Abstract
Objective
Breakfast skipping has been reported to be associated with type 2 diabetes (T2D), but the results are inconsistent. No meta-analyses have applied quantitative techniques to compute summary risk estimates. The present study aimed to conduct a meta-analysis of observational studies summarizing the evidence on the association between breakfast skipping and the risk of T2D.
Design
Systematic review and meta-analysis.
Setting
Relevant studies were identified by a search of PubMed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI) and SINOMED up to 9 August 2014. We also reviewed reference lists from retrieved articles. We included studies that reported risk estimates (including relative risks, odds ratios and hazard ratios) with 95 % confidence intervals for the association between breakfast skipping and the risk of T2D.
Subjects
Eight studies involving 106 935 participants and 7419 patients with T2D were included in the meta-analysis.
Results
A pooled adjusted relative risk for the association between exposure to breakfast skipping and T2D risk was 1·21 (95 % CI 1·12, 1·31; P=0·984; I 2 =0·0 %) in cohort studies and the pooled OR was 1·15 (95 % CI, 1·05, 1·24; P=0·770; I 2 =0·0 %) in cross-sectional studies. Visual inspection of a funnel plot and Begg’s test indicated no evidence of publication bias.
Conclusions
Breakfast skipping is associated with a significantly increased risk of T2D. Regular breakfast consumption is potentially important for the prevention of T2D.
Keywords: Breakfast skipping, Type 2 diabetes, Meta-analysis
Diabetes mellitus is considered one of the important public health challenges in modern society, both in developed and developing countries( 1 ). Type 2 diabetes (T2D), which composes more than 95 % of diabetes in the world( 2 ), is characterized by reduced insulin sensitivity and relative insulin deficiency( 3 ). It is estimated that the world prevalence of diabetes among adults will increase to 7·7 %, corresponding to 439 million patients, by the year 2030( 4 ). Therefore, the identification of modifiable risk factors for the primary prevention of T2D is of considerable public health importance.
Breakfast is defined as the first meal of the day, within 2 h of waking, typically eaten no later than 10.00 hours, which provides between 20 and 35 % of total daily energy needs( 5 ). Breakfast skipping is the behaviour that people do not consume breakfast regularly. The prevalence of breakfast skipping has increased progressively over the past decades( 6 ). It is highly prevalent in the USA and Europe (10 to 30 %) and the prevalence varies among different age and ethnic groups( 7 ). There is increasing evidence that breakfast skipping is directly associated with excess weight gain and other adverse health outcomes, including insulin resistance and T2D( 5 ). However, the associations between breakfast skipping and T2D risk have not been summarized. Thus, we performed a meta-analysis to systematically assess the association between breakfast skipping and the risk of T2D based on observational studies.
Methods
The present review was conducted in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines( 8 ).
Search strategy
We did a literature search of PubMed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI) and SINOMED up to 9 August 2014 to identify observational studies examining an association between breakfast skipping and the risk of T2D. The search terms were ‘breakfast’ or ‘eating patterns’ or ‘meal frequency’ (MeSH) or ‘diabetes’ or ‘diabetes mellitus’ (MeSH) or ‘type 2 diabetes’ or ‘impaired glucose tolerance’ or ‘impaired fasting glucose’ or ‘insulin resistance’ or ‘metabolic syndrome’. Only articles published in the English and Chinese languages were included. In addition, additional literature was reviewed by a manual search of the reference lists from original studies.
Inclusion criteria
Studies were included if they met the following criteria: (i) the study design was observational; (ii) the exposure of interest was breakfast skipping and the outcome of interest was T2D; and (iii) the study reported risk estimates of odds ratios or relative risks (RR) or hazard ratios (HR) with 95 % confidence intervals for the association between breakfast skipping and T2D risk, or provided sufficient information to allow their calculation. We excluded animal studies, clinical trials, reviews, letters, commentaries, abstracts and unpublished studies. If multiple published reports were from the same study cohort, only the most recent or informative one was included. Two authors (H.S.B. and Y.G.) independently screened the titles, abstracts and full texts of initially identified studies to determine eligibility. Disagreements were resolved through consultation with the third reviewer (Y.W.C.).
