Abstract
Objective
Polycyclic aromatic hydrocarbons (PAH) are common dietary exposures that cross the human placenta and are classified as a probable human carcinogen. The aim of the present study was to investigate the potential impact of exposure to PAH-containing meat consumed during pregnancy on birth outcomes.
Design
Prospective birth cohort study. Only non-smoking women with singleton pregnancies, who were free from chronic disease such as diabetes and hypertension, were included in the study. Maternal consumption of PAH-rich meat was estimated through FFQ. Multiple linear regression was used to assess factors related to higher intake and the association between dietary PAH and birth outcomes.
Setting
Republic of Korea, 2006–2011.
Subjects
Pregnant women (n 778) at 12–28 weeks of gestation enrolled in the Mothers and Children’s Environmental Health (MOCEH) study.
Results
The multivariable regression model showed a significant reduction in birth weight associated with higher consumption level of foods rich in PAH, such as grilled or roasted meat, during pregnancy (β=−17·48 g, P<0·05 for every 1 point higher in meat score). Further adjusting for biomarkers of airborne PAH did not alter this association. There was no evidence that higher consumption level of PAH-rich meat shortens the duration of gestation (P=0·561). Regression models performed for birth length and head circumference produced negative effects that were not statistically significant.
Conclusions
Consumption of higher levels of barbecued, fried, roasted and smoked meats during pregnancy was associated with reduced birth weight. Dietary risk of PAH exposure in Korean women is of concern.
Keywords: Biomarker, Children, Polycyclic aromatic hydrocarbons, PAH-rich meat, Pregnancy, Birth weight
Polycyclic aromatic hydrocarbons (PAH), which are ubiquitous in the environment, are well known eco-toxicants and some of them are classified as a probable human carcinogen( 1 , 2 ). Automobile exhaust gas, industrial emissions and tobacco smoke were usually regarded as sources of human exposure to PAH( 3 , 4 ). However, more than a decade ago it was reported that PAH are formed in a wide variety of foods during cooking at high temperature, especially when cooking meat over a combustion source( 5 – 7 ), and this is considered a major source of human exposure to PAH in non-occupationally exposed populations( 7 – 9 ).
PAH concentrations in food vary depending on different cooking methods and can contribute to the diet( 7 , 10 ) and to the environment( 11 ). Cooking foods at high temperature (grilling, roasting or frying) generates a large amount of PAH( 5 , 12 , 13 ). When meat is in direct contact with flame, PAH are formed due to pyrolysis of organic matter such as fat, protein, and carbohydrates, and become deposited on the outer surface of grilled or smoked meat( 14 , 15 ). Pyrolysis of fat has been shown to produce the greatest concentration of PAH( 16 ). Another possible mechanism of formation of PAH in grilled/smoked meat is the incomplete combustion of charcoal, which can generate PAH that are brought on to the surface of the meat( 17 ). The fat content of the meat and the proximity of the food to the heat source determine the PAH production in grilled or barbecued meat( 5 , 15 ). Other minor sources of exposure to PAH include house dust and contaminated soil and water( 18 , 19 ).
PAH are distributed to almost all internal organs after being absorbed into the human body, and most of them are rapidly metabolized and excreted in the urine. Therefore, it is possible to assess an individual’s internal dose of PAH by measuring PAH and/or their metabolites in urine( 20 , 21 ). PAH metabolites in urine such as 1-hydroxypyrene (1-OHP) can be used as a biomarker of an individual exposure to airborne PAH( 20 , 22 ).
Several epidemiological and experimental studies have provided evidence of adverse effects of PAH on reproductive outcomes, including low birth weight and length, preterm birth, reduced head circumference at birth and lower scores on childhood tests of neurodevelopment( 23 – 26 ). Studies have reported that PAH compounds as well as others can exert their toxic effects on the mother and her fetus through the placenta( 27 , 28 ). The transplacental transfer of PAH to the fetus may have a significant impact on fetal development( 29 ).
In view of the possible genotoxic and carcinogenic effects associated with PAH in grilled or smoked meat (PAH-rich meat)( 14 , 15 , 17 ), we investigated the association between prenatal dietary exposure due to consumption of PAH-rich meat and birth outcomes in the Republic of Korea. Only a few studies have investigated exposure to PAH from the consumption of grilled, smoked or roasted meats in pregnant women and have assessed its relationship with birth outcomes or lifestyle factors( 30 , 31 ). We assessed prenatal exposure to dietary PAH through maternal FFQ and associations between maternal dietary exposure to PAH and birth outcomes, hypothesizing that higher maternal intake of PAH-rich meat during pregnancy would be associated with reduced intra-uterine growth.
Materials and methods
Study population
The present study was conducted as part of the Mothers and Children’s Environmental Health (MOCEH) study, which is a hospital- and community-based, multicentre, prospective birth cohort study in the Republic of Korea. Details of the methods of the MOCEH study with specific eligibility criteria for the participation of mothers have been published earlier( 32 ). The study sample comprised pregnant women at mid-pregnancy (i.e. 12–28 weeks of gestation) with a normal pregnancy (not-at-risk) who were enrolled during 2006–2011 in a community-based network of university hospitals, local clinics and community public health centres located in Seoul, Ulsan and Cheonan.
In total, 1825 pregnant women were recruited between 2006 and 2011. We excluded mothers reporting multiple births (n 30), diabetes or hypertension (n 71), missing birth length (n 384) or birth head circumference (n 336) or birth weight (n 12), missing data on meat consumption (n 154) or smoking (n 7), or missing data on urinary 2-naphthol (2-NAPH) and 1-OHP (n 53). Therefore, 778 mothers were included in the final analysis. We also compared the characteristics of the sample included in the study with those of the full sample and found no substantial differences, hence enhancing the overall confidence in the data available for the study.
Outcome variables
Information on medical history, psychosocial status, health behaviour, environmental exposure as well as sociodemographic characteristics was collected. The participants were followed up until delivery, and information on birth weight, birth length, head circumference, gestational age, parity and sex of the infant was obtained from medical records. The gestational age was estimated from the interval between the last menstrual period and the date of delivery and was corrected by ultrasonography measurement if there was a discordance of >10 d between both estimates. Small-for-gestational-age (SGA) children were those who weighed less than the 10th percentile for gestational age (mean birth weight for all SGA children: 2723 g).
