Abstract
Objective
To examine and quantify the potential dose–response relationship between red and processed meat consumption and risk of all-cause, cardiovascular and cancer mortality.
Design
We searched MEDLINE, Embase, ISI Web of Knowledge, CINHAL, Scopus, the Cochrane library and reference lists of retrieved articles up to 30 November 2014 without language restrictions. We retrieved prospective cohort studies that reported risk estimates for all-cause, cardiovascular and cancer mortality by red and/or processed meat intake levels. The dose–response relationships were estimated using data from red and processed meat intake categories in each study. Random-effects models were used to calculate pooled relative risks and 95 % confidence intervals and to incorporate between-study variations.
Results
Nine articles with seventeen prospective cohorts were eligible in this meta-analysis, including a total of 150 328 deaths. There was evidence of a non-linear association between processed meat consumption and risk of all-cause and cardiovascular mortality, but not for cancer mortality. For processed meat, the pooled relative risk with an increase of one serving per day was 1·15 (95 % CI 1·11, 1·19) for all-cause mortality (five studies; P<0·001 for linear trend), 1·15 (95 % CI 1·07, 1·24) for cardiovascular mortality (six studies; P<0·001) and 1·08 (95 % CI 1·06, 1·11) for cancer mortality (five studies; P<0·001). Similar associations were found with total meat intake. The association between unprocessed red meat consumption and mortality risk was found in the US populations, but not in European or Asian populations.
Conclusions
The present meta-analysis indicates that higher consumption of total red meat and processed meat is associated with an increased risk of total, cardiovascular and cancer mortality.
Keywords: Meta-analyses, Mortality, Meat, Cohort studies
Meat is an important food group in people’s diets in many regions of the world( 1 , 2 ). Meat is a major source of protein and fat, and of also vital vitamins and nutrients such as Fe, Zn, vitamin A and B-vitamins. Overall meat consumption has continued to rise in the USA and other developed countries, although the USA remains the highest consumer of total meat( 3 ). The demand for meat in developing countries is also on the rise, due to increasing economic development( 1 , 4 ).
In recent years, there is growing evidence that red meat consumption is related to premature death, including death from CVD and cancer, but the results are not entirely consistent. Some epidemiological studies have found a positive association between red meat intake, particularly processed meat, and risk of total and cause-specific mortality( 5 – 7 ). However, others have suggested no significant association( 8 – 11 ). The magnitudes of the relationships also varied among studies. Recently, a meta-analysis( 12 ) summarized data from prospective cohort studies and reported that processed meat consumption could increase death from any cause and CVD and that red meat consumption is positively associated with CVD mortality. However, that meta-analysis did not examine cancer mortality outcome and a non-linear association. Also, that meta-analysis included several studies comparing vegetarians with non-vegetarians. In view of the fact that diet and lifestyles of vegetarians may differ from those of non-vegetarians, it is likely that the benefits are not to be ascribed to the absence of meat intake only. In another meta-analysis by O’Sullivan et al.( 13 ), some studies included white meat in the category of total meat. Besides, another study by Larsson and Orsini( 14 ) did not analyse the cause-specific mortality.
Therefore, to provide the most updated and accurate evidence on the relationship between red meat intake and total and cause-specific mortality risk, we performed a meta-analysis of prospective cohort studies and quantified the dose–response relationship.
Methods
Search strategy
We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE)( 15 ) for conducting and reporting the present study. We carried out a meta-analysis of prospective cohort studies that evaluated the association between red meat consumption and risk of all-cause, cardiovascular and cancer mortality. We systematically searched databases, including MEDLINE (since 1950), Embase (since 1980), ISI Web of Knowledge (since 1970), CINAHL (since 1981), Scopus (since 1996) and the Cochrane Library (since 1960), between May 2014 and 30 November 2014 (last date searched). We used a search strategy that included truncated free text and exploded Medical Subject Headings terms. Medical Subject Headings included ‘meat’, ‘meat products’, ‘cardiovascular diseases’, ‘coronary disease’, ‘myocardial ischemia’, ‘stroke’, ‘neoplasms’, ‘mortality’, ‘cause of death’, ‘humans’, ‘epidemiology’, ‘prospective studies’, ‘follow-up studies’ and their variants. No restrictions were imposed on language of publications. We found additional articles by manually searching the reference lists from the extracted articles and recent reviews, and also consultation of expert opinions.
Study selection
We first conducted an initial screening of all titles or abstracts and then assessed all potentially relevant studies based on full-text reviews. To be included, studies had to be prospective cohort studies that included measures of red and/or processed meat intake and the assessment of total, cardiovascular and cancer mortality. We excluded studies with an ecological, case–control or cross-sectional design, or without adjustment for potential confounders, or studies that did not report relative risks (RR) or hazard ratios (HR) and the corresponding 95 % CI.
Validity assessment
Two authors (Y.Y.O. and J.L.) independently assessed the studies for quality by using a modified scoring system. The scoring system was based on MOOSE, Quality Assessment Tool for Systematic Reviews of Observational Studies (QUATSO) and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). The system allowed a total score of 0–6 points (6 representing the highest quality)( 16 ). The system allocated 1 point each when: (i) any justification was given for the cohort; (ii) inclusion and exclusion criteria used were appropriate; (iii) diagnosis of total, cardiovascular and cancer mortality was based on accepted clinical criteria; (iv) participants’ usual red and/or processed meat consumptions was evaluated with a validated tool; (v) adjustments were made for age, sex, BMI and smoking status; and (vi) additional factors were adjusted for (such as physical activity or other dietary factors).
Data extraction
The primary exposure variable was unprocessed red meat and processed meat consumption. Unprocessed red meat was defined as any unprocessed red meat from beef, lamb or pork, and excluded poultry and fish. Processed meat refers to any meat preserved by salting, curing or smoking, or with the addition of chemical preservatives, including bacon, sausages, salami, hot dogs or processed deli meats. Processed meat was mainly processed red meat but may contain some processed white meat, for example in sausages, which could not be separately excluded. Total red meat included the sum of these two categories. Outcomes of interest in this study were all-cause, cardiovascular and cancer mortality. All outcomes were classified based on the WHO International Classification of Disease criteria.
Data extraction was conducted by using a standardized data collection form. The following characteristics of the identified papers were recorded: first author, publication year, cohort name, country, sample size of the cohort and number of outcomes, follow-up (years), age at entry, sex, assessment method of red and processed meat intake, ascertainment of outcomes and variables that entered into the multivariable model as potential confounders. RR and HR were used as a measure of the association. Two authors (J.L. and Y.Y.O.) independently selected studies and performed the data extraction. To resolve discrepancies regarding inclusion of studies and interpretation of data, a third investigator (G.Z.) was consulted. Any disagreements were checked and settled by consensus with all three authors. We also contacted the authors by email to obtain additional data for the meta-analysis.
Statistical method
We used the statistical software package STATA version 11·2 to analyse the data. The results of the original studies from multivariable models with the most complete adjustment for potential confounders were used. HR and RR were assumed to approximate the same measure of RR. We utilized the inverse-variance weighted method to calculate summary RR and 95 % CI of mortality for a one-serving-per-day increase of red and processed meat consumption. We used a random-effects model to account for inter-study variation and to provide a more conservative effect than a fixed-effects model. Between-study heterogeneity was assessed using the Cochran’s Q test (significance level at P<0·10)( 17 ). I 2 tests were also evaluated to quantify the proportion of inconsistency across studies( 18 ). We estimated the dose–response relationship using generalized least-squares trend estimation analysis, according to the methods developed by Greenland and Longnecker( 19 – 21 ). The distributions of cases and person-years, amount of red and processed meat intake, and RR and 95 % CI were extracted for the dose–response meta-analysis.
