Abstract
Objective
To summarize the best available evidence regarding the short- and long-term health effects of cow’s milk intake in healthy, full-term infants up to 3 years of age.
Design
We conducted a systematic review and meta-analysis.
Setting
We searched MEDLINE (via PubMed), EMBASE and the Cochrane Library between 1960 and July 2013 and manually reviewed reference lists of pertinent articles. Two researchers independently reviewed abstracts and full-text articles and extracted relevant data.
Subjects
We included (randomized/non-randomized) controlled trials and observational studies.
Results
We included data from twenty-three studies (one randomized controlled trial, four non-randomized controlled trials, eight case–control studies and ten cohort studies) for the evidence synthesis. Pooled results of four studies revealed a higher risk of Fe-deficiency anaemia for infants consuming cow’s milk compared with those consuming follow-on formula (relative risk=3·76; 95 % CI 2·73, 5·19). For type 1 diabetes mellitus, six out of seven case–control studies did not show a difference in the risk of developing this disease based on the age of introduction of cow’s milk. We did not find negative associations for other health effects.
Conclusions
Cow’s milk consumption in infancy is associated with an increased risk of developing Fe-deficiency anaemia. Limiting cow’s milk consumption may be important to ensure an adequate Fe intake for infants and toddlers. High-quality patient information for caregivers is needed on how infants’ Fe requirements can be met.
Keywords: Cow’s milk consumption, Infants, Systematic review
Breast-feeding is the natural and preferable way to provide ideal food for newborns and infants. Breast milk gives infants all the nutrients they need for healthy growth and development. Several nutrition committees recommend exclusive breast-feeding for the first 6 months of life( 1 – 3 ). Complementary feeding (i.e. solid foods and liquids other than breast milk or infant formula, and follow-on formula) should not be introduced before 17 weeks and after 26 weeks of age( 2 ).
One relevant aspect of infant feeding is the timing of the introduction of unmodified cow’s milk. Current recommendations on the optimal age for the introduction of cow’s milk into the infant diet in industrialized countries are conflicting. The WHO allows introducing cow’s milk simultaneously with complementary foods during the weaning period when babies are about 6 months or older( 4 ). Some recommendations allow limited cow’s milk consumption (i.e. small volumes to be added to complementary foods) before the age of 12 months( 2 , 5 , 6 ). However, there are other recommendations that strongly advise against cow’s milk consumption before 9–12 months of age( 7 ) or the age of 12 months( 8 , 9 ).
Although cow’s milk is a good source of protein, Ca and other nutrients( 10 , 11 ), concerns exist about cow’s milk consumption in early infancy due to possible negative health effects. Fe deficiency is one of the main issues to be considered when weighing the pros and cons of the effects of unmodified cow’s milk v. formula. Fe is a critical nutrient in the first year of life and besides the fact that cow’s milk is a poor source of Fe, it is known to inhibit the absorption of non-haem Fe through components like Ca and casein( 12 , 13 ). In addition, a child may require even more Fe than usual if he/she suffers from occult gastrointestinal blood loss which can occur after cow’s milk ingestion( 14 ). How much cow’s milk an infant ingested daily can become quite a significant question in light of Fe deficiency concerns. This is especially relevant if the infant is consuming greater amounts of cow’s milk in place of other complementary nutrient-rich foods that would be better suited to ensure adequate intake of all required nutrients( 8 , 15 ).
The main aim of the present review was to summarize the best available evidence for short- and long-term health effects of cow’s milk intake and to identify areas for future research. Our main research question was: what are the health effects of cow’s milk consumption in healthy, full-term infants up to 3 years of age? Further questions related to the timing of introduction and dose–response relationships.
Methods
We followed the guidelines of the Cochrane Collaboration for undertaking and reporting the results of the present systematic review( 16 ).
Search strategy
We identified references by searching electronic databases and reference lists from pertinent articles and existing guidelines on the topic. The search strategy was developed by the authors in conjunction with a senior information specialist. We searched the electronic databases MEDLINE (via PubMed), EMBASE and the Cochrane Library, between 1960 and July 2013, with a combination of MeSH (medical subject headings) terms and free-text keywords for relevant interventions (milk, milk products, follow-on formula, infant formula, breast milk) and outcomes. We limited the search to human studies in English or German language. The detailed search strategy is available in the online supplementary material. In addition, we checked reference lists from existing guidelines on cow’s milk consumption and pertinent articles.
All citations were imported to and managed in a bibliographic database (Endnote X·6·0·1) and duplicates were deleted.
Study eligibility criteria
Criteria for the consideration of studies for the present review were: (i) healthy infants aged between 17 weeks and 3 years; (ii) pasteurized animal milk, milk products or follow-on formula; (iii) patient-relevant outcomes; and (iv) randomized and non-randomized controlled studies and observational studies. These criteria are described in more detail in Table 1.
Table 1.
Eligibility criteria for inclusion of studies in the present systematic review and meta-analysis
| Population | Healthy, term-born infants aged between 17 weeks and 3 years living in countries with a population comparable to Europe, generally affluent populations |
| Interventions | For infants between 17 weeks and 12 months: pasteurized milk of animal origin (e.g. cow’s milk, goat’s milk, ewe’s milk, mare’s milk), milk products of animal origin (e.g. cheese, yoghurt, whey), or follow-on formula with a nutrient profile conforming to the EU directive( 59 ). Studies exclusively studying infant formula were excluded; for infants from 1 to 3 years: pasteurized milk or milk products of animal origin |
| Comparisons | For infants between 17 weeks and 12 months: breast milk or infant formula; for infants from 1 to 3 years: follow-on formula. Studies investigating partially and extensively hydrolysed formulas were excluded |
| Outcomes | We focused on patient-relevant outcomes (intestinal blood loss, Fe-deficiency anaemia, dehydration, obesity, osteoporosis, failure to thrive, type 1 diabetes mellitus, gastrointestinal diseases, atopic diseases), but also included surrogate parameters if patient-relevant outcomes were not available (Fe status, renal function, bone mineral content, anthropometric measurements, neurological development, stage of development) |
| Study designs | Randomized and non-randomized controlled trials, controlled prospective and retrospective cohort studies, case–control studies or other controlled studies (e.g. cross-sectional study with a comparison of at least two exposure groups) |
Study selection
Two researchers independently reviewed abstracts and full-text articles. If both reviewers agreed that the study did not meet eligibility criteria, we excluded it. Investigators resolved disagreements about inclusion or exclusion by consensus or by involving a third reviewer. When articles contained insufficient information to assess their eligibility or to extract relevant data, the corresponding author was contacted for further information. Studies reported only in abstract form were excluded.
Data extraction
Trained reviewers abstracted data from each included study into a structured data extraction sheet and assigned an initial quality rating. Investigators extracted data relating to: (i) the aims and duration of the study; (ii) observation period; (iii) study design and sample size; (iv) inclusion and exclusion criteria; (v) the intervention; (vi) outcome measurements; (vii) description of the study population; and (viii) results of the study relating to our research questions.
A senior reviewer evaluated completeness and correctness of data abstraction and confirmed the quality rating. Study authors were contacted to obtain details concerning the formula used.
Study quality
We evaluated the study quality for different study designs separately with standardized assessment forms. To assess the risk of bias of intervention studies, we used predefined criteria based on those developed by the Cochrane Collaboration( 16 ) and the Centre for Reviews and Dissemination of the University of York( 17 ). For the assessment of observational studies we applied the characteristics that were deemed essential by Deeks et al.( 18 ).
Data analyses
A meta-analysis of included studies was not justified for most outcomes; therefore we synthesized the evidence on the majority of outcomes qualitatively according to study design, exposure and health outcome. If data were sufficient, we conducted random-effects (DerSimonian and Laird) meta-analyses to estimate pooled effects. We used relative risks (RR) as outcome measures. We tested for heterogeneity with Cochrane’s Q test and used the I 2 index to estimate the magnitude of heterogeneity. We explored the impact of study duration on Fe-deficiency anaemia (IDA) using meta-regression. We determined publication bias employing funnel plots and Kendell’s test. Because of the small number of included studies and investigated children, results of these tests have to be viewed cautiously. We conducted all statistical analyses using the Comprehensive Meta Analysis software version 2·2·064.
