Highlights
-
•
A renewed interest exists in miRNAs as biomarkers in cardiovascular disease.
-
•
miR-126–3p is associated with albuminuria, a marker of endothelial dysfunction, in hypertension.
-
•
High miR-126–3p levels are associated with cardiovascular events in general population.
Keywords: Endothelial dysfunction, Cardiovascular risk, Cardiovascular event, Albuminuria, MicroRNA, Biomarker
Abstract
Background
Endothelial dysfunction is a forerunner of atherosclerosis, leading to cardiovascular disease, and albuminuria is a marker of endothelial dysfunction. Circulating levels of microRNAs are emerging as potential biomarkers for cardiovascular disease. Here we estimate the predictive value of a plasma microRNAs signature associated with albuminuria in the incidence of cardiovascular events.
Methods
Plasma microRNAs quantified in hypertensive patients by next generation sequencing were validated in a cohort of patients and controls by real-time quantitative PCR. The microRNAs found to be associated with albuminuria were analysed for their prognostic value in predicting cardiovascular events incidence on a retrospective, population-based study (Hortega Study), using Cox proportional hazard models.
Results
A plasma microRNA profile was identified in the discovery cohort (n = 48) associated with albuminuria and three microRNAs (miR-126–3p, miR-1260b and miR-374a-5p) were confirmed in the validation cohort (n = 98). The microRNA signature discriminates urinary albumin excretion at baseline (n = 1025), and predicts the incidence of cardiovascular events and coronary heart disease and stroke in a general population retrospective study within a 14-year follow-up (n = 926). High miR-126–3p levels were associated with a shorter time free of both cardiovascular events (HR=1.48, (1.36–1.62), p < 0.0001), as well as coronary artery disease and stroke combined (HR=2.49, (2.19–2.83), p < 0.0001).
Conclusions
An increased plasma microRNAs profile was identified in hypertensive patients with albuminuria. Increased miR-126–3p suggest it may serve as a prognostic marker for cardiovascular events in a long-term general population. Further studies will assess the potential role of miR-126–3p as a guide for the status of endothelial dysfunction.
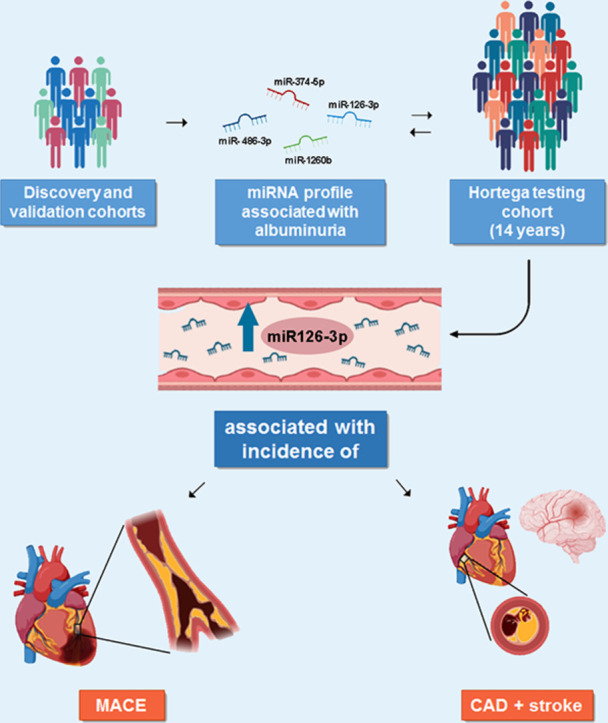
Graphical abstract
Circulating miR-126–3p as a prognostic marker of MACE and combined CAD and stroke in a long-term general population cohort. A signature of 4 miRNAs is associated with albuminuria in the discovery and validation cohorts of hypertensive patients and controls. in a general population cohort (Hortega testing cohort) followed during 14 years, increased levels of plasma circulating miR-126–3p are associated with the incidence of MACE (major adverse cardiovascular events) and combined CAD (coronary artery disease) and stroke.
1. Introduction
Endothelial dysfunction is a forerunner in the development of atherosclerosis, cardiovascular (CV) and renal disease [1]. A marker of endothelial dysfunction is an increase in urinary albumin excretion (UAE), known as albuminuria [2,3]. This condition is frequent in diabetic (DM) patients and has also been used to stratify CV and renal risk in patients with hypertension (HTN) and/or chronic kidney disease (CKD) [4,5]. Despite UAE being generated in the kidney, its value as a CV risk marker has been demonstrated in post-hoc analysis of clinical trials and prospective studies [6], [7], [8], [9], [10], [11], [12]. Advances in the knowledge of mechanisms leading to endothelial dysfunction may provide reliable new molecular biomarkers of incipient endothelial cell (EC) injury for their early detection and prognostic value.
Over the last few years, a trend has emerged supporting the use of microRNAs (miRNAs) as biomarkers for different diseases, given that their key role in disease pathogenesis and progression has been demonstrated [13], [14], [15], [16]. miRNAs are small non-coding RNAs involved in multiple cellular and molecular processes including differentiation, proliferation, fibrosis and apoptosis by regulating gene expression [17], [18], [19]. Their presence in several biofluids, such as plasma or urine, and the evidence that their changes may be a surrogate marker of cellular alterations, make them non-invasive indicators for detecting early mechanisms of cellular damage or repair. Therefore, miRNA levels have been proposed as a promising biomedical tool in precision medicine. Previous studies analysing the prognostic role of miRNAs in CV risk were based on the association with disease without analysing potential mechanisms [20]. In contrast, the present analysis relied on the impact of the miRNAs analysed compared to a well-established marker of endothelial dysfunction such as increased albuminuria.
The role and prognostic value of circulating plasma miRNAs in the development of albuminuria is not well established. In fact, there are studies assessing the effect of miRNAs on albuminuria in animal models [21], [22], [23], and those in humans are mainly association studies [24], [25], [26]. Recently, our group performed a small RNA-sequencing (RNA-seq) study on plasma and plasma derived exosomes, revealing an exosomal miRNA signature associated with albuminuria in HTN patients [27]. The prognostic role of circulating plasma miRNAs in albuminuria as a result of endothelial dysfunction remains elusive as a consequence of the scarcity of prospective studies.
Based on our previous studies about the prognostic value of albuminuria for CV risk [12] and the association of albuminuria with a plasma miRNA signature [27], the objective of the present study was to estimate the predictive value of circulating plasma miRNAs for albuminuria and CV events. To achieve this objective, we tested the prognostic value of the miRNA profile on cardiovascular disease (CVD) in a large retrospective population-based study (Hortega cohort) followed up for 14 years.
