Abstract
The pathways leading to G:C→C:G transversions and their repair mechanisms remain uncertain. C/C and G/G mismatches arising during DNA replication are a potential source of G:C→C:G transversions. The Escherichia coli mutHLS mismatch repair pathway efficiently corrects G/G mismatches, whereas C/C mismatches are a poor substrate. Escherichia coli must have a more specific repair pathway to correct C/C mismatches. In this study, we performed gel-shift assays to identify C/C mismatch-binding proteins in cell extracts of E.coli. By testing heteroduplex DNA (34mers) containing C/C mismatches, two specific band shifts were generated in the gels. The band shifts were due to mismatch-specific binding of proteins present in the extracts. Cell extracts of a mutant strain defective in MutM protein did not produce a low-mobility complex. Purified MutM protein bound efficiently to the C/C mismatch-containing heteroduplex to produce the low-mobility complex. The second protein, which produced a high-mobility complex with the C/C mismatches, was purified to homogeneity, and the amino acid sequence revealed that this protein was the FabA protein of E.coli. The high-mobility complex was not formed in cell extracts of a fabA mutant. From these results it is possible that MutM and FabA proteins are components of repair pathways for C/C mismatches in E.coli. Furthermore, we found that Saccharomyces cerevisiae OGG1 protein, a functional homolog of E.coli MutM protein, could specifically bind to the C/C mismatches in DNA.
INTRODUCTION
Base pair mismatches arise continually in DNA as a result of several mechanisms, including errors during DNA replication, spontaneous oxidation of bases and recombination between homologous but non-identical DNA sequences (1–6). The repair of such mismatched bases is essential for reducing the frequencies of spontaneous mutations and maintaining the integrity of the genome (4–10). In particular, low rates of spontaneous G:C→C:G transversions would be achieved not only by the correction of base mismatches during DNA replication but also by the prevention and removal of oxidative base damage in DNA (4,7,8,11). We previously found that MutY protein prevents the generation of G:C→C:G transversions by removing the unmodified guanine from the 8-oxoguanine:guanine mispairs in Escherichia coli (11).
In addition, C/C and G/G mispairs generated during DNA replication would be a source of the mutations. Bacteria and mammalian cells have evolved several repair mechanisms to deal with mismatches (5,7–10). In E.coli, a general repair system referred to as the mutHLS system corrects most replication errors with efficiencies reflecting the frequency and specificity of mispair formation (2,5,7,8). MutS and MutL are involved in the initiation step of mismatch repair. MutS recognizes and binds to single-base mismatches in DNA, and MutL forms a complex with MutS and MutH. Binding of MutL to MutH results in activation of the MutH endonuclease (2,4,5,7,8). Mutations in the mutS, mutL or mutH gene significantly enhance the frequencies of spontaneous mutations (4,5,7,8). However, the mutator characteristics do not result in enhancing G:C→C:G transversions (4,8,11). In E.coli, the mutHLS mismatch-repair pathway efficiently corrects G/G mismatches, but not C/C mismatches (12–14). In addition, E.coli MutS protein shows the lowest affinity for C/C mismatches in vitro (15,16). These facts suggest that E.coli has a more specific repair pathway to correct C/C mismatches for preventing G:C→C:G transversions (17). Recently Fleck et al. (18,19) reported that there are two C/C mismatch-binding proteins in Schizosaccharomyces pombe. These proteins might constitute a C/C mismatch repair system. Thus, besides the MutSL-like pathway, S.pombe has an additional pathway of mismatch repair that corrects C/C mismatches (18,19). It is of interest to know whether or not E.coli has an equivalent pathway.
In this study, we performed gel-shift assays to identify and characterize C/C mismatch-binding proteins in order to look for an additional repair pathway for C/C mismatches in E.coli. The present experiments revealed that there are two C/C mismatch-binding proteins, MutM and FabA, in cell extracts prepared from E.coli cells. The binding of these proteins to C/C mismatches was independent of MutS and MutL. Furthermore, it was also demonstrated that Saccharomyces cerevisiae OGG1 protein, a functional homolog of E.coli MutM protein (20–22), could bind specifically to C/C mismatches in DNA.
