Abstract
Objective
To evaluate the prevalence of vitamin D deficiency in pregnant women and their newborns in Beijing, China and the influence of vitamin D deficiency on birth size.
Design
A cross-sectional study.
Setting
Data were collected from pregnant women who delivered during April to May 2010 at 306 Hospital of PLA in Beijing, China.
Subjects
Participants in the study were seventy healthy nulliparous pregnant women with singleton pregnancies who delivered healthy babies at full term and their newborns.
Results
Severe vitamin D deficiency (25-hydroxyvitamin D (25(OH)D) < 25 nmol/l) was detected in 54·5 % of mothers and 46·6 % of newborns. Neither mothers nor newborns had serum 25(OH)D concentrations that reached the normal level (>75 nmol/l). The concentration of 25(OH)D in mothers was positively correlated with that in cord blood (r = 0·89, P < 0·001). Newborns of mothers with severe vitamin D deficiency had lower birth length and birth weight. The head circumference and birth weight were lower in vitamin D-deficient newborns.
Conclusions
The study indicates that pregnant women and neonates residing in Beijing are at high risk of vitamin D deficiency. Neonatal 25(OH)D concentrations are dependently related to maternal 25(OH)D levels. Maternal and neonatal vitamin D status influences newborn size.
Keywords: Vitamin D deficiency, Pregnancy, Newborn, Birth size
Vitamin D is an essential fat-soluble vitamin and has multiple functions. Vitamin D insufficiency has been suggested to influence bone health as well as increase the risk of a number of non-skeletal conditions such as autoimmune diseases, cancers, diabetes, CVD, infectious diseases, skin disorders and schizophrenia( 1 ). The discovery of the function of vitamin D in growth and development has led to growing concern about the functional impacts of maternal vitamin D on their offspring. Although evidence is inconsistent, considerable research has shown that low maternal levels of 25-hydroxyvitamin D (25(OH)D) are associated with adverse outcomes for the fetus. Maternal vitamin D deficiency has been linked with a number of fetal and neonatal health problems such as impaired bone development, multiple sclerosis, cancer, insulin-dependent diabetes mellitus, impaired function of the immune system, asthma and atopy( 2 , 3 ). The long-term effects of maternal vitamin D deficiency on offspring remain unknown. A prospective longitudinal study reported that no associations were observed between maternal 25(OH)D concentrations and a child's intelligence, psychological health or cardiovascular system and that concentrations of 25(OH)D in pregnancy above 75 nmol/l may result in an increased risk of atopic disorders( 4 ). Vitamin D supplementation during pregnancy has been suggested as an intervention to protect against the adverse outcomes of vitamin D insufficiency. Some interventional trials have shown that vitamin D supplementation can be effective at raising circulating 25(OH)D levels in women and their neonates( 5 , 6 ). However, there is not enough evidence to evaluate the effects of vitamin D supplementation on improving neonatal outcomes.
The effect of maternal vitamin D deficiency on birth size is inconclusive. Some studies have demonstrated shorter knee–heel length, lower birth weight or poorer intra-uterine long bone growth( 7 – 10 ) in infants born to mothers with low 25(OH)D concentrations. Some studies suggest that vitamin D supplementation in pregnant women may lead to an increase in birth weight( 11 ) or head circumference( 12 ). In contrast, other studies of vitamin D supplementation during pregnancy do not demonstrate a difference in birth weight( 13 , 14 ).
During the past several decades, there has been growing interest in the prevalence of vitamin D insufficiency during pregnancy. The prevalence of vitamin D deficiency in pregnant women has been reported in many countries such as India( 15 ), Greece( 16 ), Iran( 17 ) and the UK( 4 ). To date, little is known about the vitamin D status of pregnant Chinese women. Therefore the aim of the present study was to investigate the vitamin D status in pregnant women and their newborns living in the Beijing urban area (39·9°N; northern China). The correlations between newborn size and maternal or neonatal vitamin D status were also evaluated.
