Abstract
Objective
To investigate biomarkers of nutrition associated with chronic disease absence for an Aboriginal cohort.
Design
Screening for nutritional biomarkers was completed at baseline (1995). Evidence of chronic disease (diabetes, CVD, chronic kidney disease or hypertension) was sought from primary health-care clinics, hospitals and death records over 10 years of follow-up. Principal components analysis was used to group baseline nutritional biomarkers and logistic regression modelling used to investigate associations between the principal components and chronic disease absence.
Setting
Three Central Australian Aboriginal communities.
Subjects
Aboriginal people (n 444, 286 of whom were without chronic disease at baseline) aged 15–82 years.
Results
Principal components analysis grouped twelve nutritional biomarkers into four components: ‘lipids’; ‘adiposity’; ‘dietary quality’; and ‘habitus with inverse quality diet’. For the 286 individuals free of chronic disease at baseline, lower adiposity, lower lipids and better dietary quality components were each associated with the absence at follow-up of most chronic diseases examined, with the exception of chronic kidney disease. Low ‘adiposity’ component was associated with absence of diabetes, hypertension and CVD at follow-up. Low ‘lipid’ component was associated with absence of hypertension and CVD, and high ‘dietary quality’ component was associated with absence of CVD at follow-up.
Conclusions
Lowering or maintenance of the factors related to ‘adiposity’ and ‘lipids’ to healthy thresholds and increasing access to a healthy diet appear useful targets for chronic disease prevention for Aboriginal people in Central Australia.
Keywords: Aboriginal, Nutrition, Chronic disease, Prevention
Prior to European contact, Aboriginal populations are thought to have lived a life free of chronic disease( 1 ). Aboriginal people were fit, healthy and had high levels of control( 2 ). In Central Australia, as elsewhere around Australia, Aboriginal people relied on a diet centred on labour-intensive foods that required much physical exertion to collectively obtain and prepare( 3 – 5 ). However, with European contact came a drastic change to diets including but not limited to the introduction of foods of limited antioxidant content (sugar, flour, salt) and tobacco, the exclusion from land as a food source, the loss of food preparation knowledge and the introduction of community stores( 4 ). Each of these changes has contributed to the emergence of a pro-oxidant, pro-inflammatory and low-nutrient diet that is characterised by high levels of saturated fat, refined carbohydrates and salt, and low levels of fibre, folate, Ca, carotene and vitamins A and E( 2 – 6 ). This modern diet has emerged as a result of convenience, cost and limited availability of healthy alternatives and is associated with the high mortality and morbidity from chronic disease in Aboriginal people( 7 ). Such ‘nutritional transitions’ marked by a change in dietary quality have been described on a global scale for Indigenous populations in relation to chronic disease( 8 ).
Chronic conditions such as CVD, chronic kidney disease (CKD) and diabetes are thought to manifest as a result of oxidative and inflammatory stress. Stressors such as chronic and repeated infections, hyperglycaemia, dyslipidaemia and smoking are believed to mediate the transition to chronic disease via blood-vessel endothelium damage and atherosclerosis( 9 ). Regular exercise and diets rich in antioxidants derived from plants and n-3 fats derived from plant and animal sources are thought to mitigate the effect of these stressors( 10 ). In recent decades there has been ongoing interest generated by the associations of dietary antioxidants including folate (vegetables, fruits, legumes, whole grains), retinol (vegetables, eggs, dairy products), α-tocopherol (vegetable oils) and carotenoids such as lutein + zeaxanthin (leafy greens), cryptoxanthin (citrus fruits) and lycopene (tomatoes) with a lower risk of chronic disease in large-scale epidemiological cohorts( 11 – 13 ).
It has previously been reported that Aboriginal people in Central Australia have uniformly low circulating levels of diet-derived antioxidants, in addition to an unusual dyslipidaemic profile (including very low HDL cholesterol), high prevalence of obesity and high incidence of chronic disease-related mortality( 5 , 14 , 15 ). Primary health-care (PHC) organisations in remote areas have attempted to address this with health promotion and chronic disease prevention activities, including education, food supply policies, community infrastructure and homelands living( 16 – 19 ).
Here we investigate what biomarkers of nutrition are associated with chronic disease absence over a 10-year period, which has important implications for chronic disease prevention. In doing this we use a framework focusing on the absence of chronic disease and its associations with biomarkers of nutrition and dietary quality. Much like the ‘positive deviance’ approach, this framework is underpinned by an assumption that beneficial health characteristics of some members of a community can inform health interventions involving the whole community( 20 ). Although this is an unusual approach in Aboriginal health, we see this as better aligning with an Aboriginal worldview that centres health around ‘well-being’ as opposed to ‘disease’, as being a step towards a more salutogenic approach, and as offering resistance to a history of research in Aboriginal health that typically describes the problems, ‘risks’ and deficits of Aboriginal people. This approach is also consistent with core activities of PHC in Central Australia that focus on health promotion and provides PHC clinics with information on where population-level chronic disease prevention activities might be best aligned.
Methods
The study was conducted in accordance with the project agreements established with three Aboriginal communities. Ethical approval was obtained from by the Central Australian and University of Melbourne Human Research Ethics Committees.
Participant recruitment, health assessment and diagnosis
In 1995, Aboriginal people aged 15–82 years participated in risk factor screening at three PHC clinics in Central Australia. In addition to clinically diagnostic variables (blood pressure, blood glucose, urinary albumin:creatinine), screening included measurements for biomarkers of diet and nutrition: lipids (total cholesterol, TAG); lipid-soluble antioxidant vitamins (retinol, γ-tocopherol, α-tocopherol); carotenoids as biomarkers of fruit and vegetable intake (α-carotene, β-carotene, lutein+zeaxanthin, cryptoxanthin, lycopene); homocysteine as a marker of folate intake (inversely related); growth (height); and adiposity (weight, waist and hip circumferences, and biceps, triceps, subscapular and suprailiac skinfold thicknesses). These measurement procedures have been described elsewhere( 5 , 21 ). Waist-to-hip ratio (WHR) was calculated as [waist circumference (cm)]/[hip circumference (cm)] and BMI was calculated as [weight (kg)]/[height (m)]2. A correlation matrix revealed strong associations between nutritional biomarkers, so γ-tocopherol, α-carotene and β-carotene were excluded from subsequent analyses. The former two are quantitatively minor dietary components and β-carotene is a non-specific marker of fruit and vegetable intake.
