Abstract
Objective
A high intake of sugar-sweetened beverages (SSB) has been linked to weight gain, obesity and type 2 diabetes; however, the influence on CVD risk remains unclear. Therefore, our objective was to summarize current evidence for an association between SSB consumption and cardiovascular risk factors and events.
Design
The article search was performed in August 2013. Two independent researchers performed the article search and selection, data extraction and quality assessment. Eligible studies reported the intake of SSB and one of the following outcomes: change in blood pressure, blood lipid or blood sugar, or CVD events such as stroke or myocardial infarction. Only intervention and longitudinal studies were included.
Subjects
Only studies in adults (aged 18+ years old) were considered.
Results
Two of four prospective studies found clear direct associations between SSB consumption and CHD, while two of three studies, including both men and women, found direct associations between SSB consumption and stroke; however, the association was significant among women only. All included studies examining vascular risk factors found direct associations between SSB consumption and change in blood pressure, blood lipid or blood sugar.
Conclusions
The reviewed studies generally showed that SSB intake was related to vascular risk factors, whereas associations with vascular events were less consistent. Due to a limited number of published papers, especially regarding vascular events, the strength of the evidence is still limited and hence more studies are needed before firm conclusions can be made.
Keywords: Sugar-sweetened beverages, Vascular disease, Review
Dietary carbohydrates are essential for body functions as they are the main source of energy. To ensure a balanced diet, the Institute of Medicine, Food and Nutrition Board recommends that 45–55 % of the total energy intake is provided by carbohydrates( 1 ). Added sugar (table sugar) is added to foods and beverages during industrial processing, and hence refers to sugars that are not naturally occurring( 2 ).
Studies show that added sugar (over-) consumption, specifically in the form of sugar-sweetened beverages (SSB), seems to be linked to different harmful health outcomes such as obesity and diabetes( 3 – 5 ). SSB may also potentially increase the risk of CVD through their high amount of rapidly absorbable carbohydrates that may, via an elevated hepatic de novo lipogenesis, result in hypertension, accumulation of visceral and ectopic fat, and increased TAG and LDL cholesterol (LDL-C) and decreased HDL cholesterol (HDL-C) levels( 6 , 7 ). The increased glycaemic load caused by a high SSB intake may lead to inflammation, β-cell dysfunction and insulin resistance, as suggested by Malik et al. (2010) who, in their pooled meta-analysis from three prospective cohorts, reported that participants in the highest category of SSB intake had a 20 % greater risk of developing metabolic syndrome than those in the lowest category of intake( 4 ). Most previous literature reviews on potential health consequences of a high intake of SSB have focused on obesity, metabolic syndrome or diabetes as their outcome of interest( 8 – 11 ). Furthermore, of those previous reviews that assessed associations between SSB consumption and CVD risk and events, most were not systematic( 3 , 12 , 13 ) or did not include a quality assessment of the included studies. For instance, the systematic review by Sonestedt et al. (2012) examined the association between sugar intake (SSB, sucrose and fructose) and type 2 diabetes, CVD and related metabolic risk factors, but included five primary studies (four prospective cohort studies and one randomised controlled trial (RCT)) regarding SSB consumption and CVD, only( 5 ). Furthermore, Althuis and Weed (2013) in their more recent review, which included just four prospective cohort studies on SSB consumption and CHD/stroke, stressed the need for more updated reviews specifically based on results from studies on SSB intake and CHD and stroke( 14 ). Finally, in a recent review of reviews that assessed the quality of published reviews regarding SSB and health, it was concluded that systematic literature reviews assessing the quality of included studies are generally lacking( 11 ).
The primary aim of the present systematic review was therefore to review the results from published studies examining the association between SSB consumption and related vascular events and risk factors until 2013. The secondary aim was to assess the quality of the original papers included in the review using a validated quality assessment tool from the Academy of Nutrition and Dietetics. Finally, we wanted to examine if there was evidence that the association between SSB consumption and CVD was mediated by diabetes, hypertension, BMI or energy intake.
Method
Search methods and terms used
The literature search was performed through the platforms PubMed/MEDLINE and Web of Knowledge by two independent researchers in August 2013. The terms used for the article search combined key terms for SSB and CVD: Metabolic Syndrome X, Glucose, Insulin, Subcutaneous Fat, Abdominal, Intra-Abdominal Fat, Cardiovascular Diseases, Blood Pressure, Hypertension, Inflammation, Protein, Cholesterol, Triglycerides, Lipoproteins, HOMA, Waist Circumference, Carbonated Beverages, Soda, Dietary Sucrose, Sucrose, Fructose, Sweetening Agents, Glucose, Energy Drinks, Beverages, Adult.
Selection of articles
The selection of articles was performed in three steps. First, papers in the database search were selected based on their title. Second and third, all abstracts and full texts of papers identified in the first step were screened by the two researchers. (For further details, see online supplementary material: Expanded Method.)
Data extraction and quality assessment
The quality of each study included in the review was independently assessed by the same two researchers who selected the papers using the Academy of Nutrition and Dietetics’ (formerly the American Dietetic Association (ADA)) Quality Criteria Checklist: Primary Search from the ADA Evidence Analysis Manual ( 15 ). (For further details, see online supplementary material: Expanded Method.)
Selection criteria
The following criteria were used to include or exclude articles for this systematic literature review.
