Abstract
Context
Bone turnover markers (BTMs) are lower in type 2 diabetes mellitus (T2D). The relationships between bone turnover, β-cell function, and insulin sensitivity in T2D are uncertain.
Objective
To investigate if fasting levels of BTMs in persons with T2D are associated with β-cell function or insulin sensitivity.
Methods
We defined three T2D phenotypes, the insulinopenic (low β-cell function, high insulin sensitivity), the classical (low β-cell function, low insulin sensitivity), and the hyperinsulinemic (high β-cell function, low insulin sensitivity) phenotypes, in the Danish Centre for Strategic Research T2D cohort using the homeostatic model assessment. We selected age- and gender-matched subgroups to represent the three T2D phenotypes, yielding 326 glucose-lowering treatment–naïve persons with T2D. Median values of BTMs between the three T2D phenotypes were compared. Regression models were applied to assess the association between BTMs, β-cell function, and insulin sensitivity adjusted for potential confounders.
Results
Median serum levels of procollagen type I N-terminal propeptide, C-terminal telopeptide of type I collagen, and osteocalcin were higher in the insulinopenic phenotype (52.3 μg/L, IQR 41.6, 63.3; 259.4 ng/L, IQR 163.4, 347.7; and 18.0 μg/L, IQR 14.4, 25.2, respectively) compared with the classical (41.4, IQR 31.0, 51.4; 150.4 IQR 103.5, 265.1; 13.1, IQR 10.0, 17.6, respectively) and the hyperinsulinemic (43.7, IQR 32.3, 57.3; 163.3, IQR 98.9, 273.1; 15.7 IQR 10.2, 20.8, respectively) phenotypes (all P < .01). These differences persisted after adjustment for age, sex, waist to hip ratio, or fasting plasma glucose (P < .01).
Conclusion
BTMs are lower in newly diagnosed persons with T2D characterized by low insulin sensitivity.
Keywords: Type 2 diabetes mellitus, PINP, CTX, insulin, β-cell function, sensitivity
Bone is a dynamic organ that is resorbed and formed in a continuous, coupled, and tightly regulated process known as bone remodeling, which conserves skeletal integrity and maintains calcium homeostasis (1). With age, bone resorption surpasses bone formation resulting in bone loss and higher risk of osteoporosis in men and women (2). Circulating levels of bone turnover markers (BTMs), including serum N-terminal propeptide of type I procollagen (PINP) as a reference marker of bone formation and serum C-terminal telopeptide of type I collagen (CTX) as a reference marker of bone resorption, recommended by the International Osteoporosis Foundation and the International Federation of Clinical Chemistry (3), reflect overall bone resorption and formation. Also, measures of BTMs are commonly used clinically to assess the response to antiresorptive therapies in persons with osteoporosis (2).
Although a reduction in BTMs following initiation of antiresorptive treatment in persons with osteoporosis indicates a favorable effect (1), lower levels of BTMs may not uniformly associate with a lower fracture risk. In spite of lower BTMs in type 2 diabetes mellitus (T2D) (4), persons with T2D have a 1.3-fold increased risk of hip fractures (5) and a 1.3 times higher risk of incident vertebral fractures (6). The higher fracture risk is not explained by accelerated age-related bone loss (7) or by low bone mineral density (BMD) considering that BMD is normal or even high in persons with T2D (8). Even though BTMs do not predict fracture risk in persons with T2D (9), long-term suppressed bone turnover in T2D may negatively affect bone quality by impairing microdamage repair resulting in accumulation of bone microcracks (10, 11). The mechanisms explaining the diminution in bone turnover in T2D remain unsettled. Preclinical investigations show that hyperglycemia promotes adipogenesis rather than osteogenesis, impairs osteoblast growth and apoptosis (12-14), and inhibits osteoclastogenesis (15), corresponding to a state of low bone turnover. Also, high levels of sclerostin, an osteocyte-secreted endogenous inhibitor of bone formation, may in part explain the lower levels of bone formation markers in persons with T2D (16).
T2D is a heterogeneous disease caused by insulin resistance and inadequate insulin secretion (17). Clinical characteristics such as insulin sensitivity, β-cell function, age, hemoglobin A1C (HbA1c), and body mass index (BMI) have recently been used to identify T2D phenotypes that differ regarding β-cell function and insulin sensitivity, treatment response, and risk of diabetes complications (18, 19). One of these classifications included three T2D phenotypes based on the homeostatic model assessment (HOMA): the insulinopenic phenotype, characterized primarily by low β-cell function and high insulin sensitivity, the classical phenotype, defined by a combination of low β-cell function and low insulin sensitivity, and the hyperinsulinemic phenotype, marked by low insulin sensitivity and high β-cell function (18, 20). Meanwhile, it remains to be determined whether variations in β-cell function or insulin sensitivity explain abnormalities in levels of BTMs, BMD, and fracture risk in persons with T2D.
Insulin is anabolic to bone due to stimulatory effects on osteoblast proliferation and differentiation (21-23), suggesting that insulin levels may correlate with biochemical markers of formation and subsequently resorption. However, insulin resistance in bone-forming cells could blunt the anabolic effect of insulin (24), leading to lower levels of BTMs in persons with T2D characterized by severe insulin resistance. In a cross-sectional study including persons with dysglycemia (25), BTMs including CTX, PINP, and osteocalcin (OC) were negatively associated with insulin resistance and were positively associated with β-cell function. However, in the same study, HbA1c levels were different across the quartiles compared, which may in part influence the associations observed between BTMs and insulin resistance or β-cell function across the different quartiles. The relationship between insulin levels and BTMs is challenging to investigate, as medications used to control glucose levels in T2D such as metformin and insulin may increase bone turnover indirectly by improving glycemic control (26) and directly through interaction with bone cells (27).
In order to elucidate the relationship between bone turnover and β-cell function and insulin sensitivity, we investigated whether serum levels of PINP and OC, as markers of bone formation (3), and CTX, as a marker of bone resorption (3), are higher in persons with T2D characterized by high β-cell function than in persons with T2D characterized by low β-cell function. Furthermore, we investigated whether increased fasting plasma glucose levels are associated with lower levels of biochemical markers of bone formation and resorption in persons with T2D.