2. Methods
2.1. Study design
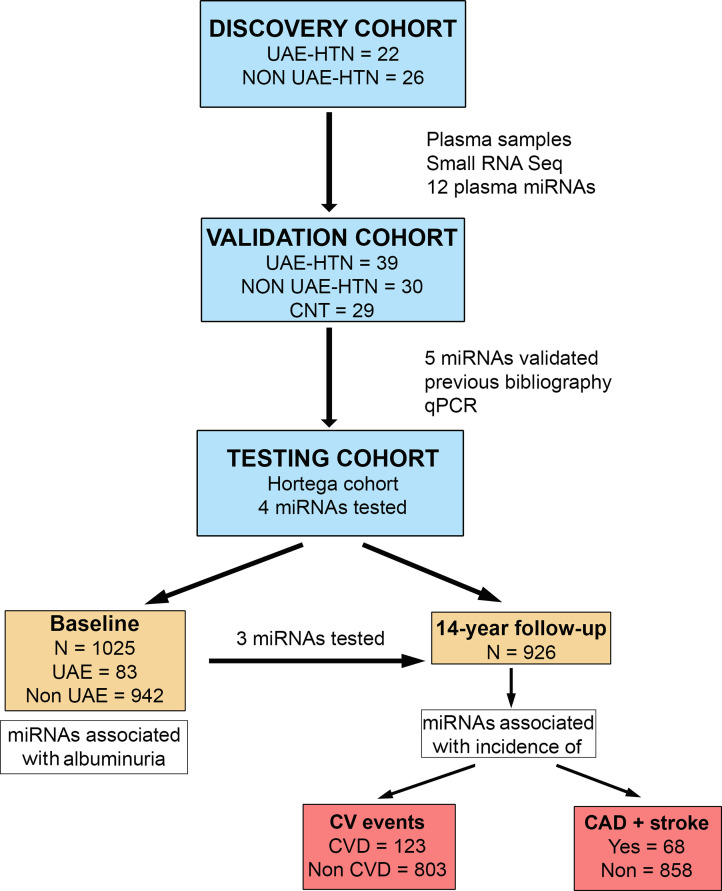
The first phase of the study was to identify the plasma miRNAs associated with albuminuria in patients with HTN and controls. In a second step, a predictive model of miRNAs to assess the risk to develop CV events was analysed in a retrospective study of the general population, on those who did not have previous CVD and had complete basal information on relevant covariates (Fig. 1).
Fig. 1.
Study design for miRNA identification associated with albuminuria and cardiovascular events. The study design involved a discovery phase including hypertensives (HTN) (n = 48) with urinary albumin excretion (UAE) or normoalbuminuric (Non-UAE) by small RNA sequencing (Small RNA-Seq). In a second validation stage with a large hypertensive cohort (n = 69), including UAE or Non-UAE, and healthy controls (CNT, n = 29), 5 microRNA (miRNAs) were validated by real-time quantitative polymerase chain reaction (RT-qPCR). Finally, in a testing population cohort (Hortega's cohort), at baseline (n = 1025), the associations with 3 miRNAs (miR-374a-5p, miR-1260b and miR-126–3p) and albuminuria were tested. Then, at the 14-year follow-up (n = 926), the predictive power of miRNA levels with cardiovascular disease (CVD) events and combined coronary artery disease (CAD) + stroke incidence was investigated.
2.2. Study populations
The first step of the study involved two groups: a discovery cohort (miRNA screening by RNA-seq) and a validation cohort (miRNA validated by quantitative PCR), both consisting of hypertensive patients and controls from the Internal Medicine section of the Hospital Clinico of Valencia. For the second step, a general population, the Hortega testing cohort (n = 1502), was followed up during 14 years [28]. For cross-sectional analysis (n = 1025), we excluded 44 subjects from the total Hortega cohort due to no sample availability, 67 due to a previous cancer diagnosis, 232 due to no renal function follow-up and 37 for no clinical data availability. Moreover, 97 subjects had miRNA expression levels above 35 cycles, rendering the miRNA data unreliable for the analysis. Finally, for the follow-up study we excluded 94 subjects due to a previous CVD and 5 were lost, thus, this cohort numbered 926 subjects (Supplemental Fig. 1). The research protocol and the different phases of the study have been approved by the Ethics Committee of Clinical Research of the University Hospital Rio Hortega (UHRH) (Health Department of Eastern Valladolid, Spain). Data bases have been posted with the Spanish Data Protection Agency. Moreover, the study protocol performed in the discovery and confirmation groups was approved by the Ethics Committee of the Hospital Clinico Universitario of Valencia. All subjects signed written informed consent.
2.3. Biological samples
Human blood samples for both cohorts were collected in EDTA tubes, immediately centrifuged at 4 °C to separate the plasma fraction, aliquoted in RNAse free tubes and stored at -80 °C for further analysis. The biological samples from the Hortega Study participants were stored at -80oC as private collections within University Hospital Rio Hortega of Valladolid and Biomedical Research Institute of the Hospital Clinico of Valencia (INCLIVA). One discovery cohort plasma aliquot was used for small RNA-sequencing and further validation results by quantitative real-time polymerase chain reaction (qRT-PCR). One testing cohort plasma aliquot was used to extract RNA and validate the sequencing miRNA results also by qRT-PCR.
2.4. Evaluation of variables at baseline and follow-up
At baseline, the following CV risk factors were gathered: Body Mass Index (BMI) was calculated by kg/m2; HTN was defined as an office mean systolic blood pressure ≥140 mm Hg, a mean diastolic blood pressure ≥90 mm Hg, a recorded physician diagnosis, or medication use. Diabetes was defined as a non-fasting glucose ≥200 mg/dl, a recorded physician diagnosis, medication use or an HbA1c ≥6.5%. Analytical parameters were: serum total cholesterol was measured enzymatically using the Cholesterol High Performance reagent (Roche Diagnostics); High density-lipoprotein (HDL) cholesterol was measured using a direct HDL reagent (Roche Diagnostics); LDL cholesterol was calculated by using the Friedwald formula; serum creatinine was measured, and estimated glomerular filtration rate (eGFR) was calculated from creatinine, age and sex using the CKD-EPI [29], expressed as ml/min/1.73 m2; UAE and/or proteinuria was assessed in first voiding urine in the morning and expressed as the ratio with urinary creatinine (mg/g). UAE >30 mg/g was considered albuminuria. Treatment with statins was also registered.
2.5. Follow-up and clinical end points
Adjudicated incident events during the 14-year follow-up study were obtained from the hospital health records. The primary outcome was CVD incidence (n = 123), defined as the first episode of hospitalization for any CV cause, coronary artery disease (CAD), stroke or heart failure, including both fatal and non-fatal events . The second endpoint was to consider the incidence of CAD (n = 37) and stroke (n = 31) separately. Time to event was calculated as the difference between the date of the baseline examination and the date of the event, the date of death or 30 November 2015 (administrative censoring), whichever occurred first.
