Abstract
Objective
To review the literature on bisphenol A (BPA) exposure and obesity in human populations.
Design
Systematic review of the literature via searches of PubMed, EMBASE, Web of Science and reference lists for articles published to 1 August 2014.
Setting
China, Italy, Japan, Republic of Korea, Sweden, UK, USA.
Subjects
Adults (≥18 years).
Results
Eighteen articles were identified and included in the review. Twelve studies included secondary evaluations of BPA exposure and BMI, and six studies evaluated body composition as the primary outcome. All analyses were cross-sectional and no study included in the review received a positive quality rating (twelve negative, six neutral). Eight studies observed a statistically significant positive association between urinary or serum BPA levels and BMI, and ten studies observed no association. Studies where BMI was a primary outcome and studies of neutral quality were more likely to observe an association.
Conclusions
Study results are conflicting and significant methodological issues limit the ability to draw conclusions from these studies. Prospective studies that measure BPA exposure and changes in body weight and composition are needed to establish temporality, causality and the direction of any observed associations.
Keywords: Obesity, Environmental, Bisphenol A
Being overweight or obese contributes to an increased risk for many chronic diseases, including CVD, type 2 diabetes and some cancers( 1 , 2 ). Since 1980, worldwide prevalence of obesity has almost doubled( 3 ). While excess energy intake and a sedentary lifestyle are known risk factors for gaining weight, there has been increasing interest in the effects that environmental chemicals may have on the development of obesity( 4 ). Food and water provide us with essential nutrients; however, food and water are also sources of exposure to environmental chemicals, including pesticides( 5 , 6 ), food packaging and processing-derived contaminants such as bisphenol A (BPA) and phthalates( 7 – 15 ), and naturally occurring contaminants such as arsenic( 16 , 17 ). Chemicals detected in the food and water supply include endocrine-disrupting chemicals, a class of chemicals that interfere in some way with the normal functioning of the endocrine system and includes chemicals that may alter hormonal regulation of body weight.
First synthesized in 1891, BPA is now one of the highest-volume chemicals produced( 18 ), resulting in widespread human exposure( 19 ). BPA is used as a component of polycarbonate plastics and in epoxy resins( 18 ). The list of products currently made with polycarbonate plastics or lined with epoxy resins is extensive, and includes food and beverage storage containers and packaging( 20 ). The use of BPA in food packaging, along with the ability of BPA to leach into the food( 7 ), has led many to believe that diet is a major route of human BPA exposure( 7 – 12 ). BPA has also been found in products made from recycled paper( 21 ), dust particles( 22 – 24 ), thermal receipt paper( 25 , 26 ), soil, tap water and surface water( 27 – 32 ).
In 2002, Baillie-Hamilton( 33 ) put forth a hypothesis that endocrine-disrupting chemicals could contribute to weight gain and that the historical toxicological emphasis on weight loss as an indicator of toxicity could have resulted in weight gain going largely unnoticed as an adverse effect of exposure to endocrine-disrupting chemicals. These observations, and results from animal and in vitro studies, have led to increased interest in evaluating the potential for environmental exposures to act as ‘obesogens’. Obesogens were defined by Grun and Blumberg as ‘molecules that inappropriately regulate lipid metabolism and adipogenesis to promote obesity’( 34 ).
In vitro studies have shown that BPA has the ability to bind to thyroid hormone receptors( 35 ) and human studies have observed associations between higher BPA levels and altered levels of thyroid hormones( 36 – 40 ). Thyroid hormones regulate basal metabolism and the impact of even small alterations to thyroid hormone levels on body composition is evidenced by weight changes in patients with thyroid dysfunction( 41 – 43 ). Another mechanism by which BPA exposure may lead to weight gain is through activation of PPARγ. PPARγ is highly expressed in adipose tissue and regulates adipocyte differentiation and lipid metabolism( 44 ). In vitro data suggest BPA has the ability to bind to PPARγ, which could trigger increased adipocyte differentiation and/or uptake of lipids by adipocytes, thus influencing body composition( 45 – 47 ).
Findings from animal studies on the association between BPA exposure and weight gain have been inconsistent( 4 ), which can likely be attributed to variability in methodologies, doses, exposure routes and outcomes, and differences between species and genders( 48 ). A 2012 report from the National Institute of Environmental Health Sciences concluded there is suggestive evidence that BPA may act as an obesogen, but that further research is required( 49 ). The majority of research on the association between BPA and weight gain to date has focused on in utero and early-life exposures. Exposure to low levels of BPA perinatally( 50 – 58 ), and during adolescence( 59 , 60 ), has been shown to result in increased weight in rodents. Few studies have evaluated the risk of obesity associated with BPA exposure in adult animals.
It is important for practitioners and researchers working to reduce obesity rates to be aware of the presence of non-nutrient exposures in the diet and the potential for these exposures to contribute to risk for becoming overweight or obese. The present systematic review summarizes the currently available literature evaluating the association between BPA levels and risk of overweight or obesity in adult human populations (≥18 years). Limitations of current studies and recommendations for future studies will also be addressed.
Methods
The Population, Intervention, Comparison, and Outcome (PICO) method( 61 ) was used to construct a focused research question for the systematic review, which was ‘What is the risk or prevalence of obesity (outcome) among human adults (≥18 years) (population) who have higher BPA exposure (intervention/exposure) compared with those who have low BPA exposure (comparison)?’
To be included in the systematic review, a study had to be published in a peer-reviewed journal, written in English and report data on the association between urine or serum BPA levels and BMI (kg/m2) in an adult population (≥18 years). BMI was not required to be the primary outcome evaluated in the study. Studies were excluded if they did not present data for the association (with corresponding P value and/or confidence interval) between BPA exposure and BMI (e.g. correlation, linear regression, logistic regression). Studies that included pregnant women were excluded.
For the review, systematic searches of PubMed, EMBASE and Web of Science to 1 August 2014 were performed using the keywords ‘body weight’, ‘body size’, ‘body composition’, ‘BMI’, ‘fat mass’, ‘overweight’ or ‘obesity’ and ‘Bisphenol A’ or ‘BPA’. The search was limited to English articles, excluded conference abstracts and identified 901 articles. Data on associations with other markers of obesity and weight gain, such as waist circumference (WC) and weight, are included in the results presented in the current systematic review. However, data on these outcomes were not a requirement for inclusion because very few studies evaluated these outcomes. Studies that presented duplicate data from the same study population from an already included study were excluded. In each case, the article where BMI was a primary outcome of interest was included in the review( 62 – 64 ). Two articles evaluated US National Health and Nutrition Examination Survey (NHANES) data with BMI as the primary outcome. Both studies were included in the systematic review because of differences in analysis approaches, study years included in the analyses and no obvious reason to justify including one over the other( 62 , 63 ).
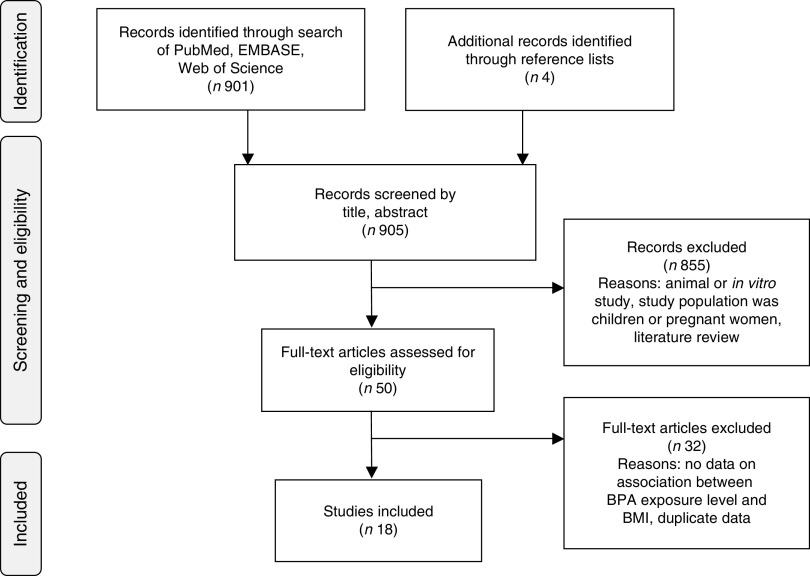
Titles, abstracts and articles were then reviewed for relevance to the research question. Fourteen articles were found to be eligible for inclusion( 62 – 75 ). Four additional articles were identified in reference lists( 76 – 79 ), for a final total of eighteen included articles. Reasons for exclusion included: not the study population of interest, no data on association between BPA levels and BMI, duplicate data, animal or in vitro study, review article and other (e.g. laboratory methods validation, did not evaluate BPA, etc.; Fig. 1).
Fig. 1.
Article identification flow diagram (BPA, bisphenol A)
Using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist as a guide( 80 ), the following information, if available, was abstracted from each study: first author; year of publication; study location; study design; study population (age, gender, health status); exposure assessment; body composition measurement methods; data analysis approach; and study results related to body composition and BPA levels. Data analysis approach was included to evaluate consistencies in analysis methods and to evaluate assessments of potential confounders. For exposure assessment methods, article abstraction focused on the type of biospecimens collected, reported materials used in collection, storage and processing, and BPA assay methodology.
