Abstract
Objective
Food neophobia has been associated with decreased consumption of vegetables mainly among children. We hypothesized that food neophobia in adults is also associated with lower overall dietary quality and higher BMI.
Design
Data for the present cross-sectional analyses were derived from parents in a follow-up family study.
Setting
The STEPS study, a longitudinal study of health and development of a cohort of children born in south-west Finland.
Subjects
The parents, 1178 women (age 19–45 years, mean 32·2 years) and 1013 men (age 18–57 years, mean 34·1 years), completed a questionnaire at home when their child was 13 months old. The questionnaire included the Food Neophobia Scale (FNS; range 10–70), the Index of Diet Quality (IDQ; range 0–16) and a measure of fruit and vegetable consumption. At that time the participants’ height and weight were also measured by a research nurse to calculate BMI.
Results
Compared with the food neophilics (FNS score 10–24), the food neophobics (FNS score 40–70) consumed fewer vegetables (women: 15 v. 10 portions/week; men: 13 v. 7 portions/week), scored lower on the IDQ (women: 9·7 v. 8·5; men: 8·8 v. 7·8) and had higher BMI (women: 24·2 v. 26·0 kg/m2; men: 26·5 v. 27·5 kg/m2) as tested by one-way ANOVA, with all P values <0·001 in women and <0·05 in men. The food neophobics followed a diet lower in nutritional quality than did the food neophilics, especially regarding vegetables.
Conclusions
Food neophobia may complicate adaptation to dietary recommendations and predispose to overweight.
Keywords: Food neophobia, Diet quality, STEPS study, Vegetable consumption
Food neophobia refers to unwillingness to try unfamiliar foods( 1 ). It is related to, but theoretically distinct from, picky eating (fussy eating, pickiness, finickiness), which denotes reluctance to eat familiar foods that are not liked( 2 ). Further, food neophobia can refer to the actual, observable behaviour, as well as the trait of an underlying predisposition (tendency) to avoid novel foods. Trait food neophobia resembles personality traits by being rather heritable( 3 – 6 ) and stable( 7 ), and appears to be more resistant to interventions than behavioural food neophobia( 8 – 10 ).
Food neophobia protects omnivores against unknown risks, whereas its opposite, seeking novel foods (food neophilia), helps to maximize the advantages of omnivory( 1 ). From an evolutionary point of view, large inter-individual variability in food neophobia within a hunter-gatherer community could have been beneficial for the population as a whole, because neophobia and neophilia can each offer advantages in certain food environments. Indeed, the level of food neophobia varies widely from person to person in present-day populations( 5 , 11 , 12 ).
Unsurprisingly, food neophobia restricts the variety of one’s diet( 13 – 15 ), but the effects of neophobia on the consumed amounts of specific foods are less obvious. In children, however, several studies have shown that high food neophobia is associated with low consumption of fruit and vegetables( 16 – 20 ) or specifically vegetables( 2 , 21 ). Results from the studies that measured consumption of fruit and vegetables separately( 16 , 17 , 19 , 21 ) suggest that child food neophobia correlates more strongly with vegetable intake than with fruit intake. On the other hand, no correlation has been found between food neophobia and the consumption of snacks or starchy staple foods( 16 , 18 ). Child food neophobia was also consistently associated with low preference for vegetables and less healthful food preferences in general( 22 ). In addition, the overall dietary quality of neophobic children was lower than that of neophilic children( 14 ). Recently, modest but significant negative correlations between food neophobia and vegetable consumption have also been reported in adults aged 20–25 years( 5 ) and 21–99 years( 23 ).
Food neophobia may restrict not only the variety of the diet, but also the quantity, and thereby the energy content. A study with 4–5-year-old children reported lower energy intake for neophobic than for neophilic children( 18 ). On the other hand, given that food neophobics who limit their consumption of fruit and vegetables may replace them with foods that are more energy-dense, neophobia may also increase energy intake. Indeed, a recent study observed higher food neophobia in overweight/obese children than in those of normal weight( 24 ). A small correlation (r=0·15) between food neophobia and BMI has been observed consistently in young adult women, although not in men( 5 ).
The aim of the present study was to investigate associations of trait food neophobia with fruit and vegetable consumption, overall dietary quality and BMI in adults. We hypothesized that food neophobic adults not only consume fewer vegetables than do food neophilics, but also that the overall quality of neophobics’ diet is lower and their BMI is higher compared with neophilics.
Methods
Participants and procedure
The present cross-sectional analyses were based on data from 1178 women and 1013 men (Table 1). The data were derived from an ongoing longitudinal study of children and their parents in south-west Finland, the Steps to the Healthy Development and Well-being of Children (the STEPS study)( 25 ). We strove to minimize recruitment-based selection bias by collecting the data from participants who represented the general population of parents of infants and thus were not selected based on eating habits.
Table 1.
Participant characteristics: parents in the STEPS longitudinal cohort study, south-west Finland
| Women (n 1178)† | Men (n 1013)‡ | |||
|---|---|---|---|---|
| Characteristic | Mean or n | sd or % | Mean or n | sd or % |
| Age (years)§ | ||||
| Minimum–maximum | 19–45 | 18–57 | ||
| Mean and sd | 32·2 | 4·4 | 34·1 | 5·3 |
| Median | 32·1 | 33·5 | ||
| Educational level|| | ||||
| Basic/vocational¶ | 410 | 34·8 | 504 | 49·8 |
| Advanced/academic†† | 735 | 62·4 | 466 | 46·0 |
| Not reported | 33 | 2·8 | 43 | 4·2 |
| Relationship status|| | ||||
| Married | 715 | 60·7 | 633 | 62·5 |
| Cohabiting | 407 | 34·6 | 356 | 35·1 |
| Single | 31 | 2·6 | 13 | 1·3 |
| Divorced/living separately | 2 | 0·2 | 0 | – |
| In the registered relationship with a same-sex partner | 8 | 0·7 | 0 | – |
| Not reported | 15 | 1·3 | 11 | 1·1 |
| Prior deliveries (mothers only)‡‡ | ||||
| No | 656 | 55·9 | – | – |
| Yes | 502 | 42·8 | – | – |
| Data not available | 16 | 1·4 | – | – |
In addition to 1174 biological mothers, includes four women who lived in a registered relationship with a female partner who was an included biological mother.
Male partners of the women who were mothers; not necessarily biological fathers.
Age when most of the data for the present analyses were collected (13 months after the delivery of the child included in the STEPS study).
According to self-reports, collected when the participants were enrolled in the STEPS study (at first trimester of pregnancy or soon after delivery).
Vocational/technical training (programme/course) from basic to intermediate level, ‘other’ training or no professional training.
Highest-level vocational training (e.g. polytechnic) or an academic degree (bachelor’s, master’s, licentiate or doctoral).
Data from the National Birth Register.
