Abstract
Introduction
Kukoamine B (KB) is a novel sepsis therapeutic drug targeting lipopolysaccharide and CpG DNA. This study aims to evaluate the safety, tolerability, and pharmacokinetic (PK) profile of multiple doses of KB in healthy volunteers.
Methods
Healthy volunteers were randomized at a 1:1:1:1 ratio to receive multiple intravenous infusion doses of KB 0.06 mg/kg, 0.12 mg/kg, 0.24 mg/kg or placebo (administered every 8 h per day) for 7 days and subsequently followed up for another 7 days at Peking Union Medical College Hospital. Primary endpoints were adverse events (AEs), and the secondary endpoints were PK parameters of the first administration and the last administration.
Results
Data of the 18 health volunteers in KB groups and 6 in the placebo group were pooled and analyzed. AEs occurred in 12 (66.67%) volunteers in the KB groups and 4 (66.67%) volunteers in the placebo group. Treatment-related adverse events (TRAEs) occurred in 8 (44.44%) volunteers in the KB groups and 2 (33.33%) volunteers in the placebo group. Hypertriglyceridemia (4 [22.22%] vs. 2 [33.33%]) and sinus bradycardia (3 [16.67%] vs. 0) were the most common AEs. The mean elimination half-life, clearance, and distribution volume of KB were 3.40–4.88 h, 9.35–13.49 L/h, and 45.74–101.90 L, respectively. The average accumulation ratios of area under the plasma concentration–time curve and maximum plasma concentration were 1.06 and 1.02, respectively.
Conclusion
Single and multiple intravenous infusions of KB at a dose range of 0.06–0.24 mg/kg are safe and tolerable in healthy volunteers.
Trial Registration
ClinicalTrials.gov identifier, NCT02690961.
Keywords: Sepsis, Kukoamine B, Pharmacokinetics, Tolerability, Safety
Key Summary Points
| Why carry out this study? |
| This phase I trial showed that KB was safe and well tolerated after a single intravenous infusion dose of KB in healthy volunteers. Our clinical trial is the first to report the safety, tolerability, and pharmacokinetic characteristics of single and multiple doses of KB in healthy volunteers |
| The hypothesis of the study was that KB with single and multiple doses may be safe and tolerable in healthy volunteers |
| What was learned from the study? |
| Kukoamine B at a dose range of 0.06 mg/kg to 0.24 mg/kg by both single and multiple intravenous infusions is safe and tolerable for healthy volunteers |
| The plasma concentration of KB increased gradually after the start of intravenous infusion and decreased rapidly after drug withdrawal in healthy volunteers. It is necessary to further evaluate the pharmacokinetics in patients with sepsis |
Introduction
Sepsis is a systemic inflammatory response syndrome (SIRS) caused by infectious factors [1, 2]. As a common cause of death and multimorbidity in clinical practice [3, 4], sepsis is a fatal complication caused by severe infection, including bacterial [5]. The hospital mortality for septic shock approaches 40–60% [6, 7]. The treatment of sepsis mainly includes etiological treatment, supportive treatment, and immune-conditioning treatment. Medications mainly focus on anti-infection treatment, prevention of deep venous thrombosis, and vasoactive drug treatment [2, 8, 9]. At present, there is no specific therapeutic drug. Among the pathogen-associated molecules known to induce sepsis, lipopolysaccharide (LPS) is the first to be recognized. LPS exists in the outer membrane of Gram-negative bacteria and is the most virulent pathogen-associated molecule that induces sepsis [10, 11]. CpG DNA, on the other hand, is also a pathogen-associated molecule widely present in Gram-negative and Gram-positive bacteria but only rarely in mammalian genomes, which related to inflammatory response [11–14]. LPS and CpG DNA synergistically activate signaling pathways, induce the release of large amounts of pro-inflammatory cytokines, and eventually cause more serious sepsis [15, 16]. Currently available drugs for treating sepsis act only against either LPS or CpG DNA. Hence, they are not particularly efficient at combating sepsis as both aforementioned molecules are usually involved during sepsis. Therefore, LPS and CpG DNA could be used as co-targets for sepsis drugs in the development of novel sepsis therapies such as kukoamine B (KB) [17].