Materials and Methods
Study Design and Research Subjects
The study is based on data and blood samples from the Danish Centre for Strategic Research in T2D (DD2) project cohort. The DD2 cohort has enrolled persons with newly diagnosed T2D by general practitioners and hospital outpatient clinics throughout Denmark since 2010 (28). Participants in the DD2 cohort were clinically diagnosed with T2D according to World Health Organization criteria (29). The implementation and design of the cohort has previously been described (30). Persons with newly clinically diagnosed T2D went through a detailed interview and clinical examination at enrollment. Supplementary data such as cardiovascular events, microvascular diseases, and treatments were obtained through linkage with the Danish National Patient Registry (31) and the Danish National Health Service Prescription Registry (32) by using the civil registration number, which is a unique person identifier assigned to all inhabitants in Denmark. In addition, fasting blood samples were obtained for the participants (33, 34).
For the present study, we first identified 5988 persons with T2D included in the DD2 cohort between November 2010 and February 2015. Secondly, persons with no available measurements of glutamic acid decarboxylase antibodies (GADA) were excluded to ensure there was no misclassification of persons with type 1 diabetes (T1D) (GADA-positive persons with age <30 years and fasting C-peptide <300 pmol/L). Then, persons with latent autoimmune diabetes in adults (GADA-positive >20 U/mL and >30 years old), secondary diabetes (history of pancreatitis, hemochromatosis, cystic fibrosis, or pancreas resection prior to diabetes debut), and glucocorticoid-induced diabetes (persons who were prescribed oral glucocorticoids within 3 months before inclusion) were excluded. In addition, persons with no available measurements of fasting plasma glucose or serum C-peptide were excluded. This left 4285 persons with T2D eligible for phenotype characterization. For this study, we excluded persons treated with glucose-lowering medications resulting in 668 glucose-lowering treatment–naïve persons with T2D. Then, we excluded persons treated with osteoporosis medications, and persons who suffered from conditions known to affect serum BTMs levels such as primary hyperparathyroidism or other known calcium metabolic disorders, thyrotoxicosis, celiac disease, inflammatory bowel disease, recent fractures (<6 months), chronic kidney disease, liver cirrhosis, and pregnant women. Subsequently, persons who had low β-cell function and high insulin sensitivity were selected and matched to persons who either had low β-cell function and low insulin sensitivity or high β-cell function and low insulin sensitivity in a 1:2:2 manner based on age and gender, which yielded 326 glucose-lowering treatment–naïve persons with T2D.
Biochemical Tests
Fasting plasma glucose, fasting serum C-peptide, and GADA measurements were performed in the ISO 15189 accredited laboratory at the Center Hospital Lillebaelt, Region of Southern Denmark. An enzymatic hexokinase method (Glucoquant Glucose/HK, Roche Diagnostics, Mannheim, Germany) was used to analyze fasting plasma glucose in mmol/L. Fasting serum C-peptide (pmol/L) was measured by using the Roche C-Peptide assay (Roche Diagnostics, Mannheim, Germany). BTM measurements were carried out at Glostrup (Rigshospitalet) Hospital, Copenhagen, Denmark. BTMs were measured in fresh samples that had not undergone a previous thaw cycle by use of an automated immunoassay system (iSYS, Immunodiagnostic Systems Ltd., Boldon, UK) in a single run with the same batch of the reagents. PINP (μg/L), CTX (ng/L), and OC (μg/L) were measured using a chemiluminescence method. The intra-assay and inter-assay coefficients of variation (CVs) were 1.0% and 1.7%, respectively, for fasting plasma glucose. For fasting serum C-peptide, the intra-assay and inter-assay CVs were 3.2% and 4.7%, respectively. For PINP, the intra-assay and inter-assay CVs were 3% and 5% to 8%, respectively (normal range in men and women 27.7-127.6 μg/L). For CTX, the intra-assay and inter-assay CVs were <5% and 7% to 10%, respectively (normal range in men 115-748 ng/L, in premenopausal women 112-738 ng/L, and in postmenopausal women 142-1351 ng/L). For OC, the intra-assay and inte-rassay CVs were 3% and 6% to 9%, respectively (normal range in men and women 10.4-45.6 μg/L).
Type 2 Diabetes Phenotyping
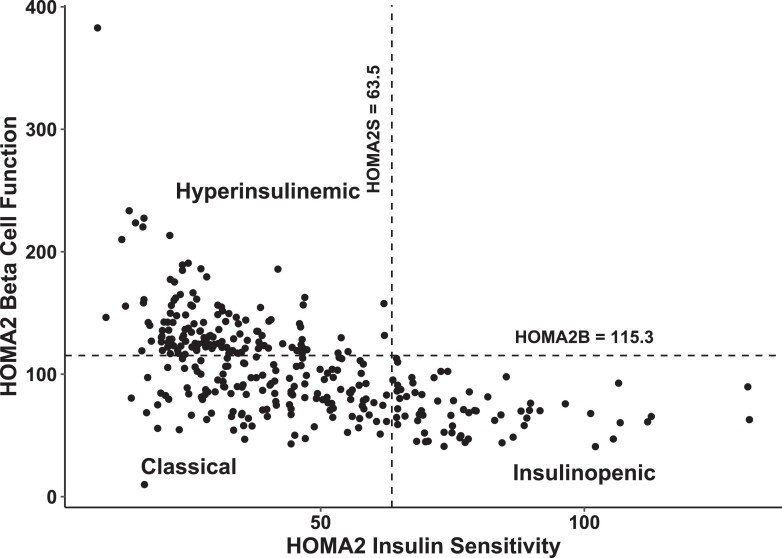
We estimated insulin sensitivity (HOMA of insulin sensitivity, HOMA2S) and β-cell function (HOMA of β-cell function, HOMA2B) based on fasting serum C-peptide and fasting plasma glucose values using the revised HOMA version 2 (HOMA2) (35). Persons were classified into three T2D phenotypes: (1) classical, low β-cell function (HOMA2B < 115.3%) and low insulin sensitivity (HOMA2S < 63.5%); (2) insulinopenic, low β-cell function (HOMA2B < 115.3%) and high insulin sensitivity (HOMA2S > 63.5%); or (3) hyperinsulinemic, high β-cell function (HOMA2B > 115.3%) and low insulin sensitivity (HOMA2S < 63.5%). The classification was done according to the median of β-cell function and insulin sensitivity in a Danish background population with normal fasting plasma glucose levels (cut-offs: HOMA2B 115.3% and HOMA2S 63.5%) (18).
Statistical Analyses
Data are presented as number (percentage) for categorical variables and median (interquartile range [IQR]) for continuous variables. To compare the median values of BTMs between the three T2D phenotypes all together, we used the Kruskal–Wallis test. The median values of BTMs between the individual T2D phenotypes were compared using a 2-sided Wilcoxon test.