The cohort profile of the STEPS study has been described in detail by Lagström et al. ( 25 ). Briefly, all Finnish- and Swedish-speaking mothers who delivered a living child between 1 January 2008 and 31 April 2010 in the Hospital District of Southwest Finland formed the cohort population (9811 mothers and their 9936 children). Of them, 1797 mothers (18·3 %) volunteered as participants for the intensive follow-up group of the STEPS study during the first trimester of pregnancy (1387 mothers, recruited at maternity health-care clinics) or soon after delivery (410 mothers, recruited at delivery wards). Together with these mothers, their 1658 partners and 1827 children (including thirty pairs of twins) enrolled in the follow-up group.
Data on food neophobia, dietary quality and BMI were collected from the parents when their STEPS-enrolled child was about 13 months old. Demographic information, including self-reported relationship status and education, were requested upon recruitment. Self-reported height and weight before pregnancy were also collected upon recruitment for an additional analysis of pre-pregnancy BMI.
In the present study we included all the mothers and partners for whom usable information about food neophobia was available. Consequently, we analysed data from 1174 mothers and 1017 partners (including four women), who represented 65 % and 61 % of the mothers and partners, respectively, of the initial follow-up group of the STEPS study. These rates were similar to the overall response rates for the questionnaires presented 13 months after the birth of the child( 25 ). The ethical considerations of the STEPS study have been reported in detail by Lagström et al. ( 25 ).
Measures
Data for the present analyses were collected using printed questionnaires that were completed by the participants at home, except data on BMI and prior deliveries. BMI data were based on the weight and height measured at the research unit as detailed below, and information about prior deliveries was drawn from the National Birth Register.
Food neophobia
We measured trait food neophobia using the Food Neophobia Scale (FNS) developed by Pliner and Hobden( 1 ). The FNS consists of ten statements, such as ‘I don’t trust new foods’, with a seven-category response scale ranging from ‘disagree strongly’ (scored 1) to ‘agree strongly’ (scored 7). As a result, the potential range of the FNS score is from 10 to 70, with higher scores indicating higher food neophobia. Half of the statements (i.e. five) are worded in reverse (e.g. ‘I am constantly sampling new and different foods’) and thus their scoring is reversed when calculating the total FNS score. Therefore, we considered the FNS to be a bipolar scale and low FNS scores to indicate food neophilia rather than mere lack of food neophobia.
We used the Finnish translation of the FNS, validated in a representative Finnish sample (n 1083, 53 % women, age 16–80 years)( 11 ), with subsequent minor revisions in wording, as published in a Finnish textbook( 26 ). Internal consistency of the FNS in the present data, as measured by Cronbach’s α, was 0·88. This value is similar to those reported when the FNS was developed (0·88)( 1 ), validated in the Finnish sample (0·85)( 11 ) and used with a large sample of Finns (n 1175) aged 20–25 years (0·87)( 5 ).
No standardized or widely followed diagnostic cut-off values exist for classifying individuals as ‘food neophobics’ and ‘food neophilics’ based on their FNS score( 12 ). Often the sample mean (or median) FNS score( 18 , 24 , 27 – 29 ), or the score 35( 30 ), has been used as the cut-off value to categorize study participants into two groups. Another common practice is to use the mean plus/minus one standard deviation as cut-off values( 11 , 14 , 31 , 32 ) to form three groups. However, we defined novel criteria for classification based on the rating scale as follows. First, we classified ‘food neophobics’ as the participants who responded, on average, affirmatively to the statements of the FNS (relative to food neophobia) and thus scored 40–70. Then, we classified the participants whose average response to the statements was negative into two groups, with equal score ranges: those who scored 25–39, named ‘the median group’, and those who scored 10–24, considered the ‘food neophilics’.
Vegetable consumption and fruit consumption
We quantified vegetable consumption and fruit consumption (including berry consumption) as the number of portions (of specified size) eaten per week. Consumption of both food groups was assessed with separate questions, which were a part of the Index of Diet Quality (IDQ) questionnaire( 33 ). Vegetable consumption was calculated as the product of responses to the questions ‘How many days per week do you eat vegetables?’ and ‘How many portions of vegetables do you consume daily?’ Fruit consumption was calculated similarly, based on responses to two corresponding questions with the word ‘vegetables’ replaced by ‘fruit and/or berries’. The word ‘berries’ was explicitly included, because otherwise participants might not count them as fruit, although berries are an important subgroup of fruit in the Finnish diet. Three examples of what was meant by a ‘portion’ were given in the questionnaire for vegetables (‘1 tomato, 1 dl of grated vegetables, 1 carrot’) and fruit and berries (‘1 apple, 1 banana, 1 dl of berries’)( 33 ). The questions were meant to measure general, average consumption; no specific time period or season was specified.
Dietary quality
We assessed overall dietary quality using the IDQ( 33 ). The IDQ consists of eighteen items designed to measure adherence to the key aspects of the (Finnish/Nordic) dietary recommendations. Each of the six sub-scores of the IDQ (whole-grain products; fat-containing products; dairy products; vegetables, fruit and berries; sugar; meal pattern) includes one to four items. A typical item consists of a question that is answered with a quantity (number), such as ‘How many days a week do you eat fish?’ In this example, if the respondent indicates eating fish twice per week or more often (consistent with the Finnish dietary recommendations), the researcher codes the raw answer as 1 (otherwise 0), to make a dyadic sub-score. The scoring scheme for the IDQ has been described in detail by Leppälä et al. ( 33 ). No IDQ score could be calculated for 115 individuals (5·2 %) because they had provided inadequate responses to one or more of the IDQ sub-scores. The potential range of the IDQ score was 0–16; higher score indicated higher diet quality (i.e. higher adherence to the dietary recommendations).
In addition to the IDQ score in its original form, we also used the IDQ score without the sub-score for the consumption of fruit and vegetables (potential range: 0–13). We did this because fruit consumption and vegetable consumption were also analysed as separate variables and because we were interested in how food neophobia is related to dietary quality outside of fruit and vegetable consumption.
BMI
To assess BMI (weight in kilograms divided by the square of height in metres), a research nurse measured the participants’ weight and height during their visit to the research unit (Turku Institute for Child and Youth Research, University of Turku, Turku, Finland) for data collection when their child was about 13 months old (at which time data for FNS and IDQ scores were collected). Weights were measured to the nearest 0·1 kg with an electronic scale (Tanita WB110MA; Tanita Corporation, Tokyo, Japan) and standing heights were measured to the nearest millimetre with a wall-mounted Harpenden stadiometer (Holtain, Crymych, UK). BMI could not be determined for 336 participants (15·3 %) who did not complete the visit to the research unit.
For additional analyses regarding FNS score and pre-pregnancy BMI, the participating mothers were asked to report their weight and height before their pregnancy as a part of the baseline questionnaire of the STEPS study, which the mothers completed when they were recruited to the study (i.e. during the first trimester of pregnancy or soon after delivery). In addition, because about 43 % of the women had had prior deliveries, which potentially had long-term effects on weight( 34 ), we took delivery history (as recorded in the National Birth Register) into account in a further analysis.
Educational level
We classified the participants into two broad categories based on the level of professional education they had completed (i.e. schools and degrees completed after basic education such as elementary and high schools). The educational level of those who had no professional training or a basic to intermediate level of vocational training was classified as ‘basic/vocational’. The educational level of those who had the highest level of vocational training (such as a 4-year programme at a polytechnic institute) or any academic degree (bachelor’s, master’s, licentiate or doctoral degree) was regarded as ‘advanced/academic’.