Of the people screened in 1995, 444 (59·4 %) had a complete profile for all measurements listed above. Participants with an incomplete profile did not differ in most measurements with the exception of having a greater prevalence of baseline diabetes (21·1 v. 11·3 %) and obesity (greater BMI (28·4 v. 24·8 kg/m2), sum of skinfolds (94·2 v. 80·6 mm) and WHR (0·89 v. 0·87)) at the 95% CI level. Not all people with known diabetes at baseline had 2 h blood samples measured. Hence, people with a missing profile are not representative of the total population as they were more likely at baseline to have diabetes and greater adiposity. In addition, this survey sample represents a sub-cohort of the larger study and was designed to examine the associations of nutritional biomarkers and risk, rather than to provide estimates of incidence and prevalence of chronic conditions( 14 ).
Participants were recorded as having chronic disease in 1995 based on a baseline health check or PHC or hospital records for CVD, hypertension, CKD or diabetes. CVD included CHD, stroke, peripheral vascular disease or chronic heart failure. The absence of at least one pedal pulse collected by physical examination at baseline provided further evidence of peripheral vascular disease. Hypertension was defined by baseline antihypertensive medication use, systolic blood pressure reading ≥140 mmHg or diastolic blood pressure reading ≥90 mmHg. Diabetes was defined by PHC diagnosis prior to 1995, use of oral hypoglycaemic agents or insulin, baseline fasting glucose ≥7·0 mmol/l or 2 h glucose ≥11·1 mmol/l. CKD was recorded on the basis of urinary albumin:creatinine >3·4 mg/mmol or PHC record.
Follow-up data were available up to 2004 for two communities and up to 2005 for one community. At least one follow-up record was available for 99·1% of the cohort. Incident chronic disease was recorded based on PHC, hospital or death records of CVD, hypertension, diabetes or CKD as previously described( 22 ). CVD was recorded on the basis of clinical diagnosis of CHD, stroke, peripheral vascular disease or chronic heart failure. Hypertension was recorded on the basis of three or more recordings ≥140/90 mmHg or evidence of antihypertensive medication use in PHC records, use of ICD (International Statistical Classification of Diseases and Related Health Problems, 9th revision (ICD-9) or 10th revision (ICD-10)) codes (ICD-9: 401–405 or ICD-10: I10–I15) in hospital records or on death records. Diabetes was recorded on the basis of two or more random glucose recordings ≥11·1 mmol/l or initiation of oral hypoglycaemic agents or insulin use in PHC records, hospital ICD codes (ICD9: 250 or ICD-10: E10–E14) or on death records. CKD was coded on the basis of two or more urinary albumin:creatinine readings >3·4 mg/mmol in PHC records, hospital records of end-stage renal failure, dialysis or transplant, or on the basis of death records.
Statistical analysis
All continuous data are reported as means and 95% CI, and categorical data are expressed as percentages and 95% CI. Normality of clinical baseline data was assessed using histogram frequencies, with non-normality assumed on the basis of asymmetry. Non-normally distributed data for total cholesterol, TAG, homocysteine, retinol, α-tocopherol, lutein + zeaxanthin, cryptoxanthin and lycopene were log-transformed before analysis. A correlation matrix was used to identify nutritional biomarkers that were highly correlated and could be dropped from the regression analysis.
Principal components analysis (PCA) was used to group highly correlated nutritional variables into a smaller number of ‘components’( 23 ). PCA used a correlation matrix and components were extracted on the basis of an eigenvalue greater than 1. An Oblimin rotation was used given the correlated nature of the variables. In interpreting factors, absolute loadings (correlations) greater than 0·4 were considered meaningful. These components were used as continuous variables in predictive modelling.
For continuous variables, ANOVA was used to assess univariate differences between groups with or without diseases and the χ 2 test was used for categorical data. Longitudinal analyses were limited to Aboriginal people with complete profiles, free of chronic disease, not pregnant and aged 15 years or older at baseline. Logistic regression was used to calculate the odds ratios for absence of chronic disease at follow-up, for each PCA component, with adjustment for age, sex, smoking status and community of residence. Logistic regression models were also used to calculate odds ratios for the absence of specific chronic diseases (diabetes, CKD, CVD and hypertension) associated with each PCA component. For this analysis, specific chronic disease status at follow-up (e.g. diabetes) was used irrespective of other co-morbidities (i.e. CKD, CVD or hypertension). That is, the groups shown in Tables 3 and 4 are not mutually exclusive.
Table 3.