For each study, the median or mean level of meat intake in each category was assigned to each corresponding RR of that category. If the highest category of the studies was open-ended, the difference from the lowest range to the median was considered to be equivalent to the same difference in the closest adjacent category. The serving size varied across studies; we converted it into the standard serving for the dose–response analysis, which was defined as 100 g (3·5 oz) for total and unprocessed red meat and 50 g (1·8 oz) for processed meat. If the number of person-years was not available, we used the RR comparing the highest v. lowest categories of unprocessed red meat intake to obtain a summary estimate.
In addition, we assessed a potential curvilinear relationship by using restricted cubic splines with five knots at percentiles 5 %, 25 %, 50 %, 75 % and 95 % of the distribution( 22 ). A test for a non-linear relationship was calculated by setting the coefficient of the second spline equal to zero.
To explore the sources of heterogeneity among studies, we carried out subgroup analyses. We evaluated potential publication bias for each outcome with the Egger linear regression test (by performing the regression of log RR v. its se)( 23 ) and the Begg rank correlation test at the P<0·10 level of significance( 24 ). Except where otherwise specified, a P value <0·05 was considered statistically significant.
Results
Literature search
Overall, the search initially identified 5536 reports (see online supplementary material, Fig. S1). After exclusion of duplicates and papers that did not meet the inclusion criteria, we obtained twenty full articles of potentially relevant studies. After full-text reviews, eleven out of the twenty articles were excluded for the following reasons: seven studies( 25 – 31 ) were excluded due to insufficient data for estimation of relative risks or unclear definition of meat; three reports compared vegetarians v. non-vegetarians( 7 , 32 , 33 ); and one further study( 34 ) was excluded because the cohort was included in another study( 35 ). Finally, nine articles( 5 , 6 , 9 , 10 , 35 – 39 ) with seventeen independent cohorts fulfilled our inclusion criteria. Among these nine studies, Pan et al.’s report( 6 ) and Lee et al.’s report( 35 ) included data from two and eight independent cohorts, respectively.
Study characteristics
Table 1 illustrates the characteristics of the included studies, all of which had a prospective cohort design. All included studies consisted of both men and women. The age of participants ranged from 17 to 87 years. The follow-up duration ranged from 5 to 28 years. Three studies( 5 , 6 , 10 ) were conducted in the USA, one in Australia( 38 ), two( 9 , 35 ) in Asia and three( 36 , 37 , 39 ) in Europe.
Table 1.
Characteristics of studies included in meta-analysis of the associations of red and processed meat consumption with risk of total, cardiovascular and cancer mortality
| Study | No. of participants | Age (years) | End points (no. of cases) | Follow-up period and person-time | Exposure and assessment method (exposure) | Outcome assessment | Covariates in fully adjusted model | Quality score |
|---|---|---|---|---|---|---|---|---|
| Kappeler et al. (2013)( 10 ); NHANES III, USA | 17 611 men and women | 20+ | All-cause mortality (683), CVD mortality (1554), cancer mortality (794) | 22 years; 219 759 person-years | Assessed by self-administered 81-item FFQ (unprocessed and processed red meat) | Confirmed by the National Center for Health Statistics through a rigorous process of probabilistic matching and death certificate review | Age, race, sex, smoking, alcohol, PA, SES, BMI, marital status, FV intake, history of hypertension, diabetes, hypercholesterolaemia, use of aspirin and ibuprofen, use of mineral and vitamin supplements, family history of diabetes or hypercholesterolaemia; HRT and OC use (women) | 4 |
| Lee et al. (2013)( 35 ); Asian cohorts*, Asian countries | 296 721 men and women | 17–87 | All-cause mortality (24 283), CVD mortality (6373), cancer mortality (9558) | 6·6–15·6 years (NA for person-years) | Assessed by a validated FFQ (total and unprocessed red meat) | NA | Age, BMI, education, smoking habit, rural/urban residence, alcohol intake, FV intake, TEI | 4 |
| Rohrmann et al. (2013)( 36 ); EPIC, Europe | 448 568 men and women | 35–69 | All-cause mortality (26 344), CVD mortality (5556), cancer mortality (9861) | 17·8 years; 7 984 510 person-years† | Assessed by self-administered FFQ, personal interview FFQ or 7 d food record. Validation study by using 24 h dietary recalls (unprocessed and processed red meat) | Confirmed by record linkages with cancer registries, Boards of Health and death indices or active follow-up | Age, sex, study centre, education, body weight, body height, TEI, alcohol consumption, PA, smoking status, smoking duration | 5 |
| Nagao et al. (2012)( 9 ); JACC, Japan | 51 683 men and women | 40–79 | CVD mortality (2685) | 18·4 years; 820 076 person-years | Assessed by 33-item FFQ. Validity study by using four 3 d dietary records (total, unprocessed and processed red meat) | Based on death certificates and the registration of residence and death | Age, BMI, ethanol intake, perceived mental stress, walking, sports participation time, education, history of hypertension and diabetes, TEI, energy-adjusted food (rice, fish, soya, vegetable and fruit) intakes | 4 |
| Pan et al. (2012)( 6 ); HPFS, USA | 37 698 men | 40–75 | All-cause mortality (8926), CVD mortality (2716), cancer mortality (3073) | 22 years; 758 524 person-years | Assessed by 131- to 166-item FFQ. Validation study by using two 1 week diet records (total, unprocessed and processed red meat) | Based on reports from next of kin, via postal authorities or searching NDI. The cause of death was based on review by physicians and medical records and death certificates | Age, BMI, alcohol, PA, smoking, race, family history of DM, MI or cancer; history of DM, hypertension or hypercholesterolaemia; intakes of total energy, whole grains, fruits, vegetables | 5 |
| Pan et al. (2012)( 6 ); NHS, USA | 83 644 women | 34–59 | All-cause mortality (15 000), CVD mortality (3194), cancer mortality (6391) | 28 years; 2 199 892 person-years | Same as above | Same as above | Age, BMI, alcohol, PA, smoking, race, menopausal status and hormone use, family history of DM, MI or cancer; history of DM, hypertension or hypercholesterolaemia; intakes of total energy, whole grains, fruits, vegetables | 5 |
| Sinha et al. (2009)(5); NIH-AARP, USA (men) | 322 263 men | 50–71 | All-cause mortality (47 967), CVD mortality (14 221), cancer mortality (16 433) | 10 years; 2 369 370 person-years | Assessed by 124-item FFQ. Validation study by using two 24 h recalls (total and processed red meat) | Based on annual linkage of the cohort to the Social Security Administration Death Master File. Cause of death is based on follow-up searches of NDI Plus with the current follow-up for mortality covered until 2005 | Age, race, TEI, education, marital status, family history of cancer, BMI, smoking status, PA, alcohol, vitamin supplement, FV consumption | 5 |
| Sinha et al. (2009)( 5 ); NIH-AARP, USA (women) | 223 390 women | 50–71 | All-cause mortality (23 276), CVD mortality (5356), cancer mortality (8929) | 10 years; 1 912 540 person-years | Same as above | Same as above | Same as above | 5 |
| Fortes et al. (2000)( 39 ); Elderly Cohort Study, Italy | 161 elderly men and women | 65+ | All-cause mortality (53) | 5 years; 805 person-years† | Assessed by 114-item FFQ (total red meat) | Examining the Registry Office of the Municipality of Rome | Sex, age, EL, BMI, smoking status, cognitive function, chronic diseases | 4 |
| Jamrozik et al. (2000)( 38 ); The Perth Community Stroke Study, Australia | 817 elderly men and women | 75+ | CVD mortality (198) | 5 years; 4085 person-years† | Personal interviews (total red meat) | Linkage to name-identified unit mortality and to the Hospital Morbidity data system | Sex, age, Barthel score, Frenchay score, Rankin score, history of MI, TIA or stroke, DM, AC, smoking | 4 |
| Whiteman et al. (1999)( 37 ); OXCHECK, UK | 11 090 men and women | 35–64 | All-cause mortality (598), CVD mortality (144), cancer mortality (257) | 9 years; 93 464 person-years | Assessed by self-completed simple FFQ (unprocessed and processed red meat) | Confirmed by the Office for National Statistics | Age, sex, smoking | 3 |
NHANES III, Third National Health and Nutrition Examination Survey; EPIC, European Prospective Investigation into Cancer and Nutrition; JACC, Japan Collaborative Cohort; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; NIH-AARP, National Institutes of Health–AARP Diet and Health Study; OXCHECK, Oxford and Collaborators Health Check; NA, not available; NDI, National Death Index; PA, physical activity; SES, socio-economic status; FV, fruit and vegetable; HRT, hormone replacement therapy; OC, oral contraceptives; TEI, total energy intake; DM, diabetes mellitus; MI, myocardial infarction; EL, education level; TIA, transient ischaemic attack; AC, alcohol consumption.
