Abstract
Objective
To assess the relationship between the degree of severity of eating disorders (ED) and energy and nutrient intakes and nutritional risk in a mixed-sex adolescent population without clinical symptoms.
Design
Cross-sectional study.
Setting
Data were collected in schools.
Subjects
Adolescents (n 495) aged 14·2 (sd 1·0) years. The Eating Attitudes Test was used to detect adolescents at risk of ED (rED) and a structured interview based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, was used to diagnose eating disorder not otherwise specified (EDNOS). Dietary intake was quantified using the 24 h recall method over three days and the probability of inadequate intake was determined.
Results
Females presented lower intakes of energy, macronutrients and micronutrients (Ca, Fe, Mg, K, P, Na, thiamin, vitamins E, C, B6, B12, pantothenic acid, folic acid) because the severity of their ED was greater. These lower dietary intakes led to nutritional risk (for Ca, Fe, Mg, P, vitamins A, D, B6) in more than 80 % and 60 % of females with EDNOS and rED, respectively. The multiple linear regression models showed that the rED and EDNOS groups presented a lower energy intake of 1597·4 kJ/d (381·8 kcal/d) and 3153·0 kJ/d (753·6 kcal/d), respectively. In contrast, little difference was observed in the nutritional intakes of males.
Conclusions
The female adolescents showed lower energy and nutrient intakes as the ED became more severe, which led to energy, vitamin and mineral deficiencies in a high percentage of females with ED. These nutritional risks could hinder adequate physical and psychological development and lead to chronic ED.
Keywords: Eating disorders, Intake, Nutrition, Adolescence, Eating disorder not otherwise specified
In recent decades, eating disorders (ED) have become a significant public health problem principally impacting on adolescents, especially females, although a certain percentage of males have also been affected( 1 – 3 ). In parallel with this, there has been an exponential increase in overweight which may have contributed to the increase in ED( 4 ).
Scientific research has indicated that cases of anorexia and bulimia represent only a small proportion of ED cases (0·3 % and 0·5 %, respectively)( 5 , 6 ), whereas cases of eating disorder not otherwise specified (EDNOS) are much more frequent (3–10 %)( 2 , 5 ), as are individuals at risk of ED (rED), who account for 13·5 %( 1 ) in adolescents with no clinical symptoms.
Among the risk factors identified in the multifactorial aetiology of ED are restrictive diets, high body weight, body dissatisfaction, and sociocultural and psychological factors( 4 , 7 , 8 ). However, although dietary restriction of nutritional intake is one of the principal predictors of ED, it has rarely been the object of real and quantitative study in schoolchildren at rED. As far we can ascertain, there are only four studies that have quantitatively evaluated dietary intake in adolescent females at rED, and none that have looked at boys. Of these studies, the Brazilian( 9 ) and Taiwanese( 10 , 11 ) studies showed that girls at rED consumed less energy than the control group. However, two of them identified lower intakes of macronutrients, vitamin B6, vitamin B12 and Zn( 10 , 11 ); whereas one of them observed lower intakes of energy and Fe compared with the control group( 9 ). Only one other study has evaluated different types of symptoms of ED, but it did not evaluate the most severe and frequent stages of ED. That study found no significant decrease in energy intake, but did find decreased intakes of carbohydrates, lipids, Na and vitamins A, E and C( 12 ).
No research has been done on European populations or males. Consequently, we set ourselves the goal of evaluating the relationship between the severity of ED and intakes of energy and nutrients and nutritional risk in a mixed-sex adolescent population without clinical symptoms. Specifically, we sought to answer the following questions: (i) Are energy and nutrient intakes different between the population with EDNOS, the population at rED and the control group? (ii) Are there gender differences? (iii) Is the percentage of adolescents at risk of insufficient intake higher as the ED becomes more severe?
Methods
Design and participants
The present study was a cross-sectional descriptive study of a representative sample of 495 adolescent schoolchildren with a mean age of 14·2 (sd 1·0) years, analysing data obtained from a pooling of two surveys of school-based populations of the same age range that were carried out by the same research group using the same methodology but over different periods( 4 , 13 ). The initial populations of the first and second studies were 2967 and 1336 adolescents, respectively, all of whom were from the city of Tarragona (Spain), including the twenty-six schools in the city proper, its suburbs and a selection of five rural zones from the surrounding province of Tarragona. Both studies had a double phase design. The first phase sorted the participants by means of a test that classified them as at rED or not at rED. In the second phase we selected the participants at rED and the control group, the latter being randomly chosen from those whose score was below the cut-off and whose characteristics of age, gender and type of school were similar. During this phase, the EDNOS diagnosis was confirmed using the criteria established in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). For the purpose of the present analysis, only participants with available data on psychopathology and energy and nutrient intakes were included. The data from both studies were brought together to increase the sample size. The first study provided 329 adolescents and the second study provided 166 adolescents. In this way we obtained a sample of 495 adolescents, of whom 307 were controls, 146 were at rED and forty-two were diagnosed with EDNOS. Only one male was diagnosed with EDNOS, which meant that we could not include a male group of EDNOS.
Procedure
We obtained permission from the Department of Education of the Catalan Government and from the schools. We sought the consent of the parents. The study was approved by the Ethics Committee for Clinical Research at the Sant Joan University Hospital in Reus (Spain). The study took place in the classroom and the researchers were psychologists and nutritionists. The first study was conducted during the academic years 1999/2000 and 2000/2001; the second study was conducted during academic years 2004/2005 and 2005/2006. In the first phase of both studies, we administered in the classroom a questionnaire regarding the participants’ sociodemographic data (based on the Hollingshead index( 14 )), a test to sort the participants according to ED symptoms and a test to assess body satisfaction. We took a sample of participants who scored at least 25 (the cut-off score for the Eating Attitudes Test (EAT)) and a control group of participants who scored below the cut-off and whose characteristics of age, gender and type of school were similar. In the second phase the chosen participants were individually interviewed in order to: (i) confirm the diagnosis of EDNOS using a structured interview; (ii) assess their dietary intake by means of a 24 h recall method using a photograph album of dishes and portion sizes with standard household measurements; (iii) measure their weight and height (and calculate BMI); and (iv) identify any symptoms of emotional disorder.
Measures
Assessment of risk of eating disorders: the Eating Attitudes Test
The EAT( 15 ) is a self-administered questionnaire used as a screening tool for ED that has good psychometric properties of reliability and validity. We administered the Spanish validated version of the EAT-40 (internal consistency α=093)( 16 ). A score greater than or equal to 25 was used as the cut-off because it has been shown to provide high-quality data on the sensitivity (87·5 %) and specificity (93·9 %) of young populations( 17 ).
Assessment of diagnoses of eating disorders
Schedules for Clinical Assessment in Neuropsychiatry. The Schedules for Clinical Assessment in Neuropsychiatry( 18 ) is a semi-structured interview for psychopathological diagnosis according to DSM-IV criteria, validated and adapted to our population( 19 ). The interviews were performed by professional psychologists.
Diagnostic Interview for Children and Adolescents. The Diagnostic Interview for Children and Adolescents (DICA; W Reich, JJ Shayka and Ch Taibleson, unpublished results) is a semi-structured interview for children and adolescents that follows DSM-IV diagnostic criteria. The Spanish computerised adaptations of the DICA-R (Diagnostic Interview for Children and Adolescents, Revised) and the DICA-IV were administered. The κ statistics for the test–retest reliability of the Spanish version for ED were between 0·74 and 1·0( 20 ). The interviews were performed by professional psychologists.
Symptoms of emotional disorders
Youth’s Inventory-4. The Youth’s Inventory-4( 21 ) is a self-report rating scale that evaluates symptoms of eighteen DSM-IV emotional and behavioral disorders in youth. We used an adapted Spanish version with satisfactory internal consistency in the several categories of disorders (α=0·66 to 0·87). We only used the following categories of disorders: major depression, dysthymia, anxiety disorder and social phobia categories.
Body Areas Satisfaction Test. The Body Areas Satisfaction Test( 22 ) is a self-evaluation test that ranks dissatisfaction with different parts of the body on a scale from 1 (‘very dissatisfied’) to 5 (‘very satisfied’). We used the adapted Spanish version, which has good reliability (α=0·879).
Dietary consumption and the risk of insufficient intake
Twenty-four-hour recall. This is a personal interview method for determining an individual’s dietary intake during the previous day( 23 ). It was carried out on three non-consecutive days including a weekend day. This method involved professional trained dietitians administering previously standardised interviews to the adolescents. We used an extensive collection of photographs with a variety of dishes and portion sizes to obtain a more accurate assessment of the amount of food consumed. The interviews were conducted at school during the academic year. The period of time between each 24 h recall was approximately two weeks. To calculate nutrient intakes, we created our own SPSS database using the French and Spanish food composition tables( 24 , 25 ).