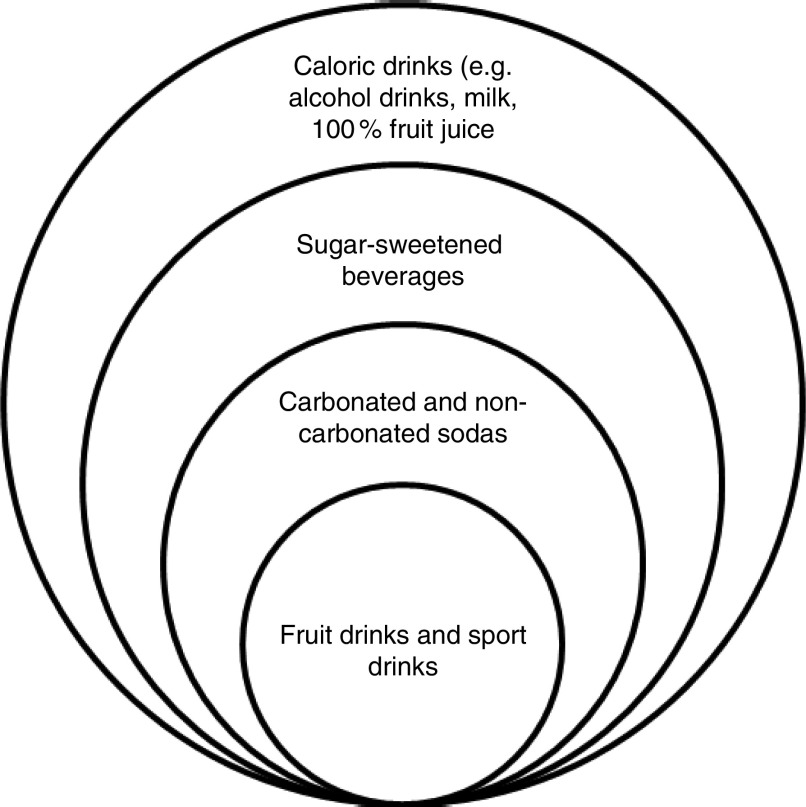
Exposure. There is no official definition for SSB; however, as a convention, SSB are defined as beverages containing added sugar( 14 , 16 ). In the present review, SSB include carbonated or non-carbonated sodas, fruit drinks and sport drinks (Fig. 1). The definition of SSB used in each study is given in the online supplementary material, Supplemental Table 1.
Fig. 1.
Classification of caloric beverages used in the present review
Table 1.
Description of studies on SSB intake, mediators and risk of vascular events
| Reference (authors and date) | Study design | Data set used and country | Gender | No. of participants analysed | Exposure† | Dietary data collection method(s) | Mediators | Outcome | Follow-up | Results: association‡ and RR/HR/OR (95 % CI) | Mediation analysis | Quality | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHD | de Koning et al. (2012)( 26 ) | Prospective | HPFS, USA | M | n 42 883 | Baseline SSB | FFQ | DB, HT, TAG, chol | Incident CHD (fatal and non-fatal) | 22 years | + (RR 1·19 (1·11, 1·26))** (for each additional serving/d) | No effect (DB & HT) | A |
| Eshak et al. (2012)( 18 ) | Prospective | Japan Public Health Centre-based cohort I, Japan | M+F | n 39 786 | Baseline SSB | FFQ | EI, BMI | IHD | 18 years | × (M: HR 1·10 (0·77, 1·57; P=0·54)) F: HR 0·89 (0·32, 2·45; P=0·98)) (daily intake v. never) | No effect (BMI & TEI) | A | |
| Fung et al. (2009)( 27 ) | Prospective | NHS, USA | F | n 88 520 | Change in SSB | FFQ | DB | CHD | 24 years | + (RR 1·28 (1·14, 1·44)) (2 servings/d increase) | ÷ | A | |
| Baseline SSB | + (RR 1·39 (1·11, 1·75))** (>2 SSB/d v. <1 SSB/month) | ||||||||||||
| Gardener et al. (2012)( 17 ) | Prospective | Northern Manhattan Study, USA | M+F | n 2564 | Baseline SSB | FFQ | Confounders | MI | 10 years | × (HR 1·26 (0·89, 1·78)) | ÷ | B | |
| Combined events (stroke, MI, vascular death) | + (non-obese: HR 1·57 (1·05, 2·35))* (daily intake v. never) | ||||||||||||
| Stroke | Bernstein et al. (2012)( 19 ) | Prospective | NHS and HPFS, USA | M+F | NHS: n 84 085 HPFS: n 43 371 | Baseline SSB | FFQ | DB, HT, EI, BMI | Total stroke | NHS: 28 years HPFS: 22 years | + (women: RR 1·19 (1·00, 1·42))* × (men: RR 1·08 (0·82, 1·41)) (daily intake v. never) | Attenuation from RR 1·19 to 1·14 (P<0·05) (DB & HT) No effect (BMI & TEI) | A |
| Eshak et al. (2012)( 18 ) | Prospective | Public Health Centre-based cohort I, Japan | M+F | n 39 786 | Baseline SSB | FFQ | EI, BMI | Total stroke (ischaemic and haemorrhagic) | 18 years | + (women: HR 1·39 (1·01, 1·91))** − (men: HR 0·74 (0·59, 0·96; P=0·01)) (daily intake v. never) | No effect (BMI & TEI) | A | |
| Gardener et al. (2012)( 17 ) | Prospective | Northern Manhattan Study, USA | M+F | n 2564 | Baseline SSB | FFQ | Confounders | Total stroke | 10 years | × (HR 1·00 (0·65, 1·54)) (daily intake v. never) | ÷ | B |
SSB, sugar-sweetened beverages; RR, relative risk; HR, hazard ratio; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; M, male; F, female; DB, diabetes; HT, hypertension; chol, cholesterol; EI, energy intake; MI, myocardial infarction; TEI, total energy intake; ÷, no mediation analysis.
*P<0·05, **P<0·01.
SSB included drinks sweetened with sugar; artificially sweetened beverages and 100 % fruit juice were not included.
+, direct association; ×, no association; −, inverse association.
Study design. Longitudinal and intervention studies were included. Cohort studies had to have a minimum length of follow-up of 1 year and intervention studies had to have a follow-up of at least 4 weeks.
Outcome. We included studies looking at SSB consumption in relation to CVD and CVD risk factors such as change in blood pressure (hypertension), HDL-C, LDL-C, TAG, blood glucose and insulin resistance, and in relation to CVD events such as stroke and CHD as they were the only end points that could be reviewed. (For further details, see online supplementary material: Expanded Method.)