Asian countries cohort studies from Bangladesh, mainland China, Japan, Korea and Taiwan.
Person-time estimated by multiplying number of participants by average follow-up time.
Red and processed meat intake was measured by FFQ in all studies. One study( 5 ) did not report the results for unprocessed red meats. Fish and seafood consumption was included in total meat consumption in one study( 35 ), thus this study was not included in the total meat analysis. Total numbers of participants (from 161 to 448 568) and events (from fifty-three to 47 967) varied widely across the cohorts. Dietary variables and/or energy intake were controlled for in six studies, but not in three studies( 37 – 39 ). No study scored the highest level of quality (maximum 6), with three of the nine studies scoring 5, five scoring 4 and one scoring 3.
Table 2 shows the results of the pooled analysis for all included studies. We describe the results according to processed, unprocessed and total red meat below. Two articles( 38 , 39 ) were not included in the dose–response analysis owing to lack of sufficient data.
Table 2.
Meta-analysis of red and processed meat intake and risk of all-cause, cardiovascular and cancer mortality
| Comparison | No. of studies | No. of cohorts | No. of cases/subjects | Pooled RR | 95 % CI | P | Heterogeneity, I 2 (%) | P | P for Begg’s test, Egger’s test |
|---|---|---|---|---|---|---|---|---|---|
| Processed meat | |||||||||
| All-cause mortality | 5 | 6 | 125 794/1 144 264 | 1·15 | 1·11, 1·19 | <0·001 | 75·0 | <0·001 | 0·90, 0·63 |
| CVD mortality | 6 | 7 | 33 278/1 195 947 | 1·15 | 1·07, 1·24 | <0·001 | 75·4 | <0·001 | 0·37, 0·85 |
| Cancer mortality | 5 | 6 | 45 738/1 144 264 | 1·08 | 1·06, 1·11 | <0·001 | 0·0 | 0·450 | 1·00, 0·54 |
| Unprocessed red meat* | |||||||||
| All-cause mortality | 5 | 13 | 78 834/895 332 | 1·05 | 0·93, 1·19 | 0·429 | 90·2 | <0·001 | 0·90, 0·49 |
| CVD mortality | 6 | 14 | 22 222/947 015 | 1·06 | 0·88, 1·28 | 0·523 | 84·5 | <0·001 | 0·72, 0·63 |
| Cancer mortality | 5 | 13 | 29 934/895 332 | 1·03 | 0·89, 1·18 | 0·460 | 78·8 | <0·001 | 0·92, 0·81 |
| Total red meat | |||||||||
| All-cause mortality | 2 | 3 | 95 169/666 995 | 1·17 | 1·14, 1·20 | <0·001 | 78·5 | 0·003 | 1·00, 0·84 |
| CVD mortality | 3 | 4 | 28 172/718 678 | 1·19 | 1·14, 1·25 | <0·001 | 60·3 | 0·027 | 1·00, 0·92 |
| Cancer mortality | 2 | 3 | 34 826/666 995 | 1·12 | 1·10, 1·14 | <0·001 | 0·0 | 0·614 | 0·73, 0·75 |
RR, relative risk.
Owing to lack of data, results are RR (95 % CI) comparing highest with lowest consumption.
Processed meat intake and mortality risk
