Abstract
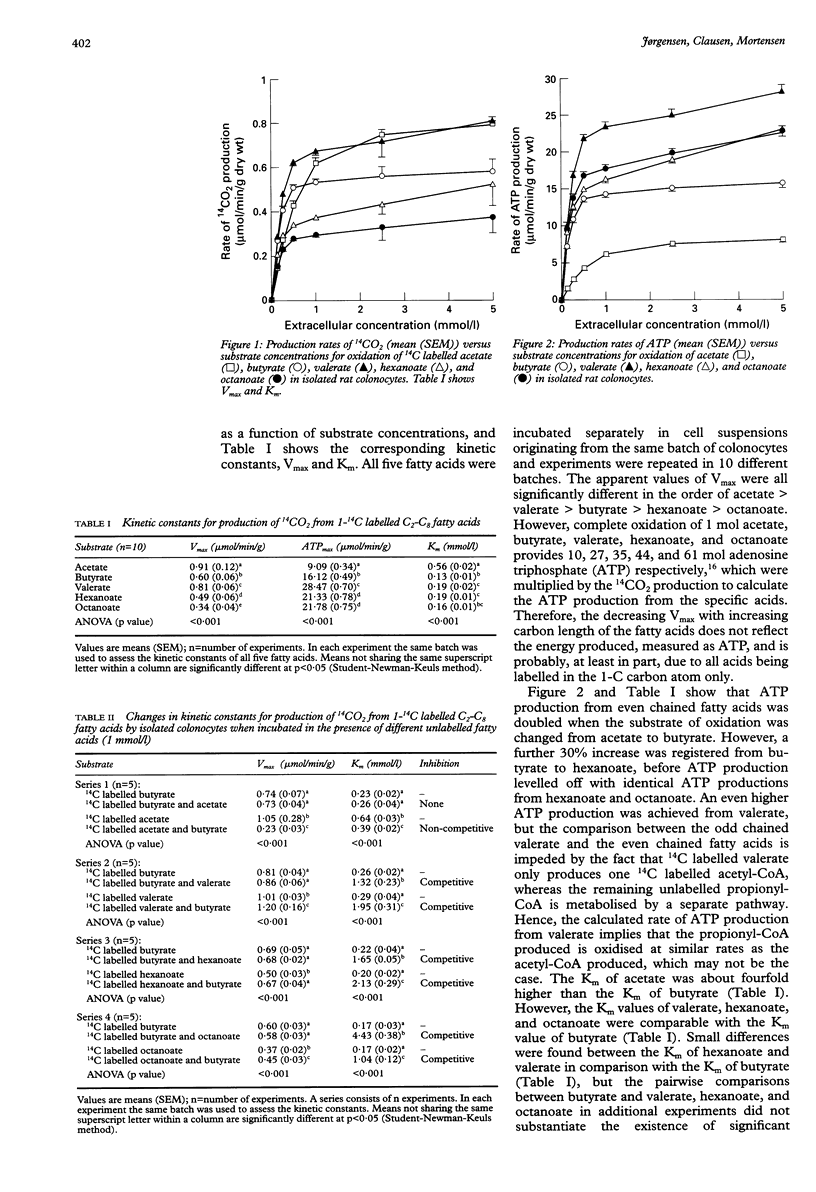
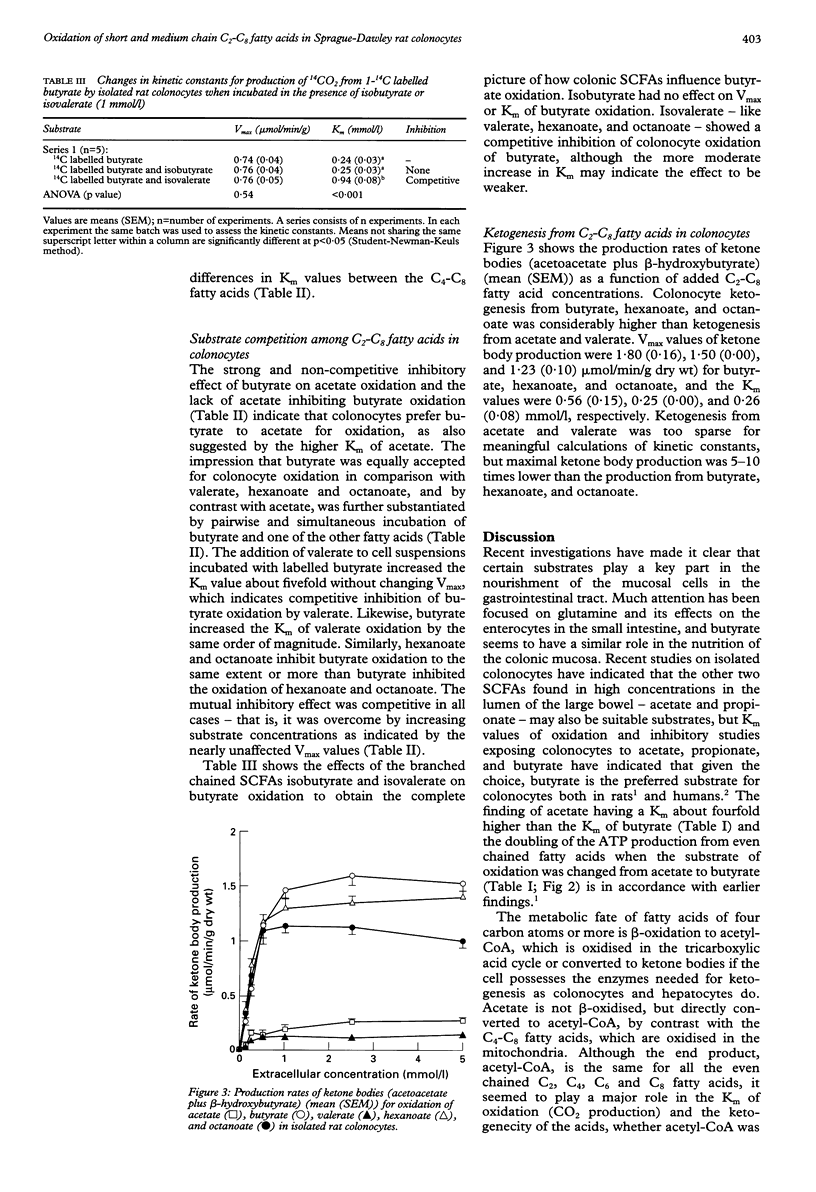
BACKGROUND: The predominant colonic short chain fatty acids, acetate, propionate, and butyrate, are oxidised into CO2 in colonocytes from rat and humans in the preferred order of butyrate (C4) > propionate (C3) > acetate (C2)- hence butyrate is considered to be the principal oxidative substrate for colonocytes. AIMS: To compare colonocyte oxidation of valerate (C5), hexanoate (C6), and octanoate (C8) with that of butyrate. METHODS: Isolated rat colonocytes were incubated in the presence of a concentration range of 1-14C labelled C2-C8 fatty acids. Oxidation rates were obtained by quantifying the production of 14CO2, and Vmax (maximum velocity) and K(m) (Michaelis-Menten constant) were calculated by computer fitting of the data to a Michaelis-Menten plot. RESULTS: The K(m) value of acetate (0.56 (SEM 0.02) mmol/l) was about fourfold higher than the K(m) of butyrate (0.13 (0.01) mmol/l), whereas the K(m) values of valerate (0.19 (0.01) mmol/l), hexanoate (0.19 (0.01) mmol/l), and octanoate (0.16 (0.01) mmol/l) were of the same order of magnitude as the K(m) of butyrate. Acetate did not influence butyrate oxidation, whereas butyrate strongly inhibited the oxidation of acetate. By contrast, valerate, hexanoate, and octanoate inhibited colonocyte oxidation of butyrate equally or more than the reverse inhibitory effect of butyrate on valerate, hexanoate, and octanoate oxidation. The maximum rates of ATP production were in the order of valerate > octanoate = hexanoate > butyrate > acetate (28.47 (0.70), 21.78 (0.75), 21.33 (0.78), 16.12 (0.49), 9.09 (0.34) (mumol/min/g) respectively). CONCLUSIONS: Valerate, hexanoate, and octanoate seem to be excellent substrates for colonocyte oxidation, similar to butyrate. These results may influence