Article quality was assessed using a quality rating system adapted from the rating system developed by the Academy of Nutrition and Dietetics( 81 ). Study design classification was based on the data analysis approach. For example, studies that evaluated data collected at the same time point from a cohort study were classified as cross-sectional. To receive a positive quality study rating, at least five of the validity criteria (questions) had to be met. Specifically, methods had to be appropriate and adequately described for subject selection, comparability of study subjects (appropriate control of confounders) and measurement of BPA levels (exposure) and body composition (BMI – outcome). A study with five or more ‘no’ or ‘unclear’ answers to the validity questions was rated as negative quality. When an article contained insufficient information to ascertain a clear ‘yes’/‘no’ for meeting a criterion, it was classified as ‘unclear’ and was considered as not meeting the criterion. All other studies were classified as neutral quality.
Results
To date, eighteen studies have presented data on associations between urinary or serum BPA levels and body composition( 62 – 79 ). Table 1 summarizes the studies and their findings. Thirteen studies were cross-sectional( 62 – 64 , 67 , 68 , 71 – 73 , 75 – 78 ), two were case–control( 65 , 70 ) and two were prospective cohort studies( 66 , 74 ), but all data analyses were cross-sectional. One of the cohort studies did prospectively evaluate rate of weight change, but only evaluated the association between BMI and urinary BPA levels at baseline( 74 ). The studies were conducted in the USA (n 5)( 62 , 63 , 70 , 74 , 77 ), Republic of Korea (n 5)( 71 , 72 , 75 , 76 , 78 ), China (n 2)( 64 , 67 ), Italy (n 2)( 65 , 66 ), Japan (n 2)( 68 , 73 ), Sweden (n 1)( 79 ) and the UK (n 1)( 70 ). For six of the articles, body composition (BMI, WC and/or weight) was the primary outcome of interest( 62 – 64 , 67 , 71 , 74 ). Seven studies included only women( 65 , 67 , 69 , 73 , 74 , 77 , 78 ), nine of the studies were in general adult populations( 62 – 64 , 66 , 67 , 70 , 71 , 75 , 76 ), one study included men and women ≥60 years( 72 ) and another included only 70-year-old men and women( 79 ).
Table 1.
Human studies evaluating BMI in relation to bisphenol A in adult populations (≥18 years)
| Study and country | Study objective | Population | Study design | Exposure assessment | Results | Covariates | Study quality* |
|---|---|---|---|---|---|---|---|
| Serum BPA measurement studies (n 6) | |||||||
| Bloom et al.( 77 ) USA (California) | To evaluate associations between serum BPA, peak oestradiol levels, and number of oocytes retrieved during IVF | Women undergoing IVF Age: 28–44 years n 41 No additional exclusion/inclusion criteria specified except for having serum BPA and serum oestradiol levels or number of oocytes retrieved | Cross-sectional | Spot blood samples Serum BPA HPLC with electrochemical detector LOD=0·3 ng/ml Collection and storage materials: PET serum separator Vacutainer tubes, PP storage cryovials | Mean (sd) BMI (kg/m2): 24·3 (4·9) Mean (sd) BPA (ng/ml): 7·22 (14·2) Median BPA (ng/ml): 2·53 Pearson correlation (BPA, BMI): r=−0·12, P=0·44 | None | D – negative |
| Kim et al.( 78 ) Republic of Korea | To evaluate associations between BPA levels and BMD and biochemical bone markers related to osteoporosis | Postmenopausal women receiving treatment for osteoporsis Age: 50–82 years n 51 Excluded women taking medications that alter bone or Ca metabolism, and women with cancer or other systemic disease | Cross-sectional | Spot blood samples Serum BPA Competitive ELISA Quantitative range: 0·50–10 ng/ml Collection and storage materials: No information provided | Mean (sd) BMI (kg/m2): 23·0 (2·7) Mean (sd) BPA (ng/ml): 1·44 (0·52) Pearson correlation (BPA, BMI): r=0·008, P=0·96 | None | D – negative |
| Olsen et al.( 79 ) Sweden | To evaluate whether there is an association between circulating BPA and phthalate levels and Framingham Risk Score and/or cardiovascular risk factors included in the score | Men and women Age: 70 years n 1016 No additional exclusion/inclusion criteria specified except age and location of residence | Cross-sectional | Fasting spot blood samples Serum BPA Isotope LC–MS/MS LOD=0·2 ng/ml (unclear if this is for BPA, phthalates or all chemicals analysed) Collection and storage materials: No information provided | Mean (sd) BMI (kg/m2): 27·0 (4·3) WHR (sd): 0·90 (0·075) Mean/median BPA: Not provided Linear regression, β (95 % CI) (ng/ml): BPA=0·085 (−0·19, 0·36) | Gender, serum cholesterol, serum TAG, hypertension, smoking, diabetes | D – negative |
| Takeuchi et al.( 73 ) Japan | To evaluate whether serum BPA levels are associated with serum hormone levels in women with ovarian dysfunction and obesity | Non-obese women with normal menstrual cycles: Mean (se) age =27·5 (0·7) years n 19 Obese women with normal menstrual cycles: Mean (se) age =28·8 (2·0) years n 7 Women with hyperprolactinaemia: Mean (se) age =27·7 (2·6) years n 7 Women with hypothalamic amenorrhoea: Mean (se) age =25·1 (1·0) years n 21 Non-obese women with PCOS: Mean (se) age =26·5 (1·5) years n 13 Obese women with PCOS: Mean (se) age =24·7 (1·9) years n 6 Exclusions were unclear, but it appears use of medications and abnormal thyroid hormone levels were exclusionary criteria | Cross-sectional | Fasting spot blood samples Serum BPA Competitive ELISA LOD: Not provided Collection and storage materials: No information provided | Mean (se) BMI (kg/m2): Non-obese women with normal menstrual cycles =19·7 (0·3) Obese women with normal menstrual cycles =28·5 (1·7) Patients with hyperprolactinaemia=20·8 (1·0) Women with hypothalamic amenorrhaea =19·2 (0·6) Non-obese PCOS =19·1 (0·6) Obese PCOS =31·3 (3·0) Mean (se) BPA (ng/ml): Normal menstrual cycles =0·71 (0·09) Obese women with normal menstrual cycles =1·04 (0·09) Patients with hyperprolactinaemia=0·83 (0·12) Women with hypothalamic amenorrhoea =0·84 (0·10) Non-obese PCOS =1·05 (0·10) Obese PCOS =1·17 (0·16) Correlation (BPA, BMI): r=0·50, P<0·001 Among normal women serum BPA levels were higher in obese women v. non-obese women, P<0·05 | None | D – negative |
| Takeuchi and Tsutsumi( 68 ) Japan | To evaluate associations between urinary BPA levels and gender and sex-hormone levels | Healthy women: Mean (se) age =28·7 (0.7) years n 14 PCOS women: Mean (se) age =25·7 (1·4) years n 16 Healthy men: Mean (se) age =29·4 (1·1) years n 11 No exclusion/inclusion criteria specified | Cross-sectional | Spot blood samples Serum BPA Competitive ELISA LOD: Not provided Collection and storage materials: No information provided | Mean (se) BMI (ng/ml): Healthy women =19·4 (0·3) PCOS women =22·4 (0·9) Healthy men =21·2 (1·1) Mean (se) BPA (ng/ml): Healthy women =0·64 (0·10) PCOS women =1·49 (0·11) Healthy men =1·04 (0·10) Correlation – women only (BPA, BMI): r=0·30, P>0·05 Correlation – all participants (BPA, BMI): r=0·32, P>0·05 | None | D – negative |
| Tarantino et al.( 65 ) Italy | To evaluate whether serum BPA levels are associated with insulin resistance, hepatic steatosis, hyperandrogenism severity and spleen size in women with PCOS | Women Cases: Mean (sd) age =27·7 (6·8) years n 40 women with PCOS Controls: Mean (sd) age =26·2 (3·9) years n 20 age-matched healthy, normal-weight women who worked at hospital Excluded smoking, alcohol consumption, pregnancy, hypothyroidism, hyperprolactinaemia, Cushing's disease, non-classical congenital adrenal hyperplasia, use of oral contraceptives in previous 6 months, also use of insulin-sensitizing agents, glucocorticoids, anti-androgens, ovulation agents, anti-obesity drugs, presence of any acute viral, bacterial or fungal infection, any type of chronic liver disease, arthritis, bronchial asthma, IBS, cancer | Case–control *Analysis is cross-sectional | Spot blood sample Serum BPA Competitive ELISA LOD: Not provided. Compared increased levels (>0·45 ng/ml) with lower levels (<0·45 ng/ml) Chose cut-off based on 95th percentile in controls Collection and storage materials: No information provided | Mean (sd) BMI (kg/m2): Cases =28·1 (7·7) Controls =22·1 (1·8) Median (range) BPA (ng/ml): Cases =0·7 (0·1–6·0) Controls =0·1 (0·1–0·6) Among women with PCOS, BMI did not differ between those with BPA levels <0·45 ng/ml v. those with levels about the cut-off point (P=0·30) BMI was significantly correlated (r=0·27, P=0·04) with serum BPA measurements Only one control had BPA level above 0·45 ng/ml | None | D – negative |