Nutrient intake inadequacy. We calculated the probability of intake inadequacy for fourteen nutrients using the Recommended Dietary Intake of energy and nutrients created for the Spanish population( 26 ). By using the probability approach( 27 ), we calculated the probability of intake inadequacy for protein, Ca, Fe, Mg, P, vitamins A, D, E, C, B6, B12, thiamin, riboflavin, niacin and folic acid for each participant. We calculated the risk of inadequate intake of micronutrients using the mean of the probability of intake inadequacy for all micronutrients( 28 ); that is, we calculated it by adding together the probability of intake inadequacy for each micronutrient and dividing the total by the number of micronutrients. Then we categorised the sample according to the percentage of the population whose probability of micronutrient intake inadequacy was higher than 50 %. For energy we used two approaches to estimate the frequency of inadequate intake. First, we compared energy intake with the standard reference values( 29 ). Second, we compared energy intake with the estimated energy requirement, which was calculated with equations that included age, weight, height and physical activity as described by the Institute of Medicine( 30 ). In both approaches, participants were considered to have inadequate intake when energy intake was less than two-thirds (67 %) of the reference values( 29 ).
Anthropometry
The anthropometric parameters evaluated were weight and height, measured with participants wearing light clothing, without heavy objects in pockets and barefoot. BMI (kg/m2) was calculated. Weight was measured using the Tanita® TBF-305 scale, which has an accuracy of 100 g. Height was measured to the nearest ±1 mm using a non-extensible tape measure.
Physical activity
We used the version modified for the Spanish population( 31 ) of the questionnaire proposed by Baecke et al. ( 32 ) to quantify the physical activity of the general population based on three domains: work, sport and leisure. This was a self-administered questionnaire with several questions scored on a five-point Likert scale. Based on the mean of these three domains, we obtained a physical activity index score and participants were divided into three groups according to the degree of physical activity (light, moderate, vigorous).
Pubertal maturity
We used images of the specific stages of pubertal maturity for each sex( 33 ). From the images of different stages of maturation, adolescents during the interview had to indicate what pubertal stage they were according to their own perception. According to means of scores of pubic hair and development of breasts and genitals, we obtained a scale of 1 (pre-puberty) to 5 (maturity).
Statistical methods
We analysed the data using the statistical software package IBM SPSS Statistics version 19·0 for Windows. The results were expressed as means and standard deviations and percentages. We verified compliance with the statistical tests’ conditions of use. The degree of non-independence of observations from adolescents nested within the same school can be estimated using intra-class correlation coefficients (ICC)( 34 , 35 ). We found no evidence to suggest that observations were non-independent for the outcome variables ‘energy intake’ (ICC=0·002), ‘protein intake’ (ICC=0·020), ‘carbohydrate intake’ (ICC=0·020), ‘lipid intake’ (ICC=0·017) and ‘risk of micronutrient intake inadequacy’ (ICC<0·001; all P>0·05). Therefore, we applied traditional statistical analysis. We used the χ 2 test, Fisher’s Student t test and ANOVA adjusted using the Bonferroni correction for multiple comparisons. Also, we wanted to determine the influence of ED on energy and nutrient intakes taking into account several confounding factors that, according to the literature, may increase or decrease dietary intake; and at the same time, these factors could influence adequate micronutrient intake. Among other factors that may influence energy and nutrient intakes, the following are particularly prominent: sociodemographic status( 36 ), physical activity( 37 ), emotional symptoms( 38 ), body satisfaction( 39 , 40 ), BMI( 41 ) and pubertal stage( 42 ). For example, a higher dietary intake was associated with higher physical activity, body satisfaction and anxiety and depressive symptoms, pubertal stage, and with lower or higher BMI. Consequently, we carried out several multiple linear regression models using the ENTER method to determine the manner in which eating disorders (rED and EDNOS) adjusted for different factors (emotional symptoms, physical activity, sociodemographic status, stage of puberty, body satisfaction and BMI) are associated with energy and macronutrient intakes and risk of inadequate micronutrient intake. For this, we designed five regression linear models and in each one we introduced a dietary variable as dependent variable: in model 1, the dependent variable was energy intake (kcal); in model 2, it was protein intake (g); in model 3, it was carbohydrate intake (g); in model 4, it was lipid intake (g); and in model 5, the dependent variable was risk of micronutrient intake inadequacy (%). Also, we performed these models in the whole sample, in the control group and in the groups with rED and EDNOS. We accepted P<0·05 as the minimum level of statistical significance.
Results
Of the 495 adolescents, 120 were male and 375 were female. Of the males, 79·6 % were controls and 20·3 % were at rED. Only one male was diagnosed with EDNOS. Among the females, 56·5 % were controls and 43·5 % were at rED, of whom 34·7 % were diagnosed with EDNOS. Table 1 shows the sociodemographic, pubertal and anthropometric characteristics, the psychological states and the physical activity of the participants. The females at rED and with EDNOS presented higher BMI and lower body satisfaction compared with the controls (P<0·05). Furthermore, we observed a higher percentage of females with emotional disorders in the rED and EDNOS groups compared with the controls (P<0·05). Specifically in females, anxious symptoms affected 32 % and other emotional symptoms such as depression affected 19·5 % (data not shown in the table).
Table 1.
Sociocultural, anthropometric and psychological characteristics in relation to the severity of eating disorder among 495 adolescents, mean age 14·2 (sd 1·0) years, Tarragona, Spain
| Females | Males | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n 212) | rED (n 121) | EDNOS (n 42) | Controls (n 95) | rED (n 25) | ||||||
| Mean or % | sd | Mean or % | sd | Mean or % | sd | Mean or % | sd | Mean or % | sd | |
| Age (years)* | 14·2a | 1·1 | 14·3a | 1·1 | 14·3a | 1·1 | 13·9a | 0·9 | 14·2a | 1·1 |
| Socio-economic status† | ||||||||||
| Low | 26·0a | – | 26·4a | – | 26·8a | – | 25·8a | – | 28·0a | – |
| Medium | 48·1a | – | 47·9a | – | 46·3a | – | 41·9a | – | 48·0a | – |
| High | 26·0a | – | 25·6a | – | 26·8a | – | 32·3a | – | 24·0a | – |
| Stage of puberty† | ||||||||||
| Stage I | 0·0a | – | 0·0a | – | 0·0a | – | 0·0a | – | 0·0a | – |
| Stage II | 0·0a | – | 0·0a | – | 0·0a | – | 1·1a | – | 0·0a | – |
| Stage III | 11·1a | – | 9·3a | – | 7·3a | – | 13·8a | – | 26·1a | – |
| Stage IV | 57·0a | – | 60·2a | – | 48·8a | – | 46·6a | – | 47·8a | – |
| Stage V | 31·9a | – | 30·5a | – | 43·9a | – | 42·6a | – | 26·1a | – |
| Weight (kg)* | 56·3a | 10·4 | 59·0b | 12·4 | 62·1b | 11·1 | 60·3a | 12·4 | 62·6a | 19·1 |
| Height (m)* | 161·6a | 6·2 | 161·0a | 6·3 | 160·9a | 5·1 | 168·1a | 8·9 | 164·0a | 11·7 |
| BMI (kg/m2)* | 21·5a | 3·4 | 22·9b | 4·1 | 24·0b | 4·0 | 21·2a | 3·4 | 22·1a | 5·2 |
| Body satisfaction (score)* | 27·2a | 7·4 | 24·8b | 7·8 | 19·8c | 6·4 | 24·9a | 7·0 | 28·0a | 6·6 |
| Presence of anxiety or depression† | ||||||||||
| No | 71·7a | – | 49·6 | – | 31·0b | – | 69·1a | – | 58·3a | – |
| Yes | 28·3a | – | 50·4 | – | 69·0b | – | 30·9a | – | 41·7a | – |
| Index of physical activity (score)* | 2·5a | 0·5 | 2·5a | 0·5 | 2·7a | 0·6 | 2·9a | 0·4 | 3·1a | 0·5 |
| Degree of physical activity† | ||||||||||
| Light | 30·7a | – | 27·3a | – | 31·0a | – | 6·3a | – | 12·0a | – |
| Moderate | 37·7a | – | 40·5a | – | 31·0a | – | 22·1a | – | 8·0a | – |
| Vigorous | 31·6a | – | 32·2a | – | 38·1a | – | 71·6a | – | 80·0a | – |
rED, risk of eating disorders; EDNOS, eating disorder not otherwise specified.
a,b,cValues within a row with unlike superscript letters were significantly different (P<0·05).
Two-sample t test or ANOVA adjusted using the Bonferroni correction for multiple comparisons of continuous variables Values are presented as mean and standard deviation.
χ 2 test for categorical variables. Values are presented as percentage.
Energy and nutrient intakes
Among the females, energy and nutrient intakes were significantly lower in those at rED and/or with EDNOS compared with the controls, except for intake of vitamin D. In contrast, for males significant differences were observed between the controls and the group at rED only for PUFA, Fe, vitamin E and vitamin C (Table 2).
Table 2.