Materials and methods
Participants
Participating in this study were seventy healthy nulliparous pregnant women with singleton pregnancies who delivered healthy babies at full term between April and May 2010 at 306 Hospital of PLA in Beijing. All women were from surrounding communities (Beijing urban area, latitude 39·9°N) without daily additional Ca and vitamin D intake. Women with a history of any disease including renal, bone and gastrointestinal disorders and medications influencing Ca or vitamin D metabolism were excluded. The study design and protocol were approved by the hospital ethics committee.
Biochemical measurements
Blood samples were collected from all women prior to labour and cord blood was drawn from the newborn's side of the severed cord after birth. Twelve samples of cord blood were excluded due to technical reasons. Thus, a total of seventy blood samples from the mothers and fifty-eight cord blood samples were included in the analyses. The blood samples were centrifuged and the serum stored at −80°C until use.
Serum concentration of 25(OH)D was determined using an ELISA kit following the manufacturer's instructions (Immunodiagnostic Systems Ltd, Boldon, UK). Vitamin D deficiency has been defined as 25(OH)D concentration below 50 nmol/l (20 ng/ml); vitamin D insufficiency as 25(OH)D concentration of 52·5–72·5 nmol/l (21–29 ng/ml)( 18 ). In our study, participants with a serum 25(OH)D concentration less than 25 nmol/l (10 ng/ml) were considered to have severe vitamin D deficiency; between 25 and 50 nmol/l as mild vitamin D deficiency; above 75 nmol/l as normal; and 25(OH)D level between 50 and 75 nmol/l as insufficiency. Serum parathyroid hormone (PTH) levels were measured using a commercially available ELISA kit (BIOMERICA, Inc., Newport Beach, CA, USA). Serum alkaline phosphatase (ALP), Ca and inorganic P were examined on an automatic analyser (model 7600; Hitachi High-Technologies Corporation, Tokyo, Japan) with commercial standard kits (Biosino Bio-Technology and Science Inc., Beijing, China).
Newborns’ anthropometric measurements
Anthropometry of the newborns, including weight, length and head circumference, was measured at birth.
Statistical analysis
Statistical analysis was carried out using the SPSS statistical software package version 16·0 (SPSS Inc.). Data were expressed as means with their standard errors or as numbers and percentages of participants. Comparisons were conducted using Student's two-tailed t test, with statistical significance set for P < 0·05. The Pearson correlation statistic was used to investigate correlations between variables.
Results
General characteristics
In total, seventy pregnant women in the Beijing urban area participated in the study. The mean age of the women was 29·9 (se 0·3; range 22–37) years and their mean weight was 73·9 (se 1·1) kg before delivery. In all women gravidity ranged from zero to three.
Maternal/neonatal serum 25-hydroxyvitamin D concentrations and prevalence of vitamin D deficiency or insufficiency in pregnant women/newborns
The mean serum 25(OH)D concentration in pregnant women (n 70) and cord blood (n 58) was 28·64 (se 1·41; range 13·4–64·3) nmol/l and 27·89 (se 1·43; range 14·85–64·72) nmol/l, respectively. The percentage of vitamin D deficiency or insufficiency is shown in Table 1. Severe vitamin D deficiency (25(OH)D < 25 nmol/l) was detected in 54·5 % of mothers and 46·6 % of newborns. More than 90 % of mothers and newborns had 25(OH)D < 50 nmol/l, and neither mothers nor newborns had serum 25(OH)D concentration >75 nmol/l which was considered the optimal level.
Table 1.