MATERIALS AND METHODS
Bacterial strains and media
Bacterial strains used in this study were derivatives of E.coli K-12. The strains and their relevant genotypes were AB1157 (wild-type), 21336 (uvrD260::Kan), BW313 (ung-1 dut-1), RPC501 (nfo-1::Tn5), JC7623 (recB21 recC22 sbcB15), WD8014 (mutD5), CC104mutM (mutM::Tet), SYT5 (mutT::Cm), CC104mutL (mutL::Kan), BMH71-18 (mutS215::Tet), CSH117 (mutY::Tet), NJK2004 (nth::Cm nei::Kan), GC4468hupAB (hupA16::Kan hupB::Cm), WU3610-45 (mfd), IC3126 [Δ(umuDC)::Kan], MS23 (alkA1), CS101 (recA269::Tet), CH1692 (topA57 tolC::Tet), N3055 (uvrA277::Tet), MG1655 (dps::Kan), TM41 (topB::Kan), RP4182 [Δ(dcm vsr)], C218 [Δ(ada25 alkB)::Cm ogt-1::Kan] and GW3773 (mutH471::Kan). Escherichia coli CC104 was ara Δ(gpt-lac)5 rpsL/F′ (lacI378 lacZ461 proA+B+) (23). Bacterial cells were grown in LB medium (4) at 37°C with aeration. The final concentrations of tetracycline, kanamycin, chloramphenicol and ampicillin were 15, 50, 20 and 50 µg/ml, respectively.
Enzymes and chemicals
Tetracycline hydrochloride, kanamycin and chloramphenicol were purchased from Wako Pure Chemicals (Osaka, Japan). Ampicillin was the product of Meiji Seika (Tokyo, Japan). T4 polynucleotide kinase was obtained from New England Biolabs (Beverly, MA). Glutathione–Sepharose 4B and thrombin protease were purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). Isopropyl-1-thio-β-d-galactopyranoside (IPTG), EcoRI, HindIII and KOD Plus DNA polymerase were obtained from TOYOBO (Osaka, Japan). [γ-32P]ATP (>259 TBq/mmol) was the product of ICN Biomedicals Inc. (Costa Mesa, CA).
Construction of recombinant S.cerevisiae OGG1 gene
The plasmid overproducing S.cerevisiae OGG1 protein was constructed according to the method of Girard et al. (24). We synthesized two PCR primers, yOGG1-For (5′-CCGGAATTCATGTCTTATAAATTCGG-3′) and yOGG1-Rev (5′-GCCCAAGCTTCTAATCTATTTTTGCTTC-3′). Saccharomyces cerevisiae genomic library, kindly supplied by Dr M. Ajimura (National Institute of Radiological Sciences, Japan), was used as a template for the PCR reaction to amplify the S.cerevisiae OGG1 gene. The primer yOGG1-For was used to engineer an EcoRI restriction site at the beginning of OGG1 gene. Primer yOGG1-Rev was used to introduce a HindIII restriction site at the end of OGG1 gene. The PCR was performed with a high-fidelity KOD Plus DNA polymerase, initiated by a 3 min incubation at 94°C, followed by 30 cycles of 94°C/30 s, 50°C/30 s and 68°C/90 s, with a final extension of 4 min at 72°C. The 1.1 kb amplified fragment containing the whole coding region of the OGG1 gene was inserted into plasmid vector pKK223-3 (Amersham Pharmacia Biotech) after digestion by EcoRI and HindIII. The resulting plasmid was named pKK223-3-yOGG1. To assure activity of protein encoded by the recombinant gene, E.coli CC104mutMmutY was transformed with the plasmid pKK223-3-yOGG1. The plasmid could suppress the frequency of spontaneous G:C→T:A transversions in E.coli CC104mutMmutY.
Preparation of cell extracts
Overnight cultures of E.coli were harvested by centrifugation, and the cell pellets (~1 g wet weight) were resuspended in 3 ml of buffer A (50 mM Tris–HCl, pH 8.0, and 1 mM EDTA) and stored at –80°C. The samples were diluted 3-fold with buffer A and then sonicated in an ice-water bath, followed by centrifugation at 8000 r.p.m. for 30 min and subsequently 12 000 r.p.m. for 30 min at 4°C.
Gel shift assays
The synthetic 34mer oligonucleotides 5′-AGCTTGGCTGCAGGTXGACGGATCCCCGGGAATT-3′ were 32P-labeled at the 5′-end with [γ-32P]ATP by T4 polynucleotide kinase and then annealed with their complementary oligonucleotides 5′-AATTCCCGGGGATCCGTCYACCTGCAGCCAAGCT-3′, where X and Y represent purine (A, G) and pyrimidine (T, C) bases, respectively.
Binding of proteins to the duplex oligonucleotides was monitored by gel shift assays according to the method of Fleck et al. (18) with a slight modification. Protein extracts were incubated for 30 min at 4°C with 23.5 fmol of 32P-labeled oligonucleotides in 25 mM Tris–HCl (pH 8.0) containing 0.5 mM dithiothreitol, 4 mM spermidine, 0.5 mM EDTA, 10% glycerol, 25 mM NaCl, 25 mM KCl, 0.01 mM ZnCl2 and 0.125 mM each dNTP. Sonicated calf thymus DNA (5 µg) was added to the reaction mixture. Electrophoresis was performed at 100 V on non-denaturing 12% polyacrylamide gels in TBE buffer (89 mM Tris–borate, pH 8.0, and 2.5 mM EDTA). The gels were dried and then autoradiographed at –80°C using Fuji RX film.
