Abstract
Objective
The present study aimed to review the association of conjugated linoleic acid (CLA) consumption in two forms, foods enriched in CLA and CLA supplements, with serum lipid profile in human studies.
Design
Systematic review and meta-analysis.
Setting
Search process was conducted in PubMed, Cochrane Library, Google Scholar, Scopus and Science Direct. Clinical trials that investigated the association of CLA intakes either in the form of supplements or enriched foods with lipid profile in healthy adults were included. All outcomes were recorded as continuous variables, and the effect size was measured by analysis of the mean and standard deviation before and after the intervention for case and control groups.
Subjects
Healthy adult population.
Results
CLA supplementation was associated with a significant decrease in LDL cholesterol (mean difference=−0·218; 95 % CI −0·358, −0·077; P=0·002), a non-significant decrease in HDL cholesterol (mean difference=−0·051; 95 % CI −0·188, 0·086; P=0·468), a non-significant increase in total cholesterol (mean difference=0·009; 95 % CI −0·128, 0·146; P=0·896) and a non-significant decrease in TAG (mean difference=−0·065; 95 % CI −0·20, 0·07; P=0·344). Foods enriched with CLA were associated with significantly decreased LDL cholesterol (mean difference=−0·231; 95 % CI −0·438, −0·024; P=0·028), non-significantly increased HDL-C (mean difference=0·075; 95 % CI −0·121, 0·270; P=0·455), non-significantly decreased total cholesterol (mean difference=−0·158; 95 % CI −0·349, 0·042; P=0·124) and non-significantly decreased TAG (mean difference=−0·078; 95 % CI −0·274, 0·117; P=0·433).
Conclusions
According to our analysis, consumption of foods enriched with CLA or CLA supplements has favourable effects on LDL cholesterol levels.
Keywords: Conjugated linoleic acid, TAG, Total cholesterol, HDL cholesterol, LDL cholesterol
Dyslipidaemia consists of different abnormalities in lipid profile and is one of the main risk factors for several diseases such as CVD, diabetes mellitus, hypertension, stroke and acute pancreatitis( 1 ). The prevalence of dyslipidaemia depends on socio-economic status and ethnicity( 2 ). It is increasing in most developed( 3 ) and developing countries owing to unhealthy diets and lifestyle changes( 4 , 5 ). The main factors for dyslipidaemia are genetic, diet and lifestyle. According to previous studies, trans-fatty acids (TFA) play an important role in lipid profile disorders( 6 ).
There are two sources of dietary TFA: (i) industrial TFA, which are produced technologically during the partial hydrogenation of vegetable oils; and (ii) ruminant TFA, such as vaccenic acid and conjugated linoleic acid (CLA) that are synthesized by rumen bacteria via the metabolism of MUFA and PUFA( 7 – 9 ). Clinical studies have reported that dietary intake of industrial TFA has a deleterious effect on lipoprotein concentrations; however, ruminant TFA may be less detrimental to blood lipid levels than industrial TFA( 10 ). Two isomers of CLA are cis-9, trans-11 (c9,t11) and trans-10, cis-12 (t10,c12)( 11 – 17 ). The abundance of these isomers is different in foods and industrial supplements( 11 – 13 , 18 – 22 ).
CLA is produced naturally by the rumen bacteria of ruminants( 14 , 19 , 23 – 27 ) or by bioconversion of vaccenic acid in the ruminant mammary gland( 26 , 28 ). Moreover, it can even be produced synthetically by partial hydrogenation of linoleic acid( 20 , 25 ). The main dietary sources of CLA are ruminant meats such as beef and lamb, and dairy products such as milk and cheese( 11 , 14 – 16 , 19 , 20 , 23 , 24 , 29 ). The mean CLA intake is estimated at 0·3–2·6 g/d and daily intake of CLA through natural sources is 160 mg/d approximately( 22 , 30 ).
Animal studies have shown that CLA might have various beneficial effects, e.g. prevention of carcinogenesis, decrease body fat, enhancement of lean body mass, empowering the immune system and prevention of diabetes and CVD( 14 , 15 , 21 , 31 – 33 ). However, the findings of human studies are controversial( 17 , 25 , 26 , 30 , 34 ). These differences may be related to the different forms and doses of CLA, study populations and duration of trials( 25 ).
Some human studies have reported that CLA supplementation had no significant effect on plasma lipid concentrations( 18 , 21 ); whereas another study found that CLA supplementation could significantly reduce total cholesterol (TC) and LDL cholesterol (LDL-C) in both genders and HDL cholesterol (HDL-C) only in women( 12 ). Moreover, there are inconsistent findings on foods enriched in CLA. Some studies have claimed that CLA-rich dairy products significantly increased TC and LDL-C and decreased HDL-C; however, they had no significant effect on TAG concentration( 34 ). On the other hand, another study indicated that the consumption of skimmed milk enriched with CLA had no significant effect on plasma lipid variables such as TAG, TC, LDL-C and HDL-C levels( 22 ).
Studies on different forms of CLA, i.e. commercial natural products enriched in CLA or supplement forms, showed various findings; therefore it is necessary to summarize the controversial findings. The present study aimed to review the association of CLA consumption in two forms, foods enriched in CLA or CLA supplements, with serum lipid profile in human studies.
Methods
Literature search
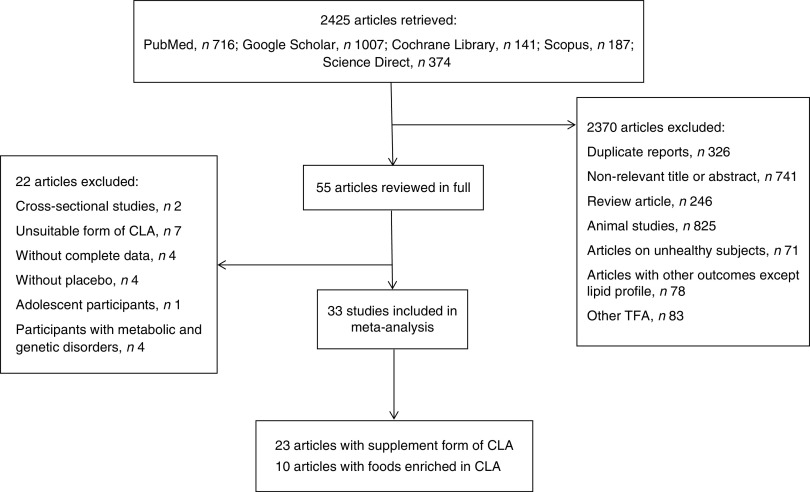
The search was conducted in the following databases: PubMed, Cochrane Library, Google Scholar, Scopus and Science Direct, from 1 June to 23 November 2013. Keywords such as ‘trans-10 cis-12-conjugated linoleic acid’, ‘cis-9 trans-11-conjugated linoleic acid’, ‘CLA fatty acid’, ‘CLA’, ‘conjugated linoleic acid’, ‘trans fatty acid’, ‘TFA’, ‘Triglycerides’, ‘lipoprotein triglyceride’, ‘Lipoproteins, HDL’, ‘Cholesterol, HDL’, ‘Cholesterol, LDL’, ‘Lipoproteins, LDL’, ‘LDL’, ‘HDL’, ‘Total cholesterol’, ‘TG’, ‘triglyceride’, ‘triacylglycerol’, ‘TAG’, ‘lipid profile’, ‘low density lipoprotein’ and ‘high density lipoprotein’ were used. Keywords and medical subject heading (MeSH) terms are presented in Table 1. Age, gender and language were not limited during the search. Clinical trials that investigated the association of CLA intakes either in the form of supplements or enriched foods with lipid profile in healthy adults were included. Animal studies, studies on unhealthy individuals, study designs other than clinical trial, studies that investigated the effect of TFA other than CLA and studies that investigated outcomes other than lipid profile were excluded. Inappropriate forms of CLA, such as CLA plus n-3 fatty acid, CLA plus amino acid, CLA plus chromium picolinate, CLA plus creatine monohydrate or CLA plus exercise were excluded because these forms did not permit us to isolate the precise effect of CLA. Articles without complete data or placebo and articles on participants with metabolic and genetic disorders were excluded. Title and abstract of papers were screened and relevant papers were selected. Then, full texts of relevant papers were read and findings were re-screened. A flowchart of the literature search is shown in Fig. 1.
Table 1.
Search strategy for PubMed, Cochrane Library, Google Scholar, Scopus and Science Direct databases
| No. | |
|---|---|
| 1 | ‘trans-10, cis-12-conjugated linoleic acid’ (Supplementary Concept) OR ‘cis-9, trans-11-conjugated linoleic acid’ (Supplementary Concept) OR ‘CLA fatty acid’ (Supplementary Concept) OR ‘CLA’ (tiab) OR ‘conjugated linoleic acid’ (tiab) OR ‘trans fatty acid’ (tiab) OR ‘TFA’ (tiab) |
| 2 | ‘Triglycerides’ (MeSH) OR ‘lipoprotein triglyceride’ (tiab) OR ‘Lipoproteins, HDL’ (MeSH) OR ‘Cholesterol, HDL’ (MeSH) OR ‘Cholesterol, LDL’ (MeSH) OR ‘Lipoproteins, LDL’ (MeSH) OR ‘LDL’ (tiab) OR ‘HDL’ (tiab) OR ‘Total cholesterol’ (tiab) OR ‘TG’ (tiab) OR ‘triglyceride’ (tiab) OR ‘triacylglycerol’ (tiab) OR ‘TAG’ (tiab) OR ‘lipid profile’ (tiab) OR ‘low density lipoprotein’ (tiab) OR ‘high density lipoprotein’ (tiab) |
| 3 | 1 AND 2 |
Fig. 1.
Flowchart of the literature search
Relevant papers were selected according to the title and abstract by three authors (S.-M.D.-R. and M.H.-B., R.K.). Two independent reviewers (S.-M.D.-R. and M.H.-B.) screened papers and read full texts of relevant papers. They assessed full texts for inclusion criteria and extracted data. Statistical analysis was done (M.M.) and cases of disagreement were resolved in consultation with a fourth arbitrating investigator (R.K.). Summaries of the clinical trials that investigated the association of CLA supplementation and foods enriched in CLA with lipid profile in human studies are shown in Tables 2 and 3, respectively.
Table 2.