Subjects. Only studies in adults (aged 18+ years old) were considered.
Language. English.
Article types. Original articles published up to 31 August 2013.
Time period. The article search was performed in August 2013.
Results
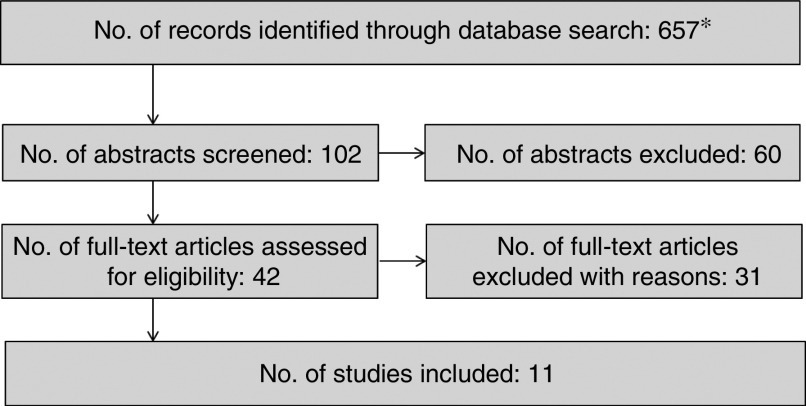
The initial literature search resulted in 657 papers. After the titles and abstracts were screened, the full-text papers of forty-two articles were screened for the final inclusion. The evaluation of full-text articles resulted in the inclusion of ten prospective studies and one RCT in the review (Fig. 2)( 17 – 27 ). Nine studies included both men and women( 17 – 25 ), one study included men only( 26 ) and another one women only( 27 ). Most studies were from the USA, one was from Japan( 18 ), one from Spain( 25 ) and another from Denmark( 24 ). The number of participants ranged from 810 to 97 991 among prospective studies, with five studies including more than 30 000 participants( 18 , 19 , 21 , 26 , 27 ) and five less than 10 000 participants( 17 , 20 , 22 , 23 , 25 ). The RCT included forty-seven participants( 24 ).
Fig. 2.
Results of the search. *Four systematic reviews and one meta-analysis were identified. However, they were not included in our analysis as only original data were included
Regarding dietary intake, nine of the eleven included studies assessed it using an FFQ, one used a 24 h recall( 23 ) and the RCT used a 7 d record( 24 ). Among prospective studies, three studies examined change in SSB( 23 , 25 , 27 ) and the others used either a single dietary assessment at baseline or a cumulative average of SSB intake collected by multiple FFQ.
In total, five studies examined associations between intake of SSB and vascular events; three of these studies further examined mediating influences of various vascular risk factors. In addition, six studies examined associations between intake of SSB and vascular risk factors.
The list of excluded articles after full-text screening and reasons for exclusion are given in the online supplementary material, Supplemental Table 2. The characteristics of each of the included studies are presented in Tables 1 and 2.
Table 2.
Description of studies on SSB intake and associations with vascular risk factors
| Reference (authors and date) | Study design | Data set used and country | Gender | No. of participants analysed | Exposure† | Dietary data collection method(s) | Outcome(s) | Follow-up | Results: association‡ and RR/HR/OR (95 % CI) | Quality | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypertension/high BP | Duffey et al. (2010)( 20 ) | Prospective | CARDIA, USA | M+F | n 2774 | Baseline SSB | FFQ+DH | BP | 20 years | + * (RR 1·06 (1·01, 1·12)) (from one quartile to the next) | A | |
| Cohen et al. (2012)( 21 ) | Prospective | NHS I and II and HPFS, USA | M+F | NHS I: n 88 540 NHS II: n 97 991 HPFS: n 37 360 | Baseline SSB | FFQ | High BP | NHS I: 38 years NHS II: 16 years HPFS: 22 years | + (HR 1·13 (1·09, 1·17)) (daily intake v. never) | A | ||
| Dhingra et al. (2007)( 22 ) | Prospective | Framingham Offspring Study, USA | M+F | n 6039 | Baseline SSB | FFQ | BP | 4 years | × (OR 1·18 (0·96, 1·44)) (daily intake v. never) | B | ||
| Chen et al. (2010)( 23 ) | Prospective | PREMIER (intervention) study), USA | M+F | n 810 | Change in SSB | 24 h recall | BP | 18 months | + * (decrease of 0·7 (0·12, 1·25) mmHg in SBP and 0·4 (0·02, 0·75) mmHg in DBP) (decrease of 1 SSB/d) | A | ||
| Baseline SSB | + (DBP; P=0·01) × (SBP; P=0·57) | |||||||||||
| Barrio-Lopez et al. (2013)( 25 ) | Prospective | SUN cohort, Spain | M+F | n 8157 | Change in SSB | FFQ | BP | 6–8 years | + ** (OR 1·6 (1·3, 2·1)) (highest v. lowest quintile) | A | ||
| Baseline SSB | × (P>0·05) | |||||||||||
| Mærsk et al. (2012)( 24 ) | RCT | N/A, Denmark | M+F | n 47 | Intervention | Control/comparison group | 7 d dietary record | BP | 6 months | × (P>0·05) (% change from baseline to 6 months SSB v. milk, diet cola, water (data not shown)) | A | |
| Consumption of 1 litre SSB/d | Consumption of 1 litre/d of (i) ASB (ii) milk (iii) water | |||||||||||
| TAG | Duffey et al. (2010)( 20 ) | Prospective | CARDIA, USA | M+F | n 2774 | Baseline SSB | FFQ+DH | TAG | 20 years | + * (RR 1·06 (1·01, 1·13; P<0·05)) (one quartile to the next) | A | |
| Dhingra et al. (2007)( 22 ) | Prospective | Framingham Offspring Study, USA | M+F | n 6039 | Baseline SSB | FFQ | Hypertriacylglycerolaemia | 4 years | + (OR 1·25 (1·04, 1·51)) (≥1 SSB/d) | B | ||
