Abstract
Objective
To assess the association between maternal caffeine intake and risk of pregnancy loss using a systematic review and meta-analysis.
Design
Categorical and dose–response meta-analysis of prospective studies.
Setting
Relevant articles were identified by searching MEDLINE and SCOPUS databases through 30 January 2015. Two authors independently extracted information from eligible studies. Random-effects models were used to derive the summary relative risks (RR) and corresponding 95 % CI for specific categories of caffeine consumption and for a continuous association using generalized least-squares trend estimation.
Subjects
A total of 130 456 participants and 3429 cases in fourteen included studies.
Results
Compared with the reference category with no or very low caffeine intake, the RR (95 % CI) of pregnancy loss was 1·02 (0·85, 1·24; I 2=28·3 %) for low intake (50–149 mg/d), 1·16 (0·94, 1·41; I 2=49·6 %) for moderate intake (150–349 mg/d), 1·40 (1·16, 1·68; I 2=18·6 %) for high intake (350–699 mg/d) and 1·72 (1·40, 2·13; I 2=0·0 %) for very high intake (≥700 mg/d). In the dose–response analysis, each 100 mg/d increment in maternal caffeine intake (~1 cup of coffee) was associated with 7 % (95 % CI 3 %, 12 %) higher risk of pregnancy loss. Our results may have been affected by publication bias, but the association remained significant for the subset of larger studies. Furthermore, adjustment for smoking and pregnancy symptoms may have been incomplete, potentially resulting in residual confounding.
Conclusions
Albeit inconclusive, higher maternal caffeine intake was associated with a higher risk of pregnancy loss and adherence to guidelines to avoid high caffeine intake during pregnancy appears prudent.
Keywords: Miscarriage, Spontaneous abortion, Stillbirth, Pregnancy loss, Pregnancy, Coffee, Caffeine
Pregnancy loss or fetal death is defined as the death of the fetus before complete expulsion from its mother( 1 ) and is subdivided into spontaneous abortion (or miscarriage) and stillbirth. The estimated global incidence of spontaneous abortion was 12 to 15 % among clinical pregnancies( 2 ), while the stillbirth rate has been estimated to be 19 per 1000 births( 3 ).
Caffeine is the most commonly used psychoactive substance, found mainly in coffee, tea, cola soft drinks and cocoa( 4 , 5 ). Caffeine is absorbed rapidly upon ingestion and readily passes the placental barrier( 6 , 7 ). Accumulation of caffeine metabolites in the fetal brain has been documented, probably due to the absence of the main caffeine-metabolizing enzyme, cytochrome P450 1A2 (CYP1A2), in both the placenta and the fetus( 6 , 8 , 9 ). The rate of caffeine metabolism decreases from the first to third trimester and the half-life of caffeine doubles in the mother during pregnancy( 10 , 11 ), leading to higher exposure for the fetus to maternally ingested caffeine. Exposure to caffeine may lead to vasoconstriction in the uteroplacental circulation, which can in turn affect fetal growth and development( 12 , 13 ).
Spontaneous abortion and stillbirth may have different underlying physiology and risk factors. For instance, while spontaneous abortion is caused mainly by implantation defects and chromosomal, autoimmune and endocrine abnormalities, the important causes of stillbirth include placental insufficiency and abruption, and pre-eclampsia( 14 , 15 ). However, spontaneous abortion and stillbirth share some common risk factors such as advanced maternal age, smoking and BMI( 15 ). It is currently unclear if caffeine has a differential influence on spontaneous abortion and stillbirth through different mechanisms.
The possible harmful effects of caffeine intake on fetal and birth outcomes warrant evaluation as many women consume caffeine-containing foods and beverages during pregnancy( 4 , 16 , 17 ). Considerable inconsistency exists in the literature concerning the relationship of maternal caffeine intake and pregnancy loss( 17 ). While high maternal caffeine intakes were more consistently associated with a higher risk of pregnancy loss, results have been mixed for moderate and low caffeine use, probably due to difficulties in measuring caffeine intakes and differences in study settings and participants. A recent meta-analysis summarized the effect of maternal caffeine intake during pregnancy on a variety of birth outcomes( 18 ). However, that analysis included only a dose–response analysis and not a categorical analysis and evaluated only a limited number of potential sources of heterogeneity in study results. We therefore conducted a categorical and dose–response meta-analysis on the association of maternal caffeine intake during pregnancy and risk of pregnancy loss in prospective studies.
Methods
The present meta-analysis was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)( 19 ) (see online supplementary Table S1) and Meta-analysis of Observational Studies in Epidemiology (MOOSE)( 20 ) guidelines (see online supplementary Table S2).
Search strategy
Two investigators searched MEDLINE and SCOPUS databases through 30 January 2015 with no language restriction. SCOPUS is an abstract and citation database of peer-reviewed literature that includes all the contents from the EMBASE database( 21 ). The search was based on combinations of synonyms for caffeine (including its chemical name, coffee and tea) and pregnancy loss (including spontaneous abortion, miscarriage, stillbirth and fetal death). The detailed search strategy is shown in online supplementary Material S1.
Selection criteria
Studies were included if they met the following criteria: (i) the study was an original, peer-reviewed study (i.e. not review articles or meeting abstracts); (ii) the study was a prospective cohort study or nested case–control study; and (iii) the authors reported the risk estimates of pregnancy loss associated with maternal caffeine intake (estimated total caffeine intake or coffee intake as a proxy for total caffeine intake) during pregnancy or the periconceptional period. We excluded studies that presented crude estimates only or did not consider potential confounding by smoking, and studies conducted in unhealthy populations (e.g. type 1 diabetes or infertility).
Pregnancy loss included both spontaneous abortion and stillbirth. Spontaneous abortion was defined as spontaneous loss of a fetus before the 20th week of pregnancy and stillbirth was defined as the death of a fetus during birth or during the last half of pregnancy. However, the definitions for stillbirth and miscarriage are not standard across the world, with miscarriage being defined as pregnancy loss occurring at less than 20–28 weeks of gestation.
The study selection was conducted independently by two authors. We also considered non-English articles with help from colleagues who are proficient in these languages. The inter-rater agreement was fairly good (κ=0·66; κ values that range from 0·61 to 0·80 indicate ‘substantial agreement’( 22 )). Discrepancies were resolved by discussion with a third investigator.
Data extraction
For included studies, information on participants, study design, measurement of exposure and outcome, effect estimates and their se (or related statistics) were extracted independently by two investigators using a standardized extraction form. Discrepancies were resolved by discussion with a third investigator. Study quality assessment was conducted by considering characteristics such as study design, number of cases and participants, method of exposure assessment and adjustment for confounders. Klebanoff et al.( 23 ) did not report numbers for effect estimates and we estimated the effect estimates and corresponding CI from their figure.
