Abstract
Context
Prader-Willi syndrome (PWS) is a rare neurobehavioral-metabolic disease caused by the lack of paternally expressed genes in the chromosome 15q11-q13 region, characterized by hypotonia, neurocognitive problems, behavioral difficulties, endocrinopathies, and hyperphagia resulting in severe obesity if not controlled.
Objective
The primary end point was change from baseline in hyperphagia using the Hyperphagia Questionnaire for Clinical Trials (HQ-CT). Other end points included Global Impression Scores, and changes in body composition, behaviors, and hormones.
Methods
In DESTINY PWS, a 13-week, randomized, double-blind, placebo-controlled, phase 3 trial, 127 participants with PWS aged 4 years and older with hyperphagia were randomly assigned 2:1 to diazoxide choline extended-release tablet (DCCR) or placebo.
Results
DCCR did not significantly improve hyperphagia (HQ-CT least-square mean (LSmean) [SE] −5.94 [0.879] vs −4.27 [1.145]; P = .198), but did so in participants with severe hyperphagia (LSmean [SE] −9.67 [1.429] vs −4.26 [1.896]; P = .012). Two of 3 secondary end points were improved (Clinical Global Impression of Improvement [CGI-I]; P = .029; fat mass; P = .023). In an analysis of results generated pre-COVID, the primary (HQ-CT; P = .037) and secondary end points were all improved (CGI-I; P = .015; Caregiver Global Impression of Change; P = .031; fat mass; P = .003). In general, DCCR was well tolerated with 83.3% in the DCCR group experiencing a treatment-emergent adverse event and 73.8% in the placebo group (not significant).
Conclusion
DCCR did not significantly improve hyperphagia in the primary analysis but did in participants with severe baseline hyperphagia and in the pre-COVID analysis. DCCR treatment was associated with significant improvements in body composition and clinician-reported outcomes.
Keywords: Prader-Willi syndrome, hyperphagia, DCCR
Prader-Willi syndrome (PWS) is a rare, complex genetic neurobehavioral/metabolic disorder with an estimated birth incidence of 1:15 000 to 1:20 000 (1, 2). PWS arises from lack of expression of paternally inherited imprinted genes on chromosome 15q11-q13 caused by a paternal deletion, maternal uniparental disomy 15, or an imprinting center defect, resulting in hypothalamic dysfunction (3). Clinical features of PWS include hypotonia and feeding difficulties in infancy and sustained accumulation of excess body fat beginning in early childhood (4). Hyperphagia presents as food obsession, aggressive food seeking, and lack of satiety, with progression to severe obesity if energy intake is not restricted, and occurs around a mean age of 9 years (4). PWS is also associated with intellectual disability, low muscle mass, neuroendocrine abnormalities including growth hormone and gonadotropin deficiency, behavioral problems including aggression, anxiety, and compulsivity, and elevated risk for early mortality (3, 5-7). According to a 2014 survey of caregivers of PWS patients, reducing hunger and improving food-related behaviors were the most important unmet needs in PWS that should be addressed in the development of a new therapeutic (8). There are no approved treatments for hyperphagia in PWS.
Diazoxide is a potent activator of the adenosine triphosphate–sensitive potassium (KATP) channel, and is capable of crossing the blood-brain barrier (9). Activating the KATP channel in neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons in the hypothalamus results in reduced secretion of NPY and AgRP, potent endogenous appetite-stimulatory neuropeptides, potentially contributing to a reduction in hyperphagia (10). These actions of the drug are complemented by activating the KATP channel in the dorsal motor nucleus of vagus, pancreatic β cells, and adipocytes to reduce hyperinsulinemia and excess body fat, and improve insulin and leptin resistance, and satiety (10). These effects have been confirmed in animal models of hyperphagic obesity including a model of PWS (Magel-2 null mouse) (10).
The increased solubility of diazoxide choline and its slow release from the extended-release once-per-day tablet formulation, DCCR, results in stable plasma concentrations compared to those seen with diazoxide oral suspension, which is usually given 2 to 3 times daily. Target daily doses of DCCR evaluated in PWS may be better tolerated than those of diazoxide equivalent doses, and efficacy in PWS may be achievable at diazoxide equivalent doses in the low range of the labeled dose range.
DCCR was tested in a phase 2 study (clinical study PC025) in participants with PWS in whom DCCR treatment significantly reduced hyperphagia, aggressive behavior, and body fat, and increased lean body mass (11). We therefore hypothesized that in people with PWS, DCCR compared with placebo would reduce hyperphagia and aggressive behaviors, and improve body composition.
Materials and Methods
DESTINY PWS was an international, randomized, double-blind, placebo-controlled, parallel-group, phase 3 study comparing DCCR to placebo in individuals with PWS. The study enrolled males and females with genetically confirmed PWS, aged 4 years and older with moderate to severe hyperphagia (Hyperphagia Questionnaire for Clinical Trials [HQ-CT] score ≥ 13), weighing between 20 and 135 kg, in a stable care setting, at 29 sites in the United States and United Kingdom. The enrolled participants who continued to meet the inclusion criteria at the end of a 2-week single-blind, placebo run-in were randomly assigned 2:1 to treatment with DCCR or matching placebo stratified by growth hormone treatment (currently treated, not currently treated) and baseline HQ-CT score (scores from 13-19 and from 20-36). Participants were included in 1 of 5 weight bands and titrated to target dose of 3.3 to 5.8 mg/kg within 6 weeks. Daily dose of DCCR ranged from 100 to 450 mg. Randomization codes were centrally generated and all study site staff, the sponsor and sponsor's on-site representatives, participants, and their caregivers were blinded until completion of the study. The primary end point was hyperphagia change from baseline through week 13 measured using the HQ-CT, a 9-item, validated questionnaire that assessed food-related behaviors associated with hyperphagia in people with PWS (range, 0-36) (12). Secondary end points included Clinical Global Impression of Improvement (CGI-I), Caregiver Global Impression of Change (GI-C), and body fat measured using dual-energy x-ray absorptiometry. Exploratory end points included other body composition parameters, behavioral complications of PWS assessed using the Prader-Willi Syndrome Profile (PWSP) Questionnaire and the Developmental Behavior Checklist version 2 parent edition (DBC2-P), and hormones involved in appetite, satiety, and fat metabolism (leptin, adiponectin, insulin, and acylated ghrelin), all measured at a central laboratory. The PWSP is a 59-item caregiver-completed questionnaire designed to assess a participant's non–food-related behaviors across 6 domains: aggressive behaviors, anxiety, rigidity/irritability, compulsivity, depression, and disordered thinking. The DBC includes 96 items used to assess the behavior and emotional problems in people aged 4 and older with developmental and intellectual disabilities. The questionnaire includes a subscale associated with PWS and subscales encompassing disruptive behaviors, self-absorbed, communication disturbance, anxiety, and social relating. The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was calculated using fasting insulin and glucose measurements from the central laboratory as [fasting insulin (μIU/mL) × fasting glucose (mmol/L)]/22.5.