Exposure assessment
Maternal diet
A well-trained dietitian administered a semi-quantitative FFQ to record usual dietary intake over a 1-year period prior to the interview. Maternal dietary intake was assessed during the first trimester of pregnancy using a 107-item FFQ. The FFQ method was previously validated in the Korean population( 33 , 34 ). The questionnaire had twenty-nine items on intake of processed meat, chicken and fish. The twenty-nine items also elicited information on the frequency of consumption of meats and fish products that involved grilling, roasting or smoking. The response categories for the frequency of consumption were divided into nine levels: ‘3 times daily’, ‘twice daily’, ‘once daily’, ‘5 or 6 times weekly’, ‘3 or 4 times weekly’, ‘once or twice weekly’, ‘2 or 3 times monthly’, ‘once monthly’ and ‘never or seldom’. Information about portion sizes was collected according to an appropriately defined unit (e.g. cup or bowl) and the sizes were classified into three categories: ‘small’, ‘medium’ and ‘large’. For the present analysis we focused on commonly consumed meat and fish products and grouped these products into nine groups known to contain potentially high levels of PAH( 35 ) based on similarities in the composition and processing of the meats in each group, specifically: ‘barbecued beef’, ‘smoked beef’, ‘roasted/fried beef’, ‘barbecued pork’, ‘smoked pork’, ‘roasted/fried chicken’, ‘smoked chicken’, ‘roasted fish’ and ‘smoked fish’.
We created a PAH-rich meat score by giving 1 point for each of the meats and fish products that were consumed at least once in a week. The nine-item scale used a 1-week recall period. The food score was developed using the same procedures as used for creating an acrylamide food score for the assessment of maternal diet on birth outcomes( 36 ). The responses on the nine items were summed to create the food score, with a minimum score of 0 indicating the lowest intake of PAH-rich meat and a maximum score of 9 indicating the highest intake. We excluded women with missing FFQ (n 154) in all dietary analyses.
Biomarkers
The urinary 1-OHP and 2-NAPH levels were used as biomarkers of personal exposure to airborne PAH, as also reported in a previous study( 37 ). Maternal urine samples were collected in the morning and sent to a specialized laboratory for analysis. The 1-OHP and 2-NAPH levels were analysed by HPLC with fluorescence detection( 38 ) and the levels that were measured during the first trimester of pregnancy were used for the present study. All data were adjusted to the urinary creatinine level to correct for urine volume. The biomarker data were expressed as median values or were natural log-transformed in the multiple regression models due to the skewed distribution of the data.
Potential confounders
The selection of confounders included in the adjusted models was based on a directed acyclic graph (see online supplementary material, Fig. S1)( 39 ) and variables were chosen which seemed to have potential risk factors for the association of interest. Covariates examined in the analysis included the following: age at delivery (years), parity (≥1 v. 0); gestational age (continuous in completed weeks); weight gain in pregnancy (kg); maternal height (cm); maternal education (low, middle, high); sex of the infant (girl v. boy); high or low maternal consumption of fruits and vegetables during pregnancy (dichotomized by median intake); maternal intake of Fe (dichotomized by total median intake of Fe, dietary and supplemental, during pregnancy); and higher consumption of fish (>150 v. ≤150 g/d).
Statistical analysis
We used linear regression models for main birth outcomes assessed on a continuous scale (birth weight, length and head circumference at birth) and estimated β coefficients and 95 % confidence intervals. Logarithmic binomial regression models were used to determine relative risks (RR) and 95 % confidence intervals for SGA. We constructed several multivariable linear regression models, following the estimation of crude effects. Model 1 was adjusted for maternal age at delivery, education, maternal height, parity, gestational age, weight gain in pregnancy and sex of the infant. Model 2 was further adjusted for high or low maternal consumption of fruits and vegetables, maternal intake of Fe and higher consumption of fish. Models were robust to heteroscedasticity, and multicollinearity was not observed in our analysis as assessed by the variance inflation factor (value >10).
We used generalized additive models with cubic regression splines that were adjusted for gestational age, maternal height and maternal age to characterize the relationship between the consumption of PAH-rich meat and birth outcomes. These models were fitted in the statistical software R (www.r-project.org) using the mgcv package for generalized additive modelling. Other analyses were done in the statistical software package STATA 12. The significance level was set at P≤0·05.
Results
Sample characteristics
Table 1 describes the characteristics of the sample. The participating mothers were 30·5 years old and had a weight gain during pregnancy of 12·9 kg. About 52 % of the pregnant women reported the consumption of barbecued or roasted or fried or smoked meats at least once in a week. Analysis of urinary 2-NAPH and 1-OHP from pregnant women included in the study showed the average concentration of 10·0 ng/g creatinine and 0·33 ng/g creatinine, respectively. The consumption of the meat and the biomarkers of PAH exposure were not correlated (Spearman’s r=0·005, P=0·838 for the consumption of PAH-rich meat and 2-NAPH; Spearman’s r=−0·031, P=0·442 for the consumption of the meat and 1-OHP), indicating that these biomarkers did not represent the recent consumption of barbecued, roasted, fried and smoked meats. The mean weight, length, head circumference at birth and gestational age of the infants under study was 3263·5 g, 50·5 cm, 34·0 cm and 38·8 weeks, respectively.
Table 1.