Statistical analysis
The multivariable-adjusted OR, relative risks (RR) or hazard ratios (HR) have been used in different studies. We chose RR as a measure of risk estimates in the meta-analysis because the incident rate was low (0·4 to 25·1 %; <20 % in twelve out of fourteen included studies), and OR and HR thus approximated RR( 24 ).
Different studies used different cut-off points for the caffeine intake categories. To combine the risk estimates from different categories in different studies, we assigned the median value for each category of caffeine intake. When lower and upper boundaries were presented for the category, we assigned the midpoint as an estimate of the median caffeine intake. If the upper boundary of the highest category was not provided, we assumed that the boundary had the same amplitude as the second-highest category( 25 ). If the lower boundary of the lowest category was not provided, we assumed the lower boundary to be zero( 25 ). Two studies reported coffee consumption in cups only but not total caffeine intake( 12 , 26 ) and we estimated caffeine intake based on the commonly cited conversion method (107 mg caffeine per cup of coffee)( 27 ). Klebanoff et al.( 23 ) reported paraxanthine concentration (a metabolite of caffeine) as the exposure and we estimated caffeine intake based on the conversion method proposed in that article. Briefly, for a 60-kg woman, caffeine intakes of 600 mg in non-smokers and 1100 mg in smokers were estimated to translate to serum paraxanthine of 1845 ng/ml. The conversion was extrapolated from the authors’ pilot data in a pregnant population with an overall lower caffeine intake( 28 ). Several studies reported results for more than one period of maternal caffeine exposure (Table 1). We used the results for the assessment period most frequently used (first trimester) in included studies. Nevertheless, we also included results from the other assessment periods in stratified analyses where possible. Although stillbirth and miscarriage can have different aetiology, as only one included study focused specifically on stillbirth and the incidence of stillbirth is low compared with miscarriage in included studies combining both outcomes, we did not separate these outcomes in our main analysis.
Table 1.
Characteristics of prospective studies on caffeine intake in relation to pregnancy loss
| Study | Country | Study design | No. of cases | Total no. of participants | Incidence (%) | Age | Exposure | Method of exposure assessment | Period of exposure assessed | Outcome | Adjustments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Srisuphan and Bracken (1986)( 38 ) * | USA | Cohort | 68 | 3135 | 2·2 | ≤30 years: 71 % >30 years: 29 % | Caffeine | Interview | First trimester | Spontaneous abortion | Gestational age at interview, maternal age, prior gynaecological surgery, member of Jewish religion, last pregnancy ending with a spontaneous abortion (maternal smoking was not adjusted for as it did not significantly predict the outcome) |
| Wilcox et al. (1990)( 39 ) | USA | Cohort | 43 | 171 | 25·1 | <29 years: 49 % ≥29 years: 51 % | Caffeine | Interviewer-administered questionnaire | Pre-pregnancy | Spontaneous abortion (<42 d) | Maternal age (only minor change after adjustments for smoking, alcohol intake) |
| Mills et al. (1993)( 40 ) | USA | Cohort | 59 | 423 | 13·9 | <30 years: 48 % ≥30 years: 52 % | Caffeine | Interview | First trimester | Spontaneous abortion | Smoking, maternal age, parity, prior spontaneous abortion, alcohol use, maternal education, income |
| Dlugosz et al. (1996)( 41 ) | USA | Cohort | 135 | 2967 | 4·6 | Mean 31 years | Caffeine | Interviewer-administered questionnaire | First month of pregnancy | Spontaneous abortion | Maternal age, gestational stage at interview, smoking, alcohol intake |
| Fenster et al. (1997)( 42 ) | USA | Cohort | 499 | 5144 | 9·7 | Mean 28 years | Caffeine | Interview (telephone) | Pre-pregnancy, first trimester† | Spontaneous abortion | Maternal age, smoking, alcohol intake, gestational age, pregnancy history, ethnicity, employment, marital status, socio-economic status |
| Klebanoff et al. (1999)( 23 ) | USA | Nested case–control | 591 | 3149 | 18·8 | Cases: mean 27 years Controls: mean 25 years | Paraxanthine | Serum paraxanthine | NA but before the occurrence of spontaneous abortion | Spontaneous abortion | Smoking, maternal age, ethnicity |
| Wen et al. (2001)( 43 ) | USA | Cohort | 75 | 650 | 11·5 | Median 29 years | Caffeine | Self-administered FFQ | Pre-pregnancy, first trimester† | Spontaneous abortion | NA (only unadjusted RR presented as they found no important confounding) |
| Tolstrup et al. (2003)( 44 ) | Denmark | Nested case–control | 303 | 1684 | 18·0 | Mean age 24·5–26·0 years according to caffeine consumption | Caffeine | Self-administered FFQ | Pre-pregnancy | Spontaneous abortion | Maternal age, marital status, smoking, alcohol intake |
| Wisborg et al. (2003)( 26 ) | Denmark | Cohort | 82 | 18 478 | 0·4 | <30 years: 57 % ≥30 years: 43 % | Coffee | Self-administered questionnaire | First trimester | Stillbirth | Smoking, alcohol intake, parity, maternal age, marital status, education, employment status, maternal BMI |
| Bech et al. (2005)( 12 ) | Denmark | Cohort | 1102 | 88 482 | 1·2 | <30 years: 54 % ≥30 years: 46 % | Coffee | Interview (telephone) | First to early second trimester | All pregnancy loss†, stillbirth | Maternal age, parity, smoking, pre-pregnancy BMI, alcohol intake, socio-occupational status |
| Savitz et al. (2008)( 45 ) | USA | Cohort | 258 | 2407 | 10·7 | <30 years: 61 % ≥30 years: 39 % | Caffeine | Interview (telephone) | Pre-pregnancy, 4 weeks post LMP, and before 16 weeks’ gestation† | Spontaneous abortion | Maternal age, ethnicity, education, marital status, alcohol use, vitamin use, symptoms of nausea and vomiting during early pregnancy (excluding smoking did not change results substantially) |
| Weng et al. (2008)( 46 ) | USA | Cohort | 172 | 1063 | 16·2 | <30 years: 42 % ≥30 years: 58 % | Caffeine | Interview | Early pregnancy (soon after pregnancy is confirmed) | Spontaneous abortion | Maternal age, ethnicity, education, family income, marital status, previous miscarriage, nausea and vomiting since LMP, smoking, alcohol intake, Jacuzzi use, exposure to magnetic fields |
| Greenwood et al. (2010)( 47 ) | UK | Cohort | 28 | 2635 | 1·1 | Mean 30 years | Caffeine | Self-administered questionnaire (validated) | First trimester | Late spontaneous abortion (12–24 weeks) and stillbirth | Maternal age, parity, smoking (cotinine concentration), alcohol intake |
| Pollack et al. (2010)( 36 ) | USA | Cohort | 14 | 68 | 20·6 | NA | Caffeine | Daily diaries | Pre-pregnancy | All pregnancy loss | Maternal age, alcohol intake, smoking |
NA, not available; LMP, last menstrual period; RR, relative risk.