the choice of fatty acid composition in enemas used for treatment of patients in whom deficient colonocyte oxidation is suspected-for example, patients with ulcerative colitis and diversion colitis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartram H. P., Scheppach W., Englert S., Dusel G., Richter A., Richter F., Kasper H. Effects of deoxycholic acid and butyrate on mucosal prostaglandin E2 release and cell proliferation in the human sigmoid colon. JPEN J Parenter Enteral Nutr. 1995 May-Jun;19(3):182–186. doi: 10.1177/0148607195019003182. [DOI] [PubMed] [Google Scholar]
- Chapman M. A., Grahn M. F., Boyle M. A., Hutton M., Rogers J., Williams N. S. Butyrate oxidation is impaired in the colonic mucosa of sufferers of quiescent ulcerative colitis. Gut. 1994 Jan;35(1):73–76. doi: 10.1136/gut.35.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen M. R., Mortensen P. B. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut. 1995 Nov;37(5):684–689. doi: 10.1136/gut.37.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen M. R., Mortensen P. B. Kinetic studies on the metabolism of short-chain fatty acids and glucose by isolated rat colonocytes. Gastroenterology. 1994 Feb;106(2):423–432. doi: 10.1016/0016-5085(94)90601-7. [DOI] [PubMed] [Google Scholar]
- Dexter D. L., Lev R., McKendall G. R., Mitchell P., Calabres P. Sodium butyrate-induced alteration of growth properties and glycogen levels in cultured human colon carcinoma cells. Histochem J. 1984 Feb;16(2):137–149. doi: 10.1007/BF01003545. [DOI] [PubMed] [Google Scholar]
- Finnie I. A., Taylor B. A., Rhodes J. M. Ileal and colonic epithelial metabolism in quiescent ulcerative colitis: increased glutamine metabolism in distal colon but no defect in butyrate metabolism. Gut. 1993 Nov;34(11):1552–1558. doi: 10.1136/gut.34.11.