Abstract
Objective
Non-pharmacological approaches to the treatment of depression and anxiety are of increasing importance, with emerging evidence supporting a role for lifestyle factors in the development of these disorders. Observational evidence supports a relationship between habitual diet quality and depression. Less is known about the causative effects of diet on mental health outcomes. Therefore a systematic review was undertaken of randomised controlled trials of dietary interventions that used depression and/or anxiety outcomes and sought to identify characteristics of programme success.
Design
A systematic search of the Cochrane, MEDLINE, EMBASE, CINAHL, PubMed and PyscInfo databases was conducted for articles published between April 1971 and May 2014.
Results
Of the 1274 articles identified, seventeen met eligibility criteria and were included. All reported depression outcomes and ten reported anxiety or total mood disturbance. Compared with a control condition, almost half (47 %) of the studies observed significant effects on depression scores in favour of the treatment group. The remaining studies reported a null effect. Effective dietary interventions were based on a single delivery mode, employed a dietitian and were less likely to recommend reducing red meat intake, select leaner meat products or follow a low-cholesterol diet.
Conclusions
Although there was a high level of heterogeneity, we found some evidence for dietary interventions improving depression outcomes. However, as only one trial specifically investigated the impact of a dietary intervention in individuals with clinical depression, appropriately powered trials that examine the effects of dietary improvement on mental health outcomes in those with clinical disorders are required.
Keywords: Diet, Diet intervention, Depression, Mental health
The prevention and treatment of the high prevalence mental disorders, depression and anxiety are of increasing global importance due to the substantial health, social and economic burden they impose. Major depressive disorders and anxiety disorders are among the leading causes of years lived with disability( 1 ); in 2010, the global cost of these conditions was estimated to be $US 2·5 trillion( 2 ). Although pharmacotherapy and psychotherapy are considered first-line treatments for depression, fewer than half of those treated achieve remission( 3 ). Thus, there is a need to develop further strategies to effectively treat depression.
Over recent years, evidence has emerged to support a relationship between habitual diet quality and the risk for depression. Epidemiological studies have suggested that a healthy dietary pattern including fruits, vegetables, fish, olive oil, nuts and legumes is protective against depression( 4 , 5 ). Conversely, a dietary pattern that comprises a high consumption of processed foods and sugary products may increase the risk of depression( 4 , 6 ). While the observational evidence generated to date is suggestive of a relationship between dietary intake and depression, only randomised controlled trials (RCT) that elucidate the effects of dietary change on mental health outcomes can verify whether the relationship between diet and mental health is causal in nature.
Previously, intervention studies evaluating the role of dietary improvement on disease outcomes have been conducted in populations with (somatic) chronic illnesses rather than mental disorders, largely focusing on those: overweight and obese; with elevated risk factors for metabolic disorders; with other medical illnesses; in the general population. These studies have often employed metabolic primary end points including obesity and risk factors for type 2 diabetes mellitus (T2DM) and CVD. Further, there has been wide variance in the mode of delivery and theoretical models underpinning these interventions. Those interventions successful in achieving dietary behaviour change and compliance have been characterised by the provision of written information (e.g. dietary guidelines, shopping lists, meal plans and recipes), free provision of key food items, self-monitoring, goal setting, individual contracts, group sessions( 7 ) and dietary counselling (e.g. motivational interviewing and mindfulness)( 8 ). In some instances, frequent contact during intensive interventions has been shown to be beneficial( 7 ).
Indeed, the existing evidence base provides support for physical health benefits as a result of dietary interventions and it stands to reason that these effects may extend beyond physical benefits to impact upon mental health outcomes. However, the impact of dietary improvement on mental health currently remains unclear.
The purpose of the present systematic review was thus to synthesise data from existing RCT of dietary interventions (with a whole-of-diet approach) that have investigated depression and/or anxiety outcomes (as either primary or secondary outcomes) in psychiatric and other populations. Furthermore, we sought to determine which components of dietary interventions (e.g. interventionist, mode of delivery, session frequency) are associated with programme success. To our knowledge, this is the first systematic literature review of its kind.
Methods
Literature search
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines( 9 ) were used as a methodological template for this review. Please see online supplementary material 1 for the PRISMA checklist.
Relevant studies were identified by systematic search from the Cochrane, MEDLINE, EMBASE, CINAHL, PubMed and PsycInfo databases for articles published between April 1971 and May 2014. Relevant keywords relating to diet in combination as MeSH (Medical Subject Heading) terms and text words (‘diet’ or ‘Mediterranean diet’ or ‘diet therapy’ or ‘diet education’ or ‘diet counselling’ or ‘diet intervention’ or diet ‘treatment’ and their variants) were used in combination with words relating to depression and anxiety (‘anxiety’ or ‘anxiety disorder’ or ‘depression’ or ‘depressive disorder’ or ‘major depressive disorder’) and intervention styles (‘randomised controlled trial’ or ‘random allocation’ or ‘clinical trial’ or ‘control groups’ and their variants). The search was limited to studies written in the English language and undertaken in human subjects. Additional publications were identified from references published in the original papers. Please see online supplementary material 2, Supplemental Table 1 for the full electronic search strategy.
Eligibility criteria
All articles that evaluated diet as a whole were included; studies which evaluated single food items or nutrients or meal replacement products (e.g. liquid shakes) were excluded. In order to be eligible for inclusion, the dietary intervention needed to be described in sufficient detail, highlighting the main components of the diet.
Only RCT were considered for inclusion, including a dietary intervention v. a control condition (e.g. usual (standard) care, non-dietary modification). Dietary studies with combined interventions (e.g. exercise, stress management) were eligible, as were dietary studies that included a placebo control. Composite interventions (e.g. those that use diet as a component of a multifaceted intervention) were also included. Where a study employed a multiple-treatment-arm design (e.g. diet and exercise v. diet), the results from the condition considered most comparable to other studies were selected.
Only studies that included adults (≥18 years of age) and reported validated depression or anxiety outcome measures were eligible for inclusion. Studies were not deemed ineligible if they included those with a chronic condition (e.g. CVD, T2DM, cancer, hypertension); however, those comprising participants with eating disorders or pregnant and breast-feeding women were excluded as these conditions were considered to be potentially confounding factors.
Data extraction
We screened potentially relevant articles for eligibility based on titles and abstracts. If deemed potentially eligible, the full text publication was retrieved and reviewed (R.S.O., S.D.). Where areas of uncertainty occurred co-authors were consulted (C.I., F.N.J.). To prevent duplication and allow for unique analysis, only empirical studies were included in the present review (e.g. systematic reviews were not included). Data were extracted from included studies and details are presented in Tables 1 and 2, using the following parameters: study aims, study design, study location, sample size, participant characteristics, length of follow-up, programme components (dietary and co-interventions), control group protocol, research outcomes and results.
Table 1.
Characteristics and results of diet interventions, categorised by composite interventions
| Reference, country (study ID), study name | Population, eligibility criteria | Intervention, sample size | Intensity, frequency, timing, delivery mode, intervention/follow-up length | Individuals delivering intervention | Details of diet intervention, dietary components, co-intervention | Control, sample size | Relevant outcomes (anxiety or depression, diet measures) | Results (depression and/or anxiety) | Method of presenting results |
|---|---|---|---|---|---|---|---|---|---|
| Diet only interventions | |||||||||
| McMillan et al. (2011)( 23 ), Australia (McMillan 2011) | Females, aged 19–30 years | Diet-change group, n 12 | Familiarised with eating plan at the 1st study visit. Participants began the new diet the following day 10 d | ‘Experimenter’ | Pilot study: provided with an eating plan outlining the foods to include and exclude during the 10 d. Required to increase intake of fruits, vegetables, fatty fish, nuts and seeds, low-fat natural dairy and wholegrain cereals At each main meal, instructed to combine a form of protein, healthy fat (providing EFA) and carbohydrates. Red meat, refined sugars, refined flour, pre-packaged and processed foods, caffeinated products, soft drinks and condiments were all excluded. Energy intake was not restricted | ‘No change’ control group: instructed to continue their usual daily diet, n 13 | The 65-item POMS. Measured depression and anxiety outcomes Daily food diary to aid compliance | (NS) There were no significant treatment × time interaction effects for depression (P=0·58) and anxiety (P=0·51) | Mean (sd) |
