Abstract
Objective
There have been inconsistent results published regarding the relationship between dyslipidaemia and an increased risk of colorectal neoplasia (CRN), including colorectal adenoma (CRA) and colorectal cancer (CRC). We conducted a meta-analysis to explore the relationship between dyslipidaemia and CRN.
Design
We identified studies by performing a literature search using PubMed, EMBASE and the Science Citation Index through October 2013.
Setting
We analysed thirty-three independent studies reporting the association between CRN and at least one of the selected lipid components, including total cholesterol (TC), TAG, HDL-cholesterol (HDL-C) and LDL-cholesterol (LDL-C).
Subjects
CRN cases (n 21 809) were identified.
Results
Overall, people with high levels of serum TAG (risk ratio (RR)=1·08; 95 % CI 1·05, 1·12, P<0·00001) and LDL-C (RR=1·07; 95 % CI 1·00, 1·14, P=0·04) presented an increased prevalence of CRN. Subgroup analyses revealed that high levels of serum TC (RR=1·04; 95 % CI 1·01, 1·09, P=0·02), TAG (RR=1·06; 95 % CI 1·03, 1·10, P=0·0009) and LDL-C (RR=1·11; 95 % CI 1·04, 1·19, P=0·003) increased the risk of CRA but not of CRC. No association between serum HDL-C and risk for CRN (including CRA and CRC) was observed.
Conclusions
Both TAG and LDL-C were significantly associated with an increasing prevalence of CRN. High levels of serum TC, TAG and LDL-C were positively associated with CRA but not with CRC. No significant association was observed between levels of serum HDL-C and CRN.
Keywords: Serum lipids, Colorectal adenoma, Colorectal cancer, TAG, Meta-analysis
Colorectal cancer (CRC) is reported to be the fourth most commonly diagnosed cancer and is the second most common cause of cancer deaths in North America( 1 ). CRC is believed to arise from colorectal adenoma (CRA) through a sequence from adenoma to adenocarcinoma as a consequence of a limited set of molecular events that largely originate with a relatively benign adenoma that progresses to cancer( 2 ). Accumulated data indicate that metachronous lesions occur at a rate of 20 to 30 % per year in post-polypectomy patients. The propensity to develop CRA identifies a sizeable subgroup of the population at an enhanced risk for subsequent adenoma formation and colorectal carcinoma. This indicates that identifying risk factors associated with CRN is essential for the reduction of colorectal carcinoma.
The positive association between obesity and CRA prevalence demonstrates an underlying dose–response relationship according to BMI( 3 ). Timely screening of obese patients for CRA is thus recommended. Dyslipidaemia is an important component of metabolic syndrome and is demonstrated to contribute to colorectal tumorigenesis through insulin resistance, oxidative stress and inflammatory pathways( 4 ). Alteration of serum lipids (high TAG and low HDL-cholesterol (HDL-C)) has been linked to an increased risk of CRA( 5 , 6 ) and several types of malignancy including CRC( 7 ). A recent large case–control study indicated that a high level of serum TAG was significantly associated with a larger number of adenomas( 8 ). Although several studies have explored the dyslipidaemia component, e.g. lipid and lipoprotein concentrations individually in relation to CRN risk, there is still inconsistency for this issue. More importantly, the association between components of serum lipids and CRN risk is largely unknown.
An increased understanding between the development of CRN and dyslipidaemia can clarify the mechanistic steps linking components of serum lipids and CRN and may be useful in determining the benefits of early CRN screening. Unfortunately, data on the relationship of serum lipid levels with CRA/CRC are contradictory. Some studies have established a positive association between serum total cholesterol (TC) level and CRN. If this theory were correct, it may contribute to an excess in mortality in individuals with dyslipidaemia compared with those without the disorder. However, other studies showed no significant association between CRN and serum lipids( 9 , 10 ). Because the association between dyslipidaemia and CRN formation has not yet been systematically assessed, we conducted a systematic review and meta-analysis of all available studies evaluating this issue to investigate the association between components of serum lipids and CRN risk.
Materials and methods
The current review and meta-analysis follows the recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement( 11 ).
Search strategy
For the present analysis, a systematic literature search through October 2013 was performed in PubMed, EMBASE and the Science Citation Index to identify relevant studies. Studies investigating the relationship between serum lipids and CRN (including CRA and CRC) were carried out by searching for articles written in English. The search term comprised the following keywords: ‘serum lipids’, ‘triglycerides, TG’, ‘total cholesterol, TC’, ‘high-density lipoprotein-cholesterol, HDL-C, or ‘low-density lipoprotein-cholesterol, LDL-C’ combined with ‘colorectal cancer, CRC’ ‘colorectal adenoma, CRA’ or ‘colorectal neoplasm, CRN’ (see online supplementary material, Supplemental Table 1 for the search strategy in PubMed). All references of the selected articles were checked, including manual searches. Additionally, to find any additional published studies, we interrogated references of all the articles. The titles and abstracts were scanned to exclude any clearly irrelevant studies. The full texts of the remaining articles were read to determine whether they contained information on the topic of interest. All searches were conducted independently by two authors (Y.T. and Y.L.). The results were then compared; any questions or discrepancies were resolved by iteration and consensus.
Study selection
Only publications that fulfilled all of the following criteria were selected for the meta-analysis: (i) the study subjects were adults (older than 18 years); (ii) publication with a case–control, cross sectional, nested case–control or cohort study design; (iii) CRN incidence as the outcome of interest; (iv) reported an estimate of the association of CRN (defined as colorectal cancer or adenoma or both) in individuals with at least one of the selected lipid components (TC, TAG, HDL-C and LDL-cholesterol (LDL-C)); and (v) reported risk ratio (RR; or OR estimates in case–control studies) or hazard ratios (HR) with estimates of their corresponding 95 % CI (or sufficient data to evaluate the above effects).
Data extraction
Information extracted from the extensive review of each publication included: (i) publication data (first author’s name, year of publication and country of the population studied); (ii) type of study design; (iii) study participants’ age range; (iv) sample size (cases and controls or cohort size); (v) type of lesion; (vi) risk estimates with their corresponding CI; (viii) method used to confirm the presence or absence of CRN; and (ix) colonoscopy examination at the time of diagnosis. OR from case–control studies were considered as an estimate of RR( 12 ). If a study provided several risk estimates, the most completely adjusted estimate was extracted and used in the meta-analysis. The information from each study was extracted by two independent researchers (Y.T. and Y.L.), with disagreements resolved with a majority vote by all authors.
Statistical analysis
All analyses, including publication bias, were performed using the computer program Review Manage version 5·1 (Oxford, UK). Study-specific risk estimates were extracted from each study and log risk estimates were weighted by the inverse of their variances to obtain a pooled risk estimate. The heterogeneity of all publications was evaluated with the Cochran Q test and I 2 statistic( 13 ). An I 2 value of <30 %, 30–50 % and >50 % was considered as little or no heterogeneity, moderate heterogeneity and severe heterogeneity, respectively. For the Q statistic, a P value of <0·1 was considered to have significant heterogeneity. Summaries of RR estimates were evaluated using both fixed-effects and random-effects methods. Random effects are used when heterogeneity is present. Initial analysis, including all studies, was performed to look for an association between serum lipids and CRN. Subgroup analyses were also carried out to estimate the components of serum lipids and the risk of CRC and CRA. For meta-analysis results, the P value of <0·05 indicated statistical significance. A funnel plot for potential publication bias analysis was conducted using the statistical software package Stata 11·0 with Begg( 14 ) and Egger tests( 15 ).
Results
Study characteristics
Two hundred and eighty-five publications relevant to the words searched were retrieved using the methodology and the search terms described above. Of these, eighty-two duplicated publications were excluded. The remaining 203 studies were selected for further evaluation based on information from abstracts and titles. After screening abstracts and titles, forty-seven studies were considered to be relevant to our study subject. Fourteen articles were excluded as they did not investigate the association of serum lipids with risk of CRN. In total, thirty-three records that met the detailed inclusion criteria were included in the present meta-analysis( 4 , 6 , 8 – 10 , 16 – 43 ). All studies reported on at least one of the serum lipid components (TC, TAG, HDL-C and LDL-C) and the risk of CRN. Details of these studies are described in Tables 1 and 2.
Table 1.