We used educational level as a proxy measure for socio-economic status. We assumed the participants’ educational level to be more stable than their actual profession or family income and therefore the most appropriate measure of socio-economic status for the present analyses.
Statistical analyses
We analysed the variables of interest (FNS score, vegetable consumption, fruit consumption, IDQ score, IDQ score excluding the sub-score for fruit and vegetables, and BMI) using mostly parametric statistics (t test, ANOVA, Pearson correlation), because the distributions of the variables were roughly Gaussian and we assumed the underlying phenomena to be approximately normally distributed. A nominal criterion for statistical significance (α=0·05) was considered for multiple comparisons using Bonferroni correction where appropriate. The statistical analyses were run using the statistical software package IBM SPSS Statistics version 20.
First, we compared women and men using an independent-samples t test (two-tailed, not assuming equal variances) regarding the variables listed above and calculated Pearson correlations between each pair of variables in women and men. Second, we used one-way ANOVA and Tukey’s post hoc test to compare participants at the three FNS score levels (fixed factor) for the variables of interest (one at a time as the dependent variable), separately in women and men. Finally, we conducted a two-way ANOVA to explore associations and potential interactions of the FNS score level and educational level (fixed factors) and the variables listed above, again separately in women and men. We performed this analysis to separate the effects of food neophobia from the effects of education (as a proxy measure for socio-economic status), because we assumed socio-economic position to be associated to food neophobia( 12 ), consumption of vegetables( 35 ) and BMI( 36 ).
Women and men were analysed separately for associations of FNS scores with the other variables because the genders differed significantly in age (t test, t
(1910)=−8·93, P<0·001) and educational level (Pearson 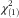 =55·82, P<0·001): women were, on average, younger and more highly educated than men (Table 1).
=55·82, P<0·001): women were, on average, younger and more highly educated than men (Table 1).
Correlational analysis indicated that age had only a weak, if any, correlation with the studied variables, and thus age was not considered in the later analyses. While in a few cases the Pearson correlation between age and a studied variable reached statistical significance, the absolute values of the correlation coefficients (r) were smaller than 0·10 and 0·12 in women and men, respectively. In addition, the age distributions were roughly normal and the standard deviation of age was only about 5 years (Table 1), indicating that the age of most participants was within a rather narrow range around the mean.
Study size
We estimated that about 1000 women and men provided an adequate size for the present study. To determine this, we first set a goal to detect differences as small as 1 kg/m2 in BMI between food neophobics (FNS score ≥40) and others, because even this small BMI difference has been reported to have a meaningful effect on the burden of chronic disease at the population level(
37
). Next, we set the criterion for statistical significance at α=0·05 (two-tailed) and power (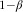 ) at 0·80. For a conservative estimate of the required sample size, we compared food neophobics and others (within a gender) using an independent-samples t test, assuming that more sophisticated methods would provide at least the same power. Then, based on results from a study with a similar sample of Finnish young adults from the general population(
5
), we estimated that the sd of BMI would be 4 kg/m2, and thus we aimed to detect effects as small as d=1/4=0·25. According to previous studies of similar samples(
5
,
11
), the mean and sd of FNS score are about 30 and 10, respectively. Therefore, our definition of a food neophobic roughly coincides with an individual FNS score higher than the mean plus sd (30+10=40). Given that the distribution of FNS scores is approximately normal, 15·8 % of individuals were predicted to be food neophobics. Finally, we used the statistical power analysis program G*Power version 3 (Heinrich-Heine-Universität, Düsseldorf, Germany) to calculate that the required total sample size would be 946 individuals (149 neophobics and 797 others). In practice, our data had enough power to detect relevant differences when we classified the participants into three groups by FNS score and used ANOVA to compare the groups, as detailed below in Results.
) at 0·80. For a conservative estimate of the required sample size, we compared food neophobics and others (within a gender) using an independent-samples t test, assuming that more sophisticated methods would provide at least the same power. Then, based on results from a study with a similar sample of Finnish young adults from the general population(
5
), we estimated that the sd of BMI would be 4 kg/m2, and thus we aimed to detect effects as small as d=1/4=0·25. According to previous studies of similar samples(
5
,
11
), the mean and sd of FNS score are about 30 and 10, respectively. Therefore, our definition of a food neophobic roughly coincides with an individual FNS score higher than the mean plus sd (30+10=40). Given that the distribution of FNS scores is approximately normal, 15·8 % of individuals were predicted to be food neophobics. Finally, we used the statistical power analysis program G*Power version 3 (Heinrich-Heine-Universität, Düsseldorf, Germany) to calculate that the required total sample size would be 946 individuals (149 neophobics and 797 others). In practice, our data had enough power to detect relevant differences when we classified the participants into three groups by FNS score and used ANOVA to compare the groups, as detailed below in Results.
Results
Food neophobia
The mean FNS score was virtually equal in women (28·48) and men (28·50). By our classification, 16·1 % of women and 17·9 % of men were food neophobic (FNS score 40–70), while 41·4 % of women and 40·0 % of men were food neophilic (FNS score 10–24). The remaining 42·4 % of women and 42·2 % of men formed the median group (FNS score 25–39).
Vegetable consumption
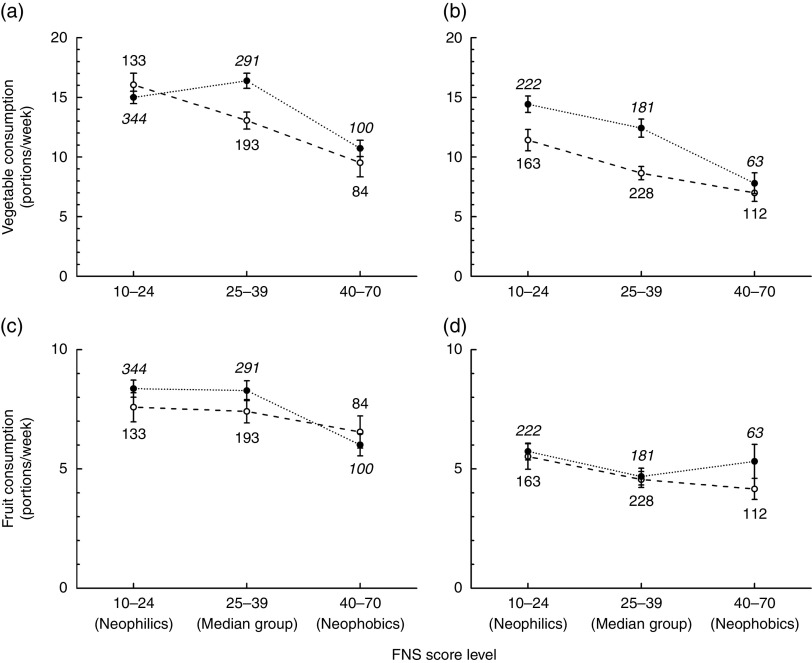
Measured as the number of specified-size portions consumed per week, women ate 32 % more vegetables than did men (Table 2). A modest, but significant, negative correlation existed between vegetable consumption and FNS score in both women and men (Table 3). In women, the food neophobics consumed 68 % of the amount of vegetables that the food neophilics did (10·3 v. 15·2 portions/week). In men, the food neophobics ate only 56 % of the amount of vegetables that the food neophilics did (7·3 v. 13·0 portions/week; Table 4). When we took educational level into account, the association between FNS score and vegetable consumption was still significant in both women and men (Fig. 1(a) and (b)) and the main effect of educational level was significant in men: the participants at the higher educational level consumed more vegetables than those at the lower educational level, regardless of FNS score (Fig. 1(b)). In women, this trend was similar but not as obvious as in men because the main effect of educational level did not quite reach significance, whereas the interaction between FNS and educational level did (Fig. 1(a)).