Baseline characteristics of the population stratified by chronic condition status in 2004/5; people from three Central Australian Aboriginal communities
| Diabetes (n 40; 14·0 %) | Hypertension (n 48; 16·8 %) | CVD (n 18; 6·3 %) | Chronic kidney disease (n 69; 24·1 %) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95 % CI | Mean | 95 % CI | Mean | 95 % CI | Mean | 95 % CI | |
| Age (years) | 31 | 27, 35 | 39† | 34, 43 | 42† | 35, 49 | 33 | 30, 37 |
| Sex (% male) | 32·5 | 18·0, 47·0 | 70·8† | 58·0, 83·6 | 61·1 | 38·6, 83·6 | 46·4 | 34·6, 58·1 |
| Smoking (%) | 35·0 | 20·2, 49·8 | 29·2 | 16·3, 42·0 | 27·8 | 7·1, 48·5 | 31·9 | 20·9, 42·9 |
| Height (cm) | 1·66 | 1·63, 1·68 | 1·68 | 1·66, 1·70 | 1·69 | 1·66, 1·72 | 1·67 | 1·65, 1·69 |
| Weight (kg) | 72·2† | 66·8, 77·7 | 73·1† | 68·9, 77·2 | 76·5† | 68·8, 84·1 | 67·0, | 63·5, 70·6 |
| BMI (kg/m2) | 26·2† | 24·5, 27·9 | 25·9† | 24·5, 27·4 | 26·7† | 24·1, 29·3 | 24·0 | 22·8, 25·4 |
| Sum of skinfolds (mm) | 91·3† | 79·2, 103·5 | 84·3 | 72·8, 95·8 | 83·6 | 62·1, 105·2 | 75·7 | 65·3, 86·2 |
| Waist-to-hip ratio | 0·89† | 0·87, 0·92 | 0·89† | 0·87, 0·91 | 0·91† | 0·87, 0·95 | 0·86 | 0·85, 0·88 |
| Cholesterol (mmol/l)* | 4·99 | 4·63, 5·37 | 5·16† | 4·88, 5·45 | 5·44 | 4·84, 6·12 | 4·85 | 4·59, 5·13 |
| TAG (mmol/l)* | 1·65 | 1·41, 1·95 | 2·09† | 1·80, 2·42 | 2·51† | 1·98, 3·19 | 1·79 | 1·57, 2·05 |
| Homocysteine (µmol/l)* | 12·1 | 10·8, 13·5 | 15·2† | 13·7, 16·8 | 16·2† | 13·5, 19·5 | 13·8 | 12·5, 15·3 |
| Retinol (µg/dl)* | 41·2 | 37·0, 45·8 | 49·4† | 45·3, 53·8 | 49·0 | 41·4, 57·9 | 44·5 | 41·0, 48·3 |
| α-Tocopherol (µg/dl)* | 708 | 634, 790 | 692 | 639, 750 | 775 | 669, 897 | 702 | 651, 7·57 |
| Lutein + zeaxanthin (µg/dl)* | 7·38 | 6·59, 8·26 | 7·29 | 6·61, 8·04 | 7·30 | 5·74, 9·28 | 6·91 | 6·31, 7·57 |
| Cryptoxanthin (µg/dl)* | 2·98 | 2·43, 3·66 | 2·70 | 2·28, 3·21 | 2·01† | 1·51, 2·68 | 2·64 | 2·28, 3·05 |
| Lycopene (µg/dl)* | 11·23 | 9·35, 13·48 | 8·67 | 7·21, 10·44 | 6·93 | 4·99, 9·61 | 9·34 | 7·98, 10·94 |
Data are mean and 95 % CI unless otherwise indicated.
Geometric mean and 95 % CI.
Difference between those with chronic condition and those without is statistically significant at the 95 % CI level.
Table 4.
Odds ratios for chronic disease prevention associated with components from principal components analysis (PCA); people from three Central Australian Aboriginal communities
| Absence of diabetes | Absence of hypertension | Absence of CVD | Absence of chronic kidney disease | |||||
|---|---|---|---|---|---|---|---|---|
| PCA component | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI |
| Component 1: ‘lipids’ | 0·73 | 0·40, 1·33 | 0·45 | 0·26, 0·75 | 0·43 | 0·21, 0·89 | 0·76 | 0·49, 1·19 |
| Component 2: ‘adiposity’ | 0·43 | 0·28, 0·66 | 0·52 | 0·34, 0·79 | 0·41 | 0·21, 0·80 | 0·97 | 0·70, 1·33 |
| Component 3: ‘dietary quality’ | 0·74 | 0·47, 1·14 | 1·50 | 0·98, 2·27 | 2·13 | 1·08, 4·17 | 1·31 | 0·93, 1·84 |
| Component 4: ‘habitus with inverse quality diet’ | 0·97 | 0·48, 1·94 | 0·59 | 0·30, 1·14 | 1·25 | 0·40, 3·90 | 0·97 | 0·56, 1·67 |
Data are odds ratio and 95 % CI adjusted for age, sex, smoking status and community of residence; values in bold font indicate significance at the 95 % CI level.
All analyses were completed using the statistical software package IBM SPSS Statistics version 21·0.
Results
Absence of chronic disease
In total, 444 Aboriginal people had complete profiles for nutritional biomarkers. Of these, 286 individuals (64·4 %) were free of chronic disease at baseline (Table 1). Those free of chronic disease were more likely to smoke (26·6 v. 15·2 %), were younger (32 v. 42 years) and had lower mean adiposity (BMI: 23·7 v. 26·9 kg/m2; weight: 65·6 v. 75·1 kg; skinfold sum: 76·1 v. 88·8 mm; WHR: 0·86 v. 0·91), cholesterol (4·1 v. 5·4 mmol/l), TAG (1·72 v. 2·67 mmol/l), homocysteine (12·9 v. 15·2 µmol/l), retinol (43·7 v. 50·6 µg/dl) and α-tocopherol (656 v. 826 µg/dl). Of the 158 people with a record of chronic disease at baseline, most (70 %) had a single chronic disease and 9% had a combination of three or more.
Table 1.
Characteristics of the population free of chronic disease at baseline (1995) and stratified by chronic disease status at follow-up (2004/5); people from three Central Australian Aboriginal communities
| Baseline status | Follow-up status | |||||
|---|---|---|---|---|---|---|
| Chronic disease absent (n 286; 64·4 %) | Chronic disease absent (n 167; 58·4 %) | Chronic disease present (n 119; 41·6 %) | ||||
| Mean | 95 % CI | Mean | 95 % CI | Mean | 95 % CI | |
| Age (years)* | 32‡ | 30, 34 | 30§ | 28, 32 | 35§ | 32, 37 |
| Sex (% male) | 40·9 | 35·2, 46·6 | 37·7 | 30·4, 45·1 | 45·4 | 36·4, 54·3 |
| Smoking (%) | 26·6‡ | 21·5, 31·7 | 21·6 | 15·3, 27·8 | 33·6 | 25·1, 42·1 |
| Height (cm)* | 1·66 | 1·65, 1·67 | 1·66 | 1·64, 1·67 | 1·67 | 1·66, 1·69 |
| Weight (kg)* | 65·6‡ | 63·9, 67·3 | 62·9§ | 60·8, 65·0 | 69·4§ | 66·7, 72·0 |
| BMI (kg/m2)* | 23·7‡ | 23·1, 24·3 | 22·9§ | 22·2, 23·6 | 24·8§ | 23·9, 25·8 |
| Sum of skinfolds (mm)* | 76·1‡ | 71·7, 80·6 | 73·7 | 68·1, 79·4 | 79·5 | 72·2, 86·7 |
| Waist-to-hip ratio* | 0·86‡ | 0·85, 0·86 | 0·84§ | 0·83, 0·85 | 0·88§ | 0·86, 0·89 |
| Cholesterol (mmol/l)† | 4·81‡ | 4·70, 4·93 | 4·71 | 4·57, 4·84 | 4·96 | 4·76, 5·18 |
| TAG (mmol/l)† | 1·72‡ | 1·62, 1·83 | 1·64 | 1·53, 1·77 | 1·84 | 1·67, 2·04 |
| Homocysteine (µmol/l)† | 12·9‡ | 12·3, 13·5 | 12·5 | 11·8, 13·1 | 13·6 | 12·6, 14·6 |
| Retinol (µg/dl)† | 43·7‡ | 42·2, 45·3 | 43·2 | 41·4, 45·2 | 44·4 | 41·8, 47·3 |
| α-Tocopherol (µg/dl)† | 656‡ | 633, 679 | 623§ | 596, 652 | 704§ | 666, 744 |
| Lutein+zeaxanthin (µg/dl)† | 6·90 | 6·60, 7·22 | 6·90 | 6·48, 7·34 | 6·91 | 6·46, 7·39 |
| Cryptoxanthin (µg/dl)† | 2·97 | 2·76, 3·20 | 3·20 | 2·90, 3·52 | 2·69 | 2·40, 3·01 |
| Lycopene (µg/dl)† | 9·80 | 9·08, 10·58 | 9·76 | 8·80, 10·83 | 9·86 | 8·80, 11·04 |
Mean and 95 % CI.