All-cause and cancer mortality was evaluated in five studies( 5 , 6 , 10 , 36 , 37 ) with a total of 1 144 264 subjects and 125 794 total deaths and 45 738 cancer deaths; cardiovascular mortality was evaluated in six studies( 5 , 6 , 9 , 10 , 36 , 37 ) involving a total of 1 195 947 subjects and 35 426 events.
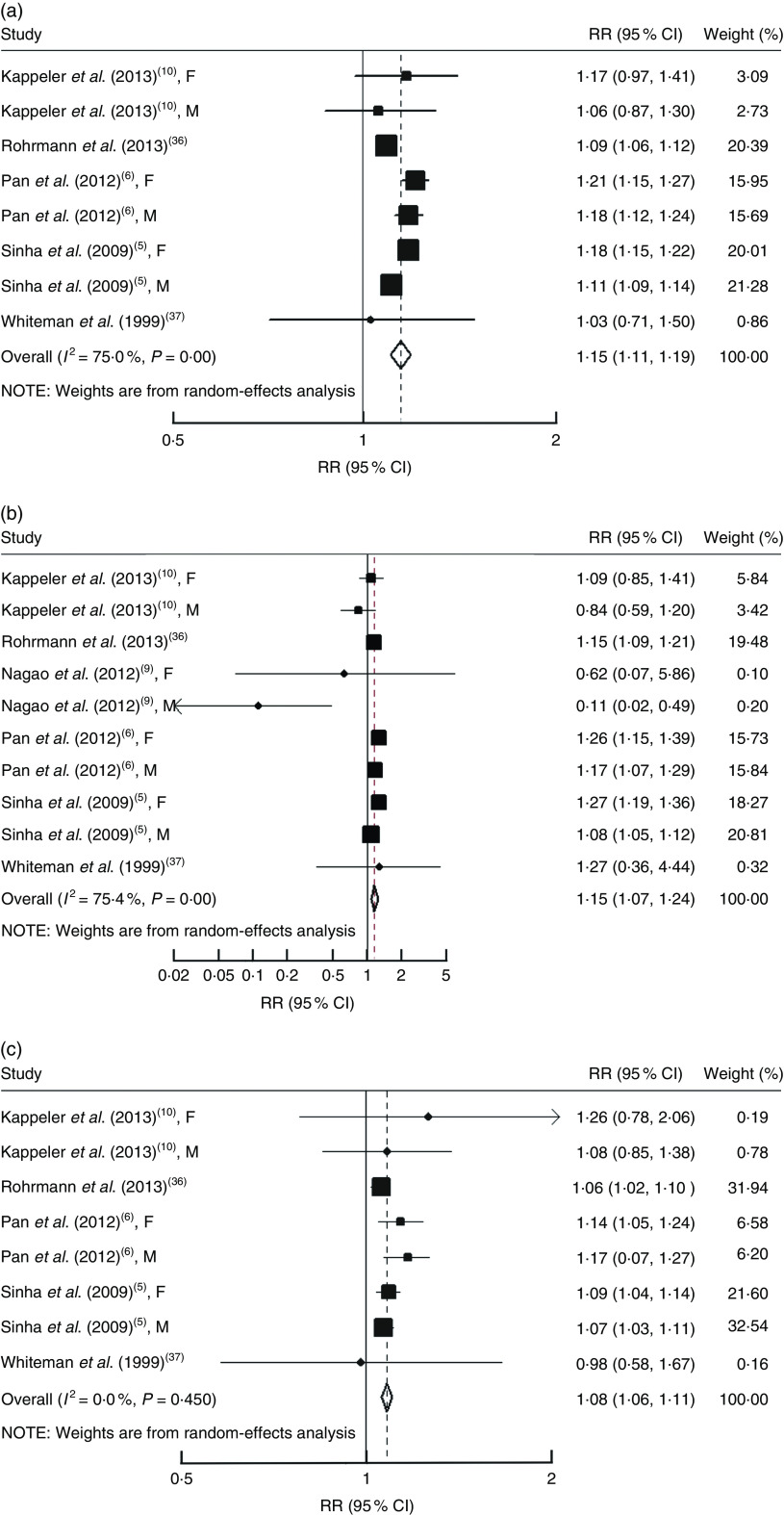
Each serving per day of processed meat consumption was associated with a 15 % (RR=1·15, 95 % CI 1·11, 1·19; Fig. 1(a)) higher risk of all-cause mortality, a 15 % (RR=1·15, 95 % CI 1·07, 1·24; Fig. 1(b)) higher risk of cardiovascular mortality and an 8 % (RR=1·08, 95 % CI 1·06, 1·11; Fig. 1(c)) higher risk of cancer mortality. All P values for linear trend were <0·05. Significant heterogeneity was found for total mortality (I 2=75·0 %; P<0·01) and cardiovascular mortality (I 2=75·4 %; P<0·01), but not cancer mortality (I 2=0·0 %; P=0·45). No significant publication bias for all these associations was found (Begg test, all P≥0·37; Egger test, all P≥0·54; Table 2).
Fig. 1.
Risk of (a) all-cause mortality, (b) cardiovascular mortality and (c) cancer mortality associated with each serving per day of processed meat. The study-specific relative risk (RR) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond presents the pooled RR and its width represents the pooled 95 % CI. Weights are from random-effects analysis (F, female; M, male)
Using a restricted cubic splines model, there was evidence of a non-linear association between processed meat consumption and risk of all-cause mortality (P for non-linearity <0·01; online supplementary material, Fig. S2) and cardiovascular mortality (P for non-linearity <0·01; Fig. S3), but not for cancer mortality (P for non-linearity=0·24; Fig. S4). However, for all-cause and cardiovascular mortality, the overall trend was still linear and the risk of all-cause and cardiovascular mortality was increased with increasing processed meat consumption. Compared with those with no/little consumption of processed meat, the estimate of all-cause mortality was RR=1·11 (95 % CI 1·07, 1·16) for 1 serving/d, RR=1·19 (95 % CI 1·13, 1·24) for 2 servings/d, RR=1·27 (95 % CI 1·19, 1·34) for 3 servings/d and RR=1·35 (95 % CI 1·23, 1·46) for 4 servings/d (Fig. S2).
Unprocessed meat intake and mortality risk
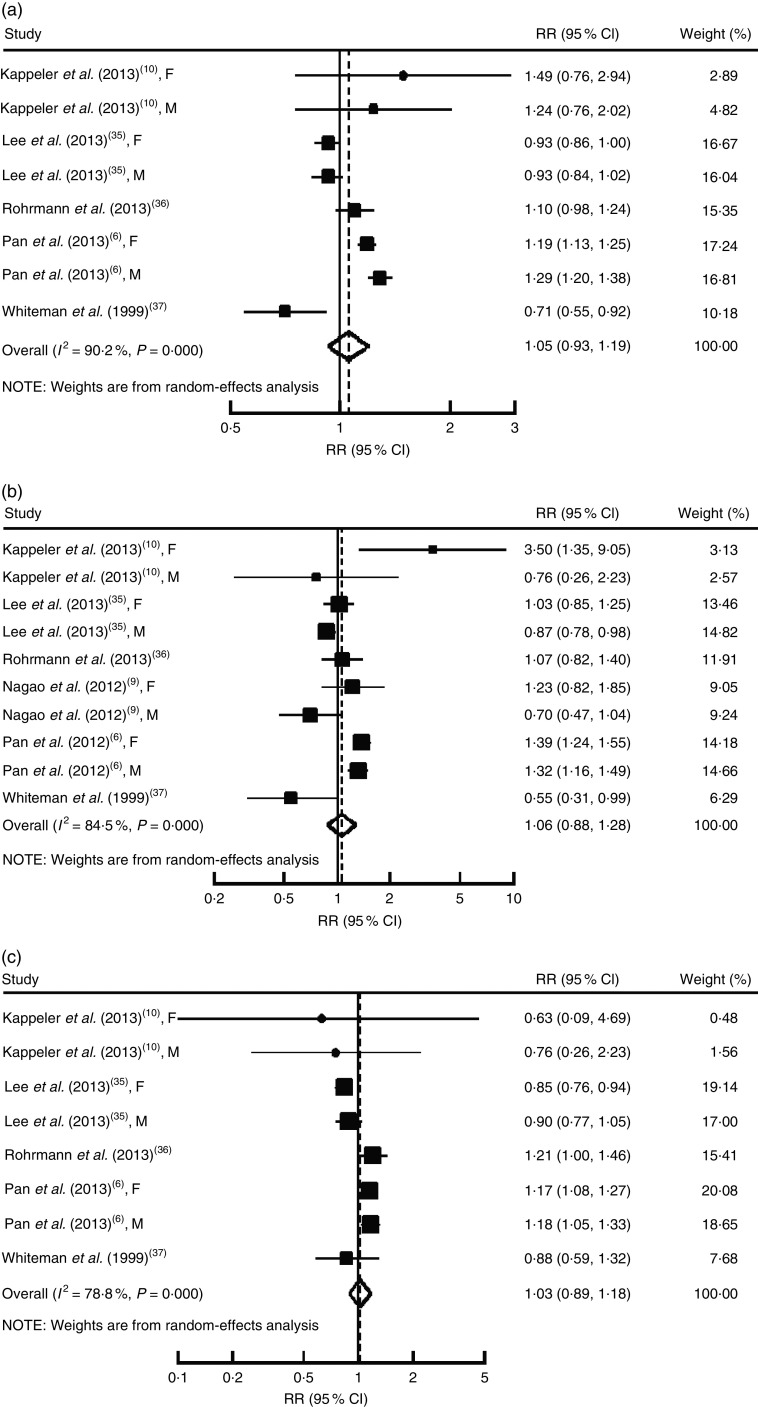
All-cause and cancer mortality was assessed in five studies( 6 , 10 , 35 – 37 ) and cardiovascular mortality was evaluated in six studies( 6 , 9 , 10 , 35 , 36 ). We cannot obtain person-years data from a study involving eight Asian cohorts( 35 ), thus we evaluated the summary RR comparing the highest with the lowest unprocessed red meat consumption for mortality risk in the total population. We also did a dose–response analysis in the US populations where data on person-years are available.