Studies contributing to the analysis of serum lipids and colorectal cancer
| Author/reference and country | Study population | Age (years) | Type of lesion | Serum lipid measurement, RR (95 % CI), start to end | Controlled variables | |
|---|---|---|---|---|---|---|
| Cohort studies | ||||||
| Ahmed et al. (2006)( 16 ), USA | 15 792 general American population | 45–64 | 194 colorectal cancers, including 139 colon cancers | TAG, 1·08 (0·8, 1·5) | Gender, race, education, sports index, age | |
| HDL-C, 1·19 (0·9, 1·6) | ||||||
| Inoue et al. (2009)( 24 ), Japan | 27 724 general Japanese population | 40–69 | 155: 102 colon cancers, 53 rectal cancers | HDL-C, 1·13 (0·8, 1·6) | Age, smoking habits, physical activity, weekly ethanol intake | |
| TAG, 0·84 (0·6, 1·2) | ||||||
| Iso et al. (2009)( 25 ), Japan | 33 368 general Japanese population | 40–69 | 320 colorectal cancers (217 men and 103 women) | TC, 1·03 (0·9, 1·2) | Age, smoking, ethanol intake, BMI, blood pressure, diabetes, vegetable consumption, coffee | |
| Schoen et al. (1999)( 33 ), USA | 5849 general American population | 74 | 102 colorectal cancers | HDL-C, 0·6 (0·3, 1·2) | Gender, race, age, income, marital status, smoking, alcoholic consumption, aspirin use, physical activity, fat and vegetable consumption | |
| LDL-C, 0·5 (0·3, 0·8) | ||||||
| Tsushima et al. (2005)( 40 ), USA | 7619 Japanese-American | Not reported | 500: 376 colon and 124 rectal cancers | TAG, 1·07 (0·8, 1·4) | BMI, heart rate, cigarette smoking, alcohol use, total energy | |
| Wulaningsih et al. (2012)( 42 ), Sweden | 540 309 general Swedish population | 44 | 3982: 2472 colon cancers, 1510 rectal cancers | TC, 1·12 (1·0, 1·3) | Age, gender, socio-economic status | |
| LDL-C, 0·81 (0·5, 1·3) | ||||||
| TAG, 1·18 (1·1, 1·3) | ||||||
| HDL-C, 1·3 (1·0, 1·7) | ||||||
| Bowers et al. (2006)( 21 ), Finland | 28 983 Finnish male smokers | 57 | 227 colon cancer cases 183 rectal cancer cases | HDL-C, 1·09 (0·80, 1·49) | Age, smoking, TC | |
| TC, 0·74 (0·54, 1·01) | ||||||
| Stocks et al. (2010)( 34 ), Norway, Austria, Sweden | 578 700 general population | 65 (men) | 2834 men and 1861 women with colorectal cancer | TC, 1·00 (0·94, 1·06) | Age, smoking, and individual components of metabolic abnormalities | |
| 64 (women) | TC, 1·08 (1·00, 1·17) | |||||
| TAG, 1·17 (1·06, 1·29) | ||||||
| TAG, 1·02 (0·91, 1·14) | ||||||
| Stürmer et al. (2006)( 35 ), USA | 22 071 healthy male physicians | 40–84 | 494 colorectal cancers | TAG, 0·9 (0·7, 1·2) | Age, exercise, smoking, alcohol use, non-steroidal anti-inflammatory drugs | |
| Trevisan et al. (2001)( 37 ), USA, Italy | 21 311 men and 15 991 women | 20–69 | 41 men and 13 women with colorectal cancer | HDL-C, 0·92 (0·48, 1·76) | Age, drinking alcoholic beverages, smoking | |
| TAG, 1·01 (0·83, 1·23) | ||||||
| Ulmer et al. (2009)( 41 ), Austria | 71 693 men and 84 460 women | Not reported | 600 colon cancers | TAG, 1·08 (0·81, 1·44) | Age and gender | |
| 273 rectal cancers | TAG, 1·56 (1·00, 2·43) | |||||
| Author/reference and country | No. of cases/controls | Source of controls | Age (years) | Type of lesion | Serum lipid measurement, RR (95 % CI), start to end | Controlled variables |
| Case–control studies | ||||||
| Chung et al. (2006)( 22 ), Korea | 105/105 | Hospital | 59 | 105 colorectal cancers | TAG, 0·6 (0·3, 1·2) | Age and gender |
| TC, 0·3 (0·1, 0·9) | ||||||
| Trichopoulou et al. (1992)( 38 ), USA | 100/100 | Hospital | Not reported | 100 colorectal cancers | TC, 0·53 (0·4, 0·7) | Age and gender |
| HDL-C, 0·45 (0·3, 0·7) | ||||||
| TAG, 0·81 (0·5, 1·3) | ||||||
| van Duijnhoven et al. (2011)( 4 ), European counties | 1238/1238 | Hospital | 59 | 1238 colorectal cancers | TC, 0·92 (0·8, 1·0) | Age, gender, centre, follow-up time, time of blood collection and fasting status |
| HDL-C, 0·83 (0·7, 1·0) | ||||||
| LDL-C, 0·93 (0·8, 1·1) | ||||||
| TAG, 1·03 (0·9, 1·2) | ||||||
| Aleksandrova et al. (2011)( 17 ), Germany, Denmark, France, Greece, Italy, Spain, Netherlands, UK | 1093/1093 | Registry data | 59 | 689 colon cancers | TAG, 1·36 (0·95, 1·95) | Study centre, gender, age at blood collection, follow-up time since blood collection, time of the day at blood collection and fasting status, (among women) menopausal status |
| 404 rectal cancers | TAG, 1·00 (0·70, 1·43) | |||||
| HDL-C, 1·36 (1·04, 1·78) | ||||||
| HDL-C, 0·91 (0·63, 1·31) | ||||||
| Saydah et al. (2003)( 32 ), USA | 173/346 | CLUE II cohort | Not reported | 132 colon cancers | TAG, 0·69 (0·41, 1·16) | Age |
| 41 rectal cancers | ||||||
| Yamada et al. (1998)( 43 ), Japan | 129/258 | Hospital | 55 | 129 colorectal cancers | TC, 2·0 (1·0, 4·1) | Gender, age, date of examination, history of prior health check-up |
| TAG, 3·0 (1·4, 6·43) | ||||||
RR, risk ratio; HDL-C, HDL-cholesterol; TC, total cholesterol; LDL-C, LDL-cholesterol.
Table 2.
Studies contributing to the analysis of serum lipids and colorectal adenoma
| Author/reference and country | No. of cases/controls | Source of controls | Age (years) | Type of lesion | Serum lipid measurement, RR (95 % CI), start to end | Controlled variables |
|---|---|---|---|---|---|---|
| Case–control studies | ||||||
| Bird et al. (1996)( 20 ), USA | 486/520 | Hospital | 62 | 486 left colon and rectum adenomas | TC, 1·2 (0·8, 1·8) | Gender and age |
| TAG, 1·5 (1·0, 2·3) | ||||||