2.6. Statistical analysis
Data are expressed as qualitative or quantitative values with average and standard deviation (SD). Differences were sought by Chi-square, Student's t-test, respectively, or Mann Whitney-U test for urinary UAE and miRNA levels. Logistic regression was used to combine two or three miRNAs to a score which was interpreted as a diagnostic marker for discrimination of cases and controls. The accuracy of miRNA alone or signature for albuminuria was investigated by the receiver operating characteristic (ROC) curve, and the area under curve (AUC) was calculated in the testing cohort. In the follow-up study, the estimated hazard ratios (HR) and 95% confidence intervals (CI) for CVD and combined CAD and stroke incidence were calculated by using weighted multivariate Cox proportional hazards regression with a robust variance specification to obtain representative estimates from the underlying source population. MiRNAs were introduced into the models to assess the incidence of CV events, as a dichotomous variable, according to AUC cut-off values. Models were adjusted for age, gender, BMI (kg/m2), smoking status (never, former, current smoker), estimated glomerular filtration rate (eGFR) (ml/min/1.73 m2), HDL (mg/dL), LDL (mg/dl), DM (no, yes), systolic blood pressure (mmHg), lipid-lowering medication (no, yes) and UAE (low <30 mg/g, high >30 mg/g). The analysis was performed using SPSS 26 package (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
Extended methods for the molecular analyses performed are explained in detail in the Supplemental Methods section.
3. Results
3.1. General characteristics of the study population
The discovery cohort included 48 essential hypertensive subjects, 22 with and 26 without albuminuria (Supplemental Table 1). Similarly, a second set of subjects which included 69 patients with HTN, with and without albuminuria, and 29 healthy controls were used for validation analysis (Table 1). The Hortega study population, included 1025 participants in the absence of previous CV events of which 94 had increased UAE at baseline that were older, with higher systolic blood pressure, fasting glucose, plasma creatinine and percentage of obesity, Table 2. Among the 926 subjects during the follow-up, 16 developed albuminuria and 123 developed a CV event, of which 68 had combined CAD and stroke.
Table 1.
Clinical characteristics of the validation cohort.
| Variables | Albuminuria (UAE) (n = 39) | Normoalbuminuric (Non UAE) (n = 30) | Controls (CNT) (n = 29) |
|---|---|---|---|
| Age (years) | 58.0 ± 11.2* | 54.6 ± 5.6* | 42.0 ± 10.8* |
| Gender (male) | 70.6%* | 64.6%* | 37.9% |
| Systolic blood pressure (mmHg) | 138 ± 19 | 134 ± 24 | 115 ± 16 |
| Diastolic blood pressure (mmHg) | 83 ± 11 | 88 ± 15 | 75 ± 5 |
| Pulse pressure (mmHg) | 54 ± 15 | 48 ± 6 | 40 ± 14 |
| Glucose (mg/dL) | 130 ± 51* | 118 ± 40 | 100 ± 12 |
| Glycated hemoglobin (%) | 6.9 ± 1.2 | 6.1 ± 0.8 | Nd |
| Total Cholesterol (mg/dL) | 190 ± 35† | 174 ± 28 | 198 ± 16 |
| LDL (mg/dL) | 120 ± 31† | 109 ± 23 | 115 ± 13 |
| HDL (mg/dL) | 47 ± 13 | 49 ± 11 | 54 ± 18 |
| Triglycerides (mg/dL) | 171 ± 114 | 129 ± 59 | 131 ± 13 |
| Plasma creatinine (mg/dL) | 1.0 ± 0.38 | 0.89 ± 0.21 | 0.70 ± 0.10 |
| eGFR (mL/min/1.73 m2) | 86 ± 30 | 88 ± 19 | 104 ± 15 |
| BMI (kg/m2) | 33 ± 7 | 30 ± 6 | 24 ± 3 |
| Obesity (%) | 56** | 41** | 0 |
| Obesity grade (%) Grade I Grade II Grade III |
22 22 12 |
19 11 11 |
nd nd nd |
| Diabetes (%) | 43** | 32** | 0 |
| Dyslipidemia (%) | 92*** | 82*** | 7 |
| Smoking (%) | 38* | 48* | 11 |
| Ex-Smoking (%) | 32* | 14 | 11 |
| Urinary albumin excretion/Creatinine (mg/g) | 166 (66–406)***†† | 2.7 (1.8–4.2) | 2.2 (1.5–4.0) |
| Antihypertensive treatment (%) ARB CCB Diuretics |
90 38 64 |
93 32 64 |
nd nd nd |
Data are expressed as mean ± SD, unless otherwise noted. Glomerular filtration rate calculated by MDRD formula. Comparisons between groups: * p < 0.05, ** p < 0.01, *** p < 0.001 vs control group; † p < 0.05, †† p < 0.01 vs. normoalbuminuric. ARB, angiotensin II receptor antagonists; BMI, body mass index; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, high-density lipoprotein.
Table 2.
Clinical characteristics of testing Hortega cohort by albumin excretion status and cardiovascular disease status.