Table 2.
Mean values of the studied variables by gender and comparison between the genders: parents in the STEPS longitudinal cohort study, south-west Finland
| Women† | Men‡ | Comparison statistics§ | |||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | sd | Mean | sd | t | P | d |
| FNS score (10–70) | 28·5 | 11·0 | 28·5 | 11·1 | −0·06 | 0·95 | <0·01 |
| Vegetable consumption (portions/week) | 14·3 | 10·3 | 10·8 | 9·9 | 8·07 | <0·001 | 0·35 |
| Fruit consumption (portions/week) | 7·8 | 6·7 | 5·0 | 5·4 | 10·87 | <0·001 | 0·46 |
| IDQ score (0–16) | 9·4 | 2·5 | 8·5 | 2·4 | 8·80 | <0·001 | 0·39 |
| IDQ score excl. the sub-score for fruit and vegetables (0–13) | 8·2 | 2·0 | 7·8 | 2·0 | 4·66 | <0·001 | 0·21 |
| BMI (kg/m2)|| | 25·0 | 5·0 | 26·6 | 3·9 | −7·96 | <0·001 | 0·37 |
FNS, Food Neophobia Scale (theoretical range in parentheses); IDQ, Index of Diet Quality (theoretical range in parentheses).
n 1054–1178, depending on the number of missing values.
n 801–1013, depending on the number of missing values.
Independent-samples t test (two-tailed, equal variances not assumed); d, Cohen’s d (effect size). All nominally significant differences remained significant after adjusting the criterion for multiple comparisons (Bonferroni correction for fourteen tests; α=0·004). Non-parametric Mann–Whitney U test confirmed the results.
Calculated based on weight and height measured by a research nurse when the participants visited the research unit 13 months after the delivery of their child.
Table 3.
Pearson correlation coefficients from pairwise comparisons of the studied variables among women (lower left triangle) and men (upper right triangle)†: parents in the STEPS longitudinal cohort study, south-west Finland
| FNS score | Vegetable consumption | Fruit consumption | IDQ score | IDQ score excl. fruit and vegetables | BMI‡ | |
|---|---|---|---|---|---|---|
| FNS score | 1·00 | −0·22** | −0·09* | −0·14** | −0·08* | 0·05 |
| Vegetable consumption | −0·18** | 1·00 | 0·30** | 0·49** | 0·21** | −0·04 |
| Fruit consumption | −0·10** | 0·39** | 1·00 | 0·39** | 0·17** | −0·03 |
| IDQ score | −0·18** | 0·51** | 0·47** | 1·00 | 0·92** | −0·03 |
| IDQ score excl. fruit and vegetables | −0·13** | 0·17** | 0·19** | 0·89** | 1·00 | −0·01 |
| BMI | 0·14** | −0·06 | −0·09* | −0·08* | −0·06 | 1·00 |
FNS, Food Neophobia Scale; IDQ, Index of Diet Quality.
Correlation is significant at level α=0·05 (two-tailed).
Correlation is significant at level α=0·0014 (the criterion α=0·05 adjusted for thirty-six tests using Bonferroni correction).
For women n 1023–1178; for men n 730–1013.
Calculated based on weight and height measured by a research nurse when the participants visited the research unit 13 months after the delivery of their child.
Table 4.
Mean values of the studied variables by FNS score level and gender, and comparison between the groups within gender†: parents in the STEPS longitudinal cohort study, south-west Finland
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| Variable | FNS score (10–70)‡ | n | Mean | sd | n | Mean | sd |
| Vegetable consumption (portions/week) | 10–24 | 488 | 15·2a | 10·0 | 404 | 13·0a | 10·8 |
| 25–39 | 500 | 15·0a | 10·6 | 426 | 10·3b | 9·4 | |
| 40–70 | 190 | 10·3b | 9·1 | 181 | 7·3c | 7·4 | |
| F[2,1177]=17·76, P<0·001 | F[2,1010]=22·74, P<0·001 | ||||||
| Fruit consumption (portions/week) | 10–24 | 488 | 8·1a | 6·7 | 405 | 5·6a | 5·9 |
| 25–39 | 500 | 8·0a | 7·0 | 426 | 4·6ab | 4·9 | |
| 40–70 | 190 | 6·3b | 5·4 | 181 | 4·5b | 5·0 | |
| F[2,1177]=6·05, P=0·002 | F[2,1011]=4·79, P=0·008 | ||||||
| IDQ score (0–16) | 10–24 | 472 | 9·7a | 2·4 | 374 | 8·8a | 2·5 |
| 25–39 | 485 | 9·4a | 2·5 | 393 | 8·4a | 2·4 | |
| 40–70 | 186 | 8·5b | 2·5 | 166 | 7·8b | 2·1 | |
| F[2,1142]=18·53, P<0·001 | F[2,932]=11·46, P<0·001 | ||||||
| IDQ score excl. fruit and vegetables (0–13) | 10–24 | 472 | 8·5a | 1·9 | 374 | 8·0a | 2·1 |
| 25–39 | 485 | 8·2a | 2·0 | 393 | 7·8a | 2·0 | |
| 40–70 | 186 | 7·7b | 2·0 | 166 | 7·4b | 1·8 | |
| F[2,1142]=10·79, P<0·001 | F[2,932]=4·42, P=0·012 | ||||||
| BMI (kg/m2)§ | 10–24 | 441 | 24·2a | 4·3 | 324 | 26·5a | 3·8 |
| 25–39 | 453 | 25·4b | 5·0 | 334 | 26·4a | 3·7 | |
| 40–70 | 160 | 26·0b | 6·2 | 143 | 27·5b | 4·5 | |
| F[2,1051]=11·03, P<0·001 | F[2,798]=4·14, P=0·016 | ||||||
FNS, Food Neophobia Scale (theoretical range in parentheses); IDQ, Index of Diet Quality (theoretical range in parentheses).
a,b,cMean values within a column (for each variable and gender) with unlike superscript letters were significantly different (Tukey, P<0·05).
One-way ANOVA with Tukey’s post hoc test.
The participants who scored 10–24, 25–39 and 40–70 were regarded as ‘food neophilics’, ‘median group’ and ‘food neophobics’, respectively.