The sample size was calculated using hyperphagia response data from the previous phase 2 study PC025, and the estimate of SD of HQ-CT change from a prior PWS obesity pharmacotherapy study (13). Based on the use of a 2-sided, 2-sample t test at α = .05 level of significance and a 2:1 randomization ratio, a total sample of 105 participants provided 90% power to detect an HQ-CT score difference of 5.0 between the groups. The primary end point was assessed as change from baseline at study week 4 (during titration to target dose) and at weeks 8 and 13 and was analyzed using a linear mixed model for repeated measurements including fixed effects for treatment group, time, the interaction between treatment and time, and the stratification variable (growth hormone use) using baseline HQ-CT score as a covariate and an unstructured covariance model. Hormonal, endocrine, and body composition parameters were analyzed using an analysis of covariance model with baseline as a covariate, and treatment group and the stratification variables as factors. CGI-I and Caregiver GI-C were compared between treatment groups using the Cochran-Mantel-Haenszel mean score test with modified ridit scores, stratified by the stratification variables. Changes in PWSP scores were tested using a Wilcoxon-Mann-Whitney test. If the primary end point was statistically significant, then each of the secondary end points was tested in order, with each subsequent analysis reported as significant only if the preceding analysis was significant. Consequently, no multiplicity adjustment was made to α and all analyses following the first nonsignificant ordered end point yielded nominal results. Data for most parameters are expressed as least-square means (LSmean) and standard errors (SEs).
The primary analysis was conducted on the full dataset consisting of all data from randomized participants (intent-to-treat [ITT] population). Since the COVID-19 pandemic resulted in considerable changes in environment and behavior for patients with PWS and their caregivers (14-17), potentially affecting qualitative data such as hyperphagia and other behaviors, a post hoc pre-COVID analysis using the same statistical methods was conducted using windowed visits and including data for all participants obtained before March 1, 2020. Although the United States and World Health Organization had both declared COVID-19 a health emergency on January 31, 2020, the use of masks, social distancing, closures of schools and clinical sites, and supply chain issues and other disruptive events did not begin to occur until late February 2020. As a consequence, a date of March 1, 2020, was selected since it precedes the aspect of the pandemic that more markedly affected the lives of participants in the study.
Post hoc exposure response analyses were conducted to explore trends in the relationship between circulating drug level and clinical end points using simple linear regression. Placebo recipients were included in the regression at a circulating drug concentration of 0 to anchor the regression.
Results
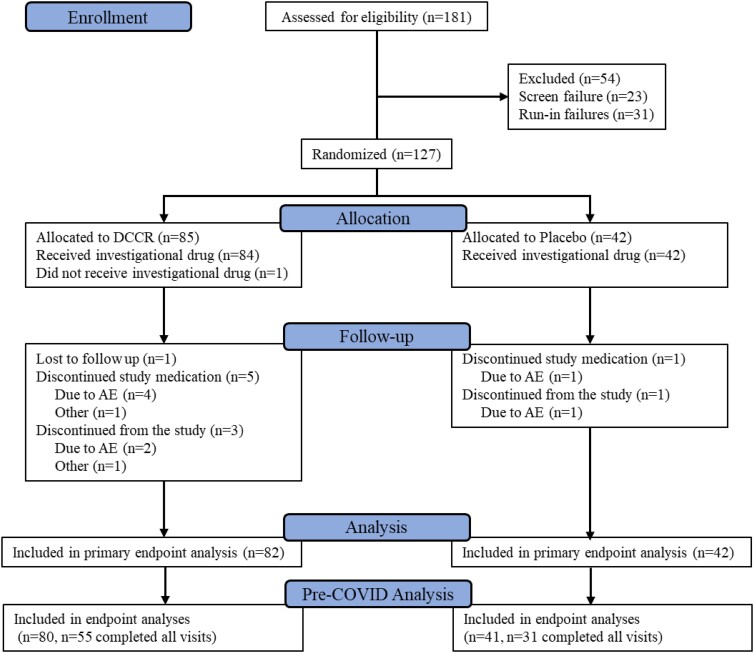
Prior to initation of the study, ethics committee review and approval of the protocol and study related materials was completed. Participants or their parents/guardians provided informed consent and, as appropriate, assent, prior to being enrolled in the study. From May 2018 until January 2020, 181 people with PWS were screened for the study, 158 of whom were enrolled; among these, 31 were not eligible for randomization because of failure to continue to meet inclusion criteria after the placebo run-in, and 127 were randomly assigned (Fig. 1). The ITT population consisted of 82 DCCR- and 42 placebo-treated participants who received study medication and had at least one postbaseline assessment of the primary end point (excluding 3 randomly assigned participants who either did not receive study medication or who lacked a postbaseline measurement of HQ-CT). Overall, the 2 groups were balanced with respect to baseline characteristics (Table 1).
Figure 1.
CONSORT diagram for the DESTINY PWS (C601) study.
Table 1.