| Wardle et al. (2000)( 21 ), UK (Wardle 2000) | Adults with raised serum cholesterol levels | 1. Low-fat diet, n 59 2. Mediterranean diet, n 61 | Delivered in 8 sessions during a 12-week period. Combination of individual and group sessions 12-week intervention | Dietitian and psychologist | Provided education about the recommended dietary changes and a cognitive-behavioural intervention that was concerned with implementing changes in eating behaviour Given free-spreading fats and oils that were high in PUFA (low-fat diet) or MUFA (Mediterranean diet) to encourage compliance Low fat group: shift away from foods containing saturated fats – to reduce energy from fats to <20 %E, largely polyunsaturated Mediterranean diet: increase fruit, vegetables and oily fish intake, and reduce fat to 30 %E, with substitution by MUFA for saturated fats | 3. Waiting list control condition, n 56 Control group were not given specific dietary advice, but were not discouraged from making dietary changes. Offered treatment at the end of their waiting list period | BDI; the depression subscale of the POMS 7 d diet diary | (NS) All three groups had stable or improved psychological well-being during the study, with no significant differences among the three groups Depression and anxiety declined significantly | Mean reduction (95 % CI) |
| Scheier et al. (2005)( 13 ), USA (Scheier 2005) | Women with stage 0, I or II breast cancer, ≤50 years of age | 3. Active treatment (nutritional intervention), n 85 | Each active treatment arm received 4 group sessions that met once a month for 4 consecutive months. Each monthly session lasted 2 h 4-month intervention | Presented by a professional trained in nutritional science | Nutrition intervention: provided with strategies to adopt and adhere to a low-fat, high-fruit-and-vegetable eating pattern Emphasis placed on the helpfulness of accomplishing any change in reducing dietary fat, not necessarily in adopting the suggested dietary changes completely | 1. Control: received standard medical care, n 84 2. Active treatment (education intervention): provided information about their illness (and treatment) and its adverse effects. Sessions were led by 2 professionals with expertise in the topic of that session, n 83 | Abbreviated 10-item version of the CES-D Nutritional arm: 4 d food diary, self-report dietary questionnaire | Participants exhibited significantly fewer depressive symptoms over time (F 2440=4·18; P<0·02). There was also a significant treatment × time interaction (F 440=2·45; P<0·05) (NS) At time 2, neither the nutrition arm nor the education arm differed from the control arm (all P>0·09) (☺) At time 3, participants in the nutrition arm reported significantly fewer depressive symptoms (β=−0·23; P<0·001) than participants in the control arm. The difference between the two active treatment arms was not significant (P>0·05) | Mean (sd) |
| Endevelt et al. (2011)( 16 ), Israel (Endevelt 2011) | Community-dwelling older adults aged ≥75 years at nutritional risk. Excluded individuals with clinical depression | DIT group – intensive nutritional intervention, n 35 | Each patient had 5 nutritional treatment meetings in the clinic or at home as required. First 2 visits lasted 45 min and other visits lasted 30 min 6-month follow-up | Nutritional intervention led by a registered dietitian | Individualised treatment strategy for each patient was designed by the dietitian. Intensity of intervention varied according to severity of undernutrition Patients and their families received guidance on how to improve the quality of their diet by getting subsidised prepared meals or by making easy and healthy meals at home | MT group – led by the primary care physician. Received treatment from the physician and a nutrition information booklet regarding nutritional needs of older adults; n 33 Non-randomised UNG group – did not receive nutritional assessment or treatment, n 59 | GDS-sf (depression score) MNA, FFQ – developed and validated for use in the older adult population in Israel | (☺) There was a significant improvement in the depression scores among the DIT group compared with the MT group and the UNG group, respectively (5·4 (sd 3·9) v. 6·3 (sd 4·0) v. 6·6 (sd 5·9); P=0·04) after 6 months of intensive intervention and follow-up | Mean (sd) |
| Forster et al. (2012)( 20 ), UK (Forster 2012) | Community-living older adults aged 65–85 years and leading an active independent life. Excluded individuals with severe medical or psychiatric illness (including Alzheimer’s and senile dementia) | 1. Food intervention, n 73 | Provided with food weekly. Researchers visited and contacted all groups at the same frequency to minimize any potential confounding 3-month intervention and 6-month follow-up | Postdoctoral researcher | Provided with a selection of foods each week (approx. $20) to help meet specific dietary targets. Asked to consume at least five portions of fruit and vegetables per day, eat only wholegrain bread, consume fish at least twice per week and consume nuts at least once per week The researcher chose the specific foods provided in consultation with each participant, taking into account taste preferences and the intention of increasing intakes of Zn, Se, carotenoids, vitamins C and E. A supermarket home delivery service delivered the food directly to participants | 2. Micronutrient supplement: capsule contained 1500 μg β-carotene, 2 mg vitamin E, 80 mg vitamin C, 2 mg Zn and 25 μg Se, n 73 3. Placebo, n 71 Groups 2 and 3 asked to consume one capsule per day | GDS Groups 1, 2 and 3: 4 d food diaries and plasma micronutrient status Food intervention: 24 h recalls | (NS) No significant difference was observed in the change in GDS score between baseline and end of intervention | Data not shown |
| Diet only (one intervention arm) + diet and exercise (other intervention arm) | |||||||||
| Nieman et al. (2000)( 19 ), USA (Nieman 2000) | Obese females; 25–70 years of age; in good health with no known diseases; BMI of 25–50 kg/m2; not experiencing salient emotional or mood problems | 1. Diet (D), n 26 2. Exercise and diet (ED), n 22 | Attended a weekly 45-min class and received instructions on weight-loss principles and nutrition guidelines 12-week study | Dietitians | Diet group: placed on a 4·19–5·44 MJ/d diet for 12 weeks. Menu was based on dietary exchanges (2 fruit, 3 vegetables, 2 milk, 6 bread, 2 fat, 5 lean protein and 0·42 MJ of optional foods). Instructed on portion sizes, food exchanges, and how to record dietary intake using a daily exchange checklist Subjects in the two exercise groups (ED and E): required to walk 5 times/week, 45 min/session, at 60–80 % of maximum heart rate, for 12 weeks (60 total exercise sessions) | 3. Obese control (C), n 22 Subjects in the two non-walking groups (C and D) reported to the exercise facility 4 d/week for 45 min of stretching and callisthenic exercises 4. Exercise (E), n 21 | GWBS consists of subscale scores that indicates cheerful–depressed. POMS consists of scales to assess anxiety, depression–dejection 3 d food record. Compliance measured by weekly 24 h dietary recalls (11 per subject) | (☺) ED; (NS) D. The GWBS total score improved significantly in ED, but not E or D, relative to C. Improvement was not significant until the 12-week study was completed (☺) ED; (NS) D. The GWBS subscale (cheerful v. depressed mood) showed significant improvement in ED (P=0·035) (NS) There was no significant improvement in the POMS global score or for any of the six POMS scores | Mean (se) |
| Imayama et al. (2011)( 24 ), USA (Imayama 2011) NEW Trial | Postmenopausal women 50–75 years of age; BMI ≥25·0 kg/m2 (if Asian-American, BMI ≥23·0 kg/m2); physical activity <100 min/week; no serious medical conditions | 1. Dietary weight loss, n 118 2. Combined exercise+diet, n 117 | Received individual sessions at least twice, then met weekly in small groups (avg. 5–10 women) until week 24. Afterwards, communicated with the dietitian at least twice per month via group sessions or email/phone 12-month intervention | Intervention conducted by dietitians with training in behaviour modification | Diet group: reduced-energy weight-loss intervention (a modification of the Diabetes Prevention Program Lifestyle and Look AHEAD Trial interventions) with goals of total energy intake of 5021–8368 kJ/d (1200–2000 kcal/d) based on baseline weight, ≤30 %E from fat, and 10 % weight loss within the first 24 weeks with maintenance for the remainder of the intervention Exercise intervention: 45 min/d, 5 d/week including 3 exercise physiologist-supervised sessions per week at the facility | 3. Moderate-to-vigorous intensity aerobic exercise for 45 min/d, 5 d/week, n 117 4. Control group: controls were not given an intervention during the trial, n 87 | Depression and anxiety assessed by Brief Symptom Inventory-18 Women’s Health Initiative 120-item FFQ | (NS) The overall and pairwise comparisons among 4 study arms did not reach statistical significance (due to Bonferroni correction for multiple comparison; P≤0·017 was considered statistically significant in the pairwise comparison). The diet + exercise group reduced depression (Δ diet + exercise=−1·7 points, P=0·03; v. Δ control=0·7 points) (NS) Change in anxiety levels did not differ by intervention arms | Mean (sd) |