Calculated based on weight and height measured by a research nurse when the participants visited the research unit 13 months after the delivery of their child.
Fig. 1.
Vegetable consumption (a, b) and fruit consumption (c, d) as a function of Food Neophobia Scale (FNS) score level by educational level (Edu;  , basic/vocational, group sizes in standard font;
, basic/vocational, group sizes in standard font;  , advanced/academic, group sizes in italic font) and gender (a, c, women; b, d, men); analyses based on data from 1178 women and 1013 men, parents in the STEPS longitudinal cohort study, south-west Finland. Values are means, with their standard errors represented by vertical bars; numbers next to the symbols denote group sizes. Statistics from the two-way ANOVA: (a) FNS: F[2,1139]=18·65, P<0·001; Edu: F[1,1139]=2·87, P=0·091; FNS×Edu: F[2,1139]=4·91, P=0·007. (b) FNS: F[2,963]=19·22, P< 0·001; Edu: F[1,963]=13·68, P<0·001; FNS × Edu: F[2,963]=1·37, P=0·25. (c) FNS: F[2,1139]=4·53, P=0·011; Edu: F[1,1139]=0·68, P=0·41; FNS×Edu: F[2,1139]=0·79, P=0·45. (d) FNS: F[2,963]=3·72, P=0·025; Edu: F[1,963]=1·69, P=0·19; FNS×Edu: F[2,963]=0·58, P=0·56
, advanced/academic, group sizes in italic font) and gender (a, c, women; b, d, men); analyses based on data from 1178 women and 1013 men, parents in the STEPS longitudinal cohort study, south-west Finland. Values are means, with their standard errors represented by vertical bars; numbers next to the symbols denote group sizes. Statistics from the two-way ANOVA: (a) FNS: F[2,1139]=18·65, P<0·001; Edu: F[1,1139]=2·87, P=0·091; FNS×Edu: F[2,1139]=4·91, P=0·007. (b) FNS: F[2,963]=19·22, P< 0·001; Edu: F[1,963]=13·68, P<0·001; FNS × Edu: F[2,963]=1·37, P=0·25. (c) FNS: F[2,1139]=4·53, P=0·011; Edu: F[1,1139]=0·68, P=0·41; FNS×Edu: F[2,1139]=0·79, P=0·45. (d) FNS: F[2,963]=3·72, P=0·025; Edu: F[1,963]=1·69, P=0·19; FNS×Edu: F[2,963]=0·58, P=0·56
Fruit consumption
The women consumed 56 % more fruit than did men (Table 2). A small negative correlation between fruit consumption and FNS score was significant after Bonferroni correction in women, but not in men (Table 3). Similarly to vegetable consumption, both male and female food neophobics ate less fruit than did the food neophilics (Table 4). According to the two-way ANOVA, the main effect of FNS level was nominally significant in both genders, whereas the effect of educational level, and the interaction between FNS and educational levels, were not (Fig. 1(c) and (d)).
Dietary quality
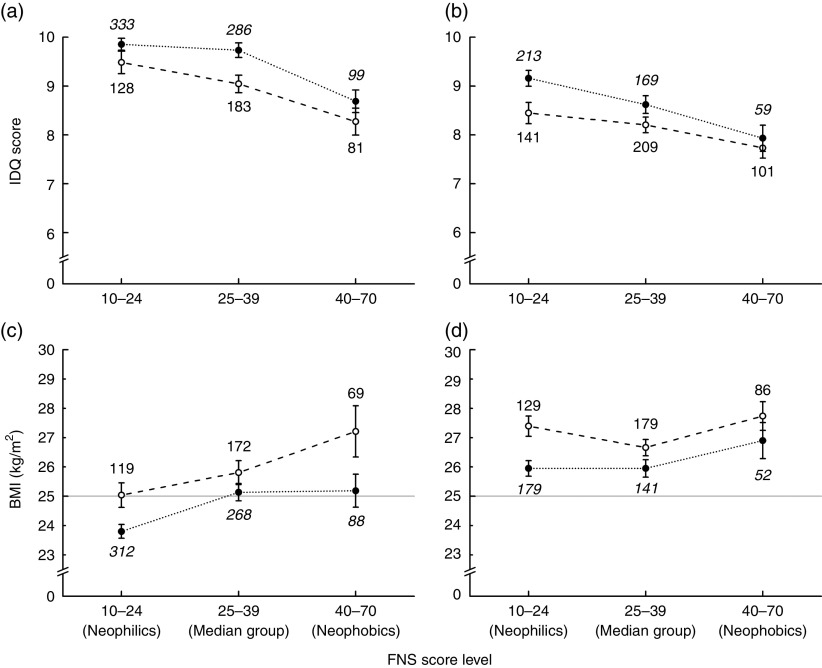
Women scored higher than men on the original IDQ, as well as when the IDQ was calculated excluding the sub-score for fruit and vegetables (Table 2). In both women and men, FNS scores correlated with the original IDQ score and the score that did not include the sub-score for fruit and vegetables (Table 3). According to the one-way ANOVA, the two IDQ scores followed a similar pattern in both women and men: the food neophobics had lower IDQ scores than the food neophilics and median group (Table 4). The two-way ANOVA showed that the main effects of both FNS score level and educational level (but not their interaction) were significant in both women and men: low FNS score and high education were associated with high IDQ score (Fig. 2(a) and (b)). The pattern of effects was similar with the IDQ score that was calculated without the sub-score for fruit and vegetables, but in men the effects hardly reached significance.
Fig. 2.