Prevalence of vitamin D deficiency and insufficiency in pregnant women (n 70) and newborns (n 58), Beijing, China, April–May 2010
| Mothers | Newborns | |||
|---|---|---|---|---|
| 25(OH)D level | n | % | n | % |
| Severe deficiency (<25 nmol/l) | 38 | 54·5 | 27 | 46·6 |
| Mild deficiency (25–<50 nmol/l) | 25 | 35·7 | 27 | 46·6 |
| Insufficiency (50–<75 nmol/l) | 7 | 10·0 | 4 | 6·9 |
| Sufficiency (≥75 nmol/l) | 0 | 0·0 | 0 | 0·0 |
| Total | 70 | 100·0 | 58 | 100·0 |
Association between maternal and neonatal 25-hydroxyvitamin D concentrations
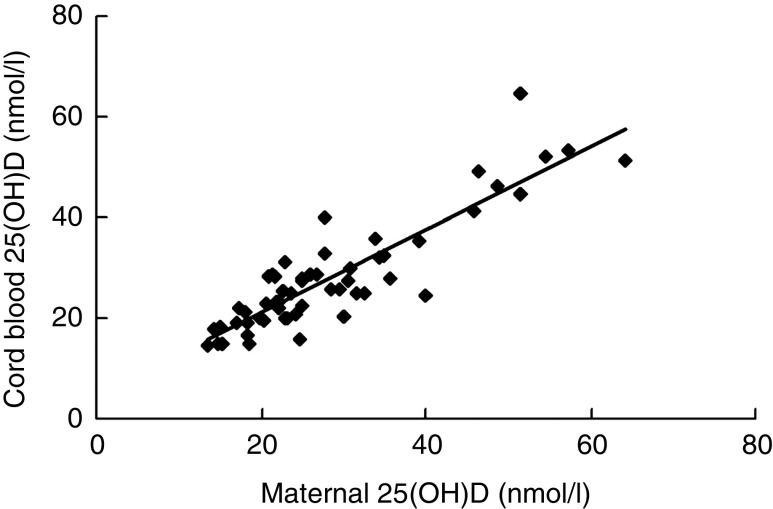
Maternal serum 25(OH)D concentration showed a significant positive correlation with cord blood 25(OH)D (r = 0·890, n 58, P = 0·000; Fig. 1). The concentrations of 25(OH)D were lower in newborns whose mothers had severe vitamin D deficiency than those whose mothers had 25(OH)D ≥ 25 nmol/l (mean 21·7 (se 0·78) nmol/l v. mean 36·06 (se 2·29) nmol/l; P = 0·000). The percentage of severe vitamin D deficiency was much higher in newborns of mothers with severe vitamin D deficiency (82·4 %) than in newborns of mothers with 25(OH)D ≥ 25 nmol/l (4 %).
Fig. 1.
Serum 25-hydroxyvitamin D (25(OH)D) concentrations in mothers and cord blood: scatter plot showing the relationship between maternal and cord blood serum 25(OH)D concentrations (n 58; r = 0·890, P = 0·000) among women and their newborns in Beijing, China, April–May 2010. Regression equation (by linear regression analysis): y = 0·8255x + 4·6145
Blood biochemical parameters in pregnant women and newborns and their relationships
The mean serum concentrations of PTH, Ca, inorganic P and ALP for mothers were 46·37 (se 3·35) pg/ml, 2·28 (se 0·01) mmol/l, 1·45 (se 0·08) mmol/l and 137·36 (se 4·92) μg/l, respectively, and for newborns were 4·92 (se 0·58) pg/ml, 2·53 (se 0·05) mmol/l, 1·60 (se 0·03) mmol/l and 126·09 (se 5·44) μg/l, respectively. Maternal PTH levels were much higher than PTH in cord blood (P = 0·000). Serum Ca levels in mothers were lower than those in cord blood (P = 0·000). The normal upper limit for ALP was considered 110 μg/l. The mean ALP levels in both maternal and cord blood were slightly higher than normal values and the ALP levels in 64·3 % of mothers and 65·5 % of newborns were elevated.
Serum PTH levels were inversely related to the concentrations of 25(OH)D (r = −0·266, P = 0·026), Ca (r = −0·381, P = 0·001) and inorganic P (r = −0·313, P = 0·008) in mothers. There were no significant correlations between maternal 25(OH)D and Ca, inorganic P or ALP. A significant correlation was observed between PTH and ALP levels in cord blood (r = 0·296, P = 0·024). No correlations were seen between 25(OH)D and PTH, ALP, Ca or inorganic P in newborns. There were no significant correlations between maternal Ca, inorganic P or PTH concentrations and these biochemical factors in cord blood.
Relationships between maternal/neonatal biochemical parameters and newborns’ anthropometric measurements
A significant correlation was seen between maternal 25(OH)D levels and the length of newborns (r = 0·247, P = 0·039). The mean length and birth weight were lower in newborns of mothers with severe vitamin D deficiency than in newborns of mothers with mild vitamin D deficiency (Table 2). No correlations were observed between maternal PTH, ALP, Ca or inorganic P and newborns’ anthropometric measurements.