Summary of clinical trials on the association of conjugated linoleic acid (CLA) supplementation and lipid profile in human studies
| Age (years) | Duration | CLA dose and | Placebo dose and form | |||||
|---|---|---|---|---|---|---|---|---|
| Reference | Population | Mean | SD | (weeks) | form (g/d) | Isomers | (g/d) | Results |
| Iwata et al. (2007)( 57 ) | Sixty males, healthy overweight and obese | 41·5 | 9·6 | 12 | 3·4 g/d, CLA-TAG 6·8 g/d, CLA-TAG | c9,t11–t10,c12 (50:50) | 10·8 g/d, high-linoleic safflower oil | TAG, HDL-C, LDL-C and TC levels did not change significantly among three groups |
| Watras et al. (2007)( 51 ) | Forty males and females, healthy overweight | 33 | 7·5 | 24 | 3·2 g/d, CLA-mix | c9,t11–t10,c12 (39·2:38·5) | 4 g/d, safflower oil | No significant changes in TC, LDL-C, HDL-C or TAG concentrations were observed between groups |
| Gaullier et al. (2004)( 47 ) | 180 males and females, healthy overweight | 45·83 | 10·3 | 48 | 3·4 g/d, CLA-TAG 3·6 g/d, CLA-NEFA | c9,t11–t10,c12 (50:50) c9,t11–t10,c12 (50:50) | 4·5 g/d, olive oil | No effect on TC or TAG concentrations; CLA-TAG group had lower HDL-C concentrations and CLA-NEFA group had higher LDL-C concentrations than at baseline of the study |
| Gaullier et al. (2005)( 32 ) | 134 males and females, healthy overweight | 46·26 | 9·96 | 96 | 3·4 g/d, CLA-TAG 3·4 g/d, CLA-NEFA | c9,t11–t10,c12 (50:50) c9,t11–t10,c12 (50:50) | 3·4 g/d, placebo | Plasma TC and LDL-C were reduced, whereas HDL-C and TAG were unchanged |
| Blankson et al. (2000)( 13 ) | Sixty males and females, healthy overweight and obese | 44·35 | 12·95 | 12 | 1·7 g/d, CLA-TAG 3·4 g/d, CLA-TAG 5·1 g/d, CLA-TAG 6·8 g/d, CLA-TAG | c9,t11–t10,c12 (50:50) | 9 g/d, olive oil | No significant differences were observed in blood lipids among the groups |
| Steck et al. (2007)( 33 ) | Forty-eight males and females, healthy obese | 34·50 | 4·85 | 12 | 3·2 g/d, CLA-TAG 6·4 g/d, CLA-TAG | c9,t11–t10,c12 (50:50) | 8 g/d, safflower oil | HDL-C decreased significantly in placebo and 6·4 g CLA/d groups; other clinical laboratory values did not change across all groups |
| Noone et al. (2002)( 23 ) | Fifty-one males and females, healthy normal-weight and overweight | 31·37 | 6·31 | 8 | 3 g/d, CLA-TAG 3 g/d, CLA-TAG | c9,t11–t10,c12 (50:50) c9,t11–t10,c12 (80:20) | 3 g/d, linoleic acid | Plasma TAG concentrations were significantly decreased in the 50:50 CLA supplement group but not in the 80:20 CLA or control groups; TC had no changes in all supplementation groups; HDL-C concentrations increased non-significantly in the control group; LDL-C concentrations decreased non-significantly in both CLA supplementation groups |
| Lambert et al. (2007)( 12 ) | Sixty-two males and females, healthy regularly exercising non-obese | 32 | 7 | 12 | 3·9 g/d, CLA-TAG | c9,t11–t10,c12–other isomers(29·7:30·9:2·9) | 3·9 g/d, high-oleic-acid sunflower oil | TC and LDL-C reduced significantly in both genders; HDL-C decreased significantly only in women; TAG did not change significantly |
| Gaullier et al. (2007)( 31 ) | 118 males and females, healthy overweight and obese | 47·25 | 9·6 | 24 | 3·4 g/d, CLA-TAG | c9,t11–t10,c12 (37·5:38·0) | 4·5 g/d, olive oil | HDL-C decreased slightly in the CLA group; other blood lipids were not significantly changed in either group |
| Berven et al. (2000)( 14 ) | Sixty males and females, healthy overweight and obese | 47·05 | 3·9 | 12 | 3·4 g/d, CLA-TAG | c9,t11–t10,c12 (50:50) | 4·5 g/d, olive oil | No significant changes were observed in blood lipid parameters |
| Mougios et al. (2001)( 15 ) | Twenty-four males and females, healthy normal-weight and overweight | 22·2 | 1·5 | 4–8 | (0·7–1·4) g/d, CLA-mix | c9,t11–t10,c12 (50:50) | 0·7–1·4 g/d, soyabean oil | HDL-C significantly reduced in all groups of CLA; TAG and TC tended to decrease in the CLA group during the low CLA intake but not during the high CLA intake |
| Petridou et al. (2003)( 16 ) | Sixteen females, healthy sedentary normal-weight and overweight | 22·30 | 1·80 | 6·5 | 2·1 g/d, CLA-mix | c9,t11–t10,c12 (50:50) | 2·1 g/d, soyabean oil | CLA supplementation had no significant effect on TAG, TC, HDL-C and TC:HDL-C |
| Kamphuis et al. (2003)( 19 ) | Sixty males and females, healthy overweight | 35·1 | 8·35 | 13 | 1·8 g/d, CLA-TAG 3·6 g/d, CLA-TAG | c9,t11–t10,c12 (50:50) | 1·8 g/d, oleic acid 3·6 g/d, oleic acid | CLA supplementation did not have any significant effect on plasma TAG concentrations |
| Pfeuffer et al. (2011)( 21 ) | Eighty-five males, healthy overweight and obese | 45–68 | – | 4 | 3·4 g/d, CLA-TAG | c9,t11–t10,c12 (50:50) | 4·5 g/d, safflower oil | CLA decreased TC and LDL-C concentrations not significantly more than safflower oil. HDL-C, fasting and postprandial TAG did not change |
| Colakoglu et al. (2006)( 29 ) | Forty-four females, healthy exercising normal-weight | 21·15 | 1·85 | 6 | 3·6 g/d, CLA-mix | c9,t11–t10,c12 | Control | CLA supplementation with or without exercise did not change serum lipid profile (TC, LDL-C, HDL-C, TAG) |
| Benito et al. (2001)( 58 ) | Seventeen females, healthy normal-weight | 28·15 | 6·2 | 9 | 3·9 g/d, CLA-TAG | c9,t11–t10,c12–c11,t13–t8,c10–cc–tt (11·4:14·7:15·3:10·8:6·74:5·99) | 3·9 g/d, high-linoleic sunflower oil | CLA supplementation did not change the levels of plasma TC, LDL-C, HDL-C and TAG |
| Tavakoli-Darestani et al.( 59 ) | Seventy-six females, healthy menopausal overweight women | 55 | 6·65 | 12 | 3·2 g/d, CLA-TAG | c9,t11–t10,c12 (50:50) | 4 g/d, high-oleic-acid sunflower oil | CLA supplementation had no significant effect on TC, TAG, LDL-C and HDL-C |
| Risérus et al. (2004)( 11 ) | Twenty-five males, healthy overweight and obese | 55 | 5·75 | 12 | 3 g/d, CLA-TAG | c9,t11–t10,c12–c9,c11–c10,c12–t9,t11+t10,t12 (83·3:7·3:0·46:0·2:1·4) | 3 g/d, olive oil | CLA had no significant effects on lipoprotein or TAG concentrations compared with placebo |
| Sluijs et al. (2010)( 18 ) | 401 males and females, healthy overweight and obese | 58·4 | 0·45 | 24 | 3·1 g/d, CLA | c9,t11–t10,c12 (80:20) | 4 g/d, 80 % palm oil + 20 % soyabean oil | There was no effect of CLA supplementation on concentrations of lipids such as TAG, LDL-C, HDL-C and TC |
| Whigham et al. (2004)( 20 ) | Sixty-four males and females, healthy overweight and obese | 42·3 | 5·35 | 24 | 6 g/d, CLA-TAG | c9,t11–t10,c12–tt (37·3:37·6:1·3) | 7·5 g/d, high-oleic acid sunflower oil | CLA increased TAG. Other lipids did not change |
| Song et al. (2005)( 54 ) | Twenty-eight males and females, healthy normal-weight | 31·35 | 7·01 | 12 | 3 g/d, CLA-TAG | c9,t11–t10,c12 (50:50) | 3 g/d, high-oleic-acid sunflower oil | CLA supplementation did not change TC level. HDL-C level decreased significantly after 12 weeks of supplementation. LDL-C did not alter. Plasma TAG levels were increased in the two groups, however; significantly in the CLA group |
| Taylor et al. (2006)( 52 ) | Forty males, healthy overweight and obese | 46 | 7 | 12 | 4·5 g/d, CLA-mix | c9,t11–t10,c12 (35:36) c9,c11–c10,c12 (1–2 %) t9,t11–t10,t11 (1·5 %) t8,c10–c11,t13 (<1 %) | 4·5 g/d, olive oil | There was no change in TC, TAG, LDL-C and HDL-C |
| Smedman and Vessby (2001)( 53 ) | Fifty-three males and females, healthy | 45·2 | 11·65 | 12 | 4·2 g/d, CLA-mix | c9,t11–t10,c12 (50:50) | 4·2 g/d, olive oil | TC, LDL-C, HDL-C increased and TAG decreased |
| Kim (2008)( 48 ) | Fifty-one females, healthy overweight Korean women | 28·24 | 20·39 | 12 | 2·25 g/d, CLA-NEFA 2·25 g/d, CLA-TAG | c9,t11-CLA–t10,c12-CLA–c9,c11-CLA–t9,t11-CLA (37·95:38·84:0·96:1·35) c9,t11–CLA–t10,c12–CLA–c9,c11-CLA–t9,t11-CLA (37·83:38·55:0·98:1·86) | 3 g/d, olive oil | No significant changes were observed within and between treatment groups in blood lipid parameters (TAG, TC, LDL-C or HDL-C) |
| Tholstrup et al. (2008)( 35 ) | Seventy-five females, healthy postmenopausal women | 60·16 | 4·46 | 16 | 4·6 g/d, CLA-mix 5·1 g/d, CLA-TAG | c9,t11–t10,c12–other CLA (41·17:39·90:1·79) c9,t11–t10,c12–other CLA (85·03:7·11:0·47) | 5·5 g/d, olive oil | CLA mixture decreased HDL-C, increased TC:HDL-C compared with other groups and increased TAG levels compared with control. Plasma LDL-C concentrations did not differ among the three groups |
| Sahin et al. (2008)( 39 ) | Twenty females, healthy overweight or obese premenopausal | 22–48 (range) | 8 | 1·8 g/d, CLA-NEFA | c9,t11–t10,c12 (80–84:37–42) | Without placebo | CLA reduced TC, TAG and LDL-C significantly and non-significantly increased HDL-C level | |
| Tricon et al. (2004)( 41 ) | Forty-nine males, healthy normal-weight | 30·95 | 1·7 | 8 | 0·59, 1·19, 2·38 g/d, CLA-TAG 0·63, 1·26, 2·52 g/d, CLA-TAG | c9,t11–t10,c12 (79·3:7·8) c9,t11–t10,c12 (10·6:84·1) | Without placebo | CLA supplementation had significant effects on TC, LDL-C and no effect on HDL-C in all isomers and doses |
| von Loeffelholz et al. (2003)( 40 ) | Fourteen males and females, healthy bodybuilders | 26 | 4 | 24 | 3·78 g/d, CLA-TAG | c9,t11–t10,c12–t8,c10–c11,t13–cc–tt (8·3:7·9:6·0:7·1:4·7:17·7) | Without placebo | CLA supplementation significantly increased LDL-C and TC concentration only in the beginners, not in the advanced athletes, and had no significant effect on TAG or HDL-C levels in either intervention group |
| Albers et al. (2003)( 36 ) | Seventy-one men, healthy overweight or obese | 52·33 | 9 | 12 | 1·7 g/d, CLA-NEFA 1·6 g/d, CLA-TAG | c9,t11–t10,c12 (50:50) c9,t11–t10,c12 (80:20) | Sunflower oil fatty acids | CLA supplementation did not affect fasting serum lipids |
Table 3.