| Total urinary BPA measurement studies (n 12) | |||||||
| Carwile and Michels( 62 ) USA | To evaluate whether urinary BPA levels were associated with general (BMI) and central (WC) obesity | US NHANES 2003–2006 Men and women Age: 18–74 years n 2747 Excluded women who were pregnant and participants missing urinary BPA or creatinine data | Cross-sectional | Fasting spot urine samples Total BPA HPLC–MS/MS LOD 2003–2004=0·36 ng/ml LOD 2005–2006=0·40 ng/ml Urinary BPA levels were evaluated as quartiles Q1: ≤1·1 ng/ml Q2: 1·2–2·3 ng/ml Q3: 2·4–4·6 ng/ml Q4: ≥4·7 ng/ml Collection and storage materials: According to CDC Laboratory Procedure Manual efforts are made to avoid contamination of samples, including using PP collection containers, and borosilicate glass or PP storage containers and vials | Mean BMI: Not provided Mean (IQR) BPA (µg/g creatinine): 2·05 (1·18–3·33) BMI (kg/m2) definitions: Recommended = BMI<25·0 Overweight =25·0<BMI≤29·9 Obese = BMI≥30·0 Overweight BMI v. Recommended BMI, Q4 BPA v. Q1 BPA: OR=1·31 (95 % CI 0·80, 2·14) Obese BMI v. Recommended BMI, Q4 BPA v. Q1 BPA: OR=1·76 (95 % CI 1·06, 2(.94) BMI and urinary BPA (continuous) change in BMI by BPA quartile, kg/m2 (95 % CI): Q1= Reference Q2=1·48 (0·46–2·51) Q3=1·69 (0·62, 2·76) Q4=1·56 (0·25, 2·87) (P trend=0·18) WC definitions for elevated: ≥102 cm (men) ≥88 cm (women) Elevated WC v. Normal WC, Q4 BPA v. Q1 BPA: OR=1·58 (95 % CI 1·03, 2·42) | Sex, age, race/ethnicity, education, smoking, creatinine | D – neutral |
| Galloway et al.( 66 ) Italy | To evaluate whether urinary BPA levels were associated with serum oestrogen and testosterone levels | InCHIANTI prospective cohort study Men and women Age: 20–74 years n 715 No additional exclusion/inclusion criteria specified except age and location of residence | Cohort *Analysis is cross-sectional | 2 h collection urine samples Total BPA HPLC–MS/MS LOD/LOQ=0·50 µg/l Evaluated as covariate, not exposure/outcome association Collection and storage materials: No information provided. Unspecified type of plastic container was used for urine sample collection | Mean BPA (95 % CI) (ng/ml): 3·59 (3·42, 3·77) Mean BMI: Not provided Mean BPA (95 % CI) (μg/d): BMI 18·5–25·0 kg/m2: 5·67 (5·22, 6·16) reference BMI 25·0–30·0 kg/m2: 5·84 (5·43, 6·27) (P=0·30) BMI 30·1–34·9 kg/m2: 5·66 (5·04, 6·34) (P=0·37) BMI≥35·0 kg/m2: 4·85 (3·94, 5·98) (P=0·73) β (95 % CI): WC (cm) =0·006 (0·002, 0·011) (P=0·013) Weight (kg) =0·006 (0·002, 0·010) (P=0·003) | Age, sex, study site *24 h urine sample-concentration adjustment not needed | D – neutral |
| Ko et al.( 71 ) Republic of Korea | To evaluate whether urinary BPA levels are associated with WC | Men and women Mean (sd) age baseline: 44·3 (14·6) years n 1030 Inclusion/exclusion criteria not provided | Cross-sectional | Unclear type of urine sample. Appears to be spot urine sample Total BPA HPLC–MS/MS LOD/LOQ: Not provided BPA quartiles: Q1:<0·85 µg/ml Q2: 0·85–1·41 µg/ml Q3: 1·41–2·59 µg/ml Q4: ≥2·59 µg/ml Collection and storage materials: No information provided. Unspecified type of plastic container was used for urine sample collection | Mean (range) BPA (µg/ml): 1·04 (0·2–198·7) Mean (sd) BMI (kg/m2): 24·0 (3·4) Mean (sd) BMI (kg/m2) by BPA quartile: Q1=24·0 (3·6) Q2=23·7 (3·2) Q3=24·0 (3·4) Q4=24·2 (3·6) (P=0·16) BMI (continuous), Β (se) P value: 0·19 (0·03) 0·01 Mean (sd) body fat (%) by BPA quartile: Q1=27·3 (6·7) Q2=26·4 (5·7) Q3=26·2 (6·3) Q4=26·2 (6·5) (P=0·74) Body fat (continuous), Β (se) P value: 0·11 (0·05) 0·04 Mean (sd) weight (kg) by BPA quartile: Q1=62·8 (10·9) Q2=63·0 (11·6) Q3=64·4 (12·0) Q4=65·3 (12·5) (P=0·07) Weight (continuous), Β (se) P value: 0·04 (0·03) 0·08 Mean (sd) WC (cm) by BPA quartile: Q1=84·0 (8·5) Q2=84·0 (8·6) Q3=84·5 (8·3) Q4=85·6 (10·7) (P=0·007) WC (continuous), Β (se) P value: 0·56 (0·03) 0·05 WC definitions for elevated: ≥90 cm (men) ≥85 cm (women) Elevated WC v. Normal WC, Q4 BPA v. Q1 BPA: OR=1·93 (95 % CI 1·31, 2·86) Mean (sd) HC (cm) by BPA quartile: Q1=94·5 (6·1) Q2=94·3 (6·2) Q3=95·1 (6·4) Q4=95·9 (7·0) (P=0·03) HC (continuous), Β (se) P value: 0·06 (0·04) 0·13 | Age, sex, urinary creatinine (WC logistic regression model additionally adjusted for education, income, alcohol intake and smoking status) | D – negative |
| Lee et al.( 72 ) Republic of Korea | To evaluate whether urinary BPA levels were associated with liver enzyme concentrations using repeated measures | Men and women Age: ≥60 years n 478 Excluded participants who visited study centre only once, with no blood and urine samples available, and those who reported currently having viral hepatitis, fatty liver disease, liver cancer or any other type of liver disease | Cross-sectional *Analysis of BMI is cross-sectional | Multiple spot urine samples were collected, but BMI was compared only with spot urine sample collected at baseline Total BPA HPLC–MS/MS LOD=0·005 µg/l Collection and storage materials: Unspecified type of container was used for urine sample collection and storage | Mean BMI: Not provided Mean (sd) BPA (µg/g creatinine): Men =0·88 (1·33) Women =1·28 (1·95) Mean (sd) BPA (µg/g creatinine) by BMI category: <23·0 kg/m2 =1·09 (1·31) 23·0–24·9 kg/m2 =1·10 (1·87) >25·0 kg/m2 =1·28 (2·02) | None | D – negative |
| Melzer et al.( 70 ) UK | To evaluate the association between urinary BPA levels and incident CAD | EPIC-Norfolk cohort Men and women Age: 40–74 years Cases: n 758 Controls: n 861 Excluded participants with diabetes and/or a history of MI or stroke at baseline | Nested case–control *Analysis is cross-sectional | Spot urine sample Total BPA HPLC–MS/MS LOQ=0·50 ng/ml Collection and storage materials: No information provided | Mean (sd) BMI (kg/m2): Cases =27·2 (3·8) Controls =26·2 (3·4) Mean (sd) BPA (ng/ml): Cases =1·23 (2·95) Controls =1·39 (3·02) BPA v. BMI category BPA≤1·243 ng/ml: BMI<18·4 kg/m2, 0·43 % BMI 18·4–24·9 kg/m2, 38·2 % BMI 25·0–29·9 kg/m2, 47·0 % BMI 30·0–34·9 kg/m2, 12·9 % BMI>35 kg/m2, 1·5 % BPA>1·243 ng/ml: BMI<18·4 kg/m2, 0·0 % BMI 18·4–24·9 kg/m2, 33·7 % BMI 25·0–29·9 kg/m2, 52·8 % BMI 30·0–34·9 kg/m2, 11·3 % BMI>35 kg/m2, 2·2 % χ 2 test: P=0·21 | None *Did not adjust for urine concentration | D – neutral |
| Mok-Lin et al.( 69 ) USA (Massachusetts) | To evaluate pre- and peri-conception urinary BPA concentrations with oocyte and oestradiol production among women undergoing IVF | Women partners of couples seeking infertility evaluation and treatment Age: 21–44 years n 84 Excluded women using donor oocytes or embryos or who underwent cryo–thaw cycles | Cross-sectional | Two spot urine samples from same IVF cycle; used geometric mean of the two SG-adjusted urinary BPA concentrations Total BPA HPLC–MS/MS LOD=0·4 ng/ml Collection and storage materials: PP collection containers. Storage container details not provided | Mean (sd) BMI (kg/m2): 24·0 (5·1) Mean (sd) BPA (ng/ml): 3·97 (5·9) Unspecified model correlation: r=−0·06, P=0·61 | None BPA levels were SG-adjusted | D – negative |