In addition, we used simple and multiple linear regression models to investigate the relationship between the log transformed BTMs as a dependent outcome and the T2D phenotypes, after adjustment for potential confounders. The first model included the following covariates: sex (reference = female), age at recruitment (in years), and the three glucose-lowering treatment–naïve T2D phenotypes (insulinopenic, hyperinsulinemic, and classical) with the classical phenotype as a reference. The second model included an additional adjustment for waist to hip ratio, hypothyroidism (reference = none), oral glucocorticoids use within 3 to 12 months prior to study enrollment (reference = none), number of comorbidities beyond diabetes, known but nontreated osteoporosis (reference = none), and microvascular diseases defined as neuropathy, retinopathy, and nephropathy (reference = none). The third model included an additional adjustment for fasting plasma glucose levels (mmol/L) instead of adjusting for the T2D phenotypes.
An alpha level of <.05 was considered statistically significant. Statistical analyses were conducted using R-programming (version 4.0.3) and R-studio (version 4.1.1). Before signing an informed consent, persons received oral and written information. The Regional Ethics Committee on Health Research (record number S-20100082) and the Danish Data Protection Agency (record number 2008-58-0035) approved this study.
Results
Patient Characteristics
The baseline characteristics of the whole glucose-lowering treatment–naïve T2D study population (n = 326) and the T2D phenotypes are presented in Table 1, where we calculated number (percentage) for categorical variables and median (interquartile range [IQR]) for continuous variables. The distribution of β-cell function and insulin sensitivity in the three HOMA2-based T2D phenotypes is shown in Fig. 1. Among the 326 persons with T2D, 39.6% had the classical, 40.5% had the hyperinsulinemic, and 19.9% had the insulinopenic T2D phenotype. The median value of waist to hip ratio was lower in the insulinopenic T2D phenotype 0.90 (IQR 0.86, 0.98) and higher in the hyperinsulinemic T2D phenotype (0.98; IQR 0.92, 1.03) than in the classical T2D phenotype (0.97; IQR 0.91, 1.02). Also, the median value of the waist circumference was lower in the insulinopenic T2D phenotype (90.0; IQR 84.0, 99.0) and higher in the hyperinsulinemic T2D phenotype (109.5; IQR 102.0, 119.2) than in the classical T2D phenotype (102.0; IQR 94.0, 112.0). The insulinopenic T2D phenotype had a lower fasting plasma glucose level (6.2; IQR 5.7,6.9) than the classical (7.2; IQR 6.6, 8.2) and the hyperinsulinemic (6.5; IQR 5.8, 7.0) T2D phenotypes. However, the difference in fasting plasma glucose levels between the insulinopenic phenotype and the hyperinsulinemic phenotype did not reach statistical significance (P = .22). The prevalence of cardiovascular events was higher in the hyperinsulinemic T2D phenotype (25%) than in the classical (12.4%) and the insulinopenic (10.8%) T2D phenotypes. In addition, the percentage of hypothyroidism was higher in the insulinopenic T2D phenotype (6.2%) than the hyperinsulinemic (3.0%) and the classical (0.0%) T2D phenotypes.
Table 1.
Baseline characteristics of the 326 glucose-lowering treatment–naïve persons with type 2 diabetes and the three type 2 diabetes phenotypes
| All | Classical | Hyperinsulinemic | Insulinopenic | |
|---|---|---|---|---|
| Number of participants (%) | 326 (100.0) | 129 (39.6) | 132 (40.5) | 65 (19.9) |
| Age, median in years (IQR) | 65.3 (58.2,69.6) | 65.4 (58.2, 69.3) | 65.2 (58.5, 70.1) | 65.1 (57.9, 69.2) |
| Sex (%) | ||||
| Men | 172 (52.8) | 67 (51.9) | 70 (53.0) | 35 (53.8) |
| Women | 154 (47.2) | 62 (48.1) | 62 (47.0) | 30 (46.2) |
| Waist to hip ratio, median (IQR) | 0.97 (0.90, 1.02) | 0.97 (0.90, 1.02) | 0.98 (0.92, 1,03) | 0.90 (0.86, 0.98) |
| Fasting plasma glucose, median in mmol/L (IQR) | 6.6 (6.0, 7.4) | 7.2 (6.6, 8.2) | 6.5 (5.8, 7.0) | 6.2 (5.7, 6.9) |
| Waist circumference, median in cm (IQR) | 103.0 (93.0, 114.0) | 102.0 (94.0, 112.0) | 109.5 (102.0, 119.2) | 90.0 (84.0, 99.0) |
| Any cardiovascular disease (%) | ||||
| Yes | 56 (17.2) | 16 (12.4) | 33 (25.0) | 7 (10.8) |
| No | 270 (82.8) | 113 (87.6) | 99 (75.0) | 58 (89.2) |
| Microvascular diseases (%) | ||||
| Yes | 39 (12.0) | 12 (9.3) | 21 (15.9) | 6 (9.2) |
| No | 287 (88.0) | 117 (90.7) | 111 (84.1) | 59 (90.8) |
| Diabetic retinopathy (%) | ||||
| Yes | 3 (0.9) | 0 (0.0) | 2 (1.5) | 1 (1.5) |
| No | 323 (99.1) | 129 (100.0) | 130 (98.5) | 64 (98.5) |
| Acute myocardial infarction (%) | ||||
| Yes | 11 (3.4) | 4 (3.1) | 6 (4.5) | 1 (1.5) |
| No | 315 (96.6) | 125 (96.9) | 126 (95.5) | 64 (98.5) |
| Nontreated osteoporosis (%) | ||||
| Yes | 19 (5.8) | 7 (5.4) | 9 (6.8) | 3 (4.6) |
| No | 307 (94.2) | 122 (94.6) | 123 (93.2) | 62 (95.4) |
| Hypothyroidism (%) | ||||
| Yes | 8 (2.5) | 0 (0.0) | 4 (3.0) | 4 (6.2) |
| No | 318 (97.5) | 129 (100.0) | 128 (97.0) | 61 (93.8) |
| Oral glucocorticoids use within 1 year prior to enrollment (%) | ||||
| Yes | 6 (1.8) | 0 (0.0) | 6 (4.5) | 0 (0.0) |
| No | 320 (98.2) | 129 (0.0) | 126 (95.5) | 65 (100.0) |
| Physical activity (%) | ||||
| Yes | 150 (46.0) | 63 (48.8) | 52 (39.4) | 35 (53.8) |
| No | 176 (54.0) | 66 (51.2) | 80 (60.6) | 30 (46.2) |
Figure 1.