Rating the quality of evidence
We rated the quality of evidence for each outcome based on the approach suggested by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group( 19 ). GRADE specifies four categories for the quality of a body of evidence: high, moderate, low and very low. According to the study design the basic rating is high for randomized controlled studies (RCT) and low for observational studies. Several criteria were then taken into account for down- or upgrading of the quality of evidence. For downgrading: risk of bias, inconsistency, indirectness, imprecision and publication bias; for upgrading: dose–response gradient, effect size and confounding in opposite direction( 20 – 23 ).
Public posting of protocol
The study protocol was posted on the project website (www.richtigessenvonanfangan.at) for two weeks in November 2010 to gather public comments.
Results
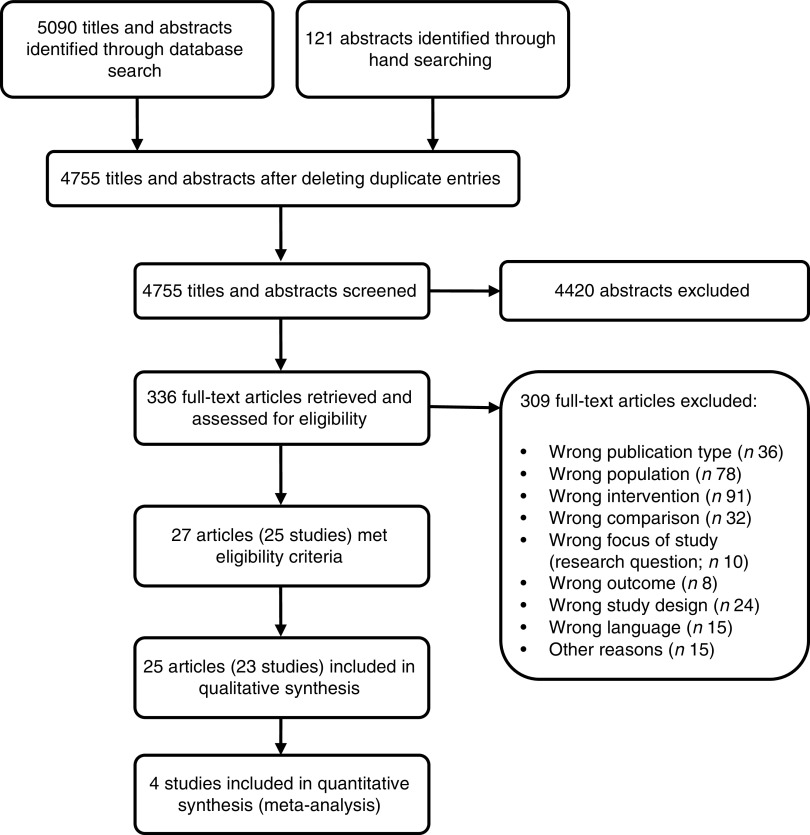
After screening 4755 titles and abstracts and assessing 336 full-text articles for eligibility, we identified twenty-seven publications (describing twenty-five studies) that met our inclusion criteria (see Fig. 1). Two studies were excluded from the qualitative analysis because of high risk of bias( 24 , 25 ). The remaining twenty-five publications (describing twenty-three studies of fair quality)( 26 – 50 ) investigated various health effects of cow’s milk intake and are presented in Table 2. Out of the twenty-three studies included in the review, one study was a randomized controlled trial (RCT)( 26 , 27 ), four were non-randomized controlled trials (nRCT)( 28 – 30 , 48 ), seven studies were prospective cohort studies( 31 , 34 – 36 , 45 , 47 , 49 , 50 ), three studies were retrospective cohort studies( 32 , 33 , 46 ) and eight were case–control studies( 37 – 44 ). In the following sections, we present the most important results. Table 2 provides a more detailed summary of the results of individual studies.
Fig. 1.
Flow diagram for the study selection process
Table 2.
Description of the studies investigating the health effects of cow’s milk intake
| Reference | Study design | Sample size/intervention and control group | Location | Duration | Outcome measures/assessment methods | Main results |
|---|---|---|---|---|---|---|
| IDA/Fe status | ||||||
| Capozzi et al. (2010)( 31 ) | Prospective cohort study | 385 E1 (cow’s milk*): 63 E2 (breast-feeding): 102 E3 (follow-on formula): 220† | Italy | 12 months (from birth to 12 months of age) | IDA (Hb<110 g/l & SF<15 ng/ml) | At 8 months: incidence of anaemia 48 % in E1, 39 % in E2, 11 % in E3 (P<0·05) At 12 months: incidence of anaemia 38 % in E1, 36 % in E2, 15 % in E3 (P<0·05) No stat. sign. diff. between E1 and E2 at 8 and 12 months |
| Daly et al. (1996)( 26 ) | RCT | 100 IG (cow’s milk): 50 CG (Fe-supplemented follow-on formula): 50 | UK | 12 months (from 6 to 18 months of age) | IDA (Hb<110 g/l) | At 18 months: frequency of anaemia 33 % in IG v. 2 % in CG (P<0·0001) |
| Fomon et al. (1981)( 30 ) | nRCT | 41‡ IG (cow’s milk): 21 CG (follow-on formula): 20 | USA | 2·8 months (from 3·7 to 6·5 months of age) | Hb levels (g/l) | No stat. sign. diff. between study groups in Hb levels at 3·7 months: IG 123 (sd 9·7) v. CG 125 (sd 10·4) g/l and at 6·5 months: IG 126 (sd 12·0) v. CG 127 (sd 11·3) g/l (P=NR) |
| Gill et al. (1997)( 28 ) | nRCT (only CG were randomized) | 406 IG (cow’s milk): 57 CG1 (Fe-supplemented follow-on formula): 264 CG2 (follow-on formula): 85 | UK and Ireland | 9 months (from 6 to 15 months of age) | IDA (Hb<110 g/l) | At 15 months: proportion of infants with anaemia 33 % in IG, 11 % in CG1, 13 % in CG2 (P=0·004) |
| Lehmann et al. (1992)( 32 ) | Retrospective cohort study | 220 Cow’s milk before 6 months: NR Cow’s milk after 6 months: NR | Canada | Comparison between cow’s milk consumption before and after 6 months of age (age of infants: 10–14 months) | IDA (Hb<110 g/l) | Introduction of cow’s milk before 6 months sign. related to increased risk of anaemia: OR=3·56 (95 % CI 1·07, 11·26) |
| Sadowitz and Oski (1983)( 33 ) | Retrospective cohort study | 122§ Cow’s milk before 6 months: 36 Cow’s milk after 6 months: 86 | USA | Comparison between cow’s milk consumption before and after 6 months of age (age of infants: 9–12 months) | IDA (Hb<110 g/l & SF<12 ng/ml & EP>30 µg/dl & MCV<70 fl) Fe depletion (Hb>110 g/l & SF<12 ng/ml & EP<30 µg/dl & MCV>70 fl) Fe deficiency (Hb>110 g/l & SF<12 ng/ml & EP>30 µg/dl & MCV<70 fl) Fe insufficient=IDA, Fe depletion and Fe deficiency | No sign. diff. in anaemia between groups (5·5 % in infants introduced to cow’s milk before 6 months and 0 % after 6 months (P=NR)) Sign. diff. in Fe-insufficient subjects between groups (47·2 % in infants introduced to cow’s milk before 6 months and 20·1 % after 6 months (P<0·01)) |
| Thorsdottir et al. (2003)( 34 ) & Thorsdottir and Gunnarson (2006)( 35 ) | Prospective cohort study | 138|| Cow’s milk intake <500 g/d: 80 Cow’s milk intake >500 g/d: 17 | Iceland | Average cow’s milk consumption between 9 and 12 months of age | Fe status: Hb levels (g/l), SF (µg/l), MCV (fl), TfR (mg/l) | No stat. sign. diff. in Hb levels between study groups; <500 g/d v. >500 g/d: Hb=114·8 v. 115·8 g/l (P=NS) Stat. sign. diff. in SF, MCV and TfR levels between study groups; <500 g/d v. >500 g/d: SF=20·1 v. 9·7 µg/l (P=0·001); MCV=77·3 v. 73·1 fl (P=0·001) and TfR=7·0 v. 8·2 mg/l (P=0·041) |
| Tunnessen and Oski (1987)( 29 ) | nRCT | 192 IG (cow’s milk): 71 CG (Fe-supplemented follow-on formula): 121 | USA | 6 months (from 6 to 12 months of age) | IDA (Hb<110 g/l) | At 12 months: incidence of anaemia 25 % in IG v. 11 % in CG (P<0·05) |
| Woodruff et al. (1972)( 48 ) | nRCT | 38 IG (cow’s milk): 13 CG (formula): 25 | USA | 10 months (from 2 to 12 months of age) | Hb (g/100 ml), MCV (cu μ) | Stat. sign. lower Hb levels in IG v. CG at 9 and 12 months (P<0·05), no stat. sign. diff. at 3 and 6 months Stat. sign. lower MCV in IG v. CG at 6 months (P<0·01) and 9 months (P<0·05), no stat. sign. diff. at 3 and 12 months |
| T1DM | ||||||
| Bodington et al. (1994)( 42 ) | Case–control study | 393 Cases (T1DM): 209 Controls: 184 | England | NA; introduction of cow’s milk from birth | Diagnosis of T1DM in children under age 15 years | Similar proportion of children were introduced to cow’s milk from birth in cases and controls |