Table 2.
Influence of maternal vitamin D deficiency on newborns’ anthropometric measurements, Beijing, China, April–May 2010
| Maternal 25(OH)D level | |||||
|---|---|---|---|---|---|
| <25 nmol/l (n 38) | ≥25 nmol/l (n 32) | ||||
| Newborn characteristic | Mean | se | Mean | se | P value |
| Birth weight (g) | 3386·1 | 66·0 | 3633·1 | 74·3 | 0·015 |
| Birth length (cm) | 50·2 | 0·2 | 51·0 | 0·3 | 0·037 |
| Head circumference (cm) | 34·9 | 0·2 | 35·4 | 0·2 | 0·082 |
There was a significant correlation between neonatal 25(OH)D concentrations and newborns’ head circumference (r = 0·317, P = 0·015). Trend associations were observed between cord blood 25(OH)D concentrations and newborns’ birth length (r = 0·239, P = 0·071) and birth weight (r = 0·202, P = 0·129). The mean head circumference and birth weight were larger in newborns with 25(OH)D concentration ≥25 nmol/l than in those with severe vitamin D deficiency (Table 3). There was a significant negative correlation between PTH levels in cord blood and birth weight (r = −0·305, P = 0·020). No relationships were observed between ALP, Ca or inorganic P and any anthropometric measurements.
Table 3.
Influence of neonatal vitamin D deficiency on newborns’ anthropometric measurements, Beijing, China, April–May 2010
| Neonatal 25(OH)D level | |||||
|---|---|---|---|---|---|
| <25 nmol/l (n 27) | ≥25 nmol/l (n 31) | ||||
| Newborn characteristic | Mean | se | Mean | se | P value |
| Birth weight (g) | 3354·8 | 18·3 | 3640·0 | 83·1 | 0·015 |
| Birth length (cm) | 50·2 | 0·3 | 50·9 | 0·3 | 0·078 |
| Head circumference (cm) | 35·0 | 0·2 | 35·7 | 0·2 | 0·026 |
Discussion
The present study showed a high prevalence of vitamin D deficiency among pregnant women and neonates in the Beijing urban area (39·9°N), northern China. Around half of mothers or newborns had severe vitamin D deficiency and neither women nor newborns reached normal 25(OH)D concentrations (>75 nmol/l). A recent study from Chengdu urban area (30·7°N; south-west China) showed that the prevalence of vitamin D deficiency was 57·1 % in mothers and 44·2 % in neonates( 19 ). The cut-off point used to define deficiency in that study was 25(OH)D < 37·5 nmol/l, and the mean concentration of 25(OH)D was 48·5 nmol/l in mothers and 41 nmol/l in cord blood( 19 ). Although the mean 25(OH)D concentrations we report in the present study from Beijing are lower than those in the aforementioned study in Chengdu, pregnant women from both northern and southern China are at high risk of vitamin D deficiency.
One study conducted at a similar latitude (40°N; Pittsburgh, northern USA)( 20 ) showed that serum mean concentrations of 25(OH)D were 80·4 nmol/l for white women and 49·4 nmol/l for black women. Mean 25(OH)D concentration in newborns was 67·4 nmol/l for white newborns and 37·0 nmol/l for black newborns. In that study, 29·2 % of black women and 45·6 % of black newborns, and 5·0 % of white women and 9·7 % of white newborns had vitamin D deficiency (cut-off point <37·5 nmol/l)( 20 ). Other studies have demonstrated that vitamin D deficiency is common in pregnant women in different countries. The prevalence of vitamin D deficiency reported in pregnant women was 31 % in southern India( 21 ), 32 % in northern India( 15 ), 19·5 % in Greece( 16 ) and 5·7 % in Iran( 17 ). One study observed vitamin D deficiency in pregnant women of several ethnic backgrounds who reside in The Hague in the Netherlands (52°N). Of all participants in that study, 8 % of Western women and most non-Western women (59–84 %) had vitamin D deficiency( 22 ). Direct comparisons between studies are not possible due to the use of different methods and inconsistent definitions of vitamin D deficiency. It seems, however, that pregnant women are at a high risk of vitamin D deficiency. Pregnant women may need additional vitamin D intake to retain normal levels.