Summary of clinical trials on the association of enriched foods with conjugated linoleic acid (CLA) and lipid profile in human studies
| Age (years) | Duration | Placebo form and | ||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Population | Mean | SD | (weeks) | CLA form and dose | Isomers | dose | Results |
| Desroches et al. (2005)( 27 ) | Sixteen males, healthy overweight and obese | 36·6 | 12·4 | 8 | Butter–CLA (4·22 g CLA/100 g fat) | c9,t11–other isomers (80:20) | Butter (0·38 g CLA/100 g fat) | Butter–CLA diet reduced TC significantly more than control. LDL-C, HDL-C and TAG levels did not change significantly between the two groups |
| Tricon et al. (2006)( 55 ) | Thirty-two males, healthy | 45·5 | 8·7 | 6 | (Butter+cheese+ milk)–CLA (1·421 g CLA/d) | c9,t11 | Butter+ cheese+milk (0·151 g CLA/d) | Dairy products enriched with CLA did not significantly affect TAG, TC, LDL-C and HDL-C. They slightly increased LDL-C:HDL-C |
| Wanders et al. (2010)(34) | Sixty-one males and females, healthy normal weight | 30·9 | 13·7 | 9 | (Margarine+ yoghurt drinks)–CLA (73·7 (sd 0·6) g CLA/100 g fat) | c9,t11–t10,c12 (80:20) | (Margarine+ yoghurt drinks)–oleic acid | TAG level did not change, LDL-C and TC:HDL-C increased, whereas HDL-C decreased in CLA group compared to control |
| Brown et al. (2011)( 26 ) | Eighteen females, healthy normal-weight and overweight | 20–40 (range) | 8 | Beef+dairy (ice cream, cheese, butter)–CLA (1·17 g CLA/d) | c9,t11–other isomers (87·5:12·5) | Beef+dairy (ice cream, cheese, butter) (0·35 g CLA/d) | No significant differences were observed in TC, TAG, LDL-C, HDL-C levels between treatment groups | |
| Sofi et al. (2010)( 24 ) | Ten males and females, healthy normal-weight and over weight | 51·5 | 20 | Pecorino cheese (1·56 g CLA/100 g lipid) | c9,t11 | Placebo cheese (0·19 g CLA/100 g lipid) | TC, TAG, LDL-C and HDL-C did not change during either intervention phases | |
| Raff et al. (2008)( 30 ) | Thirty-eight males, healthy normal-weight | 25·9 | 3·9 | 5 | Butter–CLA (4·6 g/d CLA) | c9,t11–t10,c12 (39·4:38·5) | Butter (0·3 g CLA/d) | TC, TAG, LDL-C, HDL-C and TC:HDL-C did not differ during either intervention phase |
| Chen et al. (2012)( 25 ) | Eighty males and females, healthy overweight and obese | 32·8 | 0·8 | 12 | Milk–CLA (1·7 g/d CLA) | c9,t11–t10,c12 (50:50) | Milk | CLA treatment increased levels of TC, TAG and LDL-C, decreased HDL-C concentration. None of these changes were significant |
| Naumann et al. (2006)( 17 ) | Ninety-two males and females, healthy overweight and obese with LDL phenotype B | 52·33 | 7·66 | 13 | Drinkable dairy product–CLA (3 g CLA/d) Drinkable dairy product –CLA (3 g CLA/d) | c9,t11–t10,c12 (>80:<5) t10,c12–c9,t11 (>80:<5) | Drinkable dairy product (3 g high-oleic-acid sunflower oil/d) | LDL-C, HDL-C, TAG, TC:HDL-C, LDL-C:HDL-C did not change in CLA-enriched groups |
| Laso et al. (2007)( 22 ) | Sixty males and females, healthy overweight and obese | 53·85 | 7·73 | 12 | Skimmed milk–CLA (3 g CLA/d) | c9,t11–t10,c12 | Skimmed milk | Plasma TAG, TC and LDL-C increased slightly in all CLA groups, however these changes were not significant |
| Nazare et al. (2007)( 56 ) | Forty-four males and females, healthy normal-weight and overweight | 28·9 | 1·14 | 14 | Yoghurt–CLA (3·76 g CLA/d) | c9,t11–t10,c12–tt (35:35:<1) | Yoghurt | CLA-enriched yoghurt did not alter any of the TAG, TC and HDL-C concentrations |
Data extraction
Data of thirty-three articles that investigated the effect of CLA intake in either supplement form or enriched foods on lipid profile in healthy adult populations were entered into meta-analysis. Mean and standard deviation for TC, HDL-C, LDL-C and TAG before and after placebo or CLA consumption were extracted. Data from the following studies were not extracted: four studies without complete data for analysis( 35 – 38 ), four studies without a placebo group( 32 , 39 – 41 ), one study that considered special polymorphisms (PPARγ2, Pro12Ala) of healthy adults( 42 ), one study done on adolescents( 43 ) and participants of three studies had signs of metabolic syndrome or borderline hyperlipidaemia( 44 – 46 ). Complete information about excluded studies is shown in Fig. 1.
Two structural forms of CLA, i.e. TAG and NEFA, and two isomeric forms, i.e. cis-9, trans-11 isomer (c9,t11) and trans-10, cis-12 isomer (t10,c12), were used as intervention groups( 47 , 48 ). There were different proportions (approximately 50:50 or 80:20; all proportions stated in the paper are by weight) of these isomers and we extracted results of all of them( 17 , 23 ). Two studies reported their results stratified by gender or BMI( 12 , 22 ). We entered their results into meta-analysis separately.
Statistical analysis
All outcomes were recorded as continuous variables, and the effect size was measured by analysis of the mean and standard deviation before and after the intervention for the case and control groups. Pooled meta-analyses were completed on studies that reported the same outcomes. The I 2 statistic was used to test for heterogeneity; if there was significant heterogeneity, the random-effects model was used. I 2 values of 25 %, 50 % and 75 % were used as evidence of low, moderate and high heterogeneity, respectively. Sensitivity analysis was done by successively removing a particular study that had the highest impact on the heterogeneity test. Comprehensive Meta-Analysis (CMA) software version 2 was used to carry out the data analysis. P values <0·05 were considered statistically significant. All reported P values resulted from two-sided versions of the respective tests. Potential publication bias was evaluated by Egger’s regression test( 49 ). The trim and fill method was used to assess the potential effect of any publication bias on the meta-analysis results( 50 ).
Results
Conjugated linoleic acid supplementation and LDL cholesterol
The summary mean difference and 95 % confidence interval for all fifteen clinical trial studies that investigated the effects of CLA supplementation on LDL-C are shown in Fig. 2. Heterogeneity among studies was significant (I 2=52 %; P heterogeneity=0·040). The clinical trial studies( 11 , 13 , 18 , 21 , 29 , 51 – 53 ) contributed most to heterogeneity. In an analysis excluding these studies, CLA supplementation led to a significant decrease in LDL-C level (mean difference=−0·218; 95 % CI −0·358, −0·077; P=0·002); the test for heterogeneity was not statistically significant (I 2=0 %; P heterogeneity=0·934). Publication bias was not significant (Egger’s test P value=0·17).
Fig. 2.
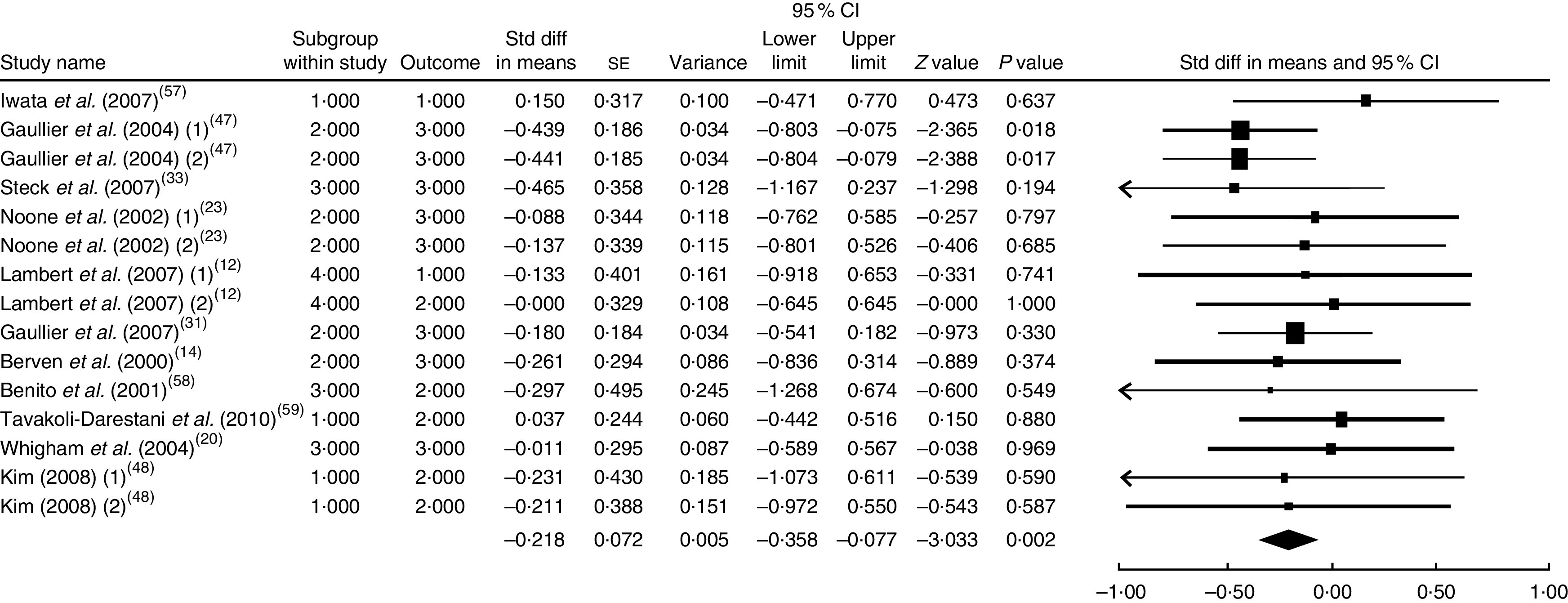
Meta-analysis of the effect of conjugated linoleic acid supplementation on LDL cholesterol in published clinical trials. The study-specific standardized difference (Std diff) in means and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the black diamond presents the pooled standardized difference in means and its width represents the pooled 95 % CI
Conjugated linoleic acid supplementation and HDL cholesterol
The summary mean difference and 95 % confidence interval for all seventeen clinical trial studies that investigated the effects of CLA supplementation on HDL-C are shown in Fig. 3. Heterogeneity among studies was significant (I 2=50 %; P heterogeneity=0·030). The clinical trial studies( 11 , 13 , 18 , 21 , 29 , 51 – 53 ) contributed most to heterogeneity. In an analysis excluding these studies, CLA supplementation led to a slight and non-significant decrease in HDL-C level (mean difference=−0·051; 95 % CI −0·188, 0·086; P=0·468); the test for heterogeneity was not statistically significant (I 2=0 %; P heterogeneity=0·649). Publication bias was not significant (Egger’s test P value=0·94).