Characteristics of the study sample and consumption of meat among pregnant women (n 778) at 12–28 weeks of gestation enrolled in the Mothers and Children’s Environmental Health (MOCEH) study, Republic of Korea, 2006–2011
| Total sample (n 778) | ||
|---|---|---|
| Sample characteristic | n or Mean | % or sd |
| Maternal age (years) | ||
| Mean and sd | 30·46 | 3·66 |
| Missing (n) | 102 | |
| Gestational age (weeks) | ||
| Mean and sd | 38·82 | 1·53 |
| Missing (n) | 1 | |
| Maternal height (cm) | ||
| Mean and sd | 161·30 | 4·55 |
| Missing (n) | 124 | |
| Maternal education, n and % | ||
| Low | 205 | 26·73 |
| Middle | 150 | 19·56 |
| High | 412 | 53·72 |
| Missing (n) | 11 | |
| Maternal weight gain in pregnancy (kg) | ||
| Mean and sd | 12·93 | 4·37 |
| Missing (n) | 123 | |
| Parity, n and % | ||
| Nulliparous | 401 | 55·93 |
| Parous | 316 | 44·07 |
| Missing (n) | 56 | |
| Sex of infant, n and % | ||
| Boy | 409 | 52·57 |
| Girl | 369 | 47·43 |
| 1-Hydroxypyrene (ng/g creatinine) | ||
| Mean and sd | 0·33 | 0·33 |
| 2-Naphthol (ng/g creatinine) | ||
| Mean and sd | 9·96 | 13·44 |
| Length at birth (cm) | ||
| Mean and sd | 50·50 | 2·51 |
| Birth weight (g) | ||
| Mean and sd | 3263·54 | 437·33 |
| Head circumference at birth (cm) | ||
| Mean and sd | 33·97 | 1·52 |
| Small for gestational age, n and % | ||
| No | 706 | 90·75 |
| Yes | 72 | 9·25 |
| Vegetables (g/d) | ||
| Mean and sd | 262·18 | 163·08 |
| Missing (n) | 36 | |
| Fruits (g/d) | ||
| Mean and sd | 345·83 | 349·19 |
| Missing (n) | 36 | |
| Total Fe intake (mg/d) | ||
| Mean and sd | 25·90 | 8·18 |
| Missing (n) | 36 | |
| Fish consumption (g/d), n and % | ||
| >150 | 70 | 9·43 |
| ≤150 | 672 | 90·57 |
| Missing (n) | 36 | |
| PAH-rich meat score, n and % | ||
| 0 | 375 | 48·20 |
| 1 | 138 | 17·74 |
| 2 | 142 | 18·25 |
| 3 | 57 | 7·33 |
| 4 | 34 | 4·37 |
| 5 | 15 | 1·93 |
| 6 | 10 | 1·29 |
| 7 | 4 | 0·51 |
| 8 | 2 | 0·26 |
| 9 | 1 | 0·13 |
PAH, polycyclic aromatic hydrocarbons.
Regression results
The results of the regression analyses that examined the association between the consumption of PAH-rich meat and birth weight are summarized in Table 2. There was a significant negative association between higher consumption level of PAH-rich meat and birth weight. The mean birth weight was reduced by 17·82 (95 % CI −35·63, −1·02) g with every 1 point higher in meat score after adjusting for sex of the infant, parity, anthropometry of mother and gestational age (model 1). After additionally adjusting for intakes of vegetables, fruits, Fe and fish (model 2), estimates remained significant (−17·48; 95 % CI −34·32, −0·64 g; P=0·042). Furthermore, inclusion of biomarkers of airborne PAH in model 2 did not substantially alter the estimated effect size or P value of dietary PAH on birth weight (−17·57; 95 % CI −34·41, −0·72 g; P=0·041; n 607; biomarkers of airborne PAH dichotomized by their median values), indicating that prenatal airborne PAH is not a potential confounder of this association. Of the total variability in birth weight (32·4 %) estimated from model 1, 0·1 % could be attributable to the consumption of PAH-rich meat. The major variability in birth weight was explained by gestational age (21·0 %), weight gain in pregnancy (7·1 %), maternal height (2·6 %) and sex of the infant (1·3 %).
Table 2.
Estimated effect of consumption of polycyclic aromatic hydrocarbons (PAH)-rich meat on birth weight adjusted for sociodemographic, reproductive and lifestyle factors among pregnant women (n 778) at 12–28 weeks of gestation enrolled in the Mothers and Children’s Environmental Health (MOCEH) study, Republic of Korea, 2006–2011
| Model 1† | Model 2‡ | |||
|---|---|---|---|---|
| Characteristic | β coefficient | 95 % CI | β coefficient | 95 % CI |
| PAH-rich meat consumption | −17·82 | −34·63, −1·02** | −17·48 | −34·32, −0·64** |
| Maternal weight gain in pregnancy | 21·43 | 14·08, 28·79**** | 21·69 | 14·24, 29·15**** |
| Gestational age | 131·65 | 107·45, 155·83**** | 131·11 | 106·82, 155·39**** |
| Maternal height | 10·11 | 3·46, 16·77*** | 9·94 | 3·26, 16·62*** |
| Maternal age | 3·35 | −5·09, 11·79 | 3·25 | −5·19, 11·69 |
| Maternal education | ||||
| Low | Reference | Reference | ||
| Middle | −73·59 | −153·07, 5·89* | −80·13 | −161·86, 1·60* |
| High | −11·78 | −83·34, 59·79 | −19·72 | −93·01, 53·58 |
| Parity (parous v. nulliparous) | 139·13 | 77·44, 200·81**** | 138·08 | 76·01, 200·14**** |
| Sex of infant (girl v. boy) | −119·69 | −175·74, −63·65**** | −121·92 | −178·14, −65·70**** |
| Fish consumption | 6·56 | −97·04, 110·17 | ||
| Vegetables§ | 20·07 | −41·08, 81·21 | ||
| Fruits§ | 46·20 | −12·52, 104·92 | ||
| Fe intake§ | −16·01 | −77·65, 45·62 | ||
| R 2 | 0·32 | 0·33 | ||
| n | 607 | 607 | ||
Statistically significant: *P≤0·1, **P≤0·05, ***P≤0·01, ****P≤0·001.
Adjusted for sex of the infant, parity, anthropometry of mother and gestational age.
Additionally adjusted for intakes of vegetables, fruits, Fe and fish. After further including biomarkers of PAH (1-hyroxypyrene and 2-naphthol; dichotomized by the median value of the distribution or natural log-transformed in separate models), the β and P values for dietary PAH were similar and significant. Moreover, the biomarkers were not significant predictors and were therefore not included in the models presented here.
Dichotomized by the median value of the distribution.