Daily energy and nutrient intakes in relation to the severity of eating disorder among 495 adolescents, mean age 14·2 (sd 1·0) years, Tarragona, Spain
| Females | Males | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n 212) | rED (n 121) | EDNOS (n 42) | Controls (n 95) | rED (n 25) | ||||||
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | Mean | sd | |
| Energy (kJ) | 9644·5a | 2946·3 | 7670·5b | 3151·3 | 5660·9c | 2356·4 | 11 480·8a | 317·9 | 11 048·1a | 4245·5 |
| Energy (kcal) | 2305·1a | 704·2 | 1833·3b | 753·2 | 1353·0c | 563·2 | 2743·9a | 765·0 | 2640·5a | 1014·7 |
| Protein (g) | 88·8a | 27·6 | 74·8b | 24·5 | 52·8c | 19·8 | 106·1a | 30·6 | 104·2a | 44·5 |
| Carbohydrate (g) | 249·6a | 82·9 | 198·0b | 87·9 | 147·4c | 60·2 | 305·6a | 102·1 | 292·8a | 118·9 |
| Starch (g) | 131·5a | 49·7 | 103·3b | 46·7 | 76·5c | 35·8 | 163·6a | 57·9 | 165·2a | 80·1 |
| Sugars (g) | 258·4a | 125·9 | 211·0b | 110·0 | 156·7c | 76·9 | 298·6a | 153·3 | 277·2a | 117·1 |
| Fibre (g) | 19·5a | 5·9 | 13·5b | 5·4 | 9·2c | 3·2 | 18·6a | 6·2 | 19·9a | 6·9 |
| Lipids (g) | 105·5a | 38·1 | 82·2b | 33·5 | 61·2c | 32·4 | 121·8a | 39·9 | 116·8a | 44·2 |
| SFA (g) | 33·4a | 13·6 | 25·7b | 12·4 | 19·2c | 11·7 | 38·8a | 14·4 | 36·7a | 14·6 |
| MUFA (g) | 47·1a | 18·0 | 36·1b | 14·1 | 27·0c | 15·4 | 51·6a | 18·4 | 51·9a | 18·0 |
| PUFA (g) | 15·5a | 7·7 | 12·8b | 8·2 | 9·3c | 5·7 | 20·9a | 9·9 | 14·8b | 7·3 |
| Cholesterol (mg) | 341·7a | 135·1 | 280·1b | 13636 | 198·0c | 102·7 | 395·6a | 164·2 | 339·0a | 171·6 |
| Ca (mg) | 847·4a | 387·2 | 721·2b | 378·1 | 545·2c | 278·1 | 922·1a | 353·9 | 1016·9a | 557·6 |
| Fe (mg) | 9·1a | 3·5 | 7·6b | 3·3 | 5·1c | 2·0 | 12·0a | 4·3 | 9·9b | 3·1 |
| Mg (mg) | 274·7a | 97·9 | 234·0b | 100·2 | 154·4c | 58·5 | 301·4a | 87·0 | 321·6a | 113·7 |
| K (mg) | 2736·2a | 783·6 | 2342·3b | 846·1 | 1715·6c | 552·1 | 3067·3a | 838·3 | 3191·1a | 1065·6 |
| P (mg) | 1167·4a | 352·6 | 1026·6b | 382·6 | 746·9c | 263·4 | 1355·2a | 368·9 | 1316·9a | 444·5 |
| Na (mg) | 3049·5a | 1227·8 | 2487·1b | 1146·3 | 1863·0c | 886·7 | 3705·7a | 1307·7 | 3674·4a | 1623·9 |
| Vitamin D (µg) | 1·5a | 1·2 | 1·5a | 1·3 | 1·1a | 0·9 | 1·8a | 2·6 | 1·8a | 2·0 |
| Vitamin E (mg) | 13·2a | 6·8 | 10·9b | 6·5 | 8·2b | 5·0 | 17·1a | 8·5 | 12·6b | 6·1 |
| Vitamin C (mg) | 80·2a | 56·9 | 61·5b | 50·0 | 50·9b | 32·5 | 72·5a | 57·7 | 105·7b | 63·9 |
| Vitamin A (mg) | 476·4a | 256·1 | 426·1a,b | 402·7 | 316·0b | 189·3 | 536·6a | 291·2 | 484·2a | 300·6 |
| Thiamin (mg) | 1·5a | 0·5 | 1·3b | 0·5 | 0·9c | 0·4 | 1·9a | 0·6 | 1·7a | 0·7 |
| Riboflavin (mg) | 2·6a | 14·4 | 2·0a | 6·3 | 1·1a | 0·4 | 2·0a | 0·8 | 1·9a | 0·8 |
| Pantothenic acid (mg) | 4·6a | 1·3 | 3·9b | 1·3 | 2·8c | 1·0 | 5·3a | 1·7 | 5·2a | 2·0 |
| Niacin (mg) | 19·5a | 6·9 | 16·4b | 6·3 | 10·9c | 4·6 | 25·3a | 10·5 | 23·5a | 11·6 |
| Vitamin B6 (mg) | 1·7a | 0·5 | 1·4b | 0·5 | 1·0c | 0·4 | 2·2a | 0·9 | 2·0a | 0·8 |
| Vitamin B12 (µg) | 3·9a | 2·0 | 3·7a | 2·9 | 2·2b | 1·2 | 5·4a | 6·7 | 4·6a | 2·6 |
| Folic acid (µg) | 253·2a | 102·8 | 205·7b | 93·3 | 159·6c | 64·1 | 300·6a | 158·9 | 294·5a | 124·7 |
rED, risk of eating disorders; EDNOS, eating disorder not otherwise specified.
a,b,cMean values within a row with unlike superscript letters were significantly different (P<0·05).
Probability of intake inadequacy in the different degrees of severity of eating disorders
Table 3 shows the percentage of adolescents with inadequate intakes according to the degree of severity of ED. In females, we can see that the percentage with micronutrient intake inadequacy was significantly higher as the ED became more severe. More than 80 % of the females with EDNOS and more than 60 % of the females at rED had intake inadequacy regarding Ca, Fe, Mg, P, vitamin D and vitamin B6. In general, 57·8 % and 83·3 % of females at rED and with EDNOS, respectively, presented more than a 50 % risk of micronutrient intake inadequacy.
Table 3.
Percentage predicted to have inadequate intake of nutrients using a probability approach according to the severity of eating disorder and gender among 495 adolescents, mean age 14·2 (sd 1·0) years, Tarragona, Spain
| Females | Males | ||||
|---|---|---|---|---|---|
| Controls (n 212) | rED (n 121) | EDNOS (n 42) | Controls (n 95) | rED (n 25) | |
| Energy* | 13·2a | 34·7b | 71·4c | 6·3a | 16·0a |
| Energy† | 9·0a | 25·0b | 59·5c | 17·9a | 24·0a |
| Protein | 1·2a | 3·6a | 20·0b | 0·1a | 0·01a |
| Ca | 62·5a | 71·7a | 89·4b | 70·3a | 44·7b |
| Fe | 88·8a | 94·2a,b | 99·9b | 38·6a | 78·4b |
| Mg | 55·1a | 69·6b | 96·6c | 47·4a | 52·4a |
| P | 44·4a | 60·7b | 87·7c | 22·9a | 35·9a |
| Vitamin D | 95·6a | 94·0a | 97·7a | 93·2a | 89·5a |
| Vitamin E | 27·6a | 47·0b | 59·5b | 12·2a | 31·7b |
| Vitamin A | 77·4a | 82·1a,b | 93·3b | 71·5a | 85·2a |
| Vitamin C | 37·1a | 53·4b | 49·8a,b | 45·2a | 29·3a |
| Thiamin | 6·6a | 14·8b | 37·0c | 2·4a | 2·2a |
| Riboflavin | 20·8a | 36·7b | 57·6c | 16·0a | 27·7a |
| Niacin | 16·7a | 32·3b | 64·7c | 5·6a | 13·0a |
| Vitamin B6 | 36·3a | 60·8b | 82·2c | 16·2a | 42·9b |
| Vitamin B12 | 8·4a | 13·3a | 34·9b | 1·8a | 0·7a |
| Folic acid | 36·3a | 46·5a,b | 62·5b | 35·0a | 13·5b |
| Risk of micronutrient intake inadequacy‡ >50 % | 31·1a | 57·8b | 83·3c | 15·8a | 33·3a |
rED, risk of eating disorders; EDNOS, eating disorder not otherwise specified.
a,b,cValues within a row with unlike superscript letters were significantly different (P<0·05).
Energy: inadequate intake was when intake was lower than two-thirds of the Recommended Dietary Intake created for the Spanish population.
Energy: inadequate intake was lower than two-thirds of the estimated energy requirement calculated using equations from the Institute of Medicine.
Risk of inadequate micronutrient intake was calculated by adding together the probability of intake inadequacy for each micronutrient and dividing the total by the number of micronutrients (mean of probability of inadequate micronutrient intake). Then we categorised the sample according to the percentage of the population whose probability of inadequate intake was above 50 %.