| Shankar et al.( 63 ) USA | To evaluate whether urinary BPA levels were associated with obesity (BMI and WC) by gender and race/ethnicity | NHANES 2003–2008 Men and women Age: ≥20 years n 3967 Excluded participants with self-reported history of CVD, missing data on education, smoking status, serum glucose levels, SBP or DBP, BMI and/or cholesterol levels | Cross-sectional | Spot urine samples Total BPA Analysis method depends on year: GC–MS or HPLC–MS/MS LOD=0·1–2 ng/ml per 100 µl urine Urinary BPA levels were evaluated as quartiles BPA quartiles: Q1: <1·10 ng/ml Q2: 1·10–2·10 ng/ml Q3: 2·11–4·20 ng/ml Q4: >4·20 ng/ml Collection and storage materials: According to CDC Laboratory Procedure Manual efforts are made to avoid contamination of samples, including using PP collection containers, and borosilicate glass or PP storage containers and vials | Mean BMI: Not provided Mean WC: Not provided Mean (sd) BPA (ng/ml): Men =3·97 (0·21) Women =3·90 (0·26) Overall population BPA v. BMI≥30·0 kg/m2, Q4 BPA v. Q1 BPA: OR=1·69 (95 % CI 1·30, 2·20) (P<0·0001) Overall population BPA v. WC ≥88 cm (women)/102 cm (men), Q4 BPA v. Q1 BPA: OR=1·59 (95 % CI: 1·21–2·09) (P=0·0009) Urinary BPA levels were significantly associated with higher odds of obesity (BMI or WC), regardless of race/ethnicity or gender | Age, sex, race/ethnicity, education, smoking, alcohol intake, physical inactivity, diabetes, hypertension, serum TC *Did not adjust for urine concentration | D – neutral |
| Song et al.( 74 ) USA | To evaluate urinary concentrations of BPA and major phthalate metabolites in relation to prospective weight change in the NHS and NHS II | Controls from nested case–control study in NHS and NHS II Women n 977 Mean (sd) baseline age (years): BPA Q1=57·6 (12·0) BPA Q2=54·8 (11·2) BPA Q3=51·1 (9·9) BPA Q4=51·7 (10·2) | Cohort (nested case–control) *Analysis of BPA and BMI association is cross-sectional | Spot first morning void urine samples Total BPA LC–MS/MS LOD: Not provided Urinary BPA levels were evaluated as quartiles BPA quartiles (median, IQR) (µg/l): Q1=0·82 (0·59–1·03) Q2=1·46 (1·32–1·66) Q3=2·39 (2·05–2·76) Q4=4·99 (3·83–8·14) Collection and storage materials: PP collection and storage containers Weight change = Weight at most recent follow-up – weight at urine sample collection (~9–14 years apart) | Median (IQR) BPA (µg/l): Q1=0·82 (0·59–1·03) Q2=1·46 (1·32–1·66) Q3=2·39 (2·05–2·76) Q4=4·99 (3·83–8·14) Mean (95 % CI) BMI (kg/m2): Q1=26·1 (25·4, 26·8) Q2=26·2 (25·5, 26·9) Q3=26·3 (25·6, 27·0) Q4=26·0 (25·3, 26·7) (adjusted for creatinine) (baseline BMI) Mean (95 % CI) BMI (kg/m2): Q1=25·7 (25·0, 26·4) Q2=26·3 (25·6, 27·0) Q3=26·2 (25·5, 26·9) Q4=26·2 (25·4, 26·9) (P trend=0·65) Weight change rate (95 % CI): Q1= Reference Q2=0·15 (0·00, 0·31) Q3=0·18 (0·03, 0·34) Q4=0·23 (0·15, 0·50) (P trend=0·02) | Creatinine, cohort origin, age at baseline, smoking, alcohol consumption, physical activity, alternative healthy eating index, total energy intake | D – neutral |
| Wang et al.( 64 ) China | To evaluate whether urinary BPA levels were associated with obesity (BMI and WC) or insulin resistance | Men and women Age: ≥40 years n 3390 Excluded participants with self-reported liver diseases (e.g. hepatitis, cirrhosis, cancer) | Cross-sectional | Spot morning urine samples Total BPA HPLC–MS/MS LOD=0·30 ng/ml (below were assigned value of 0·15) Urinary BPA levels were evaluated as quartiles BPA quartiles (ng/ml): Q1: ≤0·47 Q2: 0·48–0·81 Q3: 0·82–1·43 Q4: >1·43 Collection and storage materials: No information provided | Median (IQR) BPA (ng/ml): 0·81 (0·47–1·43) (mean not provided) Mean (sd) BMI (kg/m2): 24·9 (3·6) (adjusted for gender) BMI (kg/m2) definitions: Recommended: BMI<24·0 Overweight: 24·0≤BMI <28·0 Obese: BMI≥28·0 Overweight BMI v. Recommended BMI, Q4 BPA v. Q1 BPA: OR=1·24 (95 % CI 0·97, 1·59) Obese BMI v. Recommended BMI, Q4 BPA v. Q1 BPA: OR=1·50 (95 % CI 1·15, 1·60) BMI and urinary BPA (continuous), mean (sd) BMI (kg/m2) by BPA quartile: Q1=24·6 (3·6) Q2=24·9 (3·8) Q3=24·8 (3·6) Q4=25·1 (3·5) (P trend<0·001) WC definitions for elevated: ≥90 cm (men) ≥85 cm (women) Elevated WC v. Normal WC, Q4 BPA v. Q1 BPA: OR=1·28 (95 % CI 1·03, 1·60) WC and urinary BPA (continuous), mean (sd) WC (cm) by BPA quartile: Q1=86·6 (9·9) Q2=87·7 (9·8) Q3=87·1 (9·8) Q4=87·9 (9·6) (P trend<0·001) | Age, sex, urinary creatinine, smoking, alcohol consumption, education, SBP, HDL-C, LDL-C, TC, CRP, FPG, fasting serum insulin, serum ALT and GTT | D – neutral |
| Yang et al.( 75 ) Republic of Korea | To evaluate if associations between BPA levels and markers of oxidative stress and inflammation differ by gender and/or postmenopausal status | Men and women Age: Range not specified Mean (sd) age: Men =49·5 (8·6) years Premenopausal women =45·9 (3·9) years Postmenopausal women =57·0 (7·1) years n 485 Excluded subjects who reported a history of disease that could be associated with oxidative stress biomarker levels, such as cancer, heart disease, tuberculosis, hepatitis, arthritis and asthma | Cross-sectional | Fasting (12 h) spot morning urine samples Total BPA HPLC–MS/MS LOD=0·063 ng/ml (per 500 µl urine) Collection and storage materials: No information provided | Mean (IQR) BPA (µg/g creatinine): Men =0·52 (0·12–1·75) Premenopausal women =0·61 (0·24–1·86) Postmenopausal women =0·58 (0·11–1·82) Mean (sd) BMI (kg/m2): Men =24·6 (2·6) Premenopausal women =23·0 (2·7) Postmenopausal women =23·8 (3·0) Unspecified correlation model (BPA, BMI): r=−0·042, P=0·358 Unspecified correlation model (BPA, weight): r=−0·079, P=0·085 | Creatinine | D – negative |
| Yang et al.( 76 ) Republic of Korea | To evaluate associations between BPA levels and genetic polymorphisms (UGT, SULT), sister chromatid exchange and endocrine-related disorders | Men and women who visited the hospital for regular check-up Age: Range not specified Mean (sd) age: Men =48·9 (10·7) years Women =47·5 (12·3) years n 172 Excluded participants who were occupationally exposed to BPA, but approach for defining this was not provided | Cross-sectional | Spot morning urine samples Total BPA HPLC–fluorescence detector LOD: Not provided Collection and storage materials: No information provided | Mean (sd) BPA (ng/ml): Men =6·88 (3·72) Women =5·61 (3·16) Mean (sd) BMI (kg/m2): Men =24·5 (3·7) Women =23·6 (4·7) Unspecified model: P = 0·08 | None | D – negative |
| Zhao et al.( 67 ) China | To evaluate whether urinary BPA levels are associated with body composition, serum oestrodial, leptin and osteocalcin levels, and BMD | Healthy premenopausal women Age: 20–55 years n 282 Excluded postmenopausal women and women who reported taking medications that alter bone metabolism or body weight | Cross-sectional | Spot urine samples Total BPA HPLC–MS/MS LOD: Not provided Collection and storage materials: No information provided | Mean (se) BPA (ng/ml): 2·27 (0·32) Mean (se) BMI (kg/m2): 21·2 (0·2) BMI v. BPA: r=0·24, P<0·001 WC v. BPA: r=0·30, P<0·001 HC v. BPA: r=0·27, P<0·001 WHR v. BPA: r=0·149, P<0·001 Fat mass v. BPA: r=0·350, P<0·001 Weight v. BPA: r=0·186, P=0·001 | Age *Did not adjust for urine concentration | D – negative |
BPA, bisphenol A; IVF, in vitro fertilization; BMD, bone mineral density; PCOS, polycystic ovary syndrome; WC, waist circumference; CAD, coronary artery disease; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; UGT, UDP-glucuronosyltransferase; SULT, sulfotransferase; IBS, irritable bowel syndrome; NHANES, National Health and Nutrition Examination Survey; InCHIANTI, Invecchiare in Chianti, ageing in the Chianti area; EPIC, European Prospective Investigation on Cancer and Nutrition; MI, myocardial infarction; SBP, systolic blood pressure; DBP, diastolic blood pressure; Q, quartile; LOD, limit of detection; PET, polyethylene terephthalate; PP, polypropylene; CDC, Centers for Disease Control and Prevention; LOQ, limit of quantification; SG, specific gravity; IQR, interquartile range; HC, hip circumference; WHR, waist-to-hip ratio; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; CRP, C-reactive protein; FPG, fasting plasma glucose; ALT, alanine aminotransferase; GTT, γ-glutamyl transpeptidase.