KB is the first reported dual inhibitor for both LPS and CpG DNA, which possesses a broad spectrum of therapeutic properties, including antioxidant and anti-inflammatory properties [18]. Moreover, in vitro and in vivo pharmacodynamic studies have confirmed that KB can directly bind to LPS and CpG DNA, inhibit the binding of LPS and CpG DNA to the corresponding receptors (Toll-like receptor [TLR]4 and TLR9) on/in immune cells, and inhibit the activation of immune cells without interfering with signal pathways or cell viability in macrophages, thereby blocking or reducing the occurrence of inflammatory reactions [19]. KB has been reported to have significant protective effects against severe and extremely severe sepsis in animal models [20]. In addition, KB can effectively reduce the levels of LPS, early and late inflammatory mediators in the blood of sepsis model animals, relieve acidosis, and improve blood coagulation. Furthermore, KB has been linked to improved function of multiple organs (liver, kidney, heart, etc.) and reduced pathological damage of lung and intestinal tissue in model animals [21, 22]. The tolerability and pharmacokinetics (PK) phase I trial of single-dose KB in healthy volunteers has been completed, and the results showed that KB was safe and well tolerated after a single intravenous infusion dose of KB from 0.005 to 0.48 mg/kg. The mean elimination half-life (t1/2), clearance (CL), and distribution volume (Vd) of KB were 1.61–4.24 h, 7.75–13.40 L/h, and 29.35–57.80 L, respectively (data not published).
This study aimed to evaluate the safety, tolerability, and PK characteristics of single and multiple doses of KB in healthy volunteers.
Methods
Study Design and Participants
This study is a single-center, randomized, double-blind, placebo-controlled phase I clinical trial (ClinicalTrials.gov, NCT02690961). Healthy volunteers were enrolled in this study at Peking Union Medical College Hospital. The main inclusion criteria of the volunteers were (1) there was no restriction on gender, and the ratio of each gender did not exceed 2/3; (2) 18–45 years old; (3) body mass index (BMI) is within the range of 19–28 (including upper and lower limits), weight ≥ 50 kg (female) or ≥ 60 kg (male); (4) volunteers should fully understand the purpose, properties, method, and possible reactions in the trial and agreed to abide by the requirements of clinical protocols. Exclusion criteria: (1) primary disease in important organs that could impact the trial’s outcome or pose a risk to the participant after taking the trial drug according to the investigator’s assessment; (2) mental or physical disability; (3) familial hereditary disease; (4) abnormal results of any clinically meaningful physical examination, vital signs, electrocardiograph, or clinical laboratory; (5) history of immunodeficiency diseases, including human immunodeficiency virus antibody positive; (6) detection of positive antibodies, hepatitis B surface antigen or antibody to hepatitis C/syphilis positive; (7) alcohol and drug abusers; (8) blood donation or significant blood loss (more than 400 mL) history during the last 3 months; (9) lactating and pregnant women or those unable to take effective contraceptive measures; (10) other conditions that investigators deemed not suitable for the trial.
This clinical study was designed, conducted, and reported per the Declaration of Helsinki. The study was approved by the Ethics Committee of Peking Union Medical College Hospital, and all volunteers signed an informed consent form.
Procedure
In this study, a random incorporation, double-blind, dose escalation design was adopted. The study consisted of three dose groups of 0.06, 0.12, and 0.24 mg/kg, each containing eight volunteers. Patients were randomized and received either KB or placebo in each dose group (KB to placebo ratio 6:2). Volunteers were enrolled in sequence, starting with the low-dose group. During the trial, the setting of incremental doses was determined on the basis of the safety data of volunteers in the previous dose group. After the volunteers were enrolled, they were assigned a random number in sequence, and the investigators administered the treatment drugs to volunteers according to the random number.
KB calculated on the basis of the volunteer’s body weight was dissolved in 100 mL of 0.9% sodium chloride solution and injected intravenously at a consistent rate (infusion time was 1 h). KB was given once a day in the morning on day 1 and day 7, and three times a day with an 8-h interval on day 2 to day 6.
Volunteers were hospitalized for 48-h safety surveillance after last administration, and the last safety visit was performed 7 days after last administration.