| Kiernan et al. (2001)( 26 ), USA (Kiernan 2001) | Men and premenopausal women 25–49 years old; in generally good health Men with BMI of 28·0–34·0 kg/m2 and women with BMI of 24·0–30·0 kg/m2 | 1. Diet only, n 40 2. Diet-plus-exercise, n 39 | Diet only: weekly classes for the first 3 months, every other week for the next 3 months and monthly for the remaining 6 months 1-year trial | Registered dietitians | 1. Diet only: low-saturated-fat, low-cholesterol diet and no change in exercise behaviour. Encouraged to make dietary changes based on the National Cholesterol Education Program Step 1 recommendations 2. Diet-plus-exercise: identical low-fat diet plus group-based aerobic exercise. Attended separate but identical dietary classes and a supervised programme of aerobic exercise that met 3 d/week | 3. Assessment only (control group): no change in diet or exercise behaviour, n 40 | 21-item BDI; 20-item short form of the Taylor Manifest Anxiety Scale | (NS) For men, there was no significant main effect of programme type for depressive symptoms (P>0·23) and anxiety symptoms (P>0·07) (NS) For women, there was no significant main effect of programme type for depressive symptoms (P>0·35) and anxiety symptoms (P>0·18) | ‘Change scores’ |
| Jenkinson et al. (2009)( 25 ), UK (Jenkinson 2009) | Men and women aged 45 years and over with knee pain and BMI ≥28·0 kg/m2 | 1. Dietary intervention plus quadriceps-strengthening exercises, n 109 2. Dietary intervention alone, n 122 | Visited at home once a month for the first 6 months and then every other month for the remainder of follow-up 24-month follow-up | Dietitian conducted the first home visit. The dietitian, dietetic assistant or a research interviewer carried out follow-up home visits | Individualised dietary advice that would help to create a deficit of 2·5 MJ (600 kcal) a day, in line with healthy eating recommendations (reducing fat and sugar intake, eating more fruit and vegetables, and reducing portion sizes) and achieve a weight loss of 0·5 to 1 kg a week. Newsletters from the dietitian containing recipe ideas and advice for eating healthily when eating out or at holiday times were sent every few months For those randomised to diet and exercise group: the trial dietitian also taught the programme of exercises at the initial home visit | 3. Quadriceps-strengthening exercises alone, n 82 4. Advice leaflet only (control group), n 76 Visited every 4 months for 24 months but received a support telephone call in between their visits. Calls were not used to reinforce the exercise programme | HADS Dietary groups completed the EPIC 7 d food diary | (☺) There was a statistically significant reduction in depression score in the diet group (–0·67 (se 0·32); 95 % CI −1·30, −0·04; P=0·037). Absolute effect size=0·19 (NS) There was no evidence of an effect of dietary intervention on anxiety (P=0·807) | Mean (se) |
| Diet and placebo (one intervention arm) + diet and supplementation/medication (other intervention arm) | |||||||||
| Hyyppa et al. (2003)( 22 ), Finland (Hyyppa 2003) | Untreated hypercholesterolaemic men, aged 35–64 years; BMI ≤32·0 kg/m2 | 3. Mediterranean type diet + simvastatin 20 mg/d, n 30 4. Mediterranean-type diet + placebo, n 30 | 1 individual session and 2 group counselling sessions at the beginning of treatment. 5 subsequent monthly group ‘brush-up’ sessions 4- to 6-week placebo run-in period plus 12-week intervention | Supervised by a nutritionist | First entered a 4- to 6-week open placebo run-in period – randomly allocated to Mediterranean-type diet or habitual diet. Then, a second randomisation was performed where the subjects received simvastatin or placebo No more than 10 %E from saturated fat and trans fats; cholesterol no more than 250 mg/d; n-3 fatty acids (of plant and marine origin) at least 4 g/d, ratio of n-6:n-3 PUFA less than 4; and increased intake of fruits, vegetables and soluble fibre( 41 ) Advised to use leaner meat products, low-fat dairy, fish as a main meal once or twice per week, rapeseed margarine in place of butter. Rapeseed margarine and oil, oat bran and frozen berries were supplied free to study subjects( 41 ) | 1. Habitual diet + placebo, n 30 2. Habitual diet + simvastatin 20 mg/d, n 30 Those on habitual diet were advised to continue their usual diet | BSI – assessed psychological distress. Encompassing somatisation, depression, anxiety, hostility, phobic anxiety and obsessive-compulsive behaviour | (NS) No significant (mean) changes in anxiety or depression were seen after dietary intervention (☹) Simvastatin treatment resulted in a statistically significant increase in depression (P=0·016) | Mean (se) |
| Einvik et al. (2010)( 38 ), Norway (Einvik 2010) Screening programme: Oslo diet and Antismoking Study Follow-up: DOIT | Recruited from a screening programme of men aged 40–49 years with elevated cholesterol levels and systolic BP. At baseline of DOIT, men were a mean of 70 years of age | Received traditional lifestyle advice + 5 years of dietary counselling (1972–1977), n 604 In 1997 (after 25 years’ follow-up), survivors from the original trial were invited to participate in DOIT 1. Diet only (dietary counselling and placebo), n 139 2. Combined (dietary counselling and n-3 PUFA supplementation), n 142 | Counselling occurred for 30–45 min at baseline and after 3 months. Subjects visited the nutritionist every 6 months in the remaining study period 3 years of dietary counselling | Counselling given by a clinical nutritionist | Individual education: advised to increase the use of vegetable oils and margarines (rapeseed oil, olive oil and sunflower oil), vegetables, fruit and fish; decrease the use of meat and fat from animal sources; overweight subjects to adopt an energy-restricted diet Additional follow up was offered to subjects with poor compliance | Received traditional lifestyle advice, including advice on cessation of smoking, n 628 DOIT: 3. Control (placebo and no dietary counselling), n 142 4. n-3 PUFA only (n-3 PUFA supplementation and no dietary counselling), n 140 | Self-completed HADS Compliance was monitored by the FFQ and serum n-3 PUFA | (NS) There were no differences in depression and anxiety during the intervention period between the diet and the non-diet groups All variables showed significant negative within-group trends | Mean (sd) |
| Diet and exercise | |||||||||
| Ghroubi et al. (2009)( 17 ), Tunisia (Ghroubi 2009) | Obese patients, BMI ≥30·0 kg/m2. Excluded patients with severe psychiatric disorders and those who had not diligently followed 8 consecutive weeks of training | G2: received dietary and health advice and also underwent aerobic, treadmill-based training, n 26 G3: as per G2+arm and leg muscle strength exercises, n 28 | All three groups underwent dietary monitoring programme, with an initial consultation, a check-up in the middle and another during the final sessions 2-month programme | Interview-based food survey performed for all patients by dietitians in the Endocrinology department | A 25–30 % decrease in energy intake was applied in most cases. The diet was balanced with 15 % protein, 30–35 % fat, 50–55 % carbohydrate, on average. Checked that food was eaten as three daily meals and emphasised the need to have a substantial breakfast. The prescribed low-energy diet introduced an energy shortfall of 2092 to 2510 kJ/d All patients were advised to start their diet and lose 2–3 kg before starting the physical training sessions | Controls (G1): did not receive dietary and health advice and did not perform the physical exercise programme, n 29 | HADS Interview-based food survey with the objective of specifying previous food habits and possible anomalies in dietary behaviour | (☺) Improvement in the anxiety score on the HADS in the intervention groups; mean value fell from 12 (sd 1·8) to 8·3 (sd 2·7) in G3 (a 30 % improvement, P<0·001) and from 12·34 (sd 1·67) to 9·92 (sd 3·4) in G2 (a 19 % improvement, P<0·001). No change in the anxiety score was seen in G1 (☺) Improvement in the depression score on the HADS, with the mean values falling from 9·4 (sd 2·7) to 4·9 (sd 1·7) in G3 (a 47 % improvement, P<0·001) and from 8·8 (sd 4·2) to 5·6 (sd 3·4) in G2 (a 36 % improvement, P=0·002). No improvement in depression was seen in G1 | Mean (sd) |
| Glasgow et al. (2006)( 28 ), USA (Glasgow 2006) | Adults diagnosed with T2DM for at least 6 months; ≥25 years of age | TSM intervention, n 174 | ~1 week and 1 month after the 1st visit, participants received a follow-up call (averaging 10–15 min) from their health coach. A tailored health newsletter was also mailed ~6 weeks after the first visit 2-month follow-up | Health educators (i.e. health coaches), with no formal training and little or no experience in diabetes, answered any questions and helped to ensure that the final plan was appropriate for the individual | TSM intervention: the CD-ROM programme focused on healthy eating and physical activity. TSM incorporated key components of the chronic care model self-management programme. Tailored follow-up letters reinforcing the patients’ selected goals were automatically generated by the computer program The computer program and the health-coaching session emphasised motivating factors and barriers to healthy eating and physical activity Coaches reviewed proper food portion sizes, nutritional balance, and fat, fruit and vegetable intakes | The ‘usual care’ comparison group received computer-assisted generic health risk appraisal, n 161 | The PHQ – depression severity measure. The PHQ-9 scores each of the nine DSM-IV depression criteria Block fat screener – estimates dietary fat intake. All Day NCI Fruit and Vegetable Screener – assesses fruit and vegetable intake | (NS) There were no significant between-group differences for PHQ-9 scores (P=0·23) | Mean (sd) |
| Diet, exercise and smoking/tobacco use | |||||||||