Geometric mean and 95 % CI interval.
Indicates that the characteristic of participants without chronic disease in 1995 differs from that of participants with chronic disease (data not shown) at the 95 % CI level.
Indicates that the characteristic of participants without chronic disease in 2005 differs from that of participants with chronic disease at the 95% CI level.
At follow-up, 167 people remained free of chronic disease. The characteristics of people who remained free of chronic disease were different from those of people who developed chronic disease at the 95% CI level with regard to age (5 years younger on average), adiposity (lower weight, BMI and WHR) and lower α-tocopherol concentration (Table 1). Other variables did not differ significantly.
PCA produced four components from the twelve nutritional biomarkers that were modelled. These four components explained 67·8% of the variance in the population (Table 2). These components were classified as: (i) a ‘lipid’ component (cholesterol, TAG, the lipid-soluble antioxidants retinol, α-tocopherol, lutein+zeaxanthin, and WHR); (ii) an ‘adiposity’ component (weight, sum of skinfolds, WHR); (iii) a ‘dietary quality’ component (homocysteine, lutein+zeaxanthin, cryptoxanthin, lycopene); and (iv) a ‘habitus with inverse quality diet’ (i.e. body size with low quality diet) component (height, sum of skinfolds (inversely), cryptoxanthin (inversely)).
Table 2.
Principal components analysis (n 444; includes all participants with complete profile at baseline from three Central Australian Aboriginal communities)
| Component 1: ‘lipids’ | Component 2: ‘adiposity’ | Component 3: ‘dietary quality’ | Component 4: ‘habitus with inverse quality diet’ | |
|---|---|---|---|---|
| Height | 0·158 | 0·042 | 0·043 | 0·878 |
| Weight | 0·193 | 0·840 | 0·022 | 0·335 |
| Sum of skinfolds | −0·051 | 0·799 | 0·087 | −0·449 |
| Waist-to-hip ratio | 0·419 | 0·613 | −0·139 | 0·103 |
| Cholesterol | 0·746 | 0·152 | 0·109 | 0·057 |
| TAG | 0·777 | 0·233 | −0·049 | 0·068 |
| Homocysteine | 0·290 | −0·106 | −0·617 | 0·118 |
| Retinol | 0·691 | −0·138 | −0·236 | 0·142 |
| α-Tocopherol | 0·749 | 0·299 | 0·218 | −0·044 |
| Lutein+zeaxanthin | 0·554 | −0·064 | 0·468 | −0·372 |
| Cryptoxanthin | 0·240 | −0·109 | 0·614 | −0·509 |
| Lycopene | 0·150 | −0·051 | 0·735 | 0·307 |
| Eigenvalue | 2.91 | 1·94 | 1·66 | 1·63 |
| % of variance | 24·2 | 16·1 | 13·9 | 13·6 |
| Total variance (%) | 40·4 | 54·3 | 67·8 |
Data are loadings (correlations); values in bold font indicate variables characteristic of the respective component.
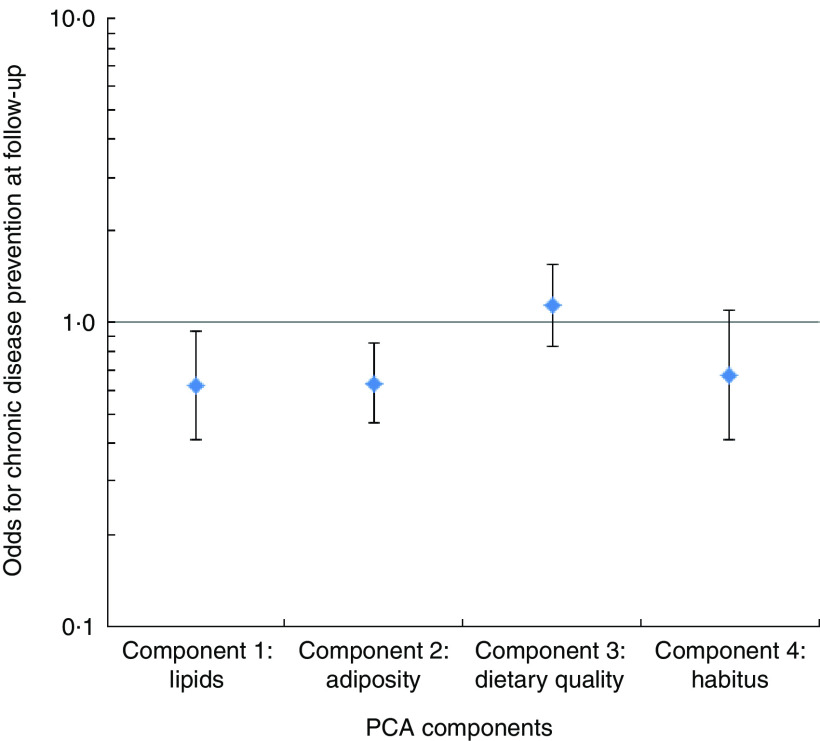
Logistic regression modelling revealed that the ‘lipid’ and ‘adiposity’ components were significantly associated with lower odds of being free of chronic disease at follow-up (Fig. 1). With each unit increase in value of the ‘lipid’ component, a person was 38% less likely to be free of chronic disease; for the ‘adiposity’ component this was 37 %. The ‘dietary quality’ component was positively associated with the absence of chronic disease at follow-up, with each unit increase in this component corresponding to a 13% increased odds of being free of chronic disease, albeit this did not reach statistical significance. There appeared to be no important associations between the ‘habitus with inverse quality diet’ component and the incidence of chronic disease.