We observed that energy adequacy was significantly and progressively lower in the control, rED and EDNOS groups (mean 96·6 (SD 30·3) %, mean 76·3 (SD 22·6) % and mean 55·9 (SD 29·0) %, P<0·001; data not shown in the table) and that there were more females with an energy adequacy below two-thirds of the recommended level in the rED group and the EDNOS group than in the control group (Table 3). In relation to energy adequacy, when energy intake was compared with the estimated energy requirement, there were also differences between the control (mean 110·8 (SD 37·4) %), rED (mean 87·0 (SD 36·2) %) and EDNOS groups (mean 62·0 (SD 25·4) %, P<0·001; data not shown in the table).
Relationship between energy and macronutrient intakes and the presence of some form of eating disorder
We designed various multiple linear regression models to determine the association of ED with energy and macronutrient intakes and the risk of micronutrient intake inadequacy adjusted by different psychosocial and anthropometric factors. We show five linear regression models according to the dependent variable: energy intake (model 1), protein intake (model 2), carbohydrate intake (model 3), lipid intake (model 4) and risk of micronutrient intake inadequacy (model 5).
Table 4 shows that rED and EDNOS in the female adolescents was associated with a lower intake of energy (model 1), protein (model 2), carbohydrate (model 3), lipids (model 4) and a higher risk of inadequate micronutrient intake (model 5). Among the controls, the presence of some form of anxiety or depression was associated with a carbohydrate intake 27·9 g/d higher than in the absence of anxiety and depression (model 3). In contrast, among the male adolescents (Table 5), rED was not related to lower intakes or to a higher risk of intake inadequacy of micronutrients.
Table 4.
Factors related to the intakes of energy and macronutrients and with the risk of micronutrient deficiency in relation to the risk of eating disorders in female adolescents, Tarragona, Spain
| All | Controls | rED and EDNOS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | se | P | B | se | P | B | se | P | |
| Model 1: Energy intake factors (kcal) | |||||||||
| Constant | 2093·36 | 384·68 | <0·001 | 1855·78 | 526·79 | 0·001 | 2094·86 | 607·114 | 0·001 |
| Control v. at rED (0=control, 1=rED) | −381·82 | 75·29 | <0·001 | – | – | – | – | – | – |
| Control v. with EDNOS (0=control, 1=EDNOS) | −753·63 | 115·48 | <0·001 | – | – | – | – | – | – |
| Anxiety and/or depression (0=no, 1=yes) | 140·48 | 69·97 | 0·045 | 155·49 | 99·76 | 0·121 | 17·48 | 106·45 | 0·870 |
| Activity index (score) | 74·13 | 67·58 | 0·273 | 157·50 | 94·36 | 0·097 | 102·54 | 102·88 | 0·320 |
| Socio-economic status (score) | −59·26 | 46·84 | 0·207 | −41·77 | 63·88 | 0·514 | −191·48 | 75·42 | 0·012 |
| Stages of puberty (score) | 153·98 | 57·21 | 0·007 | 214·88 | 76·66 | 0·006 | 66·07 | 91·47 | 0·471 |
| Body satisfaction (score) | 20·02 | 4·76 | <0·001 | 19·23 | 6·41 | 0·003 | 31·26 | 7·64 | 0·000 |
| BMI (kg/m2) | −50·89 | 9·32 | <0·001 | −62·60 | 13·50 | <0·001 | −51·99 | 13·61 | 0·000 |
 =29·3, =29·3, 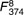 =20·39, =20·39, |
 =15·6, =15·6,  =27·49, =27·49, |
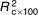 =18·9, =18·9,  =8·20, =8·20, |
|||||||
| P<0·001 | P<0·001 | P<0·001 | |||||||
| Model 2: Protein intake factors (g) | |||||||||
| Constant | 71·75 | 15·16 | <0·001 | 69·43 | 21·56 | 0·001 | 65·84 | 24·28 | 0·007 |
| Control v. at rED (0=control, 1=rED) | −10·94 | 2·96 | <0·001 | – | – | – | – | – | – |
| Control v. with EDNOS (0=control, 1=EDNOS) | −29·36 | 4·55 | <0·001 | – | – | – | – | – | – |
| Anxiety and/or depression (0=no, 1=yes) | 1·73 | 2·75 | 0·530 | 1·49 | 4·08 | 0·715 | −1·73 | 4·25 | 0·684 |
| Activity index (score) | 3·85 | 2·66 | 0·148 | 5·11 | 3·86 | 0·187 | 6·06 | 4·11 | 0·142 |
| Socio-economic status (score) | −0·50 | 1·84 | 0·783 | 0·68 | 2·61 | 0·793 | −5·88 | 3·01 | 0·053 |
| Stages of puberty (score) | 3·88 | 2·25 | 0·086 | 6·48 | 3·13 | 0·040 | 1·40 | 3·65 | 0·702 |
| Body satisfaction (score) | 0·66 | 0·18 | <0·001 | 0·60 | 0·26 | 0·023 | 1·14 | 0·30 | <0·001 |
| BMI (kg/m2) | −1·24 | 0·36 | 0·001 | −1·82 | 0·55 | 0·001 | −1·26 | 0·54 | 0·021 |
 =22·3, =22·3,  =14·44, =14·44, |
 =8·1, =8·1,  =4·09, =4·09, |
 =13·7, =13·7,  =5·92, =5·92, |
|||||||
| P<0·001 | P<0·001 | P<0·001 | |||||||
| Model 3:·Carbohydrate intake factors (g) | |||||||||
| Constant | 263·58 | 46·05 | <0·001 | 231·57 | 63·15 | <0·001 | 250·76 | 72·31 | 0·001 |
| Control v. at rED (0=control, 1=rED) | −42·31 | 9·01 | <0·001 | – | – | – | – | – | – |
| Control v. with EDNOS (0=control, 1=EDNOS) | −81·21 | 13·82 | <0·001 | – | – | – | – | – | – |
| Anxiety and/or depression (0=no, 1=yes) | 19·40 | 8·37 | 0·021 | 27·93 | 11·96 | 0·020 | −2·50 | 12·67 | 0·843 |
| Activity index (score) | 2·98 | 8·09 | 0·713 | 16·25 | 11·31 | 0·152 | 6·77 | 12·25 | 0·581 |
| Socio-economic status (score) | −7·73 | 5·60 | 0·169 | −8·75 | 7·65 | 0·255 | −19·74 | 8·99 | 0·029 |
| Stages of puberty (score) | 15·43 | 6·85 | 0·025 | 17·19 | 9·19 | 0·063 | 11·60 | 10·89 | 0·288 |
| Body satisfaction (score) | 2·02 | 0·57 | <0·001 | 2·03 | 0·76 | 0·009 | 3·26 | 0·91 | <0·001 |
| BMI (kg/m2) | −6·12 | 1·11 | <0·001 | −6·61 | 1·61 | <0·001 | −6·81 | 1·62 | <0·001 |
 =25·8, =25·8, 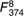 =17·21, =17·21, |
 =12·7, =12·7, 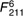 =6·10, =6·10, |
 =17·3, =17·3, 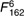 =7·46, =7·46, |
|||||||
| P<0·001 | P<0·001 | P<0·001 | |||||||
| Model 4:· Lipid intake factors (g) | |||||||||
| Constant | 82·13 | 20·66 | <0·001 | 70·18 | 28·75 | 0·016 | 91·45 | 29·49 | 0·002 |
| Control v. at rED (0=control, 1=rED) | −18·64 | 4·04 | <0·001 | – | – | – | – | – | – |
| Control v. with EDNOS (0=control, 1=EDNOS) | −34·29 | 6·20 | <0·001 | – | – | – | – | – | – |
| Anxiety and/or depression (0=no, 1=yes) | 5·97 | 3·75 | 0·113 | 3·80 | 5·44 | 0·486 | 3·59 | 5·17 | 0·488 |
| Activity index (score) | 5·59 | 3·63 | 0·124 | 9·07 | 5·15 | 0·080 | 5·64 | 4·99 | 0·260 |
| Socio-economic status (score) | −3·00 | 2·51 | 0·233 | −1·29 | 3·48 | 0·710 | −9·83 | 3·66 | 0·008 |
| Stages of puberty (score) | 8·51 | 3·07 | 0·006 | 13·09 | 4·18 | 0·002 | 1·72 | 4·44 | 0·699 |
| Body satisfaction (score) | 1·04 | 0·25 | <0·001 | 1·00 | 0·35 | 0·005 | 1·51 | 0·37 | <0·001 |
| BMI (kg/m2) | −2·37 | 0·50 | <0·001 | −3·20 | 0·73 | <0·001 | −2·19 | 0·66 | 0·001 |
 =24·9, =24·9,  =16·47, =16·47, |
 =14·3, =14·3,  =6·85, =6·85, |
 =17·6, =17·6, 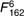 =7·61, =7·61, |
|||||||
| P<0·001 | P<0·001 | P<0·001 | |||||||
| Model 5:·Risk of micronutrient intake inadequacy factors (%)* | |||||||||
| Constant | 46·79 | 13·32 | 0·001 | 51·96 | 16·90 | 0·002 | 45·40 | 20·16 | 0·026 |
| Control v. at rED (0=control, 1=rED) | 11·01 | 2·60 | <0·001 | – | – | – | – | – | – |
| Control v. with EDNOS (0=control, 1=EDNOS) | 26·92 | 4·00 | <0·001 | – | – | – | – | – | – |
| Anxiety and/or depression (0=no, 1=yes) | −2·44 | 2·42 | 0·315 | −1·90 | 3·20 | 0·552 | −0·12 | 3·53 | 0·973 |
| Activity index (score) | −4·24 | 2·34 | 0·071 | −6·73 | 3·02 | 0·027 | −0·44 | 3·41 | 0·897 |
| Socio-economic status (score) | 0·005 | 1·62 | 0·997 | −2·30 | 2·05 | 0·263 | 4·21 | 2·50 | 0·094 |
| Stages of puberty (score) | −1·97 | 1·98 | 0·320 | 4·46 | 2·46 | 0·071 | 1·89 | 3·03 | 0·533 |
| Body satisfaction (score) | −0·36 | 0·16 | 0·029 | −0·35 | 0·20 | 0·087 | −0·79 | 0·25 | 0·002 |
| BMI (kg/m2) | 1·08 | 0·32 | 0·001 | 1·82 | 0·433 | <0·001 | 0·60 | 0·45 | 0·189 |
 =20·7, =20·7,  =13·22, =13·22, |
 =12·70, =12·70,  =6·11, =6·11, |
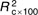 =7·00, =7·00,  =3·34, =3·34, |
|||||||
| P<0·001 | P<0·001 | P=0·004 | |||||||
rED, risk of eating disorders; EDNOS, eating disorder not otherwise specified; B, unstandardised coefficient; level of statistical significance, P<0·05.