Included for qualitative review only.
Data used for the main analysis.
We first conducted analyses based on different levels of caffeine consumption. We identified five levels of caffeine consumption based on assigned median caffeine consumption level: (i) reference category (<50 mg/d); (ii) low caffeine consumption (50–149 mg/d); (iii) moderate caffeine consumption (150–349 mg/d); (iv) high caffeine consumption (350–699 mg/d); and (v) very high caffeine consumption (≥700 mg/d). Effect estimates of the individual studies were combined using the random-effects method as described by DerSimonian and Laird( 29 ), which considers both within-study and between-study variations. The Cochran Q test and I 2 statistic were used to evaluate statistical heterogeneity among studies( 30 , 31 ), and I 2 values of 25 %, 50 % and 75 % correspond to low, moderate and high degrees of heterogeneity, respectively( 31 ).
We further conducted a dose–response analysis using the generalized least-squares trend estimation (GLST) method as described by Greenland and Longnecker( 32 , 33 ), which computes the trend from the correlated log RR estimates across caffeine consumption categories. The caffeine dose used in this analysis was based on median caffeine consumption level derived using the same methods as category-based analysis described above. We performed a two-stage GLST method that first estimates study-specific slopes before deriving an overall average slope( 33 ), because this method allowed us to include effect estimates from studies that reported results for caffeine intake only as a continuous variable. We tested for a potential non-linear relationship between maternal caffeine intake and pregnancy loss using a restricted cubic spline random-effects model with three knots; the P value for non-linearity was obtained by testing the null hypothesis that the spline term is equal to 0.
We conducted stratified analyses and meta-regression analyses to assess potential sources of heterogeneity by different study-level characteristics. In addition, we conducted sensitivity analysis to assess the influence of each individual study by omitting one study at a time and calculating the summary RR for the remaining studies. We also excluded studies focusing on stillbirth or a combination of miscarriage and stillbirth to evaluate the influence of pooling effect estimates from different outcomes. Publication bias was evaluated with Egger’s regression test, Begg’s adjusted rank correlation test and visual inspection of the funnel plot( 34 , 35 ). We excluded one small study( 36 ) with implausibly small se for effect estimates in the assessment of publication bias. We further conducted trim-and-fill analysis to generate summary effect estimates adjusted for publication bias( 37 ). All tests were performed using the statistical software package STATA version 11·2 and two-sided P values <0·05 were considered statistically significant.
Results
The flow diagram with details of the study selection is shown in Fig. 1. We included fourteen prospective studies( 12 , 23 , 26 , 36 , 38 – 47 ) on caffeine intake and pregnancy loss involving 130 456 participants and 3429 cases of pregnancy loss (Table 1). One of the studies( 38 ) was included for only qualitative review because it did not report data for more than two categories of caffeine intake. All studies in the present meta-analysis were conducted in the USA or Europe.
Fig. 1.
Flowchart of study selection
Figure 2 shows the RR for the association between maternal caffeine intake and pregnancy loss. The summary RR was 1·72 (95 % CI 1·40, 2·13) for very high caffeine intake (≥700 mg/d), 1·40 (95 % CI 1·16, 1·68) for high caffeine intake (350–699 mg/d), 1·16 (95 % CI 0·94, 1·41) for moderate caffeine intake (150–349 mg/d) and 1·02 (95 % CI 0·85, 1·24) for low caffeine intake (50–149 mg/d), as compared with the reference category with no or very low caffeine intake. The heterogeneity in study results was low to moderate: the I 2 was 28·3 % for low caffeine intake, 49·6 % for moderate caffeine intake, 18·6 % for high caffeine intake and 0 % for very high caffeine intake. Srisuphan and Bracken( 38 ) also reported a significant association between caffeine intake and risk of late spontaneous abortion, but their study could not be included in the meta-analysis because it considered only two categories of intake.
Fig. 2.
Relative risk (RR) of pregnancy loss according to maternal caffeine intake: low caffeine intake, 50–149 mg/d; moderate caffeine intake, 150–349 mg/d; high caffeine intake, 350–699 mg/d; very high caffeine intake, ≥700 mg/d. The study-specific estimates and 95 % CI are indicated by the black dots and the horizontal lines, respectively; the size of the grey squares corresponds to the weight of the studies in the meta-analysis. The centre of the diamonds indicates the summary estimates and the width of the diamonds the corresponding 95 % CI
We assumed a linear relationship in the dose–response meta-analysis between maternal caffeine intake and pregnancy loss, because there was no evidence of statistically significant departure from linearity (P=0·44). The summary RR was 1·07 (95 % CI 1·03, 1·12; Table 2 and Fig. 3) per 100 mg/d. To facilitate comparison with the categorical analysis this can also be expressed as 1·23 (95 % CI 1·09, 1·39) per 300 mg/d or 1·42 (95 % CI 1·16, 1·74) per 500 mg/d increment of maternal caffeine intake. The heterogeneity in study results was higher (I 2=80·9 %) for this analysis than for the comparisons of specific categories of caffeine consumption.
Table 2.