Fig. 1.
Logistic regression modelling of the odds of chronic disease prevention (odds ratios with their 95 % confidence intervals represented by vertical bars) at follow-up in 2004/5 associated with ‘components’ from principal components analysis (PCA) for people (n 286) from three Central Australian Aboriginal communities who were free of chronic disease at baseline in 1995
Absence of specific chronic disease: diabetes, chronic kidney disease, CVD, hypertension
Of the 286 people free of chronic disease at baseline, 86·0% remained free of diabetes, 75·9% free of CKD, 83·2% free of hypertension and 93·7% free of CVD at follow-up. Baseline characteristics of people without diabetes, CVD or hypertension differed from those of people with these chronic diseases, particularly with respect to adiposity and homocysteine. Nutritional indicators did not differ significantly between persons with CKD and those without CKD.
Diabetes
Low adiposity protected against the development of diabetes. In univariate analysis, lower biomarkers for adiposity, including weight, BMI, WHR and sum of skinfolds, were all associated with the absence of diabetes at follow-up (Table 3). Multivariate analysis revealed that the PCA-derived ‘adiposity’ component had a strong inverse association with the absence of incident diabetes (Table 4).
Hypertension
Low adiposity and lipids were associated with the absence of hypertension at follow-up. In univariate analysis the characteristics of those who were not hypertensive at follow-up included younger age, female sex, lower adiposity (weight, WHR, BMI), lower blood lipids (cholesterol, TAG) and lower homocysteine (marker of increased folate; Table 3). In multivariate analysis the ‘lipid’ and ‘adiposity’ components were associated with 55% and 48% reduced odds for the absence of incident hypertension. There was moderate evidence that higher ‘dietary quality’ component was also important (Table 4).
CVD
Lower adiposity and lipids and greater dietary quality intake were most closely associated with the absence of CVD at follow-up. Univariate associations were seen between the absence of CVD and younger age, lower adiposity (weight, BMI, WRH), lower TAG, and better dietary quality with lower homocysteine (higher folate) and higher cryptoxanthin (Table 3). Persons who remained free of CVD were on average 12 kg lighter (Table 3). In adjusted logistic regression models, the ‘dietary quality’ component was associated with twofold increased odds for absence of incident CVD (Table 4). Conversely, every unit increase in the ‘lipid’ or ‘adiposity’ components was associated with 57% and 59% decreased odds, respectively, of absence of incident CVD.
Chronic kidney disease
None of the nutritional biomarkers appeared to be strongly protective against CKD. In univariate analysis no nutritional variables were associated with incident CKD.
Discussion
Consistent with data for many Aboriginal populations( 14 , 24 ), we saw that a high proportion of this healthy young population went on to develop chronic disease. These data support the need to identify potential targets for chronic disease prevention. The key finding from the present study is that lower adiposity, lower lipids and better dietary quality were associated with the absence at 10-year follow-up of most chronic diseases examined, with the exception of CKD. While it is somewhat unorthodox to frame an analysis in terms of ‘targets for chronic disease prevention’ and investigate the ‘absence of disease’, this framework has practical application for PHC clinics in Central Australia whose core activities centre around chronic disease prevention. This is a relatively novel epidemiological approach and it is only in recent years that researchers in Aboriginal health have sought to frame analysis in a way that aligns more closely with the definitions of Aboriginal health and wellness( 25 ).
In terms of interesting findings, our unadjusted baseline statistics demonstrated a nearly twofold higher prevalence of smoking among individuals free of chronic disease at baseline as opposed to those with chronic disease, this despite smoking being a known risk factor for chronic disease. We believe several factors may explain this unconventional cross-sectional association, including higher rates of smoking among younger adults who are more likely to be free of chronic disease; the possibility of people with chronic disease ceasing smoking following chronic disease diagnosis; and higher mortality associated with smoking and chronic disease.
Low adiposity (as characterised by low BMI, weight and WHR) appears a useful marker in the prevention of most chronic diseases. With regard to distribution of adipose tissue our findings are consistent with the extensive literature that demonstrates central adiposity (indicated here by WHR) has a greater association with CVD than does peripheral and subcutaneous adiposity (indicated by skinfold thickness) and that both intra-abdominal and subcutaneous adipose tissue distributions are predictors of diabetes( 26 , 27 ). However, in terms of clinical assessment, it appears that BMI as a marker of non-specific adiposity may be of particular utility as in the present study it was associated with the absence of diabetes, CVD and hypertension. Lower BMI was previously demonstrated to be associated with lower diabetes in Central Australian and Top End Aboriginal communities( 28 , 29 ). From the data presented here, it is likely that the traditional ‘healthy’ BMI threshold of <25·0 kg/m2 will not be overly specific in chronic disease prediction as the average BMI of those who developed chronic disease (24·8 kg/m2) fell below the ‘healthy’ BMI range as specified by the WHO( 30 ). A study by Brimblecombe et al. in the Top End Aboriginal community revealed that a stringent BMI threshold of >22·0 kg/m2 was associated with a 24·1 increased odds ratio for diabetes, and findings from Daniel et al. suggested a similar threshold is applicable in Central Australia( 28 , 29 ). The absence of any meaningful association between chronic disease and the ‘habitus with inverse quality diet’ component after adjustment for sex suggests that height (despite being a marker of nutrition during infancy and childhood) may be less useful in predicting the development of chronic disease when height is uniformly low across the whole population.
Lipids, including total cholesterol and TAG, are established risk factors for CVD( 31 ) and for this cohort lower concentrations were associated with the absence of chronic disease, particularly CVD and hypertension. The grouping of lipids (cholesterol and TAG) with lipid-soluble vitamins in PCA is likely to explain why low α-tocopherol and retinol were associated with CVD and hypertension, respectively, in univariate analysis. Despite α-tocopherol intake being associated with the absence of CVD in some studies( 32 , 33 ), the fact that it is found in some processed foods may explain why high concentrations were not associated with the absence of CVD in our unadjusted univariate analysis. Further to this, α-tocopherol is a lipid-soluble molecule strongly associated with LDL, a known CVD risk factor.