Endpoints and Assessments
The primary endpoint of the study was to evaluate the safety, while the secondary endpoint was PK characteristics. Safety endpoint included adverse events (AEs) and treatment-related adverse events (TRAEs), which were evaluated according to the National Cancer Institute’s (NCI) Common Terminology Criteria for Adverse Event (CTCAE) version 5.0 and classified by the system organ classification (SOC). The incidences of all AEs were calculated and classified according to severity and relationship to study drug.
Blood samples were collected pre-dose and at 30 min, 1 (immediately after the end of infusion), 2, 4, 7, 9, 13, 17, and 24 h after the first administration; pre-dose of the last three administrations; and at 30 min, 1 (immediately after the end of infusion), 2, 4, 7, 9, 13, 17, and 24 h after the last administration to detect the KB concentration in the plasma (a total of 22 time points). Blood samples were collected into 4-mL vacutainer tubes which were kept in an ice bath, and then centrifuged (4 °C, 1500g, 15 min) within 1 h after sample collection. Plasma samples were stored in a freezer at − 80 °C until bioassay. The concentration of KB in plasma samples was determined by ultra-high-performance liquid chromatography–tandem mass spectrometry (UPLC-MSMS); the lower limit of quantification (LLOQ) of KB in plasma samples was 0.1 ng/mL [23, 24].
Statistical Methods
The safety analysis was conducted on a population-based sample of all volunteers who received at least one study medication safety set. The PK analysis was based on the per protocol set, which was defined as all volunteers without protocol deviation (such as no medication) that impacted PK parameters, including area under the plasma concentration–time curve (AUC) and maximum plasma concentration (Cmax). No PK analysis was performed for the placebo group. The blood drug concentration–time curve (C–T curve) was drawn from the plasma drug concentration of each volunteer at each time point, and then the PK parameters were calculated by the WinNonlin program (Version 6.3, Pharsight, a Certara Company, Mountain View, CA, USA) using the non-compartmental analysis method (linear up/log down) according to the blood drug concentration–time data. The Cmax and time to peak plasma concentration (tmax) were obtained directly from the concentration–time data. The terminal phase rate constant for KB (λz) and the corresponding plasma terminal phase t1/2 were calculated. λz for all volunteers was determined by linear least-squares regression using logarithmically transformed points in the terminal phase. The number of points included in the terminal phase was confirmed by visual inspection. AUC0–t, defined as the AUC from zero (pre-dose) up to the time of the last quantifiable concentration, was calculated using the trapezoidal rule and extrapolated to infinite time for AUCinf. AUC0–8h and AUC0–24h, defined as AUC from the time of dosing up to 8 h and 12 h, respectively. The CL was calculated as the dose divided by AUCinf, and Vd was estimated by dividing the apparent CL by λz. Plasma PK parameters were obtained for each volunteer. All the data were analyzed descriptively. Continuous data was expressed in mean ± standard deviation (SD) or median (range) according to the normality of the data, and categorical data was expressed in n (%).
Results
Demographics
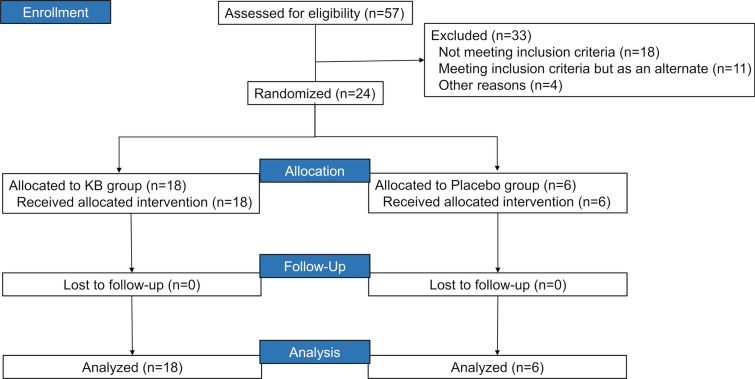
Of the 57 healthy volunteers who were screened, 33 failed the screening, and the remaining 24 volunteers were eligible for this study. Among the eligible 24 volunteers, 18 volunteers receiving KB were subdivided into three treatment groups (0.06 mg/kg [N = 6], 0.12 mg/kg [N = 6], and 0.24 mg/kg [N = 6]), and six volunteers received a placebo treatment. All the eligible healthy volunteers completed the trial according to the protocol and entered the safety set and per protocol set (Fig. 1). Generally, the average BMI was compared across the treatment groups (23.4–24.1 kg/m2). Of the 24 eligible volunteers, 14 and 10 were male and female, respectively. Nonetheless, the mean age for volunteers in the placebo group was slightly high than that in the treatment groups (36.0 years vs. 30.0–32.0 years) (Table 1).