| Andersen et al. (2004)( 18 ), USA (Andersen 2004) | Women diagnosed with stage II or III breast cancer, surgically treated awaiting adjuvant therapy; aged 20–85 years. Exclusion criteria: severe or untreated psychopathology (e.g. schizophrenia) | Intervention group, n 114 | Met weekly for 1·5 h for 18 sessions (27 therapy hours during 4 months) provided in small cohorts 4-month intervention | Conducted by 2 clinical psychologists | Topics and techniques were consistent with psychological interventions but also included diet, exercise, smoking and adherence components. 4 sessions (sessions 13 to 16) on health behaviours (diet, exercise and smoking) Diet intervention components included: food intake diary, low fat/high fibre information, food substitution, intake and energy balance information, sampling of low-fat snacks | Assessment only, n 113 Psychological, behavioural, medical and treatment information data were gathered. 60 ml blood sample was drawn. Paid $US 25 per assessment | POMS assesses negative mood. The Total Mood Disturbance score is the sum of five scales (Anxiety, Depression, Anger, Fatigue and Confusion) The Food Habits Questionnaire. Included 5 scales: 1. avoiding fat; 2. food substitution with lower-fat alternatives; 3. modification of food preparation; 4. replacing high-fat with low-fat foods; 5. fruit and vegetable intake | (NS) The two-way interaction for Total Mood Disturbance was not significant (☺) A significant three-way interaction was found (F 1,192=4·55, P=0·03), such that Total Mood Disturbance decreased more in the intervention arm than the assessment arm (control group) (F 1,93=4·13, P=0·04) for subjects with high initial cancer stress (☺) The two-way interaction was significant (F 1,193=4·15, P=0·04), such that there was a greater reduction of anxious moods in the intervention arm than in the assessment arm (NS) The three-way interaction was not significant; the intervention was equally effective in reducing anxiety for patients with low or high cancer stress | F value |
| Merrill et al. (2008)( 14 ), USA (Merrill 2008) CHIP | At least 18 years of age. Encouraged to participate in the programme with a spouse or significant other | CHIP: health education intervention that promotes better choices in nutrition, physical activity and tobacco use, n 174 | Intensive 4-week class, which met 2 h/d, 4 times/week, totalling approx. 32 h in class 6 weeks and 6-month follow-up | Dietitians and medical professionals spoke to the group weekly | Topics covered included: atherosclerosis, coronary risk factors, obesity, dietary fibre, smoking, diabetes, hypertension, hypercholesterolaemia, dietary fat and cholesterol, exercise, osteoporosis, cancer, lifestyle and health, the optimal diet, behavioural change and self-worth Participants made dietary and exercise goals. Dietary goals involved adopting a diet that emphasised unrefined food, more whole grains, legumes, vegetables and fresh fruits. Recommended diet was largely unrefined complex carbohydrates (65–70 %E); low in fat (<20 %E), animal protein, sugar and salt; and virtually free of cholesterol The exercise goal consisted of exercising at least 30 min/d | Control group: started the class 6 months later and were instructed to continue with their current lifestyle habits during the 6-month wait, n 174 | BDI-SF Block 98 full-length dietary questionnaire | (☺) Repeated-measures analysis indicated a significant time effect (P<0·0001) and group × time effect (P<0·0001) for BDI. Decrease in mean BDI through 6 weeks was significantly greater for those in the intervention group than in the control group (−2·6 v. −0·4, P<0·0001), with those in the intervention group 63 % more likely to show a decrease (P<0·0001). After 6 months, those in the intervention group continued to show a significantly greater decrease in BDI (−2·4 and −0·7, P<0·0001), with those in the intervention group 34 % more likely to show a decrease (P<0·0001) | Raw data not presented |
| Diet, exercise, sleeping and exposure to light | |||||||||
| Garcia-Toro et al. (2012)( 15 ), Spain (Garcia-Toro 2012) Four hygienic–dietary recommendations | Aged ≥18 years with a depressive episode (major depressive disorder, dysthymic disorder or bipolar disorder according to DSM-IV). All participants were on antidepressant treatment | Active treatment, n 40 | Received an envelope containing a sheet of paper 6-month evaluation | Written information only | Active treatment group received an envelope containing a sheet of paper with 4 hygienic–dietary recommendations. Topics were: eating, sleeping, exercise and exposure to light Diet recommendations: ‘Try to eat a healthy and balanced diet. Eat at regular hours without snacking between meals. Avoid especially sweet or sugary drinks. Eat fish at least three times per week, plus fruit, cereals, nuts and vegetables daily’ | The control group received an identical envelope, but the advice was to perform the pattern of eating, sleeping, exercise and exposure to light which they thought would make them feel better. ‘Try to eat a healthy and balanced diet’, n 40 | HAM-D; BDI 21-item; CGI scale | All scales indicated a statistically significant improvement of depressive symptoms in the active treatment group: (☺) final HAM-D score (P=0·00) (☺) final Beck score (P=0·03) (☺) final GCI score (P=0·00) Psychopharmacological prescription in the active group was decreased in 8 patients, while in the control group such a decrease only took place in 3 cases (P=0·03) | Mean (sd) |
| Diet, exercise and stress management | |||||||||
| Toobert et al. (2007)( 29 ), USA (Toobert 2007) MLP | Postmenopausal women; T2DM for at least 6 months; living independently; ≤75 years of age; not developmentally disabled | MLP condition, n 163 After 6 months of intervention, MLP participants were further randomised to 1 of 2 maintenance conditions: 1. a faded schedule of weekly meetings led by lay leaders; or 2. 4 meetings over 18 months to complete a personalised, computer-assisted programme | Programme started with 2½ d non-residential retreat, followed by 4 h weekly meetings consisting of 1 h each of Mediterranean-style potluck, physical activity, stress management and support groups 24-month follow-up | MLP delivered by a registered dietitian, an exercise physiologist, a stress-management instructor, and a combination of professional and lay support group leaders | Combined social cognitive theory, goal systems and social ecological theory Dietitian taught participants the Mediterranean α-linolenic acid-rich diet – low in saturated fat but moderately high in monounsaturated fats. Individualised carbohydrate and fat recommendations were provided to optimise blood glucose and lipid concentrations The diet recommended more bread, root vegetables, green vegetables, legumes and fish; less red meat replaced by poultry; daily fruit; avoidance of butter and cream to be replaced by olive/canola oil or olive/canola-based margarine Physical activity: the initial physical activity goal was 30 min of moderate physical activity on most days of the week Stress management: participants were instructed in yoga, progressive deep relaxation, meditation, and directed or receptive imagery. Participants were asked to practise these techniques for at least 1 h/d | Usual care: received no additional intervention beyond usual care from their physicians, n 116 | CES-D Semi-quantitative FFQ (developed at Fred Hutchinson Cancer Research Centre). Measures %E from saturated fat. MLP group completed a self-monitoring log of adherence to diet components | (NS) The separate repeated-measures MANCOVA revealed no significant effects for depression | Mean (sd) |
DOIT, The Diet and Omega-3 Intervention Trial; CHIP, Coronary Health Improvement Project; MLP, Mediterranean Lifestyle Program; BP, blood pressure; T2DM, type 2 diabetes mellitus; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition; DIT, dietary intensive treatment; TSM, tailored self-management; EFA, essential fatty acids; %E, percentage of total daily energy intake; CD-ROM, compact disk – read only memory; MT, medical treatment; UNG, untreated nutrition group; POMS, Profile of Mood States; BDI, Beck depression Inventory; CES-D, Center for Epidemiologic Studies Depression Scale; GDS-sf, Geriatric Depression Screening Scale; MNA, Mini Nutritional Assessment; GDS, Geriatric Depression Scale; GWBS, General Well-Being Schedule; HADS, Hospital Anxiety and Depression Scale; EPIC, European Prospective Investigation into Cancer and Nutrition; BSI, Brief Symptom Inventory; PHQ, Patient Health Questionnaire; PHQ-9, Patient Health Questionnaire nine-item scale; NCI, National Cancer Institute; BDI-SF, Beck Depression Inventory – Short Form; HAM-D, Hamilton Depression seventeen-item scale; CGI, Clinical Global Impression scale; MANCOVA, multivariate ANCOVA.
Table 2.
Study aims, primary and secondary outcomes of included studies
| Study ID | n | Population | Primary outcomes (based on sample size calculation) | Secondary outcomes | Study aims and goals |
|---|---|---|---|---|---|
| Endevelt 2011 | 127 | Community-dwelling older adults aged ≥75 years at nutritional risk. Excluded individuals with clinical depression | Weight change | Nutritional status, cognitive function, depression score (GDS-sf), functional status | To test a newly developed nutritional intervention led by a clinical dietitian and to compare outcomes with a standard care intervention led by a family physician as well as a group of untreated participants To compare the effectiveness of two modes of nutritional intervention for community-dwelling older adults at nutritional risk |
| Forster 2012 | 217 | Community-living older adults aged 65–85 years. Excluded individuals with severe medical or psychiatric illness (including Alzheimer’s and senile dementia) | Reported changes in incidence of infection | Symptoms and illness, quality of life, depression (GDS) | To determine the effects of a food intervention and micronutrient supplements with levels that are realistically achievable by food alone on self-reported infection, nutritional status and immune function in community-living older adults |
| McMillan 2011 | 25 | Females, aged 19–30 years | Mood (e.g. POMS) and cognitive performance* | To examine the effects of 10 d of changing to a nutrient-rich diet on mood and cognitive performance | |
| Scheier 2005 | 252 | Women (≤50 years of age) with breast cancer | Mental functioning, physical functioning and depressive symptoms (CES-D)* | To conduct a clinical trial to determine if an educational intervention and a nutritional intervention could enhance physical and psychological functioning among younger women completing treatment for early-stage breast cancer | |
| Scheier et al.( 12 ) | Examines whether the main intervention effects reported in Scheier 2005 were moderated or conditioned by other factors | ||||