Risk of inadequate micronutrient intake was calculated using the mean of probability of inadequate intake.
Table 5.
Factors related to the intake of energy and macronutrients and with the risk of micronutrient deficiency in relation to the risk of eating disorders in male adolescents, Tarragona, Spain
| All | Controls | rED and EDNOS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | se | P | B | se | P | B | se | P | |
| Model 1: Energy intake factors (kcal) | |||||||||
| Constant | 2254·08 | 738·77 | 0·003 | 1901·44 | 840·60 | 0·026 | 3947·31 | 1601·76 | 0·024 |
| Control v.· at rED (0=control, 1=rED) | −57·16 | 184·63 | 0·757 | – | – | – | – | – | – |
| Anxiety and/or depression (0=no, 1=yes) | −79·35 | 153·95 | 0·607 | −11·19 | 163·02 | 0·945 | −457·71 | 446·05 | 0·318 |
| Activity index (score) | 144·91 | 145·82 | 0·322 | 119·08 | 161·43 | 0·463 | 152·97 | 342·50 | 0·660 |
| Socio-economic status (score) | −18·80 | 94·88 | 0·843 | 130·57 | 98·04 | 0·186 | −712·58 | 252·97 | 0·011 |
| Stages of puberty (score) | 200·97 | 99·77 | 0·046 | 185·46 | 101·86 | 0·072 | 348·24 | 286·20 | 0·239 |
| Body satisfaction (score) | 18·42 | 11·02 | 0·097 | 21·56 | 11·23 | 0·058 | 6·01 | 32·74 | 0·856 |
| BMI (kg/m2) | −55·82 | 18·96 | 0·004 | −51·73 | 22·15 | 0·022 | −77·56 | 42·79 | 0·087 |
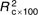 =13·4, =13·4, 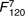 =3·62, =3·62, |
 =13·3, =13·3,  =3·49, =3·49, |
 =28·3, =28·3,  =2·57, =2·57, |
|||||||
| P<0·001 | P=0·004 | P=0·056 | |||||||
| Model 2: Protein intake factors (g) | |||||||||
| Constant | 69·97 | 31·35 | 0·028 | 58·26 | 33·77 | 0·088 | 136·13 | 79·12 | 0·102 |
| Control v. at rED (0=control, 1=rED) | 0·25 | 7·83 | 0·974 | – | – | – | – | – | – |
| Anxiety and/or depression (0=no, 1=yes) | −1·78 | 6·53 | 0·785 | 0·08 | 6·54 | 0·990 | −18·73 | 22·03 | 0·406 |
| Activity index (score) | 12·07 | 6·18 | 0·054 | 13·01 | 6·48 | 0·048 | 6·06 | 16·91 | 0·724 |
| Socio-economic status (score) | 4·02 | 4·02 | 0·319 | 9·98 | 3·93 | 0·013 | −23·31 | 12·49 | 0·078 |
| Stages of puberty (score) | 6·60 | 4·02 | 0·122 | 4·56 | 4·09 | 0·267 | 19·95 | 14·13 | 0·175 |
| Body satisfaction (score) | 0·20 | 0·46 | 0·656 | 0·35 | 0·45 | 0·431 | −0·48 | 1·61 | 0·769 |
| BMI (kg/m2) | −1·90 | 0·80 | 0·020 | −1·85 | 0·89 | 0·040 | −2·79 | 2·11 | 0·203 |
 =8·3, =8·3,  =2·53, =2·53, |
 =13·3, =13·3, 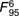 =3·40, =3·40, |
 =9·1, =9·1, 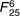 =1·40, =1·40, |
|||||||
| P=0·018 | P=0·005 | P=0·268 | |||||||
| Model 3: Carbohydrate intake factors (g) | |||||||||
| Constant | 262·43 | 94·60 | 0·006 | 229·69 | 12·44 | 0·044 | 411·40 | 192·76 | 0·047 |
| Control v. at rED (0=control, 1=rED) | −10·18 | 23·64 | 0·667 | – | – | – | – | – | – |
| Anxiety and/or depression (0=no, 1=yes) | −7·75 | 19·71 | 0·695 | 3·41 | 21·80 | 0·876 | −68·62 | 53·68 | 0·217 |
| Activity index (score) | 10·74 | 18·67 | 0·566 | 7·15 | 21·80 | 0·876 | 93·17 | 41·21 | 0·826 |
| Socio-economic status (score) | −10·57 | 12·15 | 0·386 | 4·43 | 13·11 | 0·736 | −80·55 | 30·44 | 0·016 |
| Stages of puberty (score) | 24·08 | 12·77 | 0·062 | 23·19 | 13·62 | 0·092 | 38·04 | 34·44 | 0·284 |
| Body satisfaction (score) | 3·15 | 1·41 | 0·028 | 3·56 | 1·50 | 0·020 | 1·90 | 3·94 | 0·635 |
| BMI (kg/m2) | −6·82 | 2·42 | 0·006 | −6·71 | 2·96 | 0·026 | −7·30 | 5·15 | 0·173 |
 =14·1, =14·1,  =3·78, =3·78, |
 =13·3, =13·3,  =3·39, =3·39, |
 =24·4, =24·4,  =2·20, =2·20, |
|||||||
| P<0·001 | P=0·005 | P=0·810 | |||||||
| Model 4: Lipid intake factors (g) | |||||||||
| Constant | 101·42 | 37·90 | 0·009 | 81·41 | 45·70 | 0·078 | 194·90 | 66·46 | 0·009 |
| Control v. at rED (0=control, 1=rED) | −2·01 | 9·47 | 0·832 | – | – | – | – | – | – |
| Anxiety and/or depression (0=no, 1=yes) | −4·17 | 7·89 | 0·598 | −2·28 | 8·86 | 0·797 | −12·33 | 18·50 | 0·513 |
| Activity index (score) | 7·20 | 7·48 | 0·338 | 5·66 | 8·77 | 0·520 | 11·08 | 14·21 | 0·445 |
| Socio-economic status (score) | 0·88 | 4·86 | 0·857 | 8·13 | 5·33 | 0·130 | −32·20 | 10·49 | 0·006 |
| Stages of puberty (score) | 8·82 | 5·11 | 0·088 | 8·46 | 5·53 | 0·130 | 12·85 | 11·87 | 0·293 |
| Body satisfaction (score) | 0·54 | 0·56 | 0·341 | 0·64 | 0·61 | 0·292 | <0·01 | 1·35 | 1·000 |
| BMI (kg/m2) | −2·45 | 0·97 | 0·013 | −2·09 | 1·20 | 0·086 | −4·19 | 1·77 | 0·030 |
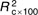 =7·6, =7·6,  =2·84, =2·84, |
 =6·2, =6·2,  =2·04, =2·04, |
 =35·1, =35·1,  =3·16, =3·16, |
|||||||
| P=0·026 | P=0·068 | P=0·027 | |||||||
| Model 5: Risk of micronutrient intake inadequacy factors (%)* | |||||||||
| Constant | 14·51 | 18·60 | 0·437 | 12·52 | 22·97 | 0·587 | 8·66 | 35·90 | 0·812 |
| Control v. at rED (0=control, 1=rED) | 0·55 | 4·65 | 0·905 | – | – | – | – | – | – |
| Anxiety and/or depression (0=no, 1=yes) | 6·83 | 3·87 | 0·081 | 7·92 | 4·45 | 0·079 | 10·12 | 9·99 | 0·325 |
| Activity index (score) | 3·72 | 3·67 | 0·313 | 2·30 | 4·41 | 0·603 | 9·02 | 7·67 | 0·255 |
| Socio-economic status (score) | −0·22 | 2·39 | 0·926 | −2·12 | 2·68 | 0·431 | 7·92 | 5·67 | 0·179 |
| Stages of puberty (score) | −6·98 | 2·51 | 0·006 | −6·27 | 2·78 | 0·027 | −10·39 | 6·41 | 0·123 |
| Body satisfaction (score) | 0·11 | 0·27 | 0·688 | 0·16 | 0·31 | 0·583 | −0·19 | 0·73 | 0·795 |
| BMI (kg/m2) | 1·39 | 0·47 | 0·004 | 1·63 | 0·60 | 0·008 | 1·15 | 0·95 | 0·246 |
 =10·2, =10·2,  =2·94, =2·94, |
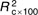 =8·7, =8·7,  =2·48, =2·48, |
 =5·9, =5·9,  =1·25, =1·25, |
|||||||
| P=0·007 | P=0·029 | P=0·327 | |||||||
rED, risk of eating disorders; EDNOS, eating disorder not otherwise specified; B, unstandardised coefficient; level of statistical significance, P<0·05.