Quality score does not necessarily reflect the study quality for the primary outcomes of the study. Outcome evaluations and data analysis quality evaluations were evaluated specifically for the presented associations between BMI and BPA, which were often not the primary outcome of interest in some of these studies.
Academy of Nutrition and Dietetics Evidence Analysis Manual( 81 ).
All studies evaluated associations using a single cross-sectional measurement of BPA, but biospecimen collection, laboratory assay methodology and data analysis approaches varied across the studies. Six studies measured serum BPA levels; four using a competitive ELISA( 65 , 68 , 73 , 78 ), one using HPLC paired with an electrochemical detector( 77 ) and one using LC and tandem MS (LC–MS/MS)( 79 ). Ten studies measured total BPA in spot urine samples( 62 – 64 , 67 , 70 – 72 , 74 – 76 ), one study used the geometric mean for two spot samples collected during the same in vitro fertilization cycle( 69 ) and one study measured total BPA in a 24 h urine sample( 66 ). Urinary BPA levels were measured using GC–MS( 63 ) and/or HPLC and tandem MS (HPLC–MS/MS)( 62 – 64 , 66 , 67 , 71 , 72 , 74 ).
Data analysis approaches included correlation( 65 , 67 – 69 , 73 , 75 – 78 ), χ 2 tests( 65 , 70 ), ANOVA( 73 ), linear regression( 62 , 64 , 66 , 71 , 74 , 79 ), logistic regression( 62 – 64 ) and unspecified analysis approach( 72 ). Differences in data analysis approaches limited the ability to perform meta-analyses.
The six studies that measured serum BPA concentrations observed wide ranges of serum BPA levels across study participants. While Tarantino et al. observed means <0·70 ng/ml( 65 ), Bloom et al. observed a mean of 7·22 ng/ml and a median of 2·53 ng/ml( 77 ). Three of the studies reported a limit of detection (LOD) or limit of quantification (LOQ)( 77 – 79 ), but only Bloom et al. reported the number of participants below the LOD/LOQ (13·6 %)( 77 ).
Mean or median urinary BPA levels in spot urine samples were difficult to compare across studies given that some studies reported means( 62 , 63 , 67 , 69 , 70 , 75 , 76 ), some reported medians( 64 , 74 ) and some reported levels unadjusted for concentration( 63 , 67 , 69 , 70 , 76 ), while others reported creatinine-adjusted concentrations( 62 , 64 , 75 ). LOD/LOQ were relatively similar across studies and ranged from 0·063 to 0·50 ng/ml. Four studies did not report an LOD/LOQ( 67 , 71 , 74 , 76 ). Most studies reported frequent detection of BPA in urine samples. Seven studies did not report the number of samples below the LOD/LOQ. In three of these studies, data presented in the paper indicated detectable urinary BPA levels in at least 75 %( 64 ), 90 %( 63 ) and 95 %( 66 ) of the population. Among studies that reported the frequency of BPA detection in participants’ urine, detection ranged from 77·9 to 97·5 % of samples( 62 , 69 , 70 , 75 , 76 ).
Among the studies that measured serum BPA, results were conflicting. Two studies( 65 , 73 ) observed positive correlations between serum BPA levels and BMI. The other four studies did not observe an association between serum BPA concentrations and BMI( 68 , 77 – 79 ). Only one of these studies reported adjusting for potential confounders, such as age or health status( 79 ).
Among the six studies that did not evaluate BMI as the primary outcome compared with urinary BPA levels, one study observed associations between body composition and urinary BPA levels( 66 ) and five observed no association( 69 , 70 , 72 , 75 , 76 ). Galloway et al. did not find BMI to be statistically significantly associated with urinary BPA levels measured in a 24 h urine collection. However, WC (continuous) and weight (continuous) were statistically significantly positively associated with 24 h urinary BPA levels( 66 ). Melzer et al. did not find an association between BMI and urinary BPA category (≤1·243 ng/ml v. >1·243 ng/ml), although there was a higher percentage of overweight and obese participants (66·3 %) in the high BPA category compared with the low BPA category (61·8 %)( 70 ).
Four of the six studies where body composition was the primary outcome observed statistically significant positive associations between BMI and urinary BPA levels( 62 – 64 , 67 ). Zhao et al. ( 67 ) observed statistically significant positive correlations (adjusted for age) between urinary BPA levels and BMI, WC and hip circumference in premenopausal women. Wang et al. found a small, but statistically significant, positive trend between spot urine BPA levels and both BMI (continuous) and WC (continuous)( 64 ). That study observed marginally statistically higher odds of being overweight (24 kg/m2≤BMI<30·0 kg/m2, OR=1·24; 95 % CI 0·97, 1·59) among participants in the highest BPA quartile (total urinary BPA>1·43 ng/ml) compared with those in the lowest quartile (total urinary BPA≤0·47 ng/ml), and being in the highest BPA quartile was associated with 50 % higher odds of being obese (BMI≥30·0 kg/m2; 95 % CI 1·15, 1·60) compared with those in the lowest BPA quartile. Wang et al. also observed higher odds of elevated WC (≥85 cm in women, ≥90 cm in men) among participants in the highest BPA quartile (OR=1·20; 95 % CI 1·03, 1·60) compared with those in lowest BPA quartile. The study by Song et al. found no association between cross-sectional BMI and urinary BPA levels measured at baseline. However, that study did observe a statistically significant positive association between urinary BPA levels and annual weight change rate (quartile 4 v. quartile 1: β=0·23 kg/year, 95 % CI 0·07, 0·38)( 74 ). Similarly, Ko et al. observed inconsistent results depending on analysis approach and measure of body composition used( 71 ). Regardless of analysis approach, WC was associated with urinary BPA levels. Conversely, hip circumference and weight were not associated with urinary BPA levels, regardless of analysis approach. Percentage body fat and BMI were associated with urinary BPA when evaluated as continuous variables only.
Using NHANES 2003–2008 data, Shankar et al. found higher odds of general obesity (BMI≥30·0 kg/m2; OR=1·69; 95 % CI 1·30, 2·20) and central obesity (WC≥88 cm in women, ≥102 cm in men; OR=1·59; 95 % CI 1·21, 2·09) among those in the highest BPA quartile compared with those in the lowest BPA quartile( 63 ). Carwile and Michels performed analyses using NHANES 2003–2006 data and the findings were consistent with those of Shankar et al. Those in the highest urinary BPA level quartiles (total urinary BPA>4·20 ng/ml) had higher odds of being overweight (25·0 kg/m2≤BMI<30·0 kg/m2; OR=1·31; 95 % CI 0·80, 2·14) or obese (BMI≥30·0 kg/m2; OR=1·76; 95 % CI 1·06, 2·94) and having an elevated WC (≥88 cm in women, ≥102 cm in men; OR=1·58; 95 % CI 1·03, 2·42) compared with participants in the first quartile (total urinary BPA<1·10 ng/ml). While higher urinary BPA levels were associated with general and central obesity in this study, there was not a clear linear pattern to the association( 62 ).
Twelve of the eighteen studies were rated as negative quality( 65 , 67 – 69 , 71 – 73 , 75 – 79 ), six were neutral quality( 62 – 64 , 66 , 70 , 74 ) and none were positive quality. The lack of positive quality studies was typically due to limitations and issues related to BPA exposure measurement (validity question 5), temporality problems (validity question 6) and/or lack of evaluation of confounding factors (validity question 7). Among the twelve negative quality studies, only three observed statistically significant associations between urinary or serum BPA levels and any measure of body composition( 67 , 71 , 73 ), while four( 62 – 64 , 66 ) of the six neutral quality studies observed statistically significant associations. All six of the serum BPA studies received a negative quality rating( 65 , 68 , 73 , 77 – 79 ), while four of the twelve urinary BPA studies received a negative quality rating( 67 , 69 , 75 , 76 ). No study, regardless of whether serum BPA or urinary BPA was measured, met the criteria for adequate exposure measurement (question 6).