| Albumin excretion status |
CVD status |
||||||
|---|---|---|---|---|---|---|---|
| Total (n = 1025) | UAE (n = 83) | Non UAE (n = 942) | Total (n = 926) | CVD (n = 123) | Non CVD (n = 803) | ||
| Age (years) | 52.1 ± 18.8 | 62.1 ± 19.7b | 51.3 ± 18.5 | 49.9 ± 18.2 | 69.0 ± 13.1b | 46.9 ± 17.0 | |
| Gender (male) | 49.5% | 45.8% | 49.8% | 48.2% | 51.2% | 47.7% | |
| SBP (mmHg) | 130.2 ± 22.2 | 142.0 ± 34.1b | 129.1 ± 20.5 | 128.7 ± 21.6 | 144.9 ± 22.1b | 126.3 ± 20.4 | |
| DBP (mmHg) | 79.4 ± 10.9 | 81.8 ± 13.5 | 79.2 ± 10.5 | 79.1 ± 11.4 | 83.2 ± 11.2b | 78.5 ± 11.3 | |
| PP (mmHg) | 51.1 ± 17.1 | 63.9 ± 22.3b | 50.0 ± 16.1 | 49.6 ± 16.2 | 61.7 ± 19.7b | 47.8 ± 14.8 | |
| Glucose (mg/dL) | 92.6 ± 20.4 | 103.8 ± 30.9b | 91.4 ± 18.6 | 91.0 ± 18.6 | 94.7 ± 19.5a | 90.5 ± 18.4 | |
| Total Cholesterol (mg/dL) | 202.0 ± 37.6 | 205.9 ± 41.6 | 201.7 ± 37.2 | 202.4 ± 37.6 | 207.5 ± 38.1 | 201.7 ± 37.5 | |
| HDL (mg/dL) | 51.8 ± 14.1 | 49.9 ± 15.1 | 51.9 ± 14.0 | 52.3 ± 14.2 | 51.3 ± 13.3 | 52.5 ± 14.4 | |
| Lipid-lowering medication (%) | 6.5 | 7.2 | 6.5 | 8.9 | 13.2 | 20.3 | |
| Plasma creatinine (mg/dL) | 0.85 ± 0.28 | 1.03 ± 0.72b | 0.83 ± 0.18 | 0.83 ± 0.20 | 0.87 ± 0.27a | 0.83 ± 0.18 | |
| BMI (kg/m2) | 26.4 ± 4.2 | 27.2 ± 5.6 | 26.3 ± 4.1 | 26.4 ± 4.3 | 27.5 ± 4.2b | 25.9 ± 4.1 | |
| Obesity (%) | 17.8 | 27.7b | 16.9 | 16.2 | 26.0 | 14.7 | |
| DM (%) | 8.1 | 26.5b | 6.5 | 5.9 | 17.9b | 4.1 | |
| Smoking status (%) Never Former Current |
46.1 29.6 24.3 |
49.5 37.1a 13.4a |
45.8 29.0 25.2 |
45.8 27.9 26.3 |
54.5 30.1 15.4 |
44.5 28.0 27.5 |
|
| eGFR (mL/min/1.73 m2) | 92.1 ± 19.9 | 77.1 ± 27.2b | 93.5 ± 18.5 | 94.1 ± 19.0 | 79.9 ± 17.5b | 96.3 ± 18.2 | |
| UAE/U-Creatinine (mg/g) | 4.00 (2.19–8.68) | 77.94 (53.37–224.29)b | 3.63 (2.08–6.84) | 3.79 (2.14–8.02) | 4.47 (2.28–10.54)b | 3.74 (2.08–7.77) | |
Data are expressed as mean ± SD, median (IQR) or%. For the comparisons of the variables between albuminuric or normoalbuminuric participants, the Student's t-test was used for the comparisons of numerical variables and the chi-square test was used for the comparisons of categorical variables. BMI = body mass index; CVD = cardiovascular disease; DBP = diastolic blood pressure; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; HDL = high density lipoprotein; PP = pulse pressure; SBP = systolic blood pressure; UAE = urinary albumin excretion; U-Creatinine = urinary creatinine. ap < 0.01 and bp < 0.0001 between UAE status.
3.2. Plasma small RNA sequencing revealed a miRNA profile differentially expressed in hypertensive patients with albuminuria
Differential expression analysis of miRNA levels in patients with HTN, with and without albuminuria, identified a total of 76 significant miRNAs (p < 0.05; Supplemental Table 2). Of these, 26 miRNAs showed the highest fold change with the majority increased in the plasma of albuminuric patients (Supplemental Fig. 2a). Then, we performed a KEGG pathways over-representation analysis, and only the pathways related to endothelial dysfunction, HTN and kidney damage were considered (Supplemental Fig. 2b). This analysis showed a miRNA profile composed of 12 miRNAs that regulates critical pathways involved in endothelial dysfunction (VEGF signaling and platelet activation), endothelial and renal epithelial-mesenchymal transition and fibrosis (MAPK and TFG-β signaling), maintenance of the glomerular filtration barrier (ECM receptor interaction, focal adhesion, cell junctions) and cytoskeleton rearrangement (apoptosis, endocytosis and regulation of actin cytoskeleton) (Supplemental Figure 2c).
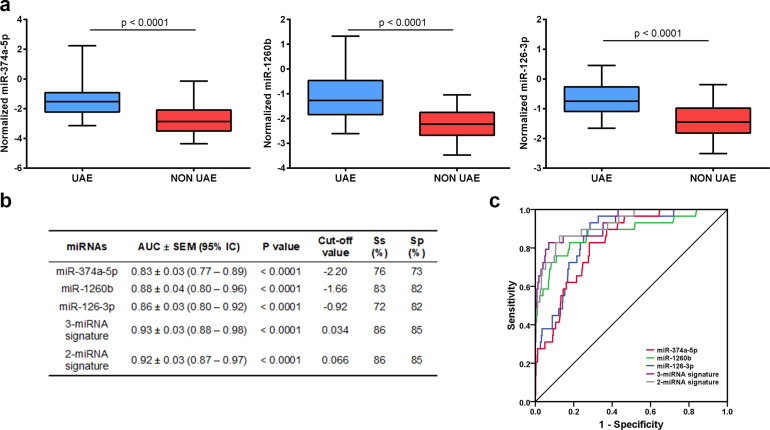
Fig. 2.
Discriminatory power of plasma miRNAs for urinary albumin excretion. (a) Plasma levels of miR-374a-5p, miR-1260b and miR-126–3p in testing cohort 1025 participants, 83 with urinary albumin excretion (UAE). The microRNA (miRNA) levels have been quantified by real-time quantitative polymerase chain reaction (RT-qPCR), expressed as log10-transformed of absolute copy number and was normalized to miR-125a and miR-186 as internal controls. The miR-374a-5p, miR-1260b and miR-126–3p were significantly increased in UAE subjects. Data was compared using the Mann-Whitney U test. The ends of the boxes indicate the quartile 1(upper) and 3 (down), the middle line represents the median, and the whiskers are the minimum and maximum values of the data series (b) The areas under the curve (AUC) and receiver operating characteristics (ROC) curves were calculated for plasma miRNAs in the testing cohort. The diagnostic signature was calculated by a logistic regression model. The AUC of 3-miRNAs and 2-miRNAs signatures were significantly higher when compared to individual miRNAs (p < 0.05). (c) ROC curves are shown for individual and miRNA signatures.
RT-qPCR analysis was performed in a validation cohort and a control group (CNT), which confirmed the increase in plasma levels of four miRNAs in albuminuric patients: miR-374a-5p, miR-1260b, miR-486–3p and miR-126–3p (Supplemental Figure 2d). Conversely, miR-654–3p, miR-486–5p, miR-224–5p, miR-23b-3p and miR-199a-3p values did not show statistical significate changes between UAE groups (Supplemental Fig. 3). In addition, miR-154–3p, miR-411–3p and miR-548ax were detected in less than 50% of the samples.
Moreover, to assess the discriminatory power of the four miRNAs in plasma for UAE presence in hypertensive patients, c-statistics analysis and AUC were calculated. The analysis showed that miR-1260b and miR-126–3p showed the highest AUC (both 0.79 ± 0.05, p < 0.0001). Conversely, miR-486–3p did not have a significant AUC (p = 0.06) (Supplemental Table 3).
3.3. Plasma miRNA signature is associated with UAE at baseline in a testing general population study
To study whether the selected three miRNAs from the validation cohort are specific and able to discriminate UAE in a general population, we tested baseline plasma miRNAs using a large testing general population cohort (Hortega cohort).