| HDL-C, 1·6 (0·9, 2·8) | ||||||
| Chung et al. (2006)( 22 ), Korea | 105/105 | Hospital | 58 | 105 colorectal adenomas | TAG, 1·2 (0·5, 2·9) | Age and gender |
| TC, 0·7 (0·3, 1·6) | ||||||
| Park et al. (2000)( 9 ), Korea | 134/134 | Hospital | Not reported | 134 male colorectal adenomas | TAG, 2·98 (1·2, 7·4) | Age and gender |
| TC, 2·44 (0·6, 10·0) | ||||||
| HDL-C, 2·23 (0·8, 6·2) | ||||||
| LDL-C, 1·31 (0·4, 4·3) | ||||||
| Hu et al. (2011)( 23 ), Taiwan | 397/2709 | Hospital | 52 | 379 colorectal adenomas | TAG, 1·18 (0·91, 1·53) | Non-polyp/cancer control |
| HDL-C, 0·94 (0·71, 1·24) | ||||||
| Kim et al. (2007)( 6 ), Korea | 731/1800 | Hospital | 54 | 731 colorectal adenomas | TAG, 1·16 (0·96, 1·40) | Non-polyp/cancer control |
| HDL-C, 1·07 (0·84, 1·36) | ||||||
| Morita et al. (2005)( 31 ), Japan | 756/1751 | Hospital | 49–57 | 756 male colorectal adenomas | TAG, 1·18 (0·98, 1·42) | Not reported |
| HDL-C, 0·85 (0·61, 1·18) | ||||||
| Otani et al. (2006)( 8 ), Japan | 782/738 | Hospital | 61 | 782 colorectal adenomas | TAG, 1·5 (1·1, 2·05) | Not reported |
| Tsilidis et al. (2010)( 39 ), USA | 132/260 | CLUE II cohort | 55 | 132 colorectal adenomas | TC, 0·97 (0·49, 1·92) | Age, gender, race, date of blood draw, fasting |
| HDL-C, 0·92 (0·45, 1·90) | ||||||
| TAG, 0·85, 0·40, 1·81 | ||||||
| Author/reference and country | Enrolment | Study population | Age (years) | Colonoscopy | Type of lesion | Serum lipid measurement, RR (95 % CI), start to end |
| Cross-sectional studies | ||||||
| Bayerdorffer et al. (1993)( 19 ), Germany | 1988–1989 | 822 consecutive patients | 52·45 | Yes | 194 colorectal adenomas | HDL-C, 0·61 (0·4, 0·9) |
| LDL-C, 1·91 (1·6, 2·3) | ||||||
| TC, 1·36 (0·8, 2·3) | ||||||
| Kono et al. (1990)( 28 ), Japan | 1986–1988 | 1297 men | 49–56 | Yes | 88 male colorectal adenomas | TC, 1·3 (0·7, 2·4) |
| HDL-C, 0·6 (0·3, 1·2) | ||||||
| TAG, 1·5 (0·8, 2·8) | ||||||
| Kono et al. (1993)( 29 ), Japan | 1988–1990 | 1363 men | 48–56 | Yes | 138 left-sided colon adenomas | TC, 0·8 (0·4, 1·6) |
| HDL-C, 1·1 (0·6, 2·0) | ||||||
| LDL-C, 0·8 (0·5, 1·3) | ||||||
| TAG, 0·8 (0·5, 1·3) | ||||||
| Tabuchi et al. (2006)( 36 ), Japan | 1995–2003 | 4887 patients | 10–94 | Yes | 3821 colorectal tubular adenomas | TC, 1·04 (1·0, 1·1) |
| TAG, 1·13 (1·0, 1·3) | ||||||
| Yang et al. (2013)( 10 ), Korea | 2006–2009 | 19 281 consecutive participants | 40–79 | Yes | 5958: 5504 non-advanced colorectal adenomas and 454 advanced colorectal adenomas | TC, 1·13 (0·9, 1·4) |
| HDL-C, 1·09 (1·0, 1·2) | ||||||
| LDL-C, 1·19 (1·1, 1·3) | ||||||
| TAG, 1·36 (1·2, 1·5) | ||||||
| Author/reference and country | Study population | Age (years) | Type of lesion | Serum lipid measurement, RR (95 % CI), start to end | Controlled variables | |
| Cohort studies | ||||||
| Ashbeck et al. (2009)( 18 ), USA | 392 individuals from Wheat Bran Fiber trial and the Ursodeoxycholic Acid trial | 66 | 770 non-advanced neoplasia | TAG, 0·97 (0·78, 1·21) | Age and study | |
| 333 advanced neoplasia | TAG, 0·87 (0·64, 1·18) | |||||
| TAG, 1·28 (0·92, 1·78) | ||||||
| TAG, 0·88 (0·56, 1·38) | ||||||
| HDL-C, 0·94 (0·74, 1·19) | ||||||
| HDL-C, 0·87 (0·63, 1·20) | ||||||
| HDL-C, 1·28 (0·89, 1·84) | ||||||
| HDL-C, 0·86 (0·52, 1·42) | ||||||
| Kim et al. (2012)( 27 ), Korea | 6438 Korean participants in the Colorectal Polyp Registry | Not reported | 1771 patients with colorectal adenomatous polyps | HDL-C, 1·13 (0·8, 1·6) | Age, education, occupation, household income, marital status | |
| TAG, 0·84 (0·6, 1·2) | ||||||
| Liu et al. (2010)( 30 ), China | 4872 general Chinese population | 50 | 719 colorectal adenoma | TAG, 1·26 (1·04, 1·53) | Age, gender, smoking, alcohol | |
| HDL-C, 1·30 (1·10, 1·54) | ||||||
| Kaneko et al. (2010)( 26 ), Japan | 727 Japanese population | Not reported | 727 colon tumours | TAG, 1·00 (0·99, 1·01) | Age, dietary intake | |
| TAG, 1·01 (1·00, 1·02) | ||||||
| LDL-C, 1·00 (0·98, 1·02) | ||||||
| LDL-C, 1·00 (0·99, 1·01) | ||||||
| HDL-C, 1·01 (1·00, 1·02) | ||||||
| HDL-C, 1·02 (1·00, 1·04) | ||||||
RR, risk ratio; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol.
Overall analyses on the association of serum lipids and colorectal neoplasm
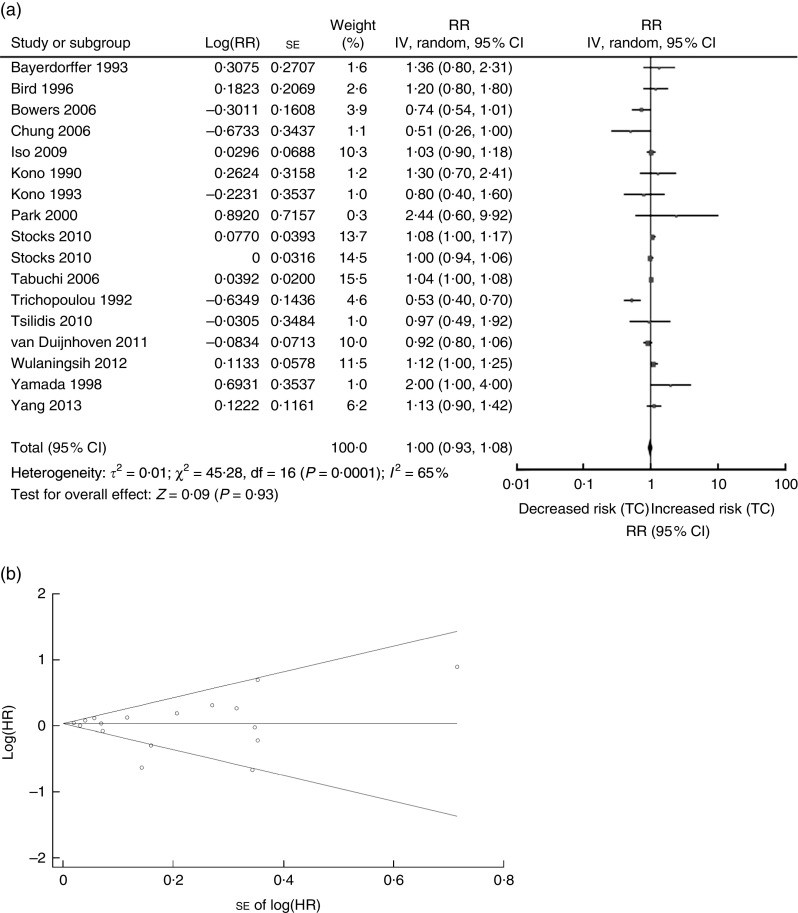
The association between serum TC and risk of CRN was analysed first. The sixteen studies on TC (four cohort, five cross-sectional and seven case–control studies) were published between 1990 and 2013 (Tables 1 and 2) and involved a total of 21809 CRN cases( 4 , 9 , 10 , 19 – 22 , 25 , 28 , 29 , 34 , 36 , 38 , 39 , 42 , 43 ). Three studies were conducted in the USA( 20 , 38 , 39 ), one in Sweden( 42 ), three in Korea( 9 , 10 , 22 ), five in Japan( 25 , 28 , 29 , 36 , 43 ), one in Finland( 21 ), one in Germany( 19 ) and two in European countries( 4 , 24 ) (Tables 1 and 2). A random-effects model was considered for a summary RR as there was evidence of heterogeneity for the included sixteen studies (Q=45·28, P value for heterogeneity =0·00001, I 2=65 %). The overall RR for CRN (adenoma or colon cancer) associated with serum TC was 1·00 (95 % CI 0·93, 1·08, P=0·93; Fig. 1(a)), while the funnel plot for potential publication bias was also analysed (Fig. 1(b)). Our result indicated that there was no statistical evidence of publication bias (Egger’s P=0·638, Begg’s P=0·837).
Fig. 1.