Index of Diet Quality (IDQ) score (a, b) and BMI (c, d) as a function of Food Neophobia Scale (FNS) score level by educational level (Edu;  , basic/vocational, group sizes in standard font;
, basic/vocational, group sizes in standard font;  , advanced/academic, group sizes in italic font) and gender (a, c, women; b, d, men); analyses based on data from 1178 women and 1013 men, parents in the STEPS longitudinal cohort study, south-west Finland. Values are means, with their standard errors represented by vertical bars; numbers next to the symbols denote group sizes. Note that breaks are added to the vertical axes to show off differences between the groups (theoretical range of the IDQ score is 0–16). Lines
, advanced/academic, group sizes in italic font) and gender (a, c, women; b, d, men); analyses based on data from 1178 women and 1013 men, parents in the STEPS longitudinal cohort study, south-west Finland. Values are means, with their standard errors represented by vertical bars; numbers next to the symbols denote group sizes. Note that breaks are added to the vertical axes to show off differences between the groups (theoretical range of the IDQ score is 0–16). Lines  in (c) and (d) highlight the conventional cut-off for overweight (BMI≥25 kg/m2). Statistics from the two-way ANOVA: (a) FNS: F[2,1104]=14·87, P<0·001; Edu: F[1,1104]=8·87, P=0·003; FNS×Edu: F[2,1104]=0·50, P=0·61. (b) FNS: F[2,886]=9·08, P<0·001; Edu: F[1,886]=6·53, P=0·011; FNS×Edu: F[2,886]=0·72, P=0·49. (c) FNS: F[2,1022]=8·09, P<0·001; Edu: F[1,1022]=13·42, P<0·001; FNS × Edu: F[2,1122]=1·10, P=0·33. (d) FNS: F[2,760]=3·21, P=0·041; Edu: F[1,760]=10·63, P=0·001; FNS×Edu: F[2,760]=0·75, P=0·47
in (c) and (d) highlight the conventional cut-off for overweight (BMI≥25 kg/m2). Statistics from the two-way ANOVA: (a) FNS: F[2,1104]=14·87, P<0·001; Edu: F[1,1104]=8·87, P=0·003; FNS×Edu: F[2,1104]=0·50, P=0·61. (b) FNS: F[2,886]=9·08, P<0·001; Edu: F[1,886]=6·53, P=0·011; FNS×Edu: F[2,886]=0·72, P=0·49. (c) FNS: F[2,1022]=8·09, P<0·001; Edu: F[1,1022]=13·42, P<0·001; FNS × Edu: F[2,1122]=1·10, P=0·33. (d) FNS: F[2,760]=3·21, P=0·041; Edu: F[1,760]=10·63, P=0·001; FNS×Edu: F[2,760]=0·75, P=0·47
BMI
Women had lower average BMI than did men (Table 2). The prevalence of overweight (BMI≥25 kg/m2) was 39 % in women and 62 % in men. The food neophobics tended to have higher BMI than did the food neophilics. In women, 43 % of the neophobics and 42 % of the median group were overweight, but only 34 % of the neophilics were overweight (Pearson 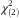 =7·40, P=0·025). In men, the respective proportions were 67 %, 60 % and 62 %, but these differences were non-significant (Pearson
=7·40, P=0·025). In men, the respective proportions were 67 %, 60 % and 62 %, but these differences were non-significant (Pearson 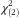 2·06, P=0·36). Similarly, the modest correlations between BMI and FNS score remained significant after the Bonferroni correction in women, but not in men (Table 3).
2·06, P=0·36). Similarly, the modest correlations between BMI and FNS score remained significant after the Bonferroni correction in women, but not in men (Table 3).
The one-way ANOVA, however, showed that food neophobics had significantly higher BMI than did food neophilics among both women and men (Table 4). According to the two-way ANOVA, the main effects of both FNS score and educational level (but not their interaction) were significant in both women and men: high FNS score (i.e. food neophobia) and low educational level were associated with high BMI (Fig. 2(c) and (d)).
In women, we ran additional analyses to compare the follow-up BMI (measured at 13 months after delivery) with pre-pregnancy BMI (based on self-reported weight and height) in relation to FNS scores. On average, the follow-up BMI was 0·95 kg/m2 higher than the pre-pregnancy BMI (paired-samples t test: t
(1037)=−16·7, P<0·001). Specifically, prevalence of overweight had increased from 30 % to 39 % (Pearson 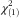 =552·4, P<0·001). However, both BMI showed a similar relationship with FNS score: the food neophobics tended to have higher BMI than did the food neophilics. According to the pre-pregnancy BMI, in women, 37 % of the neophobics and 32 % of the median group were overweight, but only 25 % of the neophilics were overweight (Pearson
=552·4, P<0·001). However, both BMI showed a similar relationship with FNS score: the food neophobics tended to have higher BMI than did the food neophilics. According to the pre-pregnancy BMI, in women, 37 % of the neophobics and 32 % of the median group were overweight, but only 25 % of the neophilics were overweight (Pearson 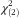 =11·74, P=0·003). Consistently, the one-way ANOVA showed that food neophobics had significantly higher pre-pregnancy BMI than did the food neophilics (F[2,1155]=9·55, P<0·001).
=11·74, P=0·003). Consistently, the one-way ANOVA showed that food neophobics had significantly higher pre-pregnancy BMI than did the food neophilics (F[2,1155]=9·55, P<0·001).
The magnitude of increase in BMI from pre-pregnancy to 13 months post-delivery showed an inverse U-shaped relationship with food neophobia (F[2,1035]=5·2, P=0·006): the average increase in BMI was lower in the neophilics (0·77 kg/m2) than in the median group (1·16 kg/m2), whereas the neophobics (0·88 kg/m2) did not differ from either group (Tukey’s post hoc test).
The women who had had one or more prior deliveries had slightly higher average pre-pregnancy BMI than did the women without prior deliveries (24·5 v. 23·9 kg/m2; t (968)=2·16, P=0·03). In spite of that, FNS score level was significantly associated with pre-pregnancy BMI in both groups (no prior deliveries: F[2,642]=6·2, P=0·002; at least one prior delivery: F[2,489]=3·17, P=0·043).
Discussion
We found that adult food neophobia was associated with lower vegetable consumption, poorer overall dietary quality and higher BMI. Our findings suggest that food neophobia complicates adaptation to dietary recommendations and may predispose to overweight.
As expected, we replicated the recent findings of a negative correlation between food neophobia (FNS score) and vegetable consumption in adults( 5 , 23 ). A similar relationship has previously been established in children by many studies( 2 , 16 – 21 ). However, we investigated the associations beyond correlations and also provided quantitative estimates for the differences between food neophilics (FNS score 10–24) and food neophobics (FNS score 40–70). The neophilic women and men consumed almost 50 % and 80 % more vegetables, respectively, than did the neophobic women and men. Our results regarding adults were consistent with those observed previously in children in two additional aspects. First, food neophobia was more clearly associated with vegetable consumption than with fruit consumption( 19 ). Second, the association between food neophobia and vegetable intake was more obvious in males than in females( 19 ). These findings suggest that food neophobia has similar effects on children and adults, or that the outcomes of food neophobia experienced during childhood remain into adulthood.
We used the IDQ not only to measure overall dietary quality but also to quantify the consumption of fruit and vegetables. Therefore, to avoid overlap (redundancy) of the variables, we also calculated the IDQ score without the sub-score for fruit and vegetables. However, regardless of the way the scores were calculated, there was an at least nominally significant relationship between the FNS and IDQ scores: higher food neophobia was consistently associated with lower dietary quality. This finding parallels the result of a study with schoolchildren (n 70, mean age about 10 years) by Falciglia et al. ( 14 ). They showed that the average dietary quality (measured by the Healthy Eating Index) of food neophobic children (by FNS) was lower than that of food neophilic children. According to the present data, the association between FNS and IDQ scores was more salient when the latter included the sub-score for fruit and vegetables, suggesting relative importance of these food categories for associations between food neophobia and dietary quality.
Our data also indicated that the food neophobics had, on average, higher BMI than did the food neophilics. This relationship appeared to be clearer in women than in men, consistent with a previous study that observed a significant correlation between FNS score and BMI in women but not in men( 5 ). The average BMI of the food neophobics were 1·8 and 1·0 kg/m2 higher in women and men, respectively, than those of the food neophilics. This means that, for example, a 1·65 m tall neophobic woman weighed 4·9 kg more than her neophilic peer of the same height. Similarly, a 1·80 m tall neophobic man was 3·3 kg heavier than an equally tall neophilic man. The association of food neophobia with BMI remained significant after taking educational level into account. The results suggest that the effects of food neophobia and education on BMI may be independent and additive, especially in the case of women.