| Barrio-Lopez et al. (2013)( 25 ) | Prospective | SUN cohort, Spain | M+F | n 8157 | Change in SSB | FFQ | Hypertriacylglycerolaemia | 6–8 years | + * (OR 1·7 (1·1, 2·6)) (highest v. lowest quintile) | A | ||
| Baseline SSB | × (P>0·05) | |||||||||||
| Mærsk et al. (2012)( 24 ) | RCT | N/A, Denmark | M+F | n 47 | Intervention | Control/comparison group | 7 d dietary record | TAG | 6 months | + * (% change (SD) from baseline to 6 months: SSB 32·7 (8·6) v. milk | A | |
| Consumption of 1 litre SSB/d | Consumption of 1 litre/d of (i) ASB (ii) milk (iii) water | −0·301(8·1), diet cola −14·1(8·1), water −14·2(7·7); P=0·001) | ||||||||||
| Cholesterol | Duffey et al. (2010)( 20 ) | Prospective | CARDIA, USA | M+F | n 2774 | Baseline SSB | FFQ+DH | HDL-C, LDL-C | 20 years | + * (high LDL-C (RR 1·18 (1·02, 1·36; P<0·05), low HDL-C (RR 1·06 (1·02, 1·10; P<0·05)) (one quartile to the next) | A | |
| Dhingra et al. (2007)( 22 ) | Prospective | Framingham Offspring Study, USA | M+F | n 6039 | Baseline SSB | FFQ | LDL-C | 4 years | + (OR 1·32 (1·06, 1·64)) (≥1 SSB/d) | B | ||
| Barrio-Lopez et al. (2013)( 25 ) | Prospective | SUN cohort, Spain | M+F | n 8157 | Change in SSB | FFQ | HDL-C | 6–8 years | × (OR 1·0 (0·7, 1·6)) (highest v. lowest quintile) | A | ||
| Baseline SSB | × (P>0·05) | |||||||||||
| Mærsk et al. (2012)( 24 ) | RCT | N/A, Denmark | M+F | n 47 | Intervention | Control/comparison group | 7 d dietary record | Cholesterol | 6 months | + (total cholesterol) * (% change (SD) from baseline to 6 months: | A | |
| Consumption of 1 litre SSB/d | Consumption of 1 litre/d of (i) ASB (ii) milk (iii) water | SSB 11·4 (3·2) v. milk 0·634(3·0), diet cola −5·89(3·0), water −0·159(2·8); P=0·004) | ||||||||||
| Blood sugar | Dhingra et al. (2007)( 22 ) | Prospective | Framingham Offspring Study, USA | M+F | n 6039 | Baseline SSB | FFQ | FBG | 4 years | + (OR 1·25 (1·05, 1·48)) | B | |
| Duffey et al. (2010)( 20 ) | Prospective | CARDIA, USA | M+F | n 2774 | Baseline SSB | FFQ+DH | FBG | 20 years | × (RR 1·03 (0·95, 1·12)) | A | ||
| Barrio-Lopez et al. (2013)( 25 ) | Prospective | SUN cohort, Spain | M+F | n 8157 | Change in SSB | FFQ | FBG | 6–8 years | + * (OR 1·6 (1·1, 2·2)) (highest v. lowest quintile) | A | ||
| Baseline SSB | × (P>0·05) | |||||||||||
SSB, sugar-sweetened beverages; RR, relative risk; HR, hazard ratio; BP, blood pressure; RCT, randomised controlled trial; CARDIA, Coronary Artery Risk Development in Young Adults; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; SUN, Seguimiento Universidad de Navarra (University of Navarra Follow-up); N/A, not applicable; M, male; F, female; ASB, artificially sweetened beverages; DH, diet history; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; FBG, fasting blood glucose; SDP, systolic blood pressure; DBP, diastolic blood pressure.
*P<0·05, **P<0·01.
SSB included drinks sweetened with sugar; artificially sweetened beverages and 100 % fruit juice were not included.
+, direct association; ×, no association.
Studies on vascular events
Four papers reported results for CHD, which was defined as fatal and non-fatal myocardial infarction (MI) in three of the papers( 17 , 26 , 27 ), and as IHD in one paper( 18 ). One paper reported results for combined vascular events which included stroke, MI and vascular death( 17 ).
Three studies examined baseline (or cumulative average) SSB intake and CHD( 17 , 18 , 26 ) and one study examined both baseline SSB intake and change in SSB intake and CHD( 27 ).
Among studies examining baseline (or cumulative average) SSB intake and CHD, de Koning et al. (2012) reported that for each additional serving of SSB, the relative risk (RR) of CHD was 1·19 (95 % CI 1·11, 1·26)( 26 ), while Fung et al. (2009) found that women who consumed more than two SSB daily had a 39 % greater risk of developing CHD over the following 24 years (RR=1·39; 95 % CI 1·11, 1·75; P<0·001) compared with women who drank SSB less than once monthly( 27 ). In contrast, the studies by Eshak et al. (2012) and Gardener et al. (2012) did not find any association between baseline SSB intake and CHD( 17 , 18 ). In their analysis of change of SSB intake and CHD, Fung et al. (2009) found that a 2 servings/d increase in SSB increased the risk of developing CHD by 28 % (RR=1·28; 95 % CI 1·14, 1·44; P<0·001) among women.
Three of five papers reported results for stroke, which were classified either as ischaemic or haemorrhagic or of unknown type, fatal or non-fatal( 17 – 19 ). All studies examined baseline (or cumulative average) SSB intake and stroke.