Association between TC and CRN (adenoma and cancer combined): (a) forest plot; (b) funnel plot. In (a), the study-specific RR and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond presents the pooled RR risk and its width represents the pooled 95 % CI. In (b), Begg’s funnel plot with pseudo 95 % CI is presented (TC, total cholesterol; CRN, colorectal neoplasm; RR, risk ratio; IV, fixed-effects model; HR, hazard ratio)
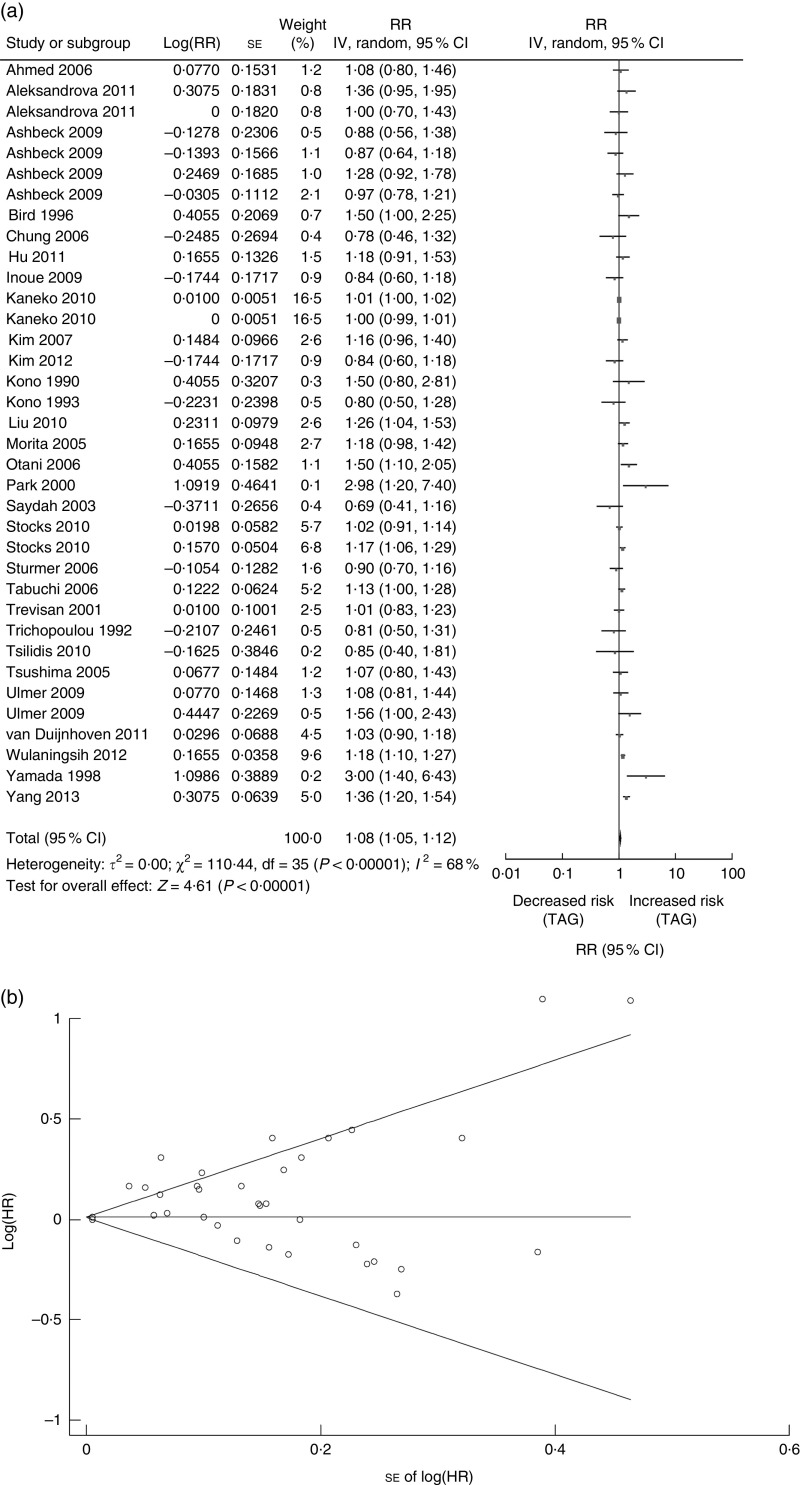
There were twenty-nine studies reported on TAG (twelve cohort studies, four cross-sectional and thirteen case–control studies) published between 1990 and 2013 and involved a total of 31546 CRN cases (Tables 1 and 2)( 4 , 6 , 8 – 10 , 16 – 18 , 20 , 22 – 24 , 26 – 32 ). Nine studies were conducted in the USA( 16 , 18 , 20 , 32 , 35 , 37 – 40 ), one in Sweden( 42 ), five in Korea( 6 , 9 , 10 , 22 , 27 ), two in China( 23 , 30 ), eight in Japan( 8 , 24 , 26 , 28 , 29 , 31 , 36 , 43 ), one in Austria( 41 ) and three in European countries( 4 , 17 , 34 ) (Tables 1 and 2). Pooled analysis showed a significant association between serum TAG and CRN (n 29 studies; summary RR=1·08; 95 % CI 1·05, 1·12, P<0·00001; Fig. 2(a)). A random-effects model was considered for a summary RR because of the significant heterogeneity of the included twenty-nine studies (Q=110·44, P value for heterogeneity<0·00001, I 2=68 %). The funnel plot for potential publication bias was also implemented (Fig. 2(b)); the results of the statistical analysis showed a potential publication bias (Egger’s P=0·006, Begg’s P=0·225).
Fig. 2.
Association between TAG and CRN (adenoma and cancer combined): (a) forest plot; (b) funnel plot. In (a), the study-specific RR and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond presents the pooled RR risk and its width represents the pooled 95 % CI. In (b), Begg’s funnel plot with pseudo 95 % CI is presented (CRN, colorectal neoplasm; RR, risk ratio; IV, fixed-effects model; HR, hazard ratio)
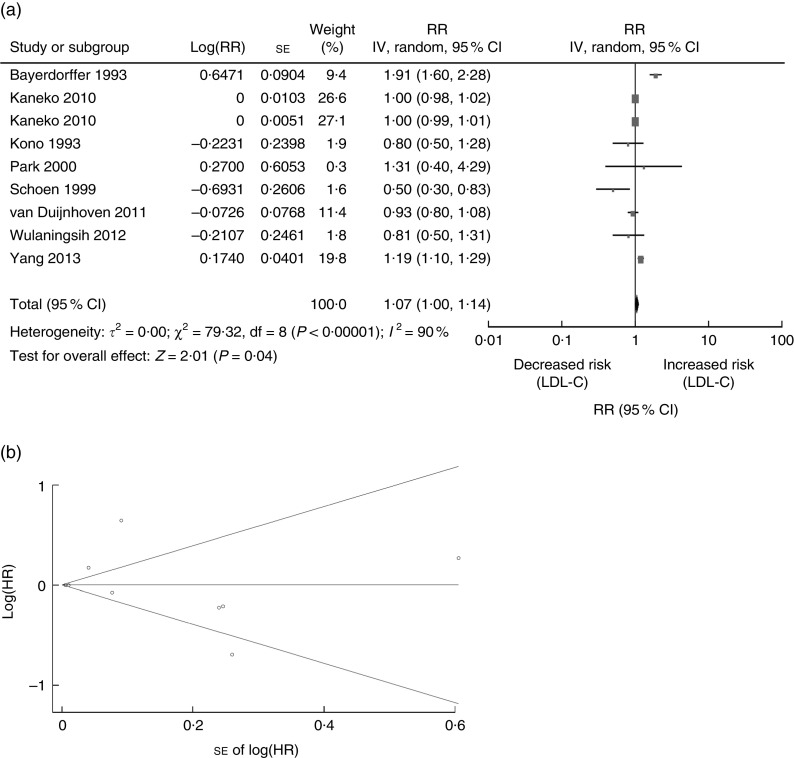
Next, we performed an analysis to evaluate the association between serum LDL-C and risk of CRN. There were a total of eight studies carried out on LDL-C (three cohort studies, three cross-sectional and two case–control studies) published between 1993 and 2013 which included 12 473 CRN cases (Tables 1 and 2)( 4 , 9 , 10 , 19 , 26 , 29 , 33 , 42 ). One study was conducted in the USA( 33 ), one in Sweden( 42 ), one in Germany( 19 ), two in Korea( 9 , 10 ), two in Japan( 26 , 29 ) and one in European countries( 4 ) (Tables 1 and 2). Data provided evidence that LDL-C is an increased risk factor of CRN development (summary RR=1·07; 95 % CI 1·00, 1·14, P=0·04; Fig. 3(a)). A random-effects model was considered for summary RR due to the existence of heterogeneity of the included eight studies (Q=79·32, P value for heterogeneity <0·00001, I 2=90 %), while the funnel plot for potential publication bias was also conducted (Fig. 3(b)). Results of the statistical analysis showed no significant publication bias (Egger’s P=0·573, Begg’s P=0·835).
Fig. 3.