It is generally agreed that serum 25(OH)D levels depend on sunshine exposure, latitude, clothing habit, skin pigmentation and the use of sunscreen as well as diet. Although the reasons for the high rate of vitamin D deficiency in pregnant women in Beijing were not investigated in the present study, there are several possible explanations. Beijing lies at a latitude of 39·9°N and the weather is windy and dusty in the spring. These conditions limit the time that women spend outdoors and decrease UVB exposure. In addition, there is a cultural reason for young Chinese women having less sun exposure, as they prefer fair skin to tanned skin. Therefore they avoid direct sun exposure and regularly use sunscreen. Another reason could be that the Chinese diet is low in vitamin D. There is no regular vitamin D supplementation in China even though maternal vitamin D requirements increase during pregnancy. Further studies are needed to investigate the reasons for the high prevalence of vitamin D deficiency.
The present study showed that cord blood 25(OH)D levels correlated strongly with maternal 25(OH)D concentrations. This is consistent with previous reports( 23 ). Our findings demonstrate that among mothers with vitamin D deficiency, their newborns are more likely to have vitamin D deficiency. This suggests that vitamin D sources in newborn infants are mainly from transplacental stores. It has been reported that maternal vitamin D status plays a key role in determining the amount of vitamin D transported across the placenta during fetal life and thereafter the size of vitamin D reserves at birth( 24 ). Adequate 25(OH)D levels are important for women who become pregnant.
Lower maternal 25(OH)D levels have been considered to be related to many conditions of the fetus and child such as osteomalacia or rickets, poor dental health, diabetes, autoimmune diseases and cancers( 2 , 3 ). However, the effects of vitamin D deficiency on birth size are inconsistent. Our results add to the evidence that 25(OH)D concentrations during pregnancy are correlated with neonatal measurements, as reported by other studies( 7 – 10 ). The Amsterdam ABCD (Amsterdam Born Children and their Development) cohort including 3730 pregnant women demonstrated that women with vitamin D deficiency (25(OH)D < 29·9 nmol/l) had infants with lower birth weights and a higher risk of being small-for-gestational-age compared with women with 25(OH)D > 50 nmol/l( 9 ). Randomized controlled trials suggested that supplementation of vitamin D in pregnant women could lead to an increase in birth weight( 11 ). A recent study showed birth weight, length and head circumference were greater in groups supplemented with vitamin D during pregnancy than control groups( 25 ). In contrast, other studies reported no such correlation( 4 , 21 ) or even a negative association( 26 ) between birth size and 25(OH)D level. These conflicting findings may be related to the varied populations studied or methods used. Variants in the vitamin D receptor gene may influence the associations between maternal 25(OH)D concentrations and birth size measurements. Studies have shown that infant( 27 ) or maternal( 28 ) vitamin D receptor polymorphisms modify the effect of maternal 25(OH)D on offspring size. Moreover, different maternal 25(OH)D levels may contribute to the inconsistent results. It could be that the influence of maternal vitamin D on offspring size may be more obvious at lower 25(OH)D levels rather than at relatively higher levels. Further studies will be needed to investigate the influence of vitamin D on newborn size.
In the present study, birth length significantly correlated with maternal 25(OH)D level. This could be due to the influence of vitamin D on bone development and growth in the fetus. A previous study demonstrated a significant association between maternal vitamin D deficiency and reduced intra-uterine long bone growth( 10 ). It was also reported that impaired fetal bone ossification correlated with maternal vitamin D deficiency( 26 ). Reduced maternal 25(OH)D concentration during late pregnancy was associated with decreased knee–heel length, mid-upper arm and calf circumferences at birth( 10 ). Mahon et al. ( 29 ) found that the decrease of maternal 25(OH)D levels correlated with an increase in the cross-sectional area of the fetal femoral metaphysis at both 19 and 34 weeks’ gestation. In addition, the present study showed a smaller head circumference and birth length in newborns with vitamin D deficiency. All of these findings suggest that maternal vitamin D status influences fetal bone development.
