Abstract
Objective
We aimed to evaluate the DHA and arachidonic acid (AA) levels in human breast milk worldwide by country, region and socio-economic status.
Design
Descriptive review conducted on English publications reporting breast-milk DHA and AA levels.
Setting
We systematically searched and identified eligible literature in PubMed from January 1980 to July 2015. Data on breast-milk DHA and AA levels from women who had given birth to term infants were included.
Subjects
Seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals.
Results
Worldwide mean levels of DHA and AA in breast milk were 0·37 (sd 0·11) % and 0·55 (sd 0·14) % of total fatty acids, respectively. The breast-milk DHA levels from women with accessibility to marine foods were significantly higher than those from women without accessibility (0·35 (sd 0·20) % v. 0·25 (sd 0·14) %, P<0·05). Data from the Asian region showed the highest DHA concentration but much lower AA concentration in breast milk compared with all other regions, independent of accessibility to marine foods. Comparison was made among Canada, Poland and Japan – three typical countries (each with sample size of more than 100 women) from different regions but all with high income and similar accessibility to fish/marine foods.
Conclusions
The current review provides an update on worldwide variation in breast-milk DHA and AA levels and underlines the need for future population- or region-specific investigations.
Keywords: Human breast milk, DHA, Arachidonic acid, Infant nutrition
Human breast milk is universally considered an optimal source of nutrition for term newborns( 1 ). The concentrations of DHA and arachidonic acid (AA) in breast milk have been extensively studied owing to their fundamental roles in neural and visual development( 2 , 3 ). Human milk is being used as a model to define acceptable intakes or recommendations for fatty acids in early life for normal infants( 4 ). According to recommendations made by the WHO/FAO, term infants should be exclusively breast-fed for the first 6 months of life and regular n-3 long-chain PUFA (LCP) should be provided to lactating women to ensure adequate LCP intake for infants( 5 ). The WHO/FAO recommended LCP intake for infants of 20 mg/kg body weight in 1994( 6 ); however, no optimal amount of LCP intake for infants was specified thereafter. The International Society for the Study of Fatty Acids and Lipids suggested the AA intake for infants should be above 0·5 % of total fat in 2008( 7 ). A Joint WHO/FAO Expert Consultation in the same year recommended that the minimum level of DHA+EPA intake was 0·3 g/d for pregnant or lactating women to ensure normal growth and development of the fetus or infant. AA was not essential for adults if the percentage of energy intake from linoleic acid was more than 2·5 % of total energy, and the upper limit for AA intake was 0·8 g/d for pregnant or lactating women( 8 ). In 2007, Brenna et al. reported that worldwide means of DHA and AA were 0·32 (sd 0·22) % and 0·47 (sd 0·13) % by extensively reviewing peer-reviewed publications( 1 ). However, it is evident that DHA and AA levels in human breast milk vary by country or population( 9 ). People residing near the sea may have higher breast-milk DHA levels than those living more inland( 10 ). This could be partly explained by disparate accessibility to fish or marine foods, a major source of DHA. Socio-economic status also contributes to food accessibility and therefore also shapes the dietary pattern of lactating women( 11 ). Additionally, genetic background has been linked with breast-milk fatty acid composition. For instance, variants of the FADS gene cluster were associated with different DHA and AA levels in breast milk at 1 month postpartum( 12 ). Hence, a worldwide mean of breast-milk fatty acid level may be not applicable for a specific population. In the current comprehensive literature review, we aim to estimate the DHA and AA levels in human breast milk by country, region, as well as country-specific income level, and discuss the possible factors contributing to discrepancies and similarities of data reported.
Methods
Inclusion criteria
A comprehensive literature search was performed in PubMed using ‘breast milk’ and ‘fatty acid’ as keywords. Publications in English from January 1980 to July 2015 were considered. Data included breast-milk DHA and AA levels from women who had given birth to term infants, and excluded those who met any of the following conditions: (i) participants under any dietary or lifestyle intervention; (ii) sample size ≤5; (iii) samples obtained from milk bank or pooled samples; or (iv) no information on lactation stage.
Data extraction and quality assessment