Data extraction
We extracted the following information from studies included: (i) name of the first author; (ii) year of publication; (iii) country of origin; (iv) characteristics of the study population at baseline; (v) duration of follow-up (for cohort studies); (vi) exposure assessment; (vii) outcome assessment; (viii) number of participants; (ix) number of cases; (x) risk estimates (including RR, OR and HR) and corresponding 95 % CI; and (xi) covariates adjusted for in the statistical analysis. Data extraction was conducted independently by two authors (H.S.B and Y.G). Inter-observer agreement was assessed using Cohen’s kappa (κ) and any disagreements were resolved by discussion with the third author (Y.W.C.).
Quality assessment
Two reviewers (H.S.B. and Y.G.) independently performed the quality assessment by applying the Newcastle–Ottawa Scale( 9 ) (for cohort and case–control studies), which is a nine-point scale that allocates points based on the selection process of cohorts (0–4 points), the comparability of cohorts (0–2 points) and the identification of the exposure and the outcomes of study participants (0–3 points). Studies with scores of 0–3, 4–6 and 7–9 were considered as low, moderate and high quality, respectively.
Assessment involving the eleven items recommended by the Agency for Healthcare Research and Quality was applied for cross-sectional studies( 10 ). The quality of the studies was first evaluated according to the established questions, which were scored by the following three values: (i) 1 point if the item was considered in the study; (ii) 0 point if the item was not considered; and (iii) 0 point if we were unable to determine that the item had been considered. Each study was rated independently by two authors (H.S.B. and Y.G.).
Statistical analyses
The RR/OR was considered as the common measure of the association across studies, and HR were directly considered as RR( 11 ). Heterogeneity across studies was assessed using the Cochrane Q statistic (significance level at P<0·10) and the I 2 statistic( 12 ). The heterogeneity was considered statistically insignificant if P>0·10 and I 2<50 %, then the Mantel–Haenszel fixed-effect model was performed to calculate pooled RR among studies. Otherwise, the DerSimonian and Laird( 13 ) random-effect model was conducted to compute the results. Sensitivity analysis was performed to investigate the influence of various exclusion criteria on the pooled result. Subgroup analyses were conducted to explore the potential sources of heterogeneity across studies. Potential publication bias was evaluated by using the Begg’s test (significance level P<0·05)( 14 ). All analyses were performed with the STATA statistical software package version 11·0.
Results
Literature search
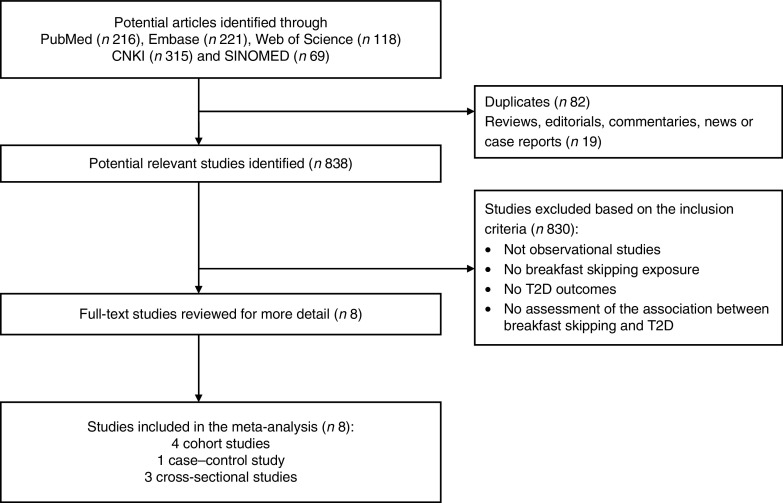
The results of the literature search and study selection process are shown in Fig. 1. Among 939 articles selected from all the databases, 838 articles were identified as potentially relevant. After retrieving the full text for detailed evaluation, eight studies examining the association between breakfast skipping and T2DM risk were identified. Inter-observer agreement between reviewers for study inclusion was outstanding (κ=0·95).
Fig. 1.