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harig J. M., Soergel K. H., Komorowski R. A., Wood C. M. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989 Jan 5;320(1):23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- Hove H., Mortensen P. B. Influence of intestinal inflammation (IBD) and small and large bowel length on fecal short-chain fatty acids and lactate. Dig Dis Sci. 1995 Jun;40(6):1372–1380. doi: 10.1007/BF02065554. [DOI] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980 Sep;21(9):793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet. 1980 Oct 4;2(8197):712–715. doi: 10.1016/s0140-6736(80)91934-0. [DOI] [PubMed] [Google Scholar]
- Roediger W. E., Truelove S. C. Method of preparing isolated colonic epithelial cells (colonocytes) for metabolic studies. Gut. 1979 Jun;20(6):484–488. doi: 10.1136/gut.20.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982 Aug;83(2):424–429. [PubMed] [Google Scholar]
- Scheppach W., Sommer H., Kirchner T., Paganelli G. M., Bartram P., Christl S., Richter F., Dusel G., Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992 Jul;103(1):51–56. doi: 10.1016/0016-5085(92)91094-k. [DOI] [PubMed] [Google Scholar]
- Senagore A. J., MacKeigan J. M., Scheider M., Ebrom J. S. Short-chain fatty acid enemas: a cost-effective alternative in the treatment of nonspecific proctosigmoiditis. Dis Colon Rectum. 1992 Oct;35(10):923–927. doi: 10.1007/BF02253492. [DOI] [PubMed] [Google Scholar]
- Steinhart A. H., Brzezinski A., Baker J. P. Treatment of refractory ulcerative proctosigmoiditis with butyrate enemas. Am J Gastroenterol. 1994 Feb;89(2):179–183. [PubMed] [Google Scholar]
- Whitehead R. H., Young G. P., Bhathal P. S. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215). Gut. 1986 Dec;27(12):1457–1463. doi: 10.1136/gut.27.12.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]