| EURODIAB Study Group (2002)( 40 ), Substudy 2 | Case–control study | 2226 Cases (T1DM): 610 Controls: 1616 | Austria, Latvia, Lithuania, Luxembourg, UK/Northern Ireland | NA; introduction of cow’s milk before 3 months of age | Population-based register of T1DM, diabetes onset before the age of 15 years | No association between risk of T1DM and introduction of cow’s milk before 3 months |
| Rosenbauer et al. (2008)( 44 ) | Case–control study | 2631 Cases (T1DM): 760 Controls: 1871 | Germany | NA; current level of cow’s milk consumption Cases: <5 years of age (mean 3 years) Controls: <6 years of age (mean 3·3 years) | Population-based register of newly diagnosed T1DM, under 5 years of age | Current cow’s milk consumption is associated with lower risk of T1DM; no cow’s milk v. <200 ml/d: OR=0·65 (95 % CI 0·49, 0·88), no cow’s milk v. ≥200 ml/d: OR=0·60 (95 % CI 0·46, 0·79) |
| Sadauskaite-Kuehne et al. (2004)( 39 ) | Case–control study | Overall n: NR Cases (T1DM): 803 Controls: NR | Sweden and Lithuania | NA; introduction of cow’s milk at or after 7 months | Diagnosis of T1DM at a hospital between 5 and 9 years | Introduction of cow’s milk at or after 7 months is protective of T1DM, OR=0·62 (95 % CI 0·39, 0·99) |
| Savilahti and Saarinen (2009)( 37 ) | Nested case–control study | 6209 Cases (T1DM): 45 Controls: 6164 | Finland | NA; first introduction of cow’s milk in the first 12 months of age | Children with T1DM at mean age of 11·5 years reported to national registry | Age of introduction of cow’s milk similar between cases and controls; no diff. in OR for development of T1DM related to introduction of cow’s milk before or after 11 months |
| Sipetic et al. (2005)( 38 ) | Case–control study | 136 Cases (T1DM): 68 Controls (siblings): 68 | Serbia | NA; age at introduction of cow’s milk | Diagnosis of T1DM according to WHO at a hospital at 16 years of age or younger | Introduction of cow’s milk at younger than 5 months v. later than 5 months: no relation after adjustment for other relevant factors |
| Thorsdottir et al. (2000)( 41 ) | Case–control study | 220 Cases: (T1DM): 55 Controls: 165 | Iceland | NA; age at introduction of cow’s milk (between 1 and 6 months) | Children with T1DM with average age of 12·5 years | No association between age of introduction of cow’s milk and development of T1DM later in life |
| Verge et al. (1994)( 43 ) | Case–control study | 475 Cases (T1DM): 217 Controls: 258 | Australia | NA; age at introduction of cow’s milk (<7 months, 7–12 months, >12 months) | Population-based incidence register of T1DM, diabetes onset before age of 15 years | No association between T1DM and age at introduction of cow’s milk |
| Asthma | ||||||
| Van Asperen et al. (1984)( 49 ) | Prospective cohort study | 79¶ Cow’s milk before 4 months: 51 No cow‘s milk before 4 months: 28 | Australia | Comparison between cow’s milk consumption before 4 months and no cow’s milk | Wheeze (any history of wheezing, doctor-diagnosed); rhinitis (for at least 4 weeks, doctor-diagnosed) | Wheeze: Cow’s milk: 16/22 (31·3 %) No cow’s milk: 6/22 (21·4 %) (P=NS) Rhinitis: Cow’s milk: 27/44 (52·9 %) No cow’s milk: 17/44 (60·7 %) (P=NS) |
| Wijga et al. (2003)( 47 ) | Prospective cohort study | 2978 Consumption frequency: daily (6–7 d/week), regularly (1–5 d/week), rarely (less than once per week); n=NR | Netherlands | NA; cow’s milk consumption at 2 years of age; asthma diagnosis at 3 years of age | Asthma (‘ever asthma’: doctor’s diagnosis of asthma; ‘recent asthma’: doctor’s diagnosis plus asthma symptoms or use of asthma medication in the last 12 months) | Full-cream milk: Daily v. rare consumption associated with ever asthma: adj. OR=0·54 (95 % CI 0·34, 0·88) and recent asthma: adj. OR=0·53 (95 % CI 0·30, 0·92) Semi-skimmed milk: No sign. associations between consumption frequency and asthma |
| Growth | ||||||
| Daly et al. (1996)( 26 ) | RCT | 100 IG (cow’s milk): 50 CG (Fe-supplemented follow-on formula): 50 | UK | 12 months (from 6 to 18 months of age) | Growth | No sign. diff. in Z-scores for weight-for-age, height-for-age, weight-for-height |
| Wiley (2010)( 46 ) | Retrospective cohort study | 1493 Quartiles of milk intake; n=NR | USA | NA; children aged 2–4 years | BMI percentiles | Diff. in BMI percentiles across milk quartiles: higher BMI in Q4 v. Q1, Q2, Q3 (β adj. for energy=−6·3, −11·8, −6·7; P<0·05) |
| Development | ||||||
| Williams et al. (1999)( 27 ) | RCT | 100 IG (cow’s milk): 50 CG (Fe-supplemented follow-on formula): 50 | UK | 12 months (from 6 to 18 months of age) | Psychomotor development (Griffiths scale) | Griffiths general quotient scores declined in both groups, but no sign. diff. between groups |
| Atopic dermatitis (eczema) | ||||||
| Roduit et al. (2012)( 50 ) | Prospective cohort study | 724** Cow’s milk between 3 and 12 months: 247 No cow’s milk within 12 months: 477 | Austria, France, Finland, Germany, Switzerland | Comparison between cow’s milk consumption before 12 months and no cow’s milk | Atopic dermatitis with onset after the first year | Lower risk for atopic dermatitis with onset after the first year when cow’s milk introduced within the first year of life: adj. OR=0·52 (95 % CI 0·30, 0·92) |
| Van Asperen et al. (1984)( 49 ) | Prospective cohort study | 79¶ Cow’s milk before 4 months: 51 No cow‘s milk before 4 months: 28 | Australia | Comparison between cow’s milk consumption before 4 months and no cow’s milk | Atopic dermatitis (doctor-diagnosed) | Atopic dermatitis: Cow’s milk: 22/38 (43·1 %) No cow’s milk: 16/38 (57·1 %) (P=NS) |
| Zutavern et al. (2004)( 45 ) | Prospective cohort study | 620†† Cow’s milk before 6 months: 407 Cow’s milk after 6 months: 213 | UK | Comparison between cow’s milk consumption before and after 6 months of age (age of infants 1 year) | Eczema (doctor-diagnosed), atopy (skin-prick test), wheezing defined at 5 to 5·5 years of age | Higher risk for eczema when cow’s milk introduced after 6 months, adj. OR=1·7 (95 % CI 1·1, 2·5); prevalence of eczema 32·2 % v. 41·2 % when cow’s milk introduced before v. after 6 months (P=0·032); no diff. in other outcomes (pre-school wheezing, transient wheezing, atopy) |
| Gastrointestinal blood loss | ||||||