Purification of GST–MutM proteins
A plasmid expressing GST–MutM fusion protein was kindly supplied by Dr J. Yokota (National Cancer Center Research Institute, Japan). The GST–mutM fusion gene contains the whole coding region of the E.coli mutM gene (amino acids 1–269) (25,26). The fusion protein was expressed in E.coli strain BL21 with 1 mM IPTG and purified on a glutathione–Sepharose 4B column. The GST–MutM fusion protein was further treated with thrombin protease to remove the GST-tag.
Purification of C/C mismatch-binding protein
The cell extracts of E.coli mutM mutant were divided into four fractions: 0–20, 20–40, 40–60 and 60–80% saturation of ammonium sulfate, and the binding activity to mismatch-containing oligonucleotides in each fraction was tested. The 40–60% ammonium sulfate saturation fraction exhibited the highest binding activity. The precipitates obtained with 40–60% ammonium sulfate saturation were dissolved in buffer A and dialyzed against the same buffer (fraction I). Fraction I was loaded on a HiTrapQ anion exchange column (Amersham Pharmacia Biotech, 5 ml ×2), and the column was washed with buffer B (20 mM Tris–HCl, pH 8.0, and 150 mM NaCl). The C/C mismatch-binding activity was not retained under these conditions. The active fraction (fraction II) was then applied to a HiTrap-Heparin column (Amersham Pharmacia Biotech, 5 ml) equilibrated with buffer C (20 mM HEPES, pH 7.6, and 150 mM NaCl) and eluted with a linear salt gradient (150–650 mM NaCl in buffer C). The active fraction eluted at 300–375 mM NaCl was concentrated and pooled (fraction III). Fraction III was then loaded on a gel filtration column (HiPrep 16/60 Sephacryl S-200HR, Amersham Pharmacia Biotech, 5 ml) equilibrated with buffer B. The active fraction was concentrated and pooled (fraction IV).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and amino acid sequencing
Fraction IV was analyzed by SDS–PAGE on a 17.5% polyacrylamide gel, transferred to an Immobilon-PSQ membrane (Millipore, Bedford, MA) by electroblotting and then stained with Coomassie blue. The 20 kDa protein band was excised from the membrane, and the sequence of 10 amino acids from the N-terminus of the purified protein was determined by using a gas-phase amino acid sequencer.
RESULTS
Identification of C/C mismatch-binding proteins in E.coli
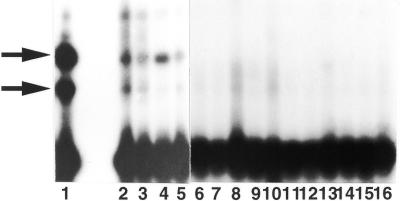
To detect C/C mismatch-binding proteins in E.coli, we carried out gel shift assays. Cell-free extract prepared from wild-type strain AB1157 was incubated with 5′-32P-labeled oligonucleotides (34mers) that contained a defined single-base mismatch, and the reaction mixtures were subsequently separated on non-denaturing polyacrylamide gels. Binding of a protein to one of the 34mers retards its migration through the gel and results in a shifted band. The results are shown in Figure 1. We found two dense bands produced by binding to the C/C mismatch-containing oligonucleotides, and weak bands produced by binding to the C/T and C/A substrates. No shifted bands were found for non-cytosine-containing mismatches, such as G/T. The binding activity was not affected by the sequence context around the mismatches (data not shown). The C/C mismatch-binding activities disappeared upon boiling the reaction mixtures (data not shown). Thus, the two band shifts were due to mismatch-specific binding of proteins present in the extracts.
Figure 1.
Gel shift assays for mismatch-binding proteins. 32P-labeled double-stranded oligonucleotides containing all possible single-base mismatches (23.5 fmol) were incubated with cell extracts prepared from wild-type E.coli. After incubation for 30 min at 4°C, the reaction mixtures were separated by an electrophoresis on 12% non-denaturing polyacrylamide gels in TBE buffer at 100 V. The gels were dried and then autoradiographed using Fuji RX films at –80°C. Lane 1, C/C; lane 2, C/A; lane 3, C=G; lane 4, C/T; lane 5, A/C; lane 6, A/A; lane 7, A/G; lane 8, A=T; lane 9, G=C; lane 10, G/A; lane 11, G/G; lane 12, G/T; lane 13, T/C; lane 14, T=A; lane 15, T/G; lane 16, T/T.