The results in Tables 3 and 4 demonstrate that coefficients on the meat scores for length and head circumference at birth were negative but not statistically significant. We further examined the combined association of dietary PAH and airborne PAH (dichotomized by the median value of the sum of two biomarkers of PAH) with birth length; the combined exposure produced a birth length deficit of 0·7 cm (P=0·005). A test of the interaction term between dietary PAH and airborne PAH was not significant (P=0·390). A similar statistical approach for birth weight produced a birth weight deficit of 69 g for the combined exposure to dietary PAH and airborne PAH. The estimated effects were of borderline significance, and the test of the interaction term between dietary PAH and airborne PAH was not statistically significant (P=0·209; see online supplementary material, Table S1).
Table 3.
Estimated effect of consumption of polycyclic aromatic hydrocarbons (PAH)-rich meat on length at birth adjusted for sociodemographic, reproductive and lifestyle factors among pregnant women (n 778) at 12–28 weeks of gestation enrolled in the Mothers and Children’s Environmental Health (MOCEH) study, Republic of Korea, 2006–2011
| Model 1† | Model 2‡ | |||
|---|---|---|---|---|
| Characteristic | β coefficient | 95 % CI | β coefficient | 95 % CI |
| PAH-rich meat consumption | −0·07 | −0·17, 0·03 | −0·07 | −0·17, 0·03 |
| Maternal weight gain in pregnancy | 0·06 | 0·02, 0·10*** | 0·06 | 0·02, 0·11*** |
| Gestational age | 0·72 | 0·54, 0·89**** | 0·71 | 0·53, 0·89**** |
| Maternal height | 0·06 | 0·02, 0·10*** | 0·05 | 0·02, 0·09*** |
| Maternal age | 0·01 | −0·05, 0·06 | 0·004 | −0·05, 0·06 |
| Maternal education | ||||
| Low | Reference | Reference | ||
| Middle | −0·23 | −0·72, 0·26 | −0·24 | −0·74, 0·26 |
| High | 0·22 | −0·18, 0·63 | 0·13 | −0·27, 0·54 |
| Parity (parous v. nulliparous) | 0·51 | 0·12, 0·89*** | 0·53 | 0·15, 0·91*** |
| Sex of infant (girl v. boy) | −0·71 | −1·06, −0·37**** | −0·73 | −1·07, −0·39**** |
| Fish consumption | 0·32 | −0·31, 0·95 | ||
| Vegetables§ | 0·26 | −0·10, 0·63 | ||
| Fruits§ | 0·30 | −0·06, 0·65* | ||
| Fe intake§ | 0·06 | −0·32, 0·44 | ||
| R 2 | 0·24 | 0·25 | ||
| n | 607 | 607 | ||
Statistically significant: *P≤0·1, **P≤0·05, ***P≤0·01, ****P≤0·001.
Adjusted for sex of the infant, parity, anthropometry of mother and gestational age.
Additionally adjusted for intakes of vegetables, fruits, Fe and fish. After further including biomarkers of PAH (1-hyroxypyrene and 2-naphthol; dichotomized by the median value of the distribution or natural log-transformed in separate models), the β and P values for dietary PAH were not substantially changed and were therefore not included in the models presented here.
Dichotomized by the median value of the distribution.
Table 4.
Estimated effect of consumption of polycyclic aromatic hydrocarbons (PAH)-rich meat on head circumference adjusted for sociodemographic, reproductive and lifestyle factors among pregnant women (n 778) at 12–28 weeks of gestation enrolled in the Mothers and Children’s Environmental Health (MOCEH) study, Republic of Korea, 2006–2011
| Model 1† | Model 2‡ | |||
|---|---|---|---|---|
| Characteristic | β coefficient | 95 % CI | β coefficient | 95 % CI |
| PAH-rich meat consumption | −0·01 | −0·08, 0·06 | −0·01 | −0·08, 0·06 |
| Maternal weight gain in pregnancy | 0·04 | 0·01, 0·06*** | 0·04 | 0·01, 0·06*** |
| Gestational age | 0·30 | 0·17, 0·43**** | 0·30 | 0·17, 0·42**** |
| Maternal height | 0·03 | 0·01, 0·06*** | 0·03 | 0·01, 0·05** |
| Maternal age | 0·06 | 0·03, 0·09**** | 0·06 | 0·03, 0·09**** |
| Maternal education | ||||
| Low | Reference | Reference | ||
| Middle | −0·28 | −0·57, 0·02* | −0·32 | −0·62, −0·02** |
| High | 0·14 | −0·12, 0·41 | 0·11 | −0·16, 0·38 |
| Parity (parous v. nulliparous) | 0·34 | 0·11, 0·58*** | 0·34 | 0·10, 0·57*** |
| Sex of infant (girl v. boy) | −0·55 | −0·76, −0·33**** | −0·55 | −0·76, −0·33**** |
| Fish consumption | −0·10 | −0·46, 0·27 | ||
| Vegetables§ | −0·06 | −0·30, 0·18 | ||
| Fruits§ | 0·20 | −0·03, 0·42* | ||
| Fe intake§ | 0·08 | −0·16, 0·31 | ||
| R 2 | 0·17 | 0·18 | ||
| n | 607 | 607 | ||
Statistically significant: *P≤0·1, **P≤0·05, ***P≤0·01, ****P≤0·001.
Adjusted for sex of the infant, parity, anthropometry of mother and gestational age.
Additionally adjusted for intakes of vegetables, fruits, Fe and fish. After further including biomarkers of PAH (1-hyroxypyrene and 2-naphthol; dichotomized by the median value of the distribution or natural log-transformed in separate models), the β and P values for dietary PAH were not substantially changed and were therefore not included in the models presented here.
Dichotomized by the median value of the distribution.