| Fomon et al. (1981)( 30 ) | nRCT | 41‡ IG (cow’s milk): 21 CG (follow-on formula): 20 | USA | 2·8 months (from 3·7 to 6·5 months of age) | Infants with guaiac-positive stools;Total number of guaiac-positive stools | At 4·7 months: proportion of infants with guaiac-positive stools 35 % in IG v. 0 % in CG (P=0·01) After 4·7 months: no stat. sign. diff. At 4·7 months: total number of guaiac-positive stools 12·5 % in IG v. 0 % in CG (P<0·003) After 4·7 months: no stat. sign. diff. |
| Thomas et al. (1986)( 36 ) | Prospective cohort study | 820 E1 (cow’s milk): 146 E2 (breast-feeding): 354 E3 (formula): 320 | USA | 6 months (from 6 to 12 months of age) | Intestinal bleeding: FH positivity, tetramethylbenzidine-Hematest reactions, orthotolidine-Hematest method | No stat. sign. diff. in FH positivity rates between groups: E1: 2·9 %, E2: 2·1 %, E3: 1·9 % (P=NS) No stat. sign. diff. in tetramethylbenzidine-Hematest reactions between groups: E1: 2·7 %, E2: 3·5 %, E3: 5·2 % (P=NS) Slightly increased incidence of orthotolidine-Hematest reactions for E1 v. E2+E3 (combined) (P<0·05): E1: 9·6 %, E2: 4·1 %, E3: 0·1 % |
| Tunnessen and Oski (1987)( 29 ) | nRCT | 192 IG (cow’s milk): 71 CG (Fe-supplemented follow-on formula): 121 | USA | 6 months (from 6 to 12 months of age) | Total number of guaiac-positive stools | No stat. sign. diff. between study groups |
| Woodruff et al. (1972)( 48 ) | nRCT | 38 IG (cow’s milk): 13 CG (formula): 25 | USA | 10 months (from 2 to 12 months of age) | Proportion of infants with at least 1 guaiac-positive stool in 3 months (2 stools per month tested for guaiac determination, rated positive when at least 2 or more were reported positive) | 3 months: IG 11/12 (91·7 %), CG 11/25 (44 %) 6 months: IG 9/13 (69·2 %), CG 13/25 (52 %) 9 months: IG 10/12 (83·3 %), CG 16/24 (66·7 %) 12 months: IG 7/11 (63·6 %), CG 12/22 (54·5 %) No stat. sign. diff. at any time point |
| Exudative protein loss | ||||||
| Thomas et al. (1986)( 36 ) | Prospective cohort study | 820 E1 (cow’s milk): 146 E2 (breast-feeding): 354 E3 (formula): 320 | USA | 6 months (from 6 to 12 months of age) | Exudative protein loss (FA1AT concentration) | Small diff. in FA1AT levels (P<0·0001): E2>E3>E1 |
IDA, Fe-deficiency anaemia; T1DM, type 1 diabetes mellitus; RCT, randomized controlled trial; nRCT, non-randomized controlled trial; CG, control group; E, exposure group; IG, intervention group; NR, not reported; NA, not applicable; SF, serum ferritin; EP, erythrocyte porphyrin; MCV, mean corpuscular volume; TfR, serum transferrin receptors; cu μ, cubic micron calculated according to Wintrobe; FH, faecal Hb; FA1AT, faecal α1-antitrypsin; stat., statistically; sign., significant; diff., difference; Q, quartile; adj., adjusted.
Cow’s milk consumption is always whole cow’s milk unless otherwise stated.
Children in both groups also received breast milk during the first 2 months of life.
We report only data on boys, because girls received either pasteurized cow’s milk or extensively heat-treated cow’s milk, but no infant formula.
We report only data on white infants.
Blood samples were only available for ninety-seven children.
The original cohort included ninety-two children.
In total, 1041 children were included in the study, but the analyses were restricted only to children having no atopic dermatitis in the first year (n 724).
The original cohort included 642 children.
Fe-deficiency anaemia
Nine studies, one RCT( 26 ), four nRCT( 28 – 30 , 48 ), two prospective cohort studies( 31 , 34 , 35 ) and two retrospective cohort studies( 32 , 33 ), addressed the risk of developing IDA in infants who regularly consumed cow’s milk over a longer period of time, providing data on 1642 children between the ages of 0 and 18 months. Overall, seven out of eight studies reported a substantially greater risk of developing IDA in infants who were fed cow’s milk compared with those who received Fe-fortified follow-on formula. Another nRCT( 48 ) showed lower Hb levels at 9 and 12 months in infants fed cow’s milk starting from 2 months of age compared with infants who received non-Fe-fortified formula. The only study that did not find a statistically significant difference in Fe status markers compared twenty-one infants who received cow’s milk v. twenty infants who received formula with low Fe content. However, all infants in that study received Fe supplementation of 12 mg/d and vitamin C supplementation( 30 ). Four of the included studies indicated that the amount of cow’s milk or/and formula consumed was measured. Values were reported in three studies and ranged between 538 and 983 ml/d in infants between 3·7 and 18 months( 26 , 30 , 32 ). In one study all study participants received an Fe supplement of 12 mg/d( 30 ) and in another study 11 % of infants reported the use of Fe supplements as drops( 32 ). In one study the use of non-dietary Fe supplements was not permitted( 28 ). Only a single study specifically reported Fe intake for each study group( 26 ); it concluded that follow-on formula contributed a substantial proportion of total Fe intake( 26 ).
Two retrospective cohort studies( 32 , 33 ) (342 infants) investigated the age of introduction of cow’s milk and the associated risk for IDA, finding an increased risk of IDA and Fe insufficiency in children exposed to cow’s milk before 6 months of age compared with those who were exposed after this age. One prospective cohort study( 34 , 35 ) explored a possible dose–response relationship in 138 infants before 12 months of age and found higher levels of the Fe status markers serum ferritin and mean corpuscular volume in infants consuming <500 g cow’s milk/d compared with those consuming >500 g/d, but no difference in Hb levels.
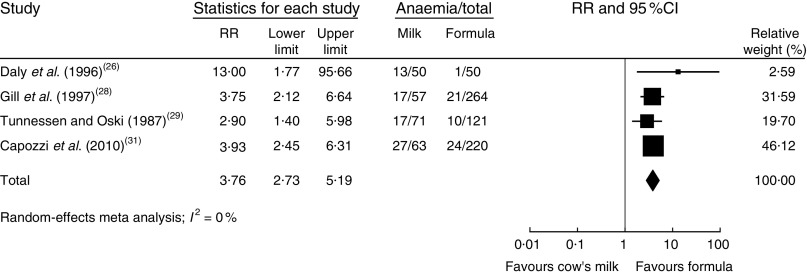
In a meta-analysis, we pooled data from one RCT( 26 ), two nRCT( 28 , 29 ) and one prospective cohort study( 31 ) (with a total of 1083 study participants). The duration of cow’s milk exposure varied between 6 and 12 months, starting at birth or at 6 months of age. Pooled results rendered a more than three times higher risk of IDA for infants consuming cow’s milk compared with those drinking follow-on formula (RR=3·76; 95 % CI 2·73, 5·19; Fig. 2). In these four studies 25–38 % of infants consuming cow’s milk developed IDA compared with 2–15 % of those fed with Fe-fortified formula.