Flowchart of study selection (T2D, type 2 diabetes)
Study characteristics
The characteristics of the eight studies included are summarized in Table 1, including four prospective cohort studies( 6 , 15 – 17 ), one case–control study( 18 ) and three cross-sectional studies( 19 – 21 ). These studies were published between 1998 and 2013. The study sample size ranged from 493 to 46 289, with a total of 106 935 participants, and the follow-up durations ranged from 6 to 18 years for cohort studies. The number of T2D cases ranged from sixty-one to 2423, with a total of 7419 reported T2D outcomes. With regard to study location, three studies were conducted in the USA( 6 , 16 , 17 ), two in China( 18 , 20 ), two in Japan( 15 , 21 ) and one in Russia( 19 ). Six studies( 15 , 17 – 21 ) reported results for both men and women; the RR of one study( 15 ) was available only for women; one study( 6 ) reported results for men only; and one study( 16 ) reported results for women only. Multivariable adjusted RR (HR/OR) were reported in all studies. The major adjustment confounding factors included age, BMI, alcohol intake, smoking status, energy intake, family history of diabetes and physical activity.
Table 1.
Characteristics of studies included in the meta-analysis
| Study ID | Study design (time period) | Country | Population (sampling procedures) | No. of cases | Exposure assessment | Outcome assessment | Breakfast consumption categories (lowest v. highest) | Adjusted confounding factors | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| Sugimori et al. (1998)( 15 ) | Cohort (follow-up at 16 years) | Japan | 2573 men and women aged 18–69 years (population-based) | 296 | Questionnaire | FBS of 110 mg/dl or higher or the initiation of diabetic therapy | Never v. every day or sometimes | Age, BMI, smoking status, drinking habit, dairy intake, hypertension, hypercholesterolaemia, hyperuricaemia, family history of diabetes, FBS | 8 |
| Zhi (2007)( 20 ) | Cross-sectional | China | 21 240 men and women aged 14–74 years (population-based) | 2423 | Questionnaire | Criterion published by WHO and IDF in 1999: FPG ≥7·0 mmol/l or 2hPG ≥11·2 mmol/l | 4–6 v. 7 times/week | Age, gender, education, occupation, income of family, proportion of diet expenditure, per capita income | 5 |
| Nishiyama et al. (2009)( 21 ) | Cross-sectional | Japan | 493 men and women aged 20–99 years (population-based) | 61 | Questionnaire | Ever been diagnosed as having DM or borderline diabetes | 0–4 v. 5–7 times/week | Age, gender, BMI, smoking | 4 |
| Xiao et al. (2010)( 18 ) | Case–control | China | 1205 men and women, case mean age=54·47 (sd 9·92) years, control mean age=53·99 (sd 10·28) years (population-based) | 585 | Questionnaire | Criterion published by WHO and IDF in 1999: FPG ≥7·0 mmol/l or 2hPG ≥11·1 mmol/l | 0 v. 1–3 times/week | Unadjusted | 6 |