The composition of breast milk changes across the lactating period, with the most significant changes occurring in the first two weeks( 13 ). Therefore, only DHA and AA data obtained >14 d after delivery were included in the present study. In studies reporting data from multiple time points, each time point was included independently and the mean of these data was used. For studies examining the effects of dietary or lifestyle intervention on DHA and AA distribution in breast milk, only the baseline and control groups were included for analysis. In most circumstances, the means of DHA and AA were expressed as a percentage of total fatty acids by weight, while those in other units were excluded. All countries were divided into European region (EUR), African region (AFR), Eastern Mediterranean region (EMR), Asian region (ASR), North American region (NAR), South American region (SAR) and Oceania region (OCR) according to geographic location. The income level of each country was defined according to the World Bank income groups based on per capita Gross Domestic Product: low (≤$US 1005), lower-middle ($US 1006–3975), higher-middle ($US 3976–12 275) and high (≥$US 12 276)( 14 ). Accessibility to fish/marine foods was defined as ‘accessible’ if: (i) regular fish consumption was clearly stated in the original article; or (ii) sample collection was from island countries or other countries adjacent to lakes, rivers or sea. Otherwise, it was defined as ‘not accessible’. Unweighted means of fatty acid level for a specific country were calculated from single means reported from each study conducted in the country. Subsequently, the calculated mean for each specific country was used to get the unweighted fatty acid level for a specific region. The differences in unweighted means between different countries, regions or income levels were analysed using one-way ANOVA. All analyses were performed using the statistical software package SPSS for Windows version 16.0. Two-tailed P value <0·05 was considered significant. Weighted means were calculated by weighting according to the number of samples for each result.
Results
The characteristics of included studies are shown in Table 1. In total, seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals. Studies included thirty-three from Europe, seven from Africa, five from the Eastern Mediterranean, fourteen from Asia, seventeen from North America, five from South America and five from Australia. Twenty-one countries were classified as high income level, twelve countries as higher-middle income level, six countries as lower-middle income level, and two countries as low income level. Thirty-seven mean levels of DHA were obtained from participants with accessibility to fish or marine foods, while ninety-eight were from those without accessibility. The lactation stage of participants ranged from 2 weeks to 18 months postpartum.
Table 1.
Characteristics of the studies included in the present review
| Author and reference | WHO region* | Country | World Bank income level | Accessibility to fish or marine foods† | No. of samples | Lactation stage | AA (% of total fatty acids) | DHA (% of total fatty acids) |
|---|---|---|---|---|---|---|---|---|
| Rocquelin et al.( 31 ) | AFR | Congo | Lower-middle | A | 102 | 5 months | 0·44 | 0·55 |
| Glew et al.( 32 ) | Nigeria | Lower-middle | A | 13 | 1–4 months | 0·6 | 0·3 | |
| Ogunleye et al.( 33 ) | Nigeria | Lower-middle | A | 20 | 60–90 d | 0·56 | 0·34 | |
| Okolo et al.( 34 ) | Nigeria | Lower-middle | A | 15 | 6–7 months | 0·44 | 0·33 | |
| Nyuar et al.( 10 ) | Sudan | Lower-middle | N | 32 | 25–30 d | 0·6 | 0·1 | |
| van der Westhuyzen et al.( 35 ) | South Africa | Higher-middle | A | 12 | 6 months | 0·6 | 0·2 | |
| A | 18 | 6 months | 1·0 | 0·1 | ||||
| Luxwolda et al.( 19 ) | Tanzania | Low | A | 47 | 3 months | – | 0·68 | |
| A | 20 | 3 months | – | 0·96 | ||||
| Marín et al.( 36 ) | SAR | Argentina | Higher-middle | A | 21 | 1–3 months | 0·45 | 0·13 |
| Martin et al.( 37 ) | Bolivia | Lower-middle | A | 35 | 1–3 months | 1·06 | 0·69 | |
| Nishimura et al.( 38 ) | Brazil | Higher-middle | N | 47 | 15 weeks | 0·48 | – | |
| Silva et al.( 39 ) | Brazil | Higher-middle | N | 80 | 4–13 weeks | 0·53 | 0·14 | |
| Yuhas et al.( 9 ) | Chile | Higher-middle | A | 50 | ≥1 month | 0·42 | 0·43 | |
| Innis et al.( 40 ) | NAR | Canada | High | A | 17 | 1–3 months | 0·5 | 0·2 |
| Ratnayake et al.( 41 ) | Canada | High | A | 198 | 3–4 weeks | 0·35 | 0·14 | |
| Yuhas et al.( 9 ) | Canada | High | A | 48 | ≥1 month | 0·37 | 0·17 | |
| Krasevec et al.( 42 ) | Cuba | Higher-middle | A | 52 | 2 months | 0·67 | 0·43 | |
| Van Beusekom et al.( 43 ) | Dominica | Higher-middle | A | 7 | 20–22 d | 0·5 | 0·4 | |
| Yuhas et al.( 9 ) | Mexico | Higher-middle | A | 46 | ≥1 month | 0·42 | 0·26 | |
| Rueda et al.( 44 ) | Panama | – | A | 8 | 16–35 d | 0·52 | 0·32 | |
| Auestad et al.( 45 ) | USA | High | N | 43 | 4 months | 0·51 | 0·12 | |
| Bitman et al.( 46 ) | USA | High | N | 6 | 42 d | 0·60 | 0·23 | |
| Bopp et al.( 47 ) | USA | High | A | 22 | 12 months | 0·41 | 0·21 | |
| A | 30 | 12 months | 0·38 | 0·43 | ||||
| Francois et al.( 48 ) | USA | High | A | 14 | 6 months | 0·5 | 0·2 | |