Fig. 3.
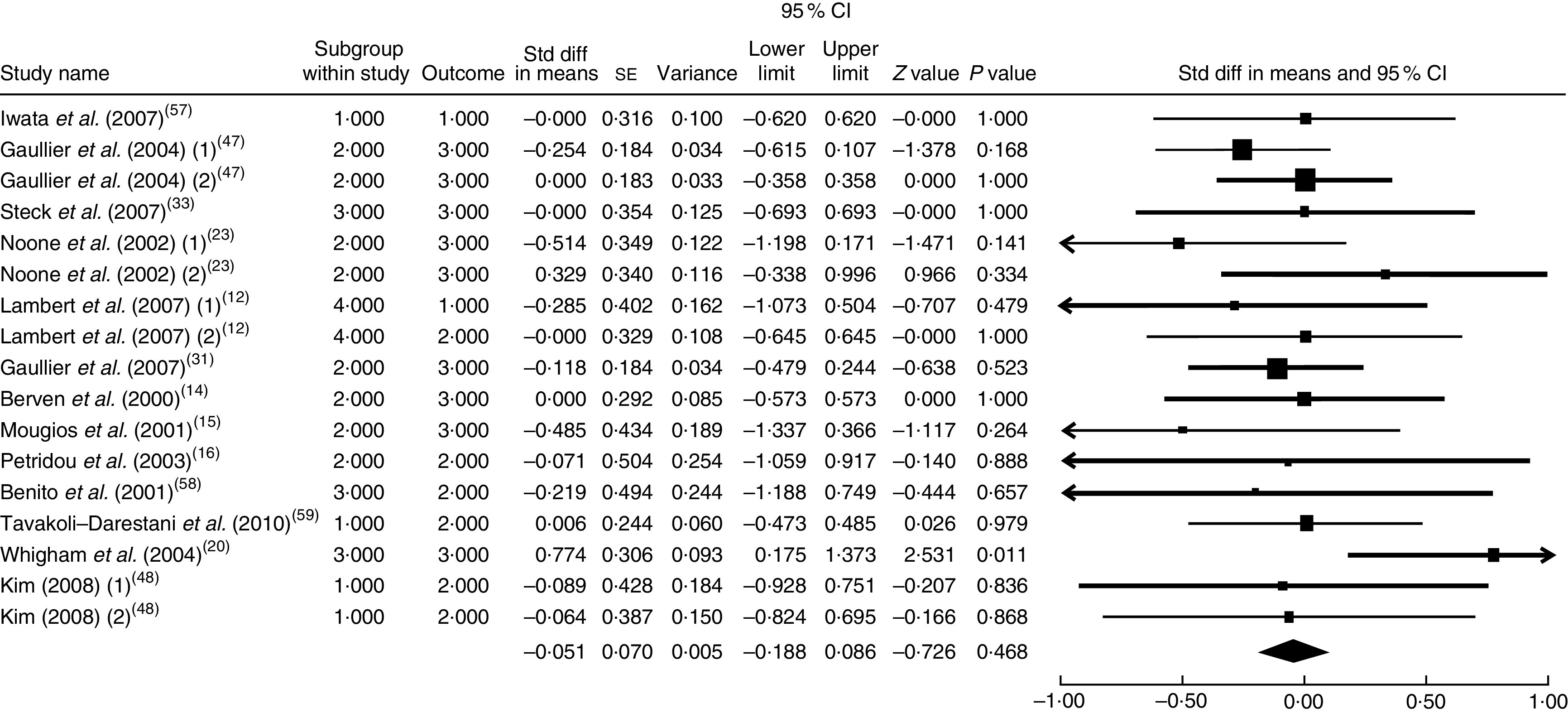
Meta-analysis of the effect of conjugated linoleic acid supplementation on HDL cholesterol in published clinical trials. The study-specific standardized difference (Std diff) in means and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the black diamond presents the pooled standardized difference in means and its width represents the pooled 95 % CI
Conjugated linoleic acid supplementation and total cholesterol
The summary mean difference and 95 % confidence interval for all seventeen clinical trial studies that investigated the effects of CLA supplementation on TC are shown in Fig. 4. Heterogeneity among studies was significant (I 2=55 %; P heterogeneity=0·034). The clinical trial studies( 11 , 13 , 18 , 21 , 29 , 51 – 54 ) contributed most to heterogeneity. In an analysis excluding these studies, CLA supplementation led to a slight and non-significant increase in TC level (mean difference=0·009; 95 % CI −0·128, 0·146; P=0·896); the test for heterogeneity was not statistically significant (I 2=0 %; P heterogeneity=0·956). Since publication bias existed, we tried to evaluate the effect of publication bias by the trim and fill method. After eliminating the effect of publication bias, the combined mean difference was 0·0089 (95 % CI −0·125, 0·152), which remained consistent with previous results.
Fig. 4.
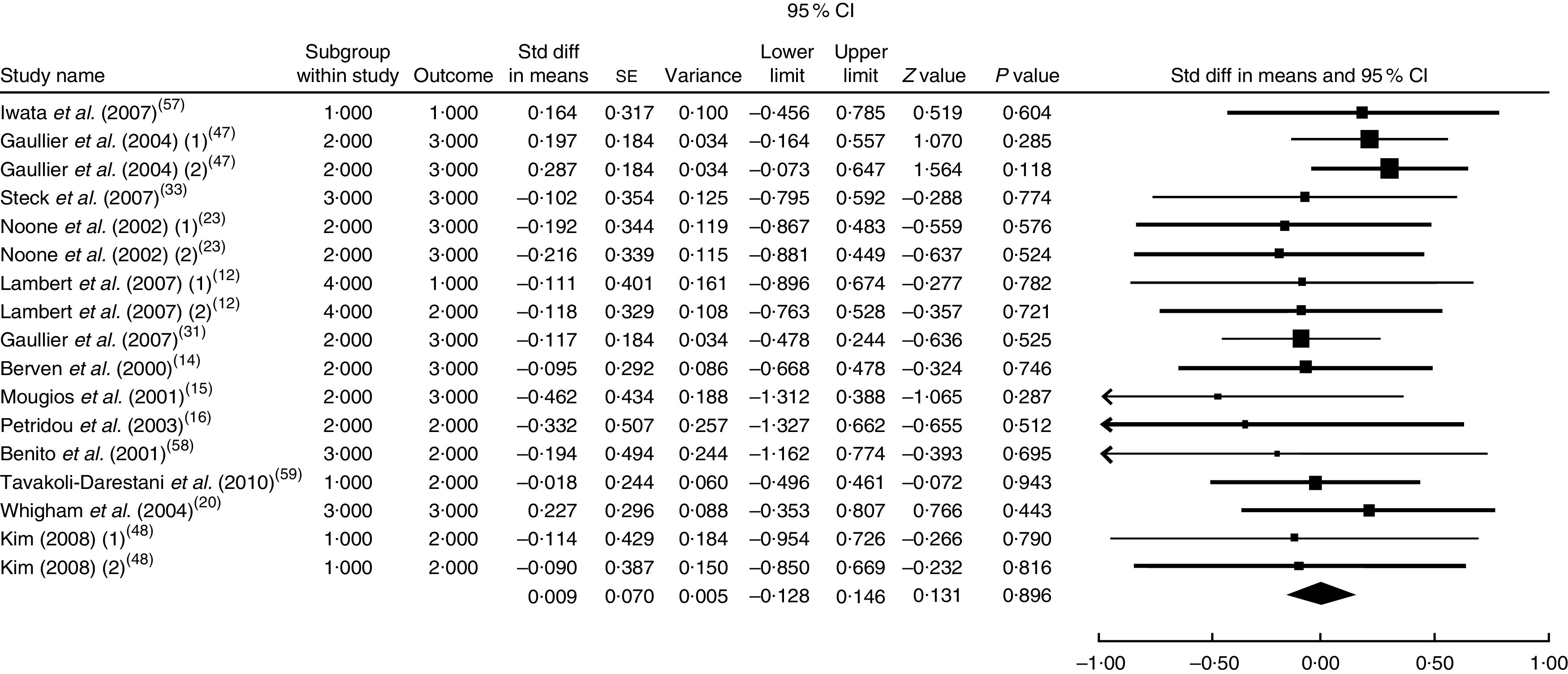
Meta-analysis of the effect of conjugated linoleic acid supplementation on total cholesterol in published clinical trials. The study-specific standardized difference (Std diff) in means and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the black diamond presents the pooled standardized difference in means and its width represents the pooled 95 % CI
Conjugated linoleic acid supplementation and TAG
The summary mean difference and 95 % confidence interval for all eighteen clinical trial studies that investigated the effects of CLA supplementation on TAG are shown in Fig. 5. Heterogeneity among studies was significant (I 2=54 %; P heterogeneity=0·041). The clinical trial studies( 11 , 13 , 18 , 21 , 29 , 51 – 53 ) contributed most to heterogeneity. In an analysis excluding these studies, CLA supplementation led to a non-significant decrease in TAG level (mean difference=−0·065; 95 % CI −0·200, 0·070; P=0·344) and the test for heterogeneity was not statistically significant (I 2=0 %; P heterogeneity=0·954). Publication bias was not significant (Egger’s test P value=0·08).
Fig. 5.