| Voronova et al. (2012)( 19 ) | Cross-sectional | Russia | 2331 men and women aged 27–69 years (convenience) | 153 | Questionnaire | ADA criteria used to establish definitions of diabetic: FPG≥126 mg/dl or 2hOGTT ≥200 mg/dl | 0–4 v. 5–7 times/week | Age, sex (men), current smoker, late dinner, infrequent exercise, drinking alcohol, BMI, elevated BP, high TAG, low HDL-C, high CRP | 4 |
| Mekary et al. (2012)( 6 ) | Cohort (follow-up at 14 years) | USA | 29 206 men aged 40–75 years (population-based) | 1944 | FFQ | National Diabetes Data Group criteria were used to confirm a self-reported diagnosis of T2D. For T2D cases identified after 1998, ADA criteria were applied | Non-consumers v. consumers | Age, BMI, family history of T2D, energy intake, alcohol intake, cereal fibre intake, PA, smoking status, prudent dietary pattern, Western dietary pattern | 8 |
| Mekary et al. (2013)( 16 ) | Cohort (follow-up at 6 years) | USA | 46 289 females aged 30–55 years (population-based) | 1560 | FFQ | ADA criteria were used to confirm self-reported diagnosis of T2D | Irregular breakfast consumers (0–6 times/week) v. regular breakfast consumers (7 times/week) | Age, BMI, family history of T2D, alcohol intake, PA, menopausal status and hormone use, smoking status, energy intake, cereal fibre intake, AHEI-2010 | 8 |
| Odegaard et al. (2013)( 17 ) | Cohort (follow-up at 18 years) | USA | 3598 men and women aged 18–30 years (population-based) | 397 | Questionnaire | Use of diabetes medication, fasting blood glucose level ≥6·99 mmol/l (200 mg/dl), 2 h post-challenge glucose ≥11·1 mmol/l (200 mg/dl) and/or HbA1c ≥6·5 % (48 mmol/mol) | 0–3 v. 7 times/week | Age, BMI, study centre, race, gender, education, cigarette smoking, PA, alcohol consumption, fast-food restaurant use, dietary quality score, frequency of lunch/dinner and morning/afternoon/evening snacks, total energy intake | 8 |
FBS, fasting blood sugar; IDF, International Diabetes Federation; FPG, fasting plasma glucose; 2hPG, 2 h postprandial blood glucose; DM, diabetes mellitus; 2hOGTT, 2 h post oral glucose tolerance test; T2D, type 2 diabetes; ADA, American Diabetes Association; HbA1c, glycated Hb; BP, blood pressure; HDL-C, HDL cholesterol; CRP, C-reactive protein; PA, physical activity; AHEI-2010, Alternative Healthy Eating Index 2010.
Association between breakfast skipping and risk of type 2 diabetes
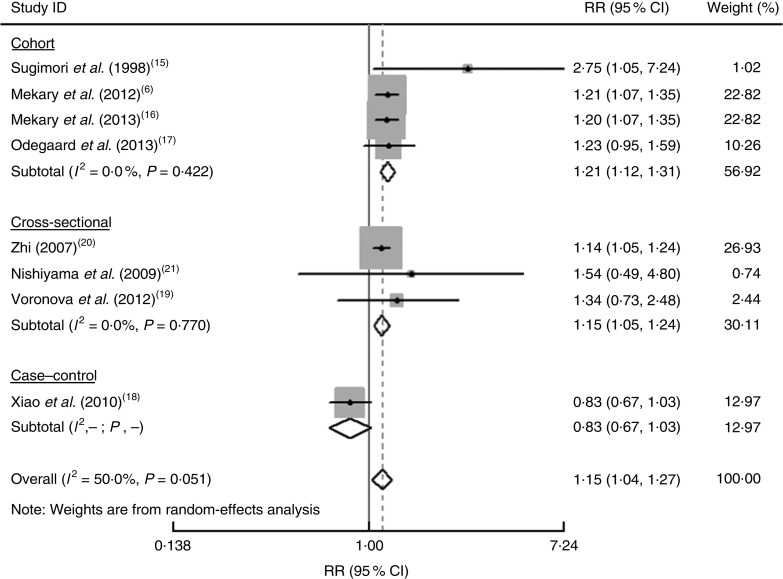
Figure 2 shows the results from the random-effects model between breakfast skipping and the risk of T2D. Four studies showed a significant positive relationship between breakfast skipping and the risk of T2D, but the others did not. The pooled RR of T2D risk for breakfast skipping was 1·21 (95 % CI 1·12, 1·31; P=0·984; I 2 =0·0 %) in cohort studies and the pooled OR was 1·15 (95 % CI 1·05, 1·24; P=0·770; I 2 =0·0 %) in cross-sectional studies. There was no heterogeneity across these studies (P=0·984, I 2 =0·0 % for cohort studies; P=0·770, I 2 =0·0 % for cross-sectional studies).
Fig. 2.