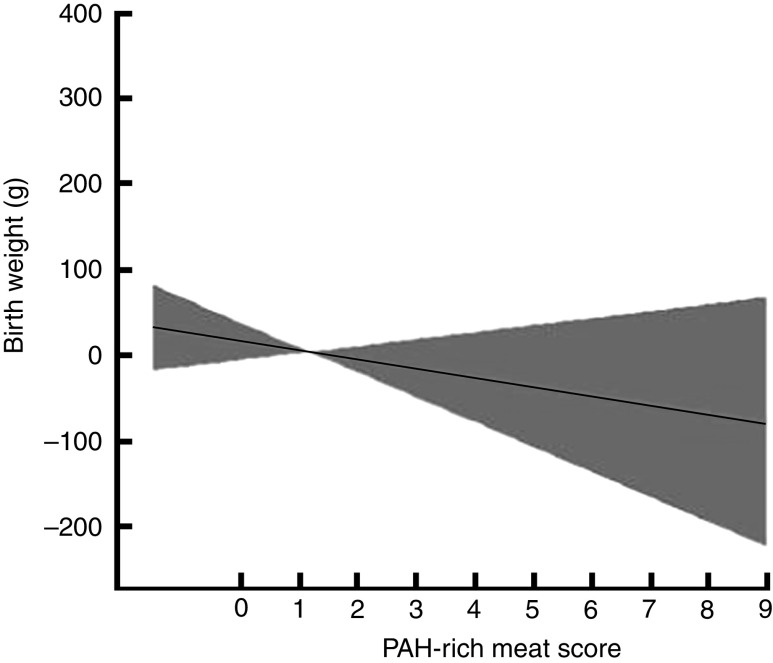
A generalized additive model revealed that a 1-unit increase in the PAH-rich meat score was associated with a decrease in birth weight after adjustment for gestational age, maternal height and maternal age (Fig. 1). This also indicated that the pooled relationship between PAH-rich meat score and birth outcomes was approximately linear, indicating the appropriateness of linear regression models. In addition, the study revealed that birth outcomes (birth weight, length and head circumference) were significantly increased in association with pregnancy weight gain, gestational age, male sex of the infant, parity and maternal height. Moreover, a quadratic relationship between gestational age and birth outcomes was observed but additional adjustment for gestational age as a quadratic term (continuous completed weeks squared) gave nearly identical results (not shown).
Fig. 1.
Association of the polycyclic aromatic hydrocarbons (PAH)-rich meat score with birth weight among pregnant women (n 778) at 12–28 weeks of gestation enrolled in the Mothers and Children’s Environmental Health (MOCEH) study, Republic of Korea, 2006–2011. Generalized additive models with cubic regression spline for PAH-rich meat score adjusted for gestational age (completed weeks), mother’s height and mother’s age, showing the fitted smoothing spline (———) and the corresponding 95 % confidence interval ( )
)
In a separate analysis, the effect of consumption of PAH-rich meat on gestational age was tested. There was no evidence that higher intake level of meat shortened the duration of pregnancy (β=0·019, P=0·561, n 607 in the model adjusted for sex of the infant, parity, anthropometry of the mother, and intakes of vegetables, fruits, Fe and fish). Therefore, the observed deficit in birth weight and other birth outcomes was not mediated by shortening the gestation period. Furthermore, SGA was increased in association with increased consumption of PAH-rich meat, but the association was of borderline significance when adjusted for gestational age, weight gain during pregnancy and parity (RR=1·27; 95 % CI 0·96, 1·68; P=0·096; n 651).
Discussion
The current study provides evidence that higher prenatal exposure to PAH through maternal consumption of PAH-rich meat during pregnancy is associated with reduced birth weight but not significantly associated with reduced birth length and head circumference. The higher consumption level of PAH-rich meat during pregnancy predicted a birth weight deficit of 17·5 g after controlling for sex of the infant, parity, anthropometry of the mother, gestational age, and maternal intakes of vegetables, fruits, Fe and fish. The relationship between prenatal dietary PAH and birth weight remained significant after controlling for biomarkers of airborne PAH. There was no significant interaction term between the exposure to dietary PAH and airborne PAH (P=0·209), suggesting that the observed effects reflect the summarized exposure to both PAH sources rather than their synergistic effects. Furthermore, the shortening of gestational age was not mediated by the reduction in birth weight which was attributed to dietary PAH. Therefore, the duration of pregnancy was not associated with the exposures in question. In addition, there was a near significant association of SGA with an increased consumption of PAH-rich meat. Given these findings and previous evidence of possible adverse birth outcomes such as low birth weight and length, and poor neurodevelopment associated with PAH exposure during pregnancy( 25 , 26 ), the dietary contribution to PAH exposure in this population is of concern.
Effects of dietary PAH exposure on birth weight in this population were in agreement with findings from a recent study examining the possible impact of co-exposure to PAH-containing grilled meat consumed during pregnancy on birth outcomes in Poland( 31 ). The authors reported that the combined effect of exposure to both airborne and dietary sources of PAH amounted to a birth weight deficit of 214 g in the Polish population. However, in our study, airborne PAH did not confound the association between dietary PAH and birth outcomes. A separate model revealed that the combined effects of dietary PAH and airborne PAH (69 g) was lower than that reported in Poland. Such differences may be due to differences in the methods used to assess exposure to dietary intake or estimates of PAH concentrations. Nevertheless, our result of a mean birth weight deficit of 17·5 g with a 1-unit increase in the PAH-rich meat score is similar to the results obtained for the acrylamide food score( 36 ). Earlier studies reported significant negative associations of maternal PAH exposure, which was estimated based on DNA adducts reflecting PAH exposure from all sources( 40 ), or personal measure of atmospheric PAH exposure, with birth weight, birth length and SGA in populations from the USA( 25 , 29 , 41 , 42 ), Poland( 25 , 43 ) and the Czech Republic( 44 ).
Although diet is recognized as the main source of human exposure to PAH in non-occupationally exposed individuals, there have been few data on the exposure to these compounds through maternal diet during pregnancy. One study in Spanish women found that elevated first-trimester dietary PAH, based on FFQ, was significantly associated with a birth weight deficit of 143 g (fourth v. first quartile)( 45 ); similarly, a study in the same cohort described the associations between prenatal exposure to dietary PAH and fetal growth( 46 ). A recent publication from a large Norwegian study demonstrated that higher prenatal exposure to dietary PAH was significantly associated with reduced birth weight( 47 ). However, these studies used the consumption of a wide range of food items (meats, seafood, dairy products, cereals/potatoes, fruit, vegetables, eggs, legumes, beverages) as indicators of total dietary exposure to PAH and reported no association with birth weight when a limited number of food items (smoked, grilled or barbequed meats) were considered as indicators of dietary PAH( 47 ).