The three miRNAs were significantly increased in subjects with UAE (p < 0.0001) (Fig. 2a), and were positively associated with log normalized albuminuria, miR-374a-5p (r = 0.23, p < 0.0001), miR-1260b (r = 0.36, p < 0.0001) and miR-126–3p (r = 0.25, p < 0.0001). In addition, we compared the miRNA levels according to CKD (eGFR <60 mL/min/1.73 m2 and/or UAE/creatinine >30 mg/g), and found a significant increase of all miRNAs in CKD subjects (p < 0.0001, Supplemental Fig. 4). To assess whether the levels of miRNAs in plasma were associated with the presence of UAE, AUCs were calculated for miRNAs individually. All of them had statistically significant AUC values for predicting UAE; miR-1260b had the highest AUC [0.88 ± 0.04, (95% IC 0.80–0.96), p < 0.0001]. A panel combining two or three miRNAs gave the highest AUC of 0.92 ± 0.03 (95% IC 0.87–0.97), p < 0.0001 and AUC of 0.93±0.03 (95% IC 0.88–0.98), p < 0.0001, respectively, with a cut-off of 0.066 and 0.034, respectively. Both had an 86% sensitivity and 85% specificity, that improved the readout of the three individual miRNAs (p < 0.05) (Fig. 2b). The ROC curves for the three individual miRNAs, the 2-miRNA- and 3-miRNA-panels are shown in Fig. 2c. These results suggest that the diagnostic signature based on the three miRNAs could be considered a biomarker with good disease specificity.
3.4. Plasma miR-126-3p is associated with the development of CV events over time: incremental value in risk prediction
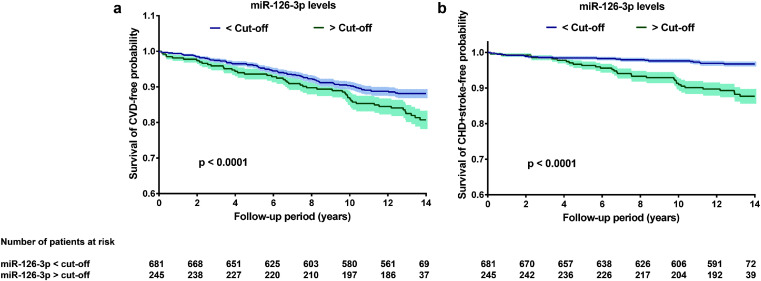
In a prospective study, a weighted Cox proportional hazard regression model was used to estimate the HR using cut-off values of miRNAs from ROC curves (Table 3). The HR (95% CI) for incident CVD of the plasma 2-miRNA signature was 1.22 (1.01–1.44, p < 0.0001) for the 14-year follow-up period, suggesting that UAE subjects with high plasma miRNA levels have a major risk of suffering a CVD event within the 14 years after sampling for measurement of the 2-miRNA signature (miR-1260b and miR-126–3p). In single-miRNA models, however, plasma miR-126–3p had the highest HR, 1.48 (1.36–1.62, p < 0.0001), miR-126–3p levels >−0.91 were associated with a significantly higher incidence rate of CVD events and by time free of CVD events within the 14-year follow-up (Fig. 3a). Furthermore, for combined CAD and stroke, plasma miRNA-126–3p had a significant HR of 2.49 (2.19–2.83, p < 0.0001). The remainder of the individual or miRNA-panels did not reach a significant risk ratio (Table 3). Higher plasma miRNA-126–3p values were also individually related to time free of combined stroke and CAD (Fig. 3b). These findings showed the predictive value of the plasma miR-126–3p levels in identifying CVD presentation in a general population.
Table 3.
Plasma miRNA hazard ratios for CVD and CAD + stroke incidence in testing group (N = 926).
| CVD |
CAD + stroke incidence |
|||
|---|---|---|---|---|
| miRNAs | HR (95% CI) | P value | HR (95% CI) | P value |
| miR-374a-5p | 1.08 (0.95–1.23) | 0.220 | 0.86 (0.72–1.01) | 0.172 |
| miR-1260b | 1.16 (1.04–1.28) | 0.005 | 1.04 (0.91–1.20) | 0.569 |
| miR-126–3p | 1.48 (1.36–1.62) | <0.0001 | 2.49 (2.19–2.83) | <0.0001 |
| 3-miRNA signature | 1.14 (0.99–1.30) | 0.055 | 1.36 (1.06–1.61) | 0.015 |
| 2-miRNA signature | 1.22 (1.01–1.44) | <0.0001 | 1.75 (1.33–2.11) | 0.002 |
Model adjusted for age, gender, body mass index, (kg/m2), smoking status (never, former, current smoker), estimated glomerular filtration rate (ml/min/1.73 m2), high density lipoprotein (mg/dL), diabetes mellitus (no, yes), systolic blood pressure (mmHg) and lipid-lowering medication (no, yes). Receiver Operating Curve (ROC) cut-offs values for microRNA (miRNA) levels: miR-374a-5p, -2.20; miR-1260b, -1.66; miR-126–3p, -0.92; 3-miRNA signature, 0.034; 2-miRNA signature (miR-1260b and miR-126–3p), 0.066. We estimated hazard ratio (HR) and 95% confidence intervals (CI) using weighted the Cox proportional hazards regression model. Cardiovascular incidence, including both fatal and non-fatal events, which was defined as mortality or the first episode of hospitalization for any cardiovascular cause (International Classification of Diseases, 10th Revision (ICD-10) codes I00-I78). CVD: cardiovascular disease; CAD: coronary artery disease.
Fig. 3.
Kaplan–Meier of cardiovascular event-free survival. Testing cohort was grouped according to plasma miR-126–3p, with the value -0.92 as the cut-off obtained from receiver operating characteristic (ROC) analysis during the follow-up period of 14-years for cardiovascular disease (CVD) incidence (a) and for combined coronary artery disease (CAD) and stroke (b). Higher levels of miR-126–3p were associated with a significantly lower incidence rate of CVD and combined CAD and stroke. Weighted long-rank test: p-values at bottom survival curves are shown.
4. Discussion
Our results evaluated the predictive value of plasma miRNA changes for albuminuria and CV events. We found that plasma miR-126–3p was associated with albuminuria in the two different hypertensive groups. Furthermore, regardless of albuminuria, miR-126–3p levels were a prognostic marker for major adverse cardiovascular events (MACE), as well as for combined CAD and stroke, after adjusting for confounding factors such as demographics, CV risk factors and albuminuria at baseline. The association of miR126–3p with CV events incidence supports their potential role as a biomarker for CV risk (Graphical abstract). The value of miRNA over albuminuria for the risk of CV events cannot be supported in the present cohort since the prevalence of albuminuria is low, but perhaps because of this it might indicate that miRNA could be a better marker.