Forest plot for the pooled relative risk (RR) of breakfast skipping and the risk of type 2 diabetes (T2D). The study-specific RR and 95 % CI are represented by the grey square and horizontal line, respectively; the area of the grey square is proportional to the study-specific weight in the overall meta-analysis. The centre of the diamond presents the pooled RR for T2M and its width represents the pooled 95 % CI
Subgroup analyses
Table 2 presents the results of subgroup analysis with the random-effects model according to the study location and adjustment for important confounding factors including BMI, smoking, drinking, cereal fibre intake, family history of diabetes, hypertension, physical activity and energy intake. For cohort studies, subgroup analysis by study location showed that the pooled RR was 1·21 (95 % CI 1·12, 1·31) in the USA and 2·75 (95 % CI 1·04, 7·23) in Japan. There was no evidence of a significant heterogeneity among US studies (I 2 =0·0 %, P=0·984). The subgroup analysis of study location in cross-sectional studies showed that the pooled RR for participants from Asia was 1·14 (95 % CI 1·05, 1·24), but there was null statistically significant risk of T2D in European populations (RR=1·34; 95 % CI 0·73, 2·47). Given the influence of energy intake on the association between breakfast skipping and T2D, we also conducted subgroup analysis by energy intake in cohort studies. The results remained materially unchanged after adjusting for energy intake (RR=1·21; 95 % CI 1·12, 1·32; P=0·984).
Table 2.
Subgroup analyses on the association between breakfast skipping and the risk of type 2 diabetes (T2M)
| Heterogeneity | ||||||
|---|---|---|---|---|---|---|
| Level | No. of studies | I 2 (%) | P value | RR | 95 % CI | |
| Cohort studies | ||||||
| Total | 4 | 0·0 | 0·422 | 1·21 | 1·12, 1·31 | |
| Location | Japan | 1 | N/A | 2·75 | 1·04, 7·23 | |
| USA | 3 | 0·0 | 0·984 | 1·21 | 1·12, 1·31 | |
| Adjustment for confounding | ||||||
| Cereal fibre intake | Yes | 2 | 0·0 | 0·921 | 1·20 | 1·11, 1·31 |
| No | 2 | 59·9 | 0·115 | 1·60 | 0·76, 3·35 | |
| Family history of T2DM | Yes | 3 | 28·5 | 0·247 | 1·22 | 1·09, 1·36 |
| No | 1 | N/A | 1·23 | 0·95, 1·59 | ||
| Hypertension | Yes | 1 | N/A | 2·75 | 1·04, 7·23 | |
| No | 3 | 0·0 | 0·984 | 1·21 | 1·12, 1·31 | |
| Energy intake | Yes | 3 | 0·0 | 0·984 | 1·21 | 1·12, 1·32 |
| No | 1 | N/A | 2·75 | 1·05, 7·22 | ||
| Physical activity | Yes | 3 | 0·0 | 0·984 | 1·21 | 1·12, 1·32 |
| No | 1 | N/A | 2·75 | 1·05, 7·22 | ||
| Cross-sectional studies | ||||||
| Total | 3 | 0·0 | 0·770 | 1·15 | 1·05, 1·24 | |
| Location | Asia | 2 | 0·0 | 0·606 | 1·14 | 1·05, 1·24 |
| Europe | 1 | N/A | 1·34 | 0·73, 2·47 | ||
| Adjustment for confounding | ||||||
| BMI | Yes | 2 | 0·0 | 0·833 | 1·38 | 0·81, 2·37 |
| No | 1 | N/A | 1·14 | 1·05, 1·24 | ||
| Smoking | Yes | 2 | 0·0 | 0·833 | 1·38 | 0·81, 2·37 |
| No | 1 | N/A | 1·14 | 1·05, 1·24 | ||
| Drinking | Yes | 1 | N/A | 1·34 | 0·73, 2·47 | |
| No | 2 | 0·0 | 0·606 | 1·14 | 1·05, 1·24 | |
| Hypertension | Yes | 1 | N/A | 1·34 | 0·73, 2·47 | |
| No | 2 | 0·0 | 0·606 | 1·14 | 1·05, 1·24 | |
| Physical activity | Yes | 1 | N/A | 1·34 | 0·73, 2·47 | |
| No | 2 | 0·0 | 0·606 | 1·14 | 1·05, 1·24 | |
N/A, not applicable.