Previous studies have reported that consumption of processed or cured meat and meat products is positively associated with the formation of PAH–DNA adducts( 48 , 49 ). It has been found that individuals who consumed barbecued food more than twice in the previous 2 weeks had fourfold increased risk of having elevated adducts level compared with individuals consuming the food two or fewer times( 41 ). Moreover, PAH–DNA adducts have been found in the placenta of women( 50 ). This suggests that exposure to PAH-rich meat could affect the functions of the placental cells and consequently be a source of genotoxicity during fetal development( 51 ). The latter study was not able to show the association between the consumption of PAH-rich meat in pregnancy and cord blood PAH–DNA adducts due to data limitation. However, DNA adducts formation is subject to a greater variability. The variability in results could be due to differences in metabolic phenotypes related to genetic polymorphisms( 52 ) or plasma antioxidants may have modulated the effect of prenatal PAH exposure on PAH–DNA adducts in cord blood( 53 ).
In addition to dietary intake of PAH, it is possible that biomarkers of airborne PAH were acting as a proxy marker for another dietary exposure or mix of other exposures such as environmental tobacco smoke that were responsible for the associations observed. Moreover, the consumption of meat might be a proxy indicator of some lifestyle factors( 54 , 55 ). However, adjusting for indicators of lifestyle factors such as intakes of fruits and vegetables, Fe and beverages, maternal characteristics, indicators of socio-economic status and environmental tobacco smoke (dichotomized based on a cut-off of 4·2 ng/g creatinine, obtained by receiver-operating characteristic curve analysis) did not substantially alter associations among the subset of the population with available data. Given the consistency of findings in different population subgroups and after adjustment for multiple potential confounders, it seems unlikely that prenatal exposure to tobacco smoke or other dietary compound(s) could fully explain the associations observed between PAH-rich meat and birth outcomes.
Findings of the present study are strengthened through statistical adjustment for multiple covariates at different levels including Fe, fruits and vegetables in the maternal diet. The findings are also largely consistent with theoretical expectations and the previous literature on this topic. Cases with missing data were excluded from our study. This may have had some effects on the findings because of a loss of information that may have affected the precision of the estimates. However, the potential bias in the study was decidedly low because the main characteristics for the full sample were almost identical to the characteristics of the sample that was available for the regression models. The exposure to PAH via food consumption was not assessed in the present study. However, the levels of PAH in food sources are considered to be consistent within the same area and exposure to PAH in most people could be similar( 56 ). The lack of precise information on timing of the consumption of processed meat during pregnancy and the estimates of PAH dietary exposure from only an FFQ based on 1-week recall may be subject to recall bias. However, there did not appear to be any differential recall bias, as the dietary survey was carried out before the pregnancy outcome( 57 ). Nevertheless, a well-trained dietary interviewer with standard protocol was used to minimize potential errors in assessing dietary intakes. Furthermore, the consumption of PAH-rich meat reported for a short period may not necessarily reflect dietary intake during the entire period of pregnancy. The questionnaire on PAH-rich meat consumption in pregnant women in our study population has not yet been validated directly; however, a previous study has shown that use of a similar FFQ could produce acceptable reproducibility and modest validity of food consumption in Korean adults living in a metropolitan area( 34 ).
Mechanisms underlying the effects of PAH on birth outcomes are not fully understood. The ability of PAH to readily damage DNA which may induce apoptosis, the anti-oestrogenic effects of PAH, and the binding of PAH to the human aryl hydrocarbon receptor to induce P450 enzymes or to receptors for placental growth factors that can result in decreased exchange of oxygen and nutrients, have been suggested to form the basis of some of PAH’s toxic effects( 44 ) and may contribute to the associations with birth outcomes in our study population. Further studies are warranted to explore the mechanisms underlying the action of antioxidants and prenatal PAH exposure on PAH–DNA adducts based on the precise time of consumption of processed meat and their implication on adverse pregnancy outcomes. Our findings have substantial public health implications because reduced birth weight is a risk factor for numerous adverse health effects early in life. In addition, it has also been associated with multiple adverse outcomes (reduced stature, increased incidence of CVD, type 2 diabetes mellitus and osteoporosis) later in life( 58 ).
Conclusion
In conclusion, the present large, population-based, multicentre, prospective cohort study has provided epidemiological evidence of a significant association between prenatal exposure to dietary PAH and reduced birth weight. The results of the study suggest that dietary PAH exposure may also be an important source of additional risk of reduced intra-uterine growth among Korean women. The findings of the study can provide public health advice to pregnant women to reduce their dietary intake of foods that may contain high concentrations of PAH; however, further investigations are warranted to validate our findings.
Acknowledgements
Acknowledgements: The authors would like to express their sincere thanks to MOCEH (Mothers and Children’s Environmental Health) project of the Ministry of Environment, Republic of Korea. Financial support: The Ministry of Environment, South Korea provided financial support. But the funder had no role in the design, analysis, or writing of this article. Conflict of interest: None declared. Authorship: D.K.L., J.-H.L. and H.-C.K. formulated the research questions, analysed the data and prepared the manuscript. J.-Y.L., M.-S.P., D.-Y.J. and J.K.K. performed the generation of data/analysis and interpretation of the data. J.-H.L., M.H., Y.K., Y.-C.H. and E.-H.H. contributed to the MOCEH study and critically revised the manuscript. All authors read and approved the final manuscript. Ethics of human subject participation: The study protocol was approved by the institutional review board at Inha University, Incheon, Republic of Korea. Trained nurses were used to interview participants after getting a written informed consent from each woman who visited the community-based network of university hospitals, local clinics and community public health centres located in Seoul, Ulsan and Cheonan.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980016000550.