Demographics and baseline characteristics of participants with Prader-Willi syndrome
| ITT population | |||
|---|---|---|---|
| DCCR | Placebo | Overall | |
| Total | 82 | 42 | 124 |
| Age, ya | 13.4 (6.82) | 13.6 (7.37) | 13.5 (6.98) |
| Race, n (%) | |||
| White | 71 (86.6) | 34 (81.0) | 105 (84.7) |
| Black or African American | 4 (4.9) | 2 (4.8) | 6 (4.8) |
| Asian | 0 (0.0) | 1 (2.4) | 1 (0.8) |
| American Indian or Alaska Native | 1 (1.2) | 0 (0.0) | 1 (0.8) |
| Native Hawaiian or Other Pacific Islander | 1 (1.2) | 0 (0.0) | 1 (0.8) |
| Other | 1 (1.2) | 2 (4.8) | 3 (2.4) |
| Multiple | 4 (4.9) | 3 (7.1) | 7 (5.6) |
| Ethnicity n (%) | |||
| Hispanic or Latino | 6 (7.3) | 7 (16.7) | 13 (10.5) |
| Not Hispanic or Latino | 75 (91.5) | 34 (81.0) | 109 (87.9) |
| Not reported | 1 (1.2) | 1 (2.4) | 2 (1.6) |
| Sex, n (%) | |||
| Male | 36 (43.9) | 19 (45.2) | 55 (44.4) |
| Female | 46 (56.1) | 23 (54.8) | 69 (55.6) |
| Height, cma | 146.3 (18.5) | 147.5 (20.1) | 146.7 (19.0) |
| HQ-CTa | 23.0 (6.03) | 21.9 (5.08) | 22.6 (5.73) |
| HQ-CT for HQ-CT > mediana | 28.0 (3.38) | 26.6 (2.65) | 27.61 (3.19) |
| Body fat mass, kga | 27.67 (16.62) | 26.47 (17.58) | 27.25 (16.9) |
| Lean body mass, kga | 29.25 (14.16) | 28.31 (12.52) | 28.92 (13.56) |
| Weight, kga | 62.2 (30.4) | 60.4 (29.6) | 61.6 (30.0) |
| BMIa | 27.7 (9.47) | 26.7 (9.88) | 27.3 (9.58) |
| Hormonal measures | |||
| Serum leptin, ng/mLa | 37.6 (28.6) | 36.3 (28.6) | 37.2 (28.5) |
| Serum adiponectin, μg/mLa | 11.21 (6.98) | 10.41 (6.09) | 10.95 (6.68) |
| Acylated ghrelin, pg/mLa | 242.0 (149.8) | 276.7 (187.7) | 253.7 (163.5) |
| Fasting insulin, μIU/mLa | 12.07 (15.25) | 9.74 (5.99) | 11.28 (12.90) |
| PWS genetic subtype, n (%) | |||
| Deletion | 48 (58.5) | 28 (66.7) | 76 (61.3) |
| Nondeletion | 33 (40.2) | 14 (33.3) | 47 (37.9) |
| Not available | 1 (1.2) | 0 (0.0) | 1 (0.8) |
| Country, n (%) | |||
| United Kingdom | 19 (23.2) | 6 (14.3) | 25 (20.2) |
| United States | 63 (76.8) | 36 (85.7) | 99 (79.8) |
| Growth hormone status, n (%) | |||
| Currently treated | 69 (84.1) | 35 (83.3) | 104 (83.9) |
| Not currently treated | 13 (15.9) | 7 (16.7) | 20 (16.1) |
Abbreviations: BMI, body mass index; DCCR, diazoxide choline extended-release tablet; HQ-CT, Hyperphagia Questionnaire for Clinical Trials; ITT, intent-to-treat; PWS, Prader-Willi syndrome.
Mean (SD).
Primary End Point
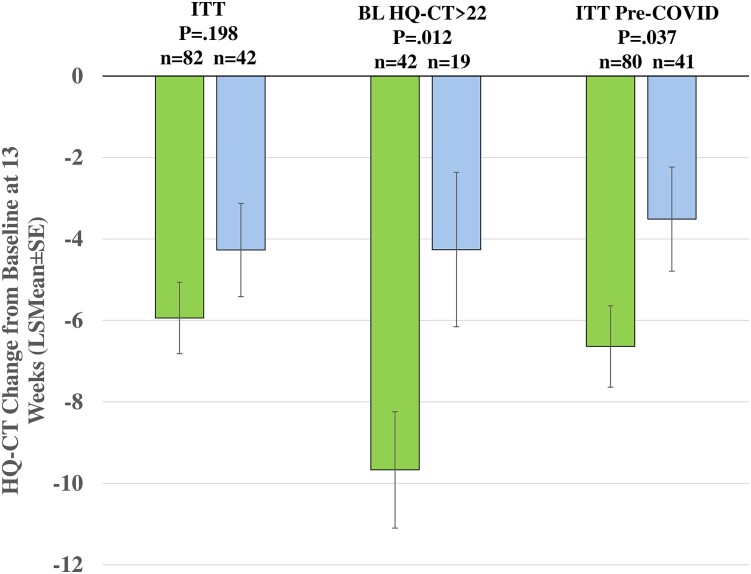
In the ITT population, DCCR, compared to placebo, was not associated with a statistically significant reduction in hyperphagia as measured by the HQ-CT (Fig. 2, LSmean [SE] −5.94 [0.879] vs −4.27 [1.145], DCCR vs placebo; P = .198). In a prespecified analysis focused on the 40 DCCR and 19 placebo participants with more severe hyperphagia (baseline HQ-CT > dichotomized median; > 22), DCCR resulted in a significantly greater reduction in HQ-CT score than placebo (see Fig. 2; LSmean [SE] −9.67 [1.429] vs −4.26 [1.896], DCCR vs placebo; P = .012).
Figure 2.
Hyperphagia Questionnaire for Clinical Trials (HQ-CT) change from baseline to week 13 for the intent-to-treat population (ITT), the preplanned analysis of participants with baseline HQ-CT score greater than median (>22) (BL HQ_CT > 22) and the post hoc analysis of HQ-CT data collected before March 1, 2020 (ITT pre-COVID). Diazoxide choline extended-release tablet (DCCR) in green, placebo in light blue.
Secondary End Points
There was a significant difference between the groups for CGI-I, with 36.7% of DCCR-treated participants minimally or much improved compared to 4.9% in the placebo group (P = .029). There was no significant difference between the groups for Caregiver GI-C, with 39.0% of DCCR-treated participants a little, moderately, or very much better and 13.0% worse, compared to 28.5% better and 23.8% worse in the placebo group (P = .409). There was a significant reduction in body fat mass in the DCCR group compared to the placebo group (Table 2, LSmean [SE] −0.80 kg [0.36] vs 0.25 kg [0.44] DCCR vs placebo; P = .023).
Table 2.