| Francois et al.( 49 ) | USA | High | A | 7 | 2–11 months | 0·4 | 0·2 | |
| Glew et al.( 50 ) | USA | High | N | 19 | 1–6 months | 0·29 | 0·1 | |
| Jensen et al.( 51 ) | USA | High | N | 77 | 4 months | 0·44 | 0·2 | |
| Jensen et al.( 52 ) | USA | High | N | 6 | 2 months | 0·53 | 0·19 | |
| Martin et al.( 37 ) | USA | High | A | 35 | 1–3 months | 0·55 | 0·16 | |
| Putnam et al.( 53 ) | USA | High | A | 9 | 8 weeks | 0·6 | 0·1 | |
| Specker et al.( 54 ) | USA | High | A | 7 | 2 months | 0·69 | 0·29 | |
| Yuhas et al.( 9 ) | USA | High | A | 49 | ≥1 month | 0·45 | 0·17 | |
| Bahrami & Rahimi( 55 ) | EMR | Iran | Higher-middle | N | 52 | 6–19 weeks | 1·4 | – |
| Hayat et al.( 56 ) | Kuwait | High | A | 19 | 6–14 weeks | 0·54 | 0·60 | |
| Budowski et al.( 57 ) | Israel | High | A | 26 | 6–10 weeks | 0·59 | 0·38 | |
| Saphier et al.( 58 ) | Israel | High | A | 29 | 3–4 months | 0·44 | 0·17 | |
| Smit et al.( 59 ) | Israel | High | A | 10 | 3–10 months | 0·46 | 0·13 | |
| Jørgensen et al.( 60 ) | EUR | Denmark | High | A | 39 | 4 months | 0·30 | 0·35 |
| Jørgensen et al.( 61 ) | Denmark | High | A | 16 | 1 months | 0·56 | 0·49 | |
| A | 17 | 2 months | 0·47 | 0·43 | ||||
| A | 14 | 4 months | 0·44 | 0·53 | ||||
| Lauritzen et al.( 62 ) | Denmark | High | A | 14 | 1 month | 0·6 | 0·51 | |
| Luukkainen et al.( 63 ) | Finland | High | A | 9 | 4 weeks | 0·49 | 0·3 | |
| A | 16 | 12 weeks | 0·33 | 0·18 | ||||
| A | 14 | 26 weeks | 0·28 | 0·18 | ||||
| Mäkelä et al.( 64 ) | Finland | High | A | 51 | 3 months | 0·37 | 0·22 | |
| A | 49 | 3 months | 0·39 | 0·46 | ||||
| Martin et al.( 65 ) | France | High | A | 24 | 30 d | 0·36 | 0·24 | |
| Maurage et al.( 66 ) | France | High | A | 15 | 6 weeks | 0·24 | 0·14 | |
| Harzer et al.( 13 ) | Germany | High | N | 15 | 3 weeks | 0·36 | 0·15 | |
| N | 14 | 4 weeks | 0·39 | 0·16 | ||||
| N | 15 | 5 weeks | 0·39 | 0·16 | ||||
| Antonakou et al.( 20 ) | Greece | High | A | 64 | 1 months | 1·08 | 0·55 | |
| A | 39 | 3 months | 0·89 | 0·45 | ||||
| A | 24 | 6 months | 0·67 | 0·52 | ||||
| Decsi et al.( 67 ) | Hungary | High | N | 15 | 4 months | 0·51 | 0·18 | |
| Kovács et al.( 68 ) | Hungary | High | N | 10 | 3 weeks | 0·33 | 0·11 | |
| Mihályi et al.( 69 ) | Hungary | High | N | 61 | 6 weeks | 0·53 | 0·14 | |
| N | 46 | 6 months | 0·46 | 0·12 | ||||
| Minda et al.( 70 ) | Hungary | High | N | 18 | 4 weeks | 0·59 | 0·19 | |
| Olafsdottir et al.( 71 ) | Iceland | High | A | 59 | 2–4 months | 0·32 | 0·30 | |
| Marangoni et al.( 72 ) | Italy | High | A | 73 | 3 months | 0·50 | 0·35 | |
| Marangoni et al.( 73 ) | Italy | High | A | 10 | 1 months | 0·64 | 0·3 | |
| A | 10 | 3 months | 0·54 | 0·25 | ||||
| A | 10 | 6 months | 0·50 | 0·28 | ||||
| A | 10 | 9 months | 0·51 | 0·25 | ||||
| A | 10 | 12 months | 0·50 | 0·34 | ||||
| Huisman et al.( 74 ) | Netherlands | High | A | 25 | 42 d | 0·34 | 0·19 | |
| A | 99 | 89 d | 0·34 | 0·19 | ||||
| Helland et al.( 75 ) | Norway | High | A | 46 | 1–3 months | 0·38 | – | |
| A | 36 | 1–3 months | 0·42 | 0·43 | ||||
| Szlagatys-Sidorkiewicz et al.( 76 ) | Poland | High | A | 136 | 17–30 d | 0·47 | 0·33 | |
| Rueda et al.( 44 ) | Spain | High | A | 8 | 16–35 d | 0·69 | 0·38 | |
| Sala-Vila et al.( 77 ) | Spain | High | A | 10 | 15–30 d | 0·49 | 0·31 | |
| Sala-Vila et al.( 78 ) | Spain | High | A | 19 | 15–30 d | 0·41 | 0·18 | |
| Sala-Vila et al.( 79 ) | Spain | High | A | 11 | 3 months | 0·41 | 0·28 | |
| Jørgensen et al.( 61 ) | Sweden | High | A | 14 | 4 months | 0·44 | 0·53 | |
| A | 17 | 2 months | 0·47 | 0·43 | ||||
| A | 16 | 1 month | 0·59 | 0·49 | ||||
| Storck Lindholm et al.( 80 ) | Sweden | High | A | 19 | 1 month | 0·33 | 0·37 | |
| A | 17 | 2 months | 0·32 | 0·41 | ||||
| A | 17 | 1 month | 0·37 | 0·24 | ||||
| A | 14 | 2 months | 0·33 | 0·22 | ||||
| Xiang et al.( 81 ) | Sweden | High | A | 19 | 3 months | 0·08 | 0·12 | |
| Xiang et al.( 82 ) | Sweden | High | A | 19 | 1 months | 0·42 | 0·28 | |
| A | 19 | 3 months | 0·38 | 0·25 | ||||
| Yu et al.( 83 ) | Sweden | High | A | 17 | 1 month | 0·46 | 0·29 | |
| A | 17 | 3 months | 0·48 | 0·24 | ||||
| A | 17 | 4 months | 0·44 | 0·23 | ||||
| A | 17 | 6 months | 0·34 | 0·18 | ||||
| Weiss et al.( 84 ) | Switzerland | High | N | 16 | 16–20 d | 1·79 | 0·71 | |
| N | 6 | 21–25 d | 1·49 | 0·59 | ||||
| N | 7 | 26–30 d | 1·48 | 0·56 | ||||
| Samur et al.( 29 ) | Turkey | Higher-middle | N | 50 | 12–16 weeks | 0·46 | 0·15 | |
| Aydin et al.( 85 ) | Turkey | Higher-middle | N | 15 | 28 d | 1·81 | 0·52 | |
| Yuhas et al.( 9 ) | UK | High | A | 44 | ≥1 month | 0·36 | 0·24 | |
| Glew et al.( 86 ) | ASR | Nepal | Low | N | 36 | 2·9 months | 0·43 | 0·23 |
| Lee et al.( 87 ) | Sri Lanka | Lower-middle | A | 47 | 6–12 weeks | 0·39 | 0·53 | |
| A | 44 | 6–12 weeks | 0·36 | 0·37 | ||||
| A | 45 | 6–12 weeks | 0·39 | 0·79 | ||||
| Chen et al.( 88 ) | China | Higher-middle | N | 33 | 4 weeks | 0·79 | 0·54 | |
| N | 33 | 6 weeks | 0·53 | 0·35 | ||||