Fig. 1.
Flowchart of patients
Table 1.
Demographics of 24 healthy volunteers
| Parameters | 0.06 mg/kg (N = 6) | 0.12 mg/kg (N = 6) | 0.24 mg/kg (N = 6) | Placebo (N = 6) |
|---|---|---|---|---|
| Age (years) | 31.0 (23.0, 43.0) | 32.0 (22.0, 45.0) | 30.0 (21.0, 42.0) | 36.0 (30.0, 38.0) |
| Weight (kg) | 67.0 (56.6, 75.0) | 64.3 (50.0, 77.0) | 64.3 (61.5, 72.4) | 69.5 (58.0, 74.8) |
| BMI (kg/m2) | 23.6 (22.8, 27.9) | 23.4 (21.4, 25.4) | 23.6 (22.4, 24.6) | 24.1 (23.4, 25.9) |
| Gender | ||||
| Male | 3 (50%) | 4 (66.7%) | 3 (50.0%) | 4 (66.7%) |
| Female | 3 (50%) | 2 (33.3%) | 3 (50.0%) | 2 (33.3%) |
Data was expressed in median (range) and n (%)
BMI body mass index
Safety Assessments
AEs occurred in 12 (66.67%) volunteers in the KB groups, and 4 (66.67%) volunteers in the placebo group (Table 2). TRAEs occurred in 8 (44.44%) volunteers in the KB groups and 2 (33.33%) volunteers in the placebo group. All AEs were judged mild by investigators. There were 4 (22.22%) “hypertriglyceridemia”, 3 (16.67%) “sinus bradycardia”, and 1 (5.56%) “pruritus” cases, which were judged by the investigator to be possibly unrelated to KB; the other AEs were judged as possibly related to KB. All volunteers who experienced AEs recovered on their own without any treatment. AEs observed in this study mainly included hypertriglyceridemia (4, 22.22%) and sinus bradycardia (3, 16.67%). In terms of SOC, the most commonly reported AEs were metabolism and nutrition disorders (6, 33.33%), investigations (4, 22.22%), and cardiac disorders (3, 16.67%). No moderate, serious, or severe AEs occurred during the study. No volunteers withdrew from the study because of AEs. The incidence rate of AEs was not significantly related to dose and systemic exposure.
Table 2.
Summary of adverse events
| Events, n (%) | Placebo (N = 6) | Kukoamine B | |||
|---|---|---|---|---|---|
| 0.06 mg/kg (N = 6) | 0.12 mg/kg (N = 6) | 0.24 mg/kg (N = 6) | All (N = 18) | ||
| With any AE, n (%) | 4 (66.67%) | 4 (66.67%) | 3 (50%) | 5 (83.33%) | 12 (66.67%) |
| With any TRAE, n (%) | 2 (33.33%) | 2 (33.33%) | 1 (16.67%) | 5 (83.33%) | 8 (44.44%) |
| AE, n (%) | |||||
| Investigations | 0 (0%) | 0 (0%) | 0 (0%) | 4 (66.67%) | 4 (22.22%) |
| γ-Glutamyl transferase increased | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.67%) | 1 (5.56%) |
| Alanine aminotransferase increased | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.67%) | 1 (5.56%) |
| Blood alkaline phosphatase increased | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.67%) | 1 (5.56%) |