Association between LDL-C and CRN (adenoma and cancer combined): (a) forest plot; (b) funnel plot. In (a), the study-specific RR and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond presents the pooled RR risk and its width represents the pooled 95 % CI. In (b), Begg’s funnel plot with pseudo 95 % CI is presented (LDL-C, LDL-cholesterol; CRN, colorectal neoplasm; RR, risk ratio; IV, fixed-effects model; HR, hazard ratio)
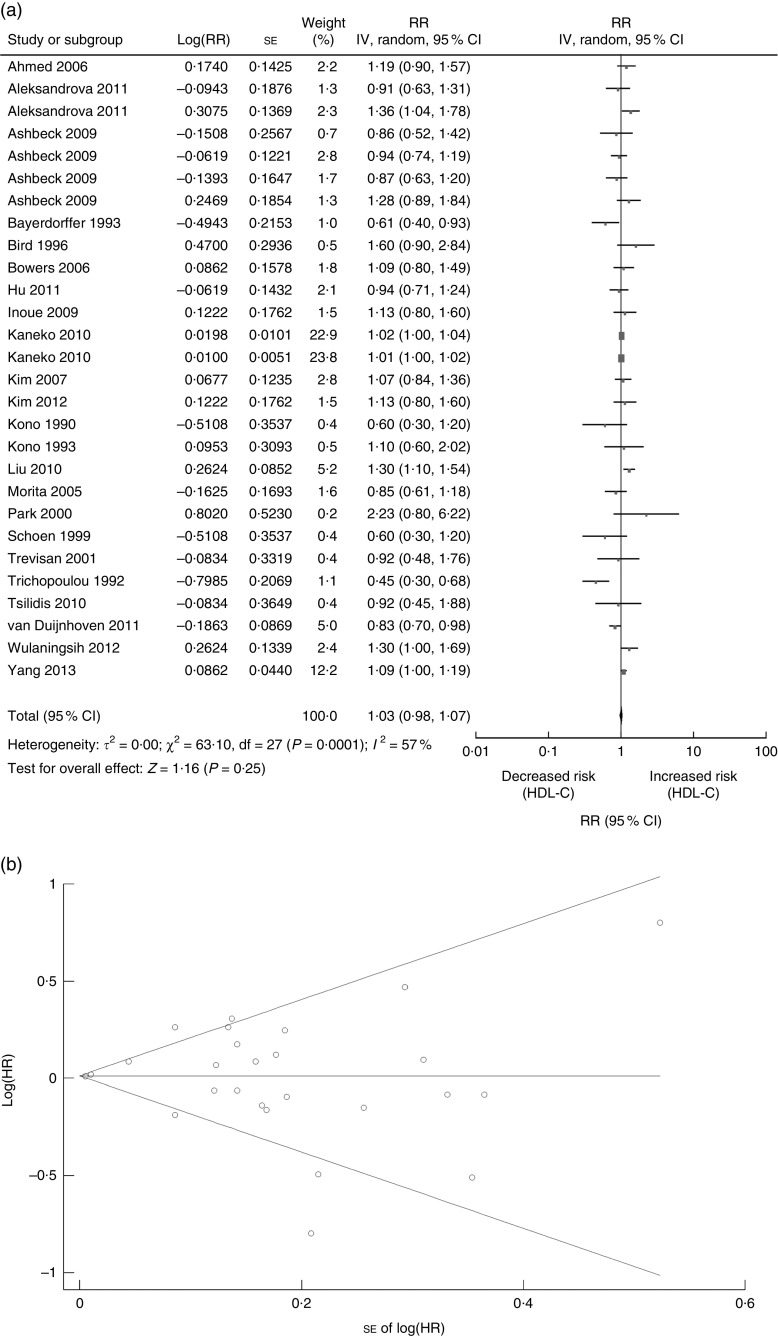
Finally, we performed analysis of studies to evaluate the association between HDL-C and CRN. The included twenty-three studies on HDL-C (ten cohort studies, four cross-sectional and nine case–control studies) were published between 1990 and 2013 (Tables 1 and 2) and involved a total of 21426 CRN cases( 4 , 6 , 9 , 10 , 16 – 21 , 23 , 24 , 26 – 31 , 33 , 37 – 39 , 42 ). Seven studies were conducted in the USA( 16 , 18 , 20 , 33 , 37 – 39 ), one in Sweden( 42 ), one in Germany( 19 ), two in China( 23 , 30 ), four in Korea( 6 , 9 , 10 , 27 ), five in Japan( 24 , 26 , 28 , 29 , 31 ), one in Finland( 21 ) and two in European countries( 4 , 17 ) (Tables 1 and 2). A random-effects model was considered for a summary RR as there was significant heterogeneity of the included twenty-three studies (Q=63·10, P value for heterogeneity=0·0001, I 2=57 %). Analysis of the twenty-three studies indicated that HDL-C was not significantly associated with CRN (RR=1·03; 95 % CI 0·98, 1·07, P=0·25; Fig. 4(a)), while the funnel plot for potential publication bias was also analysed (Fig. 4(b)). Our result indicated that there was no statistical evidence of publication bias (Egger’s P=0·983, Begg’s P=0·252).
Fig. 4.
Association between HDL-C and CRN (adenoma and cancer combined): (a) forest plot; (b) funnel plot. In (a), the study-specific RR and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond presents the pooled RR risk and its width represents the pooled 95 % CI. In (b), Begg’s funnel plot with pseudo 95 % CI is presented (HDL-C, HDL- cholesterol; CRN, colorectal neoplasm; RR, risk ratio; IV, fixed-effects model; HR, hazard ratio)
Overall analyses on the association of serum lipids and colorectal cancer
Eight publications in the present meta-analysis on TC (four cohort and four case–control studies) and risk of CRC were published between 1992 and 2012 and involved a total of 10 979 CRC cases (Table 1)( 4 , 21 , 22 , 25 , 34 , 38 , 42 , 43 ). One study was conducted in the USA( 38 ), one in Sweden( 42 ), one in Korea( 22 ), two in Japan( 25 , 43 ), one in Finland( 21 ) and two in European countries( 4 , 34 ) (Table 1). Pooled data did not support the association between TC and CRC (RR=0·95; 95 % CI 0·85, 1·06, P=0·38). There was a statistically significant heterogeneity among studies (Q=40·30, P value for heterogeneity <0·00001, I 2=80 %; Table 3).
Table 3.
Summary risk estimates of the association between serum lipids and colorectal cancer and adenoma risk
| Heterogeneity test | ||||||
|---|---|---|---|---|---|---|
| Number of studies | P | RR | 95 % CI | P | Q | I 2 (%) |
| TC and colorectal cancer risk | ||||||
| Eight | 0·38 | 0·95 | 0·85, 1·06 | <0·00001 | 40·30 | 80 |
| TAG and colorectal cancer risk | ||||||
| Fourteen | 0·10 | 1·07 | 0·99, 1·15 | 0·01 | 31·81 | 50 |
| HDL-C and colorectal cancer risk | ||||||
| Nine | 0·77 | 0·97 | 0·80, 1·18 | 0·0001 | 33·01 | 73 |
| LDL-C and colorectal cancer risk | ||||||
| Three | 0·07 | 0·88 | 0·77, 1·01 | 0·07 | 5·34 | 63 |
| TC and colorectal adenoma risk | ||||||
| Nine | 0·02 | 1·04 | 1·01, 1·09 | 0·73 | 5·26 | 0 |
| TAG and colorectal adenoma risk | ||||||
| Sixteen | 0·0009 | 1·06 | 1·03, 1·10 | <0·00001 | 62·15 | 69 |
| HDL-C and colorectal adenoma risk | ||||||
| Fourteen | 0·12 | 1·03 | 0·99, 1·06 | 0·03 | 29·88 | 43 |
| LDL-C and colorectal adenoma risk | ||||||
| Five | 0·003 | 1·11 | 1·04, 1·19 | <0·00001 | 70·43 | 93 |
RR, risk ratio; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol.
The association between serum TAG and risk of CRC was analysed. Fourteen studies on TAG (eight cohort and six case–control studies) and risk of CRC were published between 1992 and 2012 and involved a total of 13 785 CRC cases (Table 1)( 4 , 16 , 17 , 22 , 24 , 32 , 34 , 35 , 37 , 38 , 40 – 43 ). Six studies were conducted in the USA( 16 , 32 , 35 , 37 , 38 , 40 ), one in Sweden( 42 ), one in Korea( 22 ), one in Austria( 41 ) two in Japan( 24 , 43 ) and three in European countries( 4 , 17 , 34 ) (Table 1). The association of serum TAG with CRC risk was not observed (RR=1·07; 95 % CI 0·99, 1·15; P=0·10). A random-effects model was constructed as there was statistically significant heterogeneity among studies (Q=31·81, P value for heterogeneity=0·01, I 2=50 %; Table 3).
The association between serum HDL-C and risk of CRC was then analysed in our study. Nine studies on HDL-C (six cohort and three case–control studies) and risk of CRC were published between 1992 and 2012 and involved a total of 7328 CRC cases (Table 1)( 4 , 16 , 17 , 21 , 24 , 33 , 37 , 38 , 42 ). Four studies were conducted in the USA( 16 , 33 , 37 , 38 ), one in Sweden( 42 ), one in Japan( 24 ), one in Finland( 21 ) and two in European countries( 4 , 17 ) (Table 1). The pooled RR of serum HDL-C for CRC was 0·97 (95 % CI 0·80, 1·18, P=0·77), suggesting no significant relevance. Statistically significant heterogeneity existed among studies (Q=33·01, P value for heterogeneity=0·0001, I 2=73 %; Table 3).
There were three studies regarding LDL-C (two cohort and one case–control study) and risk of CRC that were published between 1990 and 2013 and involved a total of 5322 CRC cases (Table 1)( 4 , 33 , 42 ). One study was conducted in the USA( 33 ), one in Sweden( 42 ) and one in European countries( 4 ) (Table 1). The association between serum LDL-C and CRC risk was not observed (RR=0·88; 95 % CI 0·77, 1·01, P=0·07). A fixed-effects model was used as there was no statistically significant heterogeneity among studies (Q=5·34, P value for heterogeneity=0·07, I 2=63 %; Table 3).
Overall analyses on the association of serum lipids and colorectal adenoma
Nine studies reporting associations between TC (five cross-sectional and four case–control studies) and risk of CRA were published between 1990 and 2013 and involved a total of 10 935 CRA cases (Table 2)( 9 , 10 , 19 , 20 , 22 , 28 , 29 , 36 , 39 ). Two studies were conducted in the USA( 20 , 39 ), one in Germany( 19 ), three in Korea( 9 , 10 , 22 ) and three in Japan( 28 , 29 , 36 ) (Table 2). The results from nine studies showed that serum TC had a significant association with risk of CRA (RR=1·04; 95 % CI 1·01, 1·09, P=0·02) and there was no significant heterogeneity among these studies (Q=5·26, P value for heterogeneity=0·73, I 2=0 %; Table 3).