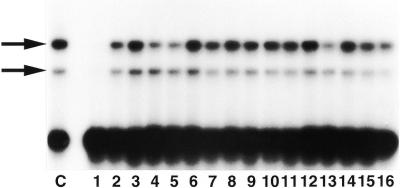
We next analyzed the competition ability of all substrates used in this study against the C/C mismatch to determine the substrate specificity of the mismatch-binding proteins. A 50-fold excess of unlabeled oligonucleotide was used as competitor. Figure 2 shows that, under these conditions, the production of both specific shifted bands was completely abolished by specific competition with unlabeled C/C mismatch-containing oligonucleotide, but unaffected when other oligonucleotides were used as competitors. These results indicated that the C/C mismatch is the best substrate for both binding proteins.
Figure 2.
Mismatch specificity of the C/C binding activities determined by competition with different heteroduplexes. Reactions were performed with radiolabeled oligonucleotides containing the C/C mismatch and a 50-fold excess of unlabeled oligonucleotide as competitor. Lane C, without competitor; lane 1, C/C; lane 2, T/C; lane 3, A/C; lane 4, C/T; lane 5, C/A; lane 6, C=G; lane 7, A/A; lane 8, A/G; lane 9, A=T; lane 10, G=C; lane 11, G/A; lane 12, G/G; lane 13, G/T; lane 14, T=A; lane 15, T/G; lane 16, T/T.
C/C binding activities in crude extracts of various mutants of E.coli
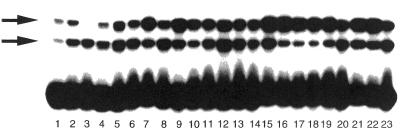
To identify genes responsible for the C/C mismatch-binding proteins, extracts of various mutants of E.coli were incubated with the duplex oligonucleotide containing the C/C mismatch. Production of the low-mobility complex was completely abolished in cell extracts of the mutM mutant (Fig. 3), indicating that the MutM protein specifically binds to the C/C mismatch-containing oligonucleotide. On the other hand, extracts from all the mutants used, including the mutM mutant, displayed the high-mobility band binding to the C/C mismatch-containing oligonucleotide (Fig. 3). Thus, the two complexes contained different proteins. Furthermore, the proteins in the complexes binding of two proteins was independent of the MutS and MutL proteins, because mutations of these genes did not affect the formation of the complexes.
Figure 3.
Cytosine-containing mismatch-binding activities in cell extracts of various mutants of E.coli. Gel shift assays were performed as described in Figure 1. Lane 1, mutD; lane 2, mutL; lane 3, mutM; lane 4, mutT; lane 5, mutY; lane 6, ung; lane 7, vsr; lane 8, uvrD; lane 9, ada and ogt; lane 10, xth and nfo; lane 11, alkA; lane 12, hupA and hupB; lane 13, dps; lane 14, mfd; lane 15, nth and nei; lane 16, recA; lane 17, mutH; lane 18, mutS; lane 19, recB, recC and sbcB; lane 20, topA; lane 21, umuC and umuD; lane 22, uvrA; lane 23, topB.
Binding activity of purified MutM protein to the C/C mismatches
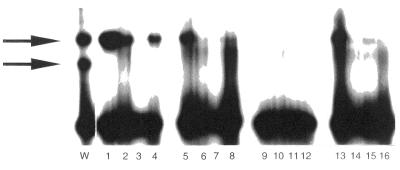
It was of interest to know whether or not MutM protein could directly bind to C/C mismatches. We used a GST-fusion system for MutM overexpression (25,26). Purified GST–MutM fusion protein was further treated with thrombin protease to remove the GST-tag. Purified MutM protein bound to the oligonucleotide containing C/C mismatches with the same specificity as the binding activity in wild-type cell extracts (Fig. 4). These results indicate that the MutM protein directly binds to the C/C mismatch. We therefore concluded that the low-mobility complex consists of the C/C mismatch-containing oligonucleotide and MutM protein.
Figure 4.
Binding of purified MutM protein to various mismatches. The GST–MutM fusion protein was expressed in E.coli strain BL21 with 1 mM IPTG and purified on glutathione–Sepharose 4B column. The GST–MutM fusion protein was further treated with thrombin protease to remove the GST-tag. Gel shift assays were performed as described in Figure 1. Lane 1, C/C; lane 2, C/A; lane 3, C=G; lane 4, C/T; lane 5, A/C; lane 6, A/A; lane 7, A/G; lane 8, A=T; lane 9, G=C; lane 10, G/A; lane 11, G/G; lane 12, G/T; lane 13, T/C; lane 14, T=A; lane 15, T/G; lane 16, T/T. Lane W, cell extracts prepared from wild-type strains.