For the total population, the highest category of unprocessed red meat consumption was not associated with an increase in the risk of all-cause mortality (RR=1·05, 95 % CI 0·93, 1·19; P=0·43; Fig. 2(a)), cardiovascular mortality (RR=1·06, 95 % CI 0·88, 1·28; P=0·52; Fig. 2(b)) and cancer mortality (RR=1·03, 95 % CI 0·89, 1·18; P=0·46; Fig. 2(c)), compared with the lowest category. There was significant heterogeneity for all these outcomes (P≤0·01, I 2≥78·8 %). The Begg rank correlation test and Egger linear regression test indicated no significant publication bias for all these associations (Begg, all P≥0·72; Egger, all P≥0·63; Table 2).
Fig. 2.
Relative risk for (a) all-cause mortality, (b) cardiovascular mortality and (c) cancer mortality for highest v. lowest intake of unprocessed red meat. The study-specific relative risk (RR) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond presents the pooled RR and its width represents the pooled 95 % CI. Weights are from random-effects analysis (F, female; M, male)
For the US populations( 5 , 6 , 10 ), a dose–response analysis showed that each serving per day of unprocessed red meat consumption was significantly and positively associated with risk of all-cause mortality (RR=1·15, 95 % CI 1·12, 1·19; P=0·001), cardiovascular mortality (RR=1·19, 95 % CI 1·13, 1·26; P=0·001) and cancer mortality (RR=1·12, 95 % CI 1·07, 1·17; P=0·001). There was no heterogeneity for all these outcomes (P≥0·36, I 2 values ≤7·1 %; online supplementary material, Fig. S5).
Total meat intake and mortality risk
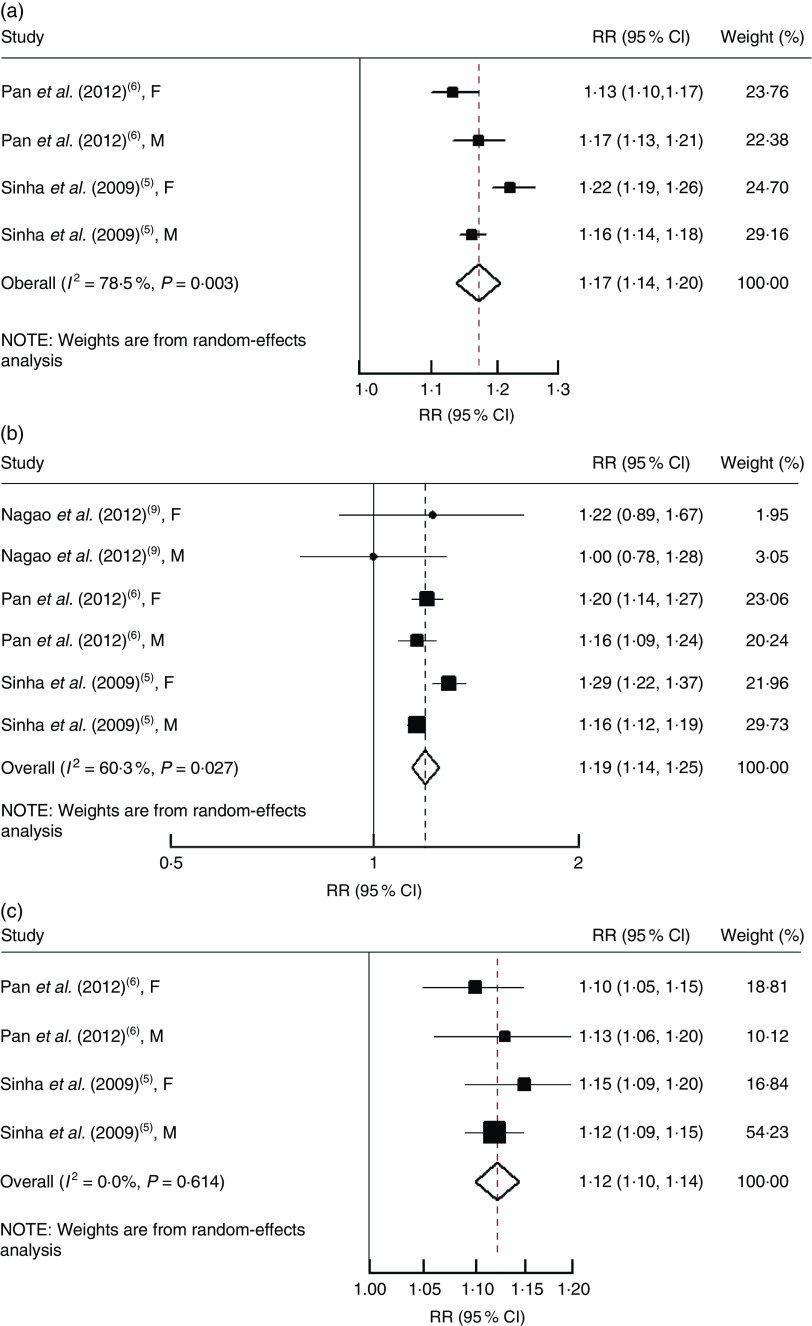
One study( 35 ) was not included in the analysis of total red meat consumption because fish and seafood were combined in total meat category. Therefore, all-cause and cancer mortality were evaluated in two studies( 5 , 6 ) and CVD mortality was examined in three studies( 5 , 6 , 9 ).
An increase of each serving per day of total red meat consumption was associated with a statistically significant increased risk of all-cause mortality (RR=1·17, 95 % CI 1·14, 1·20; Fig. 3(a)), cardiovascular mortality (RR=1·19, 95 % CI 1·14, 1·25; Fig. 3(b)) and cancer mortality (RR=1·12, 95 % CI 1·10, 1·14; Fig. 3(c)).
Fig. 3.
Risk of (a) all-cause mortality, (b) cardiovascular mortality and (c) cancer mortality associated with each serving per day of total meat. The study-specific relative risk (RR) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond presents the pooled RR and its width represents the pooled 95 % CI. Weights are from random-effects analysis (F, female; M, male)
Subgroup and sensitivity analyses
Studies included in the meta-analysis varied in some characteristics. To test the robustness of the results and explore potential sources of heterogeneity in the associations, we conducted a series of sensitivity analyses and subgroup analyses (Table 3).
Table 3.