Our additional analysis of the BMI of women showed that the average increase in BMI from pre-pregnancy (self-reported weight) to 13 months after delivery (measured weight) was almost 1 kg/m2. The increase could be due to actual weight gain and/or under-reporting of the pre-pregnancy weight. However, the food neophobic women had higher BMI than did the food neophilic women regardless of their delivery history and the method used to assess BMI. This suggests that the association between food neophobia and BMI is similar regardless of the effects of pregnancy on BMI.
Mechanisms underlying the associations we report above remain to be discovered, but we regard some behavioural, physiological and sensory factors as candidates for further studies. The preference for sweet taste is inborn, whereas people can learn to like (moderately) bitter and sour tastes only after repetitive exposure. As children, food neophobics may refuse to re-try foods that they do not like at the first bite – probably including many vegetables – whereas food neophilics may be willing to sample these foods multiple times. Therefore, neophilics may learn to like vegetables more efficiently than may neophobics. Many fruits, and some berries, are sweeter than vegetables, which may explain why the association of food neophobia with vegetable consumption was more salient than that with fruit consumption. The food preferences learned during childhood may persist into adulthood, as suggested, for example, by Vaarno et al. ( 38 ). This may explain why our data from adults showed similar associations to those previously found in children( 14 , 19 ).
Compared with the average diet of food neophilics, the diet of food neophobics may not only include fewer vegetables but also more foods that are already preferred after a few exposures. These foods are probably relatively energy-dense foods that are sweet, salty and fatty. Food neophobic adults may also be reluctant to try novel healthful alternatives to traditional food products( 39 ). These factors may underlie the differences in overall dietary quality between neophobics and neophilics. If we assume that food neophobia is not directly related to energy expenditure, we may hypothesize that neophobia increases long-term energy intake by guiding food preferences and thus predisposes to weight gain.
In addition, Raudenbush and colleagues have provided evidence for physiological responses to food neophobia, such as decreased pre-ingestive salivation( 40 ) and increased galvanic skin response( 27 ), and speculated that a physiological component may contribute to individual differences in nutrition and weight. Other potential factors that could mediate effects of food neophobia on diet and weight include chemosensory exploration and perceptions( 41 ). Food neophobics have been found to rate odours as less pleasant, sniff odour samples less vigorously( 42 ) and identify fewer odours correctly( 28 ) than food neophilics.
Limitations of the present study include the large proportion of self-reported data and the cross-sectional design of analyses. Food neophobia status and the quality of diet of the participants were not confirmed by experimental methods. Nevertheless, we used published questionnaire measures, the FNS( 1 ) and IDQ( 33 ), that have been previously validated and used with Finnish adult samples similar to that of the present study. The current participants were parents of young children (not an arbitrary group of people) from the intensive follow-up group of the STEPS study. According to a comparison of data from the National Birth Register, the mothers of the STEPS follow-up group resembled non-participating mothers of the cohort population in many aspects, importantly including BMI( 25 ). In addition, we regard parents as an important group to study for eating behaviour because, for example, maternal (or parental) fruit and vegetable consumption is a strong predictor of children’s fruit and vegetable consumption( 19 , 20 , 43 , 44 ). Furthermore, we consider our study sample unbiased in relation to eating behaviour because the participants were derived from a larger study (STEPS) for which they were recruited using criteria unrelated to eating. Importantly, for a key variable, BMI, we used objectively measured data.
Conclusions
Our results suggest that, compared with food neophilics, food neophobics comply less strictly with dietary recommendations, especially regarding the consumption of vegetables, and have higher BMI. The association between adult food neophobia and limited vegetable intake, previously established in children, supports the notion that food preferences learned in childhood persist, to a remarkable extent, into adulthood. We suggest that nutrition counselling professionals should consider food neophobia, as it may complicate adaptation to dietary recommendations and thereby also predispose to overweight.
Acknowledgements
Acknowledgements: The authors thank the families who took part in this study, the midwives for their help in recruiting them and the whole STEPS study team. Financial support: This work was supported by the Academy of Finland (M.A.S., grant numbers 252005, 256176, 263747), (H.L., grant number 121569), (A.J.K., grant number 267698); and the University of Turku. The STEPS study is mainly funded by the University of Turku, ÅboAcademi University and the Turku University Hospital. None of the funders had a role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: A.J.K. analysed the data, wrote the article and has primary responsibility for final content; M.A.S. was involved in the study conception and participated in the article preparation; J.V. participated in the study design and data collection; U.H. provided consultation regarding nutrition and assisted in the article preparation; T.P. assisted in the article preparation and proofreading; A.K. was responsible for data management of the STEPS study, contributed to the study design and provided consultation regarding statistics; H.L. was the principal investigator of the STEPS study, designed the study and assisted in the article preparation. All authors reviewed and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human participants were approved by the Ethics Committee of the Hospital District of Southwest Finland (February 2007). Written informed consent was obtained from all participants.
References
- 1. Pliner P & Hobden K (1992) Development of a scale to measure the trait of food neophobia in humans. Appetite 19, 105–120. [DOI] [PubMed] [Google Scholar]
- 2. Galloway AT, Lee Y & Birch LL (2003) Predictors and consequences of food neophobia and pickiness in young girls. J Am Diet Assoc 103, 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooke LJ, Haworth CMA & Wardle J (2007) Genetic and environmental influences on children’s food neophobia. Am J Clin Nutr 86, 428–433. [DOI] [PubMed] [Google Scholar]
- 4. Knaapila A, Tuorila H, Silventoinen K et al. (2007) Food neophobia shows heritable variation in humans. Phys Behav 91, 573–578. [DOI] [PubMed] [Google Scholar]
- 5. Knaapila A, Silventoinen K, Broms U et al. (2011) Food neophobia in young adults: genetic architecture and relation to personality, pleasantness and use frequency of foods, and body mass index – a twin study. Behav Genet 41, 512–521. [DOI] [PubMed] [Google Scholar]