Stratified meta-analysis of caffeine intake (per 100 mg/d increment) and risk of pregnancy loss
| Characteristic | No. of studies | Summary RR | 95 % CI | P for difference | P for heterogeneity | I 2 (%) | 95 % CI |
|---|---|---|---|---|---|---|---|
| All studies | 13 | 1·07 | 1·03, 1·12 | <0·01 | 80·9 | 68·4, 88·5 | |
| Region | |||||||
| USA | 9 | 1·09 | 1·02, 1·17 | Ref. | <0·01 | 79·0 | 60·6, 88·8 |
| Europe | 4 | 1·06 | 1·02, 1·10 | 0·85 | 0·07 | 57·3 | 0·0, 85·8 |
| Year of publication | |||||||
| In or after 2000 | 8 | 1·07 | 1·02, 1·13 | 0·30* | <0·01 | 86·2 | 74·9, 92·4 |
| Before 2000 | 5 | 1·07 | 1·03, 1·11 | 0·61 | 0·0 | 0·0, 79·2 | |
| Study population | |||||||
| <2500 | 7 | 1·08 | 1·00, 1·16 | Ref. | <0·01 | 78·3 | 55·0, 89·5 |
| ≥2500 | 6 | 1·06 | 1·03, 1·10 | 0·90 | 0·21 | 29·4 | 0·0, 71·2 |
| Study design | |||||||
| Cohort | 11 | 1·09 | 1·03, 1·15 | Ref. | <0·01 | 81·8 | 68·5, 89·4 |
| Nested case–control | 2 | 1·05 | 1·02, 1·08 | 0·52 | 0·39 | 0·0 | NA |
| Exposure | |||||||
| Caffeine intake | 10 | 1·10 | 1·03, 1·17 | Ref. | <0·01 | 76·8 | 57·2, 87·4 |
| Coffee intake | 2 | 1·05 | 1·03, 1·08 | 0·62 | 0·33 | 0·0 | NA |
| Paraxanthine level | 1 | 1·06 | 1·02, 1·10 | 0·69 | – | – | – |
| Outcome | |||||||
| Spontaneous abortion | 9 | 1·08 | 1·04, 1·13 | Ref. | 0·13 | 35·8 | 0·0, 70·5 |
| All pregnancy loss | 3 | 1·03 | 0·96, 1·11 | 0·31 | <0·01 | 93·1 | 83·1, 97·2 |
| Stillbirth | 1 | 1·09 | 1·02, 1·16 | 0·91 | – | – | – |
| Measure of association | |||||||
| Odds ratio | 8 | 1·06 | 1·03, 1·09 | Ref. | 0·35 | 10·7 | 0·0, 71·1 |
| Hazard ratio | 2 | 1·14 | 0·94, 1·39 | 0·73 | 0·01 | 84·4 | NA |
| Risk ratio | 3 | 1·15 | 0·89, 1·48 | 0·62 | 0·02 | 75·9 | 20·5, 92·7 |
| Age† | |||||||
| <30 years | 8 | 1·05 | 1·04, 1·07 | Ref. | 0·45 | 0·0 | 0·0, 67·6 |
| ≥30 years | 4 | 1·23 | 1·09, 1·38 | 0·02 | 0·25 | 26·7 | 0·0, 72·3 |
| NA | 1 | 0·98 | 0·97, 1·00 | <0·01 | – | – | – |
| Method of exposure assessment | |||||||
| Interviewer-based | 7 | 1·10 | 1·03, 1·17 | Ref. | 0·15 | 37·1 | 0·0, 73·5 |
| Biomarker | 1 | 1·06 | 1·02, 1·10 | 0·67 | – | – | – |
| Self-administered | 5 | 1·06 | 0·99, 1·14 | 0·58 | <0·01 | 85·2 | 67·3, 93·3 |
| Exposure period assessed‡ | |||||||
| First trimester | 9 | 1·11 | 1·05, 1·17 | Ref. | 0·04 | 49·7 | 0·0, 76·5 |
| Pre-pregnancy | 6 | 1·02 | 0·97, 1·07 | 0·05 | 0·03 | 60·6 | 0·0, 81·9 |
| NA | 1 | 1·06 | 1·02, 1·10 | 0·52 | – | – | – |
| Adjustment for nausea | |||||||
| No | 11 | 1·06 | 1·02, 1·11 | Ref. | <0·01 | 81·5 | 68·0, 89·3 |
| Yes | 2 | 1·17 | 0·96, 1·43 | 0·21 | 0·12 | 58·6 | NA |
| Adjustment for smoking§ | |||||||
| Fine | 7 | 1·05 | 1·00, 1·10 | Ref. | <0·01 | 84·8 | 70·4, 92·2 |
| Crude | 3 | 1·11 | 1·01, 1·21 | 0·40 | 0·07 | 62·9 | 0·0, 89·4 |
| Not applicable | 3 | 1·18 | 0·98, 1·43 | 0·29 | 0·23 | 31·9 | 0·0, 92·9 |
| Median population caffeine intake | |||||||
| <200 mg/d | 7 | 1·18 | 1·07, 1·30 | 0·11* | 0·11 | 41·5 | 0·0, 75·4 |
| ≥200 mg/d | 4 | 1·05 | 1·03, 1·07 | 0·63 | 0·0 | 0·0, 84·7 | |
| NA | 2 | 1·01 | 0·90, 1·13 | 0·22 | 32·2 | NA | |
RR, relative risk; NA, not available; Ref., reference.
P value was obtained by modelling year of publication and median of assigned doses as continuous variables.
Mean age <30 years or ≥30 years. If mean age is not available, classification was based on whether majority of the population (>50 %) is <30 years or ≥30 years.
Total number of studies is more than thirteen because some studies reported additional (usable) results for a different exposure period.
Fine adjustment for smoking refers to studies that adjusted for amount of smoking or studies that adjusted for smoking using a biomarker; crude adjustment refers to studies that did not adjust for amount of smoking; ‘Not applicable’ refers to studies that presented estimates without adjustment for smoking as adjustment for smoking did not change the results substantially.
Fig. 3.
Dose–response relationship between maternal caffeine intake and pregnancy loss (n 11). Adjusted relative risk (RR; ————) and 95 % CI (- - - - - -) are reported. Caffeine intake was modelled with a linear trend (P for non-linearity=0·44) in a random-effects model. The vertical axis is on a log scale; — — — — indicates RR=1. The open circles represent the effect estimates from each study and the size of the circles is proportional to the precision of the estimates. The studies by Mills et al.( 40 ) and Pollack et al.( 36 ) were not included in this plot as they did not provide results for categories of caffeine intake
The summary RR did not differ substantially in various subgroups (Table 2), except for mean baseline age of the participants. The association was stronger (P for interaction=0·02) in studies of older women (mean age ≥30 years, summary RR=1·23, 95 % CI 1·09, 1·38) than in studies of younger women (mean age <30 years, summary RR=1·05, 95 % CI 1·04, 1·07; Table 2). In addition, there was a trend of borderline significance (P for interaction=0·05) towards a stronger effect of maternal caffeine intake assessed during pregnancy (summary RR=1·11, 95 % CI 1·05, 1·17) as compared with caffeine intake assessed during the pre-pregnancy period (summary RR=1·02, 95 % CI 0·97, 1·07). However, when we modelled both study characteristics in the meta-regression analysis, only maternal age ≥30 years remained significant (P=0·02), while the interaction with period of assessment was attenuated (P=0·52).