Plot of β-cell function and insulin sensitivity of glucose-lowering treatment–naïve persons with T2D. Cut-offs illustrate the median values of HOMA2B and HOMA2S in a Danish background population without diabetes. Abbreviations: HOMA2 B, homeostatic model assessment version 2 of β-cell function; HOMA2S, homeostatic model assessment version 2 of insulin sensitivity; T2D, type 2 diabetes mellitus.
Median Levels of Serum BTMs in Different T2D Phenotypes
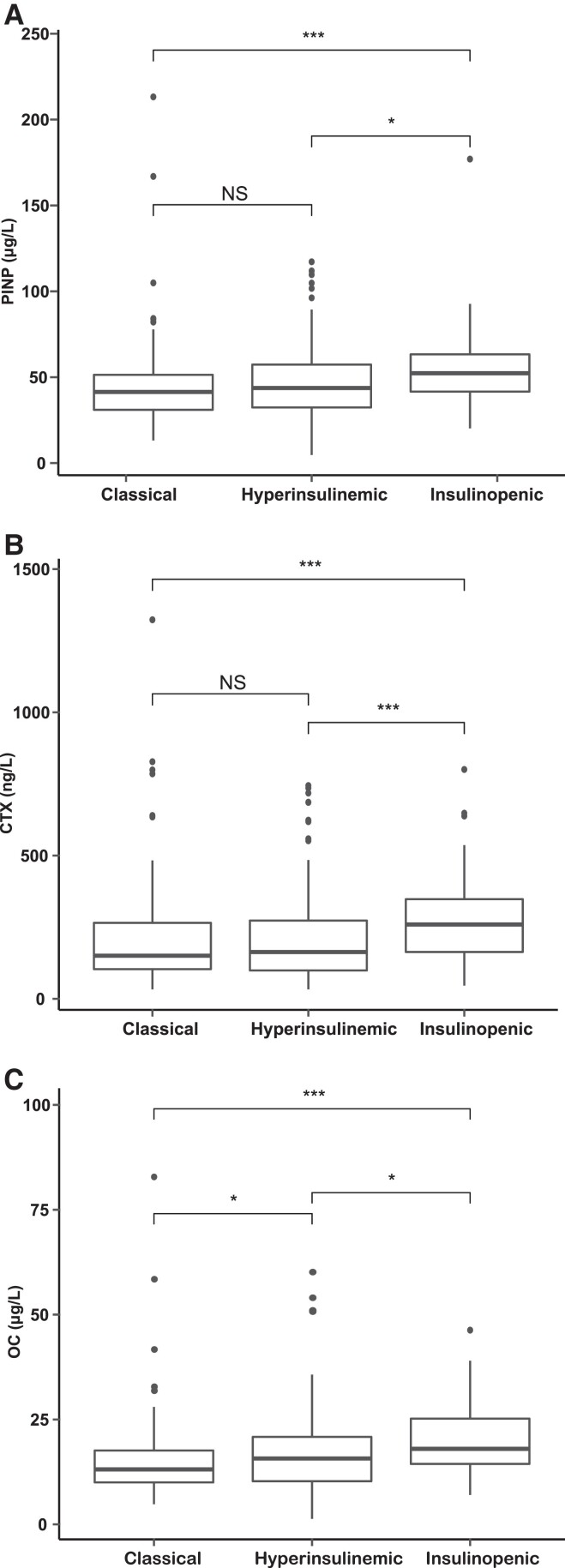
Serum BTMs differed between the three T2D phenotypes (P < .001), as illustrated in Fig. 2. Serum PINP was higher in the insulinopenic T2D phenotype 52.3 (IQR 41.6, 63.3) than in the classical (41.4; IQR 31.0, 51.4) (P < .001) and the hyperinsulinemic (43.7; IQR 32.3, 57.3) (P < .05) T2D phenotypes (Fig. 2A). Also, serum CTX was higher in the insulinopenic T2D phenotype (259.4; IQR 163.4, 347.7) than the classical (150.4 IQR 103.5, 265.1) (P < 0.001) and the hyperinsulinemic (163.3; IQR 98.9, 273.1) (P < .001) T2D phenotypes (Fig. 2B). Similarly, serum OC was higher in the insulinopenic T2D phenotype (18.0 IQR 14.4, 25.2) than in the classical (13.1; IQR 10.0, 17.6) (P < .001) and the hyperinsulinemic (15.7 IQR 10.2, 20.8) (P < .05) T2D phenotypes (Fig. 2C). Serum levels of PINP and CTX did not differ significantly between the hyperinsulinemic T2D phenotype and the classical T2D phenotype (Fig. 2A and 2B). However, serum OC was higher in the hyperinsulinemic T2D phenotype (15.7; IQR 10.2, 20.8) than in the classical T2D phenotype (13.1; IQR 10.0, 17.6) (P < .05) (Fig. 2C).
Figure 2.
Boxplots representing serum PINP (μg/L), serum CTX (ng/L), and serum OC (μg/L) levels (A, B, and C, respectively), in the three T2D phenotypes. Classical T2D phenotype, low β-cell function and insulin sensitivity; hyperinsulinemic T2D phenotype, high β-cell function and low insulin sensitivity; insulinopenic T2D phenotype, low β-cell function and high insulin sensitivity. The median values between the three T2D phenotypes all together were compared with the Kruskal–Wallis test (P < .001). Comparison of median values between the classical and the hyperinsulinemic T2D phenotypes, the classical and the insulinopenic T2D phenotypes, and between the hyperinsulinemic and the insulinopenic T2D phenotypes, was assessed using the 2-sided Wilcoxon test. Abbreviations: PINP, procollagen type I N-terminal propeptide; μg/L, micrograms per liter; CTX, C-terminal telopeptide of type 1 collagen; ng/L, nanogram per liter; OC, osteocalcin; T2D, type 2 diabetes mellitus; ***P < .001; **P < .01; NS, not significant.