Discussion
Overall, the literature evaluating the association between BPA levels and obesity is conflicting and inconclusive. Eight out of the eighteen studies observed a positive association between urinary or serum BPA levels and BMI. Among studies that indicated a lack of association, all but one were secondary analyses and all but one were rated negative quality. Significant limitations were present in all eighteen studies included in the present systematic review. No study received a positive quality rating and, thus, all results should be interpreted with caution. Of note, higher-quality studies were more likely to report a positive association between BPA levels and BMI, and all studies that evaluated BMI as a primary outcome observed statistically significant positive associations.
Our findings are consistent with another recently published systematic review by Lakind et al.( 82 ). However, the Lakind et al. review included duplicate data, pregnant women, adolescents and children, which further complicated their interpretation of the results. The previous review did not evaluate how the study results differed by quality, methods and whether BMI was the primary outcome. The present review also includes three studies( 71 , 72 , 74 ) not included in the Lakind et al. review.
The data presented in all eighteen studies included in the current systematic review were single, cross-sectional measurements of both BPA levels and body composition. The one exception was an analysis of rate of weight change, but that study also had only a single spot urine sample( 74 ). Cross-sectional data have an inherent inability to distinguish temporality, so researchers are not able to determine whether differences in observed BPA exposure levels are causally related to current body composition. An additional concern with cross-sectional data is that they only reflect very recent exposures and for many chronic health conditions (including the development of obesity) long-term exposure is most relevant. This is particularly true for BPA, which is generally considered to be absorbed, metabolized and excreted within 24 h of exposure( 48 , 83 , 84 ), and within-person BPA levels are highly variable over time( 85 – 87 ). Studies have demonstrated high intra-individual variation in urinary BPA levels from spot samples collected at multiple time points on the same day and across multiple days or years( 85 , 86 ). Single-day 24 h urine samples have been shown to also have high intra-individual variability across days. All studies included in the present review used a spot serum, spot urine or a single-day 24 h urine sample to determine BPA levels.
Biomarkers (urine or serum) are currently the only available method for assessing BPA exposure level. Actual results are difficult to compare across studies because of differences in BPA assay methodologies and observed BPA levels. Six studies measured serum BPA level which, regardless of assay method, is currently not considered to be the most appropriate method for measuring BPA exposure due to concerns regarding specimen contamination and the inability of current assay methods and equipment to accurately measure the low levels of BPA that are typically present in serum( 10 , 11 , 26 , 88 – 90 ). BPA is a non-persistent chemical that is found at nano- to picomolar concentrations in serum, which increases the potential for extraneous sample contamination to influence serum measurements( 48 , 91 , 92 ). Urinary BPA levels are much higher and consist largely of conjugated BPA, which can only be formed in vivo, thus acting as a marker for ruling out contamination by extraneous sources( 88 ). In fourteen of the studies( 64 , 66 – 73 , 75 – 79 ), including five of six studies that measured serum BPA levels, the manuscripts lacked sufficiently detailed information to determine whether BPA-free materials were used to collect, process and store biospecimens. Failure to use BPA-free laboratory materials could lead to sample contamination and inaccurate BPA measurements, particularly with serum measurements( 64 , 65 , 67 , 68 , 73 , 88 ). This could result in non-differential misclassification of exposure, which may attenuate observed associations, and should be addressed in future studies. Further complicating interpretations is the lack of reporting of LOD/LOQ and the number of participants who had undetectable levels of BPA in collected samples, which is essential information for evaluating the BPA assessment method and comparing study results.
Given the limitations in measuring low-level BPA metabolites in serum, many researchers consider total urinary BPA to be the preferred approach for measuring BPA exposure( 48 , 88 , 91 , 92 ). However, it is important to note that studies measuring total urinary BPA levels do not directly test the association between internal unconjugated BPA (biologically active) exposure and body composition. Total urinary BPA measures primarily conjugated BPA, which is no longer biologically active and is readily excreted in the urine( 20 , 48 , 93 , 94 ).
It has been suggested that BPA exhibits a non-monotonic dose–response relationship, where very low levels and very high levels are the ranges in which BPA exposure may adversely affect health( 95 ). However, the broad range of reported urinary and serum BPA levels in currently available studies and the lack of a quality control programme for BPA measurements have been a challenge for testing this hypothesis and defining the cut-off point for low-level BPA exposures. Reliable testing methodology for unconjugated serum BPA is required to fully test this hypothesis. Results were recently published from a round robin including four laboratories with previous experience in environmental chemical analysis( 96 ). The round robin was established to specifically evaluate and address improvements to serum assay methodologies and standardization of assay protocols. Results indicate that unconjugated serum BPA can be measured using strict protocols for collection, storage and processing materials and appropriate laboratory methodologies.
It is currently unclear whether there is any exposure level at which no adverse health effects occur. Adding to the complexity of evaluating BPA exposures and health outcomes in population-based studies is that nearly all human populations are exposed to low levels of BPA. There is generally no ‘unexposed’ group to compare with the ‘exposed’ population. Prospective population-based studies evaluating different levels of exposure could help elucidate if there are thresholds at which BPA is associated with health outcomes. The lack of an unexposed control group makes it difficult to interpret study findings because a lack of a statistically significant association could mean either: (i) there is no true association; or (ii) any BPA exposure is harmful.
Data analysis approaches varied widely across studies, which likely contributes to the inconclusive findings in the present systematic review. Few of the studies provided sufficient details on their approach to the data analysis. Two major issues were the use only of correlation, especially Pearson’s correlation, and a failure to adjust for relevant covariates. BPA levels generally are not normally distributed in human populations, even after log transformation, which makes Pearson’s correlation an inappropriate analysis approach. Very high outliers have the ability to skew associations, particularly in studies with smaller sample sizes. Most studies showed a range that log transformation would not have sufficiently corrected, thus complicating the interpretation of study results.
Additionally, all of the studies included in the systematic review failed to collect data and/or evaluate important potential covariates, such as dietary factors and correlated chemical exposures. Eating greater quantities of food should theoretically lead to greater potential for BPA exposure, but also higher energy intake, and thus increase the risk of being overweight or obese. Additionally, other chemical exposures found in the human environment are also suspected of being endocrine-disrupting obesogens. As an example, diet is also thought to be the primary route of exposure to phthalates( 14 , 15 ), which are often added to food packaging materials to increase flexibility and resilience( 15 , 97 – 100 ). Phthalate exposure has also been associated with increased risk of obesity( 49 , 101 , 102 ). Data indicate that most people are exposed to both BPA and phthalates, making it difficult to evaluate the individual effects of BPA without considering associations with phthalates( 103 ). Future studies should consider overall energy intake and correlated chemical exposures when evaluating the association between BPA and body weight/composition.
While cross-sectional studies have important limitations, there are challenges to evaluating the health effects of BPA exposure using other study designs. If biospecimens are collected as part of a case–control study, the biospecimen collection would occur after the health event of interest has occurred (as the presence or absence of the health effect determines study eligibility) and thus BPA measurements would not reflect long-term exposure or establish the temporality necessary for the determination of causality. In order to optimally address the association between BPA exposure and risk of obesity, new prospective cohort studies are needed in which biospecimens are collected at regular intervals using appropriate procedures and materials to minimize the risk of sample contamination. However, these studies are very expensive, require a large number of study participants for sufficient statistical power and require years of observation time. Many of the existing longitudinal, prospective cohort studies have not collected urine samples, or if they have, the samples are single spot urine samples and/or the samples were not collected using BPA-free materials.
Obesity is a multifactorial health condition and collaboration among obesity researchers from a variety of disciplines (including toxicology, nutrition and epidemiology) is essential. Future research evaluating associations between exposures to obesogenic chemicals, such as BPA, and body composition would benefit from including obesity researchers with expertise in nutrition and physical activity. Studies investigating the association between BPA exposure and changes in body weight or composition must consider the multifactorial nature of obesity and collect and evaluate additional potential confounders or effect modifiers, including dietary intake and concurrent chemical exposures. Reducing the cost of measuring BPA exposure will allow for BPA measurements in large prospective observational studies and for repeated measurements to evaluate longer-term exposure patterns. Research is needed to clarify sources of human BPA exposure and how exposure levels vary over time. This would provide insight for appropriate sample collection and may allow for the development of data collection tools, such as a questionnaire, that could be used to estimate relative BPA exposure in large population-based observational studies. Longitudinal studies that prospectively measure BPA exposures and changes in body weight and composition are needed to establish temporality and causality, and the direction of any observed associations. Finally, improving the accuracy of serum BPA measurements, especially at low levels, will allow researchers to directly test the association between unconjugated BPA levels and body composition and determine if levels of internal exposure are sufficient to adversely affect health.
Conclusion
Currently available evidence is inconclusive with regard to the association between adult BPA exposure and risk of being overweight or obese. Significant methodological issues limit the ability to draw firm conclusions from these studies. However, the lack of high-quality research findings does not mean that there are no health effects. The evidence of widespread human exposure to BPA makes it imperative that the health consequences of BPA exposure be fully evaluated.