Fig. 2.
Meta-analysis of the relative risk (RR) of iron-deficiency anaemia between cow’s milk and formula in healthy, full-term infants up to 3 years of age. The study-specific RR and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond presents the pooled RR risk and its width represents the pooled 95 % CI
In a meta-regression we explored the impact of duration of exposure to cow’s milk (4–8 months) on the risk of developing IDA. Results showed no statistically significant association (P=0·85). We could not find any relationship between duration of cow’s milk consumption and IDA. Overall, we graded the quality of evidence that cow’s milk consumption in healthy, full-term infants up to 18 months of age leads to IDA as low, indicating substantial uncertainty about the estimate of the effect. The evidence base is limited by serious risk of bias due to the lack of randomization in three of four controlled studies, missing intention-to-treat (ITT) analysis in all four controlled studies and the lack of adjustment for potential confounders in three of four observational studies. Furthermore, half of the studies were conducted in families with low or very low socio-economic background, which limits the applicability to the general population (Table 3).
Table 3.
Quality of evidence for health effects of cow’s milk consumption in infants up to 3 years old, for each outcome (according to GRADE)
| Number of studies, study designs | Sample size | Risk of bias | Inconsistency | Indirectness | Imprecision | Summary of results | Quality of evidence | Importance |
|---|---|---|---|---|---|---|---|---|
| IDA/Fe status | ||||||||
| 5 (R)CT and 4 observational studies | 1682 | Serious | No serious inconsistency | Serious indirectness | No serious imprecision | Negative association between cow’s milk consumption and IDA | Low | Critical |
| 5 (n)RCT( 26 , 28 – 30 ) | 817 | Serious Only 1 real RCT, 1 where only control groups were randomized and 3 controlled studies, no ITT analysis in any of the 5 controlled trials | No serious inconsistency | Serious indirectness 2 studies (n 192) with population with lower socio-economic background | No serious imprecision | Proportion of infants with IDA is higher in the cow’s milk group compared with the formula group | Low | Critical |
| 4 observational studies( 31 – 35 ) | 865 | Serious In 3 studies not adjusted to potential confounders, in 2 studies prognostic factors not measured accurately | No serious inconsistency | Serious indirectness 2 studies (n 342) with population with lower socio-economic background | No serious imprecision | Cow’s milk consumption is associated with increased risk of IDA, Fe sufficiency or lower Fe status | Very low | Critical |
| T1DM | ||||||||
| 8 observational studies( 37 – 44 ) | 13 093 | Serious In half of the studies not adjusted for potential confounders, in all studies not all prognostic factors reported | No serious inconsistency | No serious indirectness | No serious imprecision | No association with age of introduction of cow’s milk and T1DM | Very low | Critical |
| Asthma | ||||||||
| 2 observational studies( 47 , 49 ) | 3057 | No serious risk of bias | Serious inconsistency | No serious indirectness | No serious imprecision | Daily consumption of whole cow’s milk is protective of asthma in one study, no association with wheeze in another study | Very low | Critical |
| Growth | ||||||||
| 1 RCT and 1 observational study | 1593 | Serious | Serious inconsistency | Serious indirectness | No serious imprecision | Conflicting results | Very low | Important |
| 1 RCT( 26 ) | 100 | Serious Lack of blinding and no ITT analysis | No serious inconsistency | Serious indirectness Population with low socio-economic background | No serious imprecision | No sign. diff. in Z-scores for weight-for-age, height-for-age, weight-for-height between cow’s milk and formula group | Low | Important |
| 1 observational study( 46 ) | 1493 | No serious risk of bias | No serious inconsistency | Serious indirectness Population with large proportion of ethnic groups | No serious imprecision | Highest milk consumption associated with higher BMI percentiles | Very low | Important |
| Development | ||||||||
| 1 RCT( 27 ) | 100 | Serious Lack of blinding and no ITT analysis | No serious inconsistency | Serious indirectness Population with low socio-economic background | No serious imprecision | No diff. in psychomotor development between cow’s milk and formula group | Very low | Important |
| Atopic dermatitis (eczema) | ||||||||
| 3 observational studies( 45 , 49 , 50 ) | 1762 | No serious risk of bias | Serious inconsistency | No serious indirectness | No serious imprecision | Cow’s milk consumption in the first year is associated with lower risk of atopic dermatitis (onset after the first year) in one study; higher risk for eczema when cow’s milk introduced after 6 months v. before in another study; and no association in another study | Very low | Not important |
| Gastrointestinal blood loss | ||||||||
| 3 nRCTs and 1 observational study | 1093 | Serious | No serious inconsistency | Very serious indirectness | No serious imprecision | No diff. between cow’s milk and formula groups | Very low | Not important |
| 3 nRCTs( 29 , 30 , 48 ) | 311 | Serious Lack of blinding and no ITT analysis in all three studies | No serious inconsistency | Very serious indirectness Surrogate outcome, only low and middle socio-economic status in 1 study; bad sex distribution in 1 study | No serious imprecision | No diff. in number of guaiac-positive stools between cow’s milk group and formula group | Very low | Not important |
| 1 observational study( 36 ) | 820 | Serious No information on statistical adjustment of confounders | Serious inconsistency 3 markers for occult blood did not show similar result | Very serious indirectness Surrogate outcome, ethnic composition diverse (only 68 % Caucasian white) | No serious imprecision | No diff. in 2 of 3 markers for occult blood between cow’s milk group, breast-feeding and formula groups | Very low | Not important |
| Exudative protein loss | ||||||||
| 1 observational study( 36 ) | 820 | Serious No information on statistical adjustment of confounders | No serious inconsistency | Very serious indirectness Surrogate outcome, ethnic composition diverse (only 68 % Caucasian white) | No serious imprecision | Cow’s milk groups showed less exudative protein loss than breast-fed and formula-fed infants | Very low | Not important |
GRADE, Grading of Recommendations Assessment, Development and Evaluation; IDA, Fe-deficiency anaemia; T1DM, type 1 diabetes mellitus; RCT, randomized controlled trial; nRCT, non-randomized controlled trial; ITT, intention-to-treat; sign., significant; diff., difference.
Type 1 diabetes mellitus
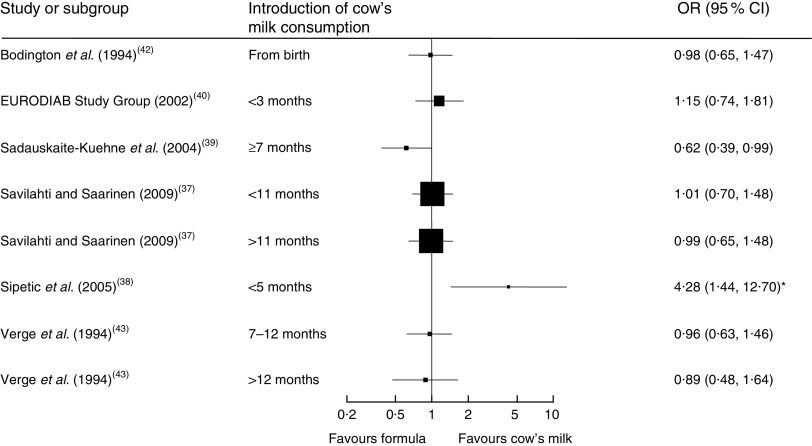
Seven case–control studies (with a total of 2007 cases and 8455 controls) investigated the association between the age of introduction of cow’s milk and type 1 diabetes mellitus (T1DM)( 37 – 43 ). In the adjusted analyses, all but one study( 39 ) consistently showed no difference in the risk for T1DM due to early introduction of cow’s milk, starting from birth or after 3, 5, 7 or 11 months. Figure 3 depicts the results of all studies reporting odds ratios in a forest plot. Because of heterogeneity regarding age of study participants and durations of exposure to cow’s milk, we did not conduct a meta-analysis of these studies.
Fig. 3.