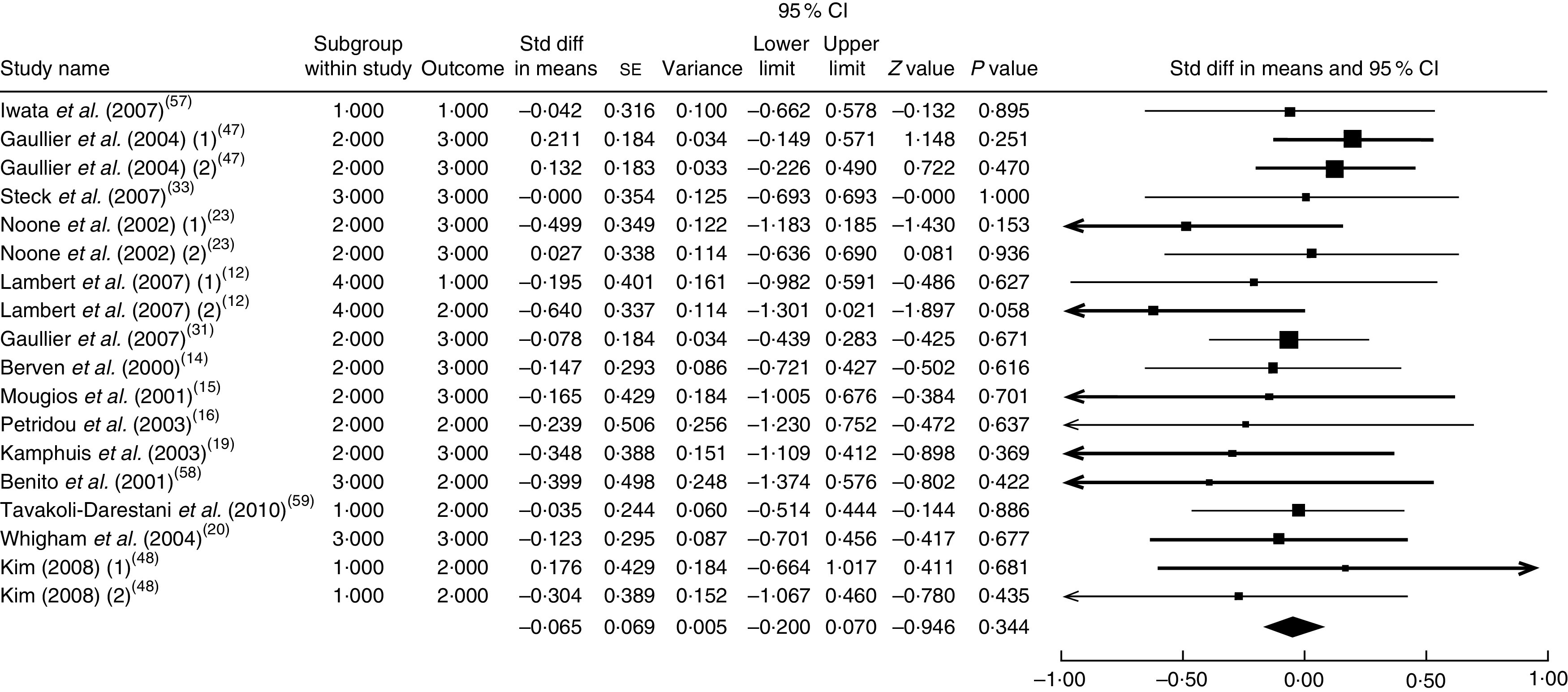
Meta-analysis of the effect of conjugated linoleic acid supplementation on TAG in published clinical trials. The study-specific standardized difference (Std diff) in means and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the black diamond presents the pooled standardized difference in means and its width represents the pooled 95 % CI
Foods enriched in conjugated linoleic acid and LDL cholesterol
The summary mean difference and 95 % confidence interval for all ten clinical trial studies that investigated the effects of foods enriched in CLA on LDL-C are shown in Fig. 6. Heterogeneity among studies was significant (I 2=51 %; P heterogeneity=0·023). One clinical trial study( 26 ) contributed most to heterogeneity. In an analysis excluding that study, we found that foods enriched in CLA led to a significant decrease in LDL-C level (mean difference=−0·231; 95 % CI −0·438, −0·024; P=0·028); the test for heterogeneity was not statistically significant (I 2=1 %; P heterogeneity=0·965). Publication bias was not significant (Egger’s test P value=0·18).
Fig. 6.
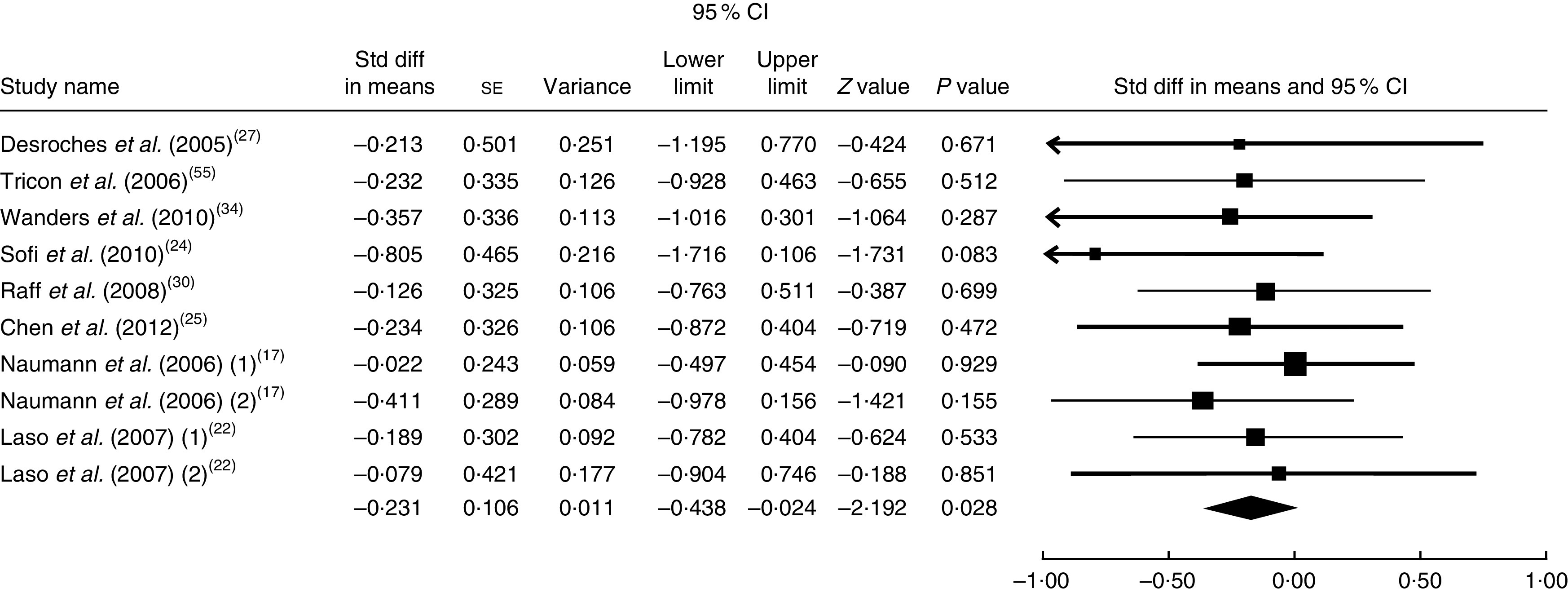
Meta-analysis of the effect of natural foods enriched with conjugated linoleic acid on LDL cholesterol in published clinical trials. The study-specific standardized difference (Std diff) in means and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the black diamond presents the pooled standardized difference in means and its width represents the pooled 95 % CI
Foods enriched in conjugated linoleic acid and HDL cholesterol
The summary mean difference and 95 % confidence interval for all eleven clinical trial studies that investigated the effects of foods enriched in CLA on HDL-C are shown in Fig. 7. Heterogeneity among studies was significant (I 2=50 %; P heterogeneity=0·045). One clinical trial study( 26 ) contributed most to heterogeneity. In an analysis excluding that study, foods enriched in CLA led to a non-significant increase in HDL-C level (mean difference=0·075; 95 % CI −0·121, 0·270; P=0·455) and the test for heterogeneity was not statistically significant (I 2=19 %; P heterogeneity=0·262). Publication bias was not significant (Egger’s test P value=0·07).
Fig. 7.
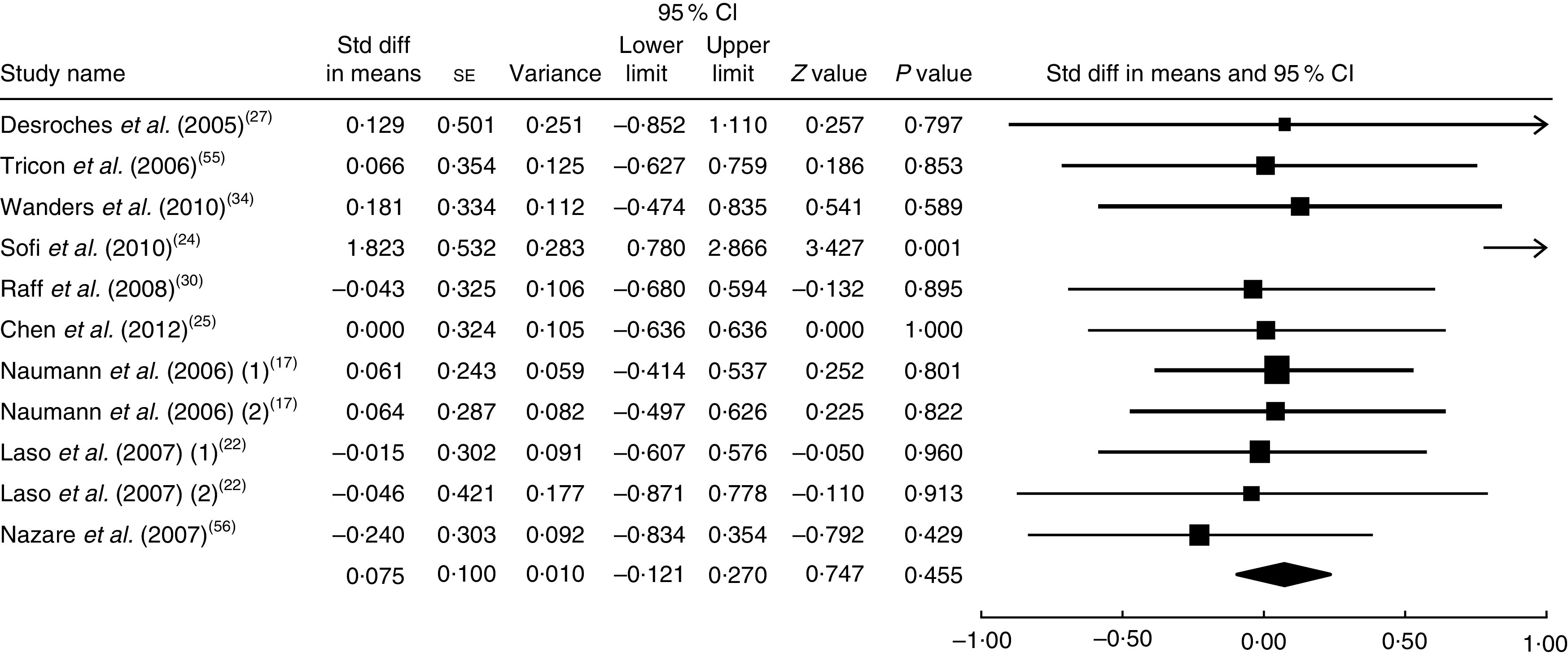
Meta-analysis of the effect of natural foods enriched with conjugated linoleic acid on HDL cholesterol in published clinical trials. The study-specific standardized difference (Std diff) in means and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the black diamond presents the pooled standardized difference in means and its width represents the pooled 95 % CI
Foods enriched in conjugated linoleic acid and total cholesterol
The summary mean difference and 95 % confidence interval for all eleven clinical trial studies that investigated the effects of foods enriched in CLA on TC are shown in Fig. 8. Heterogeneity among studies was significant (I 2=58 %; P heterogeneity=0·018). One clinical trial study( 26 ) contributed most to heterogeneity. In an analysis excluding that study, we found that foods enriched in CLA led to a non-significant decrease in TC level (mean difference=−0·158; 95 % CI −0·349, 0·042; P=0·124) and the test for heterogeneity was not statistically significant (I 2=10 %; P heterogeneity=0·345). Publication bias was not significant (Egger’s test P value=0·84).