Summary of results on secondary and exploratory end points
| Change from baseline—full data set | Change from baseline—pre-COVID | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DCCR | Placebo | P | DCCR | Placebo | P | |||||
| Parameter | n | LSmean (SE) or mean (SD) | n | LSmean (SE) or mean (SD) | n | LSmean (SE) or mean (SD) | n | LSmean (SE) or mean (SD) | ||
| Secondary | ||||||||||
| CGI-I | 82 | 3.7 (0.78) | 42 | 4.0 (0.37) | .029 | 52 | 3.7 (0.81) | 30 | 4.0 (0.41) | .015 |
| Caregiver GI-C | 82 | 3.7 (1.13) | 42 | 4.0 (1.24) | .409 | 54 | 3.6 (1.23) | 30 | 4.3 (1.18) | .031 |
| Body fat, kga,b | 78 | −0.80 (0.36) | 42 | 0.25 (0.44) | .023 | 52 | −1.12 (0.39) | 31 | 0.36 (0.45) | .003 |
| Exploratory | ||||||||||
| Body composition | ||||||||||
| Lean body mass, kga,b | 59 | 1.4 (0.36) | 36 | 0.5 (0.42) | .058 | 51 | 1.5 (0.4) | 30 | 0.6 (0.47) | .112 |
| Lean body mass/body fat massa | 59 | 0.1 (0.03) | 36 | 0.0 (0.03) | .001 | 51 | 0.1 (0.03) | 30 | 0.0 (0.03) | .002 |
| PWSP domain | ||||||||||
| Aggressive behaviors | 75 | −1.2 (3.61) | 36 | −1.3 (3.61) | .857 | 48 | −1.5 (4.35) | 25 | −0.2 (3.18) | .048 |
| Anxiety | 75 | −3.1 (4.82) | 36 | −2.3 (4.90) | .181 | 48 | −3.6 (5.39) | 25 | −1.0 (4.04) | .018 |
| Compulsivity | 75 | −2.3 (3.31) | 37 | −1.4 (3.65) | .168 | 48 | −2.8 (3.45) | 26 | −0.5 (3.11) | .008 |
| Rigidity/Irritability | 75 | −2.8 (4.12) | 37 | −1.8 (4.51) | .15 | 48 | −3.4 (4.74) | 26 | −0.4 (3.53) | .003 |
| Depression | 75 | −0.7 (1.91) | 37 | −0.7 (1.84) | .857 | 48 | −0.9 (1.97) | 26 | −0.3 (1.51) | .185 |
| Disordered thinking | 74 | −1.3 (2.21) | 36 | −0.7 (1.94) | .154 | 47 | −1.5 (2.24) | 25 | −0.3 (1.59) | .011 |
| DBC-2 | ||||||||||
| Total score | 74 | −11.3 (17.07) | 36 | −10.5 (24.69) | .173 | 47 | −10.2 (18.14) | 25 | −3.4 (21.33) | .009 |
| DBC-2 subscales | ||||||||||
| Disruptive | 74 | −3.4 (6.31) | 36 | −3.4 (8.37) | .491 | 47 | −3.3 (6.28) | 25 | −1.3 (7.32) | .056 |
| Self-absorbed | 74 | −3.1 (5.24) | 36 | −2.8 (7.21) | .295 | 47 | −2.6 (5.59) | 25 | −1.1 (6.6) | .061 |
| Communication disturbance | 74 | −1.6 (2.67) | 36 | −1.0 (3.71) | .155 | 47 | −1.7 (2.58) | 25 | 0.2 (3.1) | .003 |
| Social relating | 74 | −1.5 (2.34) | 37 | −1.0 (2.82) | .073 | 47 | −1.2 (2.54) | 26 | −0.1 (2.17) | .008 |
| Hormonal and endocrine | ||||||||||
| Serum leptin, ng/mLa,b | 63 | −10.95 (2.34) | 35 | 3.16 (2.77) | < .001 | 53 | −11.94 (2.61) | 28 | 4.32 (3.14) | < .001 |
| Serum adiponectin, μg/mLa,b | 64 | 2.72 (0.47) | 37 | −0.78 (0.57) | < .001 | 54 | 2.89 (0.53) | 29 | −0.61 (0.66) | < .001 |
| Fasting insulin μIU/mLa | 69 | −4.71 (0.71) | 38 | −1.61 (0.97) | .011 | 53 | −5.11 (0.82) | 29 | −1.50 (1.12) | .011 |
| Fasting acylated ghrelin, pg/mLa | 58 | −3.4 (20.35) | 33 | 61.5 (24.79) | .018 | 49 | −7.3 (20.05) | 28 | 65.7 (24.25) | .009 |
| HOMA-IR | 62 | −1.02 (0.15) | 38 | −0.68 (0.19) | .149 | 47 | −1.28 (0.17) | 29 | −0.89 (0.21) | .1486 |
Abbreviations: Caregiver GI-C, Caregiver Global Impression of Change; CGI-I, Clinical Global Impression of Improvement; DBC-2, Developmental Behavior Checklist version 2 parent edition; DCCR, diazoxide choline extended-release tablet; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; LSmean, least-square mean; PWSP, Prader-Willi Syndrome Profile.
Some parameters could not be obtained for visits conducted by telemedicine after the onset of the COVID-19 pandemic.
LSmean (SE).
Exploratory End Points
Lean body mass/fat mass ratio was increased significantly in the DCCR group compared to the placebo group (see Table 2; P = .001), with a significant reduction in body fat mass in the DCCR group compared to the placebo group (see Table 2; P = .003) and a nonsignificant trend toward an increase in lean body mass (see Table 2; P = .058). There were no significant changes in weight or body mass index in the DCCR group compared to placebo.
The following were significantly reduced in the DCCR group compared to placebo (see Table 2): serum leptin (P < .001), acylated ghrelin (P = .018), and insulin (P = .011). Serum adiponectin was significantly increased in the DCCR group compared to placebo (P < .001). Change in HOMA-IR, a measure of insulin sensitivity or resistance, was not statistically different in the DCCR group compared to the placebo group (see Table 2; P = not significant [NS]). There were trends toward improvement in the DCCR group compared to placebo for the anxiety, compulsivity, rigidity/irritability, and disordered thinking domains of the PWS-P and for the DBC-2 total score (see Table 2).