Purification and characterization of the second C/C mismatch-binding protein
Next, we purified the second C/C mismatch-binding protein from cell extracts of E.coli mutM strain by ammonium sulfate precipitation (fraction I), followed by chromatography through HiTrapQ (fraction II), HiTrap-Heparin (fraction III) and HiPrep Sephacryl-S200HR (fraction IV). Fraction IV showed homogeneity of molecular mass (~20 kDa) (Fig. 5). The sequence of the N-terminal 10 amino acids of the purified protein was determined to be Val-Asp-Lys-Arg-Glu-?-Tyr-Thr-Lys-Glu. This matched the sequence of a predicted polypeptide starting at the second codon of E.coli fabA gene (27). This FabA protein should have a molecular weight of 18 969 as predicted from its amino acid sequence. This value was consistent with our findings using SDS–PAGE of fraction IV.
Figure 5.
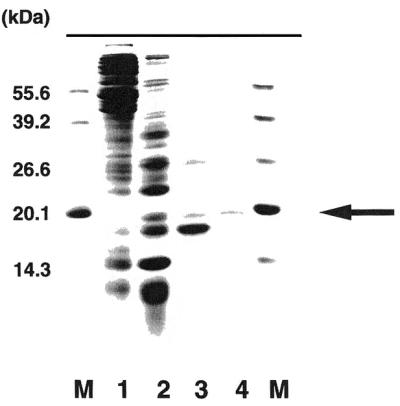
Purification of the second C/C binding protein. Proteins were separated by 17.5% SDS–PAGE and stained with Coomassie Blue. Lane M, molecular weight markers (55.6, 39.2, 26.6, 20.1 and 14.3 kDa); lane 1, 40–60% ammonium sulfate saturation fraction; lane 2, active fraction after HiTrapQ anion exchange column chromatography; lane 3, active fraction after HiTrap-Heparin column chromatography; lane 4, active fraction after gel filtration column chromatography.
C/C mismatch-binding activities in cell extracts of fabA mutant of E.coli
Extracts of an E.coli fabA mutant were tested to determine whether or not they contained the C/C mismatch-binding activities. The high-mobility complex was not found with the cell extracts of the fabA mutant, while the low-mobility complex was present (Fig. 6). These results indicated that FabA is a protein that can recognize and bind to C/C mismatches in DNA.
Figure 6.
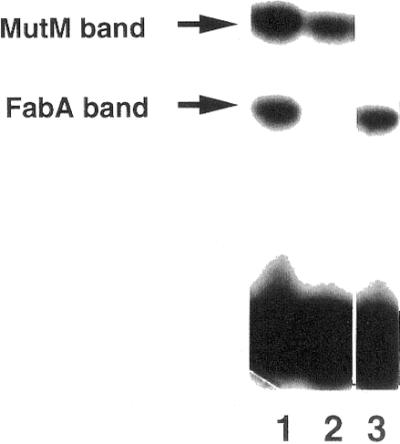
The C/C binding activity in cell extracts of fabA and mutM mutants. Cell extracts of E.coli wild-type (lane 1), fabA (lane 2) and mutM (lane 3) were incubated with the C/C mismatch-containing heteroduplex. Gel shift assays were performed as described in Figure 4.
C/C mismatch-binding activities in cell extracts of E.coli with the S.cerevisiae OGG1 gene
Saccharomyces cerevisiae and mammalian cells contain a protein, OGG1, which functions as an 8-oxoguanine-DNA glycosylase/AP lyase activity (20–22,28–30) but has no obvious structural similarity to MutM (20,21). Thus, the components that repair the 8-oxoguanine are functionally, if not structurally, conserved in bacteria, yeast and mammalian cells. So, it was of interest to examine if the S.cerevisiae OGG1 protein had a C/C mismatch-binding activity as well as the E.coli MutM protein. The plasmid pKK223-3-yOGG1 carrying the S.cerevisiae OGG1 gene was constructed and introduced into E.coli mutM mutant and extracts of the mutant cells were tested to determine whether or not they contained the C/C mismatch-binding activities. The results are shown in Figure 7. There were two dense bands produced by binding to the C/C mismatch-containing oligonucleotides, and weak bands produced by binding to the C/T and C/A substrates. The substrate specificity of yeast OGG1 protein was the same as the binding activity in E.coli MutM protein (Fig. 5). These results indicated that the S.cerevisiae OGG1 protein could also bind to the C/C mismatches in DNA.
Figure 7.