Forest plot of studies investigating the association between the age of introduction of cow’s milk and type 1 diabetes mellitus in healthy, full-term infants up to 3 years of age. Only studies reporting OR are depicted. The study-specific OR and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight. *The significant association disappeared after adjustment for other factors significantly related to diabetes
One case–control study( 44 ) (670 cases, 1871 controls) found that current consumption of cow’s milk <200 ml/d or ≥200 ml/d compared with no consumption was associated with a lower risk of T1DM (OR=0·65; 95 % CI 0·46, 0·88 and OR=0·60; 95 % CI 0·46, 0·79, respectively).
The quality of evidence is very low, mostly because of the retrospective study design and the concomitant serious risk of bias of included studies. Half of the studies did not adjust for potential confounders and none of the studies reported all relevant prognostic factors (Table 3).
Asthma
One prospective cohort study (n 2978) investigated the effect of consumption frequency of cow’s milk on the prevalence of asthma( 47 ) and found that daily v. rare consumption of full-cream milk had a protective effect on asthma (‘ever asthma’: adjusted OR=0·54; 95 % CI 0·34, 0·88 and recent asthma: adjusted OR=0·53; 95 % CI 0·30, 0·92). No significant associations between consumption and frequency of asthma were found for semi-skimmed milk (Table 2). Another prospective cohort study (n 79) showed no significant association between the occurrence of wheeze or rhinitis and the introduction of cow’s milk in the first 4 months of life( 49 ).
We rated the quality of evidence as very low, indicating great uncertainty about the validity of this finding, because it is based on a single observational study (Table 3).
Growth
One RCT( 26 ) and one prospective cohort study( 46 ) did not show a consistent effect of cow’s milk consumption on infants’ growth. The RCT reported no significant differences in growth parameters (weight-for-age, height-for-age and weight-for-height) between cow’s milk and follow-on formula intake. In the prospective cohort study a higher cow’s milk intake was associated with higher BMI percentiles. No conclusions can be drawn from these two conflicting studies. The quality of evidence is very low because of the lack of blinding and ITT analysis in the RCT, and there is limited applicability to the general population because of participants from low socio-economic background and large proportions of ethnic groups (Table 3).
Development
One RCT( 27 ) showed no statistically significant difference in psychomotor development between the cow’s milk and the formula groups. The quality of evidence is very low because of serious risk of bias due to lack of blinding and ITT analysis, and external validity is low because the investigated population had a low socio-economic background which limits the applicability to the general population (Table 3).
Atopic dermatitis (eczema)
Three prospective cohort studies( 45 , 49 , 50 ) investigated the effect of cow’s milk intake on atopic dermatitis with inconsistent results. One study (n 1041) showed that the introduction of cow’s milk in the first year of life reduced risk for the development of atopic dermatitis with onset after the first year of life compared with no cow’s milk (Table 2). Another study (n 642) reported a higher risk for eczema when cow’s milk was introduced after 6 months of age compared with before 6 months( 45 ). A third study (n 79) showed no significant association between the occurrence of atopic dermatitis and the introduction of cow’s milk in the first 4 months of life( 49 ). Because of the inconsistency of results, the inherent risk of bias and confounding, and the risk of chance findings because of low event rates, we rated the quality of evidence as very low (Table 3).
Gastrointestinal blood loss
Three nRCT( 29 , 30 , 48 ) and one cohort study( 36 ) (with a total of 1091 study participants) showed no association between cow’s milk consumption and gastrointestinal blood loss after the age of 3 and 6 months, respectively. One of the three nRCT found a higher risk of faecal blood stools when children consumed pasteurized cow’s milk compared with follow-on formula at the age of 3·7 to 4·7 months. The quality of evidence is very low because of lack of blinding and ITT analysis in the controlled studies and no statistical adjustment in the observational studies. Furthermore, surrogate markers for gastrointestinal blood loss were used and applicability is hampered because of low socio-economic status and a diverse ethnic composition (Table 3).
Discussion
To our knowledge, the present study is the first systematic review and meta-analysis on the health effects of cow’s milk consumption in infants up to 3 years of age. The results of our meta-analysis with data from four studies indicate a more than three times higher risk for IDA at ages 8 to 18 months in infants who consume cow’s milk compared with those who consume Fe-fortified formula for duration of 6–12 months starting either at birth or at 6 months of age. This confirms what has been regarded as common knowledge until now: that consumption of cow’s milk in infancy leads to an increased risk for developing IDA. The quality of evidence is low due to the fact that several methodological flaws in the available studies introduce a serious risk of bias. Furthermore, some of the studies were carried out in populations with low socio-economic status which by itself is an independent risk factor for the development of Fe deficiency( 51 ). Nevertheless, the very large increase in risk attributable to cow’s milk that our meta-analysis found is unlikely to be caused exclusively by bias and confounding. Due to the fact that the group of infants included in our meta-analysis all received Fe-fortified formula, it is likely that the observed increase in risk for IDA in the group of infants fed cow’s milk is largely explained by differences in Fe intake. However, studies comparing different formulas with high and low Fe content showed no statistically significant difference in anaemia incidence, although in most studies infants in the group fed with high-Fe formula had higher ferritin levels than the infants fed low-Fe formula( 52 ). In the study by Gill et al.( 28 ) a smaller proportion of infants receiving the non-fortified formula were anaemic compared with infants fed cow’s milk (13 % v. 33 %). It is therefore possible that cow’s milk exerts adverse effects on Fe status on its own, either by inhibition of non-haem Fe absorption or by another unknown mechanism or a combination of all( 53 ). Young children are most susceptible to Fe deficiency as a result of an increased Fe requirement related to rapid growth during the first 2 years of life( 54 ). However, little is known about whether cow’s milk is also detrimental if the diet otherwise provides sufficient Fe for the infant. Meat is a good source of Fe because it contains a high proportion of haem Fe which has a higher rate of absorption compared with non-haem Fe. Non-haem Fe found in plant foods and fortified food products has lower rates of absorption. Ascorbic acid and the concurrent consumption of meat are enhancers of non-haem Fe absorption. Tea, bran and milk tend to inhibit non-haem Fe absorption( 54 ). Cow’s milk generally tends to be less expensive than infant formula or follow-on formula, and should therefore be considered a possible alternative if an infant who consumes milk can still achieve a well-balanced diet along with Fe-rich sources. Efforts should be made to increase public awareness regarding the risk of anaemia associated with cow’s milk consumption in infants up to 18 months. Mothers who opt to feed their infants cow’s milk should be provided enough information to do so conscientiously by ensuring that the babies given cow’s milk also receive an Fe-rich diet with foods that enhance Fe absorption.
Cow’s milk exposure during infancy has been described as a possible risk factor for the later development of T1DM( 55 ). Earlier studies showed an increased risk of T1DM when an infant was exposed to cow’s milk at an early age and breast-feeding lasted less than 3 months( 56 ). A systematic review highlighted the role of exclusive breast-feeding as a protective factor against the development of T1DM( 57 ). In contrast to earlier results, our review shows no association between the age of introduction of cow’s milk and T1DM. The difference in results may be explained by the definition of cow’s milk exposure. Whereas in all studies that we included for the question regarding T1DM, the intake of pure cow’s milk was investigated, other reviews did not confine cow’s milk exposure in such a way and included all exposure to dairy proteins including cow’s milk-based formulas. Therefore, it seems likely that the duration of breast-feeding exhibits a protective effect and the concomitant introduction of cow’s milk may not be associated with the risk of developing T1DM later in life.
The evidence concerning the question if cow’s milk intake poses a risk for asthma, or is negatively associated with development, showed no negative effects of cow’s milk intake in infancy.
Although associations between high protein intake early in life and an increased risk of developing obesity later in life have been shown previously( 58 ), our study did not find evidence for an association between cow’s milk intake in infancy and growth. With regard to atopic dermatitis, we found very-low-quality evidence that showed infants exposed to cow’s milk in the first year of life were less likely to develop atopic dermatitis than those infants who were not exposed to cow’s milk.
Our systematic review did not identify conclusive evidence for a dose–response relationship between the consumption of cow’s milk and any of the described outcomes.