| Aspartate aminotransferase increased | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.67%) | 1 (5.56%) |
| Metabolism and nutrition disorders | 2 (33.33%) | 2 (33.33%) | 1 (16.67%) | 3 (50%) | 6 (33.33%) |
| Hypocalcemia | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.67%) | 1 (5.56%) |
| Hypertriglyceridemia | 2 (33.33%) | 2 (33.33%) | 1 (16.67%) | 1 (16.67%) | 4 (22.22%) |
| Hypokalemia | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.67%) | 1 (5.56%) |
| Skin and subcutaneous tissue disorders | 1 (16.67%) | 1 (16.67%) | 1 (16.67%) | 0 (0%) | 2 (11.11%) |
| Skin exfoliation | 1 (16.67%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Rash maculopapular | 0 (0%) | 1 (16.67%) | 0 (0%) | 0 (0%) | 1 (5.56%) |
| Pruritus | 0 (0%) | 0 (0%) | 1 (16.67%) | 0 (0%) | 1 (5.56%) |
| Gastrointestinal disorders | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33.33%) | 2 (11.11%) |
| Gingival pain | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.67%) | 1 (5.56%) |
| Mouth ulcer | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.67%) | 1 (5.56%) |
| Renal and urinary disorders | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.67%) | 1 (5.56%) |
| Proteinuria | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.67%) | 1 (5.56%) |
| Blood and lymphatic system disorders | 0 (0%) | 1 (16.67%) | 0 (0%) | 0 (0%) | 1 (5.56%) |
| Anemia | 0 (0%) | 1 (16.67%) | 0 (0%) | 0 (0%) | 1 (5.56%) |
| Nervous system disorders | 1 (16.67%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Orthostatic hypotension | 1 (16.67%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Cardiac disorders | 0 (0%) | 2 (33.33%) | 1 (16.67%) | 0 (0%) | 3 (16.67%) |
| Sinus bradycardia | 0 (0%) | 2 (33.33%) | 1 (16.67%) | 0 (0%) | 3 (16.67%) |
AE adverse event, TRAE treatment-related adverse event
Pharmacokinetic Analysis
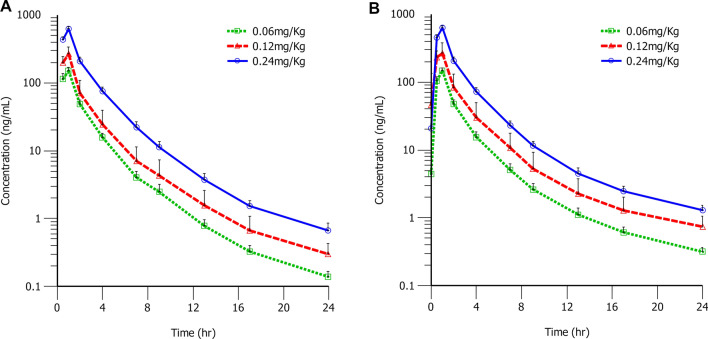
The mean plasma concentration of KB vs. time curves of volunteers who received KB at different dose levels are shown in Fig. 2 for day 1 (KB single dose administration) and day 7 (KB multiple dose administration). The corresponding PK parameters are presented in Table 3 (day 1) and Table 4 (day 7), respectively.
Fig. 2.
Mean plasma drug concentration–time semi-logarithmic plot. Eighteen healthy volunteers received multiple intravenous infusions of 0.06 mg/kg, 0.12 mg/kg, and 0.24 mg/kg kukoamine B (KB); the mean plasma drug concentration–time curves of KB after the first dose on day 1 (A) and the last dose on day 7 (B)
Table 3.