Sensitivity analyses
Sensitivity analyses were used to find potential origins of heterogeneity in the association between breakfast skipping and T2D risk, to examine the influence of various exclusions on the pooled RR/OR and to check the robustness of all results above. For cohort studies, we excluded each single study in turn and pooled the results of the remaining included studies; this did not change the overall combined RR, with a range from 1·21 (95 % CI 1·12, 1·31) to 1·25 (95 % CI 1·06, 1·47), which indicated that the pooled RR was not substantially influenced by any individual study. The fixed-effects model was also used to pool results, but we found no significant difference in the pooled RR between the two (fixed-effects model: pooled RR=1·21; 95 % CI 1·12, 1·31, random-effects model: pooled RR=1·21; 95 % CI 1·12, 1·32). For cross-sectional studies, the results of sensitivity analyses were influenced by one study( 20 ) with a range from RR=1·14 (95 % CI 1·05, 1·24) to RR=1·38 (95 % CI 0·81, 2·37).
Publication bias
No significant evidence of publication bias was found in the studies, as identified by Begg’s test (P=0·149 for the cohort studies and P=0·296 for the cross-sectional studies).
Discussion
The results of our meta-analysis confirmed the positive association between breakfast skipping and T2D risk. Both in cohort and cross-sectional studies, the pooled RR/OR showed a significant increased risk on T2D for breakfast skippers. Compared with individuals who consume breakfast regularly, the risk of T2D was increased by 21 % in cohort studies and 15 % in cross-sectional studies for breakfast skippers. Our subgroup analyses identified that the significant positive association between breakfast skipping and T2D risk was consistent in cohort studies after adjusting for confounding factors.
Some potential mechanisms might be involved in the effect of breakfast skipping on T2D risk. First, food consumption in the morning has been shown to be particularly satiating and associated with lower appetite and better weight control( 22 ). Breakfast is a unique meal since it is the time that prolonged fasting ceases. It is known that the longer the fasting time, the higher the ghrelin concentrations and the lower the insulin concentrations, which could induce hunger and eating( 6 ). As a result of breakfast skipping, people could develop obesity and chronic diseases, including T2D( 5 ). Nowadays most people have the idea that skipping breakfast could help with weight control, which could be a mediating pathway of obesity facilitating T2D risk, whereas some observational studies have shown that breakfast consumption is associated with a risk of increased BMI( 23 , 24 ). What is more, the pooled RR of cohort studies adjusting for BMI (1·21; 95 % CI 1·21, 1·32) in the present study suggested that the observed association was not completely mediated via weight control.
Besides, several studies have shown that the type of food consumed at breakfast plays an important role in affecting hormone release and activity, postprandial insulin secretion, glucose and lipid metabolism. Breakfast consumption could result in an elevated postprandial glycaemic response and insulin sensitivity( 25 – 29 ), especially when the foods consumed are fibre-rich foods (i.e. whole grains, fruit and low-fat dairy) rather than refined cereals. It could also reduce fasting total and LDL cholesterol( 30 , 31 ) and serum TAG concentrations( 32 ), which could improve lipid metabolism. A randomized crossover trial showed that men who regularly consumed breakfast had better metabolic and endocrine responses in response to foods consumed later during the day, compared with those who skipped breakfast( 33 ). Finally, energy intake was shown to be a mediating pathway which links breakfast consumption and T2D risk( 5 ). When the breakfast did not result in a significant increase in energy intake, favourable changes would be made in glycaemia and insulinaemia( 34 ). Thus, sufficient energy intake at breakfast could reduce the risk of lipid-associated chronic diseases such as CVD, obesity and T2D.
Breakfast skipping is very common in modern society( 6 , 7 ). Many people have misunderstandings about skipping breakfast, thinking it could help with keeping slim or weight loss. However, studies have suggested this behaviour may not contribute to losing weight, but increase the risk of T2D. Thus the selection of this topic is of great importance, which is necessary to further expand and deepen the research on breakfast skipping in relation to T2D risk.