| Wardle 2000 | 176 | Adults with raised serum cholesterol levels | Depression score (BDI) | Lipids levels, mood, cognitive function | Designed to evaluate the effects of cholesterol-lowering dietary treatments on mood and cognitive functioning in adults with raised serum cholesterol levels |
| Imayama 2011 | 439 | Postmenopausal women 50–75 years of age; BMI ≥25·0 kg/m2 (if Asian-American, BMI ≥23·0 kg/m2) | Physical functioning scale (HRQOL) | Psychosocial factors (depression, anxiety (BSI), perceived stress, social support) | To examine the individual and combined effects of dietary weight loss and/or exercise interventions on HRQOL and psychosocial factors (depression, anxiety, stress, social support) |
| Jenkinson 2009 | 389 | Men and women aged 45 years and over with knee pain and BMI ≥28·0 kg/m2 | Reduction in pain score | WOMAC stiffness subscale, WOMAC physical function subscale, hospital and anxiety depression rating scale, bodily pain and physical function domains of the SF-36 | To determine whether individualised interventions of diet and quadriceps-strengthening exercise reduce knee pain in community-derived overweight and obese adults aged 45 years and over. To examine the effects of these interventions on knee stiffness, physical function and QOL |
| Kiernan 2001 | 264 | Men and premenopausal women 25–49 years old; in generally good health. Men with BMI of 28·0–34·0 kg/m2 and women with BMI of 24·0–30·0 kg/m2 | Psychological outcomes (e.g. BDI, Taylor Manifest Anxiety Scale)* | Examined whether participants in a diet-plus-exercise weight-loss programme improved on psychological outcomes more than participants in a diet-only weight-loss programme or participants in an assessment-only control group | |
| Nieman 2000 | 91 | Obese females; 25–70 years of age; in good health with no known diseases; BMI of 25·0–50·0 kg/m2; not experiencing ‘salient emotional or mood problems’ | Psychological general well-being and mood state (e.g. POMS)* | To compare psychological mood state in obese and non-obese women, and then to determine the influence of 12 weeks of moderate energy restriction and/or exercise on mood state using a randomised, controlled research design | |
| Einvik 2010 | 563 | Men with elevated cholesterol levels and systolic BP | Psychosocial outcomes (e.g. HADS)* | To examine whether 25 years of awareness of high cardiovascular risk is associated with changes in symptoms of depression and anxiety compared with population norm data, and whether dietary counselling influences long-term perceptions of health behaviour and concerns, depression, anxiety or QOL | |
| Hyyppa 2003 | 120 | Untreated hypercholesterolaemic men, aged 35–64 years | Cholesterol values, testosterone levels, psychological variables (BSI and State-Trait Anger Inventory) | To study simultaneously psychological functions and steroid hormone levels in hypercholesterolaemic men | |
| Ghroubi 2009 | 83 | Obese patients, BMI ≥30·0 kg/m2; excluded patients with severe psychiatric disorders | Metabolic, cardiovascular, psychological status (HADS) and QOL parameters* | To determine the impact of a combination of physical training and dietary measures on metabolic, cardiovascular, psychological and QOL parameters in obese adults, compared with a control group | |
| Glasgow 2006 | 335 | Adults diagnosed with T2DM for at least 6 months; ≥25 years of age | Dietary change | Biological measures, QOL and depression (PHQ-9)† | To evaluate the effects of an interactive technology-assisted TSM programme on a variety of outcomes. The primary purpose of the article was to report on the short-term (2-month) dietary, biological and QOL outcome from TSM |
| Andersen 2004 | 227 | Women diagnosed with stage II or III breast cancer, aged 20–85 years. Exclusion criteria: severe or untreated psychopathology (e.g. schizophrenia) | Stress reduction, emotional distress (POMS) | Social adjustment, health behaviours, adherence, immunity | Data on the efficacy of the intervention on emotional distress, health behaviours, chemotherapy dose-intensity and immune responses are reported in this article |
| Merrill 2008 | 348 | At least 18 years of age | Depression (BDI)* | Evaluated the efficacy of the CHIP at lowering depression by modifying selected daily nutrients from food | |
| Thieszen et al.( 11 ) | To further assess the relationship between the CHIP-mediated changes in body weight and changes in selected psychological health measures: BDI, role-emotional, social functioning and mental health (nervousness and depression) | ||||
| Garcia-Toro 2012 | 80 | Aged ≥18 years with a depressive episode | Depression (HAM-D) | Depression (BDI and CGI) | To study the effects of an intervention combining sleep, exercise, diet and sunlight exposure. To determine whether modifying these components is useful in the treatment of depression |
| Toobert 2007 | 279 | Postmenopausal women; T2DM for at least 6 months; living independently; ≤75 years of age | Dietary and physical activity outcomes | Stress management, psychosocial outcomes (e.g. depression (CES-D)) | Multiple-risk-factor interventions offer a promising means for addressing the complex interactions between lifestyle behaviours, psychosocial factors and the social environment. This report examines the long-term effects of a multiple-risk-factor intervention |
BP, blood pressure; T2DM, type 2 diabetes mellitus; POMS, Profile of Mood States; CES-D, Center for Epidemiologic Studies Depression Scale; BDI, Beck Depression Inventory; HRQOL, health-related quality of life; HADS, Hospital Anxiety and Depression Scale; BSI, Brief Symptom Inventory; QOL, quality of life; HAM-D, Hamilton Depression seventeen-item scale; GDS-sf, Geriatric Depression Screening Scale; GDS, Geriatric Depression Scale; BSI, Brief Symptom Inventory; WOMAC, Western Ontario McMaster osteoarthritis index; SF-36, Short Form (36) Health Survey; PHQ-9, Patient Health Questionnaire nine-item scale; CGI, Clinical Global Impression scale; TSM, tailored self-management; CHIP, Coronary Health Improvement Project.
Power calculation not reported in article.
Did not reach statistical significance due to inadequate sample size.
Outcomes
Outcomes of interest (depression and anxiety) were those measured by validated self-report inventories, psychiatric diagnostic interview or medical records. Instruments containing a subscale from which a depression/anxiety score could be derived were eligible for inclusion.
Synthesis of results
Studies were classified into three categories in relation to their results:
-
1.
those with statistically significant improvements in depression/anxiety outcomes, compared with the comparator group (☺);
-
2.
those with non-significant improvements in depression/anxiety outcomes, compared with the comparator group (NS); and
-
3.
those with significantly worse scores, compared with the comparator group (☹).
In order to assess which programme components led to improved outcomes, a table was created to classify dietary interventions by the method of intervention delivery (four categories), interventionist (five categories), dietary components (three categories) and weight and exercise focus (two categories).
Quality assessment
Quality criteria recommended by the Academy of Nutrition and Dietetics (formerly the American Dietetic Association) were used to assess the quality of the included studies( 10 ). This tool includes criteria for assessing selection bias, allocation bias, blinding, data collection, study retention, intervention adherence and methods of handling withdrawals/dropouts. Each study was assessed as negative, neutral or positive using the ‘most important quality consideration’ questions listed in Table 3·2a of the ADA Evidence Analysis Manual to assess the quality of RCT( 10 ). All studies were included in the review regardless of quality rating. This information was used as a post hoc way of synthesising the data from high-quality studies. All studies received a score out of 12. A score of 9 or above was considered positive (+), indicating that it clearly addressed issues of inclusion/exclusion, generalisability, bias, data collection and analysis. A score of 6–8 was considered neutral (ϕ) (neither exceptionally strong nor weak). A score of 5 or below was negative (−), reflecting that these issues were not reported or addressed adequately.
Results
Study selection
The initial search yielded 1274 citations, of which 1194 were excluded upon initial screening for not meeting inclusion criteria. Of the remaining eighty articles, sixty-five were excluded for the following reasons: not a target population (e.g. bulimia nervosa; n 2); did not include a target outcome (n 11); not a relevant intervention (e.g. not a whole-of-diet approach; n 11); not a target study design (e.g. study protocol or not an RCT; n 14); control group was not usual care or non-dietary modification (n 20); insufficient detail of dietary intervention (n 6); and not a validated measure of anxiety (n 1). Fifteen studies fulfilled the inclusion criteria and an additional four studies were found through searching of the included studies’ reference lists (n 3) and forward citation (n 1). Two studies( 11 , 12 ) are not discussed separately in the present paper as they report on the same cohort and intervention as two other studies( 13 , 14 ) already included in the systematic review. Overall, seventeen RCT were included in the review. Study selection and extraction process are according to PRISMA guidelines( 9 ). Figure 1 displays the results of study selection.
Fig. 1.
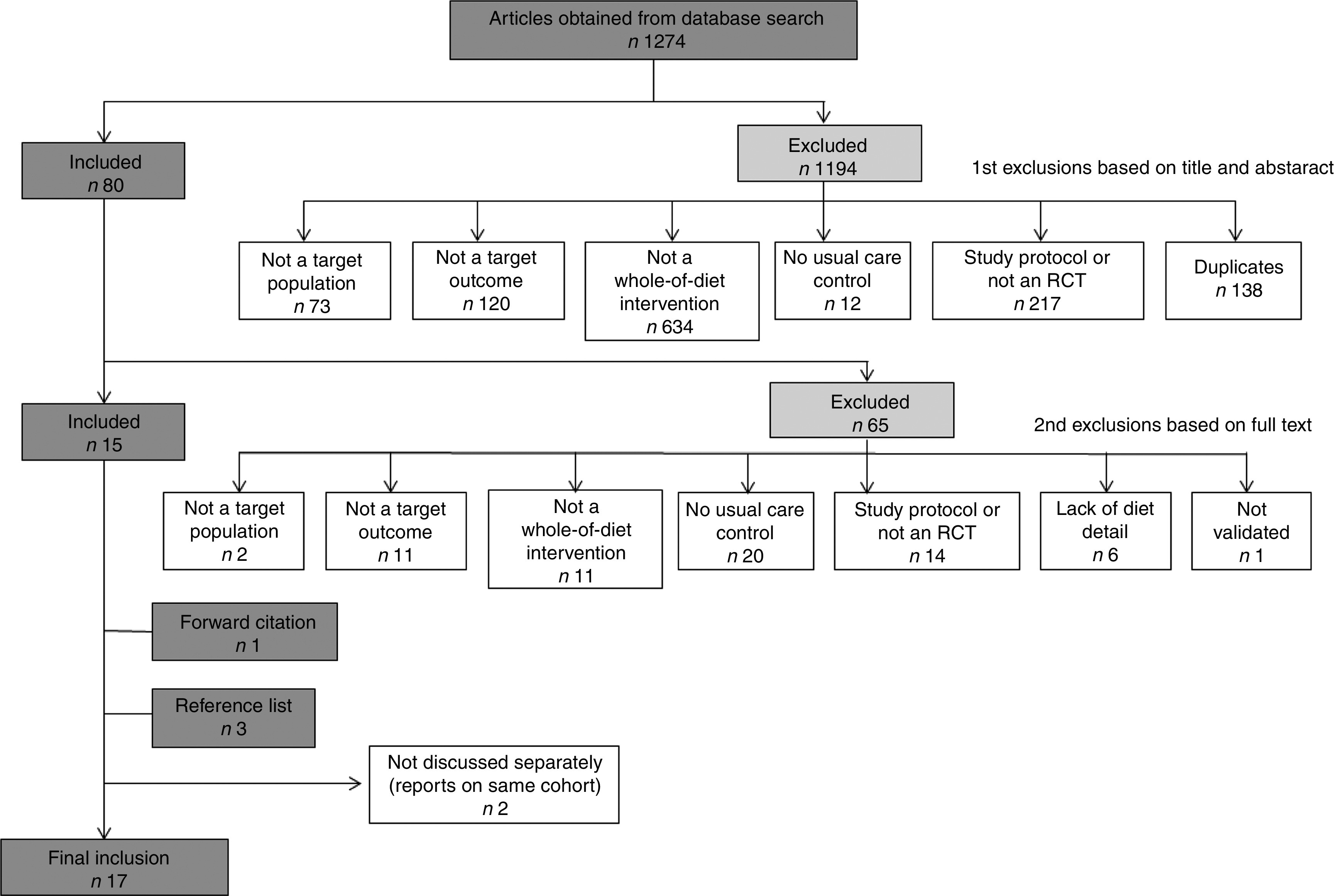
The process of study selection and the number of included and excluded studies; articles were published between April 1971 and May 2014
Study characteristics
Location and sample size
The majority of studies were conducted in the USA (n 8). Three studies were conducted in the UK and one each in Australia, Finland, Israel, Norway, Spain and Tunisia. Seventeen studies were included in the final analysis, representing a total of 4015 subjects, with sample sizes ranging from twenty-five to 563.