| A | 51 | 4 weeks | 0·56 | 0·53 | ||||
| A | 51 | 6 weeks | 0·52 | 0·48 | ||||
| Dodge et al. ( 89 ) | China | Higher-middle | N | 10 | 2–18 months | 0·52 | 0·22 | |
| N | 10 | 2–18 months | 0·63 | 0·28 | ||||
| N | 9 | 2–18 months | 0·35 | 0·15 | ||||
| Huang et al.( 21 ) | China | Higher-middle | A | 42 | 42 d | 0·93 | 0·98 | |
| Li et al.( 90 ) | China | Higher-middle | A | 25 | 3 weeks | 0·54 | 0·39 | |
| A | 25 | 3 weeks | 0·56 | 0·42 | ||||
| N | 25 | 3 weeks | 0·63 | 0·35 | ||||
| N | 11 | 3 weeks | 0·73 | 0·51 | ||||
| N | 11 | 3 weeks | 0·54 | 0·29 | ||||
| Peng et al.( 91 ) | China | Higher-middle | A | 45 | 42 d | 0·49 | 0·27 | |
| Urwin et al.( 22 ) | China | Higher-middle | N | 42 | 2–4 weeks | 0·85 | 0·53 | |
| A | 42 | 2–4 weeks | 0·8 | 0·53 | ||||
| N | 41 | 2–4 weeks | 0·82 | 0·35 | ||||
| Wan et al.( 92 ) | China | Higher-middle | N | 52 | 9–12 weeks | 0·3 | 0·19 | |
| Xiang et al.( 93 ) | China | Higher-middle | N | 18 | 4 weeks | 0·63 | 0·33 | |
| N | 23 | 12 weeks | 0·51 | 0·18 | ||||
| Yuhas et al.( 9 ) | China | Higher-middle | N | 50 | 1–12 months | 0·49 | 0·35 | |
| Ogunleye et al.( 33 ) | Japan | High | A | 53 | 70–100 d | 0·36 | 0·53 | |
| Yuhas et al.( 9 ) | Japan | High | A | 51 | ≥1 month | 0·40 | 0·99 | |
| Kneebone et al.( 94 ) | Malaysia | Higher-middle | A | 26 | 1–6 months | 0·47 | 0·90 | |
| A | 10 | 1–6 months | 0·57 | 0·9 | ||||
| A | 15 | 1–6 months | 0·64 | 0·71 | ||||
| Yuhas et al.( 9 ) | Philippines | Lower-middle | A | 54 | ≥1 month | 0·39 | 0·74 | |
| Gibson & Kneebone( 95 ) | OCR | Australia | High | A | 61 | 6 weeks | 0·40 | 0·32 |
| Makrides et al.( 96 ) | Australia | High | A | 23 | 6 weeks | 0·45 | 0·26 | |
| A | 23 | 16 weeks | 0·40 | 0·21 | ||||
| A | 23 | 30 weeks | 0·39 | 0·19 | ||||
| Makrides et al.( 97 ) | Australia | High | A | 23 | 4 months | 0·40 | 0·21 | |
| Stoney et al.( 98 ) | Australia | High | A | 36 | 1 months | 0·38 | 0·26 | |
| Yuhas et al.( 9 ) | Australia | High | A | 48 | ≥1 month | 0·38 | 0·23 |
AFR, African region; SAR, South American region; NAR, North American region; EMR, Eastern Mediterranean region; EUR, European region; ASR, Asian region; OCR, Oceania region.
A, accessible; N, not accessible.
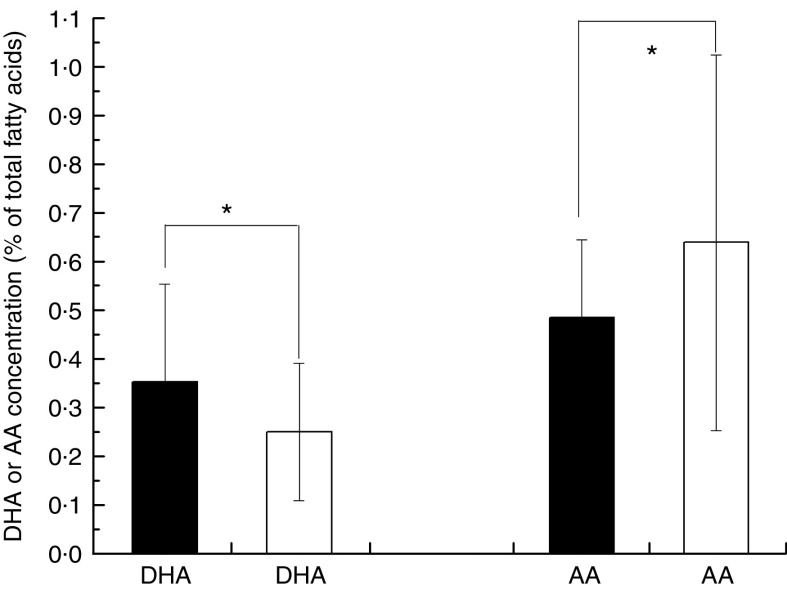
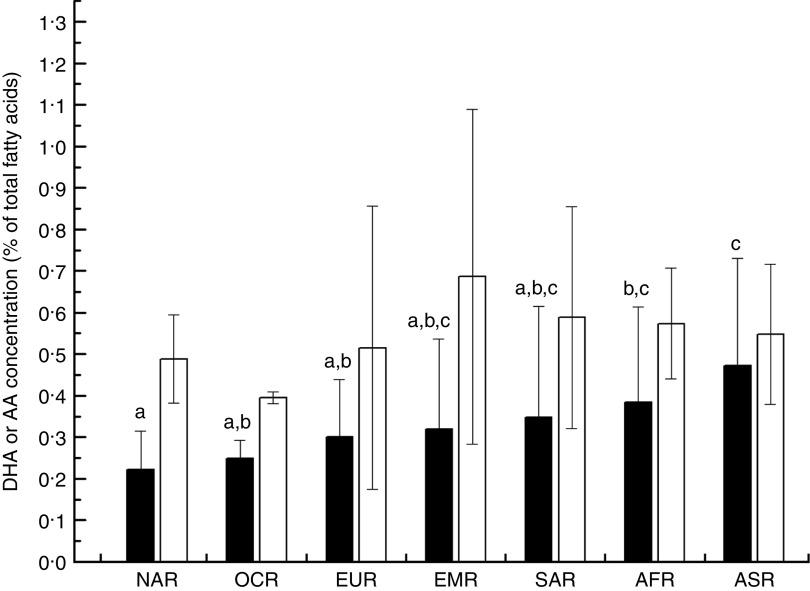
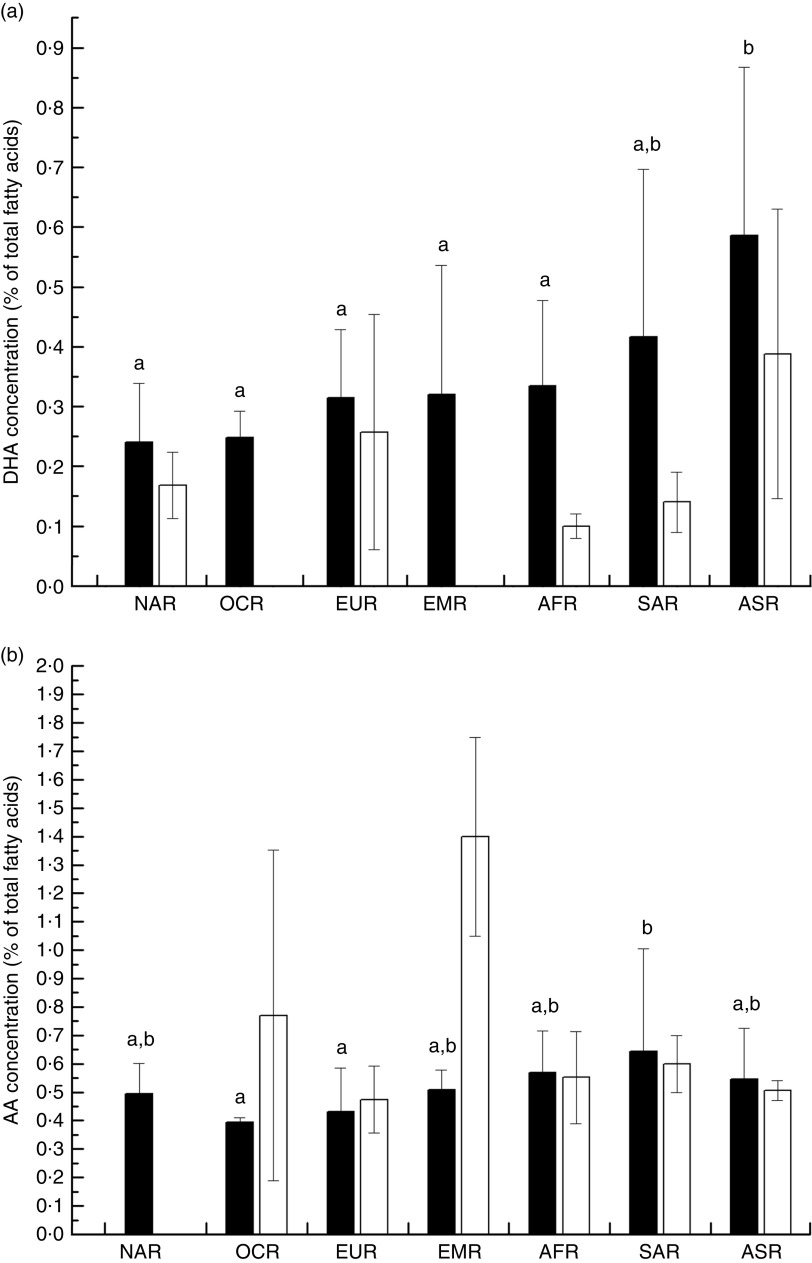
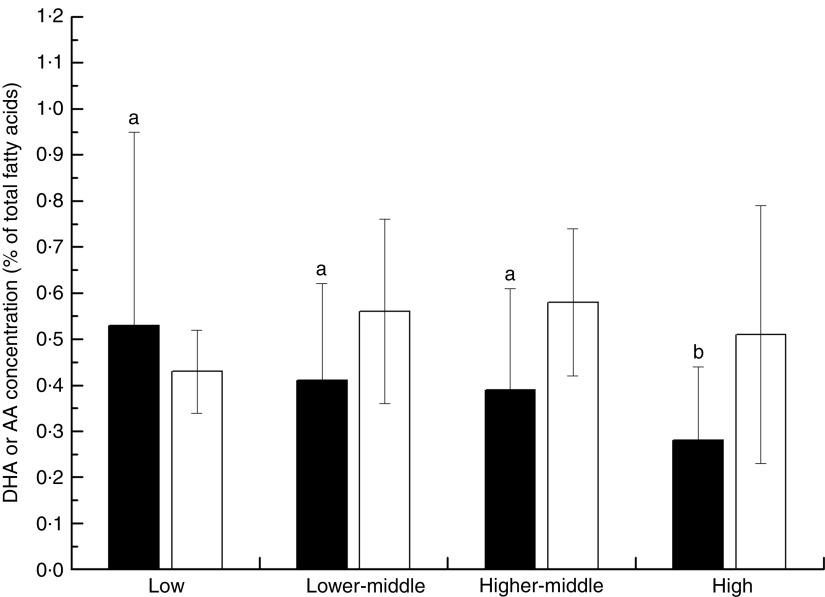
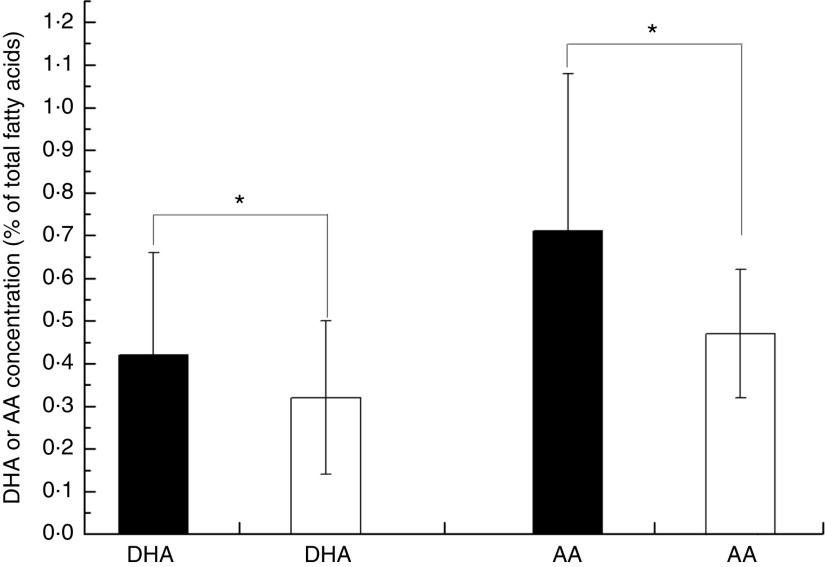
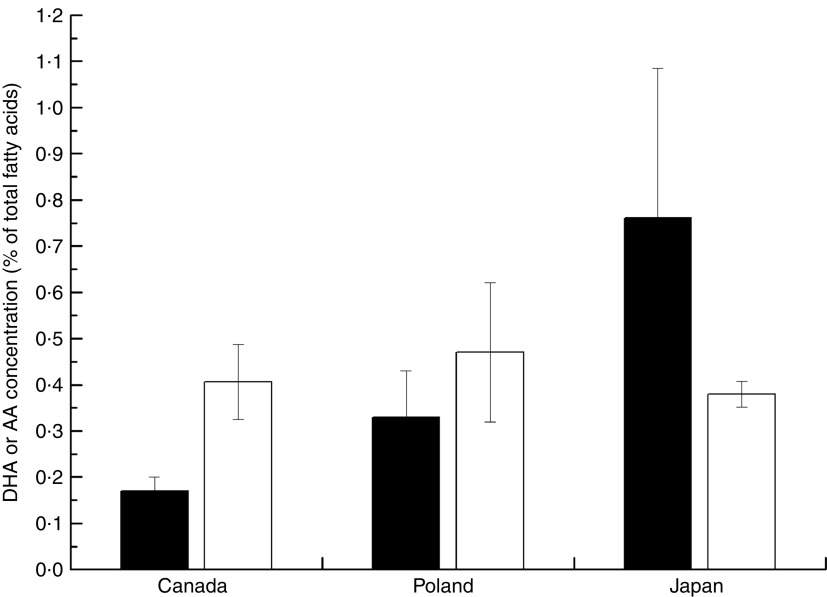
Overall, the unweighted means of DHA and AA in breast milk were 0·37 (sd 0·11) % and 0·55 (sd 0·14) %, respectively. The weighted means of DHA and AA were 0·35 (sd 0·11) % and 0·50 (sd 0·12) %, respectively, which represented a small deviation of less than 0·10 % from our reported value. The country-specific means of DHA and AA are presented in Fig. 1. The mean breast-milk DHA levels ranged from 0·10 % in Sudan to 0·84 % in Malaysia. The breast-milk DHA levels from women with accessibility to marine foods were significantly higher than those from women without accessibility (0·35 (sd 0·20) % v. 0·25 (sd 0·14) %, P<0·05; Fig. 2). The levels of AA were relatively constant among regions, with the exception of Iran and Switzerland (beyond 3 sd above the mean value). Breast milk DHA and AA levels are illustrated by region in Fig. 3. Overall, both DHA and AA levels differed regionally. DHA levels were higher for Asian women and lower for Australian and North American women, while AA levels were relatively high for Eastern Mediterranean women and lower for Australian and Asian women. In the subgroup analysis, samples from Asian countries showed the highest DHA levels but much lower AA levels compared with other regions, independent of accessibility to fish/marine foods (Fig. 4). Breast-milk DHA and AA levels are illustrated by World Bank income groups in Fig. 5. Samples from high-income countries exhibited significantly low DHA concentrations in comparison with samples from other countries (P<0·05). However, over 90 % of the data were collected from countries with high or higher-middle income, making a conclusive statement based on income variation difficult. As a previous review on this topic was published in 2007, we compared the collected data published after 2007 with those published before 2007. The means of DHA and AA concentration in breast milk published since 2007 (DHA, 0·42 (sd 0·24) %; AA, 0·71 (sd 0·37) %) were significantly higher (P<0·05) than those reported before 2007 (DHA, 0·32 (sd 0·18) %; AA, 0·47 (sd 0·15) %; Fig. 6). DHA and AA have been most extensively studied in Canada, Poland and Japan (each with samples size of more than 100 women), all three countries from three different regions but with high income and accessibility to fish/marine foods. The breast-milk fatty acid levels, especially DHA level, of women from those countries differed considerably from each other, and confirmed higher DHA level and lower AA level in Asian women’s breast milk (Fig. 7).
Fig. 1.