Fig. 8.
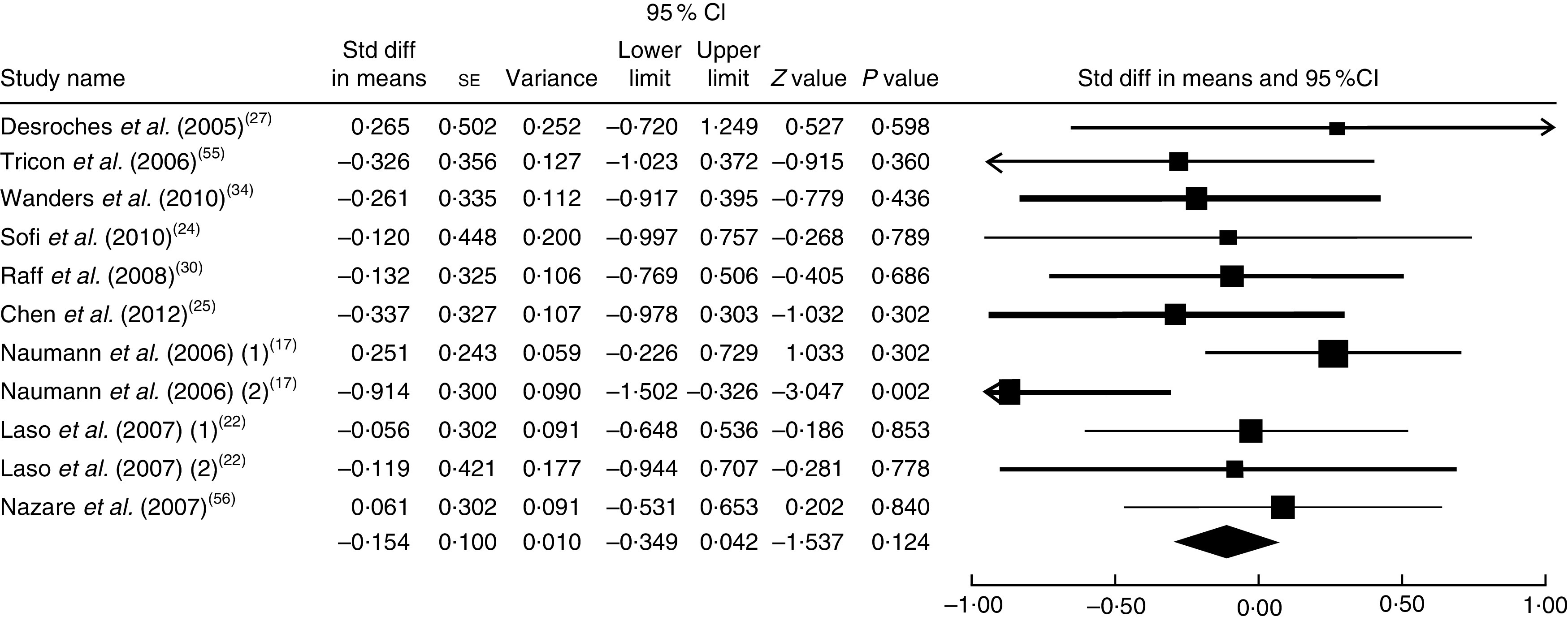
Meta-analysis of the effect of natural foods enriched with conjugated linoleic acid on total cholesterol in published clinical trials. The study-specific standardized difference (Std diff) in means and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the black diamond presents the pooled standardized difference in means and its width represents the pooled 95 % CI
Foods enriched in conjugated linoleic acid and TAG
The summary mean difference and 95 % confidence interval for all eleven clinical trial studies that investigated the effects of foods enriched in CLA on TAG are shown in Fig. 9. Heterogeneity among studies was significant (I 2=56 %; P heterogeneity=0·033). One clinical trial study( 26 ) contributed most to heterogeneity. In an analysis excluding that study, we documented that foods enriched in CLA led to a non-significant decrease in TAG level (mean difference=−0·078; 95 % CI −0·274, 0·117; P=0·433); the test for heterogeneity was not statistically significant (I 2=6 %; P heterogeneity=0·384). Publication bias was not significant (Egger’s test P value=0·71).
Fig. 9.
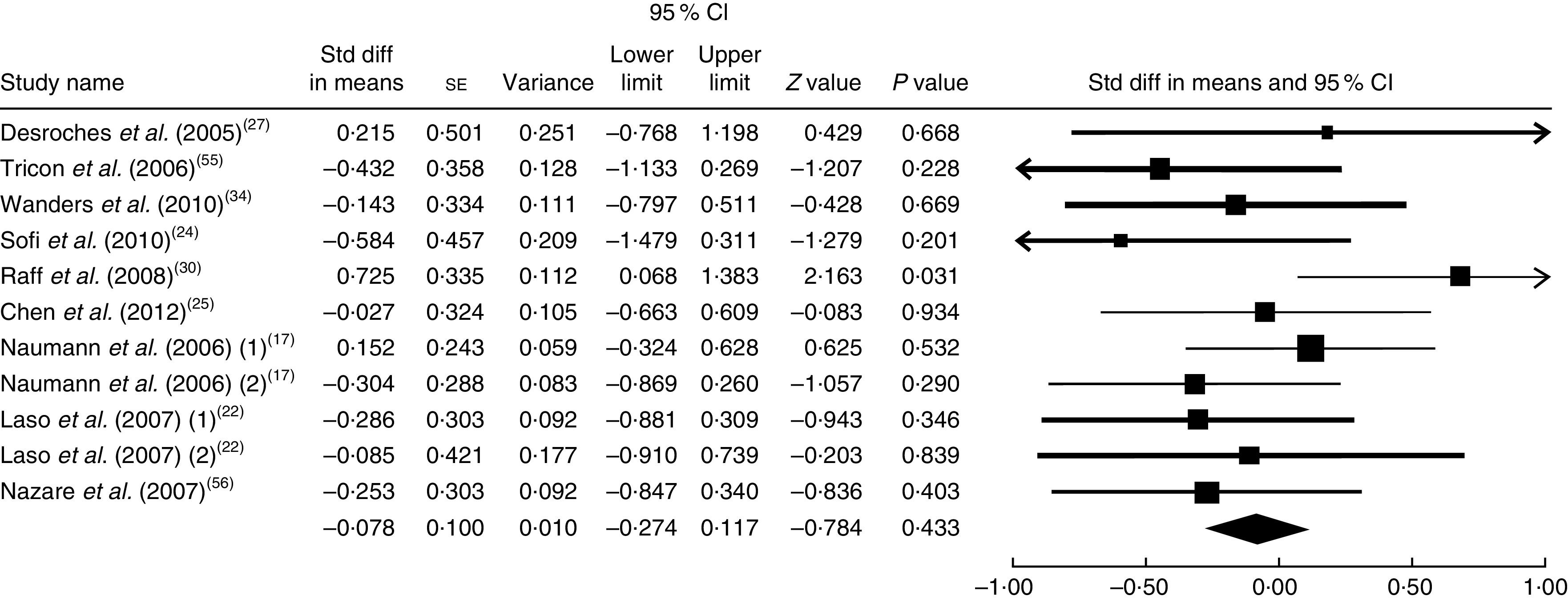
Meta-analysis of the effect of natural foods enriched with conjugated linoleic acid on TAG in published clinical trials. The study-specific standardized difference (Std diff) in means and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the black diamond presents the pooled standardized difference in means and its width represents the pooled 95 % CI
Sensitivity analyses
To identify the source of the heterogeneity between studies, we performed sensitivity analyses by including and excluding some studies. Sensitivity analyses were done sequentially for all of the lipids and all of the studies. In a sensitivity analysis excluding one study at a time, we consistently found statistically the same results. Ranges of summary mean differences were (−0·242, −0·178), (−0·097, −0·016), (0·001, 0·017) and (−0·110, −0·040) for the effect of CLA supplementation on LDL-C, HDL-C, TC and TAG, respectively. Also the sensitivity analysis results based on the effect of enriched foods with CLA for different lipids according to summary mean differences were (−0·280, −0·200), (−0·082, −0·641), (−0·460, −0·222) and (−0·156, −0·047) for LDL-C, HDL-C, TC and TAG, respectively.
Discussion
The present meta-analysis is the first quantitative review of thirty-three randomized controlled clinical studies investigating the effect of CLA supplements and foods enriched in CLA on serum lipids separately. Our meta-analysis showed that intake of foods enriched in CLA decreased LDL-C levels significantly, decreased TC and TAG concentrations non-significantly and increased HDL-C levels non-significantly. CLA supplements decreased LDL-C, HDL-C and TAG levels and increased TC level; however, only the effect on LDL-C level was statistically significant. According to our analysis, consumption of foods enriched in CLA and CLA supplements has favourable effects on LDL-C level.
Some studies, in agreement with our results, showed that a mixture of CLA isomers decreased LDL-C level significantly in healthy adults( 12 , 34 , 39 , 41 ). Noone et al.( 23 ) observed that the daily intake of 3 g CLA supplement (50:50 and 80:20) decreased LDL-C levels non-significantly in CLA groups. However, von Loeffelholz( 40 ) claimed that CLA supplementation for 6 months increased LDL-C and TC concentrations significantly. Some studies showed that CLA supplementation( 47 , 53 ) or foods enriched in CLA( 22 , 25 ) led to a slight, non-significant increase in LDL-C level.