We next performed a meta-analysis specifically for TAG and risk of CRA. The sixteen studies on TAG (four cohort studies, four cross-sectional and eight case–control studies) and risk of CRA were published between 1990 and 2013 and involved a total of 17 830 CRA cases (Table 2)( 6 , 8 – 10 , 18 , 20 , 22 , 23 , 26 – 31 , 36 , 39 ). Three studies were conducted in the USA( 18 , 20 , 39 ), five in Korea( 6 , 9 , 10 , 22 , 27 ), two in China( 23 , 30 ) and six in Japan( 8 , 26 , 28 , 29 , 31 , 36 ) (Table 2). The pooled RR for CRA was 1·06 (95 % CI 1·03, 1·10, P=0·0009) which indicated that serum TAG was significantly associated with CRA development. Significant between-study heterogeneity was found in this analysis (Q=62·15, P value for heterogeneity <0·00001, I 2=69 %; Table 3).
There were fourteen studies on HDL-C (four cohort studies, four cross-sectional and six case–control studies) and risk of CRA published between 1990 and 2013 and involved a total of 14 098 CRA cases (Table 2)( 6 , 9 , 10 , 18 – 20 , 23 , 26 – 31 , 39 ). Three studies were conducted in the USA( 18 , 20 , 39 ), one in Germany( 19 ), two in China( 23 , 30 ), four in Korea( 6 , 9 , 10 , 27 ) and four in Japan( 26 , 28 , 29 , 31 ) (Table 2). A pooled analysis of these fourteen studies showed that the RR for CRA with serum HDL-C was 1·03 (95 % CI 0·99, 1·06, P=0·12) with a significant heterogeneity indicated (Q=29·88, P value for heterogeneity=0·03, I 2=43 %; Table 3).
Finally, we performed a meta-analysis specifically for the association between LDL-C and risk of CRA. Five studies on LDL-C (one cohort study, three cross-sectional and one case-control study) and risk of CRA were published between 1990 and 2013 and involved a total of 7151 CRA cases (Table 2)( 9 , 10 , 19 , 26 , 29 ). One study was conducted in Germany( 19 ), two in Korea( 9 , 10 ) and two in Japan( 26 , 29 ) (Table 2). Because there was statistical heterogeneity among studies (Q=70·43, P value for heterogeneity<0·00001, I 2=93 %), the random-effects model was applied. The pooled RR for CRA was 1·11 (95 % CI 1·04, 1·19, P=0·003) which presented an increased risk for CRA (Table 3).
Subgroup and sensitivity analyses
The association of lipid components with risk for CRN was performed by subsets within the study design, geographic area, age, gender or the number of cases. Table 4 presents detailed results of subgroup analyses.
Table 4.
Results of subgroup analysis of serum lipids and colorectal neoplasm risk
| Heterogeneity test | ||||||
|---|---|---|---|---|---|---|
| Subgroup | No. of studies | P | RR | 95 % CI | P | I 2 (%) |
| TC and colorectal neoplasm risk | ||||||
| Study design | ||||||
| Cohort | 4 | 0·32 | 1·04 | 0·97, 1·11 | 0·07 | 53 |
| Case–control | 7 | 0·65 | 0·93 | 0·66, 1·29 | 0·0003 | 76 |
| Cross-sectional | 5 | 0·03 | 1·04 | 1·00, 1·08 | 0·64 | 0 |
| Study location | ||||||
| Asia | 9 | 0·02 | 1·04 | 1·01, 1·08 | 0·17 | 31 |
| Europe | 4 | 0·75 | 1·01 | 0·93, 1·10 | 0·03 | 64 |
| USA | 3 | 0·54 | 0·83 | 0·46, 1·50 | 0·003 | 82 |
| Age (years) | ||||||
| <60 | 9 | 0·92 | 0·99 | 0·83, 1·18 | 0·02 | 57 |
| ≥60 | 2 | 0·19 | 1·03 | 0·98, 1·08 | 0·24 | 30 |
| Gender composition | ||||||
| Male | 3 | 0·56 | 1·14 | 0·73, 1·77 | 0·32 | 13 |
| Female and male | 13 | 0·99 | 1·00 | 0·93, 1·08 | 0·0001 | 70 |
| No. of cases | ||||||
| <1000 | 11 | 0·72 | 0·96 | 0·75, 1·22 | 0·0002 | 71 |
| ≥1000 | 5 | 0·01 | 1·04 | 1·01, 1·07 | 0·18 | 34 |
| TAG and colorectal neoplasm risk | ||||||
| Study design | ||||||
| Cohort | 12 | 0·01 | 1·04 | 1·01, 1·07 | 0·0001 | 64 |
| Case–control | 13 | 0·02 | 1·16 | 1·02, 1·32 | 0·01 | 52 |
| Cross-sectional | 14 | 0·05 | 1·19 | 1·00, 1·43 | 0·05 | 62 |
| Study location | ||||||
| Asia | 15 | 0·001 | 1·07 | 1·03, 1·11 | 0·0001 | 77 |
| Europe | 4 | 0·0001 | 1·13 | 1·08, 1·18 | 0·14 | 40 |
| USA | 9 | 0·95 | 1·00 | 0·91, 1·09 | 0·43 | 2 |
| Age (years) | ||||||
| <60 | 11 | 0·0001 | 1·16 | 1·10, 1·22 | 0·10 | 36 |
| ≥60 | 4 | 0·08 | 1·11 | 0·99, 1·24 | 0·04 | 52 |
| Gender composition | ||||||
| Male | 4 | 0·24 | 1·25 | 0·86, 1·81 | 0·07 | 58 |
| Female and male | 25 | 0·0001 | 1·08 | 1·04, 1·11 | 0·0001 | 69 |
| No. of cases | ||||||
| <1000 | 20 | 0·07 | 1·03 | 1·00, 1·07 | 0·0001 | 58 |
| ≥1000 | 11 | 0·003 | 1·10 | 1·03, 1·18 | 0·02 | 48 |
| HDL-C and colorectal neoplasm risk | ||||||
| Study design | ||||||
| Cohort | 10 | 0·004 | 1·01 | 1·00, 1·02 | 0·08 | 37 |
| Case–control | 9 | 0·68 | 0·96 | 0·79, 1·17 | 0·0001 | 69 |
| Cross-sectional | 4 | 0·39 | 0·85 | 0·59, 1·23 | 0·02 | 69 |
| Study location | ||||||
| Asia | 12 | 0·10 | 1·03 | 0·99, 1·07 | 0·02 | 51 |
| Europe | 4 | 0·53 | 1·07 | 0·86, 1·34 | 0·008 | 71 |
| USA | 7 | 0·46 | 0·92 | 0·74, 1·14 | 0·005 | 62 |
| Age (years) | ||||||
| <60 | 11 | 0·79 | 1·02 | 0·88, 1·18 | 0·002 | 63 |
| ≥60 | 3 | 0·82 | 0·98 | 0·84, 1·14 | 0·17 | 35 |
| No. of cases | ||||||
| <1000 | 17 | 0·46 | 1·02 | 0·97, 1·07 | 0·0001 | 60 |
| ≥1000 | 6 | 0·47 | 1·04 | 0·93, 1·17 | 0·03 | 52 |
| LDL-C and colorectal neoplasm risk | ||||||
| Study design | ||||||
| Cohort | 3 | 0·85 | 1·00 | 0·97, 1·02 | 0·05 | 62 |
| Case–control | 2 | 0·38 | 0·94 | 0·81, 1·09 | 0·57 | 0 |
| Cross-sectional | 5 | 0·003 | 1·11 | 1·04, 1·19 | 0·0001 | 93 |
| Study location | ||||||
| Asia | 3 | 0·23 | 1·28 | 0·86, 1·90 | 0·0001 | 93 |
| Europe | 2 | 0·25 | 0·92 | 0·80, 1·06 | 0·59 | 0 |
| USA | 1 | 0·008 | 0·50 | 0·30, 0·83 | – | – |
| Age (years) | ||||||
| <60 | 4 | 0·80 | 1·06 | 0·66, 1·72 | 0·0001 | 93 |
| ≥60 | 1 | 0·008 | 0·50 | 0·30, 0·83 | – | – |
| Gender composition | ||||||
| Male | 2 | 0·48 | 0·86 | 0·55, 1·32 | 0·45 | 0 |
| Female and male | 6 | 0·03 | 1·08 | 1·01, 1·15 | 0·0001 | 92 |
| No. of cases | ||||||
| <1000 | 5 | 0·10 | 1·07 | 0·99, 1·15 | 0·0001 | 92 |
| ≥1000 | 3 | 0·87 | 1·02 | 0·82, 1·27 | 0·007 | 80 |
RR, risk ratio; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol.