BTMs in Relation to T2D Phenotypes
The associations between log-transformed BTMs and T2D phenotypes and potential confounders were investigated in multiple linear regression models (Tables 2-4). Compared with the classical T2D phenotype, the insulinopenic T2D phenotype was associated with higher mean values of PINP, CTX, and OC (P < .01) in all the simple linear models. The mean value of PINP was 17% higher (e0.16) in the insulinopenic T2D phenotype than in the classical T2D phenotype (P ≤ .01) after multivariable adjustment including age, sex, waist to hip ratio, hypothyroidism, number of comorbidities beyond diabetes, oral glucocorticoids within 1 year prior to study enrollment, nontreated osteoporosis, and microvascular diseases (model 2, Table 2). Compared with the classical T2D phenotype, no significant association between the hyperinsulinemic T2D phenotype and the mean value of PINP was found (model 2, Table 2). In addition, the mean value of CTX was 49% higher in the insulinopenic T2D phenotype than in the classical T2D phenotype (P ≤ 0.01) after multivariable adjustment of potential confounders (model 2, Table 3). Furthermore, compared with the classical T2D phenotype, the hyperinsulinemic T2D phenotype and the mean value of CTX were not significantly associated (model 2, Table 3). Also, the mean value of OC was 29% higher in the insulinopenic T2D phenotype than in the classical T2D phenotype (P < .01) after adjustment of potential confounders (model 2, Table 4). Similarly, the mean value of OC was 11% higher in the hyperinsulinemic T2D phenotype compared with the classical T2D phenotype (P < .05) after adjustment of potential confounders (model 2, Table 4).
Table 2.
Linear regression models of log (PINP) as a dependent variable, with different explanatory variables
| Dependent variable | Independent variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| Estimate | P value | Estimate | P value | Estimate | P value | ||
| Log (PINP) | Age | 0.00 | .16 | 0.00 | .16 | 0.00 | .14 |
| Sex | −0.11 | <.05 | −0.04 | .42 | −0.02 | .63 | |
| Waist to hip ratio | — | — | −0.97 | <.01 | −0.97 | <.01 | |
| Classical phenotype | 1 | — | 1 | — | — | — | |
| Insulinopenic phenotype | 0.19 | <.01 | 0.16 | ≤.01 | — | — | |
| Hyperinsulinemic phenotype | 0.04 | .39 | 0.06 | .25 | — | — | |
| Hypothyroidism | — | — | −0.30 | .05 | −0.25 | .09 | |
| Number of comorbidities beyond diabetes | — | — | 0.01 | .62 | 0.01 | .64 | |
| Oral glucocorticoids use within 1 year prior to enrollment | — | — | 0.31 | .08 | 0.32 | .06 | |
| Nontreated osteoporosis | — | — | −0.12 | .23 | −0.15 | .15 | |
| MVD | — | — | −0.02 | .74 | −0.03 | .65 | |
| Fasting plasma glucose (mmol/L) | — | — | — | — | −0.05 | <.01 | |
Model 1 was adjusted for age (in years), sex (reference = female), glucose-lowering treatment–naïve T2D phenotypes (reference = classical T2D phenotype). Model 2 was adjusted for age (in years), sex (reference = female), waist to hip ratio, glucose-lowering treatment–naïve T2D phenotypes (reference = classical T2D phenotype), hypothyroidism (reference = none), number of comorbidities beyond diabetes, oral glucocorticoids (within 3-12 months prior to enrollment, reference = none), nontreated osteoporosis (reference = none) and MVD (reference = none). Model 3 was additionally adjusted for fasting plasma glucose (mmol/L) instead of the T2D phenotypes.
Abbreviations: PINP, procollagen type I N-terminal propeptide; T2D, type 2 diabetes mellitus; MVD, microvascular diseases.
Table 4.
Linear regression models of log (OC) as a dependent variable, with different explanatory variables
| Dependent Variable | Independent variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| Estimate | P value | Estimate | P value | Estimate | P value | ||
| Log (OC) | Age | 0.01 | <.01 | 0.01 | <.01 | 0.01 | <.01 |
| Sex | −0.23 | <.01 | −0.14 | <.05 | −0.11 | .07 | |
| Waist to hip ratio | — | — | −1.24 | <.01 | −1.19 | <.01 | |
| Classical phenotype | 1 | — | 1 | — | — | — | |
| Insulinopenic phenotype | 0.31 | < .01 | 0.26 | <.01 | — | — | |
| Hyperinsulinemic phenotype | 0.09 | .13 | 0.11 | <.05 | — | — | |
| Hypothyroidism | — | — | −0.24 | .16 | −0.16 | .33 | |
| Number of comorbidities without diabetes | — | — | −0.02 | .40 | −0.02 | .38 | |
| Oral glucocorticoids use within 1 year prior to enrollment | — | — | 0.49 | ≤ .01 | 0.52 | <.01 | |
| Nontreated osteoporosis | — | ── | −0.26 | <.05 | −0.30 | <.01 | |
| MVD | — | — | −0.02 | .77 | −0.03 | .63 | |
| Fasting plasma glucose (mmol/L) | — | — | — | — | −0.09 | <.01 | |
Model 1 was adjusted for age (in years), sex (reference = female), and glucose-lowering treatment–naïve T2D phenotypes (reference = classical T2D phenotype). Model 2 was adjusted for age (in years), sex (reference = female), waist to hip ratio, glucose-lowering treatment–naïve T2D phenotypes (reference = classical T2D phenotype), hypothyroidism (reference = none), number of comorbidities beyond diabetes, oral glucocorticoids use (within 3-12 months prior to enrollment, reference = none), nontreated osteoporosis (reference = none), and MVD (reference = none). Model 3 was additionally adjusted for fasting plasma glucose (mmol/L) instead of the T2D phenotypes.
Abbreviations: OC, osteocalcin; T2D, type 2 diabetes mellitus; MVD, microvascular diseases.
Table 3.