Bernstein et al. (2012) reported that women consuming one or more serving of SSB daily were 19 % more likely to develop stroke (RR=1·19; 95 % CI 1·00, 1·42) while no significant association was found for men (RR=1·08; 95 % CI 0·82, 1·41)( 19 ). Similarly, a high intake of SSB was also directly associated with an increased risk of stroke for women (hazard ratio (HR)=1·39; 95 % CI 1·01, 1·91; P<0·01), but an inverse association was found for men (HR=0·74; 95 % CI 0·59, 0·96; P=0·01), in the study by Eshak et al. (2012)( 18 ). Finally, Gardener et al. (2012) did not find an association between SSB intake and risk of stroke; however, the authors reported a direct association, e.g. that a high SSB consumption was associated with an increased risk of combined vascular events (stroke, MI and vascular death) among healthier subjects (e.g. those without obesity, or a history of diabetes or metabolic syndrome) at baseline. In this subgroup, daily high SSB intake was associated with an increased risk of vascular events of 57 % (HR=1·57; 95 % CI 1·05, 2·35)( 17 ).
In summary, of the five identified prospective studies using vascular events as outcomes( 17 – 19 , 26 , 27 ), two found direct associations between SSB consumption and CHD( 26 , 27 ), two others between SSB consumption and stroke( 18 , 19 ), and another one between SSB consumption and combined vascular events( 17 ).
Studies on mediation of vascular events risk related to sugar-sweetened beverages consumption by vascular risk factors
Three of five studies included potential mediators of the relationships between SSB consumption and vascular events in their statistical analysis (Table 1)( 18 , 19 , 26 ).
Diabetes and hypertension
Two studies( 19 , 26 ) adjusted for diabetes in one of their models and a third study mentioned diabetes as a potential intermediate risk factor in the pathway between SSB and CHD( 18 ). Adjusting for diabetes did not influence the associations in the study by de Koning et al. (2012)( 26 ), whereas Bernstein et al. (2012) in their study found that the association between SSB intake and stroke risk was slightly attenuated when controlled for both diabetes and hypertension. The authors reported that their findings suggest that diabetes and hypertension might be mediators rather than confounders( 19 ). When adjusting for hypertension as well as for diabetes, the results remained unchanged in the study by de Koning et al. (2012)( 26 ) (Table 1).
Energy intake and BMI
Energy intake and BMI were considered as mediators and adjusted for in one model by both Bernstein et al. (2012)( 19 ) and Eshak et al. (2012)( 18 ); however, no mediation effects were reported (data not shown). The authors concluded that BMI and total energy intake were not major mediators of associations between SSB and vascular events( 18 ) (Table 1).
Studies on vascular risk factors
The six studies that explored associations between SSB and vascular risk factors considered hypertension, HDL-C and LDL-C, TAG, glucose and insulin (homeostasis model assessment of insulin resistance (HOMA-IR)) in addition to some obesity outcomes such as visceral adipose tissue (VAT) and subcutaneous abdominal adipose tissue (SAAT) in their analyses (Table 2)( 20 – 25 ).
Four studies examined baseline (or cumulative average) SSB intake and vascular risk factors( 20 – 22 , 24 ) and two others further examined change in SSB intake and vascular risk factors( 23 , 25 ).
Change in SSB consumption over a period of 18 months was strongly and directly associated with concurrent changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) in the study by Chen et al. (2010). After adjustment for change in body weight, a reduction of one serving of SSB (∼350 ml) per day was associated with a concurrent decrease of 0·7 (95 % CI 0·12, 1·25) mmHg in SBP and 0·4 (95 % CI 0·02, 0·75) mmHg in DBP( 23 ). In the study by Barrio-Lopez et al. (2013), participants in the highest quintile of increase in SSB intake over 6-year follow-up had a higher risk of developing high blood pressure compared with the lowest quintile (OR=1·6; 95 % CI 1·3, 2·1)( 25 ). Among studies examining baseline SSB and vascular risk factors, Cohen et al. (2012) reported a direct association between high SSB intake and risk of high blood pressure. In that study, the consumption of one SSB daily was associated with an adjusted HR of new-onset hypertension of 1·13 (95 % CI 1·09, 1·17), when compared with non-drinkers. When analysed separately, the association between SSB intake and incident hypertension was stronger for women than men (women: aged-adjusted HR=1·22; 95 % CI 1·18, 1·27, men: aged-adjusted HR=1·39; 95 % CI 1·34, 1·46)( 21 ). Two other studies found a direct association between SSB intake and hypertension or high blood pressure. In the study by Duffey et al. (2010), higher SSB intake (from one quartile to the next) was associated with a significant increase in the risk of hypertension (RR=1·06; 95 % CI 1·01, 1·12)( 20 ). Dhingra et al. (2007) found that the daily consumption of one or more servings of SSB was associated with increased odds of having developed high blood pressure (OR=1·18; 95 % CI 0·96, 1·44) at 4-year follow-up. However, this trend was not statistically significant, and the analysis combined SSB and artificially sweetened beverage consumption( 22 ). In contrast, no concurrent changes in blood pressure were found in the intervention study by Mærsk et al. (2012) which compared the effect of the intake of one litre of SSB with those of milk and artificially sweetened beverages on changes in total fat mass and ectopic fat deposition( 24 ). Similarly, Barrio-Lopez et al. (2013) did not find any significant association between baseline quintile of SSB and any vascular risk factor( 25 ) and only a small significant increase was reported for DBP but not SBP in the study by Chen et al. (2010)( 23 ).