We found that TC level decreased and HDL-C level increased non-significantly after intake of foods enriched in CLA and our findings are in accordance with other studies( 17 , 22 , 27 ). According to our meta-analysis, CLA supplementation led to an adverse non-significant effect on TC or HDL-C level, which is in agreement with some studies on CLA supplements( 12 , 15 , 31 , 33 , 35 , 40 , 47 , 53 , 54 ) and is in disagreement with other studies( 21 , 39 , 41 ).
Our analysis showed that TAG level decreased non-significantly after intake of either CLA supplements or CLA-enriched foods, similar to previous studies on either enriched foods or CLA supplements. Some findings suggested that CLA had no significant effect on TAG concentration( 11 – 19 , 21 , 22 , 24 – 27 , 29 – 34 , 36 , 40 , 47 – 53 , 55 – 59 ). However, Chen et al.( 25 ) reported that TAG level increased in individuals who consumed foods enriched in CLA. Some trials reported a significant increase in TAG concentration after consuming CLA supplements( 20 , 35 , 41 , 54 ).
The proportion of CLA isomers and their dosage may be important to determine the effect of CLA on lipid profile. Noone et al.( 23 ) showed that CLA supplementation with the 50:50 proportions of cis-9, trans-11 and trans-10, cis-12 isomers caused a significant reduction in plasma TAG concentrations; however, this effect disappeared with the 80:20 proportion of CLA isomers. Mougios et al.( 15 ) investigated the effect of CLA capsules that included 0·7–1·4 g CLA mixture for 4–8 weeks. They showed that low-dose CLA intake decreased TAG and TC and high CLA intake did not change TAG and TC levels.
Findings from human studies that investigated the effects of CLA mixtures or cis-9, trans-11 and trans-10, cis-12 CLA isomers separately on lipid profile in either enriched foods or supplement forms are controversial. This may be related to differences in the CLA forms (TAG or NEFA), doses of CLA (0·59–6·8 g in supplement forms and 1·17–73·7 g in enriched foods), variation in isomers and their proportions, duration of studies (from 4 weeks to 2 years in supplement forms and from 5 weeks to 5 months in enriched foods), variation in subjects’ body weight and different control groups. As placebo, most of the studies used olive oil or oleic acid extracts; some of them used safflower oil, sunflower oil or linoleic acid extracts; and a few studies used soyabean oil solely or in combination with palm oil. Studies enriched different kinds of dairy products such as cheese, milk, yoghurt, butter and ice cream with CLA. Furthermore, the CLA content of milk and other dairy products ranged from 0·34 % to 1·07 % of total fat, which is influenced by the diet of cows. In European countries, where cows are traditionally pasture grazed, their milk contains higher CLA levels than in countries where cows are mainly fed corn, such as the USA. These can lead to different results in studies( 60 ).
The mechanism of lowering cholesterol level by CLA remains to be determined( 28 ). It was suggested that CLA could decrease LDL-C particles by forbidding the secretion of apo B or by increasing the clearance rate of circulating LDL-C through increasing activity of the LDL receptor( 61 , 62 ). According to evidence, dietary CLA enhances the fecal excretion of total neutral sterols( 63 ) and inhibits cholesterol absorption through down-regulation of intestinal acyl-CoA cholesterol acyltransferase( 28 ). CLA can decrease TAG level by inhibiting the expression and activity of hepatic stearoyl-CoA desaturase. This enzyme is involved in the desaturation of substrate for the synthesis of TAG( 64 ).
According to our meta-analysis, foods enriched in CLA and CLA supplements have beneficial effect on LDL-C concentration. CLA did not affect other lipids in the profile. Foods enriched in CLA increased HDL-C and tended to decrease TC non-significantly. Nutrients such as calcium, potassium, vitamin D and vitamin B, or bioactive peptides in dairy products, have been shown to be associated with beneficial outcomes. These nutrients and CLA can influence the lipid profile synergistically( 60 ).
There are some concerns about the potential safety of CLA for human subjects. Studies have shown that supplementation with CLA or trans-10, cis-12 isomer could induce insulin resistance, lipodystrophy in animals, fatty liver, C-reactive protein enhancement and undesirable changes in lipid profile in man( 65 , 66 ).
There is no consensus on the recommended dosage of CLA; however, according to evidence, 3 g/d seems to be most desirable. Consumption of CLA supplements is not recommended in pregnancy( 67 , 68 ).
Conclusion
The present review showed that both CLA supplements and foods enriched in CLA caused a significant reduction in LDL-C level. Foods enriched in CLA, in comparison with CLA supplementation, had a beneficial effect on the whole lipid profile although only the effect on LDL-C level was statistically significant.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, private or not-for-profit sectors. Conflict of interest: None. Authorship: Relevant papers were selected according to the title and abstract by three authors (S.-M.D.-R., M.H.-B. and R.K.). Two independent reviewers (S.-M.D.-R. and M.H.-B.) screened papers and read the full text of relevant papers. They assessed full texts for inclusion criteria and extracted data. Statistical analysis was done (M.M.) and cases of disagreement were resolved in consultation with a fourth arbitrating investigator (R.K.). Ethics of human subject participation: Ethical approval was not required.
References
- 1. Reiner Z & Tedeschi-Reiner E (2013) Prevalence and types of persistent dyslipidemia in patients treated with statins. Croat Med J 54, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Souza LJ, Souto Filho JT, de Souza TF et al. (2003) Prevalence of dyslipidemia and risk factors in Campos dos Goytacazes, in the Brazilian state of Rio de Janeiro. Arq Bras Cardiol 81, 257–264. [DOI] [PubMed] [Google Scholar]
- 3. Wietlisbach V, Paccaud F, Rickenbach M et al. (1997) Trends in cardiovascular risk factors (1984–1993) in a Swiss region: results of three population surveys. Prev Med 26, 523–533. [DOI] [PubMed] [Google Scholar]
- 4. Yamada M, Wong FL, Kodama K et al. (1997) Longitudinal trends in total serum cholesterol levels in a Japanese cohort, 1958–1986. J Clin Epidemiol 50, 425–434. [DOI] [PubMed] [Google Scholar]
- 5. Hodge AM, Dowse GK, Toelupe P et al. (1997) The association of modernization with dyslipidaemia and changes in lipid levels in the Polynesian population of Western Samoa. Int J Epidemiol 26, 297–306. [DOI] [PubMed] [Google Scholar]
- 6. Ruixing Y, Qiming F, Dezhai Y et al. (2007) Comparison of demography, diet, lifestyle, and serum lipid levels between the Guangxi Bai Ku Yao and Han populations. J Lipid Res 48, 2673–2681. [DOI] [PubMed] [Google Scholar]
- 7. Rice BH, Kraft J, Destaillats F et al. (2012) Ruminant-produced trans-fatty acids raise plasma HDL particle concentrations in intact and ovariectomized female Hartley guinea pigs. J Nutr 142, 1679–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lock AL, Parodi PW & Bauman DE (2005) The biology of trans-fatty acids: implications for human health and the dairy industry. Aust J Dairy Technol 60, 134–142. [Google Scholar]
- 9. Gebauer SK, Chardigny JM, Jakobsen MU et al. (2011) Effects of ruminant trans-fatty acids on cardiovascular disease and cancer: a comprehensive review of epidemiological, clinical, and mechanistic studies. Adv Nutr 2, 332–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Motard-Belanger A, Charest A, Grenier G et al. (2008) Study of the effect of trans fatty acids from ruminants on blood lipids and other risk factors for cardiovascular disease. Am J Clin Nutr 87, 593–599. [DOI] [PubMed] [Google Scholar]
- 11. Risérus U, Vessby B, Arnlöv J et al. (2004) Effects of cis-9,trans-11 conjugated linoleic acid supplementation on insulin sensitivity, lipid peroxidation, and proinflammatory markers in obese men. Am J Clin Nutr 80, 279–283. [DOI] [PubMed] [Google Scholar]
- 12. Lambert EV, Goedecke JH, Bluett K et al. (2007) Conjugated linoleic acid versus high-oleic acid sunflower oil: effects on energy metabolism, glucose tolerance, blood lipids, appetite and body composition in regularly exercising individuals. Br J Nutr 97, 1001–1011. [DOI] [PubMed] [Google Scholar]
- 13. Blankson H, Stakkestad JA, Fagertun H et al. (2000) Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr 130, 2943–2948. [DOI] [PubMed] [Google Scholar]
- 14. Berven G, Bye A, Hals O et al. (2000) Safety of conjugated linoleic acid (CLA) in overweight or obese human volunteers. Eur J Lipid Sci Technol 102, 455–462. [Google Scholar]
- 15. Mougios V, Matsakas A, Petridou A et al. (2001) Effect of supplementation with conjugated linoleic acid on human serum lipids and body fat. J Nutr Biochem 12, 585–594. [DOI] [PubMed] [Google Scholar]
- 16. Petridou A, Mougios V & Sagredos A (2003) Supplementation with CLA: isomer incorporation into serum lipids and effect on body fat of women. Lipids 38, 805–811. [DOI] [PubMed] [Google Scholar]
- 17. Naumann E, Carpentier YA, Saebo A et al. (2006) Cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) do not affect the plasma lipoprotein profile in moderately overweight subjects with LDL phenotype B. Atherosclerosis 188, 167–174. [DOI] [PubMed] [Google Scholar]
- 18. Sluijs I, Plantinga Y, de Roos B et al. (2010) Dietary supplementation with cis-9, trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am J Clin Nutr 91, 175–183. [DOI] [PubMed] [Google Scholar]
- 19. Kamphuis MM, Lejeune MP, Saris WH et al. (2003) The effect of conjugated linoleic acid supplementation after weight loss on body weight regain, body composition, and resting metabolic rate in overweight subjects. Int J Obes Relat Metab Disord 27, 840–847. [DOI] [PubMed] [Google Scholar]
- 20. Whigham LD, O’Shea M, Mohede ICM et al. (2004) Safety profile of conjugated linoleic acid in a 12-month trial in obese humans. Food Chem Toxicol 42, 1701–1709. [DOI] [PubMed] [Google Scholar]
- 21. Pfeuffer M, Fielitz K, Laue C et al. (2011) CLA does not impair endothelial function and decreases body weight as compared with safflower oil in overweight and obese male subjects. J Am Coll Nutr 30, 19–28. [DOI] [PubMed] [Google Scholar]