Linear regression models of log (CTX) as a dependent variable, with different explanatory variables
| Dependent Variable | Independent variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| Estimate | P value | Estimate | P value | Estimate | P value | ||
| Log (CTX) | Age | 0.01 | <.01 | 0.01 | <.01 | 0.01 | <.01 |
| Sex | −0.11 | .17 | 0.02 | .77 | 0.09 | .36 | |
| Waist to hip ratio | — | — | −1.75 | ≤ .01 | −1.92 | <.01 | |
| Classical phenotype | 1 | — | 1 | — | — | — | |
| Insulinopenic phenotype | 0.44 | <.01 | 0.40 | ≤.01 | — | — | |
| Hyperinsulinemic phenotype | 0.04 | .64 | 0.11 | .20 | — | — | |
| Hypothyroidism | — | — | −0.76 | <.01 | −0.64 | ≤.01 | |
| Number of comorbidities beyond diabetes | — | — | −0.04 | .24 | −0.04 | .21 | |
| Oral glucocorticoids use within 1 year prior to enrollment | — | — | 0.26 | .38 | 0.26 | .37 | |
| Nontreated osteoporosis | — | — | −0.14 | .41 | −0.19 | .26 | |
| MVD | — | — | −0.01 | .93 | −0.03 | .80 | |
| Fasting plasma glucose (mmol/L) | — | — | — | — | −0.10 | <.01 | |
Model 1 was adjusted for age (in years), sex (reference = female), and glucose-lowering treatment–naïve T2D phenotypes (reference = classical T2D phenotype). Model 2 was adjusted for age (in years), sex (reference = female), waist to- hip ratio, glucose-lowering treatment–naïve T2D phenotypes (reference = classical T2D phenotype), hypothyroidism (reference = none), number of comorbidities beyond diabetes, oral glucocorticoids (within 3-12 months to enrollment, reference = none), nontreated osteoporosis (reference = none), and MVD (reference = none). Model 3 was additionally adjusted for fasting plasma glucose (mmol/L) instead of the T2D phenotypes.
Abbreviations: CTX, C-terminal telopeptide of type I collagen; T2D, type 2 diabetes mellitus; MVD, microvascular diseases.
BTMs and Fasting Plasma Glucose Levels
The associations between log-transformed BTMs and fasting plasma glucose levels in the whole study population are reported in multiple linear regression models (Tables 2-4). The mean values of PINP, CTX, and OC decreased by 5%, 10% and 9%, respectively, for each additional 1 mmol/L in fasting plasma glucose after adjustment of potential confounders including waist to hip ratio, hypothyroidism, number of comorbidities beyond diabetes, oral glucocorticoids use within 1 year prior to enrollment, nontreated osteoporosis, and microvascular diseases (P < .01) (model 3, Tables 2-4).
Discussion
This study demonstrates the relationships between BTMs and β-cell function and insulin sensitivity in glucose-lowering treatment–naïve newly diagnosed persons with T2D. Serum levels of PINP, CTX, and OC were higher in participants characterized by low β-cell function and high insulin sensitivity than in participants with high β-cell function and low insulin sensitivity or the classical T2D phenotype. However, serum levels of PINP and CTX did not differ between the hyperinsulinemic phenotype and the classical phenotype, suggesting that β-cell function has no substantial impact on bone turnover. Although persons with the insulinopenic phenotype had lower waist to hip ratio and lower fasting plasma glucose levels, which both correlated in a negative fashion with BTMs, the mean values of BTMs remained higher in the insulinopenic phenotype after adjustment of potential confounders including waist to hip ratio and fasting plasma glucose, indicating that the association was independent of the fasting plasma glucose levels and the body composition.
Due to higher β-cell function and preclinical studies showing anabolic effects of insulin on bone, the hyperinsulinemic phenotype was expected to be associated with the highest level of BTMs. By contrast, BTMs were highest in the persons with T2D characterized by low β-cell function but high insulin sensitivity, indicating that insulin sensitivity rather than the relative level of insulin in serum is a determinant of bone turnover in T2D. The level of evidence of direct effects of insulin secretion, exogenous insulin, and insulin sensitivity on bone turnover is limited. Insulin stimulates bone formation and improves bone microarchitecture in rat models of diabetes (36), and insulin infusion stimulates osteoblast activity in diabetic rats (37). Also, insulin sensitivity, assessed by a hyperinsulinemic–euglycemic clamp, correlated with CTX but not with PINP and OC in 14 persons with or without T2D (38). Furthermore, BTMs are lower in insulin-resistant obese persons and in persons with T2D than in lean and insulin-sensitive obese individuals (39). These relationships, alongside the results obtained in our study, suggest that the bone anabolic effect of insulin is blunted in persons with T2D if insulin sensitivity is decreased. Therefore, lower bone turnover in T2D study populations (27) may at least in part be explained by insulin insensitivity in bone cells.
To disentangle the relationship between the T2D phenotypes and other clinical characteristics on BTMs in glucose-lowering treatment–naïve persons with T2D, we performed a multiple linear regression analysis that included adjustment for potential confounders, such as body composition and fasting plasma glucose. Obesity is associated with higher BMD (40), insulin resistance (41), and lower BTMs levels (40). Furthermore, in persons with T2D, BTMs levels are lower at hyperglycemia but increase with improved glucose control (27). Accordingly, BTMs levels were negatively correlated with fasting plasma glucose levels in the present study. Hyperglycemia impairs bone cell activity and associates with lower BTMs in T2D (4), inferring that between group differences in glucose levels could influence BTMs. While glucotoxicity could explain the lower bone turnover in the classical phenotype compared to the insulinopenic phenotype, similar fasting plasma glucose levels in the insulinopenic and the hyperinsulinemic phenotypes support that lower bone turnover in the former phenotype is not related to fasting plasma glucose levels. Also, BTMs remained higher in the insulinopenic T2D phenotype after adjustment for waist to hip ratio and fasting plasma glucose levels, demonstrating that unmeasured factors influence BTMs in the insulinopenic phenotype. Compared with the classical phenotype in the regression models, the hyperinsulinemic phenotype had similar mean values of BTMs, which supports that insulin sensitivity rather than β-cell function per se is involved in the regulation of bone turnover in persons with T2D.
Importantly, these variations between different T2D phenotypes do not necessarily reflect a higher fracture risk in the hyperinsulinemic, less insulin-sensitive T2D phenotype. It remains to be investigated whether insulin insensitivity alters bone remodeling, microstructure, and ultimately fracture risk.
Provided that future studies corroborate that low insulin sensitivity impairs bone turnover leading to hypermineralized bone and subsequently higher BMD but impaired bone material properties, glucose-lowering medications with positive effects on bone could be targeted for persons with T2D. Glucagon-like peptide-1 receptor agonists promote bone formation and reduce bone resorption in ovariectomized rats (42) and reduce fracture risk in persons with T2D (43).