Stratified analysis to investigate differences between studies evaluating the relationships of red and processed meat intake and all-cause, cardiovascular and cancer mortality
| Unprocessed red meat* | Processed meat | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of cohorts | RR | 95 % CI | P for heterogeneity | I 2 (%) | No. of cohorts | RR | 95 % CI | P for heterogeneity | I 2 (%) | |
| Subgroup analyses for all-cause mortality | ||||||||||
| Location | ||||||||||
| USA | 4 | 1·23 | 1·17, 1·30 | 0·296 | 18·8 | 6 | 1·16 | 1·12, 1·21 | 0·003 | 72·3 |
| Europe | 2 | 0·90 | 0·59, 1·38 | 0·002 | 89·1 | 2 | 1·09 | 1·06, 1·12 | 0·767 | 0·0 |
| Asia | 2 | 0·93 | 0·88, 0·99 | 1·000 | 0·0 | – | – | – | – | – |
| Sex | ||||||||||
| Men and women | 2 | 0·90 | 0·59, 1·38 | 0·002 | 89·1 | 2 | 1·09 | 1·06, 1·12 | 0·767 | 0·0 |
| Men | 3 | 1·12 | 0·85, 1·48 | 0·001 | 93·1 | 3 | 1·13 | 1·08, 1·19 | 0·085 | 59·5 |
| Women | 3 | 1·09 | 0·87, 1·37 | 0·001 | 93·1 | 3 | 1·19 | 1·16, 1·22 | 0·394 | 0·0 |
| Follow-up time | ||||||||||
| <15 years | 3 | 0·90 | 0·82, 0·99 | 0·134 | 50·2 | 3 | 1·14 | 1·08, 1·21 | 0·005 | 81·3 |
| ≥15 years | 5 | 1·21 | 1·13, 1·29 | 0·162 | 38·8 | 5 | 1·15 | 1·08, 1·22 | 0·002 | 76·7 |
| Study quality | ||||||||||
| High (4/5) | 7 | 1·10 | 0·97, 1·24 | 0·001 | 81·2 | 7 | 1·15 | 1·11, 1·19 | 0·0 | 78·4 |
| Low (<4) | 1 | 0·71 | 0·55, 0·92 | – | – | 1 | 1·03 | 0·71, 1·50 | – | – |
| No. of participants | ||||||||||
| ≥50 000 | 4 | 1·03 | 0·89, 1·19 | 0·001 | 92·2 | 4 | 1·14 | 1·09, 1·19 | 0·001 | 87·8 |
| <50 000 | 4 | 1·10 | 0·76, 1·60 | 0·001 | 84·7 | 4 | 1·17 | 1·12, 1·23 | 0·686 | 0·0 |
| Subgroup analyses for CVD mortality | ||||||||||
| Location | ||||||||||
| USA | 4 | 1·37 | 1·18, 1·59 | 0·152 | 43·2 | 6 | 1·16 | 1·06, 1·27 | 0·001 | 81·9 |
| Europe | 2 | 0·81 | 0·42, 1·54 | 0·041 | 76·0 | 2 | 1·15 | 1·09, 1·21 | 0·877 | 0·0 |
| Asia | 4 | 0·93 | 0·79, 1·10 | 0·113 | 49·7 | 2 | 0·22 | 0·04, 1·15 | 0·215 | 35·1 |
| Sex | ||||||||||
| Men and women | 2 | 0·81 | 0·42, 1·54 | 0·041 | 76·0 | 2 | 1·15 | 1·09, 1·21 | 0·877 | 0·0 |
| Men | 4 | 0·94 | 0·68, 1·31 | 0·001 | 89·1 | 4 | 1·06 | 0·92, 1·23 | 0·006 | 75·9 |
| Women | 4 | 1·30 | 1·00, 1·68 | 0·011 | 73·3 | 4 | 1·26 | 1·19, 1·33 | 0·636 | 0·0 |
| Follow-up time | ||||||||||
| <15 years | 3 | 0·89 | 0·73, 1·09 | 0·083 | 59·8 | 3 | 1·17 | 1·00, 1·36 | 0·001 | 89·1 |
| ≥15 years | 7 | 1·20 | 1·01, 1·44 | 0·006 | 66·7 | 7 | 1·14 | 1·04, 1·26 | 0·020 | 60·2 |
| Study quality | ||||||||||
| High (4/5) | 9 | 1·11 | 0·92, 1·34 | 0·001 | 84·6 | 9 | 1·15 | 1·07, 1·24 | 0·001 | 78·1 |
| Low (<4) | 1 | 0·55 | 0·31, 0·99 | – | – | 1 | 1·27 | 0·36, 4·44 | – | – |
| No of participants | ||||||||||
| ≥50 000 | 4 | 1·04 | 0·71, 1·53 | 0·001 | 77·0 | 4 | 1·18 | 1·09, 1·28 | 0·001 | 87·9 |
| <50 000 | 6 | 1·08 | 0·83, 1·39 | 0·001 | 91·1 | 6 | 1·00 | 0·77, 1·29 | 0·038 | 57·5 |
| Subgroup analyses for cancer mortality | ||||||||||
| Location | ||||||||||
| US | 4 | 1·17 | 1·09, 1·25 | 0·797 | 0·0 | 6 | 1·09 | 1·06, 1·12 | 0·416 | 0·1 |
| Europe | 2 | 1·09 | 0·81, 1·46 | 0·161 | 49·2 | 2 | 1·06 | 1·02, 1·10 | 0·772 | 0·0 |
| China | 2 | 0·87 | 0·79, 0·94 | 0·551 | 0·0 | – | – | – | – | – |
| Sex | ||||||||||
| Men and women | 2 | 1·09 | 0·81, 1·46 | 0·161 | 49·2 | 2 | 1·06 | 1·02, 1·10 | 0·772 | 0·0 |
| Men | 3 | 1·02 | 0·80, 1·30 | 0·020 | 74·3 | 3 | 1·10 | 1·03, 1·18 | 0·173 | 43·0 |
| Women | 3 | 0·99 | 0·73, 1·34 | 0·001 | 91·0 | 3 | 1·10 | 1·06, 1·15 | 0·563 | 0·0 |
| Follow-up time | ||||||||||
| <15 years | 3 | 0·87 | 0·80, 0·94 | 0·835 | 0·0 | 3 | 1·08 | 1·05, 1·11 | 0·779 | 0·0 |
| ≥15 years | 5 | 1·17 | 1·10, 1·25 | 0·891 | 0·0 | 5 | 1·11 | 1·05, 1·17 | 0·188 | 35·0 |
| Study quality | ||||||||||
| High (4/5) | 7 | 1·04 | 0·90, 1·20 | 0·001 | 81·4 | 7 | 1·08 | 1·06, 1·11 | 0·352 | 10·0 |
| Low (<4) | 1 | 0·88 | 0·59, 1·32 | – | – | 1 | 0·98 | 0·58, 1·67 | – | – |
| No of participants | ||||||||||
| ≥50 000 | 4 | 1·02 | 0·84, 1·23 | 0·001 | 89·2 | 4 | 1·08 | 1·05, 1·10 | 0·415 | 0·0 |
| <50 000 | 4 | 1·15 | 0·99, 1·29 | 0·423 | 0·0 | 4 | 1·16 | 1·07, 1·25 | 0·832 | 0·0 |
RR, relative risk.
Owing to lack of data, results are RR (95 % CI) comparing highest with lowest consumption.
With stratification by ethnicity, a statistically significant positive association between unprocessed red meat consumption and mortality risk was found in the US populations, but not in European or Asian populations. Stratified analyses also showed a positive association between unprocessed red meat intake and mortality risk in the subgroup of long-term follow-up studies (≥15 years), but not in the short-term studies (<15 years).
For processed meat consumption, positive associations were consistently observed in both the US and European populations, and the associations did not differ substantially by duration of follow-up and other study-level characteristics.
Discussion
The present meta-analysis of prospective cohort studies suggested that total red and processed meat consumption was associated with a significant increase in risk of all-cause, cardiovascular and cancer mortality. The associations between unprocessed red meat consumption and mortality risk were significant in the US populations, but not in European or Asian populations.
The association between processed meat consumption and risk of cancer mortality has not been entirely consistent. In our study, the pooled results showed that higher consumption of processed meat was associated with risk of cancer mortality, particularly in the range up to 1 serving/d. However, few people ate more than 1 serving/d in the meta-analysis and it is unclear whether the risk would continue to increase in people with very high consumption levels. Nevertheless, we found that each 1 serving/d processed meat was associated with an 8% increased risk of cancer mortality. This indicates that decreasing the amount of processed meat in an individual’s diet might help to reduce the risk of cancer mortality. Eating less or no processed meat should be further emphasized besides the adverse effects of unhealthy lifestyle such as little exercise, sleep deprivation and tobacco use( 40 ).