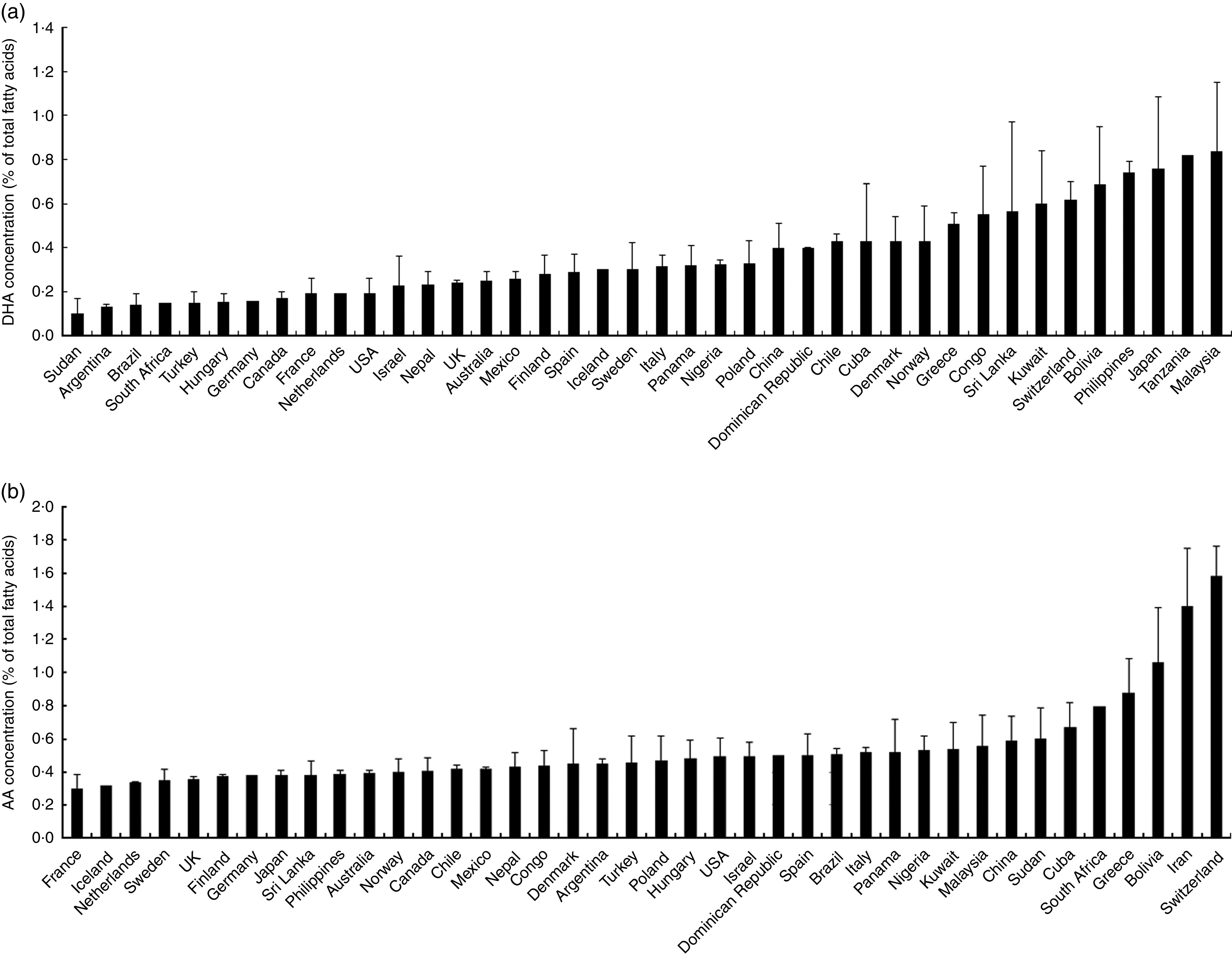
Country-specific levels of breast-milk DHA (a) and arachidonic acid (AA; b) worldwide. Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals)
Fig. 2.
Worldwide levels of breast-milk DHA and arachidonic acid (AA) by accessibility to marine foods:  , with accessibility;
, with accessibility;  , without accessibility. Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals). Mean values were significantly different according to accessibility: *P<0·05
, without accessibility. Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals). Mean values were significantly different according to accessibility: *P<0·05
Fig. 3.
Worldwide region-specific levels of breast-milk DHA ( ) and arachidonic acid (AA;
) and arachidonic acid (AA; ): NAR, North American region; OCR, Oceania region; EUR, European region; EMR, Eastern Mediterranean region; SAR, South American region; AFR, African region; ASR, Asian region. Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals). a,b,cMean DHA values with unlike superscript letters were significantly different (P<0·05)
): NAR, North American region; OCR, Oceania region; EUR, European region; EMR, Eastern Mediterranean region; SAR, South American region; AFR, African region; ASR, Asian region. Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals). a,b,cMean DHA values with unlike superscript letters were significantly different (P<0·05)
Fig. 4.
Subgroup analysis for worldwide region-specific levels of breast-milk DHA (a) and arachidonic acid (AA; b) by accessibility to marine foods:  , with accessibility;
, with accessibility;  , without accessibility; NAR, North American region; OCR, Oceania region; EUR, European region; EMR, Eastern Mediterranean region; AFR, African region; SAR, South American region; ASR, Asian region. Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals). a,bMean DHA or AA values with unlike superscript letters were significantly different (P<0·05)
, without accessibility; NAR, North American region; OCR, Oceania region; EUR, European region; EMR, Eastern Mediterranean region; AFR, African region; SAR, South American region; ASR, Asian region. Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals). a,bMean DHA or AA values with unlike superscript letters were significantly different (P<0·05)
Fig. 5.
Worldwide levels of breast-milk DHA ( ) and arachidonic acid (AA;
) and arachidonic acid (AA;  ) by World Bank income classification. Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals). a,bMean DHA values with unlike superscript letters were significantly different (P<0·05)
) by World Bank income classification. Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals). a,bMean DHA values with unlike superscript letters were significantly different (P<0·05)
Fig. 6.
Worldwide levels of breast-milk DHA and arachidonic acid (AA) reported before ( ) and after 2007 (
) and after 2007 ( ). Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals). Mean values were significantly different before and after 2007: *P<0·05
). Values are means with their standard deviation represented by vertical bars (seventy-eight studies from forty-one countries were included with 4163 breast-milk samples of 3746 individuals). Mean values were significantly different before and after 2007: *P<0·05
Fig. 7.
Breast-milk levels of DHA ( ) and arachidonic acid (AA;
) and arachidonic acid (AA;  ) in Japan, Canada and Poland. Values are means with their standard deviation represented by vertical bars (three studies with 263 samples of 263 individuals were included from Canada; one study with 136 samples of 136 individuals was included from Poland; and two studies with 104 samples of 104 individuals were included from Japan)
) in Japan, Canada and Poland. Values are means with their standard deviation represented by vertical bars (three studies with 263 samples of 263 individuals were included from Canada; one study with 136 samples of 136 individuals was included from Poland; and two studies with 104 samples of 104 individuals were included from Japan)
Discussion
Breast-milk composition, despite being one of the most important issues in infant nutrition, has not been detailed by region. Yuhas et al. reported the fatty acid composition of breast milk from nine countries located in America, Europe and the Western Pacific region( 9 ), while Brenna et al. reported a descriptive meta-analysis for worldwide breast-milk DHA and AA levels( 1 ). Both of them indicated highly variable breast-milk DHA and AA levels among different countries and that DHA concentration was much more variable than that of AA. The present review describes the variability and levels of breast-milk DHA and AA distributions by region. As global regions are characterized by differences in geographic distribution, population density/ethnicity and socio-economic status( 15 – 17 ), people from different regions may have varied dietary habits and genetic backgrounds, as well as prevalence of maternal or early-life burden of diseases( 17 , 18 ). Therefore, the reasons for the varied breast-milk levels of DHA and AA are multifactorial.
By collecting global data from 1980 to 2015, our analysis found unweighted mean levels of DHA and AA in human breast milk of 0·37 (sd 0·11) % and 0·55 (sd 0·14) %, with minimal difference from weighted means (0·02 % for DHA, 0·05 % for AA). These values are slightly higher than previously reported data by Brenna et al. in 2007( 1 ), which estimated unweighted means of 0·32 (sd 0·22) % and 0·47 (sd 0·13) % for DHA and AA, respectively. Of note, we found that the means of DHA and AA levels in studies published since 2007 (DHA, 0·42 (sd 0·24) %; AA, 0·71 (sd 0·37) %) are significantly higher (P<0·05) than those reported before 2007 (DHA, 0·32 (sd 0·18) %; AA, 0·47 (sd 0·15) %). Additionally, our review found that women with habitual fish/seafood intake, and/or dwelling near the river or sea, tend to have significantly high DHA levels compared with women without an indicator of good accessibility to fish or marine foods (P<0·05). Combining the mentioned two findings, the reportedly high consumption of fish or other seafood in Tanzania( 19 ), Greece( 20 ) and China( 21 , 22 ) after 2007 may help explain the discrepancies. On the other hand, we observed a relatively low level of DHA in the North America region (Fig. 2). This could be partly explained by the low breast-milk DHA levels of included data from inland, such as in the USA, where habitual seafood intake may be more moderate( 1 ). Therefore, these findings confirm that good accessibility to fish and seafood would likely contribute to breast-milk DHA level. It has been well accepted that maternal dietary habits can influence the nutrient composition of breast milk, especially DHA( 9 ). For those pregnant or lactating women without accessibility to marine foods, n-3 LCP supplementation should be encouraged to ensure normal growth and development of the fetus or infant( 8 ). Additionally, increased consumption of marine foods in Japanese, Malaysian, Philippine and south-eastern Chinese women who live near rivers or the sea may help explain the high DHA level in the Asian region. However, more studies are needed to explain the low level of DHA in Australia, where marine food consumption is also frequent.