To our knowledge, the current paper presents the first meta-analysis of observational studies on breakfast skipping and the risk of T2D. The results of the meta-analysis suggested that breakfast skipping was associated with a significantly increased risk of T2D. Subgroup analyses, sensitivity analyses and publication bias tests suggested that the overall result of this analysis was stable and robust. Notably, the analysis was very specific about whether people skipped breakfast or not, and the exposure of breakfast skipping was easy to confirm. Thus, except for difference in exposure dose, there was relatively less recall bias in the included studies. Both in cohort studies and in cross-sectional studies, the results showed great reliability and stability in outcomes. Thus, we also combined results of the cross-sectional studies and the prospective cohort studies in this meta-analysis. We suggest that more prospective cohort studies with a longer follow-up period and larger sample size are needed to replicate our results.
A few limitations of the meta-analysis should be acknowledged. First, although all included studies controlled for several established risk factors for T2D (including age, sex, BMI, alcohol, physical activity, etc.), the possibility of residual or unmeasured confounders attributable to other dietary factors may still influence the observed association. Second, most studies included in the meta-analysis were conducted in the USA and Asia (Japan and China), which could limit the generalizability of the findings to other populations. Finally, the limited information provided in the included studies precluded the possibility of a dose–response analysis.
For future studies, based on our findings, we suggest first that further research fully adjusting for dietary quality or other dietary factors may make the observed effects more accurate, which would more reliably attribute the reported association to breakfast skipping alone. Second, more observational and interventional studies are needed to explore the underlying mechanisms that link breakfast skipping and T2D risk. Finally, the dose–response relationship between them should be further investigated in future studies.
Conclusion
In conclusion, the current meta-analysis suggests that breakfast skipping is associated with a significantly increased risk of T2D. Given the increasing prevalence of breakfast skipping worldwide and the heavy economic burden of T2D, the results of our study provide practical and valuable clues for the prevention and aetiology of T2D.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: H.S.B. and Y.G. contributed equally to this work. H.S.B., Y.G. and Z.X.L. conceived the study. H.S.B. and Y.G. searched the databases and checked them according to the inclusion and exclusion criteria. Z.X.L. helped develop search strategies. H.S.B. and Y.G. did data extraction and quality assessment. H.S.B., Y.G., C.Y., Y.W.C. and X.Y.T. analysed the data. X.Y.T. gave advice on meta-analysis methodology. H.S.B. wrote the draft of the paper. All authors contributed to writing, reviewing or revising the paper. All authors read and approved the final manuscript. Z.X.L. is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Ethics of human subject participation: Ethical approval was not required.
These authors have contributed equally to this work.
References
- 1. Zimmet P, Alberti KG & Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414, 782–787. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33, Suppl. 1, S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCrimmon RJ, Ryan CM & Frier BM (2012) Diabetes and cognitive dysfunction. Lancet 379, 2291–2299. [DOI] [PubMed] [Google Scholar]
- 4. Shaw JE, Sicree RA & Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87, 4–14. [DOI] [PubMed] [Google Scholar]
- 5. Timlin MT & Pereira MA (2007) Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr Rev 65, 268–281. [DOI] [PubMed] [Google Scholar]
- 6. Mekary RA, Giovannucci E, Willett WC et al. (2012) Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr 95, 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rampersaud GC, Pereira MA, Girard BL et al. (2005) Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Diet Assoc 105, 743–760. [DOI] [PubMed] [Google Scholar]
- 8. Stroup DF, Berlin JA, Morton SC et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012. [DOI] [PubMed] [Google Scholar]
- 9. Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605. [DOI] [PubMed] [Google Scholar]
- 10. Rostom A, Dubé C, Cranney A et al. (2005) Appendix D, Quality Assessment Forms. In Celiac Disease. Evidence Report/Technology Assessment no. 104. Rockville, MD: Agency for Healthcare Research and Quality, DHHS. [Google Scholar]
- 11. Spruance SL, Reid JE, Grace M et al. (2004) Hazard ratio in clinical trials. Antimicrob Agents Chemother 48, 2787–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JP & Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- 13. DerSimonian R & Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7, 177–188. [DOI] [PubMed] [Google Scholar]
- 14. Begg CB & Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. [PubMed] [Google Scholar]
- 15. Sugimori H, Miyakawa M, Yoshida K et al. (1998) Health risk assessment for diabetes mellitus based on longitudinal analysis of MHTS database. J Med Syst 22, 27–32. [DOI] [PubMed] [Google Scholar]
- 16. Mekary RA, Giovannucci E, Cahill L et al. (2013) Eating patterns and type 2 diabetes risk in older women: breakfast consumption and eating frequency. Am J Clin Nutr 98, 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Odegaard AO, Jacobs DR Jr, Steffen LM et al. (2013) Breakfast frequency and development of metabolic risk. Diabetes Care 36, 3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiao H, Wang J & Zhi X (2010) A case–control study on risk factors of type 2 diabetes mellitus in suburban of Tianjin. Chin J Dis Control Prev 14, 95–98. [Google Scholar]
- 19. Voronova NV, Nikitin AG, Chistiakov AP et al. (2012) Skipping breakfast is correlated with impaired fasting glucose in apparently healthy subjects. Cent Eur J Med 7, 376–382. [Google Scholar]
- 20. Zhi X-Y (2007) A study of prevalences and risk factors of type 2 diabetes and its complications in Tianjin. PhD Thesis, Tianjin Medical University.