Higher circulating levels of carotenoids and lower homocysteine as biomarkers of dietary fruit and vegetable intake were significantly associated with the absence of CVD in this cohort, despite a very limited range of blood concentrations for these analytes. However, in interpreting the findings related to homocysteine we are mindful that although homocysteine is a useful surrogate inverse marker of folate (fruit and vegetable) intake, against a background of high kidney disease incidence it may also be influenced by subclinical renal impairment( 34 ). It is also important to note that folate from white bread (a dietary staple) may be contributing to higher folate readings, as opposed to fruits and vegetables alone( 6 ). Carotenoids have previously been reported as ubiquitously low, and homocysteine high, across this Central Australian population with only one in twenty and one in four meeting ‘adequate’ carotenoid and homocysteine concentrations, respectively( 35 ). Present-day studies indicate that low fruit and vegetable consumption remains an issue in remote communities in the Northern Territory as 2·2% of food expenditure is on fruits and 5·4% on vegetables( 6 ). Further to this, point-of-sale data from remote communities in the Northern Territory indicate that fruit and vegetable sales account for only 10·4% of all sales( 36 ).
Here we saw evidence that greater dietary quality, namely higher fruit and vegetable intake, was associated with increased odds of preventing hypertension (approaching significance) and CVD. This is supported by meta-analysis for populations elsewhere demonstrating that CHD risk decreased by 4% for each portion of fruit and vegetables per day( 37 ). The association of the PCA ‘dietary quality’ component (lower homocysteine and higher cryptoxanthin, lutein + zeaxanthin, lycopene) with hypertension and CVD, and limited univariate associations with single carotenoids, suggests that a diet high in carotenoids from a variety of sources such as leafy greens, corn, eggs, oranges, pumpkin and tomato is more important than a single ‘superfood’. However, in interpreting these findings we must also be mindful that sources of carotenoids may include bush foods, and that messages to eat fruits and vegetables originating solely from stores might not be as useful as overall messages to improve dietary quality. Messages for chronic disease prevention should advocate for increased plant-based foods (whole grains, fruits and vegetables, bush tucker) as well as lean animal foods (particularly traditional lean meat higher in n-3 fatty acids).
Fruit and vegetable intake was not associated with the absence of diabetes or CKD for this population. Other cross-sectional studies relating to this population demonstrated that carotenes were associated with impaired kidney function at baseline( 5 ). However, no significant association of carotenoids with CKD absence was seen in the current longitudinal study. The role of other oxidative stressors (infection, smoking, intake of excess catalytic transition metals) and other potential causes (fetal environment, early life, family history) may mask dietary effects. Hoy et al. previously demonstrated for a Top End Aboriginal population that the causes of renal disease are multifactorial and that a strong relationship exists between whole-of-life nutrition, infections, health behaviours, low birth weight and deteriorating metabolic profiles and renal disease( 38 ). This reported relationship with low birth weight stresses the particular importance of maternal nutrition.
Current literature is also somewhat contradictory as to the benefits of fruits and vegetables in diabetes prevention. Studies for other populations have demonstrated limited benefit of fruits and vegetables in diabetes prevention( 39 ), while a meta-analysis of six studies in 2010 suggested that increasing green leafy vegetables reduces risk of diabetes but increasing fruits or vegetables may have limited benefit for diabetes prevention( 40 ). Here biomarkers of green leafy vegetables – homocysteine (as a marker of folate) and lutein + zeaxanthin – showed no association with the absence of diabetes in univariate analysis. We reiterate, however, that the range of intakes for this population was very limited. It is clear that diets high in plant foods (and thus of low energy density) are, at a minimum, valuable for weight management and thus important for diabetes prevention.
An implication of our findings is that chronic disease prevention in Central Australia (at the individual, family, community and societal levels) can be further targeted towards the maintenance of leanness (or lowering of adiposity), lowering of lipids to a population-specific ‘healthy range’ and increasing nutritional quality. Previous Aboriginal community-driven health promotion activities have demonstrated that education messages aimed at increasing fruit and vegetable intake, increasing physical activity, cutting fat from meat and replacing sucrose/fructose drinks with water or diet drinks, combined with improving the nutritional quality of food available at the general store, led to improvements in homocysteine (via increased folate), lipids, carotenoids and antioxidant vitamin levels, and weight loss (in the younger population)( 16 , 35 ). There is also evidence that chronic disease prevention activity to decolonise the modern diet by reverting to a ‘traditional diet’ is associated with lowering hyperglycaemia and CVD risk( 2 ). Homelands living is also associated with lower incidence of chronic disease( 41 ).
For Aboriginal populations, programmes that are most successful are those that are affordable, social, flexible and held in accessible, culturally appropriate settings( 42 ). There is also evidence for community-driven health promotion interventions, as externally imposed approaches such as the income management in the Northern Territory have had no beneficial effect on tobacco, cigarette, soft drink, fruit or vegetable consumption( 43 ). In considering this, future health promotion should act beyond individual pharmacological and clinic-based interventions to lower and manage lipids and adiposity and increase fruit and vegetable intake, and really need to target larger systemic issues around access, storage and cost of healthy foods. It is also important that education messages and activities are driven from within communities so they are locally relevant and centre around Aboriginal normalities( 43 ).
As identified by Hodge et al., blood carotenoid concentrations were associated with socio-economic status (education, income, household size, home ownership and employment) for an Aboriginal population in Darwin( 44 ). Given that the Central Australian population is one of the most disadvantaged in Australia our finding of poor nutritional biomarkers is not surprising. An overarching explanation is that people in Central Australia may be likely to maximise their value for money by purchasing energy-dense nutrient-poor alternatives such as sugar, flour and oil( 3 ). This is further exacerbated by geographic isolation and remoteness that make storage of and access to fruits and vegetables expensive and difficult. This pattern of fruit and vegetable intake is a likely contributor to elevated adiposity. Here we reported very low carotenoid and homocysteine levels across the whole population, suggesting that this is not due to individual preference for low fruit and vegetable intake, but likely wider systemic issues at a population level relating to access, quality and storage. These concentrations mirror those of a remote Aboriginal population in Western Australia( 16 ), are lower than those of an urban Aboriginal population from Darwin( 44 ) and are far lower than those of a diabetic cohort from Melbourne( 45 ).
