;79(3):693–698. [PubMed] [Google Scholar]