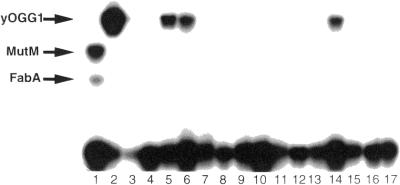
The C/C binding activity in cell extracts of E.coli mutM with pKK223-3-yOGG1 carrying S.cerevisiae OGG1 gene. 32P-labeled double-stranded oligonucleotides containing all possible single-base mismatches (23.5 fmol) were incubated with cell extract prepared from E.coli mutM mutant with pKK223-3-yOGG1 carrying S.cerevisiae OGG1 gene. Gel shift assays were performed as described in Figure 1. Lane 1, extracts from E.coli wild-type; lanes 2–17, extracts from E.coli mutM mutant with pKK223-3-yOGG1. Lanes 1 and 2, C/C; lane 3, C/A; lane 4, C=G; lane 5, C/T; lane 6, A/C; lane 7, A/A; lane 8, A/G; lane 9, A=T; lane 10, G=C; lane 11, G/A; lane 12, G/G; lane 13, G/T; lane 14, T/C; lane 15, T=A; lane 16, T/G; lane 17, T/T.
DISCUSSION
Errors during DNA replication are a potential source of mismatches such as G/G and C/C (4,5,7,8). Genetic recombination between homologous but not identical DNA sequences would be another cause of such mismatches (18,31,32). Mispairs in DNA are a substrate for mismatch repair pathways. Because C/C mispairs are less stable than other types of mispairs (33), they would be a good substrate for mismatch repair. However, the mutHLS mismatch repair pathway does not recognize C/C mismatches (4,8,11). If left unrepaired, C/C mismatches would have mutagenic consequences and should result in G:C→C:G transversions. However, spontaneous G:C→C:G transversions are rare events (4,8,11). Therefore, another specific repair pathway must operate on C/C mismatches in E.coli. The present experiments revealed the formation of two mismatch-dependent DNA–protein complexes upon incubation of oligonucleotide heteroduplexes with crude extracts of E.coli wild-type cells (Fig. 1). Both complexes were formed preferentially with C/C mismatch-containing oligonucleotides (Fig. 1). In addition, unlabeled duplex oligonucleotides containing C/C mismatches efficiently prevented the formation of C/T and C/A mismatch-protein complexes (Fig. 2). These results indicated that E.coli has at least two distinct proteins that specifically bind to C/C mismatches.
The present experiments revealed that E.coli MutM protein has the ability to specifically bind to the C/C mismatches. This conclusion was reached based on the following facts: gel shift assays showed that the low-mobility complex was completely absent in the cell extracts prepared from E.coli mutM mutant (Fig. 3). Furthermore, purified MutM protein could bind directly to the C/C mismatches (Fig. 4). MutM protein shows broad substrate specificity: formamidopyrimidine (Fapy), 8-oxoguanine, 5-hydroxycytosine and thymine glycols (34–37). The MutM protein removes these products of oxidative base damage from DNA by its DNA glycosylase activity and incises the DNA by its associated AP lyase activity (6,9,10,34–37). In this study, purified MutM protein did not incise the C/C mismatch-containing oligonucleotide (data not shown). Therefore, it is suggested that the MutM protein can recognize and bind to C/C mismatches in DNA, and requires another protein(s) to incise DNA for the repair of the mismatches. The MutM protein might be a component of a multi-enzymic pathway involved in C/C mismatch repair.
The present experiments, moreover, showed that the second protein that specifically binds to the C/C mismatch is the FabA protein of E.coli. The purified FabA protein could bind the mismatch-containing oligonucleotide to form the high-mobility complex (Figs 5 and 6). In addition, cell extracts of the fabA mutant did not display the binding activity (Fig. 6). The FabA protein is a component of the fatty acid biosynthesis system in E.coli, and defined as β-hydroxydecanoyl-acyl carrier protein dehydrase, which introduces a double bond into a growing fatty acid chain (27,38–40). However, no DNA-binding domain is found in the amino acid sequence of the FabA protein (27). Interestingly, S.pombe fatty acid synthase, p190/210 complex, binds to single- and double-stranded DNA leading to condensation of large DNA aggregates (41). Furthermore, the protein is capable of renaturing complementary single-stranded DNA and exhibits strand-exchange activity (41). Thus, some enzymes, which also catalyze fatty acid synthesis, have DNA-processing activities. The mechanisms of the involvement of the MutM and FabA proteins in C/C mismatch repair in E.coli cells are under investigation in our laboratory.