Exposure Response Analysis
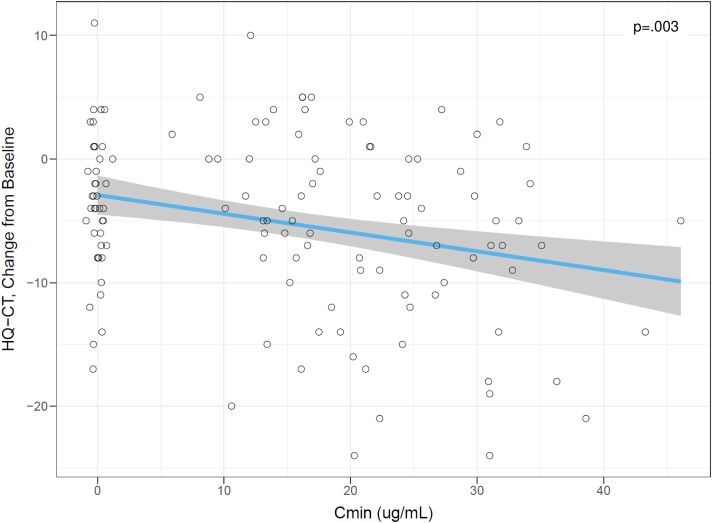
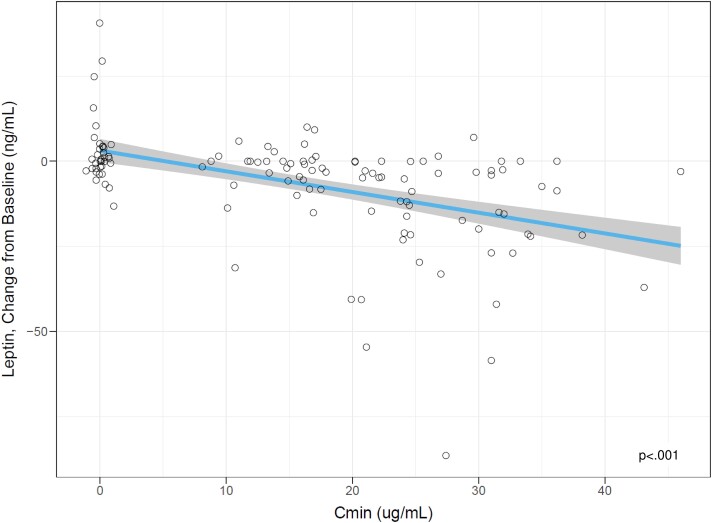
A post hoc analysis was conducted to evaluate the relationship between circulating drug levels and change from baseline for clinical end points of interest. Using measured circulating drug levels from visits at week 2, 4, 6, 8, and 13 and the corresponding HQ-CT change from baseline with placebo-treated participant data to anchor the intercept, there was a significant relationship between circulating drug level measured at trough (Cmin) and HQ-CT change from baseline, with baseline HQ-CT score as a significant covariate (Fig. 3; HQ-CT change from baseline = 4.87-0.135 × Cmin – 0.354 × baseline HQ-CT score; P = .003). Similarly, there were highly significant relationships between circulating drug levels (Cmin) and leptin change from baseline, with leptin baseline as a significant covariate (Fig. 4; leptin change from baseline = 1.46-0.364 × Cmin + 0.070 × (baseline leptin-32) – 0.012 × Cmin × (baseline leptin-32; P < .001)).
Figure 3.
Exposure-Response analysis for Hyperphagia Questionnaire for Clinical Trials) (HQ-CT change from baseline at week 13. Cmin, average trough concentration. Each point represents end-of-study results for one study participant. Placebo points are jittered for visualization purposes. The blue line is a linear regression fit. The shaded region represents 90% CI. The P value is the statistical significance level for the slope of the linear fit using a z test. Source: er-analysis.r.
Figure 4.
Exposure response analysis for leptin change from baseline at week 13. Cmin, average trough concentration; HQ-CT, Hyperphagia Questionnaire for Clinical Trials. Each point represents end-of-study results for one study participant. Placebo points are jittered for visualization purposes. The blue line is a linear regression fit. The shaded region represents 90% CI. The P value is the statistical significance level for the slope of the linear fit using a z test. Source: er-analysis.r.
Pre-COVID Results
The pre-COVID analysis included all data available before March 1, 2020. New procedures were implemented in the study in response to the COVID pandemic that provided for the collection of key questionnaire data in the absence of being able to conduct on-site visits. Some sites were closed for on-site visits before the new procedures were implemented, resulting in missing data. Because the primary end point was analyzed using a mixed model for repeated measures, it included 121 participants who had at least one postbaseline assessment of HQ-CT (n = 80 for DCCR and n = 41 for placebo), among whom 86 had completed treatment in the study before March 1, 2020 (n = 55 DCCR and n = 31 placebo). Statistically significant differences between DCCR and placebo were found for the primary, and all secondary end points, most of the objective exploratory end points, and nearly all the caregiver-assessed end points. DCCR treatment was associated with a significant reduction in HQ-CT score compared to placebo (see Fig. 2, LSmean [SE] −6.6 [1.0], n = 80 vs -3.5 [1.3] n = 41; P = .037). A sensitivity analysis, distinct from the pre-COVID analysis of the aforementioned primary end point and shown in Fig. 2, was conducted using data only from participants who had completed the end of study visit before March 1, 2020. In the sensitivity analysis, there was a significant difference between the groups for HQ-CT change from baseline at 13 weeks (LSmean [SE] −6.54 [1.083] n = 53 vs −3.33 [1.375] n = 29 DCCR vs placebo; P = .0451). Each of the secondary end points was significant (see Table 2; CGI-I P = .015; Caregiver GI-C P = .031, and fat mass P = .003). The pattern, magnitude, and significance of changes in body composition were similar in the analysis of the full data set and in the pre-COVID analysis (see Table 2), as were the changes in endocrine and hormonal markers, which were all significantly improved in the DCCR group vs placebo, with the exception of HOMA-IR. Significant improvements were also seen in 5 of 6 domains of the PWS-P in the DCCR group compared to placebo including aggressive behaviors (P = .048), anxiety (P = .018), compulsivity (P = .008), rigidity/irritability (P = .003), and disordered thinking (P = .011). Similarly, significant behavioral improvements were seen in the DCCR group compared to placebo for DBC-2 total score (P = .009) and the communication disturbance (P = .003) and social-relating (P = .008) subscales.