Acknowledgements
Financial support: S.J.O. was supported by a National Cancer Institute training grant (T32 CA13267, Principal Investigator: KE Anderson). The National Cancer Institute had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: The review question was initiated by S.J.O. The article search and evaluations were performed by both authors (S.J.O., K.R.). The manuscript was written by S.J.O. and edited/reviewed by K.R. Ethics of human subject participation: This systematic review did not require approval from an ethics committee.
References
- 1. Bailin PD, Byrne M, Lewis S et al. (2008) Public awareness drives market for safer alternatives: bisphenol A market analysis report. http://www.iehn.org/publications.reports.bpa.php (accessed December 2013).
- 2. Tang-Peronard JL, Andersen HR, Jensen TK et al. (2011) Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev 12, 622–636. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (2014) Obesity and Overweight. http://www.who.int/mediacentre/factsheets/fs311/en/ (acessed July 2014).
- 4. Thayer KA, Heindel JJ, Bucher JR et al. (2012) Role of environmental chemicals in diabetes and obesity: a National Toxicology Program Workshop Report. Environ Health Perspect 120, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu C, Barr DB, Pearson MA et al. (2008) Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect 116, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melnyk LJ, Xue J, Brown GG et al. (2014) Dietary intakes of pesticides based on community duplicate diet samples. Sci Total Environ 468–469, 785–790. [DOI] [PubMed] [Google Scholar]
- 7. Welshons WV, Nagel SC & vom Saal FS (2006) Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147, 6 Suppl., S56–S69. [DOI] [PubMed] [Google Scholar]
- 8. US Environmental Protection Agency (2010) Bisphenol A Action Plan. http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/bpa.html (accessed January 2014).
- 9. Maffini MV, Rubin BS, Sonnenschein C et al. (2006) Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol 254–255, 179–186. [DOI] [PubMed] [Google Scholar]
- 10. Lakind JS & Naiman DQ (2011) Daily intake of bisphenol A and potential sources of exposure: 2005–2006 National Health and Nutrition Examination Survey. J Expo Sci Environ Epidemiol 21, 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lakind JS & Naiman DQ (2008) Bisphenol A (BPA) daily intakes in the United States: estimates from the 2003–2004 NHANES urinary BPA data. J Expo Sci Environ Epidemiol 18, 608–615. [DOI] [PubMed] [Google Scholar]
- 12. Cao XL, Perez-Locas C, Dufresne G et al. (2011) Concentrations of bisphenol A in the composite food samples from the 2008 Canadian total diet study in Quebec City and dietary intake estimates. Food Addit Contam Part A, Chem Anal Control Expo Risk Assess 28, 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao XL, Corriveau J & Popovic S (2010) Bisphenol A in canned food products from Canadian markets. J Food Protect 73, 1085–1089. [DOI] [PubMed] [Google Scholar]
- 14. Clark K, Cousins IT & Mackay D (2003) Assessment of critical exposure pathways. In The Handbook of Environmental Chemistry. vol. 3: Part Q: Phthalate Esters , pp. 227–262 [CA Staples, editor]. New York: Springer. [Google Scholar]
- 15. Centers for Disease Control and Prevention (2009) Fourth National Report on Human Exposure to Environmental Chemicals [Department of Health and Human Services, editor]. Atlanta, GA: CDC. [Google Scholar]
- 16. Bundschuh J, Nath B, Bhattacharya P et al. (2012) Arsenic in the human food chain: the Latin American perspective. Sci Total Environ 429, 92–106. [DOI] [PubMed] [Google Scholar]
- 17. Heikens A, Panaullah GM & Meharg AA (2007) Arsenic behaviour from groundwater and soil to crops: impacts on agriculture and food safety. Rev Environ Contam Toxicol 189, 43–87. [DOI] [PubMed] [Google Scholar]
- 18. Rubin BS (2011) Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 127, 27–34. [DOI] [PubMed] [Google Scholar]
- 19. Vandenberg LN, Chahoud I, Heindel JJ et al. (2010) Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118, 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vandenberg LN, Hauser R, Marcus M et al. (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24, 139–177. [DOI] [PubMed] [Google Scholar]
- 21. Liao C & Kannan K (2011) Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure. Environ Sci Technol 45, 9372–9379. [DOI] [PubMed] [Google Scholar]
- 22. Loganathan SN & Kannan K (2011) Occurrence of bisphenol A in indoor dust from two locations in the eastern United States and implications for human exposures. Arch Environ Contam Toxicol 61, 68–73. [DOI] [PubMed] [Google Scholar]
- 23. Geens T, Roosens L, Neels H et al. (2009) Assessment of human exposure to bisphenol-A, triclosan and tetrabromobisphenol-A through indoor dust intake in Belgium. Chemosphere 76, 755–760. [DOI] [PubMed] [Google Scholar]
- 24. Rudel RA, Camann DE, Spengler JD et al. (2003) Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol 37, 4543–4553. [DOI] [PubMed] [Google Scholar]
- 25. Geens T, Goeyens L, Kannan K et al. (2012) Levels of bisphenol-A in thermal paper receipts from Belgium and estimation of human exposure. Sci Total Environ 435–436, 30–33. [DOI] [PubMed] [Google Scholar]
- 26. Geens T, Aerts D, Berthot C et al. (2012) A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol 50, 3725–3740. [DOI] [PubMed] [Google Scholar]
- 27. Santhi VA, Sakai N, Ahmad ED et al. (2012) Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Sci Total Environ 427–428, 332–338. [DOI] [PubMed] [Google Scholar]
- 28. Maggioni S, Balaguer P, Chiozzotto C et al. (2013) Screening of endocrine-disrupting phenols, herbicides, steroid estrogens, and estrogenicity in drinking water from the waterworks of 35 Italian cities and from PET-bottled mineral water. Environ Sci Pollut Res Int 20, 1649–1660. [DOI] [PubMed] [Google Scholar]
- 29. Li X, Ying GG, Su HC et al. (2010) Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ Int 36, 557–562. [DOI] [PubMed] [Google Scholar]
- 30. Dupuis A, Migeot V, Cariot A et al. (2012) Quantification of bisphenol A, 353-nonylphenol and their chlorinated derivatives in drinking water treatment plants. Environ Sci Pollut Res Int 19, 4193–4205. [DOI] [PubMed] [Google Scholar]
- 31. Barnes KK, Kolpin DW, Furlong ET et al. (2008) A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States – I) groundwater. Sci Total Environ 402, 192–200. [DOI] [PubMed] [Google Scholar]
- 32. Kang JH, Kondo F & Katayama Y (2006) Human exposure to bisphenol A. Toxicology 226, 79–89. [DOI] [PubMed] [Google Scholar]
- 33. Baillie-Hamilton PF (2002) Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med 8, 185–192. [DOI] [PubMed] [Google Scholar]
- 34. Grun F & Blumberg B (2006) Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 147, 6 Suppl., S50–S55. [DOI] [PubMed] [Google Scholar]
- 35. Zoeller RT (2005) Environmental chemicals as thyroid hormone analogues: new studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol Cell Endocrinol 242, 10–15. [DOI] [PubMed] [Google Scholar]
- 36. Meeker JD, Calafat AM & Hauser R (2010) Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol 44, 1458–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meeker JD & Ferguson KK (2011) Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in US adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ Health Perspect 119, 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sriphrapradang C, Chailurkit LO, Aekplakorn W et al. (2013) Association between bisphenol A and abnormal free thyroxine level in men. Endocrine 44, 441–447. [DOI] [PubMed] [Google Scholar]
- 39. Wang F, Hua J, Chen M et al. (2012) High urinary bisphenol A concentrations in workers and possible laboratory abnormalities. Occup Environ Med 69, 679–684. [DOI] [PubMed] [Google Scholar]
- 40. Wang T, Lu J, Xu M et al. (2013) Urinary bisphenol a concentration and thyroid function in Chinese adults. Epidemiology 24, 295–302. [DOI] [PubMed] [Google Scholar]
- 41. de Moura Souza A & Sichieri R (2011) Association between serum TSH concentration within the normal range and adiposity. Eur J Endocrinol 165, 11–15. [DOI] [PubMed] [Google Scholar]
- 42. Knudsen N, Laurberg P, Rasmussen LB et al. (2005) Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab 90, 4019–4024. [DOI] [PubMed] [Google Scholar]
- 43. Pucci E, Chiovato L & Pinchera A (2000) Thyroid and lipid metabolism. Int J Obes Relat Metab Disord 24, Suppl. 2, S109–S112. [DOI] [PubMed] [Google Scholar]
- 44. Berger JP (2005) Role of PPARγ, transcriptional cofactors, and adiponectin in the regulation of nutrient metabolism, adipogenesis and insulin action: view from the chair. Int J Obes (Lond) 29, Suppl. 1, S3–S4. [DOI] [PubMed] [Google Scholar]
- 45. Pereira-Fernandes A, Demaegdt H, Vandermeiren K et al. (2013) Evaluation of a screening system for obesogenic compounds: screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS One 8, e77481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rubin BS & Soto AM (2009) Bisphenol A: perinatal exposure and body weight. Mol Cell Endocrinol 304, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Masuno H, Iwanami J, Kidani T et al. (2005) Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci 84, 319–327. [DOI] [PubMed] [Google Scholar]
- 48. World Health Organization & Food and Agriculture Organization of the United Nations (2010) Toxicological and Health Aspects of Bisphenol A. Geneva: WHO. [Google Scholar]