After seminal studies demonstrating that an increment in UAE is dependent on vascular permeability to albumin [2], many studies have focused on the factors related to its development. Both cross-sectional and follow-up studies [30,31], have demonstrated that albuminuria is associated with a clustering of factors of which insulin resistance and high BP are the principal ones [32,33], although others too can play a role [34], [35], [36]. Changes in UAE over time are a proxy of the effect of CV risk factors on the vascular tree, and albuminuria has been linked with CV events, mainly those produced by atherosclerosis. Indeed, clinical trials have reported that a reduction in albuminuria was followed by not only a decrease in CV events, but also in renal events [9], [10], [11]. Likewise, the association of albuminuria with CV risk was observed in previous studies of patients with HTN [12], renal post-transplant [10] and CAD [11]. In a previous prospective study of 2835 hypertensive patients followed up for an average of 4.7 years, our group demonstrated that persistence or new development of albuminuria increased the incidence of CV events [12].
The renewed interest in miRNAs in CV disease, for evaluating new potential biomarkers and therapeutic tools, has produced a large amount of scientific output [37] and concerted cooperation among groups, such as the EU-CardioRNA COST (Cooperation in Science and Technology), a COST action funded in the framework of the European Commission [38]. However, over the last few years, studies assessing the prognostic value of miRNA have shown heterogeneity in terms of study population, settings, miRNAs and outcomes [39,40]. A review by Navickas et al. showed that the majority of studies determined the association of miRNA with the CV state at the time of blood sampling with a lack of follow-up. This did not allow for an assessment of the potential prognostic value [41].
The most striking finding of the present study is the prognostic value of miR-126–3p as a marker of EC dysfunction, albuminuria, and the incidence of CV disease in a general population. Few studies have analysed the prognostic value of various miRNAs [20,42,43]. Moreover, all of them in which miRNAs derived from peripheral blood predict cardiovascular mortality were in secondary prevention settings. Jakob et al. assessed the MACE incidence in patients with ST-segment elevation myocardial infarction within a one-year follow-up [44]; and Badacz et al. analyzed risk factors in a 6-year follow-up of secondary CV events during incident ischemia [45]. Karakas et al. performed the largest study so far evaluating the prognostic value of circulating miRNAs in cardiovascular disease identifying various miRNAs that predicted cardiovascular death in acute coronary syndrome [20]. To date, only one study has a similar design to ours, Zampetaki et al. screened miRNA profile at baseline in a population-based survey and was associated with the incidence of myocardial infarction over a 10-year observation period [46]. However, they focused only on platelet miRNA analysis and did not analyze any association with albuminuria or other endothelial markers.
Another strength of our study is the use of a next generation sequencing to screen a global miRNA profile in all samples from the discovery cohort. Then, we have used stringently controlled methods for absolute RT-qPCR-based quantification of miRNA copy number and we have used multistep spike-in controls to correct for any technical variation in both validation and testing cohorts. With this approach, this study has achieved greater sensitivity and reproducibility for the results. In our population-based analysis, we considered 3 miRNAs that emerged as promising targets for albuminuria and CVD in the pre-screening phases. When we tested for their prognostic value for the incidence of CV events, augmented miR126–3p levels had the highest risk for CVD and combined CAD and stroke when compared to miRNA signatures or miR-1260b. miR-126–3p is an EC miRNA which exerts a role in the maintenance of endothelial integrity and angiogenesis by suppressing VCAM-1 expression and the activity of MAPK signaling. Decreased miR-126–3p leads to: (i) an increment in the TNF-stimulated VCAM-1 expression that promotes leukocyte adherence to endothelial cells; and (ii) a reduction of Spred-1, a negative regulator of MAPK signaling, increasing VEGF- and FGF-mediated EC migration [47], [48], [49]. Although miR-126–3p is not expressed in smooth muscle cells (VSMCs), it participates in the regulation of VSMC function, downregulating insulin receptor substrate-1 (IRS-1), reducing VSMC proliferation and migration [50,51].
Finally, our results have shown that elevated values of plasma miRNA-126–3p are a prognostic marker for both MACE, as well as for a combined atherosclerosis-driven disease such are CAD and stroke, independent of albuminuria. Higher levels of circulating miR126–3p could be in line with previous reports investigating its prognostic role for myocardial infarction in a general population [46], or for implicating endothelial microparticles as biomarkers for vascular damage and increased CV risk [27,52]. It is not possible to answer the question of whether or not miRNA-126–3p could represent a diabetes/hyperglycaemia related EC dysfunction [53]. The fact that the association with albuminuria is also observed in non-diabetic hypertensive patients and the multivariate analysis of the present study, may indicate a broader association with endothelial dysfunction.
4.1. Study limitations
Care must be taken in the design of case-control studies for biomarker selection. Comparisons of circulating miRNAs among patients with manifest HTN and healthy controls, for example, are likely to be confounded by medication. In addition, the origin of the patients for discovery and validation cohorts could need further research using larger and independent cohorts to confirm the miRNAs association. Despite this, the robust study design, with an association of selected miRNA levels in a testing general population cohort, supports the results obtained, even though a fraction of the population was omitted due to the lack of assessment of UAE. Furthermore, causality cannot be inferred from associations of biomarkers in population studies. Additional investigations are warranted to underpin the association of identified miRNAs in understanding the mechanisms of the disease.
5. Conclusions
A circulating miRNA profile is associated with an increment of albuminuria levels in HTN. Increased levels of miR-126–3p serve as a prognostic marker for CV events in a long-term general population study, indicating its potential as a hallmark of EC protection to mitigate the impact of vascular insults. Future prospective studies are a relevant issue to address whether miR-126–3p could be used as therapeutic-guide assessment of endothelial protection.
Declaration of Competing Interest
None.
Acknowledgments
This study was supported by Health Sciences Research grants from the Carlos III Health Institute (grant numbers PI12/02615, PI16/01402, and PI19/01796 to J. Redon; PI18/01405 and PI21/00249 to R. Cortes and MJ Forner; PI21/00506 to FJ. Chaves; FI20/00096, PFIS to O. Martinez-Arroyo; FI22/00032, PFIS for A. Flores-Chova); FP7-HEALTH 11 (grant number no. 278249); and BIGDATA@HEART (grant number H2020-JTI-IMI2-2015-07) to J. Redon. Ministerio de Ciencia e Innovación (grant number IJC2020-045308-I to Ana Ortega. Co-funded by the European Union (the European Regional Development Fund (ERDF) and the European Social Funding (FSE)); CIBER Fisiopatologia Obesidad y Nutricion (CIBEROBN) (grant numbers CIBER-02-08-2009, CB06/03 and CB12/03/30016); CIBER Cardiovascular (CIBERCV) (grant number CB16/11/00261). Castilla-Leon Government (grant number GRS/279/A/08) The Strategic Action for Research in Health sciences, CIBEROBN is co-funded with European Funds for Regional Development (FEDER). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2023.04.013.
Contributor Information
Ana Ortega, Email: aortega@incliva.es.
Raquel Cortes, Email: raquel.cortes@uv.es.