- 21. Nishiyama M, Muto T, Minakawa T et al. (2009) The combined unhealthy behaviors of breakfast skipping and smoking are associated with the prevalence of diabetes mellitus. Tohoku J Exp Med 218, 259–264. [DOI] [PubMed] [Google Scholar]
- 22. de Castro JM (2004) The time of day of food intake influences overall intake in humans. J Nutr 134, 104–111. [DOI] [PubMed] [Google Scholar]
- 23. Song WO, Chun OK, Obayashi S et al. (2005) Is consumption of breakfast associated with body mass index in US adults? J Am Diet Assoc 105, 1373–1382. [DOI] [PubMed] [Google Scholar]
- 24. Ma Y, Bertone ER, Stanek EJ 3rd et al. (2003) Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol 158, 85–92. [DOI] [PubMed] [Google Scholar]
- 25. Clark CA, Gardiner J, McBurney MI et al. (2006) Effects of breakfast meal composition on second meal metabolic responses in adults with type 2 diabetes mellitus. Eur J Clin Nutr 60, 1122–1129. [DOI] [PubMed] [Google Scholar]
- 26. Liljeberg HG, Akerberg AK & Bjorck IM (1999) Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am J Clin Nutr 69, 647–655. [DOI] [PubMed] [Google Scholar]
- 27. Kochar J, Djousse L & Gaziano JM (2007) Breakfast cereals and risk of type 2 diabetes in the Physicians’ Health Study I. Obesity (Silver Spring) 15, 3039–3044. [DOI] [PubMed] [Google Scholar]
- 28. Song YJ, Sawamura M, Ikeda K et al. (2000) Soluble dietary fibre improves insulin sensitivity by increasing muscle GLUT-4 content in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 27, 41–45. [DOI] [PubMed] [Google Scholar]
- 29. Nestler JE, Barlascini CO, Clore JN et al. (1988) Absorption characteristic of breakfast determines insulin sensitivity and carbohydrate tolerance for lunch. Diabetes Care 11, 755–760. [DOI] [PubMed] [Google Scholar]
- 30. Farshchi HR, Taylor MA & Macdonald IA (2005) Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr 81, 388–396. [DOI] [PubMed] [Google Scholar]
- 31. Smith KJ, Gall SL, McNaughton SA et al. (2010) Skipping breakfast: longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am J Clin Nutr 92, 1316–1325. [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto R, Kawamura T, Wakai K et al. (1999) Favorable life-style modification and attenuation of cardiovascular risk factors. Jpn Circ J 63, 184–188. [DOI] [PubMed] [Google Scholar]
- 33. Astbury NM, Taylor MA & Macdonald IA (2011) Breakfast consumption affects appetite, energy intake, and the metabolic and endocrine responses to foods consumed later in the day in male habitual breakfast eaters. J Nutr 141, 1381–1389. [DOI] [PubMed] [Google Scholar]
- 34. McCrory MA & Campbell WW (2011) Effects of eating frequency, snacking, and breakfast skipping on energy regulation: symposium overview. J Nutr 141, 144–147. [DOI] [PubMed] [Google Scholar]