Both DHA and AA can be synthesized endogenously by elongation and desaturation via elongases and desaturases from the dietary precursor α-linolenic acid and linoleic acid, respectively( 23 ). However, the correlation between dietary sources and breast-milk expression of AA is suggested to be lower than that of DHA( 24 ). One human study indicated that the conversion rate of α-linolenic acid to its longer-chain metabolites was nearly ten times higher than that of linoleic acid to AA( 25 ), which may help explain the lower degree of variability of breast-milk AA level among different regions. Moreover, dietary linoleic acid may exert influences on brain function and cognition, not least because of the role it plays as a precursor of AA. Lassek and Gaulin( 26 ) reported a negative relationship in a sample of twenty-eight countries between breast-milk linoleic acid and test scores in mathematics, reading and science from the Program for International Student Assessment using population-level data, while breast-milk DHA acid in these countries was positively related to the test scores. The opposite relationships of breast-milk linoleic acid and DHA with cognitive performance in the study were attributed to the competition between them. Dietary linoleic acid may not only competitively interfere with the conversion of n-3 fatty acids into DHA, but also compete with DHA for incorporation into plasma phospholipids (immediate source of DHA in breast milk) and inclusion into brain phospholipids( 26 ). Therefore higher dietary linoleic acid among women may decrease the DHA concentration in breast milk and interfere in the beneficial effects of dietary DHA.
Socio-economic status has been considered a major factor in maternal and infant health( 17 , 27 ). However, studies addressing breast-milk composition from lower-income countries are quite limited. For example, less than 10 % of our collected data was from countries with low or lower-middle income due to unavailability of data. Interestingly, we observed that samples from high-income countries exhibited significantly lower DHA level compared with samples from low-income countries. Similarly, Michaelsen et al. found that breast-milk samples from lactating women in low- and middle-income countries generally do not have DHA levels that are considerably lower than those from high-income countries( 28 ). It should be noted that people from high-income countries tend to consume more processed foods and more trans-fat, which have been suggested to have a disturbing effect on long-chain fatty acid synthesis and metabolism( 29 ).
To control for socio-economic and dietary factors, data from Canada, Poland and Japan were used because these three countries represented different regions and were considered high income and with accessibility to marine foods. The results were consistent with a significantly higher DHA level and lower AA level in Asian countries compared with other regions. Therefore, other possible contributing factors such as the influence of different genetic backgrounds need further investigation. In a study among fifty-four Canadian lactating women, Xie and Innis observed that variations in rs174 553 and rs174 575 within the FADS gene cluster were associated with breast-milk levels of AA( 12 ). Moreover, in a later study among 463 German participants, similar associations were also reported between rs174 547 and rs174 556 within the FADS gene cluster and breast-milk AA concentration( 30 ). The effects of genetics or dietary modification on the composition of breast-milk fatty acids are worth further study.
Several limitations exist in the current study. By only including data of breast milk collected after two weeks postpartum, the DHA and AA levels in colostrum and transitional milk were not examined. Moreover, available data from lower-income countries were quite limited, so the observed DHA level among countries with different income levels may be biased.
Conclusion
Our study reports updated worldwide levels of breast-milk DHA and AA, highlighting relatively high levels of DHA and lower levels of AA in breast milk from women in the Asian region. Novel discrepancies were also confirmed among country-specific and income-stratified DHA and AA levels, which underlines global variations of DHA and AA distributions and the necessity for further population- or country-specific investigations of breast-milk DHA and AA levels.
Acknowledgements
Acknowledgements: The authors express their sincere appreciation to Jing Zhu, Huiling Wu and Quanmei Zhang from the Maternal & Infant Nutrition Research Department, Beingmate Research Institute, for their help in this study. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: L.C. and Y.F. conceived and designed the study. Y.F. and X.L. contributed to the data collection, data analyses and manuscript preparation. B.Z. and A.C.J. contributed to revising the present manuscript. All the authors approved the manuscript before submission. L.C. is responsible for the final content of the manuscript. Ethics of human subject participation: Not applicable.
References
- 1. Brenna JT, Varamini B, Jensen RG et al. (2007) Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr 85, 1457–1464. [DOI] [PubMed] [Google Scholar]
- 2. Willatts P, Forsyth JS, DiModugno MK et al. (1998) Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet 352, 688–691. [DOI] [PubMed] [Google Scholar]
- 3. Molloy C, Doyle LW, Makrides M et al. (2012) Docosahexaenoic acid and visual functioning in preterm infants: a review. Neuropsychol Rev 22, 425–437. [DOI] [PubMed] [Google Scholar]
- 4. Uauy R & Dangour AD (2009) Fat and fatty acid requirements and recommendations for infants of 0–2 years and children of 2–18 years. Ann Nutr Metab 55, 76–96. [DOI] [PubMed] [Google Scholar]
- 5. Food and Agriculture Organization of the United Nations/World Health Organization/United Nations University (2004) Human Energy Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation, Rome, 17–24 October 2001. FAO Food and Nutrition Technical Report Series no. 1. Rome: FAO. [Google Scholar]
- 6. Food and Agriculture Organization of the United Nations/World Health Organization (1994) Fats and Oils in Human Nutrition. Report of a Joint Expert Consultation, Rome, 19–26 October 1993. FAO Food and Nutrition Paper no. 57. Rome: FAO. [PubMed] [Google Scholar]
- 7. International Society for the Study of Fatty Acids and Lipids (2008) ISSFAL statement on dietary recommendations on LCPUFA in infant formula. Prostaglandins Leukot Essent Fatty Acids 78, 229. [DOI] [PubMed] [Google Scholar]
- 8. Brenna JT & Lapillonne A (2009) Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann Nutr Metab 55, 97–122. [DOI] [PubMed] [Google Scholar]
- 9. Yuhas R, Pramuk K & Lien EL (2006) Human milk fatty acid composition from nine countries varies most in DHA. Lipids 41, 851–858. [DOI] [PubMed] [Google Scholar]
- 10. Nyuar KB, Min Y, Dawood M et al. (2013) Regular consumption of Nile river fish could ameliorate the low milk DHA of Southern Sudanese women living in Khartoum City area. Prostaglandins Leukot Essent Fatty Acids 89, 65–69. [DOI] [PubMed] [Google Scholar]
- 11. Higginbottom GM, Vallianatos H, Forgeron J et al. (2014) Food choices and practices during pregnancy of immigrant women with high-risk pregnancies in Canada: a pilot study. BMC Pregnancy Childbirth 14, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie L & Innis SM (2008) Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr 138, 2222–2228. [DOI] [PubMed] [Google Scholar]
- 13. Harzer G, Haug M & Bindels JG (1986) Biochemistry of human milk in early lactation. Z Ernahrungswiss 25, 77–90. [DOI] [PubMed] [Google Scholar]
- 14. World Bank (2015) Country and lending groups. http://data.worldbank.org/about/country-and-lending-groups (accessed July 2015).