Strengths and Limitations
The participants in this study were not treated with glucose-lowering medications, preventing effects of glucose-lowering medications on insulin levels and BTMs. Medications and conditions known to influence bone metabolism such as chronic kidney disease and osteoporosis treatments were excluded, limiting the impact of potential confounders on the interpretation of differences in BTMs between T2D phenotypes. While the first two exclusion criteria were strengths, phenotyping of T2D could be questioned. Although T2D phenotyping has been suggested (18), there is currently no universal agreement on the optimal classification. For this study, classification was limited to measures of β-cell function and fasting plasma glucose levels and cut-offs based on Danish reference values, but inclusion of other factors such as age, BMI, and HbA1c have been suggested in other studies (19). It is possible that inclusion of these factors in T2D phenotyping including genotyping would result in different relationships between BTMs and specific T2D phenotypes. Moreover, the cut-offs implemented to classify persons as having low or high β-cell function may have a bearing on the outcomes. A lower HOMA2S cut-off might result in creation of new phenotypes or migration between phenotypes such as a migration from the classical T2D phenotype to the insulinopenic T2D phenotype, which could result in lower BTMs mean values in the insulinopenic T2D phenotype. In addition, some of the participants may have been misclassified as T2D due to the absence of measures of other antibodies such as islet cells autoantibodies or tyrosine phosphatase antibodies (IA-2A). However, identification of GADA but not islet cells autoantibodies or IA-2A predicted the need of insulin treatment within 3 years after diagnosis of diabetes in cases that were not initially classified as T1D (44). Thus, this misclassification, if it occurred, will be less likely to influence our study results. Also, there could be a risk of misclassification between the insulinopenic phenotype and T1D considering that both groups might have overlapping characteristics, both being insulinopenic but insulin sensitive. BTMs are reported to be lower in T1D than in healthy controls (45), limiting the likelihood that a misclassification would affect the outcomes of the present investigation. Besides, it is unknown whether differences in BTM levels across the T2D phenotypes would have a bearing on bone mass or fracture risk; however, it was not possible to investigate fracture risk due to the low number of individuals in our study cohort. In addition, a control group without diabetes was not included, as our study was mainly designed to investigate BTMs levels across different T2D phenotypes. Also, additional information on the menopausal status and T2D duration might have resulted in better adjustment for potential confounders.
This study demonstrates that BTMs were lower in persons with T2D and low insulin sensitivity, suggesting that low insulin sensitivity may impair the anabolic effect of insulin on bone, which at least in part could explain why bone turnover is lower in T2D. Importantly, it remains to be established whether bone cells are insulin resistant in T2D and whether impaired skeletal response to insulin has any bearing on bone strength and fracture risk.
Abbreviations
- BMD
bone mineral density
- BMI
body mass index
- BTM
bone turnover marker
- CTX
C-terminal telopeptide of type I collagen
- CV
coefficient of variation
- DD2
Danish Centre for Strategic Research in Type 2 Diabetes
- HbA1c
hemoglobin A1C
- HOMA
homeostatic model assessment
- GADA
glutamic acid decarboxylase antibody
- OC
osteocalcin
- PINP
procollagen type I N-terminal propeptide
- T2D
type 2 diabetes mellitus
Contributor Information
Mohamad I Nasser, Department of Endocrinology and Metabolism, Molecular Endocrinology Laboratory (KMEB), Odense University Hospital, Odense 5000, Denmark; Department of Clinical Research, University of Southern Denmark, Odense 5000, Denmark; Steno Diabetes Center Odense, Odense University Hospital, Odense 5000, Denmark.
Jacob V Stidsen, Steno Diabetes Center Odense, Odense University Hospital, Odense 5000, Denmark.
Kurt Højlund, Department of Clinical Research, University of Southern Denmark, Odense 5000, Denmark; Steno Diabetes Center Odense, Odense University Hospital, Odense 5000, Denmark.
Jens Steen Nielsen, Department of Clinical Research, University of Southern Denmark, Odense 5000, Denmark; Steno Diabetes Center Odense, Odense University Hospital, Odense 5000, Denmark.
Richard Eastell, Academic Unit of Bone Metabolism, University of Sheffield, Sheffield S10, UK; Mellanby Centre for Musculoskeletal Research, University of Sheffield, Sheffield S10, UK.
Morten Frost, Department of Endocrinology and Metabolism, Molecular Endocrinology Laboratory (KMEB), Odense University Hospital, Odense 5000, Denmark; Department of Clinical Research, University of Southern Denmark, Odense 5000, Denmark; Steno Diabetes Center Odense, Odense University Hospital, Odense 5000, Denmark.
Funding
This project has received funding from the Horizon 2020 Framework Programme - European Union research and innovation program under the H2020 Marie Skłodowska-Curie Actions - European Union grant agreement no. 860898.
Disclosures
M.N., J.S., K.H., J.N., and M.F. have nothing to declare. R.E. received consultancy and grant funding from Immunodiagnostic Systems.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Eastell R, Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5(11):908‐923. [DOI] [PubMed] [Google Scholar]
- 2. Naylor K, Eastell R. Bone turnover markers: use in osteoporosis. Nat Rev Rheumatol. 2012;8(7):379‐389. [DOI] [PubMed] [Google Scholar]
- 3. Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391‐420. [DOI] [PubMed] [Google Scholar]
- 4. Hygum K, Starup-Linde J, Harslof T, Vestergaard P, Langdahl BL. Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover—a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(3):R137‐R157. [DOI] [PubMed] [Google Scholar]
- 5. Vilaca T, Schini M, Harnan S, et al. The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: a systematic review and meta-analysis update. Bone. 2020;137:115457. [DOI] [PubMed] [Google Scholar]
- 6. Koromani F, Oei L, Shevroja E, et al. Vertebral fractures in individuals with type 2 diabetes: more than skeletal complications alone. Diabetes Care. 2020;43(1):137‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hothersall EJ, Livingstone SJ, Looker HC, et al. Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res. 2014;29(5):1054‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int. 2007;18(4):427‐444. [DOI] [PubMed] [Google Scholar]
- 9. Napoli N, Conte C, Eastell R, et al. Bone turnover markers do not predict fracture risk in type 2 diabetes. J Bone Miner Res. 2020;35(12):2363‐2371. [DOI] [PubMed] [Google Scholar]