In S.pombe, there are at least two different mechanisms for mismatch repair pathways. The major system recognizes all base mismatches except C/C, probably like the mutHLS system in E.coli. The minor system can recognize C/C mismatches and leads to short excision tracts (42,43). Furthermore, recent reports have shown that S.pombe has two distinct C/C mismatch-binding proteins, termed Cmb1 and Cmb2 (19). Purified Cmb1 protein has a molecular mass of 22 kDa. The deduced amino acid sequence contains a high mobility group (HMG) domain, a motif common to a heterogeneous family of DNA-binding proteins. Unlike the Cmb1 protein, MutM and FabA do not have the HMG domain (39,44). The Cmb1 protein recognizes C/T and C/A mismatches, and, in addition, T/T mismatch, C/Δ loop, O6-methylguanine:C mispair and 1,2 GpG intrastrand crosslinks produced by cisplatin (19). On the other hand, Cmb2 protein recognizes only the C/C mismatch (19). It is also suggested that components of the nucleotide excision repair system are involved in C/C mismatch repair systems (45). Thus, it is expected that DNA repair systems other than the mutHLS system must play a role in C/C mismatch repair in E.coli cells. It is possible that MutM and FabA are components of such repair pathways as mismatch recognition proteins like MutS.
In S.cerevisiae, rat and human cells, OGG1 proteins are identified as an 8-oxoguanine-DNA glycosylase/AP lyase (20–22). They remove 8-oxoG paired with cytosine and guanine, like E.coli MutM protein (6,9,10,20–22). The present experiments demonstrated that S.cerevisiae OGG1 protein could specifically bind to C/C mismatches like E.coli MutM protein (Fig. 7). OGG1 has no significant structural homology to the E.coli MutM protein in the amino acid sequences (20,21). It is sure that these proteins recognize and bind to the mismatches as an essential function.
Acknowledgments
ACKNOWLEDGEMENTS
We wish to express our thanks to Drs J. Yokota (National Cancer Center Research Institute, Japan), M. Ajimura (National Institute of Radiological Sciences, Japan), K. Yamamoto (Tohoku University, Japan), B. Weiss (Michigan University, USA), A. Nishimura (National Institute of Genetics, Japan), M. M. Wu (Harvard University, USA), A. Holmgren (Karolinska Institute, Sweden) and B. Bachmann (Yale University, USA) for kindly supplying E.coli strains and plasmids. This study was supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan and the Nissan Science Foundation (to Q.-M.Z.).
REFERENCES
- 1.Drake J.W. (1991) Annu. Rev. Genet., 25, 125–146. [DOI] [PubMed] [Google Scholar]
- 2.Modrich P. (1991) Annu. Rev. Genet., 25, 229–253. [DOI] [PubMed] [Google Scholar]
- 3.Smith K.C. (1992) Mutat. Res., 277, 139–162. [DOI] [PubMed] [Google Scholar]
- 4.Miller J.H. (1992) A Short Course in Bacterial Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 5.Modrich P. and Lahue,R. (1996) Annu. Rev. Biochem., 62, 101–133. [DOI] [PubMed] [Google Scholar]
- 6.Wallace S.S. (1997) In Scandalios,J.G. (ed.), Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 49–90.
- 7.Miller J.H. (1996) Annu. Rev. Microbiol., 50, 625–643. [DOI] [PubMed] [Google Scholar]
- 8.Miller J.H. (1998) Mutat. Res., 409, 99–106. [DOI] [PubMed] [Google Scholar]
- 9.Mol C.D., Parikh,S.S., Putnam,C.D., Lo,T.P. and Tainer,J.A. (1999) Annu Rev. Biophys. Biomol. Struct., 28, 101–128. [DOI] [PubMed] [Google Scholar]
- 10.Eisen J.A. and Hanawalt,P.C. (1999) Mutat. Res., 435, 171–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q.-M., Ishikawa,N., Nakahara,T. and Yonei,S. (1998) Nucleic Acids Res., 26, 4669–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer B., Kramer,W. and Fritz,H.-J. (1984) Cell, 38, 879–887. [DOI] [PubMed] [Google Scholar]
- 13.Su S.-S., Lahue,R.S., Au,K.G. and Modrich,P. (1988) J. Biol. Chem., 263, 6829–6835. [PubMed] [Google Scholar]
- 14.Schaaper R.M. (1993) J. Biol. Chem., 268, 23762–23765. [PubMed] [Google Scholar]
- 15.Wagner R., Debbie,P. and Radman,M. (1995) Nucleic Acids Res., 23, 3944–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babic I., Andrew,S.E. and Jirik,F.R. (1996) Mutat. Res., 372, 87–96. [DOI] [PubMed] [Google Scholar]