The consumption of one or more servings of SSB daily was found to be associated with increased odds of hypertriacylglycerolaemia (OR=1·25; 95 % CI 1·04, 1·51) and high LDL-C (OR=1·32; 95 % CI 1·06, 1·64) over the subsequent four years in the study by Dhingra et al. (2007)( 22 ). In the Coronary Artery Risk Development in Young Adults (CARDIA) cohort study, associations between SSB and 20-year development in HDL-C, LDL-C and TAG concentrations were examined by Duffey et al. (2010). In this latter study the authors reported that, compared with milk or fruit juice consumption, moving from one quartile of SSB intake to the next was associated with attainment of high TAG (RR=1·06; 95 % CI 1·01, 1·13; P<0·05), high LDL-C (RR=1·18; 95 % CI 1·02, 1·36; P<0·05) and low HDL-C (RR=1·06; 95 % CI 1·02, 1·10; P<0·05) levels( 20 ). In a third prospective study looking at change in SSB intake among Spanish men and women, participants who increased their SSB consumption (upper v. lower quintile) had significantly higher risk of developing hypertriacylglycerolaemia (OR=1·7, 95 % CI 1·1, 2·6)( 25 ). Finally, Mærsk et al. (2012) found that mean relative change from baseline to 6-month follow-up in TAG (32·7 (sd 8·6) %; P=0·001), total cholesterol (11·4 (sd 3·2) %; P=0·004) and VAT/SAAT (18·1 (sd 6·0) %; P=0·004) was higher in the intervention group who consumed one litre of SSB daily compared with that in the water (TAG: −14·2 (sd 7·7) %; total cholesterol: −0·159 (sd 2·8) %; VAT/SAAT: 3·90 (sd 5·7) %), milk (TG: −0·301 (sd 8·1) %; total cholesterol: 0·634 (sd 3·0) %; VAT/SAAT: −12·5 (sd 6·1) %) and artificially sweetened beverages groups (TAG: −14·1 (sd 8·1) %; total cholesterol: −5·89 (sd 3·0) %; VAT/SAAT: 4·59 (sd 5·5) %)( 24 ).
Impaired fasting blood glucose was directly associated with SSB intake in the study by Dhingra et al. (2007) (OR=1·25; 95 % CI 1·05, 1·48)( 22 ) and in the study by Barrio-Lopez et al. (2013) (OR=1·6; 95 % CI 1·1, 2·2)( 25 ), but not in the prospective study by Duffey et al. (2010)( 20 ). Finally, no changes in plasma glucose or HOMA-IR were reported by Mærsk et al. (2012)( 24 ).
In summary, six studies using vascular risk factors as outcomes found direct associations between baseline or change in SSB consumption and changes in blood pressure or lipid metabolism (Table 2)( 20 – 25 ). Whereas when examining baseline SSB intake, one study only found a small direct association with DBP but not SBP( 23 ) and another did not find any significant associations.( 25 )
Quality of studies
Among the eleven identified studies, nine were of good quality (quality score: A) and two were of medium quality (quality score: B); see online supplementary material, Supplemental Table 3. None of the studies was classified with a negative quality score. Quality scores were not related to the conclusions of the papers about SSB and CVD. The two studies with a quality score of B were prospective( 17 , 22 ). They both had non-detailed descriptions of exposure and outcome factors or procedures( 17 , 22 ), and one study( 19 ) presented some ambiguity regarding outcome definition and measurements. The strength of the evidence was graded as ‘fair’ for the association between SSB and vascular risk factors, and as ‘limited to fair’ for the association between SSB and vascular events as well as for diabetes, hypertension, BMI and energy intake as mediators (see online supplementary material, Supplemental Tables 4 and 5).
Discussion
The present review of the literature linking intake of SSB to cardiovascular risk factors and events found weak evidence for a direct association between SSB and vascular events (CHD or stroke). The restricted number of studies as well as the discrepant results for different subgroups in relation to stroke limited the strength of this evidence. However, a high intake of SSB was generally associated with vascular risk factors, e.g. increased blood pressure and hyperlipidaemia. Our findings are in accordance with the results from Malik et al. (2010) who, based on a review of ten studies on cardiovascular risk, concluded that accumulating data suggest a direct association between SSB intake and the development of hypertension, adverse lipid parameters, inflammation and clinical CHD, although the evidence is limited( 3 ). More recently, a systematic review on the impact of SSB on blood pressure from Malik et al. (2014) showed that SSB intake was directly associated with high blood pressure and consequent hypertension( 28 ).
In the present review, most studies related to vascular risk factors found direct associations. The finding of a direct association between SSB and subsequent change in CVD risk factors is further sustained by findings from a recent meta-analysis and two smaller short-term RCT that were not included in the present review as the duration of the intervention was <4 weeks. The meta-analysis by Huang et al. (2014) of four longitudinal studies on SSB consumption and CHD risk showed that an increase of one serving of SSB daily was associated with a 16 % increased risk of CHD and individuals with the highest SSB intake had 17 % greater risk of CHD than individuals with the lowest SSB intake( 29 ). The first RCT randomly assigned twenty-nine subjects to six 3-week interventions assessing the effects of SSB consumed in small to moderate quantities on lipid and glucose metabolism, and showed a direct relationship between low to moderate SSB consumption and LDL-C and fasting glucose( 30 ). The second study found that ingestion of 500 ml of SSB containing 60 g of fructose increased blood pressure from 30 min to 2 h post-ingestion significantly more than did water intake (P<0·01)( 31 ). The picture is less clear when considering vascular events rather than vascular risk factors, as some studies found a direct association( 26 , 27 ) while others did not( 17 , 18 ). Furthermore, few studies examined if differences in blood pressure, blood lipid and blood sugar were actual mediators of the associations between SSB and vascular events, and for those that did so, results were inconsistent( 19 , 27 ). Inconsistent results were also found in regard to examining diabetes and hypertension as mediators. In one of the two studies that performed mediation analyses for diabetes and hypertension, the association between SSB intake and stroke risk was slightly attenuated( 19 ) whereas the results remained unchanged in the other study( 26 ). Results regarding energy intake and BMI were more consistent as no mediation effects were reported( 18 , 19 ).
As described earlier, the sugar load contained in SSB may induce rapid increases in blood sugar, insulin resistance and lead to changes in lipid profile and blood pressure. In turn, hyperglycaemia, insulin resistance, increased LDL-C and TAG may trigger inflammation responses and the production of free radicals which affect vascular functions( 32 ). However, as highlighted in the present review, the different mediating factors in the pathway between SSB consumption and CVD have not yet been sufficiently investigated. Thus, future studies should examine potential pathways and mediations of the relationship between SSB consumption and vascular events, and consider including mediating factors such as blood pressure, lipid metabolism, blood sugar and inflammation markers to examine and better understand their role in the pathway between SSB intake and vascular events.