In considering all these findings and the implications for future health promotion, we must recognise that a limitation of the current analysis is that it still conceptualises health only in terms of physical and biological variables. As this cohort study was originally designed to investigate clinical biomarkers of CVD risk, absent from this analysis are the economic, behavioural and social determinants for chronic disease. As researchers using epidemiological methods we must appreciate that for Aboriginal populations ‘conventional science fails to recognise the true root causes of diabetes (and chronic disease more broadly): a life out of balance due to the loss of traditional lifestyles, community and spirituality’( 46 , 47 ). Social epidemiologists such as Thompson et al. stress the importance that ‘social identity, connections, sense of coherence and control play in the “physical” configuration of “risk”’ for Aboriginal populations( 46 ) (p. 726). Future research should explore and/or adjust for such behavioural, social, cultural and political determinants as opposed to clinical variables alone( 46 ).
Conclusion
In conclusion, the information presented here suggests that nutrition-based chronic disease prevention activity in Central Australia needs to focus on maintaining and preventing adiposity (maintaining or reducing BMI to <22·0 kg/m2), improving dietary quality (particularly the accessibility of fruits and vegetables) and the prevention and treatment of dyslipidaemia.
Acknowledgements
Acknowledgements: The authors thank the Elders, community members and medical service providers of the Central Australian communities involved in the project; and Leah Johnston, Ricky Tilmouth, Iris Roberts, Joseph Fitz and Paul Rickards for expert assistance. Financial support: This work was funded by the National Health and Medical Research Council of Australia (grant numbers 631947, 299852 and 974302). The National Health and Medical Research Council had no role in the design, analysis or writing of this manuscript. Conflict of interest: None. Authorship: J.N.L.: preparation of manuscript, statistical analysis, review of manuscript. R.R.: statistical advice and analysis, review of manuscript. K.O.: study design, collection and interpretation of data, review of manuscript. A.B.: study design, clinical expertise, collection of data, review of manuscript. L.S.P.: clinical expertise, collection of data, review of manuscript. A.J.J.: study design, clinical expertise, review of manuscript. K.G.R.: study design, data collection, preparation of manuscript, statistical and clinical expertise, review of manuscript. Ethics of human subject participation: The study was approved by the Central Australian and University of Melbourne Human Research Ethics Committees.
References
- 1. Elphinstone JJ (1971) The health of Australian Aborigines with no previous association with Europeans. Med J Aust 2, 293–301. [DOI] [PubMed] [Google Scholar]
- 2. O’Dea K (1991) Westernization and non-insulin-dependent diabetes in Australian Aborigines. Ethn Dis 1, 171–187. [PubMed] [Google Scholar]
- 3. Brimblecombe J & O’Dea K (2009) The role of energy cost in food choices for an Aboriginal population in northern Australia. Med J Aust 190, 549–551. [DOI] [PubMed] [Google Scholar]
- 4. Gracey M (2000) Historical, cultural, political, and social influences on dietary patterns and nutrition in Australian Aboriginal children. Am J Clin Nutr 72, 5 Suppl, 1361S–1367S. [DOI] [PubMed] [Google Scholar]
- 5. Rowley K, O’Dea K, Su Q et al. (2003) Low plasma concentrations of diet-derived antioxidants in association with microalbuminuria in Indigenous Australian populations. Clin Sci (Lond) 105, 569–575. [DOI] [PubMed] [Google Scholar]
- 6. Brimblecombe JK, Ferguson MM, Liberato SC et al. (2013) Characteristics of the community-level diet of Aboriginal people in remote northern Australia. Med J Aust 198, 380–384. [DOI] [PubMed] [Google Scholar]
- 7. Burke V, Zhao Y, Lee AH et al. (2007) Health-related behaviours as predictors of mortality and morbidity in Australian Aborigines. Prev Med 44, 135–142. [DOI] [PubMed] [Google Scholar]
- 8. Kuhnlein HV, Receveur O, Soueida R et al. (2004) Arctic Indigenous peoples experiences of the nutrition transition with changing dietary patterns and obesity. J Nutr 134, 1447–1453. [DOI] [PubMed] [Google Scholar]
- 9. Wattanapitayakul SK & Bauer JA (2001) Oxidative pathways in cardiovascular disease: roles, mechanisms, and therapeutic implications. Pharmacol Ther 89, 187–206. [DOI] [PubMed] [Google Scholar]
- 10. Steinburg D (1992) Antioxidants in the prevention of human atherosclerosis. Summary of the proceedings of a National Heart, Lung, and Blood Institute Workshop: September 5–6, 1991, Bethesda, Maryland. Circulation 85, 2337–2344. [DOI] [PubMed]
- 11. Bohm V (2012) Lycopene and heart health. Mol Nutr Food Res 56, 296–303. [DOI] [PubMed] [Google Scholar]
- 12. Ford ES, Byers TE & Giles WH (1998) Serum folate and chronic disease risk: findings from a cohort of United States adults. Int J Epidemiol 27, 592–598. [DOI] [PubMed] [Google Scholar]
- 13. Gey KF, Puska P, Jordan P et al. (1991) Inverse correlation between plasma vitamin E and mortality from ischemic heart disease in cross-cultural epidemiology. Am J Clin Nutr 53, 1 Suppl, 326S–334S. [DOI] [PubMed] [Google Scholar]
- 14. Luke JN, Brown AD, Brazionis L et al. (2013) Exploring clinical predictors of cardiovascular disease in a central Australian Aboriginal cohort. Eur J Prev Cardiol 20, 246–253. [DOI] [PubMed] [Google Scholar]
- 15. O’Neal DN, Piers LS, Iser DM et al. (2008) Australian Aboriginal people and Torres Strait Islanders have an atherogenic lipid profile that is characterised by low HDL-cholesterol level and small LDL particles. Atherosclerosis 201, 368–377. [DOI] [PubMed] [Google Scholar]