Strengths and limitations of the review
The strength of our review is that it used state-of-the-art methods to objectively and systematically assess the benefits and risks of cow’s milk intake in healthy, full-term infants. We searched multiple scientific databases, hand searched reference lists and contacted authors to receive additional data or information about their studies. Nevertheless, some potential limitations of the review process exist. Naturally, it is possible that we did not identify all relevant publications. We may have introduced bias by excluding publications written in languages other than English or German.
The main limitation of our review, however, is that the strength of our conclusions is limited by the low methodological quality of the primary studies that are available for our questions of interest. The identified articles were very heterogeneous in terms of their study designs and methods used for measurement of exposure. In particular, our search did not identify sufficient high-quality controlled trials. Although it would be unethical to randomize mothers into groups who either breast-feed or feed their babies cow’s milk, researchers can conduct high-quality prospective studies using methods such as propensity score analyses to adjust for potential confounders. Finally, the conclusions of our systematic review apply only to healthy and term-born infants living in countries with a population comparable to European populations.
Implications for research
Further high-quality studies should be conducted to contribute sufficient high-quality evidence as grounds for public recommendations. Such studies should specifically address questions concerning the kinds and amounts of complementary food necessary to ensure that infants consuming cow’s milk have sufficient Fe intakes. Additional research should compare the effects of introducing cow’s milk into the diets of children who are younger than 6 months, between 6 months and 12 months old, and older than 1 year of age.
Implications for practice
According to the results of the present review, the only currently known risk associated with introducing cow’s milk to a young infant is that the child will develop Fe deficiency. This could occur if the child is given too much cow’s milk in place of alternative food sources that would support a well-balanced infant diet. Further research is needed to make concrete public recommendations. Parents and caregivers should have access to high-quality information regarding the effects of cow’s milk consumption on their babies. Recommendations regarding cow’s milk should provide parents with information regarding the ideal age to introduce a child to cow’s milk, the proper amount for that age, as well as the most effective dietary complements (Fe-source foods). In the absence of clear evidence answering questions about the optimal age to introduce a child to cow’s milk and the proper amount for that age, expert consensus is the best way to provide guidance to parents.
Acknowledgements
Financial support: This work was supported by the Austrian Agency for Health and Food Safety (AGES); the Austrian Federal Ministry of Health; and the Main Association of Austrian Social Security Institutions. The funders had no role in the design, analysis or writing of this article. Conflicts of interest: None. Authorship: A.W., A.H. and G.G. initiated and designed the study; U.G., M.U.B., C.K., B.D., B.M., K.S. and R.E. conducted the literature review (abstract and full-text review, data abstraction) and synthesized the data; G.G. performed the meta-analysis; U.G., C.K., A.H. and G.G. prepared drafts of the manuscript; U.G. had primary responsibility for the final content of the manuscript; and all authors read, critically revised and approved the final manuscript. Ethics of human subject participation: Ethical approval was not required.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980015001354.
click here to view supplementary material
References
- 1. American Academy of Pediatrics (2012) Breastfeeding and the use of human milk. Pediatrics 129, e827–e841. [DOI] [PubMed] [Google Scholar]
- 2. Agostoni C, Decsi T, Fewtrell M et al. (2008) Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 46, 99–110. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (2013) 10 facts on breastfeeding. http://www.who.int/features/factfiles/breastfeeding/en/ (accessed January 2014).
- 4. World Health Organization (2009) Infant and young child feeding. Model Chapter for textbooks for medical students and allied health professionals. http://whqlibdoc.who.int/publications/2009/9789241597494_eng.pdf (accessed January 2014). [PubMed]
- 5. National Health and Medical Research Council (2012) Infant Feeding Guidelines. Information for Health Workers. Canberra: National Health and Medical Research Council; available at http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/n56_infant_feeding_guidelines.pdf [DOI] [PubMed] [Google Scholar]
- 6. Koletzko B, Bauer CP, Brönstrup A et al. (2013) Säuglingsernährung und Ernährung der stillenden Mutter (Infant nutrition and nutrition of the breastfeeding mother). Monatsschr Kinderheilkd 161, 237–246. [Google Scholar]
- 7. Health Canada (2005) Nutrition for Healthy Term Infants – Statement of the Joint Working Group: Canadian Paediatric Society, Dietitians of Canada and Health Canada. http://www.hc-sc.gc.ca/fn-an/pubs/infant-nourrisson/nut_infant_nourrisson_term-eng.php#other-1 (accessed February 2012).
- 8. European Commission, Karolinska Insitutet, Institute for Child Health IRCCS Burlo Garofolo et al. (2006) Infant and young child feeding: standard recommendations for the European Union. http://www.ilca.org/files/resources/international_regional_documetns/EUPolicy06English.pdf (accessed July 2011).
- 9. Baehler P, Baenziger O, Belli D et al. (2009) Empfehlungen für die Säuglingsernährung (Recommendations for infant nutrition). Paediatrica 20, 13–15. [Google Scholar]
- 10. European Union (2008) Commission Directive 2008/100/EC of 28 October 2008 amending Council Directive 90/496/EEC on nutrition labelling for foodstuffs as regards recommended daily allowances, energy conversion factors and definitions. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:285:0009:0012:EN:PDF (accessed January 2014).
- 11. Souci S, Fachmann W & Kraut H (2008) Food Composition and Nutrition Tables. Die Zusammensetzung der Lebensmittel, Nährwert-Tabellen. La composition des aliments Tableaux des valeurs nutritives. http://www.sfk-online.net/cgi-bin/sfkstart.mysql?language=english (accessed January 2014).
- 12. Fleischer Michaelsen K, Weaver L, Branca F et al. (2003) Feeding and Nutrition of Infants and Young Children. Guidelines for the WHO European Region, with Emphasis on the Former Soviet Countries. WHO Regional Publications, Euopean Series no. 87. Copenhagen: WHO Regional Office for Europe; available at http://www.euro.who.int/__data/assets/pdf_file/0004/98302/WS_115_2000FE.pdf [Google Scholar]
- 13. Ziegler EE (2007) Adverse effects of cow’s milk in infants. Nestle Nutr Workshop Ser Pediatr Program 60, 185–196. [DOI] [PubMed] [Google Scholar]
- 14. Oski FA (1985) Is bovine milk a health hazard? Pediatrics 75, 182–186. [PubMed] [Google Scholar]
- 15. Michaelsen KF, Hoppe C, Lauritzen L et al. (2007) Whole cow’s milk: why, what and when? Nestle Nutr Workshop Ser Pediatr Program 60, 201–216. [DOI] [PubMed] [Google Scholar]
- 16. Higgins J & Green S (2008) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated September 2008]. http://www.cochrane-handbook.org/ (accessed July 2011).