Population characteristics
Only one study exclusively targeted adults with a depressive episode( 15 ). Five studies targeted individuals with a chronic disease (e.g. high cholesterol, high blood pressure, T2DM), three studies targeted overweight or obese individuals and two studies targeted women with breast cancer. Five studies recruited women only and two studies recruited only men. With regard to co-morbid psychopathology, five studies( 16 – 20 ) excluded individuals with clinical depression or severe psychiatric disorders (e.g. schizophrenia) or individuals experiencing emotional or mood problems. Table 1 provides further detail of the eligibility criteria and participant characteristics for each study.
Study aims and primary outcomes
Primary outcomes varied among studies. Only four studies reported being powered to detect statistically significant differences in depression and/or anxiety scores( 15 , 18 , 21 , 22 ). A further seven studies included a depression and/or anxiety score as a primary end point; however, information as to whether the study was sufficiently powered was lacking (Table 2).
Intervention description
Programme intensity (sessions offered and length of follow-up)
There was considerable diversity in programme intensity. The number of dietary intervention sessions offered ranged from one to sixty-two sessions. Length of follow-up ranged from 10 d to 36 months with the most common length of follow-up being 6 months (n 4) and 3 months (n 3).
Intervention style and programme components
The majority of studies (n 6) delivered the intervention in a group setting, followed by individualised education/treatment (n 4) or as a combination of individual and group sessions (n 3). Other intervention styles included written recommendations (n 1), CD-ROM (compact disk – read only memory) programme and telephone call (n 1) and non-residential retreat and group meetings with/without a personalised computer-assisted program (n 1). One study( 23 ) failed to report the intervention delivery method.
Five studies( 13 , 16 , 20 , 21 , 23 ) delivered the intervention with a diet only focus. A further four studies( 19 , 24 – 26 ) included two intervention arms: a diet only arm and a diet and exercise arm. The remaining eight studies included programme components in addition to dietary improvement, including: physical activity/exercise (n 2); physical activity/exercise and education on smoking/tobacco use (n 2); physical activity and stress management, e.g. yoga and meditation (n 1); placebo or n-3 PUFA supplementation (n 1); placebo or cholesterol-lowering medication (simvastatin; n 1); and sleeping, exercise and exposure to light (n 1).
All studies used a whole-of-diet approach. Common dietary themes included increasing fruit, vegetable and fibre intake (n 13) and an increased fish intake (n 7). Approximately 41 % of studies made a specific recommendation to reduce intake of red meat, to select leaner meat products or to follow a low-cholesterol diet, while ~59 % of studies had a weight-loss focus or reported on weight change.
The majority of studies (n 9) used a dietitian for the dietary intervention. This was achieved using a dietitian in isolation (n 5) or a dietitian working with another practitioner, such as: dietitian and dietetic assistant/research interviewer (n 1); dietitian and medical professionals (n 1); dietitian and exercise physiologist and stress-managing instructor and group leaders (n 1); and dietitian and psychologist (n 1).
The remaining studies used clinical psychologists (n 1); a postdoctoral researcher (n 1); professionals trained in nutritional science (n 1); nutritionists (with no evidence of dietitian qualifications) (n 2); a lay person, e.g. health coach with no formal training (n 1); or an ‘experimenter’ (n 1). Only one study used written recommendations only and did not report on the qualifications of the individual who developed the guidelines( 15 ).
Depression and anxiety outcome measures
The depression and anxiety outcomes reported among the studies were as follows: Profile of Mood States (POMS); Hospital Anxiety and Depression Scale (HADS); Geriatric Depression Screening scale (GDS); Hamilton Depression scale (HAM-D); Beck Depression Inventory (BDI); Clinical Global Impression scale; Patient Health Questionnaire (PHQ); Brief Symptom Inventory; Taylor Manifest Anxiety scale; General Well-Being Schedule (GWBS); and Center for Epidemiologic Studies Depression Scale (CES-D).
All studies included a depression outcome. The most common depression and anxiety outcome measures used were the POMS (n 4), the BDI (n 4)( 21 ) and the HADS (n 3)( 27 ) (Table 1).
Results of individual studies
Depression and anxiety outcomes are presented separately for each paper due to the heterogeneity of included studies. These outcome measures are presented as differences in mean values from baseline scores. The heterogeneity across exposures, outcome measures and intervention period precluded a meta-analysis.
Depression
Of the seventeen studies that reported a depression outcome, eight studies( 13 – 19 , 25 ) found that the dietary intervention resulted in statistically significant improvements in depression scores when compared with a control group. The magnitude of effect, among the positive studies reporting the requisite data, ranged from 0·19 to 2·02 (Cohen’s d)( 13 , 15 – 17 , 25 ). The remaining nine studies reported no intervention effects. No study found the control group to produce superior outcomes. Table 1 provides detail of the depression scores for each study.
Anxiety/total mood disturbance
Of the ten studies that measured anxiety or total mood disturbance only two studies( 17 , 18 ) found that the intervention yielded significant improvements when compared with a comparator group. Conversely, eight studies found no significant between-group differences. No study found the control group to produce superior outcomes. Table 1 provides detail of the anxiety scores for the ten studies.
Depression outcomes categorised by composite interventions
When evidence was synthesised according to whether a study used a composite intervention (e.g. exercise, stress management) or focused solely on diet no clear differences in outcomes were detected (Table 1). We explored the relationship between weight loss, dietary intake and depression/anxiety outcomes. No obvious associations were found, thus this information is not presented.
Key characteristics of successful programmes
Table 3 provides a summary of the programme components (i.e. intervention delivery method, interventionist, dietary components, weight/exercise focus) for the seventeen studies that reported a depression outcome.
Table 3.
A summary of the programme components of the seventeen studies that reported on a depression outcome
| Intervention delivery method | Interventionist | Dietary components (specified in paper) | Weight/exercise focus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variety of delivery methods (e.g. combination of group and individual sessions) | Group only | Individual only | Written only | Trained professional (clinical psychologist or postdoctoral researcher) | Trained professional (dietitian or professional trained in nutritional science) | Combination of trained professionals (including a dietitian) | Nutritionist | Lay person | High fibre intake/more fruit and vegetables | More fish | Reduce intake of red meat/select leaner meat products/low-cholesterol diet | Weight-loss focus/reports on weight change | Diet+exercise focus | |
| Positive outcome (☺): intervention performed statistically significantly better than control group (n 8) | ||||||||||||||
| Study ID (n) | Andersen 2004 Ghroubi 2009 Merrill 2008 Nieman 2000* Scheier 2005 (n 5) | Endevelt 2011 Jenkinson 2009 (n 2) | Garcia-Toro 2012 (n 1) | Andersen 2004 (n 1) | Endevelt 2011 Ghroubi 2009 Nieman 2000* Scheier 2005 (n 4) | Jenkinson 2009 Merrill 2008 (n 2) | Andersen 2004 Garcia-Toro 2012 Jenkinson 2009 Merrill 2008 Nieman 2000* Scheier 2005 (n 6) | Garcia-Toro 2012 (n 1) | Merrill 2008 (n 1) | Ghroubi 2009 Jenkinson 2009 Nieman 2000 (n 3) | Andersen 2004 Garcia-Toro 2012 Ghroubi 2009 Jenkinson 2009 (group 1) Merrill 2008 Nieman 2000* (group 2) (n 6) | |||
| Study ID – information NA | Garcia-Toro 2012 (n 1) | |||||||||||||
| Total % | 0 | 62·5 | 25 | 12·5 | 14·3 | 57·1 | 28·6 | 0 | 0 | 75 | 12·5 | 12·5 | 37·5 | 75 |
| Total % | 0 | 100 | 14·3 | 85·7 | 0 | 0 | 75 | 50 | 75 | |||||
| Not significantly different (NS): intervention group did not perform statistically significantly better than control group (n 9) | ||||||||||||||
| Study ID (n) | Glasgow 2006 Hyyppa 2003 Imayama 2011 Toobert 2007 Wardle 2000 (n 5) | Kiernan 2001 (n 1) | Einvik 2010 Forster 2012 (n 2) | Forster 2012 (n 1) | Imayama 2011 Kiernan 2001 (n 2) | Toobert 2007 Wardle 2000 (n 2) | Einvik 2010 Hyyppa 2003 (n 2) | Glasgow 2006 McMillan 2011 (n 2) | Einvik 2010 Forster 2012 Glasgow 2006 Hyyppa 2003 McMillan 2011 Toobert 2007 Wardle 2000 (n 7) | Einvik 2010 Forster 2012 Hyyppa 2003 McMillan 2011 Toobert 2007 Wardle 2000 (n 6) | Einvik 2010 Hyyppa 2003 Kiernan 2001 McMillan 2011 Toobert 2007 Wardle 2000 (n 6) | Forster 2012 Glasgow 2006 Hyyppa 2003 Imayama 2011 Kiernan 2001 McMillan 2011 Wardle 2000 (n 7) | Glasgow 2006 Imayama 2011 (group 2) Kiernan 2001 (group 2) Toobert 2007 (n 4) | |
| Study ID – information NA | McMillan 2011 (n 1) | |||||||||||||
| Total % | 62·5 | 12·5 | 25·0 | 0 | 11·1 | 22·2 | 22·2 | 22·2 | 22·2 | 77·8 | 66·7 | 66·7 | 77·8 | 44·4 |
| Total % | 62·5 | 37·5 | 11·1 | 44·4 | 22·2 | 22·2 | 77·8 | 100 | 44·4 | |||||
Nieman 2000: reported on GWBS (General Well-Being Schedule) and POMS (Profile of Mood States) outcomes; results were only positive for GWBS scales in the exercise and diet group (ED).