Risk of inadequate micronutrient intake was calculated using the mean of probability of inadequate intake.
Discussion
Our findings showed that in a population of Mediterranean adolescents, females at rED presented substantially lower energy and nutrient intakes than the control group, which worsened in females with EDNOS. Although restricting energy intake in adolescents could be beneficial for those with EDNOS, given that the mean BMI (24·0 (sd 4·0) kg/m2) reached the level of overweight according to the classification of Cole et al. ( 43 ), when such a restriction was without the supervision of a health professional, it led to a high percentage of females at risk of inadequate micronutrient intake. In contrast, no great differences in nutritional intake were reported for the males.
Similar studies in adolescent males with anomalous dietary behaviours have also observed no significant differences in intake( 44 ). In males, overweight and underweight are known to be risk factors of body dissatisfaction. Although our results did not show that males with rED performed more vigorous physical activity than the controls, we did observe more males than females performing vigorous physical activity. Some studies indicate that this is because, rather than making significant dietary modifications, males prefer to engage in greater levels of vigorous physical activity as means of avoiding weight gain and increasing muscle, and thus conforming to the ideal of masculine beauty( 45 ). In general, our results in males should be treated with caution due to the small size of the sample.
Most of the literature on dietary intake and ED has studied clinical populations but none has studied populations with EDNOS and very little has studied populations at rED. In this regard, and in line with the findings of several authors( 9 – 11 ), our study showed lower energy and/or macronutrient intake(s) in adolescent females at rED. The energy intake of females at rED was 20·0 % lower than that of the control group, a slightly higher percentage than that shown by the previously cited authors (4·8–16·0 %). At the same time, we observed that adolescent females with EDNOS presented reductions in macronutrients and an energy intake (3983·1 kJ/d (952·0 kcal/d)) which was closer to that observed in other studies on anorexic populations (between 1288·6 and 3895·3 kJ/d (308 and 931 kcal/d))( 46 , 47 ). Based on adjusted multiple linear regression, we confirmed that rED and EDNOS was associated with a lower energy intake of 1255·2 kJ/d (300 kcal/d) and 2928·8 kJ/d (700 kcal/d), respectively. Also, our results showed that the presence of rED and EDNOS was associated with lower protein, carbohydrate and lipid intakes, as has been found by other studies on rED and clinical populations. These lower intakes of energy and macronutrients in the ED population may arise because they usually choose foods that are low in energy and fat and avoid meats, sugars, cereals and fatty foods( 44 ). However, Allen et al. ( 12 ) found that a population at rED did not restrict energy intake but did restrict intakes of carbohydrates and lipids, except in the case of females with symptoms of bulimia, who consumed a higher amount of refined sugars. In this regard, individuals with symptoms of binge eating disorders have been observed to consume greater amounts of carbohydrates and fats, even to the point of consuming an extra quantity of 1673·6 kJ/d (400 kcal/d)( 48 ).
With respect to energy adequacy, some studies in clinical ED( 46 ) and community populations with symptoms of ED( 9 ) have shown higher energy inadequacy compared with the reference energy intakes and estimated energy requirements. In this sense, our results showed that between 30 % and 70 % of females with rED and EDNOS had an energy intake below two-thirds of the recommended reference value. It is known that energy expenditure depends on several factors such as BMR and level of physical activity. Therefore, if we take into account the estimated energy requirement, we also can observe that 25–60 % of females with rED and EDNOS presented an inadequate energy intake.
With regard to micronutrient intake, previous studies have identified lower intakes of thiamin, riboflavin, niacin, vitamins B6 and B12, Se, Zn, Cu, Ca, Fe and/or P in anorexic females( 46 , 47 ). Lower intakes of Na, Fe and vitamins B12, B6, A, E and/or C have been observed in females at rED( 9 – 12 ). Compared with these studies, our results show lower intakes of a greater number of micronutrients (Ca, Fe, Mg, K, P, Na, thiamin, vitamins E, C, B6, B12, pantothenic acid and folic acid) in adolescents at rED and with EDNOS. These differences could be because these studies used different methodologies to assess dietary intake such as FFQ(12,47), a single 24 h recall( 9 – 11 ) and diet histories( 46 ).
In relation to inadequate micronutrient intake, we observed that a high percentage of females with rED (57·8 %) or EDNOS (83·3 %) presented risk of micronutrient intake inadequacy. In this vein, based on multiple linear regressions, our results showed that rED and EDNOS were associated with an 11 % and a 26 % greater risk of micronutrient intake inadequacy, respectively. This risk of energy and micronutrient deficiency becomes evident as soon as the first symptoms appear and may be sufficiently severe to interfere in the optimum development of the adolescent. Particularly, various studies have revealed that the adolescent population does not consume the recommended daily portions of dairy products and, therefore, does not meet its daily Ca requirement( 48 ). This situation worsens in the presence of rED or EDNOS, as confirmed by our study and others( 9 , 46 ). A deficient dairy intake can also lead to a deficiency in other micronutrients such as vitamin D, P and Mg. We observed that more than 50 % of females at rED and with EDNOS presented a risk of Mg and P deficiency; and that practically all of the adolescents presented a risk of intake inadequacy of vitamin D, similar to that in the general population( 49 ). All of these nutrients are essential to the mineralisation of the skeleton( 50 ). Consequently, nutrient deficiency can affect the formation of optimum bone mass or may even accelerate bone loss at a crucial moment during bone growth. Also, our results showed that Fe intake was lower as the ED became more severe. This may be due to a lower intake of meat and meat products in our participants, and has previously been observed in an anorexic population( 47 ). In our study, practically all females at rED and with EDNOS and 88·8 % of the adolescent controls presented a risk of Fe intake inadequacy, with a larger percentage of the population at risk Fe deficiency than is reported for the general population( 49 ). Fe deficiency has been associated with the risk of anaemia, reduced immune response and cognitive impairment, among other problems, because Fe is involved in multiple metabolic functions( 51 ).
Apart from the physical repercussions, deficient intake may also be related to emotional disorders and may aggravate ED. In this regard, restricting energy or lipid intake, specifically the n-3 PUFA, may be associated with depression symptoms( 6 ). Likewise, deficient intakes of other micronutrients such as vitamins A, C, B6, B2, niacin, folic acid and Mg, among others, could be related to poorer mental health and lead to negative repercussions in the medium and long term( 52 ).
Therefore, our results showed that females with rED and EDNOS had low energy and macronutrient intakes and a high risk of micronutrient intake inadequacy, and these associations remained even when adjusting for confounding factors. Also, in these multiple linear regression models, we observed that several of these confounding factors contributed significantly to energy and macronutrient intakes and risk of micronutrient intake inadequacy in concordance with other studies. For example, we observed that the presence of anxiety and depressive symptoms was associated with a carbohydrate intake higher by 467·3 kJ/d (111·7 kcal/d) in females not at risk of ED. In this regard, intake of carbohydrate and palatable foods may serve to alleviate negative affective states via the physiological activation of the opioid and dopaminergic pleasure and reward systems( 53 ). However, we did not observe that anxiety and depression affected energy and macronutrient intakes in the groups with rED and EDNOS. A possible explanation might be that ED causes a contrary effect on dietary intake. Also, it is important to highlight that high body satisfaction score is associated with high energy and macronutrient intakes and/or with low risk of micronutrient intake inadequacy. In this vein, several authors have shown that females with body dissatisfaction (with or without ED) present lower energy intake and low diet quality which could involve inadequate intakes of micronutrients( 39 , 40 , 54 ).