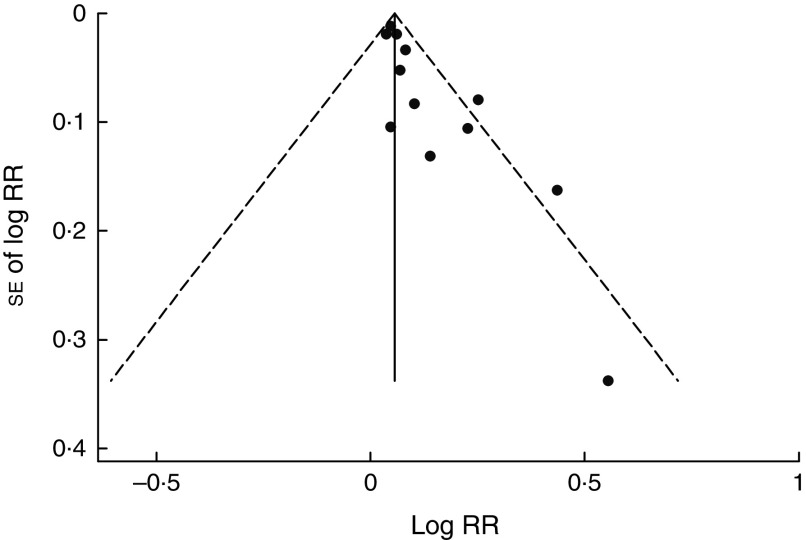
In a sensitivity analysis, the summary RR ranged from 1·06 (95 % CI 1·02, 1·10) to 1·08 (95 % CI 1·03, 1·14) per 100 mg/d increment in maternal caffeine intake when we omitted studies one at a time. When we excluded studies focusing on stillbirth or a combination of miscarriage and stillbirth, the pooled effect estimates were not materially different in both the dose–response (summary RR=1·08, 95 % CI 1·04, 1·13) and category-based meta-analysis, and the conclusion remained the same. There was a suggestion of small-study effect such as publication bias based on visual inspection of the funnel plot (Fig. 4) and in both Egger’s test (P<0·01) and Begg’s test (P=0·047). However, the summary effect estimate remained significant in the subgroup of the larger studies (RR=1·06 per 100 mg/d, 95 % CI 1·03, 1·10; Table 2) or when we conducted the trim-and-fill analysis (RR=1·06, 95 % CI 1·02, 1·10) that imputed six inferred missing studies.
Fig. 4.
Funnel plot with pseudo 95 % confidence limits (– – – – –) for maternal caffeine intake and pregnancy loss (RR, relative risk). There was a suggestion of publication bias in Egger’s (P<0·01) and Begg’s test (P=0·047)
Discussion
The findings from the current meta-analysis of prospective studies suggest that maternal caffeine intake is associated with a higher risk of pregnancy loss. High maternal intake of caffeine (350–699 mg/d) was associated with a 40 % higher risk of pregnancy loss. In our dose–response analysis, a 100 mg/d increment in maternal caffeine intake (~1 cup of coffee) was associated with a 7 % higher risk for pregnancy loss. However, our results should be interpreted with caution in consideration of methodological limitations of the original studies and other potential issues such as residual confounding and publication bias that may have influenced our results.
Most previous reviews on maternal caffeine intake and pregnancy loss included only a qualitative summary of the evidence( 17 , 48 , 49 ), used estimates that were not adjusted for potential confounders( 50 ) or included only five studies on preconception caffeine intake( 51 ). A recent meta-analysis reported significant associations stronger than those observed in our meta-analysis between higher maternal caffeine intakes and higher risks of miscarriage (summary RR=1·14 per 100 mg caffeine/d, 95 % CI 1·10, 1·19 in that study compared with 1·08, 95 % CI 1·04, 1·13 in our study) and stillbirth (summary RR=1·19, 95 % CI 1·05, 1·35 in that study compared with 1·09, 95 % CI 1·02, 1·16 in our study)( 18 ). However, that meta-analysis also included retrospective studies which are more prone to recall and selection bias. Similar to our study, high heterogeneity was reported in the dose–response analysis and publication bias may have affected the observed associations. We also conducted a complementary analysis using specific categories of caffeine consumption which resulted in substantially lower heterogeneity. This suggests that the estimation of dose–response relationships based on the results for categories of caffeine consumption reported in the original studies may have introduced additional heterogeneity. The correlation matrices of the adjusted and unadjusted log RR are assumed to be approximately equal in the GLST method proposed by Greenland and Longnecker, and approximation using this method tends to be unstable if there is small number of cases in the referent exposure level( 52 ). To date, there has been no randomized controlled trial conducted to evaluate the effect of a reduction in maternal caffeine intake on pregnancy loss( 53 , 54 ).
Our meta-analysis has several strengths. We searched databases with no language restriction to increase the completeness of the identification of studies. Moreover, we included only prospective studies and thus reduced the influence of selection bias, recall bias and reverse causation. The included studies were reasonably large, with study size of more than 1000 participants in ten out of fourteen studies. Lastly, we did not only compare the highest categories with the lowest categories but divided maternal caffeine intake into five levels and also conducted a dose–response analysis.
Our study results should be interpreted with consideration of several potential limitations. First, our results could have been affected by measurement error of caffeine intake assessment in the original studies. The included studies relied mostly on questionnaires for caffeine assessment. However, validation studies have shown that self-reports of major sources of caffeine such as coffee and tea are in general reasonably accurate and reliable( 55 , 56 ). Another potential limitation is that two of the included studies conducted in Denmark used coffee consumption as the proxy of total caffeine intake. However, coffee consumption is high in Denmark and in a similar Swedish study coffee was the predominant source (accounting for 76 %) of all caffeine ingested by pregnant women( 57 ). In our stratified analyses, the summary estimates did not differ substantially by dietary assessment method or by assessment of coffee v. total caffeine intake. The caffeine assessments in the remaining studies were reasonably comprehensive. Most studies considered at least the intakes of coffee, tea and caffeinated soft drinks( 36 , 38 – 43 , 45 – 47 ), while some also additionally considered other sources such as cocoa products( 40 , 43 , 46 , 47 ) and caffeine-containing medication( 38 , 40 , 47 ). Energy drinks containing relatively high amounts of caffeine have increased in popularity, especially since the mid-2000s( 58 , 59 ). However, most studies included in our meta-analysis were conducted before that period. Furthermore, the contribution of energy drinks to total caffeine intakes was found to be small in studies conducted in pregnant women or women of childbearing age( 60 , 61 ).
Another potential limitation of our meta-analysis is residual confounding resulting from unmeasured or imperfectly measured covariates. A majority of the included studies adjusted for potential confounders rather comprehensively. We only included studies that considered potential confounding by smoking, either by adjusting for it in multivariable models or reporting that such adjustment had minimal influence on the results. Moreover, most studies adjusted for maternal age, parity, alcohol intake and socio-economic status.
Smoking tends to correlate with caffeine intake( 9 , 40 ) and is a known risk factor for pregnancy loss( 62 , 63 ). Smoking is therefore a potentially important confounder of the association between caffeine intake and pregnancy loss. The six included studies( 12 , 26 , 36 , 40 , 44 , 46 ) that investigated a possible interaction between caffeine intake and smoking in relation to pregnancy loss either did not suggest differences in association between smokers and non-smokers( 12 , 26 , 36 , 40 , 44 ) or suggested a stronger association in non-smokers( 46 ). It is reassuring that the association between caffeine intake and pregnancy loss was observed in non-smokers, because residual confounding by intensity of smoking is not a concern in this group. However, non-disclosure rate of active smoking was higher among pregnant women compared with non-pregnant women in a study comparing self-reported and biomarker-determined smoking status( 64 ). Thus, residual confounding by smoking may still be a concern due to under-reporting of smoking in pregnant women. Nevertheless, in Greenwood et al.’s study higher maternal caffeine intake was associated with a higher risk of late miscarriage and stillbirth even after adjustment for salivary cotinine, a biomarker for smoking( 47 ).