We performed a sensitivity analyses to explore the heterogeneity among studies of serum lipids and CRN. By using a stepwise process, we determined that most of the heterogeneity was accounted for in two studies( 21 , 38 ) reporting the association between TC and CRN. After excluding the two studies, there was no study heterogeneity (P=0·15, I 2=27 %) and the RR for CRN was 1·04 (95 % CI 1·01, 1·07). Most of the heterogeneity was accounted for in eight studies( 8 – 10 , 32 , 34 , 36 , 42 , 43 ) reporting the association between TAG and CRN. After excluding these studies, there was no study heterogeneity (P=0·11, I 2=26 %) and the RR for CRN was 1·01 (95 % CI 1·00, 1·01). We found that most of the heterogeneity was accounted for in two studies( 10 , 19 ) reporting the association between HDL-C and CRN. After excluding the two studies, there was no study heterogeneity (P=0·14, I 2=38 %) and the RR for CRN was 1·00 (95 % CI 0·99, 1·01). Most of the heterogeneity was accounted for in four studies( 17 , 19 , 30 , 38 ) reporting the association between LDL-C and CRN. After excluding these studies, there was no study heterogeneity (P=0·16, I 2=23 %) and the RR for CRN was 1·01 (95 % CI 1·00, 1·02).
Discussion
Our data clearly indicated the positive association between serum TAG and LDL-C and the increased risk of CRN. Subgroup analysis indicated that serum TAG was associated with an increased risk of CRA, but not CRC. It remains uncertain whether TAG is the causal factor responsible for increasing the risk of CRA but not for CRC, although several mechanisms have tried to explain the association between increasing TAG levels and CRN risk. Serum TAG plays an important role in insulin-like growth factor-1 levels( 44 ), a hormone with proliferative and anti-apoptotic effects. It is demonstrated that the insulin/insulin-like growth factor-1 pathway increases the activity of the ras protein occurring in abnormal colonocytes and stimulates the progression of adenomas into cancers through mediating mitogenicity by activation of K-ras( 45 ). Additionally, hypertriacylglycerolaemia also increases pro-inflammatory cytokines, including IL-6 and TNF-α, while decreasing anti-inflammatory cytokines such as IL-10. The increased inflammatory response has been linked to DNA damage and to effects on the growth, apoptosis and proliferation of colorectal tumour cells. Furthermore, high levels of serum TAG may also result in oxidative stress and in the development of reactive oxygen species( 46 ), which could damage DNA and affect carcinogenesis by affecting gene expression, mutation and chromosomal rearrangement( 47 ). Serum TAG may also affect colorectal tumorigenesis by mechanisms involving modification of bile acid excretion, circulating hormones and energy supply to neoplastic cells( 48 ). Experiments with animal models indicated that Apc-deficient mice showed age-dependent hypertriacylglycerolaemia and a number of intestinal polyp formations, which could be suppressed by anti-hyperlipidaemic medicines. This was confirmed by another report that azoxymethane injection in obese rats with hypertriacylglycerolaemia resulted in an increased number of advanced colon aberrant crypt foci, which are putative precursors of colon cancer( 49 ). In Apc-deficient FAP model mice, because of the reduced activity of the lipoprotein lipase, serum TAG and intestinal polyp formation decreased significantly after systemic administration of a PPAR ligand( 50 ). Although the biological or molecular mechanism is unclear so far, experiments with animal models most likely showed a clear association between TAG and intestinal neoplasms. Further laboratory and epidemiological studies are still necessary to shed light on the association between serum TAG and CRN development.
Our meta-analysis showed no positive association between TC level and the prevalence of CRN or CRC, although a suggestive association was observed in CRA. A prospective study showed a significant association between serum TC and CRC, which also indicated the risk was higher in patients with colon cancer than with rectal cancer( 51 ). The possible association between TC and colorectal tumorigenesis may be at least partly caused by genetic factors, such as an apoE phenotype which affects both cholesterol metabolism and susceptibility to carcinoma( 52 ). However, the pooled results of our study did not provide evidence for the association between TC and CRN. In our study, we found different associations of serum TC with CRA and CRC. Further research including a large number of studies is necessary to clarify this issue.
In our meta-analysis, higher levels of serum LDL-C were significantly associated with an increasing prevalence of CRN, while serum HDL-C levels were not significantly associated with a decreasing prevalence of CRN. Subgroup analysis demonstrated the positive association between serum LDL-C and CRA, although it was not associated with CRC. HDL-C, however, did not associate with either CRA or CRC. Previous studies have demonstrated that lipids and lipoproteins have been associated with neoplastic processes such as inflammation( 53 ), insulin resistance and oxidative stress. Although there are several possible mechanisms whereby serum lipoproteins influence CRC, little is known regarding the mechanisms by which LDL-C and HDL-C participate in colorectal carcinogenesis. Further mechanistic studies are needed to understand the deferent roles of LDL-C and HDL-C in the development of CRA as well as advanced carcinoma.
The other important finding is that none of the components of serum lipids included in the present meta-analysis (TAG, TC, HDL-C and LDL-C) was significantly correlated with the development of CRC, although positive associations between serum TAG, TC and LDL-C and CRA were found. It was previously reported the significant association of LDL-C with low-grade but not with high-grade CRA( 26 ), a stronger correlation between high levels of serum TAG and the number of adenomas, and different associations of serum lipids and adenomas according to histological examination( 8 ). With the accumulated data, it is difficult to interpret the different effects of serum lipids on CRA and CRC because of the limited mechanisms that have demonstrated a clear biological plausibility for differential effects of lipids on the development of adenoma and advanced carcinoma. Because the potential effects of lipid parameters on different stages of colorectal carcinogenesis have not yet been established, this provides a potential chance for further study.
Our meta-analysis of studies with large numbers of incident cases provides high statistical power for estimating the relationship between components of serum lipids and prevalence of CRN. Despite the strength of the meta-analysis, our study also has several limitations. First, several studies we included are observational. Second, a meta-analysis is not able to solve problems with confounding factors because it did not take into account other possible confounding variables such as dietary patterns, family history of colon cancer and alcohol use, which might be associated with the risk of CRN. However, most studies in the meta-analysis adjusted for other known and potential risk factors for CRN development. Third, heterogeneity may be introduced because of methodological and demographic differences among studies, although appropriate well-motivated inclusion criteria were used. Fourth, the RR values of the baseline serum lipids were endorsed by different panels/organizations in the original studies. Finally, the individual studies may adjust for different covariates including diets, which may affect our results. It is reported that several attributes of diet (such as alcohol and high intake of saturated fats) appear to alter levels of individual lipids( 54 , 55 ). Many of these diets, which determine serum lipid levels, are established risk factors for CRN( 56 , 57 ). Therefore, dietary factors might be an important confounding factor in our study.
Conclusion
In conclusion, the present systematic review and meta-analysis demonstrated that high levels of serum TAG and LDL-C are positively associated with the prevalence of CRN. In addition, persons with high levels of the serum lipid components TC, TAG and LDL-C have a greater risk of suffering from CRA but not CRC. Neither CRA nor CRC is linked with serum HDL-C levels. Given the rise in the epidemic of dyslipidaemia worldwide, health-care providers should be more vigilant and adhere with colorectal carcinogenesis screening guidelines in subjects with dyslipidaemia, especially those with abnormal serum TAG, TC and LDL-C levels.
Acknowledgements
Sources of funding: This study was supported in part by funding from the National Natural Science Foundation of China (Y.T., grant number 81302110) (Y.L., grant number 81200263). The National Natural Science Foundation of China had no role in the design, analysis or writing of this article. Conflict of interest: The authors have declared no conflicts of interest. Authorship: Y.T., K.W. and J.L. contributed equally to this work. Y.L. and Y.T. conceived and designed the study, and were involved in drafting of the manuscript; K.W. and J.L. were involved in study concept and design; J.W., Z.W. and Y.F. were involved analysis and interpretation of data; Y.Y. was involved in acquisition of data; G.J. and Y.L. were involved critical revision of the manuscript for important intellectual content. Ethics of human subject participation: Ethical approval was not required.
The first three authors contributed equally to this work.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980015000646.