The source of added sugar in SSB comes from either sucrose or high-fructose corn syrup, both of which are composed of 50–55 % fructose (and 45–50 % glucose). As the metabolic response to fructose differs from that to glucose, this composition may be of importance in understanding the association between SSB and CVD. Fructose has been shown to cause a variety of metabolic effects such as dyslipidaemia, raised blood pressure and increased visceral adiposity( 33 – 35 ), potentially owing to the facts that (i) unlike glucose, fructose does not need insulin to be metabolised and (ii) fructose metabolism occurs more rapidly and almost exclusively in the liver( 16 , 20 ). Due to their higher fructose v. glucose content, SSB consumption may be a contributing factor to the incidence of vascular events( 33 , 36 ). In the present review, differentiation between fructose and glucose effects could not be made and more studies examining relationships between fructose or glucose and risk of developing vascular events are needed to determine whether limiting the proportion of fructose in SSB would be an adequate strategy for the prevention of vascular events and other chronic diseases.
In general, the evidence for an association between SSB and stroke seemed stronger for women than for men, with two studies finding a direct association among women and no association among men( 18 , 19 ). These results are in agreement with the findings from the review by Fried and Rao (2003), who reported that associations between high-glycaemic-index foods, including beverages such as SSB, and higher serum TAG concentrations and risk of CHD were stronger for women( 37 ). The somewhat stronger associations generally seen for women may relate to gender differences in lipid and glucose metabolism as well as hypertension between men and women. Increased CVD risks have been described primarily in postmenopausal women. Indeed, premenopausal women have a more favourable lipoprotein profile and consequently lower CVD risk relative to similarly aged men, due to the stimulatory effect of oestrogen and the inhibitory effects of androgen that disappear after menopause. Consequently, CVD death rates are two to three times higher among postmenopausal women than for women of the same age who have not yet reached menopause and furthermore seem to exceed the death rates for same-age men( 38 ). (For further details, see online supplementary material: Expanded Discussion.)
The majority of the included studies had a quality score of A, indicating a low bias level. The two studies( 17 , 22 ) which were graded level B had methodological gaps that may have affected their conclusion. Despite the majority of studies being attributed an A score, the total strength of the evidence was graded ‘limited to fair’ because of the low number of studies and inconsistent results from different studies. Regarding vascular events in particular, only two studies found a direct association between SSB intake and CHD( 26 , 27 ) and one study found a direct association for combined vascular events( 17 ). For stroke, results for women were more consistent, although no association was found among the three reviewed studies in general( 17 – 19 ). Therefore, to strengthen the evidence, more well-designed studies are needed. When only quality A studies are considered, the strength of the evidence increases from ‘limited to fair’ to ‘fair to good’ regarding the association between SSB and stroke and decreases from ‘limited to fair’ to ‘limited’ regarding the association between SSB and blood glucose. The present review is the first to systematically review published studies on SSB and CVD with a focus on mediation of associations by differences in risk factors such as blood pressure, blood lipids and blood sugar. Furthermore, the review used a validated quality assessment tool and the grading of the strength of the evidence, which provide a more critical evaluation.
One limitation to our review is the inclusion of only published articles. Publication bias, favouring studies that show an association between SSB and CVD, cannot be excluded and it is possible that the inclusion of unpublished articles and reports would have limited the positive findings further. In addition, most included studies, eight of eleven, were from the USA, and therefore generalisation to other populations of different racial and ethnic backgrounds may not be possible.
The majority of included studies (n 10) assessed dietary intake using an FFQ. Any diet assessment method, including FFQ, is prone to measurement errors or recall bias that might lead to over- or underestimation of dietary intake. Obese people tend to under-report their sugar and fat intakes more than normal-weight individuals( 39 , 40 ) and consequently this may have inflated results in some cases. Therefore, studies included in the review might have suffered from such bias.
As with any assessment tool, the quality criteria and the grading of the strength of the evidence checklists of the Academy of Nutrition and Dietetics (former ADA)( 15 ) present some limitations, and our quality assessment approach may hence also introduce some errors. In addition, assessing the degree of the evidence may be subject to interpretation and may consequently introduce some bias. In this regard, the assessment of the quality and strength of the evidence by two independent researchers rather than one limits the opportunity for bias.
Conclusion
The strength of the evidence relating SSB and CVD is still limited. The reviewed papers generally showed discrepant results for the association between SSB intake and vascular events, while the evidence for an association between SSB and vascular risk factors was stronger. However, due to the limited number of studies investigating these associations and the discrepant results, further studies are needed.
Acknowledgements
Financial support: This review received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: The question of the review was discussed and developed by A.K. and B.L.H. A.K. and N.O. performed the literature search, extracted the data and assessed quality. A.K. wrote the manuscript. B.L.H. and N.O. revised the manuscript. All authors read and approved the final draft of the manuscript. Ethics of human subject participation: Ethical approval was not needed.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980014002122.
click here to view supplementary material
References
- 1. Institute of Medicine, Food and Nutrition Board (2005) Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, DC: National Academies Press.