- 15. Hatou K (2014) Mental Health Atlas 2011 (WHO). Seishin Shinkeigaku Zasshi 116, 267. [PubMed] [Google Scholar]
- 16. Yoshikura H (2013) Population size/density dependency hypothesis for measles epidemic: application of the hypothesis to countries in five WHO regions. Jpn J Infect Dis 66, 165–171. [DOI] [PubMed] [Google Scholar]
- 17. Bilano VL, Ota E, Ganchimeg T et al. (2014) Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PLoS One 9, e91198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS et al. (2014) Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luxwolda MF, Kuipers RS, Koops JH et al. (2014) Interrelationships between maternal DHA in erythrocytes, milk and adipose tissue. Is 1 wt% DHA the optimal human milk content? Data from four Tanzanian tribes differing in lifetime stable intakes of fish. Br J Nutr 111, 854–866. [DOI] [PubMed] [Google Scholar]
- 20. Antonakou A, Skenderi KP, Chiou A et al. (2013) Breast milk fat concentration and fatty acid pattern during the first six months in exclusively breastfeeding Greek women. Eur J Nutr 52, 963–973. [DOI] [PubMed] [Google Scholar]
- 21. Huang HL, Chuang LT, Li HH et al. (2013) Docosahexaenoic acid in maternal and neonatal plasma phospholipids and milk lipids of Taiwanese women in Kinmen: fatty acid composition of maternal blood, neonatal blood and breast milk. Lipids Health Dis 12, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Urwin HJ, Zhang J, Gao Y et al. (2013) Immune factors and fatty acid composition in human milk from river/lake, coastal and inland regions of China. Br J Nutr 109, 1949–1961. [DOI] [PubMed] [Google Scholar]
- 23. Sprecher H (1999) An update on the pathways of polyunsaturated fatty acid metabolism. Curr Opin Clin Nutr Metab Care 2, 135–138. [DOI] [PubMed] [Google Scholar]
- 24. Lauritzen L, Fewtrell M & Agostoni C (2015) Dietary arachidonic acid in perinatal nutrition: a commentary. Pediatr Res 77, 263–269. [DOI] [PubMed] [Google Scholar]
- 25. Emken EA (1994) Metabolism of dietary stearic acid relative to other fatty acids in human subjects. Am J Clin Nutr 60, 6 Suppl., 1023S–1028S. [DOI] [PubMed] [Google Scholar]
- 26. Lassek WD & Gaulin SJ (2014) Linoleic and docosahexaenoic acids in human milk have opposite relationships with cognitive test performance in a sample of 28 countries. Prostaglandins Leukot Essent Fatty Acids 91, 195–201. [DOI] [PubMed] [Google Scholar]
- 27. Tzioumis E & Adair LS (2014) Childhood dual burden of under- and overnutrition in low- and middle-income countries: a critical review. Food Nutr Bull 35, 230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michaelsen KF, Dewey KG, Perez-Exposito AB et al. (2011) Food sources and intake of n-6 and n-3 fatty acids in low-income countries with emphasis on infants, young children (6–24 months), and pregnant and lactating women. Matern Child Nutr 7, 124–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Samur G, Topcu A & Turan S (2009) Trans fatty acids and fatty acid composition of mature breast milk in Turkish women and their association with maternal diets. Lipids 44, 405–413. [DOI] [PubMed] [Google Scholar]
- 30. Lattka E, Rzehak P & Szabó É (2011) Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am J Clin Nutr 93, 382–391. [DOI] [PubMed] [Google Scholar]
- 31. Rocquelin G, Tapsoba S & Dop MC (1998) Lipid content and essential fatty acid (EFA) composition of mature Congolese breast milk are influenced by mothers’ nutritional status: impact on infants’ EFA supply. Eur J Clin Nutr 52, 164–171. [DOI] [PubMed] [Google Scholar]
- 32. Glew RH, Omene JA, Vignetti S et al. (1995) Fatty acid composition of breast milk lipids of Nigerian women. Nutr Res 15, 477–489. [Google Scholar]
- 33. Ogunleye A, Fakoya AT, Niizeki S et al. (1991) Fatty acid composition of breast milk from Nigerian and Japanese women. J Nutr Sci Vitaminol (Tokyo) 37, 435–442. [DOI] [PubMed] [Google Scholar]
- 34. Okolo SN, VanderJagt TJ, Vu T et al. (2000) The fatty acid composition of human milk in northern Nigeria. J Hum Lact 16, 28–35. [DOI] [PubMed] [Google Scholar]
- 35. van der Westhuyzen J, Chetty N & Atkinson PM (1988) Fatty acid composition of human milk from South African black mothers consuming a traditional maize diet. Eur J Clin Nutr 42, 213–220. [PubMed] [Google Scholar]
- 36. Marín MC, Sanjurjo A, Rodrigo MA et al. (2005) Long-chain polyunsaturated fatty acids in breast milk in La Plata, Argentina: relationship with maternal nutritional status. Prostaglandins Leukot Essent Fatty Acids 73, 355–360. [DOI] [PubMed] [Google Scholar]
- 37. Martin MA, Lassek WD, Gaulin SJ et al. (2012) Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Matern Child Nutr 8, 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nishimura RY, Castro GS, Jordão AA et al. (2013) Breast milk fatty acid composition of women living far from the coastal area in Brazil. J Pediatr (Rio J) 89, 263–268. [DOI] [PubMed] [Google Scholar]
- 39. Silva McHL, Silva MTC, Brandão SCC et al. (2005) Fatty acid composition of mature breast milk in Brazilian women. Food Chem 93, 297–303. [Google Scholar]
- 40. Innis SM, Nelson CM, Rioux MF et al. (1994) Development of visual acuity in relation to plasma and erythrocyte omega-6 and omega-3 fatty acids in healthy term gestation infants. Am J Clin Nutr 60, 347–352. [DOI] [PubMed] [Google Scholar]
- 41. Ratnayake WM & Chen ZY (1996) Trans, n-3, and n-6 fatty acids in Canadian human milk. Lipids 31, Suppl., S279–S282. [DOI] [PubMed] [Google Scholar]
- 42. Krasevec JM, Jones PJ, Cabrera-Hernandez A et al. (2002) Maternal and infant essential fatty acid status in Havana, Cuba. Am J Clin Nutr 76, 834–844. [DOI] [PubMed] [Google Scholar]
- 43. van Beusekom CM, Nijeboer HJ, van der Veere CN et al. (1993) Indicators of long chain polyunsaturated fatty acid status of exclusively breastfed infants at delivery and after 20–22 days. Early Hum Dev 32, 207–218. [DOI] [PubMed] [Google Scholar]
- 44. Rueda R, Ramírez M, García-Salmerón JL et al. (1998) Gestational age and origin of human milk influence total lipid and fatty acid contents. Ann Nutr Metab 42, 12–22. [DOI] [PubMed] [Google Scholar]
- 45. Auestad N, Halter R, Hall RT et al. (2001) Growth and development in term infants fed long-chain polyunsaturated fatty acids: a double-masked, randomized, parallel, prospective, multivariate study. Pediatrics 108, 372–381. [DOI] [PubMed] [Google Scholar]
- 46. Bitman J, Wood L, Hamosh M et al. (1983) Comparison of the lipid composition of breast milk from mothers of term and preterm infants. Am J Clin Nutr 38, 300–312. [DOI] [PubMed] [Google Scholar]
- 47. Bopp M, Lovelady C, Hunter C et al. (2005) Maternal diet and exercise: effects on long-chain polyunsaturated fatty acid concentrations in breast milk. J Am Diet Assoc 105, 1098–1103. [DOI] [PubMed] [Google Scholar]
- 48. Francois CA, Connor SL, Wander RC et al. (1998) Acute effects of dietary fatty acids on the fatty acids of human milk. Am J Clin Nutr 67, 301–308. [DOI] [PubMed] [Google Scholar]
- 49. Francois CA, Connor SL, Bolewicz LC et al. (2023) Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr 77, 226–233. [DOI] [PubMed] [Google Scholar]
- 50. Glew RH, Wold RS, Corl B et al. (2011) Low docosahexaenoic acid in the diet and milk of American Indian women in New Mexico. J Am Diet Assoc 111, 744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jensen CL, Voigt RG, Prager TC et al. (2005) Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am J Clin Nutr 82, 125–132. [DOI] [PubMed] [Google Scholar]
- 52. Jensen CL, Maude M, Anderson RE et al. (2000) Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am J Clin Nutr 71, 1 Suppl., 292S–299S. [DOI] [PubMed] [Google Scholar]
- 53. Putnam JC, Carlson SE, DeVoe PW et al. (1982) The effect of variations in dietary fatty acids on the fatty acid composition of erythrocyte phosphatidylcholine and phosphatidylethanolamine in human infants. Am J Clin Nutr 36, 106–114. [DOI] [PubMed] [Google Scholar]
- 54. Specker BL, Wey HE & Miller D (1987) Differences in fatty acid composition of human milk in vegetarian and nonvegetarian women: long-term effect of diet. J Pediatr Gastroenterol Nutr 6, 764–768. [DOI] [PubMed] [Google Scholar]
- 55. Bahrami G & Rahimi Z (2005) Fatty acid composition of human milk in Western Iran. Eur J Clin Nutr 59, 494–497. [DOI] [PubMed] [Google Scholar]
- 56. Hayat L, al-Sughayer MA & Afzal M (1999) Fatty acid composition of human milk in Kuwaiti mothers. Comp Biochem Physiol B Biochem Mol Biol 124, 261–267. [DOI] [PubMed] [Google Scholar]
- 57. Budowski P, Druckmann H, Kaplan B et al. (1994) Mature milk from Israeli mothers is rich in polyunsaturated fatty acids. World Rev Nutr Diet 75, 105–108. [DOI] [PubMed] [Google Scholar]
- 58. Saphier O, Blumenfeld J, Silberstein T et al. (2013) Fatty acid composition of breastmilk of Israeli mothers. Indian Pediatr 50, 1044–1046. [DOI] [PubMed] [Google Scholar]