Pregnancy symptoms including nausea, vomiting, and aversions to smells and taste are more common in healthy pregnancies than in pregnancies that end in spontaneous abortion( 17 ). Women with viable pregnancies may be more likely to decrease their caffeine consumption in response to pregnancy symptoms( 17 ). Pregnancy symptoms can affect the interpretation of the relationship between caffeine and pregnancy loss in two ways. First, pregnancy symptoms are associated with both pregnancy loss and maternal caffeine intake and thus potentially are a confounder. Second, loss of fetal viability (which precedes the actual detection of pregnancy loss) can result in reduced pregnancy symptoms and subsequently increased caffeine intake; this scenario represents reverse causality. If pregnancy symptoms confound the relationship between caffeine and pregnancy loss, their influence can be studied through adjustment or stratification for these symptoms. Wen et al. reported a substantial association between caffeine intake measured after nausea occurrence and the risk of spontaneous abortion in women with nausea, but not for caffeine intake assessed earlier or in women without nausea( 43 ). In contrast, Klebanoff et al. observed similar associations in women who did or did not experience vomiting( 23 ). Other studies that investigated the influence of pregnancy signals using self-reported information on nausea, vomiting and fatigue did not support the argument that confounding by pregnancy-related symptoms results in spurious associations between maternal caffeine intake and the risk of spontaneous abortion( 45 , 46 , 57 ). Furthermore, the association between maternal caffeine intake and pregnancy loss was not significantly different for studies that did and did not adjust for nausea and vomiting in our meta-regression analysis (Table 2). Nevertheless, assessment of relevant pregnancy symptoms is unlikely to be without measurement error and residual confounding by symptoms reflecting fetal viability thus cannot be completely excluded. Moreover, the possibility that higher caffeine intake is a consequence rather than a cause of pregnancy loss (reverse causality) cannot be excluded. A study with detailed serial measurement of both caffeine intake and fetal viability based on ultrasounds may help to disentangle the cause-and-effect sequence, but such measurements are unlikely to be feasible in big epidemiological studies.
In addition to methodological limitations of the original studies, our results may have been affected by small-study effect such as publication bias as suggested by the funnel plot and statistical tests. Publication bias is only one of the possible reasons for small-study effect and there can be a real difference in effect size in smaller studies due to methodological or clinical diversity. It is possible that smaller studies have a larger effect size due to more complete and comprehensive measurement of caffeine intake. Nevertheless, in our stratified analysis the association between caffeine intake and pregnancy loss was similar in the larger studies that are less likely to be affected by this bias, but as in any meta-analysis the possibility of the bias cannot be completely excluded even for the subset of larger studies. If the observed small-study effect is really due to publication bias, our trim-and-fill analysis suggested that the bias did not affect the pooled effect estimate substantially.
The exact mechanism through which caffeine may increase risk of pregnancy loss remains unclear. Caffeine can increase the release of catecholamines, which may lead to vasoconstriction in the uteroplacental circulation and fetal hypoxia and subsequently affect fetal development and growth( 12 , 13 ). Indeed, after maternal ingestion of just 200 mg of caffeine, a 25 % reduction in intervillous placental blood flow has been documented( 6 , 13 ). Another proposed mechanism is that caffeine increases the cellular concentration of cyclic adenosine monophosphate (cAMP) by inhibiting an enzyme (phosphodiesterase) responsible for the breakdown of cAMP( 9 , 65 ). A build-up of cAMP may affect fetal growth through its influence on cell division or catecholamine-mediated vasoconstriction( 66 , 67 ). Furthermore, caffeine may also affect the fetal cardiovascular system directly by increasing fetal heart rate accelerations and causing tachycardia( 26 , 68 , 69 ). As mentioned before, spontaneous abortion and stillbirth may have different aetiology. Caffeine may influence these outcomes through a common mechanism such as vascular disorder or via different pathways, but there is limited evidence to postulate further. If maternal caffeine intake is indeed causally associated with pregnancy loss, the effect can also be possibly exerted by its metabolites. These may have important implications as wide inter-individual variation in caffeine metabolism due to either genetic variation (e.g. polymorphism in CYP1A2) or lifestyle factors (e.g. smoking) has been reported( 70 ). Thus, in order to provide a more complete picture on caffeine metabolism, future studies should consider assaying for circulating caffeine, its metabolites and genotype information on top of well-planned caffeine intake and lifestyle factors measurements.
It is not clear why the association between caffeine intake and pregnancy loss was more pronounced in studies of older women than in studies of younger women and this observation requires confirmation in further studies with individual-level data on age. It may be that older mothers drink more coffee( 46 , 71 ) or that older age inherently exacerbates the negative influence of caffeine on pathways leading to pregnancy loss. It is known that advancing maternal age increases risk of fetal chromosomal abnormalities and thus results in higher risk of spontaneous abortion. However, caffeine is generally not considered to be mutagenic and cytotoxic at usual human exposure level( 72 ).
Conclusions
The present systematic review and meta-analysis supports the hypothesis that high maternal caffeine intake during pregnancy is associated with a higher risk of pregnancy loss. The results of recent meta-analyses also indicate that higher maternal coffee consumption may increase the risk of low birth weight in offspring( 18 , 73 ). Currently, the WHO recommends a caffeine intake of less than 300 mg (~3 cups of coffee) per day. Our results suggested that high caffeine intake (~3·5 to 7 cups of coffee) and very high caffeine intake (≥7 cups of coffee) were associated with a substantially higher risk of pregnancy loss. Our results may have been affected by publication bias and the possibility of residual confounding by smoking or pregnancy symptoms cannot be ruled out. Nevertheless, in combination with the biological plausibility of adverse effects on the fetus and evidence for effects of maternal caffeine intake on reduced fetal growth, it appears prudent to adhere to public recommendations to limit caffeine intake during pregnancy to low levels.