The associations between unprocessed red meat consumption and mortality risk were inconsistent among different populations, which are unexpected and seem confusing. One possible explanation for the null findings in Asian populations is the difference in the amount of red meat consumed. Intake of unprocessed red meat in the US populations is relatively higher than that reported in study populations in Asian countries. Per capita intake of meat (excluding fish) in the USA was more than thirty-three times that in Bangladesh and more than two times that in China, Japan and South Korea in the 1990s and 2000s( 35 ). Median unprocessed red meat intake was 63 g/d among men and 49 g/d among women in the National Institutes of Health–American Association of Retired Persons cohort( 5 ). Median intake of total meat was 33·7 g/d among men and 27·0 g/d among women in Japan( 9 ). In 2007, mean meat consumption in China, Japan and South Korea ranged from 46·1 to 55·9 kg/year, whereas mean consumption in the USA was 122·8 kg/year( 35 ). A low consumption of red meat might not increase mortality risk. One cohort study( 8 ) in Japan also indicated no association between meat intake and stroke mortality risk. Moreover, fish and seafood consumption in Japan and Korea has remained higher than that in the USA( 35 ). Higher fish intake can inhibit eicosanoid biosynthesis and contribute to a reduction in prostaglandin conversion from arachidonic acid( 41 , 42 ). A reduced risk of mortality in Asia could be explained as a result of higher fish intakes and thus delayed progression of disease.
The associations between unprocessed red meat consumption and mortality risk were not statistically significant in European populations. However, the number of studies conducted in European populations was relatively small. Additionally, it is likely that compared with European habits, red meat in the USA may be often barbecued or grilled, thus contributing to higher contents of polycyclic hydrocarbons and heterocyclic amines.
Stratified analysis also suggests that there was a positive association between unprocessed red meat intake and mortality risk in the subgroup of long-term follow-up studies, but not in the short-term studies. It is possible that the follow-up period was too short to identify a meaningful association for mortality. In addition, all the Asian cohorts had relatively short follow-up time and the studies in Western populations tended to have longer follow-up years. Thus, the differences between long and short follow-up duration should be interpreted cautiously and could be due to the differences in populations.
For processed meat intake and all-cause and cardiovascular mortality, we observed significant heterogeneity between studies. In one cohort study( 36 ), there was a significant association between processed meat consumption and increased mortality in smokers, current and former, but there was no significant association in the never smokers( 36 ). However, sensitivity analyses showed that the removal of this study had little effect on the results and between-study heterogeneity. For the associations between total red meat consumption and mortality risk, due to the limited studies reporting the associations, we did not assess a potential curvilinear relationship and sensitivity analyses were not performed to explore the potential heterogeneity.
There are several possible mechanisms underlying the adverse effects of red meat consumption on mortality risk. Red meat has been found to be significantly related to increased cancer risk( 43 , 44 ). During high-temperature cooking of meat, the formation of several compounds, such as heterocyclic amines and polycyclic aromatic hydrocarbons, may pose cancer risk to humans( 44 , 45 ). High red meat intake was associated with an increased risk of CHD( 46 ). In the USA, an average of 400 % higher Na and 50 % higher nitrate levels are included in processed meats( 47 ). Dietary Na may increase CVD risk due to its effect on blood pressure( 48 , 49 ). Blood nitrite concentrations were used as a biomarker of endothelial dysfunction( 50 ). Processed meats may also contain N-nitroso compounds. N-nitroso compounds can further be converted from nitrites( 51 ), heterocyclic amines and polycyclic aromatic hydrocarbons( 52 , 53 ), which are potential carcinogens( 54 ). A recent study( 55 ) also suggested that l-carnitine enriched in red meat may increase risk of atherosclerosis.
We conducted the dose–response meta-analysis to evaluate the linear and non-linear relationships, which helps to quantify the associations and test the shape of these possible associations. We have used the estimates from the fully adjusted models from each included study in our analyses to reduce the potential for confounding, and performed sensitivity analyses to examine the potential sources of heterogeneity and examine the robustness in the subgroups. Some limitations of the meta-analysis should be addressed. First, all included studies were observational in nature. The results may be subject to residual confounding or unmeasured factors. It is possible that the associations of red and processed meat intake with mortality could relate to generally less healthy lifestyle or diet rather than causal effects of unprocessed and processed meat intake. Second, levels of red and processed meat intake were assessed by FFQ in most studies. Measurement error of meat intake is inevitable. The imprecise measurement of red and processed meat consumption is likely to attenuate the true association. Third, data transformation in meta-analysis may add another layer of error. For example, the serving size of red and processed meat was not specified in four studies( 8 , 10 , 31 , 37 ), and we had to assume all servings to 100 g for total and unprocessed red meat and 50 g for processed meat. Thus, risks could change accordingly when serving sizes are lower or higher. Finally, the limited data in certain subgroups may contribute to the reduced statistical power for detecting heterogeneity and the results should be cautiously interpreted.
In summary, the present meta-analysis suggests that higher consumption of total red meat and processed meat is associated with an increased risk of total mortality and deaths from CVD and cancer. Significant association between unprocessed red meat and mortality risk was found in US studies but not others. More studies are still needed to confirm the results and explore the underlying mechanisms.
Acknowledgements
Financial support: This work was funded by the National Natural Science Foundation (NSFC 81370966) of China. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish. Conflict of interest: The authors have declared that no competing interests exist. Authorship: X.W., G.Z., and Y.Y.O. took responsibility for data integrity and the accuracy of data analysis. X.W., X.L., G.Z., Y.Y.O. and F.B.H. were responsible for study concept and design. X.L., A.P. and G.Z acquired the data. X.W., G.Z., Y.Y.O., J.L. and A.P. were responsible for analysis and interpretation of the data. J.L., X.L. and Y.Y.O. performed the statistical analysis. X.W. and A.P. drafted the manuscript. X.W., A.P. and F.B.H. critically revised the manuscript for important intellectual content. Ethics of human subject participation: Ethical approval was not required.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980015002062.