- 17. Centre for Reviews and Dissemination (2009) Systematic Reviews. CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York, Centre for Reviews and Dissemination; available at http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf [Google Scholar]
- 18. Deeks JJ, Dinnes J, D’Amico R et al. (2003) Evaluating non-randomised intervention studies. Health Technol Assess 7, 1–173. [DOI] [PubMed] [Google Scholar]
- 19. Balshem H, Helfand M, Schunemann HJ et al. (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64, 401–406. [DOI] [PubMed] [Google Scholar]
- 20. Guyatt GH, Oxman AD, Kunz R et al. (2011) GRADE guidelines: 6. Rating the quality of evidence – imprecision. J Clin Epidemiol 64, 1283–1293. [DOI] [PubMed] [Google Scholar]
- 21. Guyatt GH, Oxman AD, Kunz R et al. (2011) GRADE guidelines: 8. Rating the quality of evidence – indirectness. J Clin Epidemiol 64, 1303–1310. [DOI] [PubMed] [Google Scholar]
- 22. Guyatt GH, Oxman AD, Kunz R et al. (2011) GRADE guidelines: 7. Rating the quality of evidence – inconsistency. J Clin Epidemiol 64, 1294–1302. [DOI] [PubMed] [Google Scholar]
- 23. Guyatt GH, Oxman AD, Vist G et al. (2011) GRADE guidelines: 4. Rating the quality of evidence – study limitations (risk of bias). J Clin Epidemiol 64, 407–415. [DOI] [PubMed] [Google Scholar]
- 24. Koopman JS, Turkish VJ & Monto AS (1985) Infant formulas and gastrointestinal illness. Am J Public Health 75, 477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skrodeniene E, Marciulionyte D, Padaiga Z et al. (2008) Environmental risk factors in prediction of childhood prediabetes. Medicina (Kaunas) 44, 56–63. [PubMed] [Google Scholar]
- 26. Daly A, MacDonald A, Aukett A et al. (1996) Prevention of anaemia in inner city toddlers by an iron supplemented cows’ milk formula. Arch Dis Child 75, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams J, Wolff A, Daly A et al. (1999) Iron supplemented formula milk related to reduction in psychomotor decline in infants from inner city areas: randomised study. BMJ 318, 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gill DG, Vincent S & Segal DS (1997) Follow-on formula in the prevention of iron deficiency: a multicentre study. Acta Paediatr 86, 683–689. [DOI] [PubMed] [Google Scholar]
- 29. Tunnessen WW Jr & Oski FA (1987) Consequences of starting whole cow milk at 6 months of age. J Pediatr 111, 813–816. [DOI] [PubMed] [Google Scholar]
- 30. Fomon SJ, Ziegler EE, Nelson SE et al. (1981) Cow milk feeding in infancy: gastrointestinal blood loss and iron nutritional status. J Pediatr 98, 540–545. [DOI] [PubMed] [Google Scholar]
- 31. Capozzi L, Russo R, Bertocco F et al. (2010) Diet and iron deficiency in the first year of life: a retrospective study. Hematology 15, 410–413. [DOI] [PubMed] [Google Scholar]
- 32. Lehmann F, Gray-Donald K, Mongeon M et al. (1992) Iron deficiency anemia in 1-year-old children of disadvantaged families in Montreal. CMAJ 146, 1571–1577. [PMC free article] [PubMed] [Google Scholar]
- 33. Sadowitz PD & Oski FA (1983) Iron status and infant feeding practices in an urban ambulatory center. Pediatrics 72, 33–36. [PubMed] [Google Scholar]
- 34. Thorsdottir I, Gunnarsson BS, Atladottir H et al. (2003) Iron status at 12 months of age – effects of body size, growth and diet in a population with high birth weight. Eur J Clin Nutr 57, 505–513. [DOI] [PubMed] [Google Scholar]
- 35. Thorsdottir I & Gunnarsson BS (2006) Dietary quality and adequacy of micronutrient intakes in children. Proc Nutr Soc 65, 366–375. [DOI] [PubMed] [Google Scholar]
- 36. Thomas DW, McGilligan KM, Carlson M et al. (1986) Fecal alpha 1-antitrypsin and hemoglobin excretion in healthy human milk-, formula-, or cow’s milk-fed infants. Pediatrics 78, 305–312. [PubMed] [Google Scholar]
- 37. Savilahti E & Saarinen KM (2009) Early infant feeding and type 1 diabetes. Eur J Nutr 48, 243–249. [DOI] [PubMed] [Google Scholar]
- 38. Sipetic S, Vlajinac H, Kocev N et al. (2005) Early infant diet and risk of type 1 diabetes mellitus in Belgrade children. Nutrition 21, 474–479. [DOI] [PubMed] [Google Scholar]
- 39. Sadauskaite-Kuehne V, Ludvigsson J, Padaiga Z et al. (2004) Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metab Res Rev 20, 150–157. [DOI] [PubMed] [Google Scholar]
- 40. EURODIAB (2002) Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care 25, 1755–1760. [DOI] [PubMed] [Google Scholar]
- 41. Thorsdottir I, Birgisdottir BE, Johannsdottir IM et al. (2000) Different β-casein fractions in Icelandic versus Scandinavian cow’s milk may influence diabetogenicity of cow’s milk in infancy and explain low incidence of insulin-dependent diabetes mellitus in Iceland. Pediatrics 106, 719–724. [DOI] [PubMed] [Google Scholar]
- 42. Bodington MJ, McNally PG & Burden AC (1994) Cow’s milk and type 1 childhood diabetes: no increase in risk. Diabet Med 11, 663–665. [DOI] [PubMed] [Google Scholar]
- 43. Verge CF, Howard NJ, Irwig L et al. (1994) Environmental factors in childhood IDDM. A population-based, case–control study. Diabetes Care 17, 1381–1389. [DOI] [PubMed] [Google Scholar]
- 44. Rosenbauer J, Herzig P & Giani G (2008) Early infant feeding and risk of type 1 diabetes mellitus – a nationwide population-based case–control study in pre-school children. Diabetes Metab Res Rev 24, 211–222. [DOI] [PubMed] [Google Scholar]
- 45. Zutavern A, Von Mutius E, Harris J et al. (2004) The introduction of solids in relation to asthma and eczema. Arch Dis Child 89, 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiley AS (2010) Dairy and milk consumption and child growth: is BMI involved? An analysis of NHANES 1999–2004. Am J Hum Biol 22, 517–525. [DOI] [PubMed] [Google Scholar]
- 47. Wijga AH, Smit HA, Kerkhof M et al. (2003) Association of consumption of products containing milk fat with reduced asthma risk in pre-school children: the PIAMA birth cohort study. Thorax 58, 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Woodruff CW, Wright SW & Wright RP (1972) The role of fresh cow’s milk in iron deficiency. II. Comparison of fresh cow’s milk with a prepared formula. Am J Dis Child 124, 26–30. [DOI] [PubMed] [Google Scholar]
- 49. Van Asperen PP, Kemp AS & Mellis CM (1984) Relationship of diet in the development of atopy in infancy. Clin Allergy 14, 525–532. [DOI] [PubMed] [Google Scholar]
- 50. Roduit C, Frei R, Loss G et al. (2012) Development of atopic dermatitis according to age of onset and association with early-life exposures. J Allergy Clin Immunol 130, 130–136.e5. [DOI] [PubMed] [Google Scholar]
- 51. Oski FA (1993) Iron deficiency in infancy and childhood. N Engl J Med 329, 190–193. [DOI] [PubMed] [Google Scholar]
- 52. Domellöf M, Braegger C, Campoy C et al. (2014) Iron requirements of infants and toddlers. J Pediatr Gastroenterol Nutr 58, 119–129. [DOI] [PubMed] [Google Scholar]
- 53. Ziegler EE (2011) Consumption of cow’s milk as a cause of iron deficiency in infants and toddlers. Nutr Rev 69, Suppl. 1, S37–S42. [DOI] [PubMed] [Google Scholar]
- 54. Kleinman RE (2009) Pediatric Nutrition Handbook, 6th ed. Chicago, IL: American Academy of Pediatrics. [Google Scholar]
- 55. Gerstein HC & VanderMeulen J (1996) The relationship between cow’s milk exposure and type 1 diabetes. Diabet Med 13, 23–29. [DOI] [PubMed] [Google Scholar]
- 56. Gerstein HC (1994) Cow’s milk exposure and type I diabetes mellitus: a critical overview of the clinical literature. Diabetes Care 17, 13–19. [DOI] [PubMed] [Google Scholar]
- 57. Patelarou E, Girvalaki C, Brokalaki H et al. (2012) Current evidence on the associations of breastfeeding, infant formula, and cow’s milk introduction with type 1 diabetes mellitus: a systematic review. Nutr Rev 70, 509–519. [DOI] [PubMed] [Google Scholar]
- 58. Michaelsen KF, Larnkjaer A & Molgaard C (2012) Amount and quality of dietary proteins during the first two years of life in relation to NCD risk in adulthood. Nutr Metab Cardiovasc Dis 22, 781–786. [DOI] [PubMed] [Google Scholar]
- 59. European Union (2006) Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae and amending Directive 1999/21/EC. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:401:0001:0033:EN:PDF (accessed July 2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980015001354.
click here to view supplementary material