- 6. Faith MS, Heo M, Keller KL et al. (2013) Child food neophobia is heritable, associated with less compliant eating, and moderates familial resemblance for BMI. Obesity (Silver Spring) 21, 1650–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards JSA, Hartwell HL & Brown L (2010) Changes in food neophobia and dietary habits of international students. J Hum Nutr Diet 23, 301–311. [DOI] [PubMed] [Google Scholar]
- 8. Hobden K & Pliner P (1995) Effects of a model on food neophobia in humans. Appetite 25, 101–114. [DOI] [PubMed] [Google Scholar]
- 9. Reverdy C, Chesnel F, Schlich P et al. (2008) Effect of sensory education on willingness to taste novel food in children. Appetite 51, 156–165. [DOI] [PubMed] [Google Scholar]
- 10. Mustonen S & Tuorila H (2010) Sensory education decreases food neophobia score and encourages trying unfamiliar foods in 8–12-year-old children. Food Qual Prefer 21, 353–360. [Google Scholar]
- 11. Tuorila H, Lähteenmäki L, Pohjalainen L et al. (2001) Food neophobia among the Finns and related responses to familiar and unfamiliar foods. Food Qual Prefer 12, 29–37. [Google Scholar]
- 12. Meiselman HL, King SC & Gillette M (2010) The demographics of neophobia in a large commercial US sample. Food Qual Prefer 21, 893–897. [Google Scholar]
- 13. Koivisto-Hursti U-K & Sjödén P-O (1997) Food and general neophobia and their relationship with self-reported food choice: familial resemblance in Swedish families with children of ages 7–17 years. Appetite 29, 89–103. [DOI] [PubMed] [Google Scholar]
- 14. Falciglia GA, Couch SC, Gribble LS et al. (2000) Food neophobia in childhood affects dietary variety. J Am Diet Assoc 100, 1474–1481. [DOI] [PubMed] [Google Scholar]
- 15. Quick V, Lipsky LM, Laffel LMB et al. (2014) Relationships of neophobia and pickiness with dietary variety, dietary quality and diabetes management adherence in youth with type 1 diabetes. Eur J Clin Nutr 68, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooke L, Wardle J & Gibson EL (2003) Relationship between parental report of food neophobia and everyday food consumption in 2–6-year-old children. Appetite 41, 205–206. [DOI] [PubMed] [Google Scholar]
- 17. Cooke LJ, Wardle J, Gibson EL et al. (2004) Demographic, familial and trait predictors of fruit and vegetable consumption by pre-school children. Public Health Nutr 7, 295–302. [DOI] [PubMed] [Google Scholar]
- 18. Cooke L, Carnell S & Wardle J (2006) Food neophobia and mealtime food consumption in 4–5 year old children. Int J Behav Nutr Phys Act 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wardle J, Carnell S & Cooke L (2005) Parental control over feeding and children’s fruit and vegetable intake: how are they related? J Am Diet Assoc 105, 227–232. [DOI] [PubMed] [Google Scholar]
- 20. Coulthard H & Blissett J (2009) Fruit and vegetable consumption in children and their mothers. Moderating effects of child sensory sensitivity. Appetite 52, 410–415. [DOI] [PubMed] [Google Scholar]
- 21. Tsuji M, Nakamura K, Tamai Y et al. (2012) Relationship of intake of plant-based foods with 6-n-propylthiouracil sensitivity and food neophobia in Japanese preschool children. Eur J Clin Nutr 66, 47–52. [DOI] [PubMed] [Google Scholar]
- 22. Russell CG & Worsley A (2008) A population-based study of preschoolers’ food neophobia and its associations with food preferences. J Nutr Educ Behav 40, 11–19. [DOI] [PubMed] [Google Scholar]
- 23. Siegrist M, Hartmann C & Keller C (2013) Antecedents of food neophobia and its association with eating behavior and food choices. Food Qual Prefer 30, 293–298. [Google Scholar]
- 24. Finistrella V, Manco M, Ferrara A et al. (2012) Cross-sectional exploration of maternal reports of food neophobia and pickiness in pre-schooler–mother dyads. J Am Coll Nutr 31, 152–159. [DOI] [PubMed] [Google Scholar]
- 25. Lagström H, Rautava P, Kaljonen A et al. (2013) Cohort profile: Steps to the healthy development and well-being of children (the STEPS study). Int J Epidemiol 42, 1273–1284. [DOI] [PubMed] [Google Scholar]
- 26. Tuorila H, Parkkinen K & Tolonen K (2008) Aistit ammattikäyttöön (Senses for professional use), 1st ed. Helsinki: WSOY Oppimateriaalit. [Google Scholar]
- 27. Raudenbush B & Capiola A (2012) Physiological responses of food neophobics and food neophilics to food and non-food stimuli. Appetite 58, 1106–1108. [DOI] [PubMed] [Google Scholar]
- 28. Demattè ML, Endrizzi I, Biasioli F et al. (2013) Food neophobia and its relation with olfactory ability in common odour identification. Appetite 68, 112–117. [DOI] [PubMed] [Google Scholar]
- 29. Barrena R & Sánchez M (2012) Neophobia, personal consumer values and novel food acceptance. Food Qual Prefer 27, 72–84. [Google Scholar]
- 30. Bajec MR & Pickering GJ (2010) Association of thermal taste and PROP responsiveness with food liking, neophobia, body mass index, and waist circumference. Food Qual Prefer 21, 589–601. [Google Scholar]
- 31. Olabi A, Najm NEO, Baghdadi OK et al. (2009) Food neophobia levels of Lebanese and American college students. Food Qual Prefer 20, 353–362. [Google Scholar]
- 32. Choe JY & Cho MS (2011) Food neophobia and willingness to try non-traditional foods for Koreans. Food Qual Prefer 22, 671–677. [Google Scholar]
- 33. Leppälä J, Lagström H, Kaljonen A et al. (2010) Construction and evaluation of a self-contained index for assessment of diet quality. Scand J Public Health 38, 794–802. [DOI] [PubMed] [Google Scholar]
- 34. Abrams B, Heggeseth B, Rehkopf D et al. (2013) Parity and body mass index in US women: a prospective 25-year study. Obesity (Silver Spring) 21, 1514–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giskes K, Avendaňo M, Brug J et al. (2010) A systematic review of studies on socioeconomic inequalities in dietary intakes associated with weight gain and overweight/obesity conducted among European adults. Obes Rev 11, 413–429. [DOI] [PubMed] [Google Scholar]
- 36. Johnson W, Kyvik KO, Skytthe A et al. (2011) Education modifies genetic and environmental influences on BMI. PLoS ONE 6, e16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kearns K, Dee A, Fitzgerald AP et al. (2014) Chronic disease burden associated with overweight and obesity in Ireland: the effects of a small BMI reduction at population level. BMC Public Health 14, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vaarno J, Leppälä J, Kaljonen A et al. (2011) Parental neophobia and children’s exposure to novel foods, is there a connection? – the STEPS Study. Eur J Public Health 21, Suppl. 1, 88. [Google Scholar]
- 39. Schickenberg B, van Assema P, Brug J et al. (2008) Are the Dutch acquainted with and willing to try healthful food products? The role of food neophobia. Public Health Nutr 11, 493–500. [DOI] [PubMed] [Google Scholar]
- 40. Raudenbush B, Corley N, Flower NR et al. (2003) Cephalic phase salivary response differences characterize level of food neophobia. Appetite 41, 211–212. [DOI] [PubMed] [Google Scholar]
- 41. Demattè LM, Endrizzi I & Gasperi F (2014) Food neophobia and its relation with olfaction. Front Psychol 5, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raudenbush B, Schroth F, Reilley S et al. (1998) Food neophobia, odor evaluation and exploratory sniffing behavior. Appetite 31, 171–183. [DOI] [PubMed] [Google Scholar]
- 43. Fisher JO, Mitchell DC, Smiciklas-Wright H et al. (2002) Parental influences on young girls’ fruit and vegetable, micronutrient, and fat intakes. J Am Diet Assoc 102, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McGowan L, Croker H, Wardle J et al. (2012) Environmental and individual determinants of core and non-core food and drink intake in preschool-aged children in the United Kingdom. Eur J Clin Nutr 66, 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]