- 16. Rowley KG, Su Q, Cincotta M et al. (2001) Improvements in circulating cholesterol, antioxidants and homocystine after dietary intervention in an Australian Aboriginal community. Am J Clin Nutr 74, 442–448. [DOI] [PubMed] [Google Scholar]
- 17. Humphery K, Dixon Japanangka M & Marrawal J (1998) From the Bush to the Store – Diabetes, Everyday Life and Critique of Health Services in Two Remote Northern Territory Aboriginal Communities. Darwin, NT: Territory Health Services and Diabetes Australia. [Google Scholar]
- 18. McDermott R, Rowley KG, Lee AJ et al. (2000) Increase in prevalance of obesity and decrease in plasma cholesterol in a central Australian Aboriginal community. Med J Aust 172, 480–484. [DOI] [PubMed] [Google Scholar]
- 19. Rowley KG, Gault A, McDermott R et al. (2000) Reduced prevalence of impaired glucose tolerance and no change in prevalence of diabetes despite increasing BMI among Aboriginal people from a group of remote homeland communities. Diabetes Care 23, 898–904. [DOI] [PubMed] [Google Scholar]
- 20. Marsh DR, Schroeder DG, Dearden KA et al. (2004) The power of positive deviance. BMJ 329, 1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piers LS, Rowley KG, Soares MJ et al. (2003) The relationship of adiposity and body fat distribution to body mass index in Australians of Aboriginal and European ancestry. Eur J Clin Nutr 57, 956–963. [DOI] [PubMed] [Google Scholar]
- 22. Luke JN, Brown AD, O’Neal D et al. (2009) Lipid treatment guidelines and cardiovascular risk for Aboriginal people in Central Australia. Med J Aust 190, 552–556. [DOI] [PubMed] [Google Scholar]
- 23. Jolliffe I (2002) Principal Component Analysis, 2nd ed. New York: Springer-Verlag New York Inc. [Google Scholar]
- 24. Wang Z & Hoy WE (2010) C-reactive protein: an independent predictor of cardiovascular disease in Aboriginal Australians. Aust N Z J Public Health 34, Suppl. 1, S25–S29. [DOI] [PubMed] [Google Scholar]
- 25. Thomas DP, Briggs V & Anderson I (2008) The social determinants of being an Indigenous non-smoker. Aust N Z J Public Health 32, 110–116. [DOI] [PubMed] [Google Scholar]
- 26. Larsson B, Svardsudd K, Welin L et al. (1984) Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 288, 1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Rimm EB, Stampfer MJ et al. (2005) Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 81, 555–563. [DOI] [PubMed] [Google Scholar]
- 28. Brimblecombe J, Mackerras D, Garnggulkpuy J et al. (2006) Leanness and type 2 diabetes in a population of indigenous Australians. Diabetes Res Clin Pract 72, 93–99. [DOI] [PubMed] [Google Scholar]
- 29. Daniel M, Rowley K, McDermott R et al. (1999) Diabetes incidence in an Australian Aboriginal 8-year follow-up study. Diabetes Care 22, 1993–1998. [DOI] [PubMed] [Google Scholar]
- 30. Alberti KG & Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15, 539–553. [DOI] [PubMed] [Google Scholar]
- 31. Wilson P, D’Agostino R, Levy D et al. (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97, 1837–1847. [DOI] [PubMed] [Google Scholar]
- 32. Rimm EB, Stampfer MJ, Ascherio A et al. (1993) Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 328, 1450–1456. [DOI] [PubMed] [Google Scholar]
- 33. Stampfer MJ, Hennekens CH, Manson JE et al. (1993) Vitamin E consumption and the risk of coronary heart disease in women. N Engl J Med 328, 1444–1449. [DOI] [PubMed] [Google Scholar]
- 34. Friedman AN, Bostom AG, Selhub J et al. (2001) The kidney and homocysteine metabolism. J Am Soc Nephrol 12, 2181–2189. [DOI] [PubMed] [Google Scholar]
- 35. Rowley K, Lee AH & Yarmirr D (2003) Homocystine concentrations lowered following dietary intervention in an Aboriginal community. Asia Pac J Clin Nutr 12, 92–95. [PubMed] [Google Scholar]
- 36. Brimblecombe J, Liddle R & O’Dea K (2013) Use of point-of-sale data to assess food and nutrient quality in remote stores. Public Health Nutr 16, 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dauchet L, Amouyel P & Hercberg S (2006) Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr 136, 2588–2593. [DOI] [PubMed] [Google Scholar]
- 38. Hoy WE, Mathews JD, McCredie DA et al. (1998) The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney Int 54, 1296–1304. [DOI] [PubMed] [Google Scholar]
- 39. Liu S, Serdula M & Janket S (2004) A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care 27, 2993–2996. [DOI] [PubMed] [Google Scholar]
- 40. Carter P, Gray LJ, Troughton J et al. (2010) Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ 341, c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rowley K, O’Dea K, Anderson I et al. (2008) Lower than expected morbidity and mortality for an Australian Aboriginal population: 10-year follow-up in a decentralised community. Med J Aust 188, 283–287. [DOI] [PubMed] [Google Scholar]
- 42. Abbott PA, Davidson JE & Moore LF (2012) Effective nutrition education for Aboriginal Australians: lessons from a diabetes cooking course. J Nutr Educ Behav 44, 55–59. [DOI] [PubMed] [Google Scholar]
- 43. Brimblecombe J, McDonnell J, Barnes A et al. (2010) Impact of income management on store sales in the Northern Territory. Med J Aust 192, 549–554. [DOI] [PubMed] [Google Scholar]
- 44. Hodge A, Cunningham J, Maple-Brown L et al. (2011) Plasma carotenoids are assoicated with socioeconomic status in an urban Indigenous population: an observational study. BMC Public Health 11, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brazionis L, Rowley K, Itsiopoulos C et al. (2009) Plasma carotenoids and diabetic retinopathy. Br J Nutr 101, 270–277. [DOI] [PubMed] [Google Scholar]
- 46. Thompson SJ, Gifford SM & Thorpe LG (2000) The social and cultural context of risk and prevention: food and physical activity in an urban Aboriginal community. Health Educ Behav 27, 725–743. [DOI] [PubMed] [Google Scholar]
- 47. Giles BG, Findlay CS, Hass G et al. (2010) Integrating conventional science and aboriginal perspectives on diabetes using fuzzy cognitive maps. Soc Sci Med 64, 562–576. [DOI] [PubMed] [Google Scholar]