Safety
Patients with PWS experience frequent complications and comorbidities. Treatment-emergent adverse events (TEAEs) occurred in 83.3% of participants in the DCCR group compared to 73.8% of the placebo group (Fisher exact test, NS, Table 3). Study drug–related TEAEs occurred in 63.1% of participants in the DCCR group compared to 52.4% of the placebo group (Fisher exact test, NS). Six participants experienced serious AEs in the study, all occurring in the DCCR group, which in only one participant was considered drug related (peripheral and pulmonary edema in the setting of an unrelated concurrent pneumonia). The most common TEAEs in the DCCR compared to the placebo group were hypertrichosis (35.7% vs 14.3%, Fisher exact test; P < .05), peripheral edema (20.2% vs 9.5%, Fisher exact test; NS), and hyperglycemia (11.9% vs 0.0%, Fisher exact test; P < .05) (Table 3). These TEAEs rarely resulted in discontinuation of study drug (1 discontinuation related to peripheral edema and 1 related to hyperglycemia), but occasionally required blinded dose adjustment or other intervention (6 in the DCCR group and 0 in placebo). Pyrexia, when it occurred, was typically associated with respiratory infection and was viewed in nearly all cases as not related to study medication. Headache, diarrhea, and viral infections were more frequent in the placebo than the DCCR group (14.3% vs 6.0%, 9.5% vs 1.2%, 7.1% vs 1.2%, respectively, for placebo vs DCCR; Fisher exact test; P < .05 for diarrhea) as were AEs commonly associated with PWS, including aggression, intentional self-injury, and premature menarche (28.6% vs 14.3%, placebo vs DCCR; odds ratio 2.4; 95% CI, 0.97-5.94) (Table 3). Consistent with the observation of hyperglycemia as an AE, fasting glucose rose through week 6 (during titration) and stabilized thereafter through the end of blinded treatment at week 13 (DCCR mean ± SD 0.29 ± 1.07 mmol/L, median 0.10 mmol/L; placebo mean ± SD −0.01 ± 0.56 mmol/L, median -0.05 mmol/L). There was a small but significant increase from baseline in glycated hemoglobin A1c in the DCCR group compared to placebo (DCCR mean ± SD 0.15 ± 0.61%, median 0.0%; placebo mean ± SD −0.09 ± 0.24%, median −0.1%; P = .023) consistent with the small rise in glucose concentration. Overall, DCCR was well tolerated with the majority of AEs being grade 1.
Table 3.
Summary of treatment-emergent adverse events
| DCCR n (%) | Placebo n (%) | |
|---|---|---|
| Total | 84 | 42 |
| TEAE | 70 (83.3) | 31 (73.8) |
| TEAE related to study druga | 53 (63.1) | 22 (52.4) |
| SAEa | 6 (7.1) | 0 (0.0) |
| SAE related to study drug | 1 (1.2) | 0 (0.0) |
| TEAE leading to premature study discontinuation | 4 (4.8) | 1 (2.4) |
| TEAEs occurring in more than 5% of participants in either group ordered by overall incidence | ||
| Hypertrichosisb | 30 (35.7) | 6 (14.3) |
| Peripheral edemab | 17 (20.2) | 4 (9.5) |
| Upper respiratory tract infections | 9 (10.7) | 5 (11.9) |
| Headache | 5 (6.0) | 6 (14.3) |
| Hyperglycemiab | 10 (11.9) | 0 (0.0) |
| Hirsutism | 6 (7.1) | 3 (7.1) |
| Blood glucose increased | 5 (6.0) | 2 (4.8) |
| Diarrhea | 1 (1.2) | 4 (9.5) |
| Pyrexia | 5 (6.0) | 0 (0.0) |
| Abdominal pain | 1 (1.2) | 3 (7.1) |
| Viral infections | 1 (1.2) | 3 (7.1) |
| TEAEs that are common complications and comorbidities of PWS | 12 (14.3) | 12 (28.6) |
Abbreviations: DCCR, diazoxide choline extended-release tablet; SAE, serious adverse event; TEAE, treatment-emergent adverse event; PWS, Prader-Willi syndrome.
Fisher exact test statistic result was not statistically significant.
Fisher exact test statistic result was significant at P less than .05.
Discussion
Hyperphagia, the hallmark of PWS, was improved significantly by DCCR among those with more severe hyperphagia and in the pre-COVID analysis, but not in the ITT population analysis. The observed improvement likely results from the combination of the direct effects of DCCR in NPY/AgRP neurons and from improvements in hyperinsulinemia and leptin resistance (10). The improvement seen with DCCR treatment in those with more severe hyperphagia at baseline was consistent with the results of the phase 2 study in PWS, in which those with more severe hyperphagia also had greater improvements (11). Individuals with more severe hyperphagia at baseline are likely to have more marked central insulin and leptin resistance and hence greater dysregulation of NPY/AgRP secretion, which consequently increases orexigenic signaling and impairs anorectic signaling through MC4R. DCCR treatment of these individuals reinforces the inhibitory action of insulin and leptin on NPY/AgRP secretion, which may have a greater impact in these individuals compared to those with less severe hyperphagia.
The observed reductions in leptin and insulin concentrations are consistent with the proposed mode of action of DCCR. The decrease in leptin appears to be in excess of what would be predicted by loss of body fat alone and may suggest a direct effect of DCCR on leptin secretion, an improvement in leptin resistance, or a reduction in leptin secondary to a reduction in insulin concentration (18, 19). Adiponectin concentration is increased in patients with PWS (20), and the further increases in adiponectin associated with DCCR treatment may result in reduced systemic inflammation, improved insulin sensitivity, and cardiovascular health (20). Levels of acylated ghrelin decreased in the DCCR group, rather than increasing, which would be expected in PWS with loss of body fat and reduction of serum leptin and insulin levels (21, 22). Acylated ghrelin concentrations are higher in PWS compared to people without PWS (19), and the prevention of the rise in acylated ghrelin by DCCR may have contributed to the observed improvements in hyperphagia, though studies aimed at reducing ghrelin action have not confirmed the role of ghrelin for the hyperphagia of PWS. Improvements in hyperphagia have the potential to allow the person with PWS to focus their attention on activities rather than food and ease the burden of care on their caregiver, typically a parent. Once a person with PWS develops hyperphagia, access to food must be strictly controlled, and situations where food is present must be closely managed. Some of these constraints on access to food and the level of supervision around food may begin to be relaxed if the improvements in hyperphagia are sustained. Increases in muscle mass and reductions in body fat were viewed by parents and caregivers as important or very important responses to a new therapeutic (8). The loss of body fat, which was baseline fat mass dependent, the trend toward increased lean body mass, and the increases in the lean body mass/fat mass ratio were all consistent with the results from the phase 2 study of DCCR in PWS (11).
There was a clear linear relationship between circulating drug levels and key therapeutic responses to treatment including HQ-CT and leptin. These results further confirm the relationship between DCCR administration and responses to treatment that are viewed as important to PWS participants and their families.