Appendix. Supplementary materials
References
- 1.Gallo G., Volpe M., Savoia C. Endothelial dysfunction in hypertension: current concepts and clinical implications. Front Med. 2021;8 doi: 10.3389/fmed.2021.798958. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deckert T., Feldt-Rasmussen B., Borch-Johnsen K., Jensen T., Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 3.Martens R.J.H., Houben A., Kooman J.P., Berendschot T., Dagnelie P.C., van der Kallen C.J.H., et al. Microvascular endothelial dysfunction is associated with albuminuria: the maastricht study. J Hypertens. 2018;36:1178–1187. doi: 10.1097/HJH.0000000000001674. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes CKDMBDUWG. KDIGO 2017 Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int. 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. Suppl (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., et al. Practice guidelines for the management of arterial hypertension of the European society of hypertension and the European society of cardiology: ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2018;36:2284–2309. doi: 10.1097/HJH.0000000000001961. 2018. [DOI] [PubMed] [Google Scholar]
- 6.Ibsen H., Olsen M.H., Wachtell K., Borch-Johnsen K., Lindholm L.H., Mogensen C.E., et al. Does albuminuria predict cardiovascular outcomes on treatment with losartan versus atenolol in patients with diabetes, hypertension, and left ventricular hypertrophy? The LIFE study. Diabetes Care. 2006;29:595–600. doi: 10.2337/diacare.29.03.06.dc05-1724. [DOI] [PubMed] [Google Scholar]
- 7.Patel A., Group A.C., MacMahon S., Chalmers J., Neal B., Woodward M., et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 8.Zoungas S., de Galan B.E., Ninomiya T., Grobbee D., Hamet P., Heller S., et al. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: new results from the ADVANCE trial. Diabetes Care. 2009;32:2068–2074. doi: 10.2337/dc09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmieder R.E., Mann J.F., Schumacher H., Gao P., Mancia G., Weber M.A., et al. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011;22:1353–1364. doi: 10.1681/ASN.2010091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bielopolski D., Rahamimov R., Zingerman B., Chagnac A., Azulay-Gitter L., Rozen Zvi B. Microalbuminuria after kidney transplantation predicts cardiovascular morbidity. Front Med. 2021;8 doi: 10.3389/fmed.2021.635847. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunimura A., Ishii H., Uetani T., Harada K., Kataoka T., Takeshita M., et al. Prognostic value of albuminuria on cardiovascular outcomes after elective percutaneous coronary intervention. Am J Cardiol. 2016;117:714–719. doi: 10.1016/j.amjcard.2015.11.054. [DOI] [PubMed] [Google Scholar]
- 12.Pascual J.M., Rodilla E., Costa J.A., Garcia-Escrich M., Gonzalez C., Redon J. Prognostic value of microalbuminuria during antihypertensive treatment in essential hypertension. Hypertension. 2014;64:1228–1234. doi: 10.1161/HYPERTENSIONAHA.114.04273. [DOI] [PubMed] [Google Scholar]
- 13.Su Y., Sun Y., Tang Y., Li H., Wang X., Pan X., et al. Circulating miR-19b-3p as a novel prognostic biomarker for acute heart failure. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.022304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perdoncin M., Konrad A., Wyner J.R., Lohana S., Pillai S.S., Pereira D.G., et al. A review of miRNAs as biomarkers and effect of dietary modulation in obesity associated cognitive decline and neurodegenerative disorders. Front Mol Neurosci. 2021;14 doi: 10.3389/fnmol.2021.756499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyoshi J., Zhu Z., Luo A., Toden S., Zhou X., Izumi D., et al. A microRNA-based liquid biopsy signature for the early detection of esophageal squamous cell carcinoma: a retrospective, prospective and multicenter study. Mol Cancer. 2022;21:44. doi: 10.1186/s12943-022-01507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Hernandez J., Martinez-Arroyo O., Ortega A., Galera M., Solis-Salguero M.A., Chaves F.J., et al. Urinary exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in lupus nephritis. J Nephrol. 2021;34:1157–1167. doi: 10.1007/s40620-020-00832-y. [DOI] [PubMed] [Google Scholar]
- 17.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Improta-Caria A.C., Aras M.G., Nascimento L., De Sousa R.A.L., Aras-Junior R., Souza B.S.F. MicroRNAs regulating renin-angiotensin-aldosterone system, sympathetic nervous system and left ventricular hypertrophy in systemic arterial hypertension. Biomolecules. 2021;11 doi: 10.3390/biom11121771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karakas M., Schulte C., Appelbaum S., Ojeda F., Lackner K.J., Munzel T., et al. Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease-results from the large AtheroGene study. Eur Heart J. 2017;38:516–523. doi: 10.1093/eurheartj/ehw250. [DOI] [PubMed] [Google Scholar]
- 21.Ma Z., Li L., Livingston M.J., Zhang D., Mi Q., Zhang M., et al. p53/microRNA-214/ULK1 axis impairs renal tubular autophagy in diabetic kidney disease. J Clin Investig. 2020;130:5011–5026. doi: 10.1172/JCI135536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y., Wang D., Wang F., Liu J., Huang B., Baker M.A., et al. Endogenous miR-204 protects the kidney against chronic injury in hypertension and diabetes. J Am Soc Nephrol. 2020;31:1539–1554. doi: 10.1681/ASN.2019101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei X., Zhang B.D., Ren J.G., Luo F.L. Astragaloside suppresses apoptosis of the podocytes in rats with diabetic nephropathy via miR-378/TRAF5 signaling pathway. Life Sci. 2018;206:77–83. doi: 10.1016/j.lfs.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 24.Riffo-Campos A.L., Perez-Hernandez J., Ortega A., Martinez-Arroyo O., Flores-Chova A., Redon J., et al. Exosomal and plasma non-coding RNA signature associated with urinary albumin excretion in hypertension. Int J Mol Sci. 2022:23. doi: 10.3390/ijms23020823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Huang Y.Q. The correlation of circulating miR-29b and inflammatory markers with albuminuria in hypertensive patients. Clin Exp Hypertens. 2020;42:743–747. doi: 10.1080/10641963.2020.1790585. [DOI] [PubMed] [Google Scholar]
- 26.Ren H., Wu C., Shao Y., Liu S., Zhou Y., Wang Q. Correlation between serum miR-154-5p and urinary albumin excretion rates in patients with type 2 diabetes mellitus: a cross-sectional cohort study. Front Med. 2020;14:642–650. doi: 10.1007/s11684-019-0719-3. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Hernandez J., Riffo-Campos A.L., Ortega A., Martinez-Arroyo O., Perez-Gil D., Olivares D., et al. Urinary- and plasma-derived exosomes reveal a distinct MicroRNA signature associated with albuminuria in hypertension. Hypertension. 2021;77:960–971. doi: 10.1161/HYPERTENSIONAHA.120.16598. [DOI] [PubMed] [Google Scholar]