Intervention delivery method
All studies (100 %) that achieved an improvement in depression score used only one mode of delivery to conduct their intervention, such as nutritional treatment meetings at home( 16 ); a weekly class on weight-loss principles and nutrition guidelines( 19 ); or written information only( 15 ). The most common forum was face-to-face treatment in an individual or group setting( 13 , 14 , 16 – 19 , 25 ).
Of the studies that found no difference between intervention and control group, most (62·5 %) used multiple delivery modes such as CD-ROM programme and telephone call( 28 ); non-residential retreat and weekly meetings and/or computer-assisted program( 29 ); or a combination of group and individual sessions( 21 , 22 , 24 ).
Interventionist
Approximately 85 % of studies that resulted in a positive outcome used a dietitian (or a professional trained in nutritional science) to conduct the intervention. Four of these studies( 13 , 16 , 17 , 19 ) used a dietitian (or a professional trained in nutritional science) in isolation, whereas two studies included a dietitian with at least one other individual( 14 , 25 ). Conversely, less than half (44 %) of the studies that produced null findings used a dietitian to facilitate the intervention. The remaining studies used a postdoctoral researcher, nutritionist (as opposed to a qualified dietitian) or a lay person.
Dietary components and weight/exercise focus
Among studies that achieved an improvement in depression score, 75 % of studies explicitly recommended a diet high in fibre and/or fruit and vegetables. The findings were similar (~78 %) for studies achieving no significant difference between intervention and control group.
With regard to fish intake, studies that produced null findings were more likely to have recommended an increase in fish consumption than studies achieving a positive outcome (66·7 % v. 12·5 %, respectively).
All studies (100 %) that resulted in non-significant between-group differences in depression outcomes recommended reducing red meat intake/selecting lean meat/following a low-cholesterol diet and/or included a weight-loss focus/reported on weight change. On the other hand, only 50 % of studies that resulted in significant improvements in depression scores made a specific reference to reducing red meat intake/selecting lean meat/following a low-cholesterol diet or had a weight-loss focus/reported on weight change.
Quality rating
A relatively high proportion of studies received a positive quality rating; twelve papers were considered to be of high methodological quality (received a positive (+) rating) and the remaining five studies were considered to be of moderate methodological quality (received a neutral (ϕ) rating). No studies received a negative (−) rating.
Common features of studies that were assessed as lower quality related to the selection of study subjects, methods of handling withdrawals, failure to employ intention-to-treat analyses (some excluded randomised participants from analyses due to non-compliance), insufficient detail/absence of blinding and randomisation techniques, and potential contamination of the comparator group. The online supplementary material 2, Supplemental Table 2 provides details of the quality assessment scores for included studies.
Discussion
Summary of evidence
The aim of the present review was to synthesise findings from existing RCT in order to evaluate the impact of dietary interventions (with a whole-of-diet approach) on depression and anxiety outcomes. Indeed, there is evidence from controlled trials that dietary interventions can result in improved depression scores among different clinical and healthy populations. Successful interventions yielded an effect size for depression scores between 0·19 and 2·02, which is a small to very large effect and is comparable to pharmacotherapy or psychotherapy( 30 ). However, the evidence was not consistent, with just over half of studies revealing no effect on mental health outcomes and a lack of evidence of treatment effects for anxiety.
We found that the interventions shown to produce positive effects shared similar characteristics including: a single delivery mode; a qualified dietitian to deliver the intervention; and being less likely to recommend reducing red meat intake/selecting lean meat/following a low-cholesterol diet.
The importance of these features is supported by previous evidence. For example, nutrition education and counselling facilitated by a registered dietitian within a cardiac rehabilitation programme has been associated with improved diet-related outcomes when compared with patients receiving general education from cardiac rehabilitation staff( 31 ). While low-cholesterol diets or those encouraging a reduction in red meat intake are frequently designed for reducing chronic disease risk, such a diet may not be the best strategy for achieving improvements in mental health. Inadequate intake of red meat has been linked to a greater likelihood of depression or anxiety in women, when compared with those consuming the recommended amount( 32 ). Moreover, low cholesterol may have a detrimental impact on mental health( 33 ).
Strengths and limitations
To the authors’ knowledge, the present systematic review is the first of its kind. A strength of the review is that it included only RCT – the highest level of evidence( 34 ). The search strategy applied was comprehensive, and methods of study selection and inclusion criteria were determined a priori. Moreover, all studies used validated measures of depression and anxiety. However, it should be noted that there were a multitude of different mental health assessment tools utilised in the included studies, making direct comparisons difficult. Further, only four authors specifically stated that their study was powered to detect a statistically significant difference in depression and/or anxiety scores. Importantly, the heterogeneous nature of the dietary interventions precluded direct comparisons and meta-analyses.
Of the ten studies that reported on dietary adherence or change in dietary intake, all studies used validated tools to measure dietary intake. The majority of these studies( 13 , 19 – 21 , 23 , 25 , 29 ) included 3 d to 7 d food diaries. In practice, diet history interview and 7 d food diaries interchangeably serve as the ‘gold standard’ dietary assessment tools( 35 ). However, it is readily acknowledged that all methods that rely on self-reported dietary intake are subject to measurement and systematic error( 36 ). Biochemical data from serum or blood samples provide a more objective measure of nutritional status and dietary intake( 37 ), yet are not commonly reported due to the associated expense and labour intensity; only two studies( 20 , 38 ) reported participants’ fatty acid levels or plasma micronutrient status.
While our findings are equivocal, substantial methodological limitations of the reviewed studies made it difficult to adequately answer the research question. In particular, the fact that only one study specifically included people with a depressive or anxiety illness( 15 ), while other studies specifically excluded those with pre-existing mental health symptoms or disorders( 16 – 19 ), made it far less likely that effects on mental health parameters by dietary interventions would be detected. The majority of studies included individuals with lifestyle diseases (e.g. cancer, T2DM, overweight/obesity or hypercholesterolaemia), some examined only one gender( 13 , 18 , 19 , 22 – 24 , 29 , 38 ) and a number of study samples consisted of primarily white adults with a high education level( 13 , 14 , 18 , 24 , 26 ). Hence, the findings may not be generalisable to other clinical and general populations. There was also substantial variation with regard to participants’ total exposure to the intervention; dietary intervention sessions offered ranged from one to sixty-two sessions and length of follow-up ranged from 10 d to 36 months. These limitations should be borne in mind when evaluating the significance of the results.
Only research articles that evaluated diet as a whole were included in the present review. This is of great relevance as not only do analyses of single nutrients ignore important interactions between components of a diet( 39 ), but individuals do not naturally consume foods or nutrients in isolation. As a result, there is heterogeneity across many of the dietary interventions included herein. The review has attempted to address this issue by carefully examining each dietary intervention and allocating each component, such as increasing vegetable intake or reducing red meat intake, to a predetermined category to allow direct comparisons to be made between studies. The dietary information collected was based on the descriptions provided in each publication. Studies were excluded from the review if they failed to highlight the main dietary components of the intervention; therefore it is possible that some studies that were potentially relevant to review were excluded due to poor reporting/inadequate detail of the intervention.
Many studies failed to provide detailed information regarding techniques that interventionists employed to assist with achieving dietary compliance (e.g. counselling, motivational interviewing). Thus, ascertaining which intervention aspects (delivery techniques, group of foods or single nutrients) led to improved outcomes was difficult, which may hamper replication of these interventions. Finally, the inclusion of composite interventions (e.g. diet and physical activity or diet and n-3 PUFA supplementation) may have hindered our ability to elucidate the direct impact of dietary improvement on mental health outcomes.
Implications
Notwithstanding the methodological limitations of the present review, there is evidence that interventions with a whole-of-diet approach can achieve improvements in depression outcomes. This is an important finding as it suggests that dietary interventions could potentially be used as a treatment and preventive approach at the clinical and population level. The potential for dietary intervention to be employed as a prevention strategy may be of benefit when considering evidence from epidemiological studies that have shown a healthy dietary pattern may be protective against depression( 4 – 6 ). These benefits are in addition to the already established evidence indicating that greater adherence to a healthy diet, such as the Mediterranean diet, can significantly decrease the risk of overall mortality, mortality from CVD, incidence of or mortality from cancer, and incidence of Parkinson’s disease and Alzheimer’s disease( 39 ). Additionally, depression appears to share common pathophysiological mechanisms with metabolic syndrome, obesity and CVD( 5 ) and several major cardiovascular risk factors are more prevalent among depressed individuals( 5 ). Thus, evidence to date from epidemiological studies and RCT indicates that incorporating lifestyle recommendations, such as dietary improvement, into clinical practice and public health messages may contribute to a reduction in depressive symptoms, as well as providing additional benefits for the prevention and management of highly prevalent chronic disease states such as CVD, obesity and T2DM. This approach has the potential to reduce the public health burden of common mental illness as well as chronic diseases.
Conclusions
The present review of RCT has demonstrated that dietary intervention studies have the potential to achieve improved depression scores. The paper provides some insight into the key components that are likely to achieve improved depression outcomes. Appropriately powered RCT evaluating the impact of dietary improvement on mental health outcomes in those with clinical disorders are required( 40 ).