In addition, similar to previous studies( 3 ), we observed that both adolescents at rED and those with EDNOS were more dissatisfied with their bodies and that a higher percentage of these presented symptoms of emotional disorders. Moreover, as has been found by other authors( 11 , 12 ), adolescents at rED and with EDNOS showed BMI values close to overweight. However, in contrast to other authors who have shown that adolescents with ED engage in vigorous physical exercise, we observed that approximately one-third of the females in all groups were sedentary. Although diet and physical exercise form the central pillars on which the treatment of overweight and obesity is founded, any changes to diet and physical exercise need to be monitored to prevent situations of risk. As we mentioned above, adolescents at rED and with EDNOS adopt more energy restrictive diets compared with the control group. Such diets are not monitored by health professionals and, even in a context of overweight, can lead to a higher risk of inadequate intakes. On the basis of this, we recommend a healthy lifestyle with a proper energy intake and physical activity.
In general, the results of our study support previous findings albeit with certain differences, which may be due to: (i) socio-economic and cultural differences; (ii) the type and degree of severity of the ED; (iii) the use of a different methodology (screening questionnaires, consumption evaluations and food composition tables). With regard to the methodology’s limitations, the 24 h recall method is limited by the flat slope syndrome (i.e. it overestimates low intake and underestimates high intake)( 55 ) and by misreporting due to various causes (voluntary omission of foods consumed, erroneous estimation of portion sizes eaten, memory lapses, etc.)( 56 ). Another limitation is that the number of days required to assess habitual intake of a nutrient is not always the same; that is, it depends on the type of nutrient. Because of this, an assessment of the habitual intake of a certain nutrient may be inaccurate. The evidence indicates that at least three days of these recalls are necessary and that they should occur on non-consecutive days in order to asses habitual dietary intake with relative accuracy and without significantly diminishing participation( 57 ); (iv) additionally, we did not record supplement intake, as in the previously cited studies of community samples with symptoms of ED( 9 – 12 ). According to a recent review, in most cases supplement intake made very little difference to the proportion of individuals with inadequate intake below the recommended level( 58 ). In Spain, the percentage of the population consuming these products is not as high as in other countries( 59 ). Therefore, supplement intake may have little influence on the assessment of micronutrient intake; and (v) finally, our study is limited by the weaknesses inherent in such cross-sectional studies and by the fact that it does not distinguish between the different types of EDNOS because intake can vary depending on the type of pathology. Further research needs to address this by studying in greater detail the relationship between intake and nutritional risk during the onset and continuation of ED.
Nevertheless, our study also has certain strengths in that: (i) it takes a representative sample of the adolescent population in order to evaluate intakes in relation to the degree of severity of ED; (ii) it uses not only self-administered tests to record the symptoms of ED, but also structured clinical interviews to obtain diagnoses of EDNOS; and (iii) furthermore, we employed the internationally used 24 h recall method to obtain real and quantitative data regarding the participants’ regular dietary intakes( 23 ).
Our study is the first that has quantitatively examined dietary intakes in a school-based population of Mediterranean adolescents with symptoms of ED and EDNOS and that has identified the percentage at nutritional risk within these groups. In addition to the new information provided by the study, our findings have significant public health implications in that they highlight the value of early detection and intervention in individuals at rED. It shows that these adolescents are not usually clinically identified, which may lead to significant risks of nutritional deficiency in early stages of the disorder. Thus, by the time the individual is diagnosed with ED, he or she may already present serious nutritional problems. Furthermore, the high level of obesity and overweight in children and adolescents may lead to risky attitudes and behaviours, such as self-imposed dietary restriction and excessive physical activity, which increase the risk of ED onset and persistence. Given that the acquisition of healthy dietary habits starts in infancy, it is essential to implement integrated educational programmes on nutrition in the pre-adolescent stages of schooling to encourage the adoption of a healthy dietary intake, increase the likelihood of this being maintained in later life and prevent the development of ED and obesity( 60 ). Families and professionals need to be aware that dietary treatments in overweight adolescents must be supervised by health professionals who specialise in prescribing diets that both restrict energy intake and ensure an adequate supply of nutrients.
Conclusion
To conclude, the female adolescents showed that energy and nutrient intakes fell as the ED became more severe, and this heightened the risk of micronutrient deficiencies. These nutritional risks combined with emotional factors could negatively affect physical and psychological development and increase the likelihood of chronic ED. Nutritional education strategies need to be implemented to correct these deficiencies and to prevent ED.
Acknowledgements
Acknowledgements: The authors acknowledge the collaboration of the participants, the schools and the Education Department of Catalonia. They also grateful to Elisabeth Biarnés, Oscar Asorey, Dolors Simón, Carolina Sancho, Nancy Babio and Marta Ferrer for their collaboration in recruitment and fieldwork. Financial support: These studies was supported by two grants from the Healthcare Research Fund (01/1364 and PI042596). The Healthcare Research Fund (FIS) of the Carlos III Institute of Health had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: The authors’ contributions to manuscript were as follows: formulation of research questions, V.A. and J.C.; design of the study, V.A. and J.C.; data collection, J.C., V.A. and S.P.; analysis and interpretation, V.A., J.C. and E.A.; writing the article, E.A.; critical revision of the article, V.A. and J.C. Ethics of human subject participation: The study was approved by the Ethics Committee for Clinical Research at the Sant Joan University Hospital in Reus (Spain).
References
- 1. Eisenberg D, Nicklett EJ, Roeder K et al. (2011) Eating disorder symptoms among college students: prevalence, persistence, correlates, and treatment-seeking. J Am Coll Health 59, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rome ES (2012) Eating disorders in children and adolescents. Curr Probl Pediatr Adolesc Health Care 42, 28–44. [DOI] [PubMed] [Google Scholar]
- 3. Micali N, Hagberg KW, Petersen I et al. (2013) The incidence of eating disorders in the UK in 2000–2009: findings from the General Practice Research Database. BMJ Open 3, e002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babio N, Canals J, Pietrobelli A et al. (2009) A two-phase population study: relationships between overweight, body composition and risk of eating disorders. Nutr Hosp 24, 485–491. [PubMed] [Google Scholar]
- 5. Sancho C, Arija MV, Asorey O et al. (2007) Epidemiology of eating disorders: a two year follow up in an early adolescent school population. Eur Child Adolesc Psychiatry 16, 495–504. [DOI] [PubMed] [Google Scholar]
- 6. Preti A, Girolamo G, Vilagut G et al. (2009) The epidemiology of eating disorders in six European countries: results of the ESEMeD-WMH Project. J Psychiatr Res 43, 1125–1132. [DOI] [PubMed] [Google Scholar]
- 7. Canals J, Carbajo G, Fernandez J et al. (1996) Biopsychopathologic risk profile of adolescents with eating disorder symptoms. Adolescence 31, 443–450. [PubMed] [Google Scholar]
- 8. Mitchison D & Hay PJ (2014) The epidemiology of eating disorders: genetic, environmental, and societal factors. Clin Epidemiol 17, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunker KL & Philippi ST (2005) Differences in diet composition of Brazilian adolescent girls with positive or negative score in the Eating Attitudes Test. Eat Weight Disord 10, e70–e75. [DOI] [PubMed] [Google Scholar]
- 10. Chang YJ, Lin W & Wong Y (2011) Survey on eating disorder-related thoughts, behaviors, and their relationship with food intake and nutritional status in female high school students in Taiwan. J Am Coll Nutr 30, 39–48. [DOI] [PubMed] [Google Scholar]
- 11. Tsai MR, Chang YJ, Lien PJ et al. (2011) Survey on eating disorders related thoughts, behaviors and dietary intake in female junior high school students in Taiwan. Asia Pac J Clin Nutr 20, 196–205. [PubMed] [Google Scholar]