Acknowledgements
Financial support: The project was supported by Saw Swee Hock School of Public Health, National University of Singapore, Singapore. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflict of interest: None. Authorship: L.-W.C., M.F.-F.C. and R.M.v.D. conceived the study. L.-W.C. and Y.W. searched the databases and screened studies according to inclusion and exclusion criteria. L.-W.C., Y.W., M.F.-F.C. and R.M.v.D. developed the search strategy. A.P. and N.N. gave advice on meta-analysis methodology. L.-W.C. analysed the data. A.P. and R.M.v.D. helped in the interpretation of the results. L.-W.C. wrote the draft of the paper. L.-W.C., Y.W., N.N., M.F.-F.C., A.P. and R.M.v.D. were involved in critical reviewing and revision of the manuscript. All authors approved the final version of the manuscript. Ethics of human subject participation: Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980015002463.
click here to view supplementary material
References
- 1. Sims MA & Collins KA (2001) Fetal death: a 10-year retrospective study. Am J Forensic Med Pathol 22, 261–265. [DOI] [PubMed] [Google Scholar]
- 2. García-Enguídanos A, Calle ME, Valero J et al. (2002) Risk factors in miscarriage: a review. Eur J Obstet Gynecol Reprod Biol 102, 111–119. [DOI] [PubMed] [Google Scholar]
- 3. Lawn JE, Blencowe H, Pattinson R et al. (2011) Stillbirths: where? When? Why? How to make the data count? Lancet 377, 1448–1463. [DOI] [PubMed] [Google Scholar]
- 4. Maslova E, Bhattacharya S, Lin SW et al. (2010) Caffeine consumption during pregnancy and risk of preterm birth: a meta-analysis. Am J Clin Nutr 92, 1120–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eteng MU, Eyong EU, Akpanyung EO et al. (1997) Recent advances in caffeine and theobromine toxicities: a review. Plant Foods Hum Nutr 51, 231–243. [DOI] [PubMed] [Google Scholar]
- 6. CARE Study Group (2008) Maternal caffeine intake during pregnancy and risk of fetal growth restriction: a large prospective observational study. BMJ 337, a2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arnaud MJ, Bracco I, Sauvageat JL et al. (1983) Placental transfer of the major caffeine metabolite in the rat using 6-amino-5[N-formylmethylamino]1,3[Me-14C]-dimethyluracil administered orally or intravenously to the pregnant rat. Toxicol Lett 16, 271–279. [DOI] [PubMed] [Google Scholar]
- 8. Aldridge A, Aranda JV & Neims AH (1979) Caffeine metabolism in the newborn. Clin Pharmacol Ther 25, 447–453. [DOI] [PubMed] [Google Scholar]
- 9. Sengpiel V, Elind E, Bacelis J et al. (2013) Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: results from a large prospective observational cohort study. BMC Med 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knutti R, Rothweiler H & Schlatter C (1982) The effect of pregnancy on the pharmacokinetics of caffeine. Arch Toxicol Suppl 5, 187–192. [DOI] [PubMed] [Google Scholar]
- 11. Brazier JL, Ritter J, Berland M et al. (1983) Pharmacokinetics of caffeine during and after pregnancy. Dev Pharmacol Ther 6, 315–322. [DOI] [PubMed] [Google Scholar]
- 12. Bech BH, Nohr EA, Vaeth M et al. (2005) Coffee and fetal death: a cohort study with prospective data. Am J Epidemiol 162, 983–990. [DOI] [PubMed] [Google Scholar]
- 13. Kirkinen P, Jouppila P, Koivula A et al. (1983) The effect of caffeine on placental and fetal blood flow in human pregnancy. Am J Obstet Gynecol 147, 939–942. [DOI] [PubMed] [Google Scholar]
- 14. Regan L & Rai R (2000) Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol 14, 839–854. [DOI] [PubMed] [Google Scholar]
- 15. O’Neill SM, Kearney PM, Kenny LC et al. (2013) Caesarean delivery and subsequent stillbirth or miscarriage: systematic review and meta-analysis. PLoS One 8, e54588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frary CD, Johnson RK & Wang MQ (2005) Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc 105, 110–113. [DOI] [PubMed] [Google Scholar]
- 17. Peck JD, Leviton A & Cowan LD (2010) A review of the epidemiologic evidence concerning the reproductive health effects of caffeine consumption: a 2000–2009 update. Food Chem Toxicol 48, 2549–2576. [DOI] [PubMed] [Google Scholar]
- 18. Greenwood DC, Thatcher NJ, Ye J et al. (2014) Caffeine intake during pregnancy and adverse birth outcomes: a systematic review and dose–response meta-analysis. Eur J Epidemiol 29, 725–734. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stroup DF, Berlin JA, Morton SC et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283, 2008–2012. [DOI] [PubMed] [Google Scholar]
- 21. Elsevier BV (2011) Scopus Content Coverage Guide. http://www.elsevier.com/__data/assets/pdf_file/0007/69451/sc_content-coverage-guide_july-2014.pdf (accessed August 2015). [Google Scholar]
- 22. Sim J & Wright CC (2005) The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther 85, 257–268. [PubMed] [Google Scholar]
- 23. Klebanoff MA, Levine RJ, DerSimonian R et al. (1999) Maternal serum paraxanthine, a caffeine metabolite, and the risk of spontaneous abortion. N Engl J Med 341, 1639–1644. [DOI] [PubMed] [Google Scholar]
- 24. Thomas DC (2009) Statistical Methods in Environmental Epidemiology. Oxford: Oxford University Press. [Google Scholar]
- 25. Larsson SC & Orsini N (2011) Coffee consumption and risk of stroke: a dose–response meta-analysis of prospective studies. Am J Epidemiol 174, 993–1001. [DOI] [PubMed] [Google Scholar]
- 26. Wisborg K, Kesmodel U, Bech BH et al. (2003) Maternal consumption of coffee during pregnancy and stillbirth and infant death in first year of life: prospective study. BMJ 326, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bunker ML & McWilliams M (1979) Caffeine content of common beverages. J Am Diet Assoc 74, 28–32. [PubMed] [Google Scholar]
- 28. Klebanoff MA, Levine RJ, Dersimonian R et al. (1998) Serum caffeine and paraxanthine as markers for reported caffeine intake in pregnancy. Ann Epidemiol 8, 107–111. [DOI] [PubMed] [Google Scholar]
- 29. DerSimonian R & Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7, 177–188. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JPT & Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- 31. Higgins JPT, Thompson SG, Deeks JJ et al. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greenland S & Longnecker MP (1992) Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol 135, 1301–1309. [DOI] [PubMed] [Google Scholar]
- 33. Orsini N, Bellocco R & Greenland S (2006) Generalized least squares for trend estimation of summarized dose–response data. Stata J 6, 40–57. [Google Scholar]
- 34. Begg CB & Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. [PubMed] [Google Scholar]
- 35. Egger M, Smith GD, Schneider M et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pollack AZ, Buck Louis GM, Sundaram R et al. (2010) Caffeine consumption and miscarriage: a prospective cohort study. Fertil Steril 93, 304–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duval S & Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. [DOI] [PubMed] [Google Scholar]