The effect on other behavioral aspects of the condition—anxiety, compulsivity, and rigidity/irritability—were more obvious in the pre-COVID analyses. Subjective end points, such as change from baseline in hyperphagia and characteristic PWS-associated behaviors, were significantly improved by DCCR in the pre-COVID analysis only, whereas objective end points such as change in body fat and various metabolic parameters remained positive regardless of the analysis data set. It may also be indicative of a strong placebo effect for these subjective measures, including the HQ-CT primary outcome measure, which decreased on average by more than 4 points in placebo-treated participants from baseline to final follow-up. The difference between pre-COVID analysis and the analysis of the full data set reflects the abrupt and extensive nature of the changes in routine associated with the onset of the pandemic, the challenges people with PWS generally face in adapting to changes in routine and environment, especially the lack of school routine, the elevated stress level of caregiver, participant, and the participant's family, and the fact that nearly all participants spent considerably more time with their caregiver(s) compared to the pre-COVID period. These effects have been confirmed by other studies typically showing worsening of characteristic symptoms but also improvements in some participants (14-17).
Further evidence of the broad-ranging effect of DCCR is supported by the AE profile, as participants on DCCR experienced AEs commonly considered as comorbidities of PWS (aggression, intentional self-injury, etc) at half the rate as those on placebo.
The safety profile of DCCR in participants with PWS was consistent with that observed in prior studies and with the known safety profile of diazoxide. Three AEs were carefully surveilled and consistently assessed: hyperglycemia, including other related AE terms, peripheral edema, and hypertrichosis/hirsutism. There were assessments of hypertrichosis/hirsutism and peripheral edema at each site visit during the physical exam. Hyperglycemia tended to occur more frequently in older, obese participants who may have been predisposed to hyperglycemia, but also tended to receive higher DCCR doses and consequently had higher circulating drug levels. To effectively manage hyperglycemia, 3 participants received concomitant metformin and 3 individuals had dose reductions in DCCR.
Although the primary efficacy outcome of this study (change in HQ-CT score at 13 weeks) was not statistically different in DCCR vs placebo in the entire cohort, DCCR reduced hyperphagia in those with more severe hyperphagia, and in the subset of data collected before the onset of the COVID-19 pandemic. The data also show improved body composition as well as adipokines, acylated ghrelin, and insulin, and suggest the potential for improvement of a range of behavioral difficulties in PWS. Many of these outcomes are aligned with what have been identified as the highest-priority unmet needs in the syndrome by parents and caregivers. If improvements in hyperphagia and other behaviors are sustained, they may result in improved quality of life for these patients and a reduced burden of care for their families and thereby may benefit people with PWS and their families.
Acknowledgments
In addition to the majority of the authors, the DESTINY PWS Investigators include Urmi Das, MD, at Alder Hey Children's Hospital NHS Foundation Trust, Liverpool, UK; Amy Fleischman, MD, at Boston Children's Hospital, Boston, Massachusetts, USA; Anthony Goldstone, MD, at Hammersmith Hospital, London, UK; Katerina Harwood, MD, at St Joseph's University Medical Center, Paterson, New Jersey, USA; Prof Anthony Holland at Fulbourn Hospital, Cambridge, UK; Virginia Kimonis, MD, at the University of California Irvine, Orange, California, USA; Shawn McCandless, MD, at Children's Hospital Colorado, Aurora, Colorado, USA; Lori Anne Schillaci, MD, at University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA; Mohammed Shaikh, MD, at The Queen Elizabeth University, Glasgow, Scotland, UK; Heidi Shea, MD, at Research Institute of Dallas, Dallas, Texas, USA; David Viskochil, MD, at the University of Utah, Salt Lake City, Utah, USA; and Prof John Wilding at Aintree University Hospital NHS Foundation Trust, Liverpool, UK.
The authors would like to acknowledge the important contributions of Tara Murray and Natasha Thorn of Queen Mary University London/Barts Health NHS Trust, Rhian Bull and Dr Isha Duraisingam of Chelsea and Westminster Hospital, Kaitlin L. Ballenger and Sheila M. Brady of the NIH, Sue Kerns of Seattle Children's, Emily Viall and Bonny Bowen of the Research Institute at Nationwide Children's Hospital, Lynette Gonzalez and Wanda Sanchez at Emory Children's, Rachel Winograd of Rady Children's Hospital of San Diego, Nathan Bingham of Vanderbilt University, Dr Waheeda Hossain of the Kansas University Medical Center, Dr Diane Stafford and Dr Hilary Seeley of Stanford University, Brittany Machus and Briana Escobar of Children's Minnesota, Dr Ruth Krone and Sr Gemma Grosvenor of Birmingham Women's and Children's Hospital NHS Foundation Trust, Susan Romie of the Indiana University School of Medicine, Jennifer Boak of the Sparrow Clinical Research Institute, and Dr June Anne Gold and Ajanta Naidu of the University of California, Irvine, to the conduct of this clinical study. The authors would also like to acknowledge the significant contributions of Kristen Yen and Patricia Hirano of Soleno Therapeutics.
Abbreviations
- AgRP
agouti-related protein
- Caregiver GI-C
Caregiver Global Impression of Change
- CGI-I
Clinical Global Impression of Improvement
- Cmin
circulating drug level measured at trough
- DBC
Developmental Behavior Checklist
- DBC2-P
Developmental Behavior Checklist version 2 parent edition
- DCCR
diazoxide choline extended-release tablet
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- ITT
intent-to-treat
- KATP
adenosine triphosphate–sensitive potassium
- LSmean
least-square mean
- NPY
neuropeptide Y
- NS
not significant
- PWSP
Prader-Willi Syndrome Profile
- TEAE
treatment-emergent adverse event
Contributor Information
Jennifer L Miller, Department of Pediatric Endocrinology, University of Florida College of Medicine, Gainesville, Florida 32608, USA.
Evelien Gevers, Queen Mary University London, London E1 4NS, UK; Barts Health NHS Trust-Royal London Children's Hospital, London E1 1FR, UK.
Nicola Bridges, Chelsea and Westminster Hospital, London, UK.
Jack A Yanovski, US Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland 20892, USA.
Parisa Salehi, Endocrinology, Seattle Children's Hospital, Seattle, Washington 98105, USA.
Kathryn S Obrynba, Endocrinology, Nationwide Children's Hospital, Columbus, Ohio 43205, USA.
Eric I Felner, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia 30322, USA.
Lynne M Bird, University of California, San Diego/Rady's Children's Hospital, San Diego, California 92123, USA.