click here to view supplementary material
References
- 1. Liu G, Niu Z, Van Niekerk D et al. (2008) Polycyclic aromatic hydrocarbons (PAHs) from coal combustion: emissions, analysis, and toxicology. Rev Environ Contam Toxicol 192, 1–28. [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer (2010) Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. IARC Monographs on the Evaluation of Carcinogenic Risks in Humans, vol. 92. Lyon: IARC. [PMC free article] [PubMed] [Google Scholar]
- 3. Rodgman A, Smith CJ & Perfetti TA (2000) The composition of cigarette smoke: a retrospective, with emphasis on polycyclic components. Hum Exp Toxicol 19, 573–595. [DOI] [PubMed] [Google Scholar]
- 4. Chang KF, Fang GC, Chen JC et al. (2006) Atmospheric polycyclic aromatic hydrocarbons (PAHs) in Asia: a review from 1999 to 2004. Environ Pollut 142, 388–396. [DOI] [PubMed] [Google Scholar]
- 5. Chen BH & Lin YS (1997) Formation of PAHs during processing of duck meat. J Agric Food Chem 45, 1394–1403. [Google Scholar]
- 6. Mottier P, Parisod V & Turesky RJ (2000) Quantitative determination of polycyclic aromatic hydrocarbons in barbecued meat sausages by gas chromatography coupled to mass spectrometry. J Agric Food Chem 48, 1160–1166. [DOI] [PubMed] [Google Scholar]
- 7. Phillips DH (1999) Polycyclic aromatic hydrocarbons in the diet. Mutat Res 443, 139–147. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki K & Yoshinaga J (2007) Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. Int Arch Occup Environ Health 81, 115–121. [DOI] [PubMed] [Google Scholar]
- 9. European Commission Scientific Committee on Food (2002) Opinion of the Scientific Committee on Food on the Risks to Human Health of Polycyclic Aromatic Hydrocarbons in Food. Background Document SCF/CS/CNTM/PAH/29 Final. Brussels: European Commission Scientific Committee on Food. [Google Scholar]
- 10. Viegas O, Novo P, Pinto E et al. (2012) Effect of charcoal types and grilling conditions on formation of heterocyclic aromatic amines (HAs) and polycyclic aromatic hydrocarbons (PAHs) in grilled muscle foods. Food Chem Toxicol 50, 2128–2134. [DOI] [PubMed] [Google Scholar]
- 11. Ding J, Zhong J, Yang Y et al. (2012) Occurrence and exposure to polycyclic aromatic hydrocarbons and their derivatives in a rural Chinese home through biomass fuelled cooking. Environ Pollut 169, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knize MG, Salmon CP, Pais P et al. (1999) Food heating and the formation of heterocyclic aromatic amine and polycyclic aromatic hydrocarbon mutagens/carcinogens. Adv Exp Med Biol 459, 179–193. [DOI] [PubMed] [Google Scholar]
- 13. Dyremark A, Westerholm R, Overvik E et al. (1995) PAH emissions from charcoal grilling. Atmosph Environ 13, 1553–1558. [Google Scholar]
- 14. Farhadian A, Jinap S, Abas F et al. (2010) Determination of polycyclic aromatic hydrocarbons in grilled meat. Food Control 21, 606–610. [Google Scholar]
- 15. Kazerouni N, Sinha R, Hsu CH et al. (2001) Analysis of 200 items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol 39, 423–436. [DOI] [PubMed] [Google Scholar]
- 16. Bartle KD (1991) Analysis and occurrence of PAHs in food. In Food Contaminants: Sources and Surveillance, 1st ed., pp. 41–60 [CS Creaser and R Purchase, editors]. Cambridge: Royal Society of Chemistry. [Google Scholar]
- 17. Wu J, Wong MK, Lee HK et al. (1997) Determination of polycyclic aromatic hydrocarbons in rougan, a tradition Chinese barbecued food, by capillary gas chromatography. Environ Monit Assess 44, 577–585. [Google Scholar]
- 18. Lambert TW & Lane S (2004) Lead, arsenic, and polycyclic aromatic hydrocarbons in soil and house dust in the communities surrounding the Sydney, Nova Scotia, tar ponds. Environ Health Perspect 112, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization (1998) Guidelines for Drinking-Water Quality. Addendum to Vol. 2. Health Criteria and Other Supporting Information. WHO/EOS/98.1, 2nd ed. Geneva: WHO. [Google Scholar]
- 20. Strickland P, Kang D & Sithisarankul P (1996) Polycyclic aromatic hydrocarbon metabolites in urine as biomarkers of exposure and effect. Environ Health Perspect 104, Suppl. 5, 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brandt HC & Watson WP (2003) Monitoring human occupational and environmental exposures to polycyclic aromatic compounds. Ann Occup Hyg 47, 349–378. [DOI] [PubMed] [Google Scholar]
- 22. Ciarrocca M, Rosati MV, Tomei F et al. (2014) Is urinary 1-hydroxypyrene a valid biomarker for exposure to air pollution in outdoor workers? A meta-analysis. J Expo Sci Environ Epidemiol 24, 17–26. [DOI] [PubMed] [Google Scholar]
- 23. Sram RJ, Binkova B, Dejmek J et al. (2005) Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect 113, 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jurisicova A, Taniuchi A, Li H et al. (2007) Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J Clin Invest 117, 3971–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi H, Jedrychowski W, Spendgler J et al. (2006) International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ Health Perspect 114, 1744–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perera FP, Rauh V, Whyatt RM et al. (2006) Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect 114, 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller KP, Borgeest C, Greenfeld C et al. (2004) In utero effects of chemicals on reproductive tissues in females. Toxicol Appl Pharmacol 198, 111–131. [DOI] [PubMed] [Google Scholar]
- 28. Barr DB, Bishop A & Needham LL (2007) Concentrations of xenobiotic chemicals in the maternal–fetal unit. Reprod Toxicol 23, 260–266. [DOI] [PubMed] [Google Scholar]
- 29. Perera FP, Rauh V, Tsai W et al. (2003) Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect 111, 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polanska K, Hanke W, Sobala W et al. (2011) Predictors of environmental exposure to polycyclic aromatic hydrocarbons among pregnant women – prospective cohort study in Poland. Int J Occup Med Environ Health 24, 8–17. [DOI] [PubMed] [Google Scholar]