- 59. Smit EN, Koopmann M, Boersma ER et al. (2000) Effect of supplementation of arachidonic acid (AA) or a combination of AA plus docosahexaenoic acid on breastmilk fatty acid composition. Prostaglandins Leukot Essent Fatty Acids 62, 335–340. [DOI] [PubMed] [Google Scholar]
- 60. Jørgensen MH, Hernell O, Hughes E et al. (2001) Is there a relation between docosahexaenoic acid concentration in mothers’ milk and visual development in term infants? J Pediatr Gastroenterol Nutr 32, 293–296. [DOI] [PubMed] [Google Scholar]
- 61. Jørgensen MH, Hernell O, Lund P et al. (1996) Visual acuity and erythrocyte docosahexaenoic acid status in breast-fed and formula-fed term infants during the first four months of life. Lipids 31, 99–105. [DOI] [PubMed] [Google Scholar]
- 62. Lauritzen L, Halkjaer LB, Mikkelsen TB et al. (2006) Fatty acid composition of human milk in atopic Danish mothers. Am J Clin Nutr 84, 190–196. [DOI] [PubMed] [Google Scholar]
- 63. Luukkainen P, Salo MK, Nikkari T et al. (1994) Changes in the fatty acid composition of preterm and term human milk from 1 week to 6 months of lactation. J Pediatr Gastroenterol Nutr 18, 355–360. [DOI] [PubMed] [Google Scholar]
- 64. Mäkelä J, Linderborg K, Niinikoski H et al. (2013) Breast milk fatty acid composition differs between overweight and normal weight women: the STEPS Study. Eur J Nutr 52, 727–735. [DOI] [PubMed] [Google Scholar]
- 65. Martin JC, Bougnoux P, Fignon A et al. (1993) Dependence of human milk essential fatty acids on adipose stores during lactation. Am J Clin Nutr 58, 653–659. [DOI] [PubMed] [Google Scholar]
- 66. Maurage C, Guesnet P, Pinault M et al. (1998) Effect of two types of fish oil supplementation on plasma and erythrocyte phospholipids in formula-fed term infants. Biol Neonate 74, 416–429. [DOI] [PubMed] [Google Scholar]
- 67. Decsi T, Oláh S, Molnár S et al. (2000) Fatty acid composition of human milk in Hungary. Acta Paediatr 89, 1394–1395. [DOI] [PubMed] [Google Scholar]
- 68. Kovács A, Funke S, Marosvölgyi T et al. (2005) Fatty acids in early human milk after preterm and full-term delivery. J Pediatr Gastroenterol Nutr 41, 454–459. [DOI] [PubMed] [Google Scholar]
- 69. Mihályi K, Györei E, Szabó É et al. (2015) Contribution of n-3 long-chain polyunsaturated fatty acids to human milk is still low in Hungarian mothers. Eur J Pediatr 174, 393–398. [DOI] [PubMed] [Google Scholar]
- 70. Minda H, Kovács A, Funke S et al. (2004) Changes of fatty acid composition of human milk during the first month of lactation: a day-to-day approach in the first week. Ann Nutr Metab 48, 202–209. [DOI] [PubMed] [Google Scholar]
- 71. Olafsdottir AS, Thorsdottir I, Wagner KH et al. (2006) Polyunsaturated fatty acids in the diet and breast milk of lactating Icelandic women with traditional fish and cod liver oil consumption. Ann Nutr Metab 50, 270–276. [DOI] [PubMed] [Google Scholar]
- 72. Marangoni F, Agostoni C, Lammardo AM et al. (2000) Polyunsaturated fatty acid concentrations in human hindmilk are stable throughout 12-months of lactation and provide a sustained intake to the infant during exclusive breastfeeding: an Italian study. Br J Nutr 84, 103–109. [PubMed] [Google Scholar]
- 73. Marangoni F, Agostoni C, Lammardo AM et al. (2002) Polyunsaturated fatty acids in maternal plasma and in breast milk. Prostaglandins Leukot Essent Fatty Acids 66, 535–540. [DOI] [PubMed] [Google Scholar]
- 74. Huisman M, van Beusekom CM, Lanting CI et al. (1996) Triglycerides, fatty acids, sterols, mono- and disaccharides and sugar alcohols in human milk and current types of infant formula milk. Eur J Clin Nutr 50, 255–260. [PubMed] [Google Scholar]
- 75. Helland IB, Smith L, Saarem K et al. (2003) Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 111, e39–e44. [DOI] [PubMed] [Google Scholar]
- 76. Szlagatys-Sidorkiewicz A, Martysiak-Żurowska D, Krzykowski G et al. (2013) Maternal smoking modulates fatty acid profile of breast milk. Acta Paediatr 102, e353–e359. [DOI] [PubMed] [Google Scholar]
- 77. Sala-Vila A, Campoy C, Castellote AI et al. (2006) Influence of dietary source of docosahexaenoic and arachidonic acids on their incorporation into membrane phospholipids of red blood cells in term infants. Prostaglandins Leukot Essent Fatty Acids 74, 143–148. [DOI] [PubMed] [Google Scholar]
- 78. Sala-Vila A, Castellote AI, Rodriguez-Palmero M et al. (2005) Lipid composition in human breast milk from Granada (Spain): changes during lactation. Nutrition 21, 467–473. [DOI] [PubMed] [Google Scholar]
- 79. Sala-Vila A, Castellote AI, Campoy C et al. (2004) The source of long-chain PUFA in formula supplements does not affect the fatty acid composition of plasma lipids in full-term infants. J Nutr 134, 868–873. [DOI] [PubMed] [Google Scholar]
- 80. Storck Lindholm E, Strandvik B, Altman D et al. (2013) Different fatty acid pattern in breast milk of obese compared to normal-weight mothers. Prostaglandins Leukot Essent Fatty Acids 88, 211–217. [DOI] [PubMed] [Google Scholar]
- 81. Xiang M, Harbige LS & Zetterström R (2005) Long-chain polyunsaturated fatty acids in Chinese and Swedish mothers: diet, breast milk and infant growth. Acta Paediatr 94, 1543–1549. [DOI] [PubMed] [Google Scholar]
- 82. Xiang M, Alfvén G, Blennow M et al. (2000) Long-chain polyunsaturated fatty acids in human milk and brain growth during early infancy. Acta Paediatr 89, 142–147. [DOI] [PubMed] [Google Scholar]
- 83. Yu G, Duchén K & Björkstén B (1998) Fatty acid composition in colostrum and mature milk from non-atopic and atopic mothers during the first 6 months of lactation. Acta Paediatr 87, 729–736. [DOI] [PubMed] [Google Scholar]
- 84. Weiss GA, Troxler H, Klinke G et al. (2013) High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis 12, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Aydin İ, Turan Ö, Aydin FN et al. (2014) Comparing the fatty acid levels of preterm and term breast milk in Turkish women. Turk J Med Sci 44, 305–310. [PubMed] [Google Scholar]
- 86. Glew RH, Huang YS, Vander Jagt TA et al. (2001) Fatty acid composition of the milk lipids of Nepalese women: correlation between fatty acid composition of serum phospholipids and melting point. Prostaglandins Leukot Essent Fatty Acids 65, 147–156. [DOI] [PubMed] [Google Scholar]
- 87. Lee PS, Wickramasinghe VP, Lamabadusuriya SP et al. (2013) Breast milk DHA levels in Sri Lankan mothers vary significantly in three locations that have different access to dietary fish. Ceylon Med J 58, 51–55. [DOI] [PubMed] [Google Scholar]
- 88. Chen ZY, Kwan KY, Tong KK et al. (1997) Breast milk fatty acid composition: a comparative study between Hong Kong and Chongqing Chinese. Lipids 32, 1061–1067. [DOI] [PubMed] [Google Scholar]
- 89. Dodge ML, Wander RC, Xia Y et al. (1999) Glutathione peroxidase activity modulates fatty acid profiles of plasma and breast milk in Chinese women. J Trace Elem Med Biol 12, 221–230. [DOI] [PubMed] [Google Scholar]
- 90. Li J, Fan Y, Zhang Z et al. (2009) Evaluating the trans fatty acid, CLA, PUFA and erucic acid diversity in human milk from five regions in China. Lipids 44, 257–271. [DOI] [PubMed] [Google Scholar]
- 91. Peng YM, Zhang TY, Wang Q et al. (2007) Fatty acid composition in breast milk and serum phospholipids of healthy term Chinese infants during first 6 weeks of life. Acta Paediatr 96, 1640–1645. [DOI] [PubMed] [Google Scholar]
- 92. Wan ZX, Wang XL, Xu L et al. (2010) Lipid content and fatty acids composition of mature human milk in rural North China. Br J Nutr 103, 913–916. [DOI] [PubMed] [Google Scholar]
- 93. Xiang M, Lei S, Li T et al. (1999) Composition of long chain polyunsaturated fatty acids in human milk and growth of young infants in rural areas of northern China. Acta Paediatr 88, 126–131. [DOI] [PubMed] [Google Scholar]
- 94. Kneebone GM, Kneebone R & Gibson RA (1985) Fatty acid composition of breast milk from three racial groups from Penang, Malaysia. Am J Clin Nutr 41, 765–769. [DOI] [PubMed] [Google Scholar]
- 95. Gibson RA & Kneebone GM (1981) Fatty acid composition of human colostrum and mature breast milk. Am J Clin Nutr 34, 252–257. [DOI] [PubMed] [Google Scholar]
- 96. Makrides M, Simmer K, Neumann M et al. (1995) Changes in the polyunsaturated fatty acids of breast milk from mothers of full-term infants over 30 wk of lactation. Am J Clin Nutr 61, 1231–1233. [DOI] [PubMed] [Google Scholar]
- 97. Makrides M, Neumann M, Simmer K et al. (1995) Are long-chain polyunsaturated fatty acids essential nutrients in infancy? Lancet 345, 1463–1468. [DOI] [PubMed] [Google Scholar]
- 98. Stoney RM, Woods RK, Hosking CS et al. (2004) Maternal breast milk long-chain n-3 fatty acids are associated with increased risk of atopy in breastfed infants. Clin Exp Allergy 34, 194–200. [DOI] [PubMed] [Google Scholar]