click here to view supplementary material
References
- 1. Jemal A, Siegel R, Ward E et al. (2006) Cancer statistics, 2006. CA Cancer J Clin 56, 106–130. [DOI] [PubMed] [Google Scholar]
- 2. Jass JR (2006) Colorectal cancer: a multipathway disease. Crit Rev Oncog 12, 273–287. [DOI] [PubMed] [Google Scholar]
- 3. Ben Q, An W, Jiang Y et al. (2012) Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology 142, 762–772. [DOI] [PubMed] [Google Scholar]
- 4. van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M et al. (2011) Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut 60, 1094–1102. [DOI] [PubMed] [Google Scholar]
- 5. Giovannucci E (2007) Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 86, issue 3, s836–s842. [DOI] [PubMed] [Google Scholar]
- 6. Kim JH, Lim YJ, Kim YH et al. (2007) Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev 16, 1543–1546. [DOI] [PubMed] [Google Scholar]
- 7. Jinjuvadia R, Lohia P, Jinjuvadia C et al. (2013) The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J Clin Gastroenterol 47, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Otani T, Iwasaki M, Ikeda S et al. (2006) Serum triglycerides and colorectal adenoma in a case–control study among cancer screening examinees (Japan). Cancer Causes Control 17, 1245–1252. [DOI] [PubMed] [Google Scholar]
- 9. Park SK, Joo JS, Kim DH et al. (2000) Association of serum lipids and glucose with the risk of colorectal adenomatous polyp in men: a case–control study in Korea. J Korean Med Sci 15, 690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang MH, Rampal S, Sung J et al. (2013) The association of serum lipids with colorectal adenomas. Am J Gastroenterol 108, 833–841. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hogue CJ, Gaylor DW & Schulz KF (1983) Estimators of relative risk for case–control studies. Am J Epidemiol 118, 396–407. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP & Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- 14. Begg CB & Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. [PubMed] [Google Scholar]
- 15. Egger M, Davey Smith G, Schneider M et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed RL, Schmitz KH, Anderson KE et al. (2006) The metabolic syndrome and risk of incident colorectal cancer. Cancer 107, 28–36. [DOI] [PubMed] [Google Scholar]
- 17. Aleksandrova K, Boeing H, Jenab M et al. (2011) Metabolic syndrome and risks of colon and rectal cancer: the European prospective investigation into cancer and nutrition study. Cancer Prev Res (Phila) 4, 1873–1883. [DOI] [PubMed] [Google Scholar]
- 18. Ashbeck EL, Jacobs ET, Martinez ME et al. (2009) Components of metabolic syndrome and metachronous colorectal neoplasia. Cancer Epidemiol Biomarkers Prev 18, 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bayerdorffer E, Mannes GA, Richter WO et al. (1993) Decreased high-density lipoprotein cholesterol and increased low-density cholesterol levels in patients with colorectal adenomas. Ann Intern Med 118, 481–487. [DOI] [PubMed] [Google Scholar]
- 20. Bird CL, Ingles SA, Frankl HD et al. (1996) Serum lipids and adenomas of the left colon and rectum. Cancer Epidemiol Biomarkers Prev 5, 607–612. [PubMed] [Google Scholar]
- 21. Bowers K, Albanes D, Limburg P et al. (2006) A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol 164, 652–664. [DOI] [PubMed] [Google Scholar]
- 22. Chung YW, Han DS, Park YK et al. (2006) Association of obesity, serum glucose and lipids with the risk of advanced colorectal adenoma and cancer: a case–control study in Korea. Dig Liver Dis 38, 668–672. [DOI] [PubMed] [Google Scholar]
- 23. Hu NC, Chen JD, Lin YM et al. (2011) Stepwise relationship between components of metabolic syndrome and risk of colorectal adenoma in a Taiwanese population receiving screening colonoscopy. J Formos Med Assoc 110, 100–108. [DOI] [PubMed] [Google Scholar]
- 24. Inoue M, Noda M, Kurahashi N et al. (2009) Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev 18, 240–247. [DOI] [PubMed] [Google Scholar]
- 25. Iso H, Ikeda A, Inoue M et al. (2009) Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer 125, 2679–2686. [DOI] [PubMed] [Google Scholar]
- 26. Kaneko R, Sato Y, An Y et al. (2010) Clinico-epidemiologic study of the metabolic syndrome and lifestyle factors associated with the risk of colon adenoma and adenocarcinoma. Asian Pac J Cancer Prev 11, 975–983. [PubMed] [Google Scholar]
- 27. Kim BC, Shin A, Hong CW et al. (2012) Association of colorectal adenoma with components of metabolic syndrome. Cancer Causes Control 23, 727–735. [DOI] [PubMed] [Google Scholar]
- 28. Kono S, Ikeda N, Yanai F et al. (1990) Serum lipids and colorectal adenoma among male self-defence officials in northern Kyushu, Japan. Int J Epidemiol 19, 274–278. [DOI] [PubMed] [Google Scholar]
- 29. Kono S, Imanishi K, Shinchi K et al. (1993) Serum lipids and left-sided adenomas of the large bowel: an extended study of self-defense officials in Japan. Cancer Causes Control 4, 117–121. [DOI] [PubMed] [Google Scholar]
- 30. Liu CS, Hsu HS, Li CI et al. (2010) Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol 10, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morita T, Tabata S, Mineshita M et al. (2005) The metabolic syndrome is associated with increased risk of colorectal adenoma development: the Self-Defense Forces health study. Asian Pac J Cancer Prev 6, 485–489. [PubMed] [Google Scholar]
- 32. Saydah SH, Platz EA, Rifai N et al. (2003) Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 12, 412–418. [PubMed] [Google Scholar]
- 33. Schoen RE, Tangen CM, Kuller LH et al. (1999) Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 91, 1147–1154. [DOI] [PubMed] [Google Scholar]
- 34. Stocks T, Lukanova A, Bjorge T et al. (2010) Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can). Cancer 117, 2398–2407. [DOI] [PubMed] [Google Scholar]
- 35. Sturmer T, Buring JE, Lee IM et al. (2006) Metabolic abnormalities and risk for colorectal cancer in the physicians’ health study. Cancer Epidemiol Biomarkers Prev 15, 2391–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tabuchi M, Kitayama J & Nagawa H (2006) Hypertriglyceridemia is positively correlated with the development of colorectal tubular adenoma in Japanese men. World J Gastroenterol 12, 1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trevisan M, Liu J, Muti P et al. (2001) Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev 10, 937–941. [PubMed] [Google Scholar]
- 38. Trichopoulou A, Tzonou A, Hsieh CC et al. (1992) High protein, saturated fat and cholesterol diet, and low levels of serum lipids in colorectal cancer. Int J Cancer 51, 386–389. [DOI] [PubMed] [Google Scholar]
- 39. Tsilidis KK, Brancati FL, Pollak MN et al. (2010) Metabolic syndrome components and colorectal adenoma in the CLUE II cohort. Cancer Causes Control 21, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsushima M, Nomura AM, Lee J et al. (2005) Prospective study of the association of serum triglyceride and glucose with colorectal cancer. Dig Dis Sci 50, 499–505. [DOI] [PubMed] [Google Scholar]
- 41. Ulmer H, Borena W, Rapp K et al. (2009) Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer 101, 1202–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wulaningsih W, Garmo H, Holmberg L et al. (2012) Serum lipids and the risk of gastrointestinal malignancies in the Swedish AMORIS study. J Cancer Epidemiol 2012, 792034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamada K, Araki S, Tamura M et al. (1998) Relation of serum total cholesterol, serum triglycerides and fasting plasma glucose to colorectal carcinoma in situ . Int J Epidemiol 27, 794–798. [DOI] [PubMed] [Google Scholar]
- 44. Ibrahim YH & Yee D (2004) Insulin-like growth factor-I and cancer risk. Growth Horm IGF Res 14, 261–269. [DOI] [PubMed] [Google Scholar]
- 45. Siddiqui AA (2011) Metabolic syndrome and its association with colorectal cancer: a review. Am J Med Sci 341, 227–231. [DOI] [PubMed] [Google Scholar]
- 46. Cowey S & Hardy RW (2006) The metabolic syndrome: a high-risk state for cancer? Am J Pathol 169, 1505–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Valko M, Izakovic M, Mazur M et al. (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266, 37–56. [DOI] [PubMed] [Google Scholar]
- 48. McKeown-Eyssen G (1994) Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev 3, 687–695. [PubMed] [Google Scholar]
- 49. Raju J & Bird RP (2003) Energy restriction reduces the number of advanced aberrant crypt foci and attenuates the expression of colonic transforming growth factor β and cyclooxygenase isoforms in Zucker obese (fa/fa) rats. Cancer Res 63, 6595–6601. [PubMed] [Google Scholar]
- 50. Niho N, Mutoh M, Takahashi M et al. (2005) Concurrent suppression of hyperlipidemia and intestinal polyp formation by NO-1886, increasing lipoprotein lipase activity in Min mice. Proc Natl Acad Sci U S A 102, 2970–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tornberg SA, Holm LE, Carstensen JM et al. (1986) Risks of cancer of the colon and rectum in relation to serum cholesterol and β-lipoprotein. N Engl J Med 315, 1629–1633. [DOI] [PubMed] [Google Scholar]
- 52. Kervinen K, Sodervik H, Makela J et al. (1996) Is the development of adenoma and carcinoma in proximal colon related to apolipoprotein E phenotype? Gastroenterology 110, 1785–1790. [DOI] [PubMed] [Google Scholar]
- 53. Esteve E, Ricart W & Fernandez-Real JM (2005) Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr 24, 16–31. [DOI] [PubMed] [Google Scholar]
- 54. Hegsted DM & Kritchevsky D (1997) Diet and serum lipid concentrations: where are we? Am J Clin Nutr 65, 1893–1896. [DOI] [PubMed] [Google Scholar]
- 55. Rietman A, Schwarz J, Blokker BA et al. (2014) Increasing protein intake modulates lipid metabolism in healthy young men and women consuming a high-fat hypercaloric diet. J Nutr 144, 1174–1180. [DOI] [PubMed] [Google Scholar]
- 56. Vargas AJ & Thompson PA (2012) Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract 27, 613–623. [DOI] [PubMed] [Google Scholar]
- 57. Vrieling A & Kampman E (2010) The role of body mass index, physical activity, and diet in colorectal cancer recurrence and survival: a review of the literature. Am J Clin Nutr 92, 471–490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980015000646.
click here to view supplementary material