- 31. Jedrychowski W, Perera FP, Tang D et al. (2012) Impact of barbecued meat consumed in pregnancy on birth outcomes accounting for personal prenatal exposure to airborne polycyclic aromatic hydrocarbons: birth cohort study in Poland. Nutrition 28, 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim BM, Ha M, Park HS et al. (2009) The mothers and children’s environmental health (MOCEH) study. Eur J Epidemiol 24, 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park MK, Noh HY, Song NY et al. (2012) Validity and reliability of a dish-based, semi-quantitative food frequency questionnaire for Korean diet and cancer research. Asian Pac J Cancer Prev 13, 545–552. [DOI] [PubMed] [Google Scholar]
- 34. Kim DW, Song S, Lee JE et al. (2015) Reproducibility and validity of an FFQ developed for the Korea National Health and Nutrition Examination Survey (KNHANES). Public Health Nutr 18, 1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim MJ, Hwang JH & Shin HS (2014) Evaluation of polycyclic aromatic hydrocarbon contents and risk assessment for fish and meat products in Korea. Food Sci Biotechnol 23, 991–998. [Google Scholar]
- 36. Pedersen M, von-Stedingk H, Botsivali M et al. (2012) Birth weight, head circumference, and prenatal exposure to acrylamide from maternal diet: the European Prospective Mother–Child Study (NewGeneris). Environ Health Perspect 120, 1739–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim H, Cho SH, Kang JW et al. (2001) Urinary 1-hydroxypyrene and 2-naphthol concentrations in male Koreans. Int Arch Occup Environ Health 74, 59–62. [DOI] [PubMed] [Google Scholar]
- 38. Bae S, Pan XC, Kim SY et al. (2010) Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in school children. Environ Health Perspect 118, 579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greenland S, Pearl J & Robins JM (1999) Causal diagrams for epidemiologic research. Epidemiology 10, 37–48. [PubMed] [Google Scholar]
- 40. Pavanello S, Pulliero A, Saia BO et al. (2006) Determinants of anti-benzo[a]pyrene diol epoxide–DNA adduct formation in lymphomonocytes of the general population. Mutat Res 611, 54–63. [DOI] [PubMed] [Google Scholar]
- 41. Choi H, Rauh V, Garfinkel R et al. (2008) Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect 116, 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perera FP, Rauh V, Whyatt RM et al. (2004) Molecular evidence of an interaction between prenatal environmental exposures and birth outcomes in a multiethnic population. Environ Health Perspect 112, 626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perera FP, Whyatt RM, Jedrychowski W et al. (1998) Recent developments in molecular epidemiology: a study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am J Epidemiol 147, 309–314. [DOI] [PubMed] [Google Scholar]
- 44. Dejmek J, Solansky I, Benes I et al. (2000) The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect 108, 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Duarte-Salles T, Mendez MA, Pessoa V et al. (2010) Smoking during pregnancy is associated with higher dietary intake of polycyclic aromatic hydrocarbons and poor diet quality. Public Health Nutr 13, 2034–2043. [DOI] [PubMed] [Google Scholar]
- 46. Duarte-Salles T, Mendez MA, Morales E et al. (2012) Dietary benzo(a)pyrene and fetal growth: effect modification by vitamin C intake and glutathione S-transferase P1 polymorphism. Environ Int 45, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duarte-Salles T, Mendez MA, Meltzer HM et al. (2013) Dietary benzo(a)pyrene intake during pregnancy and birth weight: associations modified by vitamin C intakes in the Norwegian Mother and Child Cohort Study (MoBa). Environ Int 60, 217–223. [DOI] [PubMed] [Google Scholar]
- 48. Rothman N, Poirier MC, Baser ME et al. (1990) Formation of polycyclic aromatic hydrocarbons–DNA adducts in peripheral white blood cells during consumption of charcoal broiled beef. Carcinogenesis 11, 1241–1243. [DOI] [PubMed] [Google Scholar]
- 49. Rothman N, Poirier MC, Haas RA et al. (1993) Association of PAH-adducts in peripheral while blood cells with dietary exposure to polyaromatic hydrocarbons. Environ Health Perspect 99, 265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pratt MM, John K, MacLean AB et al. (2011) Polycyclic aromatic hydrocarbon (PAH) exposure and DNA adduct semi-quantitation in archived human tissues. Int J Environ Res Public Health 8, 2675–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanyal MK, Mercan D, Belanger K et al. (2007) DNA adducts in human placenta exposed to ambient environment and passive cigarette smoking during pregnancy. Birth Defects Res A Clin Mol Teratol 79, 289–294. [DOI] [PubMed] [Google Scholar]
- 52. Godschalk RW, Van Schooten FJ & Bartsch H (2003) A critical evaluation of DNA adducts as biological markers for human exposure to polycyclic aromatic compounds. J Biochem Mol Biol 36, 1–11. [DOI] [PubMed] [Google Scholar]
- 53. Kelvin EA, Edwards S, Jedrychowski W et al. (2009) Modulation of prenatal PAH exposure on PAH–DNA adducts in cord blood by plasma antioxidants. Cancer Epidemiol Biomarkers Prev 18, 2262–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Santillan ME, Vincenti LM, Martini AC et al. (2010) Developmental and neurobehavioral effects of perinatal exposure to diets with different ω-6:ω-3 ratios in mice. Nutrition 26, 423–431. [DOI] [PubMed] [Google Scholar]
- 55. Banhidy F, Acs N, Puho EH et al. (2011) Iron deficiency anemia: pregnancy outcomes with or without iron supplementation. Nutrition 27, 65–72. [DOI] [PubMed] [Google Scholar]
- 56. Fiala Z, Vyskocil A, Krajak V et al. (2001) Environmental exposure of small children to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health 74, 411–420. [DOI] [PubMed] [Google Scholar]
- 57. Xue F, Holzman C, Rahbar MH et al. (2007) Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ Health Perspect 115, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gluckman PD, Hanson MA, Cooper C et al. (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359, 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980016000550.
click here to view supplementary material