Ashley H Shoemaker, Vanderbilt University Medical Center, Nashville, Tennessee 37240, USA.
Moris Angulo, NYU Langone Health, Mineola, New York 11501, USA.
Merlin G Butler, University of Kansas Medical Center, Kansas City, Kansas 66160, USA.
David Stevenson, Stanford University, Palo Alto, California 94305, USA.
Jennifer Abuzzahab, Children's Minnesota, Minneapolis, Minnesota 55404, USA.
Timothy Barrett, Birmingham Women's and Children's Hospital, Birmingham B15 2TG, UK.
Melissa Lah, Indiana University School of Medicine, Indianapolis, Indiana 46202, USA.
Elizabeth Littlejohn, Sparrow Clinical Research Institute, Lansing, Michigan 48912, USA.
Verghese Mathew, Hull and East Yorkshire Hospitals NHS Trust, Hull HU3 2JZ, UK.
Neil M Cowen, Soleno Therapeutics, Redwood City, California 94065, USA.
Anish Bhatnagar, Soleno Therapeutics, Redwood City, California 94065, USA.
the DESTINY PWS Investigators:
Urmi Das, Amy Fleischman, Anthony Goldstone, Katerina Harwood, Prof Anthony Holland, Virginia Kimonis, Shawn McCandless, Lori Anne Schillaci, Mohammed Shaikh, Heidi Shea, David Viskochil, and Prof John Wilding
Financial Support
This work was supported by Soleno Therapeutics.
Data Availability
Some or all of the data sets generated and/or analyzed during the present study are not publicly available but are available from Soleno Therapeutics for further research on reasonable request.
Clinical Trial Information
ClinicalTrials.gov registration No. NCT03440814 (22 February 2018).
References
- 1. Lionti T, Reid SM, White SM, Rowell MM. A population-based profile of 160 Australians with Prader-Willi syndrome: trends in diagnosis, birth prevalence and birth characteristics. Am J Med Genet A. 2015;167A(2):371‐378. [DOI] [PubMed] [Google Scholar]
- 2. Bar C, Diene G, Molinas C, Bieth E, Casper C, Tauber M. Early diagnosis and care is achieved but should be improved in infants with Prader-Willi syndrome. Orphanet J Rare Dis. 2017;12(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Butler MG, Miller JL, Forster JL. Prader-Willi syndrome—clinical genetics, diagnosis and treatment approaches: an update. Curr Pediatr Rev. 2019;15(4):207‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A. 2011;155A(5):1040‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCandless SE. Clinical report-health supervision for children with Prader-Willi syndrome. Pediatrics. 2011;127(1):195‐204. [DOI] [PubMed] [Google Scholar]
- 6. Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi Association (USA) 40-year mortality survey. Genet Med. 2017;19(6):635‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellis SA, Kuhn I, Adams S, Mullarkey L, Holland A. The consequences of hyperphagia in people with Prader-Willi syndrome: a systematic review of studies of morbidity and mortality. Eur J Med Genet. 2022;65(1):104379. [DOI] [PubMed] [Google Scholar]
- 8. Foundation for Prader-Willi Research. Summary of the impact of PWS on individuals and their families and views on treatments: results of an international online survey. Accessed October 27, 2022. https://www.fpwr.org/pws-patient-voices
- 9. Kishore P, Boucai L, Zhang K, et al. Activation of KATP channels suppresses glucose production in humans. J Clin Invest. 2011;121(12):4916‐4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cowen N, Bhatnagar A. The potential role of activating the ATP-sensitive potassium channel in the treatment of hyperphagic obesity. Genes (Basel). 2020;11(4):450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimonis V, Surampalli A, Wencel M, Gold JA, Cowen NM. A randomized pilot efficacy and safety study of diazoxide choline controlled-release in patients with Prader-Willi syndrome. PLoS One. 2019;14(9):e0221615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fehnel S, Brown TM, Nelson L, et al. Development of the hyperphagia questionnaire for use in Prader-Willi syndrome clinical trials. Value Health. 2015;18(3):PA25. [Google Scholar]
- 13. McCandless SE, Yanovski JA, Miller J, et al. Effects of MetAP2 inhibition on hyperphagia and body weight in Prader-Willi syndrome: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2017;19(12):1751‐1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wieting J, Eberlein C, Bleich S, Frieling H, Deest M. Behavioural change in Prader-Willi syndrome during COVID-19 pandemic. J Intellect Disabil Res. 2021;65(7):609‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bohonowych J. PWS registry data: impact of COVID-19 on PWS families. Foundation for Prader-Willi Research. Published November 23, 2020, Accessed December 29, 2022. https://www.fpwr.org/blog/pws-registry-data-impact-of-covid-19-on-pws-families-infographic
- 16. Mosbah H, Coupaye M, Jacques F, et al. Effects of the COVID-19 pandemic and lockdown on the mental and physical health of adults with Prader-Willi syndrome. Orphanet J Rare Dis. 2021;16(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohr AK, Laemmer C, Schulte S, Gohlke B. Effects of COVID-19 lockdown on weight, body composition, and behavior of children, adolescents, and young adults with Prader-Willi syndrome. J Clin Med. 2021;10(20):4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levy JR, Gyarmati J, Lesko JM, Adler RA, Stevens W. Dual regulation of leptin secretion: intracellular energy and calcium dependence of regulated pathway. Am J Physiol Endocrinol Metab. 2000;278(5):E892‐E901. [DOI] [PubMed] [Google Scholar]
- 19. Rissanen P, Mäkimattila S, Vehmas T, Taavitsainen M, Rissanen A. Effect of weight loss and regional fat distribution on plasma leptin concentration in obese women. Int J Obes Relat Metab Disord. 1999;23(6):645‐649. [DOI] [PubMed] [Google Scholar]
- 20. Hui X, Lam KSL, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;163(3):574‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin X, Li Y, Xu G, An W, Zhang W. Ghrelin fluctuation, what determines its production? Acta Biochim Biophys Sin (Shanghai). 2009;41(3):188‐197. [DOI] [PubMed] [Google Scholar]
- 22. Chabot F, Caron A, Laplante M, St-Pierre DH. Interrelationships between ghrelin, insulin and glucose homeostasis: physiological relevance. World J Diabetes. 2014;5(3):328‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all of the data sets generated and/or analyzed during the present study are not publicly available but are available from Soleno Therapeutics for further research on reasonable request.