Pharmacokinetic analysis of KB on day 1
| Parameters | 0.06 mg/kg | 0.12 mg/kg | 0.24 mg/kg |
|---|---|---|---|
| AUC0–24h (h·ng/mL) | 283.64 (37.90) | 465.91 (188.43) | 1216.13 (97.78) |
| AUC0–8h (h·n/mL) | 271.55 (35.86) | 443.09 (174.02) | 1158.49 (87.91) |
| AUC_%Extrap (%) | 0.32 (0.09) | 0.50 (0.57) | 0.20 (0.07) |
| AUCinf (h·n/mL) | 284.23 (37.95) | 467.76 (188.31) | 1218.63 (98.33) |
| CL (L/h) | 10.16 (1.94) | 13.49 (4.47) | 9.35 (0.73) |
| Cmax (ng/mL) | 150.72 (17.40) | 269.97 (68.89) | 622.73 (46.93) |
| Kel (1/h) | 0.19 (0.04) | 0.17 (0.06) | 0.21 (0.03) |
| MRTinf (h) | 1.88 (0.11) | 1.86 (0.52) | 2.05 (0.15) |
| Tmax (h)# | 1.00 (1.00–1.00) | 1.00 (0.50–1.00) | 1.00 (1.00–1.00) |
| Vd (L) | 55.24 (17.39) | 101.90 (84.85) | 45.74 (6.40) |
| T1/2 (h) | 3.72 (0.78) | 4.88 (2.93) | 3.40 (0.50) |
Data was expressed in mean (standard deviation)
AUC0–24h area under the plasma concentration–time curve from zero time to 24 h, AUC0–8h area under the plasma concentration–time curve from zero time to 8 h, AUC_%Extrap extrapolated area percentage, AUCinf area under the plasma concentration–time curve from zero time to inferred infinite time, CL apparent clearance rate, Cmax peak plasma concentration, Kel elimination rate constant, MRTinf average retention time, Tmax time to peak plasma concentration, Vd apparent volume of distribution, T1/2 elimination half-life
#Data was expressed in median (range)
Table 4.
Pharmacokinetic analysis of KB on day 7
| Parameter | 0.06 mg/kg | 0.12 mg/kg | 0.24 mg/kg |
|---|---|---|---|
| AUC0–24h (h·n/mL) | 284.02 (37.24) | 541.55 (238.99) | 1236.08 (76.04) |
| AUC0–8h (h·n/mL) | 267.39 (34.13) | 507.27 (217.49) | 1166.30 (73.24) |
| AUC_%Extrap (%) | 0.96 (0.32) | 1.73 (2.03) | 0.97 (0.41) |
| AUCinf (h·ng/mL) | 286.74 (37.46) | 549.80 (239.24) | 1247.97 (73.43) |
| CLss (L/h) | 10.81 (2.22) | 12.59 (4.39) | 9.76 (0.79) |
| CLav,ss (ng/h) | 33.42 (4.27) | 63.41 (27.19) | 145.79 (9.16) |
| Cmax (ng/mL) | 146.91 (14.05) | 290.35 (69.47) | 629.98 (36.27) |
| Cmin_day7 (ng/mL) | 4.42 (1.34) | 10.62 (5.66) | 20.76 (3.06) |
| Kel (1/h) | 0.12 (0.03) | 0.11 (0.04) | 0.11 (0.02) |
| MRTinf,ss (h) | 2.04 (0.09) | 2.00 (0.43) | 2.06 (0.16) |
| PTF (%) | 429.36 (37.37) | 498.90 (186.75) | 418.96 (25.26) |
| R1 | 0.94 (0.05) | 1.08 (0.09) | 0.96 (0.09) |
| RAUC1 | 1.00 (0.05) | 1.15 (0.11) | 1.02 (0.09) |
| RAUC2 | 0.99 (0.06) | 1.13 (0.11) | 1.01 (0.09) |
| RCmax | 0.98 (0.08) | 1.08 (0.10) | 1.01 (0.08) |
| Tmax (h)# | 1.00 (1.00–1.00) | 1.00 (0.50–1.00) | 1.00 (1.00–1.00) |
Data was expressed in mean (standard deviation)
#Data was expressed in median (range)
AUC0–24h area under the plasma concentration–time curve from zero time to 24 h, AUC0–8h area under the plasma concentration–time curve from zero time to 8 h, AUC_%Extrap extrapolated area percentage, AUCinf area under the plasma concentration–time curve from the zero time to the estimated infinite time, CL apparent clearance rate, Cmax peak plasma concentration, Kel elimination rate constant, MRTinf average retention time, Tmax time to peak plasma concentration, Vd apparent volume of distribution, T1/2 elimination half-life, CLss apparent drug clearance rate, CLavss steady-state mean plasma concentration, Cmin_day7 trough concentration before the last dose, MRTinf, ss mean residence time, PTF fluctuation percentage, RAUC1 steady-state accumulation ratio expressed by AUC0–24h, RAUC1 = AUC0–24h (last dose)/AUC0–24h (first dose), RAUC2 steady accumulation ratio by AUC0–8h, RAUC2 = AUC0–8h (last dose)/AUC0–8h (first dose), R1 steady state accumulation ratio expressed by AUC, R1 = AUC0–8h (last dose)/AUCinf (first dose), RCmax steady-state accumulation expressed by Cmax, RCmax = Cmax (last dose)/Cmax (first dose)