- 49. Holtcamp W (2012) Obesogens: an environmental link to obesity. Environ Health Perspect 120, a62–a68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ashby J, Tinwell H & Haseman J (1999) Lack of effects for low dose levels of bisphenol A and diethylstilbestrol on the prostate gland of CF1 mice exposed in utero . Regul Toxicol Pharmacol 30, 156–166. [DOI] [PubMed] [Google Scholar]
- 51. Howdeshell KL, Hotchkiss AK, Thayer KA et al. (1999) Exposure to bisphenol A advances puberty. Nature 401, 763–764. [DOI] [PubMed] [Google Scholar]
- 52. Markey CM, Coombs MA, Sonnenschein C et al. (2003) Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev 5, 67–75. [DOI] [PubMed] [Google Scholar]
- 53. Miyawaki J, Sakayama K, Kato H et al. (2007) Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb 14, 245–252. [DOI] [PubMed] [Google Scholar]
- 54. Newbold RR, Jefferson WN & Padilla-Banks E (2007) Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol 24, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nikaido Y, Yoshizawa K, Danbara N et al. (2004) Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod Toxicol 18, 803–811. [DOI] [PubMed] [Google Scholar]
- 56. Patisaul HB & Bateman HL (2008) Neonatal exposure to endocrine active compounds or an ERβ agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav 53, 580–588. [DOI] [PubMed] [Google Scholar]
- 57. Rubin BS, Murray MK, Damassa DA et al. (2001) Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 109, 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Somm E, Schwitzgebel VM, Toulotte A et al. (2009) Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect 117, 1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Akingbemi BT, Sottas CM, Koulova AI et al. (2004) Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 145, 592–603. [DOI] [PubMed] [Google Scholar]
- 60. Markey CM, Michaelson CL, Veson EC et al. (2001) The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect 109, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. da Costa Santos CM, de Mattos Pimenta CA & Nobre MR (2007) The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem 15, 508–511. [DOI] [PubMed] [Google Scholar]
- 62. Carwile JL & Michels KB (2011) Urinary bisphenol A and obesity: NHANES 2003–2006. Environ Res 111, 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shankar A, Teppala S & Sabanayagam C (2012) Urinary bisphenol a levels and measures of obesity: results from the national health and nutrition examination survey 2003–2008. ISRN Endocrinol 2012, 965243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang T, Li M, Chen B et al. (2012) Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 97, E223–E227. [DOI] [PubMed] [Google Scholar]
- 65. Tarantino G, Valentino R, Di Somma C et al. (2013) Bisphenol A in polycystic ovary syndrome and its association with liver–spleen axis. Clin Endocrinol 78, 447–453. [DOI] [PubMed] [Google Scholar]
- 66. Galloway T, Cipelli R, Guralnik J et al. (2010) Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ Health Perspect 118, 1603–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao HY, Bi YF, Ma LY et al. (2012) The effects of bisphenol A (BPA) exposure on fat mass and serum leptin concentrations have no impact on bone mineral densities in non-obese premenopausal women. Clin Biochem 45, 1602–1606. [DOI] [PubMed] [Google Scholar]
- 68. Takeuchi T & Tsutsumi O (2002) Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun 291, 76–78. [DOI] [PubMed] [Google Scholar]
- 69. Mok-Lin E, Ehrlich S, Williams PL et al. (2010) Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl 33, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Melzer D, Osborne NJ, Henley WE et al. (2012) Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 125, 1482–1490. [DOI] [PubMed] [Google Scholar]
- 71. Ko A, Hwang MS, Park JH et al. (2014) Association between urinary bisphenol A and waist circumference in Korean adults. Toxicol Res 30, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee MR, Park H, Bae S et al. (2014) Urinary bisphenol A concentrations are associated with abnormal liver function in the elderly: a repeated panel study. J Epidemiol Community Health 68, 312–317. [DOI] [PubMed] [Google Scholar]
- 73. Takeuchi T, Tsutsumi O, Ikezuki Y et al. (2004) Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J 51, 165–169. [DOI] [PubMed] [Google Scholar]
- 74. Song Y, Hauser R, Hu FB et al. (2014) Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes (Lond) (Epublication ahead of print version). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang YJ, Hong YC, Oh SY et al. (2009) Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ Res 109, 797–801. [DOI] [PubMed] [Google Scholar]
- 76. Yang M, Kim SY, Chang SS et al. (2006) Urinary concentrations of bisphenol A in relation to biomarkers of sensitivity and effect and endocrine-related health effects. Environ Mol Mutagen 47, 571–578. [DOI] [PubMed] [Google Scholar]
- 77. Bloom MS, Kim D, Vom Saal FS et al. (2011) Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril 96, 672–677.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim DH, Oh CH, Hwang YC et al. (2012) Serum bisphenol A concentration in postmenopausal women with osteoporosis. J Bone Metab 19, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Olsen L, Lind L & Lind PM (2012) Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol Environ Saf 80, 179–183. [DOI] [PubMed] [Google Scholar]
- 80. von Elm E, Altman DG, Egger M et al. (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61, 344–349. [DOI] [PubMed] [Google Scholar]
- 81. Academy of Nutrition and Dietetics (2012) Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process. Chicago, IL: Academy of Nutrition and Dietetics. [Google Scholar]
- 82. Lakind JS, Goodman M & Mattison DR (2014) Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit Rev Toxicol 44, 121–150. [DOI] [PubMed] [Google Scholar]
- 83. Volkel W, Bittner N & Dekant W (2005) Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography–tandem mass spectrometry. Drug Metab Dispos 33, 1748–1757. [DOI] [PubMed] [Google Scholar]
- 84. Volkel W, Colnot T, Csanady GA et al. (2002) Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem Res Toxicol 15, 1281–1287. [DOI] [PubMed] [Google Scholar]
- 85. Lassen TH, Frederiksen H, Jensen TK et al. (2013) Temporal variability in urinary excretion of bisphenol A and seven other phenols in spot, morning, and 24-h urine samples. Environ Res 126, 164–170. [DOI] [PubMed] [Google Scholar]
- 86. Townsend MK, Franke AA, Li X et al. (2013) Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: a prospective cohort study. Environ Health 12, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Braun JM, Kalkbrenner AE, Calafat AM et al. (2011) Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect 119, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Calafat AM, Koch HM, Swan SH et al. (2013) Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast Cancer Res 15, 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Calafat AM, Kuklenyik Z, Reidy JA et al. (2005) Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113, 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mahalingaiah S, Meeker JD, Pearson KR et al. (2008) Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect 116, 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Markham DA, Waechter JM Jr, Wimber M et al. (2010) Development of a method for the determination of bisphenol A at trace concentrations in human blood and urine and elucidation of factors influencing method accuracy and sensitivity. J Anal Toxicol 34, 293–303. [DOI] [PubMed] [Google Scholar]
- 92. Volkel W, Kiranoglu M & Fromme H (2008) Determination of free and total bisphenol A in human urine to assess daily uptake as a basis for a valid risk assessment. Toxicol Lett 179, 155–162. [DOI] [PubMed] [Google Scholar]
- 93. Matthews JB, Twomey K & Zacharewski TR (2001) In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β. Chem Res Toxicol 14, 149–157. [DOI] [PubMed] [Google Scholar]
- 94. Snyder RW, Maness SC, Gaido KW et al. (2000) Metabolism and disposition of bisphenol A in female rats. Toxicol Appl Pharmacol 168, 225–234. [DOI] [PubMed] [Google Scholar]
- 95. Vandenberg LN, Colborn T, Hayes TB et al. (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33, 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vandenberg LN, Gerona RR, Kannan K et al. (2014) A round robin approach to the analysis of bisphenol A (BPA) in human blood samples. Environ Health 13, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fromme H, Bolte G, Koch HM et al. (2007) Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int J Hyg Environ Health 210, 21–33. [DOI] [PubMed] [Google Scholar]
- 98. Schettler T (2006) Human exposure to phthalates via consumer products. Int J Androl 29, 134–139, discussion 181–185. [DOI] [PubMed] [Google Scholar]
- 99. Agency for Toxic Subtances and Disease Registry (2001) Toxicological profile for di-n-butyl phthalate. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=859&tid=167 (accessed January 2014). [PubMed]
- 100. Agency for Toxic Subtances and Disease Registry (2002) Toxicological Profile for di(2-ethylhexyl)phthalate (DEHP). http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=684&tid=65 (accessed January 2014). [PubMed]
- 101. Stahlhut RW, van Wijngaarden E, Dye TD et al. (2007) Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult US males. Environ Health Perspect 115, 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hatch EE, Nelson JW, Qureshi MM et al. (2008) Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Barr DB, Silva MJ, Kato K et al. (2003) Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect 111, 1148–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]