- 2. Johnson RK, Appel LJ, Brands M et al. (2009) Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 120, 1011–1020. [DOI] [PubMed] [Google Scholar]
- 3. Malik VS, Popkin BM, Bray GA et al. (2010) Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 121, 1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malik VS, Popkin BM, Bray GA et al. (2010) Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 33, 2477–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sonestedt E, Overby NC, Laaksonen DE et al. (2012) Does high sugar consumption exacerbate cardiometabolic risk factors and increase the risk of type 2 diabetes and cardiovascular disease? Food Nutr Res 2012, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tappy L & Lê K-A (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90, 23–46. [DOI] [PubMed] [Google Scholar]
- 7. Tappy L, Lê KA, Tran C et al. (2010) Fructose and metabolic diseases: new findings, new questions. Nutrition 26, 1044–1049. [DOI] [PubMed] [Google Scholar]
- 8. Vartanian LR, Schwartz MB & Brownell KD (2007) Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health 97, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pereira MA (2006) The possible role of sugar-sweetened beverages in obesity etiology: a review of the evidence. Int J Obes (Lond) 30, Suppl. 3, S28–S36. [Google Scholar]
- 10. Hu FB & Malik VS (2010) Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav 100, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weed DL, Althuis MD & Mink PJ (2011) Quality of reviews on sugar-sweetened beverages and health outcomes: a systematic review. Am J Clin Nutr 94, 1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richelsen B (2013) Sugar-sweetened beverages and cardio-metabolic disease risks. Curr Opin Clin Nutr Metab Care 16, 478–484. [DOI] [PubMed] [Google Scholar]
- 13. Brown CM, Dulloo AG & Montani J-P (2008) Sugary drinks in the pathogenesis of obesity and cardiovascular diseases. Int J Obes (Lond) 32, Suppl. 6, S28–S34. [DOI] [PubMed] [Google Scholar]
- 14. Althuis MD & Weed DL (2013) Evidence mapping: methodologic foundations and application to intervention and observational research on sugar-sweetened beverages and health outcomes. Am J Clin Nutr 98, 755–768. [DOI] [PubMed] [Google Scholar]
- 15. Academy of Nutrition and Dietetics (2012) Evidence Analysis Manual – Steps in the Academy Evidence Analysis Process. Chicago, IL: Academy of Nutrition and Dietetics. [Google Scholar]
- 16. Miller PE, McKinnon RA, Krebs-Smith SM et al. (2013) Sugar-sweetened beverage consumption in the US. Am J Prev Med 45, 416–421. [DOI] [PubMed] [Google Scholar]
- 17. Gardener H, Rundek T, Markert M et al. (2012) Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Intern Med 27, 1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eshak ES, Iso H, Kokubo Y et al. (2012) Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: the Japan Public Health Centre-based study cohort I. Am J Clin Nutr 96, 1390–1397. [DOI] [PubMed] [Google Scholar]
- 19. Bernstein AM, de Koning L, Flint AJ et al. (2012) Soda consumption and the risk of stroke in men and women. Am J Clin Nutr 95, 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duffey KJ, Gordon-Larsen P, Steffen LM et al. (2010) Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr 92, 954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen L, Curhan G & Forman J (2012) Association of sweetened beverage intake with incident hypertension. J Gen Intern Med 27, 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dhingra R, Sullivan L, Jacques PF et al. (2007) Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 116, 480–488. [DOI] [PubMed] [Google Scholar]
- 23. Chen L, Caballero B, Mitchell DC et al. (2010) Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation 121, 2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mærsk M, Belza A, Stødkilde-Jørgensen H et al. (2012) Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 95, 283–289. [DOI] [PubMed] [Google Scholar]
- 25. Barrio-Lopez MT, Martinez-Gonzalez MA, Fernandez-Montero A et al. (2013) Prospective study of changes in sugar-sweetened beverage consumption and the incidence of the metabolic syndrome and its components: the SUN cohort. Br J Nutr 110, 1722–1731. [DOI] [PubMed] [Google Scholar]
- 26. de Koning L, Malik VS, Kellogg MD et al. (2012) Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 125, 1735–1741. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fung TT, Malik V, Rexrode KM et al. (2009) Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 89, 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malik AH, Akram Y, Shetty S et al. (2014) Impact of sugar-sweetened beverages on blood pressure. Am J Cardiol 113, 1574–1580. [DOI] [PubMed] [Google Scholar]
- 29. Huang C, Huang J, Tian Y et al. (2014) Sugar sweetened beverages consumption and risk of coronary heart disease: a meta-analysis of prospective studies. Atherosclerosis 234, 11–16. [DOI] [PubMed] [Google Scholar]
- 30. Aeberli I, Gerber PA, Hochuli M et al. (2011) Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 94, 479–485. [DOI] [PubMed] [Google Scholar]
- 31. Brown CM, Dulloo AG, Yepuri G et al. (2008) Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol 294, R730–R737. [DOI] [PubMed] [Google Scholar]
- 32. Malik VS, Pan A, Willett WC et al. (2013) Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 98, 1084–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le MT, Frye RF, Rivard CJ et al. (2012) Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism 61, 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stanhope KL, Griffen SC, Bremer AA et al. (2011) Metabolic responses to prolonged consumption of glucose- and fructose-sweetened beverages are not associated with postprandial or 24-h glucose and insulin excursions. Am J Clin Nutr 94, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stanhope KL, Schwarz JM, Keim NL et al. (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119, 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stanhope KL, Bremer AA, Medici V et al. (2011) Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 96, E1596–E1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fried SK & Rao SP (2003) Sugars, hypertriglyceridemia, and cardiovascular disease. Am J Clin Nutr 78, issue 4, 873S–880S. [DOI] [PubMed] [Google Scholar]
- 38. Matthan NR, Jalbert SM, Barrett PHR et al. (2008) Gender-specific differences in the kinetics of nonfasting TRL, IDL, and LDL apolipoprotein B-100 in men and premenopausal women. Arterioscler Thromb Vasc Biol 28, 1838–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poppitt SD, Swann D, Black AE et al. (1998) Assessment of selective under-reporting of food intake by both obese and non-obese women in a metabolic facility. Int J Obes Relat Metab Disord 22, 303–311. [DOI] [PubMed] [Google Scholar]
- 40. Goris AH, Westerterp-Plantenga MS & Westerterp KR (2000) Undereating and underrecording of habitual food intake in obese men: selective underreporting of fat intake. Am J Clin Nutr 71, 130–134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980014002122.
click here to view supplementary material