click here to view supplementary material
References
- 1. Speedy AW (2003) Global production and consumption of animal source foods. J Nutr 133, 11 Suppl. 2, 4048S–4053S. [DOI] [PubMed] [Google Scholar]
- 2. Delgado CL (2003) Rising consumption of meat and milk in developing countries has created a new food revolution. J Nutr 133, 11 Suppl. 2, 3907S–3910S. [DOI] [PubMed] [Google Scholar]
- 3. Daniel CR, Cross AJ, Koebnick C et al. (2011) Trends in meat consumption in the USA. Public Health Nutr 14, 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker P, Rhubart-Berg P, McKenzie S et al. (2005) Public health implications of meat production and consumption. Public Health Nutr 8, 348–356. [DOI] [PubMed] [Google Scholar]
- 5. Sinha R, Cross AJ, Graubard BI et al. (2009) Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med 169, 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan A, Sun Q, Bernstein AM et al. (2012) Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 172, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fraser GE (1999) Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr 70, 3 Suppl., 532S–538S. [DOI] [PubMed] [Google Scholar]
- 8. Sauvaget C, Nagano J, Allen N et al. (2003) Intake of animal products and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Int J Epidemiol 32, 536–543. [DOI] [PubMed] [Google Scholar]
- 9. Nagao M, Iso H, Yamagishi K et al. (2012) Meat consumption in relation to mortality from cardiovascular disease among Japanese men and women. Eur J Clin Nutr 66, 687–693. [DOI] [PubMed] [Google Scholar]
- 10. Kappeler R, Eichholzer M & Rohrmann S (2013) Meat consumption and diet quality and mortality in NHANES III. Eur J Clin Nutr 67, 598–606. [DOI] [PubMed] [Google Scholar]
- 11. Key TJ, Appleby PN, Spencer EA et al. (2009) Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 89, issue 5, 1613S–1619S. [DOI] [PubMed] [Google Scholar]
- 12. Abete I, Romaguera D, Vieira AR et al. (2014) Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr 112, 762–775. [DOI] [PubMed] [Google Scholar]
- 13. O’Sullivan TA, Hafekost K, Mitrou F et al. (2013) Food sources of saturated fat and the association with mortality: a meta-analysis. Am J Public Health 103, e31–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larsson SC & Orsini N (2014) Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol 179, 282–289. [DOI] [PubMed] [Google Scholar]
- 15. Stroup DF, Berlin JA, Morton SC et al. (2000) Meta-analysis of observational studies in epidemiology. JAMA 283, 2008–2012. [DOI] [PubMed] [Google Scholar]
- 16. Carter P, Gray LJ, Troughton J et al. (2010) Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ 341, c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hedges LV, Olkin I & Statistiker M (1985) Statistical Methods for Meta-Analysis. New York: Academic Press. [Google Scholar]
- 18. Higgins J, Thompson SG, Deeks JJ et al. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berlin JA, Longnecker MP & Greenland S (1993) Meta-analysis of epidemiologic dose–response data. Epidemiology 4, 218–228. [DOI] [PubMed] [Google Scholar]
- 20. Greenland S & Longnecker MP (1992) Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol 135, 1301–1309. [DOI] [PubMed] [Google Scholar]
- 21. Orsini N, Bellocco R & Greenland S (2006) Generalized least squares for trend estimation of summarized dose–response data. Stata J 6, 40–57. [Google Scholar]
- 22. Harre FE, Lee KL & Pollock BG (1988) Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 80, 1198–1202. [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Smith GD, Schneider M et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Begg CB & Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. [PubMed] [Google Scholar]
- 25. Cox BD & Whichelow MJ (1997) Frequent consumption of red meat is not risk factor for cancer. BMJ 315, 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu D, Mei J, Tanihata T et al. (2003) A cohort study on cerebrovascular disease in middle-aged and elderly population in rural areas in Jiangxi Province, China. J Epidemiol 13, 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kinjo Y, Beral V, Akiba S et al. (1999) Possible protective effect of milk, meat and fish for cerebrovascular disease mortality in Japan. J Epidemiol 9, 268–274. [DOI] [PubMed] [Google Scholar]
- 28. Kelemen LE, Kushi LH, Jacobs DR et al. (2005) Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol 161, 239–249. [DOI] [PubMed] [Google Scholar]
- 29. Trichopoulou A, Bamia C & Trichopoulos D (2009) Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 338, b2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zell JA, Ziogas A, Bernstein L et al. (2010) Meat consumption, nonsteroidal anti-inflammatory drug use, and mortality among colorectal cancer patients in the California Teachers Study. Cancer Prev Res 3, 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Breslow RA, Graubard BI, Sinha R et al. (2000) Diet and lung cancer mortality: a 1987 National Health Interview Survey cohort study. Cancer Causes Control 11, 419–431. [DOI] [PubMed] [Google Scholar]
- 32. Mann JI, Appleby PN, Key TJ et al. (1997) Dietary determinants of ischaemic heart disease in health conscious individuals. Heart 78, 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang-Claude J, Hermann S, Eilber U et al. (2005) Lifestyle determinants and mortality in German vegetarians and health-conscious persons: results of a 21-year follow-up. Cancer Epidemiol Biomarkers Prev 14, 963–968. [DOI] [PubMed] [Google Scholar]
- 34. Takata Y, Shu X-O, Gao Y-T et al. (2013) Red meat and poultry intakes and risk of total and cause-specific mortality: results from cohort studies of Chinese adults in Shanghai. PLoS One 8, e56963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee JE, McLerran DF, Rolland B et al. (2013) Meat intake and cause-specific mortality: a pooled analysis of Asian prospective cohort studies. Am J Clin Nutr 98, 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rohrmann S, Overvad K, Bueno-de-Mesquita HB et al. (2013) Meat consumption and mortality-results from the European Prospective Investigation into Cancer and Nutrition. BMC Med 11, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whiteman D, Muir J, Jones L et al. (1999) Dietary questions as determinants of mortality: the OXCHECK experience. Public Health Nutr 2, 477–487. [DOI] [PubMed] [Google Scholar]
- 38. Jamrozik K, Broadhurst RJ, Forbes S et al. (2000) Predictors of death and vascular events in the elderly: the Perth Community Stroke Study. Stroke 31, 863–868. [DOI] [PubMed] [Google Scholar]
- 39. Fortes C, Forastiere F, Farchi S et al. (2000) Diet and overall survival in a cohort of very elderly people. Epidemiology 11, 440–445. [DOI] [PubMed] [Google Scholar]
- 40. Spring B, King AC, Pagoto SL et al. (2015) Fostering multiple healthy lifestyle behaviors for primary prevention of cancer. Am Psychol 70, 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calder PC & Yaqoob P (2009) Omega-3 polyunsaturated fatty acids and human health outcomes. Biofactors 35, 266–272. [DOI] [PubMed] [Google Scholar]
- 42. Tapiero H, Ba GN, Couvreur P et al. (2002) Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother 56, 215–222. [DOI] [PubMed] [Google Scholar]
- 43. World Cancer Research Fund/American Institute for Cancer Research (2007) Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR. [Google Scholar]
- 44. Skog K, Steineck G, Augustsson K et al. (1995) Effect of cooking temperature on the formation of heterocyclic amines in fried meat products and pan residues. Carcinogenesis 16, 861–867. [DOI] [PubMed] [Google Scholar]
- 45. Butler L, Sinha R, Millikan R et al. (2003) Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am J Epidemiol 157, 434–445. [DOI] [PubMed] [Google Scholar]
- 46. Bernstein AM, Sun Q, Hu FB et al. (2010) Major dietary protein sources and risk of coronary heart disease in women. Circulation 122, 876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Micha R, Wallace SK & Mozaffarian D (2010) Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 121, 2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bibbins-Domingo K, Chertow GM, Coxson PG et al. (2010) Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 362, 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cook NR, Cutler JA, Obarzanek E et al. (2007) Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334, 885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kleinbongard P, Dejam A, Lauer T et al. (2006) Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med 40, 295–302. [DOI] [PubMed] [Google Scholar]
- 51. Hughes R, Cross A, Pollock J et al. (2001) Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis 22, 199–202. [DOI] [PubMed] [Google Scholar]
- 52. Cross AJ & Sinha R (2004) Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen 44, 44–55. [DOI] [PubMed] [Google Scholar]
- 53. Sen NP, Seaman SW, Burgess C et al. (2000) Investigation on the possible formation of N-nitroso-N-methylurea by nitrosation of creatinine in model systems and in cured meats at gastric pH. J Agric Food Chem 48, 5088–5096. [DOI] [PubMed] [Google Scholar]
- 54. Cross AJ, Leitzmann MF, Gail MH et al. (2007) A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med 4, e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koeth RA, Wang Z, Levison BS et al. (2013) Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19, 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980015002062.
click here to view supplementary material