- 38. Srisuphan W & Bracken MB (1986) Caffeine consumption during pregnancy and association with late spontaneous abortion. Am J Obstet Gynecol 154, 14–20. [DOI] [PubMed] [Google Scholar]
- 39. Wilcox AJ, Weinberg CR & Baird DD (1990) Risk factors for early pregnancy loss. Epidemiology 1, 382–385. [DOI] [PubMed] [Google Scholar]
- 40. Mills JL, Holmes LB, Aarons JH et al. (1993) Moderate caffeine use and the risk of spontaneous abortion and intrauterine growth retardation. JAMA 269, 593–597. [PubMed] [Google Scholar]
- 41. Dlugosz L, Belanger K, Hellenbrand K et al. (1996) Maternal caffeine consumption and spontaneous abortion: a prospective cohort study. Epidemiology 7, 250–255. [DOI] [PubMed] [Google Scholar]
- 42. Fenster L, Hubbard AE, Swan SH et al. (1997) Caffeinated beverages, decaffeinated coffee, and spontaneous abortion. Epidemiology 8, 515–523. [DOI] [PubMed] [Google Scholar]
- 43. Wen W, Shu XO, Jacobs Jr DR et al. (2001) The associations of maternal caffeine consumption and nausea with spontaneous abortion. Epidemiology 12, 38–42. [DOI] [PubMed] [Google Scholar]
- 44. Tolstrup JS, Kjaer SK, Munk C et al. (2003) Does caffeine and alcohol intake before pregnancy predict the occurrence of spontaneous abortion? Hum Reprod 18, 2704–2710. [DOI] [PubMed] [Google Scholar]
- 45. Savitz DA, Chan RL, Herring AH et al. (2008) Caffeine and miscarriage risk. Epidemiology 19, 55–62. [DOI] [PubMed] [Google Scholar]
- 46. Weng X, Odouli R & Li D-K (2008) Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol 198, 279.e1–279.e8. [DOI] [PubMed] [Google Scholar]
- 47. Greenwood DC, Alwan N, Boylan S et al. (2010) Caffeine intake during pregnancy, late miscarriage and stillbirth. Eur J Epidemiol 25, 275–280. [DOI] [PubMed] [Google Scholar]
- 48. Matijasevich A, Santos IS & Barros FC (2005) Does caffeine consumption during pregnancy increase the risk of fetal mortality? A literature review. Cad Saude Publica 21, 1676–1684. [DOI] [PubMed] [Google Scholar]
- 49. Signorello LB & McLaughlin JK (2004) Maternal caffeine consumption and spontaneous abortion. Epidemiology 15, 229–239. [DOI] [PubMed] [Google Scholar]
- 50. Fernandes O, Sabharwal M, Smiley T et al. (1998) Moderate to heavy caffeine consumption during pregnancy and relationship to spontaneous abortion and abnormal fetal growth: a meta-analysis. Reprod Toxicol 12, 435–444. [DOI] [PubMed] [Google Scholar]
- 51. Lassi ZS, Imam AM, Dean SV et al. (2014) Preconception care: caffeine, smoking, alcohol, drugs and other environmental chemical/radiation exposure. Reprod Health 11, Suppl. 3, S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Orsini N, Li R, Wolk A et al. (2012) Meta-analysis for linear and nonlinear dose–response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jahanfar S & Jaafar SH (2013) Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome. Cochrane Database Syst Rev issue 2, CD006965. [DOI] [PubMed] [Google Scholar]
- 54. Jahanfar S & Sharifah H (2009) Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcome. Cochrane Database Syst Rev issue 2, CD006965. [DOI] [PubMed] [Google Scholar]
- 55. James JE, Bruce MS, Lader MH et al. (1989) Self-report reliability and symptomatology of habitual caffeine consumption. Br J Clin Pharmacol 27, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grosso LM, Triche E, Benowitz NL et al. (2008) Prenatal caffeine assessment: fetal and maternal biomarkers or self-reported intake? Ann Epidemiol 18, 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cnattingius S, Signorello LB, Annerén G et al. (2000) Caffeine intake and the risk of first-trimester spontaneous abortion. N Engl J Med 343, 1839–1845. [DOI] [PubMed] [Google Scholar]
- 58. Heckman MA, Sherry K & De Mejia EG (2010) Energy drinks: an assessment of their market size, consumer demographics, ingredient profile, functionality, and regulations in the United States. Compr Rev Food Sci Food Saf 9, 303–317. [DOI] [PubMed] [Google Scholar]
- 59. Oddy WH & O’Sullivan TA (2009) Energy drinks for children and adolescents. BMJ 339, b5268. [DOI] [PubMed] [Google Scholar]
- 60. Derbyshire E & Abdula S (2008) Habitual caffeine intake in women of childbearing age. J Hum Nutr Diet 21, 159–164. [DOI] [PubMed] [Google Scholar]
- 61. Knight CA, Knight I, Mitchell DC et al. (2004) Beverage caffeine intake in US consumers and subpopulations of interest: estimates from the Share of Intake Panel survey. Food Chem Toxicol 42, 1923–1930. [DOI] [PubMed] [Google Scholar]
- 62. Cnattingius S (2004) The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res 6, Suppl. 2, S125–S140. [DOI] [PubMed] [Google Scholar]
- 63. Pineles BL, Park E & Samet JM (2014) Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol 179, 807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dietz PM, Homa D, England LJ et al. (2011) Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol 173, 355–359. [DOI] [PubMed] [Google Scholar]
- 65. Weathersbee PS & Lodge JR (1977) Caffeine: its direct and indirect influence on reproduction. J Reprod Med 19, 55–63. [PubMed] [Google Scholar]
- 66. Grosso LM, Rosenberg KD, Belanger K et al. (2001) Maternal caffeine intake and intrauterine growth retardation. Epidemiology 12, 447–455. [DOI] [PubMed] [Google Scholar]
- 67. Soyka LF (1979) Effects of methylxanthines on the fetus. Clin Perinatol 6, 37–51. [PubMed] [Google Scholar]
- 68. Buscicchio G, Piemontese M, Gentilucci L et al. (2012) The effects of maternal caffeine and chocolate intake on fetal heart rate. J Matern Fetal Neonatal Med 25, 528–530. [DOI] [PubMed] [Google Scholar]
- 69. Resch BA, Papp JG, Gyöngyösi J et al. (1985) Effect of caffeine on fetal heart rate and caffeine consumption habits in pregnant patients. Zentralbl Gynakol 107, 1249–1253. [PubMed] [Google Scholar]
- 70. Grosso LM & Bracken MB (2005) Caffeine metabolism, genetics, and perinatal outcomes: a review of exposure assessment considerations during pregnancy. Ann Epidemiol 15, 460–466. [DOI] [PubMed] [Google Scholar]
- 71. Olsen J, Overvad K & Frische G (1991) Coffee consumption, birthweight, and reproductive failures. Epidemiology 2, 370–374. [DOI] [PubMed] [Google Scholar]
- 72. Brent RL, Christian MS & Diener RM (2011) Evaluation of the reproductive and developmental risks of caffeine. Birth Defects Res B Dev Reprod Toxicol 92, 152–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen L-W, Wu Y, Neelakantan N et al. (2014) Maternal caffeine intake during pregnancy is associated with risk of low birth weight: a systematic review and dose–response meta-analysis. BMC Med 12, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980015002463.
click here to view supplementary material