- 28.Tellez-Plaza M., Briongos-Figuero L., Pichler G., Dominguez-Lucas A., Simal-Blanco F., Mena-Martin F.J., et al. Cohort profile: the Hortega Study for the evaluation of non-traditional risk factors of cardiometabolic and other chronic diseases in a general population from Spain. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-024073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., Feldman H.I., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkins R.C., Briganti E.M., Zimmet P.Z., Chadban S.J. Association between albuminuria and proteinuria in the general population: the AusDiab Study. Nephrol Dial Transplant. 2003;18:2170–2174. doi: 10.1093/ndt/gfg314. [DOI] [PubMed] [Google Scholar]
- 31.Pascual J.M., Rodilla E., Miralles A., Gonzalez C., Redon J. Determinants of urinary albumin excretion reduction in essential hypertension: a long-term follow-up study. J Hypertens. 2006;24:2277–2284. doi: 10.1097/01.hjh.0000249707.36393.02. [DOI] [PubMed] [Google Scholar]
- 32.Redon J., Miralles A., Pascual J.M., Baldo E., Robles R.G., Carmena R. Hyperinsulinemia as a determinant of microalbuminuria in essential hypertension. J Hypertens. 1997;15:79–86. doi: 10.1097/00004872-199715010-00008. [DOI] [PubMed] [Google Scholar]
- 33.Redon J., Liao Y., Lozano J.V., Miralles A., Pascual J.M., Cooper R.S. Ambulatory blood pressure and microalbuminuria in essential hypertension: role of circadian variability. J Hypertens. 1994;12:947–953. [PubMed] [Google Scholar]
- 34.Thoenes M., Reil J.C., Khan B.V., Bramlage P., Volpe M., Kirch W., et al. Abdominal obesity is associated with microalbuminuria and an elevated cardiovascular risk profile in patients with hypertension. Vasc Health Risk Manag. 2009;5:577–585. doi: 10.2147/vhrm.s5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orth S.R. Effects of smoking on systemic and intrarenal hemodynamics: influence on renal function. J Am Soc Nephrol. 2004;15(1):S58–S63. doi: 10.1097/01.asn.0000093461.36097.d5. Suppl. [DOI] [PubMed] [Google Scholar]
- 36.Martinez F., Mansego M.L., Chaves F.J., Redon J. Genetic bases of urinary albumin excretion and related traits in hypertension. J Hypertens. 2010;28:213–225. doi: 10.1097/hjh.0b013e328333afb3. [DOI] [PubMed] [Google Scholar]
- 37.Jusic A., Devaux Y. Action EU-CC. Noncoding RNAs in Hypertension. Hypertension. 2019;74:477–492. doi: 10.1161/HYPERTENSIONAHA.119.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson E.L., Emanueli C., Martelli F., Devaux Y. Leveraging non-coding RNAs to fight cardiovascular disease: the EU-CardioRNA network. Eur Heart J. 2021;42:4881–4883. doi: 10.1093/eurheartj/ehab326. [DOI] [PubMed] [Google Scholar]
- 39.Dai R., Liu Y., Zhou Y., Xiong X., Zhou W., Li W., et al. Potential of circulating pro-angiogenic microRNA expressions as biomarkers for rapid angiographic stenotic progression and restenosis risks in coronary artery disease patients underwent percutaneous coronary intervention. J Clin Lab Anal. 2020;34:e23013. doi: 10.1002/jcla.23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin F., Xing J. Circulating pro-angiogenic and anti-angiogenic microRNA expressions in patients with acute ischemic stroke and their association with disease severity. Neurol Sci. 2017;38:2015–2023. doi: 10.1007/s10072-017-3071-x. [DOI] [PubMed] [Google Scholar]
- 41.Navickas R., Gal D., Laucevicius A., Taparauskaite A., Zdanyte M., Holvoet P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc Res. 2016;111:322–337. doi: 10.1093/cvr/cvw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escate R., Padro T., Suades R., Camino S., Muniz O., Diaz-Diaz J.L., et al. High miR-133a levels in the circulation anticipates presentation of clinical events in familial hypercholesterolaemia patients. Cardiovasc Res. 2021;117:109–122. doi: 10.1093/cvr/cvaa039. [DOI] [PubMed] [Google Scholar]
- 43.Schulte C., Molz S., Appelbaum S., Karakas M., Ojeda F., Lau D.M., et al. miRNA-197 and miRNA-223 predict cardiovascular death in a cohort of patients with symptomatic coronary artery disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakob P., Kacprowski T., Briand-Schumacher S., Heg D., Klingenberg R., Stahli B.E., et al. Profiling and validation of circulating microRNAs for cardiovascular events in patients presenting with ST-segment elevation myocardial infarction. Eur Heart J. 2017;38:511–515. doi: 10.1093/eurheartj/ehw563. [DOI] [PubMed] [Google Scholar]
- 45.Badacz R., Kleczynski P., Legutko J., Zmudka K., Gacon J., Przewlocki T., et al. Expression of miR-1-3p, miR-16-5p and miR-122-5p as possible risk factors of secondary cardiovascular events. Biomedicines. 2021;9 doi: 10.3390/biomedicines9081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zampetaki A., Willeit P., Tilling L., Drozdov I., Prokopi M., Renard J.M., et al. Prospective study on circulating MicroRNAs and risk of myocardial infarction. J Am Coll Cardiol. 2012;60:290–299. doi: 10.1016/j.jacc.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 47.Fish J.E., Santoro M.M., Morton S.U., Yu S., Yeh R.F., Wythe J.D., et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A., et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asgeirsdottir S.A., van Solingen C., Kurniati N.F., Zwiers P.J., Heeringa P., van Meurs M., et al. MicroRNA-126 contributes to renal microvascular heterogeneity of VCAM-1 protein expression in acute inflammation. Am J Physiol Renal Physiol. 2012;302:F1630–F1639. doi: 10.1152/ajprenal.00400.2011. [DOI] [PubMed] [Google Scholar]
- 50.Izuhara M., Kuwabara Y., Saito N., Yamamoto E., Hakuno D., Nakashima Y., et al. Prevention of neointimal formation using miRNA-126-containing nanoparticle-conjugated stents in a rabbit model. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang M., Zhang W., Zhang L., Wang L., Li J., Shu C., et al. Roles of MicroRNAs in peripheral artery in-stent restenosis after endovascular treatment. Biomed Res Int. 2021;2021 doi: 10.1155/2021/9935671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rautou P.E., Vion A.C., Amabile N., Chironi G., Simon A., Tedgui A., et al. Microparticles, vascular function, and atherothrombosis. Circ Res. 2011;109:593–606. doi: 10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- 53.Grieco G.E., Besharat Z.M., Licata G., Fignani D., Brusco N., Nigi L., et al. Circulating microRNAs as clinically useful biomarkers for type 2 diabetes mellitus: miRNomics from bench to bedside. Transl Res. 2022;247:137–157. doi: 10.1016/j.trsl.2022.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.