Acknowledgements
Acknowledgements: The authors thank Sarah Dash (S.D.) for replicating the systematic search of the literature. Financial support: R.S.O. is currently supported by a PhD scholarship from La Trobe University and Deakin University. A.O. is a recipient of a National Health and Medical Research Council (NHMRC) Postdoctoral Training Fellowship (#1052865). F.N.J. has received Grant/Research support from the Brain and Behaviour Research Institute, the NHMRC; Australian Rotary Health; the Geelong Medical Research Foundation; the Ian Potter Foundation; Eli Lilly; and The University of Melbourne. F.N.J. has been a paid speaker for Sanofi-Synthelabo; Janssen Cilag; Servier; Pfizer; Health Ed; Network Nutrition; Angelini Farmaceutica; and Eli Lilly. F.N.J., C.I. and A.O. have received funding from Meat and Livestock Australia. These funding sources had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: R.S.O. contributed to the conception and design of the work; acquisition, analysis and interpretation of the data; as well as preparation of the manuscript for publication. A.O., C.I. and F.N.J. contributed to the design of the work; analysis and interpretation of the data; and preparation of the manuscript for publication. Ethics of human subject participation: Ethical approval was not required.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980014002614.
click here to view supplementary material
References
- 1. Vos T, Flaxman AD, Naghavi M et al. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bloom DE, Cafiero ET, Jane-Llopis E et al. (2011) The Global Economic Burden of Non-communicable Diseases. Geneva: World Economic Forum. [Google Scholar]
- 3. Casacalenda N, Perry CJ & Looper K (2002) Remission in major depressive disorder: a comparison of pharmacotherapy, psychotherapy, and control conditions. Am J Psychiatry 159, 1354–1360. [DOI] [PubMed] [Google Scholar]
- 4. Sanhueza C, Ryan L & Foxcroft DR (2013) Diet and the risk of unipolar depression in adults: systematic review of cohort studies. J Hum Nutr Diet 26, 56–70. [DOI] [PubMed] [Google Scholar]
- 5. Sanchez-Villegas A & Martinez-Gonzalez MA (2013) Diet, a new target to prevent depression? BMC Med 11, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akbaraly TN, Brunner EJ, Ferrie JE et al. (2009) Dietary pattern and depressive symptoms in middle age. Br J Psychiatry 195, 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zazpe I, Sanchez-Tainta A, Estruch R et al. (2008) A large randomized individual and group intervention conducted by registered dietitians increased adherence to Mediterranean-type diets: the PREDIMED Study. J Am Diet Assoc 108, 1134–1144. [DOI] [PubMed] [Google Scholar]
- 8. Framson C, Kristal AR, Schenk JM et al. (2009) Development and validation of the mindful eating questionnaire. J Am Diet Assoc 109, 1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Dietetic Association, Scientific Affairs & Research (2007) ADA Evidence Analysis Manual, Edition V. Chicago, IL: ADA. [Google Scholar]
- 11. Thieszen CL, Merrill RM, Aldana SG et al. (2011) The Coronary Health Improvement Project (CHIP) for lowering weight and improving psychosocial health. Psychol Rep 109, 338–352. [DOI] [PubMed] [Google Scholar]
- 12. Scheier MF, Helgeson VS, Schulz R et al. (2007) Moderators of interventions designed to enhance physical and psychological functioning among younger women with early-stage breast cancer. J Clin Oncol 25, 5710–5714. [DOI] [PubMed] [Google Scholar]
- 13. Scheier MF, Helgeson VS, Schulz R et al. (2005) Interventions to enhance physical and psychological functioning among younger women who are ending nonhormonal adjuvant treatment for early-stage breast cancer. J Clin Oncol 23, 4298–4311. [DOI] [PubMed] [Google Scholar]
- 14. Merrill RM, Taylor P & Aldana SG (2008) Coronary Health Improvement Project (CHIP) is associated with improved nutrient intake and decreased depression. Nutrition 24, 314–321. [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Toro M, Ibarra O, Gili M et al. (2012) Four hygienic–dietary recommendations as add-on treatment in depression: a randomized-controlled trial. J Affect Disord 140, 200–203. [DOI] [PubMed] [Google Scholar]
- 16. Endevelt R, Lemberger J, Bregman J et al. (2011) Intensive dietary intervention by a dietitian as a case manager among community dwelling older adults: the EDIT study. J Nutr Health Aging 15, 624–630. [DOI] [PubMed] [Google Scholar]
- 17. Ghroubi S, Elleuch H, Chikh T et al. (2009) Physical training combined with dietary measures in the treatment of adult obesity. A comparison of two protocols. Ann Phys Rehabil Med 52, 394–413. [DOI] [PubMed] [Google Scholar]
- 18. Andersen BL, Farrar WB, Golden-Kreutz DM et al. (2004) Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol 22, 3570–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nieman D, Custer W, Butterworth D et al. (2000) Psychological response to exercise training and/or energy restriction in obese women. J Psychosom Res 48, 23–29. [DOI] [PubMed] [Google Scholar]
- 20. Forster SE, Powers HJ, Foulds GA et al. (2012) Improvement in nutritional status reduces the clinical impact of infections in older adults. J Am Geriatr Soc 60, 1645–1654. [DOI] [PubMed] [Google Scholar]
- 21. Wardle J, Rogers P, Judd P et al. (2000) Randomized trial of the effects of cholesterol-lowering dietary treatment on psychological function. Am J Med 108, 547–553. [DOI] [PubMed] [Google Scholar]
- 22. Hyyppa MT, Kronholm E, Virtanen A et al. (2003) Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology 28, 181–194. [DOI] [PubMed] [Google Scholar]
- 23. McMillan L, Owen L, Kras M et al. (2011) Behavioural effects of a 10-day Mediterranean diet. Results form a pilot study evaluating mood and cognitive performance. Appetite 56, 143–147. [DOI] [PubMed] [Google Scholar]
- 24. Imayama I, Alfano CM, Kong A et al. (2011) Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. Int J Behav Nutr Phys Act 8, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jenkinson CM, Doherty M, Avery AJ et al. (2009) Effects of dietary intervention and quadriceps strengthening exercises on pain and function in overweight people with knee pain: randomised controlled trial. BMJ 339, 606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiernan M, King A, Stefanick M et al. (2001) Men gain additional psychological benefits by adding exercise to a weight-loss program. Obes Res 9, 770–777. [DOI] [PubMed] [Google Scholar]
- 27. Zigmond AS & Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67, 361–370. [DOI] [PubMed] [Google Scholar]
- 28. Glasgow RE, Nutting PA, Toobert DJ et al. (2006) Effects of a brief computer-assisted diabetes self-management intervention on dietary, biological and quality-of-life outcomes. Chronic Illn 2, 27–38. [DOI] [PubMed] [Google Scholar]
- 29. Toobert DJ, Glasgow RE, Strycker LA et al. (2007) Long-term effects of the Mediterranean lifestyle program: a randomized clinical trial for postmenopausal women with type 2 diabetes. Int J Behav Nutr Phys Act 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pinquart M, Duberstein PR & Lyness JM (2006) Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry 163, 1493–1501. [DOI] [PubMed] [Google Scholar]
- 31. Holmes AL, Sanderson B, Maisiak R et al. (2005) Dietitian services are associated with improved patient outcomes and the MEDFICTS dietary assessment questionnaire is a suitable outcome measure in cardiac rehabilitation. J Am Diet Assoc 105, 1533–1540. [DOI] [PubMed] [Google Scholar]
- 32. Jacka FN, Pasco JA, Williams LJ et al. (2012) Red meat consumption and mood and anxiety disorders. Psychother Psychosom 81, 196–198. [DOI] [PubMed] [Google Scholar]
- 33. Engelberg H (1992) Low serum cholesterol and suicide. Lancet 339, 727–729. [DOI] [PubMed] [Google Scholar]
- 34. National Health and Medical Research Council (2009) NHMRC levels of evidence and grades for recommendations for developers guidelines. http://www.nhmrc.gov.au/_files_nhmrc/file/guidelines/developers/nhmrc_levels_grades_evidence_120423.pdf (accessed November 2014).
- 35. Hoidrup S, Andreasen AH, Osler M et al. (2002) Assessment of habitual energy and macronutrient intake in adults: comparison of a seven day food record with a dietary history interview. Eur J Clin Nutr 56, 105–113. [DOI] [PubMed] [Google Scholar]
- 36. Trabulsi J & Schoeller DA (2001) Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab 281, E891–E899. [DOI] [PubMed] [Google Scholar]
- 37. Bach-Faig A, Geleva D, Carrasco J et al. (2006) Evaluating associations between Mediterranean diet adherence indexes and biomarkers of diet and disease. Public Health Nutr 9, 1110–1117. [DOI] [PubMed] [Google Scholar]
- 38. Einvik G, Ekeberg O, Lavik JG et al. (2010) The influence of long-term awareness of hyperlipidemia and of 3 years of dietary counseling on depression, anxiety, and quality of life. J Psychosom Res 68, 567–572. [DOI] [PubMed] [Google Scholar]
- 39. Sofi F, Cesari F, Abbate R et al. (2008) Adherence to Mediterranean diet and health status: meta-analysis. BMJ 337, a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O’Neil A, Berk M, Itsiopoulos C et al. (2013) A randomised, controlled trial of a dietary intervention for adults with major depression (the ‘SMILES’ trial): study protocol. BMC Psychiatry 13, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jula A, Marniemi J, Huupponen R et al. (2002) Effecs of diet and simvastatin on serum lipids, insulin, and antioxidants in hypercholesterolemic men: a randomized controlled trial. JAMA 287, 598–605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980014002614.
click here to view supplementary material