- 22. Laso N, Brugué E, Vidal J et al. (2007) Effects of milk supplementation with conjugated linoleic acid (isomers cis-9, trans-11 and trans-10, cis-12) on body composition and metabolic syndrome components. Br J Nutr 98, 860–867. [DOI] [PubMed] [Google Scholar]
- 23. Noone EJ, Roche HM, Nugent AP et al. (2002) The effect of dietary supplementation using isomeric blends of conjugated linoleic acid on lipid metabolism in healthy human subjects. Br J Nutr 88, 243–251. [DOI] [PubMed] [Google Scholar]
- 24. Sofi F, Buccioni A, Cesari F et al. (2010) Effects of a dairy product (pecorino cheese) naturally rich in cis-9, trans-11 conjugated linoleic acid on lipid, inflammatory and haemorheological variables: a dietary intervention study. Nutr Metab Cardiovasc Dis 20, 117–124. [DOI] [PubMed] [Google Scholar]
- 25. Chen SC, Lin YH, Huang HP et al. (2012) Effect of conjugated linoleic acid supplementation on weight loss and body fat composition in a Chinese population. Nutrition 28, 559–565. [DOI] [PubMed] [Google Scholar]
- 26. Brown AW, Trenkle AH & Beitz DC (2011) Diets high in conjugated linoleic acid from pasture-fed cattle did not alter markers of health in young women. Nutr Res 31, 33–41. [DOI] [PubMed] [Google Scholar]
- 27. Desroches S, Chouinard PY, Galibois I et al. (2005) Lack of effect of dietary conjugated linoleic acids naturally incorporated into butter on the lipid profile and body composition of overweight and obese men. Am J Clin Nutr 82, 309–319. [DOI] [PubMed] [Google Scholar]
- 28. Aminot-Gilchrist DV & Anderson HD (2004) Insulin resistance-associated cardiovascular disease: potential benefits of conjugated linoleic acid. Am J Clin Nutr 79, 6 Suppl., 1159S–1163S. [DOI] [PubMed] [Google Scholar]
- 29. Colakoglu S, Colakoglu M & Taneli F (2006) Cumulative effects of conjugated linoleic acid and exercise on endurance development, body composition, serum leptin and insulin levels. J Sports Med Phys Fitness 46, 570–577. [PubMed] [Google Scholar]
- 30. Raff M, Tholstrup T, Basu S et al. (2008) A diet rich in conjugated linoleic acid and butter increases lipid peroxidation but does not affect atherosclerotic, inflammatory, or diabetic risk markers in healthy young men. J Nutr 138, 509–514. [DOI] [PubMed] [Google Scholar]
- 31. Gaullier JM, Halse J, Hoivik HO et al. (2007) Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br J Nutr 97, 550–560. [DOI] [PubMed] [Google Scholar]
- 32. Gaullier JM, Halse J, Høye K et al. (2005) Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr 135, 778–784. [DOI] [PubMed] [Google Scholar]
- 33. Steck SE, Chalecki AM, Miller P et al. (2007) Conjugated linoleic acid supplementation for twelve weeks increases lean body mass in obese humans. J Nutr 137, 1188–1193. [DOI] [PubMed] [Google Scholar]
- 34. Wanders AJ, Brouwer IA, Siebelink E et al. (2010) Effect of a high intake of conjugated linoleic acid on lipoprotein levels in healthy human subjects. PLoS One 5, e9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tholstrup T, Raff M, Straarup EM et al. (2008) An oil mixture with trans-10, cis-12 conjugated linoleic acid increases markers of inflammation and in vivo lipid peroxidation compared with cis-9, trans-11 conjugated linoleic acid in postmenopausal women. J Nutr 138, 1445–1451. [DOI] [PubMed] [Google Scholar]
- 36. Albers R, Van der Wielen RPJ, Brink EJ et al. (2003) Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) isomers on immune function in healthy men. Eur J Clin Nutr 57, 595–603. [DOI] [PubMed] [Google Scholar]
- 37. Ramakers JD, Plat J, Sébédio JL et al. (2005) Effects of the individual isomers cis-9, trans-11 vs. trans-10, cis-12 of conjugated linoleic acid (CLA) on inflammation parameters in moderately overweight subjects with LDL-phenotype B. Lipids 40, 909–918. [DOI] [PubMed] [Google Scholar]
- 38. Engberink MF, Geleijnse JM, Wanders AJ et al. (2011) The effect of conjugated linoleic acid, a natural trans fat from milk and meat, on human blood pressure: results from a randomized crossover feeding study. J Hum Hypertens 26, 127–132. [DOI] [PubMed] [Google Scholar]
- 39. Sahin H, Uyanik F & Inanc N (2008) Effects of conjugated linoleic acid on body composition and selected biochemical parameters in obese women. Pak J Nutr 7, 546–549. [Google Scholar]
- 40. von Loeffelholz C, Kratzsch J & Jahreis G (2003) Influence of conjugated linoleic acids on body composition and selected serum and endocrine parameters in resistance-trained athletes. Eur J Lipid Sci Technol 105, 251–259. [Google Scholar]
- 41. Tricon S, Burdge GC, Kew S et al. (2004) Opposing effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid on blood lipids in healthy humans. Am J Clin Nutr 80, 614–620. [DOI] [PubMed] [Google Scholar]
- 42. Rubin D, Herrmann J, Much D et al. (2012) Influence of different CLA isomers on insulin resistance and adipocytokines in pre-diabetic, middle-aged men with PPARγ2 Pro12Ala polymorphism. Genes Nutr 7, 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonet Serra B, Quintanar Rioja A, Viana Arribas M et al. (2008) The effects of yogurt with isomer enriched conjugated linoleic acid on insulin resistance in obese adolescents. Rev Esp Pediatr 64, 94–100. [Google Scholar]
- 44. Venkatramanan S, Joseph SV, Chouinard PY et al. (2010) Milk enriched with conjugated linoleic acid fails to alter blood lipids or body composition in moderately overweight, borderline hyperlipidemic individuals. J Am Coll Nutr 29, 152–159. [DOI] [PubMed] [Google Scholar]
- 45. Risérus U, Berglund L & Vessby B (2001) Conjugated linoleic acid (CLA) reduced abdominal adipose tissue in obese middle-aged men with signs of the metabolic syndrome: a randomised controlled trial. Int J Obes Relat Metab Disord 25, 1129–1135. [DOI] [PubMed] [Google Scholar]
- 46. Basu S, Riserus U, Turpeinen A et al. (2000) Conjugated linoleic acid induces lipid peroxidation in men with abdominal obesity. Clin Sci (Lond) 99, 511–516. [DOI] [PubMed] [Google Scholar]
- 47. Gaullier JM, Halse J, Høye K et al. (2004) Conjugated linoleic acid supplementation for 1 y reduces body fat mass in healthy overweight humans. Am J Clin Nutr 79, 1118–1125. [DOI] [PubMed] [Google Scholar]
- 48. Kim JO (2008) Supplementation of conjugated linoleic acid with γ-oryzanol for 12 weeks effectively reduces body fat in healthy overweight Korean women. J Food Sci 13, 146–156. [Google Scholar]
- 49. Egger M, Davey Smith G, Schneider M et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Duval S & Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. [DOI] [PubMed] [Google Scholar]
- 51. Watras AC, Buchholz AC, Close RN et al. (2007) The role of conjugated linoleic acid in reducing body fat and preventing holiday weight gain. Int J Obes (Lond) 31, 481–487. [DOI] [PubMed] [Google Scholar]
- 52. Taylor JS, Williams SR, Rhys R et al. (2006) Conjugated linoleic acid impairs endothelial function. Arterioscler Thromb Vasc Biol 26, 307–312. [DOI] [PubMed] [Google Scholar]
- 53. Smedman A & Vessby B (2001) Conjugated linoleic acid supplementation in humans – metabolic effects. Lipids 36, 773–781. [DOI] [PubMed] [Google Scholar]
- 54. Song HJ, Grant I, Rotondo D et al. (2005) Effect of CLA supplementation on immune function in young healthy volunteers. Eur J Clin Nutr 59, 508–517. [DOI] [PubMed] [Google Scholar]
- 55. Tricon S, Burdge GC, Jones EL et al. (2006) Effects of dairy products naturally enriched with cis-9, trans-11 conjugated linoleic acid on the blood lipid profile in healthy middle-aged men. Am J Clin Nutr 83, 744–753. [DOI] [PubMed] [Google Scholar]
- 56. Nazare J-A, Perrière ABdl, Bonnet F et al. (2007) Daily intake of conjugated linoleic acid-enriched yoghurts: effects on energy metabolism and adipose tissue gene expression in healthy subjects. Br J Nutr 97, 273–280. [DOI] [PubMed] [Google Scholar]
- 57. Iwata T, Kamegai T, Yamauchi-Sato Y et al. (2007) Safety of dietary conjugated linoleic acid (CLA) in a 12-weeks trial in healthy overweight Japanese male volunteers. J Oleo Sci 56, 517–525. [DOI] [PubMed] [Google Scholar]
- 58. Benito P, Nelson GJ, Kelley DS et al. (2001) The effect of conjugated linoleic acid on plasma lipoproteins and tissue fatty acid composition in humans. Lipids 36, 229–236. [DOI] [PubMed] [Google Scholar]
- 59. Tavakoli-Darestani A, Hosseinpanah F, Hedayati M et al. (2010) Conjugated linoleic acid and lipid profile of postmenopausal women. Pejouhesh 34, 26–34. [Google Scholar]
- 60. Smit LA, Baylin A & Campos H (2010) Conjugated linoleic acid in adipose tissue and risk of myocardial infarction. Am J Clin Nutr 92, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grundy SM & Denke MA (1990) Dietary influences on serum lipids and lipoproteins. J Lipid Res 31, 1149–1172. [PubMed] [Google Scholar]
- 62. Yotsumoto H, Hara E, Naka S et al. (1999) Trans-10, cis-12-linoleic acid reduces apolipoprotein B secretion in HepG2 cells. Food Res Int 31, 403–409. [Google Scholar]
- 63. Yeung C, Yang L, Huang Y et al. (2000) Dietary conjugated linoleic acid mixture affects the activity of intestinal acyl coenzyme A: cholesterol acyltransferase in hamsters. Br J Nutr 84, 935–941. [PubMed] [Google Scholar]
- 64. Pariza MW, Park Y & Cook ME (2001) The biologically active isomers of conjugated linoleic acid. Prog Lipid Res 40, 283–298. [DOI] [PubMed] [Google Scholar]
- 65. Gudbrandsen OA, Rodriguez E, Wergedahl H et al. (2009) Trans-10, cis-12-conjugated linoleic acid reduces the hepatic triacylglycerol content and the leptin mRNA level in adipose tissue in obese Zucker fa/fa rats. Br J Nutr 102, 803–815. [DOI] [PubMed] [Google Scholar]
- 66. Vyas D, Kadegowda AK & Erdman RA (2012) Dietary conjugated linoleic acid and hepatic steatosis: species-specific effects on liver and adipose lipid metabolism and gene expression. J Nutr Metab 2012, 932–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kelly GS (2001) Conjugated linoleic acid: a review. Altern Med Rev 6, 367–382. [PubMed] [Google Scholar]
- 68. Oleszczuk L, Oleszczuk J, Kwiecińska B et al. (2011) Use of diet supplements, synthetic drugs and herbal remedies with immunotropic activity during pregnancy. III. Conjugated linoleic acid. Centr Eur J Immunol 36, 308–310. [Google Scholar]