Even though the high prevalence of vitamin D deficiency during pregnancy has been recognized for some years, the current study indicates that the problem has still not been solved. Vitamin D supplementation in pregnant women has been recommended in some countries. All (100 %) of the pregnant women and newborns had 25(OH)D < 75 nmol/l in our study. On the basis of these findings, it seems logical that pregnant women should routinely be supplemented with vitamin D in China. We recommend the screening of pregnant women in China for vitamin D deficiency. An adequate vitamin D supplementation for Chinese pregnant women is also highly recommended.
Serum PTH was lower and Ca was higher in newborns than their mothers, which is consistent with other studies( 16 , 26 ). This could be explained by the active transport of Ca across the placenta by a calcium pump in the placenta and elevated fetal Ca levels suppressing PTH release in the fetus.
In our study we found a significant negative correlation between maternal 25(OH)D or Ca and PTH concentrations, in agreement with a previous report( 23 ). PTH is the most important regulator of the body's Ca and P levels. Release of PTH is controlled by the levels of Ca in the blood. Low blood Ca levels cause an increase in PTH release, while high blood Ca levels inhibit PTH release. Vitamin D suppresses PTH release and long-term vitamin D deficiency can result in an increase in PTH levels. Therefore, elevated maternal PTH levels further indicated vitamin D deficiency in these pregnant women.
In present study, the mean serum concentrations of ALP were above the normal value in both mothers and cord blood and more than 60 % of pregnant women and neonates had elevated ALP levels. These results are consistent with other reports( 30 ). These data indicate that the previous reference for upper limits of ALP in pregnant women or neonates may be inappropriate.
Conclusion
We found that vitamin D deficiency or insufficiency is very common in pregnant women and their newborns in Beijing. The concentration of 25(OH)D in cord blood depends on the maternal 25(OH)D level. Maternal and neonatal 25(OH)D levels influence birth size. Measures such as vitamin D supplementation should be taken to improve the vitamin D status of pregnant women in China.
Acknowledgements
Sources of funding: This work was supported by a Medical Research Grant of 306 Hospital of PLA. The study sponsors did not make any contribution to the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The authors did not receive writing assistance in preparation the manuscript. The study was carried out at the Department of Pathology and Experimental Medicine and the Department of Obstetrics & Gynecology, 306 Hospital of PLA, Beijing, China. Conflicts of interest: There are no conflicts of interest. Authors’ contributions: All authors made substantial contributions to the study. Study conception (S.J.S., J.Z., W.Z.); study design (S.J.S., W.Z., J.Z.); data collection and conduct of the experiment (S.J.S., S.S., J.L., X.C., L.Z., Y.L., G.J., Y.N., J.W.); statistical analyses (S.J.S.); analysis and interpretation of data (S.J.S., J.Z., W.Z., S.S., J.L., X.C., L.Z., Y.L., G.J., Y.N., J.W.); drafting the manuscript (S.J.S., J.Z., W.Z.); critical review for intellectual content (S.J.S., J.Z., W.Z., S.S., J.L., X.C., L.Z., Y.L., G.J., Y.N., J.W.); obtaining funding (S.J.S., J.Z., W.Z., S.S., J.L.); provision of consultation (J.Z., W.Z.). All authors have read and approved the final manuscript. Acknowledgements: The authors wish to thank Yaya Qin, Xiaoqing Tan and the staff in the Department of Obstetrics in 306 Hospital of PLA for their assistance with sample collection and recruitment for the study.