- 17.Radicella J.P., Clark,E.A. and Fox,M.S. (1988) Proc. Natl Acad. Sci. USA, 85, 9674–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleck O., Schar,P. and Kohli,J. (1994) Nucleic Acids Res., 22, 5289–5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleck O., Kunz,C., Rudolph,C. and Kohli,J. (1998) J. Biol. Chem., 273, 30398–30405. [DOI] [PubMed] [Google Scholar]
- 20.van der Kemp P.A., Thomas,D., Barbey,R., de Oliveira,R. and Boiteux,S. (1996) Proc. Natl Acad. Sci. USA, 93, 5197–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash H.M., Bruner,S.D., Scharer,O.D., Kawate,T., Addona,T.A., Spooner,E., Lane,W.S. and Verdine,G.L. (1996) Curr. Biol., 6, 968–980. [DOI] [PubMed] [Google Scholar]
- 22.Sandigursky M., Yacoub,A., Kelley,M.R., Xu,Y., Franklin,W.A. and Deutsch,W.A. (1997) Nucleic Acids Res., 25, 4557–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cupples C.G. and Miller,J.H. (1989) Proc. Natl Acad. Sci. USA, 86, 5345–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girard P.M., Guibourt,N. and Boiteux, S. (1997) Nucleic Acids Res., 25, 3204–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagashima M., Sasaki,A., Morishita,K., Takenoshita,S., Nagamachi,Y., Kasai,H. and Yokota,J. (1997) Mutat. Res., 383, 49–59. [DOI] [PubMed] [Google Scholar]
- 26.Shinmura K., Kasai,H., Sasaki,A., Sugimura,H. and Yokota,J. (1997) Mutat. Res., 385, 75–82. [DOI] [PubMed] [Google Scholar]
- 27.Cronan J.E. Jr, Li,W.-B., Coleman,R., Narasimhan,M., de Mendoza,D. and Schwab,J.M. (1988) J. Biol. Chem., 263, 4641–4646. [PubMed] [Google Scholar]
- 28.Bjørås M., Luna,L., Johnson,B., Hoff,E., Haug,T., Rognes,T. and Seeberg,E. (1997) EMBO J., 16, 6314–6322, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dherin C., Radicella,J.P., Dizdaroglu,M. and Boiteux,S. (1999) Nucleic Acids Res., 27, 4001–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishida T., Hippo,Y., Nakahori,Y., Matsushita,I., Kodama,T., Nishimura,S. and Aburatani,H. (1999) Cytogenet. Cell Genet., 85, 232–236. [DOI] [PubMed] [Google Scholar]
- 31.Fox M.S., Radicella,J.P. and Yamamoto,K. (1994) Experientia, 50, 253–260. [DOI] [PubMed] [Google Scholar]
- 32.Worth L. Jr, Clark,S., Radman,M. and Modrich,P. (1994) Proc. Natl Acad. Sci. USA, 91, 3238–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aboul-ela E., Koh,F.D. and Tinoco,I.,Jr (1985) Nucleic Acids Res., 13, 4811–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatahet Z., Kow,Y.W., Purmal,A.A., Cunningham,R.P. and Wallace,S.S. (1994) J. Biol. Chem., 269, 18814–18820. [PubMed] [Google Scholar]
- 35.Tchou J., Kasai,H., Shibutani,S., Chung,M.-H., Laval,J., Grollman,A.P. and Nishimura,S. (1991) Proc. Natl Acad. Sci. USA, 88, 4690–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tchou J., Bodepudi,V., Shibutani,S., Antoshechkin,I., Miller,J.H., Grollman,A.P. and Johnson,F. (1994) J. Biol. Chem., 269, 15318–15324. [PubMed] [Google Scholar]
- 37.David S.S. and Williams,S.D. (1998) Chem. Rev., 98, 1221–1261. [DOI] [PubMed] [Google Scholar]
- 38.Silbert D.F. and Vagelos,P.R. (1967) Proc. Natl Acad. Sci. USA, 58, 1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magnuson K., Jackowski,S., Rock,C.O. and Cronan,J.E.,Jr (1993) Microbiol. Rev., 57, 522–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito K., Hamajima,A., Ohkuma,M., Murakoshi,I., Ohmori,S., Kawaguchi,A., Teeri,T.H. and Cronan,J.E.,Jr (1995) Transgenic Res., 4, 60–69. [DOI] [PubMed] [Google Scholar]
- 41.Käslin E. and Heyer,W.-D. (1994) J. Biol. Chem., 269, 14103–14110. [PubMed] [Google Scholar]
- 42.Schär P. and Kohli,J. (1993) Genetics, 133, 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schär P., Munz,P. and Kohli,J. (1993) Genetics, 133, 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boiteux S., O’Connor,T.R. and Laval,J. (1987) EMBO J., 6, 3177–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleck O., Lemann,E., Schär,P. and Kohli,J. (1999) Nature Genet., 21, 314–317. [DOI] [PubMed] [Google Scholar]