- 10. Farr JN, Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone. 2016;82:28‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allen MR, Burr DB. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don't know. Bone. 2011;49(1):56‐65. [DOI] [PubMed] [Google Scholar]
- 12. Wang W, Zhang X, Zheng J, Yang J. High glucose stimulates adipogenic and inhibits osteogenic differentiation in MG-63 cells through cAMP/protein kinase A/extracellular signal-regulated kinase pathway. Mol Cell Biochem. 2010;338(1-2):115‐122. [DOI] [PubMed] [Google Scholar]
- 13. Mercer N, Ahmed H, Etcheverry SB, Vasta GR, Cortizo AM. Regulation of advanced glycation end product (AGE) receptors and apoptosis by AGEs in osteoblast-like cells. Mol Cell Biochem. 2007;306(1-2):87‐94. [DOI] [PubMed] [Google Scholar]
- 14. Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006;99(2):411‐424. [DOI] [PubMed] [Google Scholar]
- 15. Wittrant Y, Gorin Y, Woodruff K, et al. High d(+)glucose concentration inhibits RANKL-induced osteoclastogenesis. Bone. 2008;42(6):1122‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaudio A, Privitera F, Battaglia K, et al. Sclerostin levels associated with inhibition of the Wnt/beta-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(10):3744‐3750. [DOI] [PubMed] [Google Scholar]
- 17. Beck-Nielsen H, Henriksen JE, Vaag A, Hother-Nielsen O. Pathophysiology of non-insulin-dependent diabetes mellitus (NIDDM). Diabetes Res Clin Pract. 1995;28:S13‐S25. [DOI] [PubMed] [Google Scholar]
- 18. Stidsen JV, Henriksen JE, Olsen MH, et al. Pathophysiology-based phenotyping in type 2 diabetes: a clinical classification tool. Diabetes Metab Res Rev. 2018;34(5):e3005. [DOI] [PubMed] [Google Scholar]
- 19. Ahlqvist E, Storm P, Karajamaki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361‐369. [DOI] [PubMed] [Google Scholar]
- 20. Stidsen JV, Christensen DH, Henriksen JE, et al. Risk of cardiovascular events associated with pathophysiological phenotypes of type 2 diabetes. Eur J Endocrinol. 2022; 187(2):279‐291. [DOI] [PubMed] [Google Scholar]
- 21. Hickman J, McElduff A. Insulin promotes growth of the cultured rat osteosarcoma cell line UMR-106-01: an osteoblast-like cell. Endocrinology. 1989;124(2):701‐706. [DOI] [PubMed] [Google Scholar]
- 22. Yang J, Zhang X, Wang W, Liu J. Insulin stimulates osteoblast proliferation and differentiation through ERK and PI3K in MG-63 cells. Cell Biochem Funct. 2010;28(4):334‐341. [DOI] [PubMed] [Google Scholar]
- 23. Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei J, Ferron M, Clarke CJ, et al. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest. 2014;124(4):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo H, Wang C, Jiang B, et al. Association of insulin resistance and beta-cell function with bone turnover biomarkers in dysglycemia patients. Front Endocrinol (Lausanne). 2021;12:554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stage TB, Christensen MH, Jorgensen NR, et al. Effects of metformin, rosiglitazone and insulin on bone metabolism in patients with type 2 diabetes. Bone. 2018;112:35‐41. [DOI] [PubMed] [Google Scholar]
- 27. Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus—a systematic review. Bone. 2016;82:69‐78. [DOI] [PubMed] [Google Scholar]
- 28. Nielsen JS, Thomsen RW, Steffensen C, Christiansen JS. The Danish Centre for strategic research in type 2 diabetes (DD2) study: implementation of a nationwide patient enrollment system. Clin Epidemiol. 2012;4(Suppl 1):27‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5‐S20. [DOI] [PubMed] [Google Scholar]
- 30. Christensen DH, Nicolaisen SK, Berencsi K, et al. Danish Centre for Strategic Research in Type 2 Diabetes (DD2) project cohort of newly diagnosed patients with type 2 diabetes: a cohort profile. BMJ Open. 2018;8(4):e017273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30‐33. [DOI] [PubMed] [Google Scholar]
- 32. Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38‐41. [DOI] [PubMed] [Google Scholar]
- 33. Thomsen RW, Nielsen JS, Ulrichsen SP, Pedersen L, Hansen AM, Nilsson T. The Danish Centre for Strategic Research in Type 2 Diabetes (DD2) study: collection of baseline data from the first 580 patients. Clin Epidemiol. 2012;4:43‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christensen H, Nielsen JS, Sorensen KM, Melbye M, Brandslund I. New national biobank of the Danish Center for Strategic Research on Type 2 Diabetes (DD2). Clin Epidemiol. 2012;4:37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hill NR, Levy JC, Matthews DR. Expansion of the homeostasis model assessment of beta-cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: iHOMA2. Diabetes Care. 2013;36(8):2324‐2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thrailkill KM, Lumpkin CK Jr, Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289(5):E735‐E745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verhaeghe J, Suiker AM, Visser WJ, Van Herck E, Van Bree R, Bouillon R. The effects of systemic insulin, insulin-like growth factor-I and growth hormone on bone growth and turnover in spontaneously diabetic BB rats. J Endocrinol. 1992;134(3):485‐492. [DOI] [PubMed] [Google Scholar]
- 38. Basu R, Peterson J, Rizza R, Khosla S. Effects of physiological variations in circulating insulin levels on bone turnover in humans. J Clin Endocrinol Metab. 2011;96(5):1450‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tonks KT, White CP, Center JR, Samocha-Bonet D, Greenfield JR. Bone turnover is suppressed in insulin resistance, independent of adiposity. J Clin Endocrinol Metab. 2017;102(4):1112‐1121. [DOI] [PubMed] [Google Scholar]
- 40. Evans AL, Paggiosi MA, Eastell R, Walsh JS. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J Bone Miner Res. 2015;30(5):920‐928. [DOI] [PubMed] [Google Scholar]
- 41. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840‐846. [DOI] [PubMed] [Google Scholar]
- 42. Ma X, Meng J, Jia M, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, prevents osteopenia by promoting bone formation and suppressing bone resorption in aged ovariectomized rats. J Bone Miner Res. 2013;28(7):1641‐1652. [DOI] [PubMed] [Google Scholar]
- 43. Al-Mashhadi Z, Viggers R, Fuglsang-Nielsen R, et al. Glucose-lowering drugs and fracture risk-a systematic review. Curr Osteoporos Rep. 2020;18(6):737‐758. [DOI] [PubMed] [Google Scholar]
- 44. Torn C, Landin-Olsson M, Ostman J, et al. Glutamic acid decarboxylase antibodies (GADA) is the most important factor for prediction of insulin therapy within 3 years in young adult diabetic patients not classified as type 1 diabetes on clinical grounds. Diabetes Metab Res Rev. 2000;16(6):442‐447. [DOI] [PubMed] [Google Scholar]
- 45. Starup-Linde J, Hygum K, Harslof T, Langdahl B. Type 1 diabetes and bone fragility: links and risks. Diabetes Metab Syndr Obes. 2019;12:2539‐2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.