- 12. Allen KL, Mori TA, Beilin L et al. (2012) Dietary intake in population-based adolescents: support for a relationship between eating disorder symptoms, low fatty acid intake and depressive symptoms. J Hum Nutr Diet 26, 459–469. [DOI] [PubMed] [Google Scholar]
- 13. Arija V, Ferrer-Barcala M, Aranda N et al. (2010) BDNF Val66Met polymorphism, energy intake and BMI: a follow-up study in schoolchildren at risk of eating disorders. BMC Public Health 10, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hollingshead AB (2011) Four factor index of social status. Yale J Sociol 8, 21–52. [Google Scholar]
- 15. Garner DM & Garfinkel PE (1979) The Eating Attitudes Test: an index of the symptoms of anorexia nervosa. Psychol Med 9, 273–279. [DOI] [PubMed] [Google Scholar]
- 16. Castro J, Toro J, Salamero M et al. (1991) The Eating Attitudes Test: validation of the Spanish version. Psychol Assess 7, 175–190. [DOI] [PubMed] [Google Scholar]
- 17. Canals J, Carbajo G & Fernandez-Ballart J (2002) Discriminant validity of the Eating Attitudes Test according to American Psychiatric Association and World Health Organization criteria of eating disorders. Psychol Rep 91, 1052–1056. [DOI] [PubMed] [Google Scholar]
- 18. Wing JK, Barbor T, Brugha T et al. (1990) SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 47, 589–593. [DOI] [PubMed] [Google Scholar]
- 19. Vazquez-Barquero J, Herrera S & Gaitel L (1991) La entrevista estructurada en psiquiatría. Rev Asoc Esp Neuropsiq 44, 19–28. [Google Scholar]
- 20. Ezpeleta L, de la Osa N, Domenech J et al. (1997) Diagnostic agreement between clinicians and the Diagnostic Interview for Children and Adolescents – DICA-R – in an outpatient sample. J Child Psychol Psychiatry 38, 431–440. [DOI] [PubMed] [Google Scholar]
- 21. Gadow K & Sprafkin J (1999) Youth’s Inventory-4 Manual. Stony Brook, NY: Checkmate Plus, Ltd. [Google Scholar]
- 22. Cash T (1997) The Body Image Workbook: An 8-step Program for Learning to Like Your Looks. Oakland, CA: New Harbingen Publications. [Google Scholar]
- 23. Beaton GH, Milner J, Corey P et al. (1979) Sources of variance in 24-h dietary recall data: implications for nutrition study design and interpretation. Am J Clin Nutr 32, 2546–2559. [DOI] [PubMed] [Google Scholar]
- 24. Favier JC, Ireland-Ripert J, Toque C et al. (1997) Répertoire general des aliments. Table de composition. Paris: TEC & Lavoiseir-INRA. [Google Scholar]
- 25. Mataix J (1995) Tabla de Composición de los Alimentos Española. Granada: Servicio de publicaciones de la Universidad de Granada. [Google Scholar]
- 26. Ortega RM, Requejo AM & Navia B (2004) Ingestas Diarias Recomendadas de Energía y Nutrientes para la Población Española. Madrid: Universidad Computense de Madrid. [Google Scholar]
- 27. Institute of Medicine (2000) Dietary Reference Intakes: Applications in Dietary Assessment. Washington, DC: National Academy Press. [Google Scholar]
- 28. Foote JA, Murphy SP, Wilkens LR et al. (2004) Dietary variety increases the probability of nutrient adequacy among adults. J Nutr 134, 1779–1785. [DOI] [PubMed] [Google Scholar]
- 29. Serra-Majem L & Aranceta-Bartrina J (2006) Requerimientos nutricionales e ingestas recomendadas: ingestas dietéticas de referencia. In Nutrición y Salud Pública, pp. 20–30 [L Serra-Majem and J Aranceta-Bartrina, editors]. Barcelona: Masson. [Google Scholar]
- 30. Institute of Medicine of the National Academies (2005) Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press; availabale at http://www.nap.edu/books/0309085373/html [Google Scholar]
- 31. Sarriá A, Selles H, Cañedo-Argüelles L et al. (1987) Un autotest como método de cuantificación de la actividad física en adolescentes. Med Clin (Barc) 7, 56–61. [Google Scholar]
- 32. Baecke JA, Burema J & Frijters JE (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36, 936–942. [DOI] [PubMed] [Google Scholar]
- 33. Tanner JM (1969) Growth and endocrinology of the adolescent. In Endocrine and Genetic Diseases of Childhood, pp. 16–60 [L Gardner, editor]. Philadelphia, PA/London: Saunders. [Google Scholar]
- 34. Kenny DA, Mannetti L, Pierro A et al. (2002) The statistical analysis of data from small groups. J Pers Soc Psychol 83, 126–137. [PubMed] [Google Scholar]
- 35. Pardo A, Ruiz MA & San Martin R (2007) How to fit and interpret multilevel models using SPSS. Psicothema 19, 308–321. [PubMed] [Google Scholar]
- 36. Zarnowiecki DM, Dollman J & Parletta N (2014) Associations between predictors of children’s dietary intake and socioeconomic position: a systematic review of the literature. Obes Rev 15, 375–391. [DOI] [PubMed] [Google Scholar]
- 37. Bales CW, Hawk VH, Granville EO et al. (2012) Aerobic and resistance training effects on energy intake: the STRRIDE-AT/RT study. Med Sci Sports Exerc 44, 2033–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mooreville M, Shomaker LB, Reina SA et al. (2014) Depressive symptoms and observed eating in youth. Appetite 75, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Babio N, Arija V, Sancho C et al. (2008) Factors associated with body dissatisfaction in non-clinical adolescents at risk of eating disorders. J Public Health 16, 107–115. [Google Scholar]
- 40. Bibiloni M, Pich J, Pons A et al. (2013) Body image and eating patterns among adolescents. BMC Public Health 13, 1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bisset S, Gauvin L, Potvin L et al. (2007) Association of body mass index and dietary restraint with changes in eating behaviour throughout late childhood and early adolescence: a 5-year study. Public Health Nutr 10, 780–789. [DOI] [PubMed] [Google Scholar]
- 42. Shomaker LB, Tanofsky-Kraff M & Savastano DM (2010) Puberty and observed energy intake: boy, can they eat! Am J Clin Nutr 92, 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cole TJ, Bellizzi MC, Flegal KM et al. (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320, 1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neumark-Sztainer D, Hannan PJ, Story M et al. (2004) Weight-control behaviors among adolescent girls and boys: implications for dietary intake. J Am Diet Assoc 104, 913–920. [DOI] [PubMed] [Google Scholar]
- 45. Calzo JP, Sonneville KR, Haines J et al. (2012) The development of associations among body mass index, body dissatisfaction, and weight and shape concern in adolescent boys and girls. J Adolesc Health 51, 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hadigan CM, Anderson EJ, Miller KK et al. (2000) Assessment of macronutrient and micronutrient intake in women with anorexia nervosa. Int J Eat Disord 28, 284–292. [DOI] [PubMed] [Google Scholar]
- 47. Jáuregui I & Bolaños P (2009) Choice of diet in patients with anorexia nervosa. Nutr Hosp 24, 682–687. [PubMed] [Google Scholar]
- 48. Mirch MC, McDuffie JR, Yanovski SZ et al. (2006) Effects of binge eating on satiation, satiety, and energy intake of overweight children. Am J Clin Nutr 84, 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Serra-Majem L, Ribas-Barba L, Perez-Rodrigo C et al. (2006) Nutrient adequacy in Spanish children and adolescents. Br J Nutr 96, Suppl. 1, S49–S57. [DOI] [PubMed] [Google Scholar]
- 50. Price CT, Langford JR & Liporace FA (2012) Essential nutrients for bone health and a review of their availability in the average North American diet. Open Orthop J 6, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eden AN & Sandoval C (2012) Iron deficiency in infants and toddlers in the United States. Pediatr Hematol Oncol 29, 704–709. [DOI] [PubMed] [Google Scholar]
- 52. Davison KM & Kaplan BJ (2012) Nutrient intakes are correlated with overall psychiatric functioning in adults with mood disorders. Can J Psychiatry 57, 85–92. [DOI] [PubMed] [Google Scholar]
- 53. Gosnell BA & Levine AS (2009) Reward systems and food intake: role of opioids. Int J Obes (Lond) 33, Suppl. 2, S54–S58. [DOI] [PubMed] [Google Scholar]
- 54. Mäkinen M, Puukko-Viertomies L, Lindberg N et al. (2012) Body dissatisfaction and body mass in girls and boys transitioning from early to mid-adolescence: additional role of self-esteem and eating habits. BMC Psychiatry 12, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gibson RS (1993) Nutritional Assessment. A Laboratory Manual. New York: Oxford University Press. [Google Scholar]
- 56. Poslusna K, Ruprich J, de Vries JHM et al. (2009) Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr 101, Suppl. 2, S73–S85. [DOI] [PubMed] [Google Scholar]
- 57. Thompson F & Byers T (1994) Dietary assessment resource manual. J Nutr 124, 11 Suppl., S2245–S2317. [DOI] [PubMed] [Google Scholar]
- 58. Mensink GB, Fletcher R, Gurinovic M et al. (2013) Mapping low intake of micronutrients across Europe. Br J Nutr 110, 755–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Serra-Majem L, Román-Viñas B & Salvador G (2007) Knowledge, opinions and behaviours related to food and nutrition in Catalonia, Spain (1992–2003). Public Health Nutr 10, 1396–1405. [DOI] [PubMed] [Google Scholar]
- 60. Sanchez-Carracedo D, Neumark-Sztainer D & Lopez-Guimera G (2012) Integrated prevention of obesity and eating disorders: barriers, developments and opportunities. Public Health Nutr 15, 2295–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]