References
- 1. Holick MF (2011) Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets 12, 4–18. [DOI] [PubMed] [Google Scholar]
- 2. Mulligan ML, Felton SK, Riek AE et al et al. (2010) Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol 202, 429, e 1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dror DK & Allen LH (2010) Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutr Rev 68, 465–477. [DOI] [PubMed] [Google Scholar]
- 4. Gale CR, Robinson SM, Harvey NC et al et al. (2008) Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 62, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu CK, Sykes L, Sethi M et al et al. (2009) Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf) 70, 685–690. [DOI] [PubMed] [Google Scholar]
- 6. Hollis BW, Johnson D, Hulsey TC et al et al. (2011) Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 26, 2341–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mannion CA, Gray-Donald K, Koski KG et al et al. (2006) Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ 174, 1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scholl TO & Chen X (2009) Vitamin D intake during pregnancy: association with maternal characteristics and infant birth weight. Early Hum Dev 85, 231–234. [DOI] [PubMed] [Google Scholar]
- 9. Leffelaar ER, Vrijkotte TG, van Eijsden M et al et al. (2010) Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br J Nutr 104, 108–117. [DOI] [PubMed] [Google Scholar]
- 10. Morley R, Carlin JB, Pasco JA et al et al. (2006) Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 91, 906–912. [DOI] [PubMed] [Google Scholar]
- 11. Marya RK, Rathee S, Lata V et al et al. (1981) Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest 12, 155–161. [DOI] [PubMed] [Google Scholar]
- 12. Marya RK, Rathee S, Dua V et al et al. (1988) Effect of vitamin D supplementation during pregnancy on foetal growth. Indian J Med Res 88, 488–492. [PubMed] [Google Scholar]
- 13. Mallet E, Gugi B, Brunelle P et al et al. (1986) Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol 68, 300–304. [DOI] [PubMed] [Google Scholar]
- 14. Brooke OG, Brown IR, Bone CD et al et al. (1980) Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. BMJ 280, 751–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sahu M, Bhatia V, Aggarwal A et al et al. (2009) Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol (Oxf) 70, 680–684. [DOI] [PubMed] [Google Scholar]
- 16. Nicolaidou P, Hatzistamatiou Z, Papadopoulou A et al et al. (2006) Low vitamin D status in mother newborn pairs in Greece. Calcif Tissue Int 78, 337–342. [DOI] [PubMed] [Google Scholar]
- 17. Salek M, Hashemipour M, Aminorroaya A et al et al. (2008) Vitamin D deficiency among pregnant women and their newborns in Isfahan, Iran. Exp Clin Endocrinol Diabetes 116, 352–356. [DOI] [PubMed] [Google Scholar]
- 18. Holick MF, Binkley NC, Bischoff-Ferrari HA et al et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96, 1911–1930. [DOI] [PubMed] [Google Scholar]
- 19. Wang J, Yang F, Mao M et al et al. (2010) High prevalence of vitamin D and calcium deficiency among pregnant women and their newborns in Chengdu, China. World J Pediatr 6, 265–267. [DOI] [PubMed] [Google Scholar]
- 20. Bodnar LM, Simhan HN, Powers RW et al et al. (2007) High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 137, 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farrant HJ, Krishnaveni GV, Hill JC et al et al. (2009) Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr 63, 646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Meer IM, Karamali NS, Boeke AJ et al et al. (2006) High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr 84, 350–353. [DOI] [PubMed] [Google Scholar]
- 23. Sachan A, Gupta R, Das V et al et al. (2005) High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr 81, 1060–1064. [DOI] [PubMed] [Google Scholar]
- 24. Hatun S, Ozkan B, Orbak Z et al et al. (2005) Vitamin D deficiency in early infancy. J Nutr 135, 279–282. [DOI] [PubMed] [Google Scholar]
- 25. Kalra P, Das V, Agarwal A et al et al. (2012) Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr (Epublication ahead of print version). [DOI] [PubMed] [Google Scholar]
- 26. Weiler H, Fitzpatrick-Wong S, Veitch R et al et al. (2005) Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. CMAJ 172, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morley R, Carlin JB, Pasco JA et al et al. (2009) Maternal 25-hydroxyvitamin D concentration and offspring birth size: effect modification by infant VDR genotype. Eur J Clin Nutr 63, 802–804. [DOI] [PubMed] [Google Scholar]
- 28. Bodnar LM, Catov JM, Zmuda JM et al et al. (2010) Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr 140, 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahon P, Harvey N, Crozier S et al et al. (2010) Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res 25, 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kazemi A, Sharifi F, Jafari N et al et al. (2009) High prevalence of vitamin D deficiency among pregnant women and their newborns in an Iranian population. J Womens Health 18, 835–839. [DOI] [PubMed] [Google Scholar]