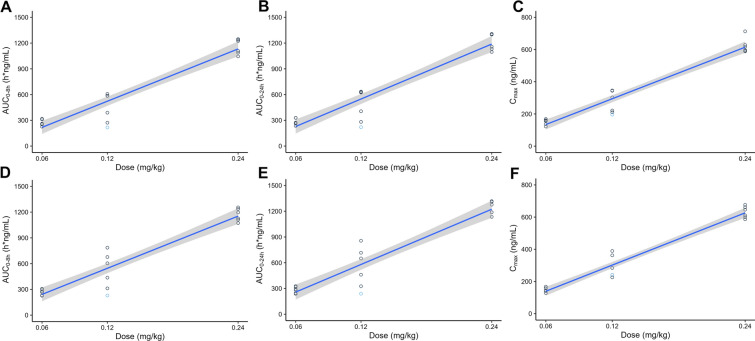
Plasma concentration of KB gradually increased after the start of intravenous infusion and drastically decreased following drug withdrawal. Moreover, the trend and shape of the plasma concentration–time curve of KB in each dose group were similar whether single administration or multiple administration. The peak level of KB was reached approximately 1.0 h after dosing. After Cmax was reached, concentrations of KB declined with a t1/2 of 4 h. The CL was 11.0 L/h. The Vss was 67.6 L across all dose levels. Based on 8-h intervals dosing plan, the AUC and Cmax obtained from day 7 (the last administration) were close to those from day 1 (the first administration), which suggested that there was no accumulation following multiple administrations; the average accumulation ratios of AUC and Cmax were 1.06 and 1.02, respectively. The maximum Cmax/minimum Cmax ratios of each dose group were 1.1–2.5, which suggested a relatively low inter-volunteer variability on the exposure level of KB. The mean AUC0–8h and Cmax increased by 4.3-fold and 4.1-fold on day 1, and increased by 4.4-fold and 4.3-fold on day 7 over the approximately 4-fold dose range, respectively, indicating a dose-proportional PK (Fig. 3).
Fig. 3.
Scatter plot of AUC and Cmax versus dose after intravenous infusions of 0.06 mg/kg, 0.12 mg/kg, and 0.24 mg/kg (N = 18) kukoamine B (KB). A Single dose (AUC0–8h); B single dose (AUC0–24h); C single dose (Cmax); D multiple dose (AUC0–8h); E multiple dose (AUC0–24h); F multiple dose (Cmax). Blue dots indicate an abnormal administration process (see “Discussion” for details)
During day 1 infusion in the 0.12 mg/kg group, one volunteer experienced a pump failure 52 min after the start of administration, resulting in the termination of infusion at approximately 86.3 mL of KB injected. After re-administration, the remaining drug was administered 1 h later and stopped at 1.13 h. As a result, the blood collection time for this volunteer’s PK samples was delayed by 8 min from the planned time (excluding 24 h), and an additional time point was collected at the actual withdrawal time of 1.13 h. The investigators determined that this was a minor deviation that did not affect the results and included it in the PK analysis. Figure 3 shows the AUC and Cmax of this subject, represented by blue dots.
Discussion
Sepsis is a fatal complication caused by severe infection [5], and it is among the most prevalent causes of death and multimorbidity in clinical practice [3, 4]. KB possesses a broad spectrum of therapeutic capabilities, including antioxidant and anti-inflammatory properties [18], and has demonstrated significant protective effects against severe and extremely severe sepsis in animal models [20]. Thus, this phase I clinical trial was designed to evaluate the safety and PK of KB administration in healthy adult volunteers following single- and multiple-dose administration.
All AEs that occurred during the study were mild, and volunteers who experienced AEs recovered on their own without any treatment. In addition, none of the eligible volunteers withdrew from the trial because of AEs. The incidence rate of AEs was not significantly related to dose and systemic exposure. The results of our study showed that the multiple intravenous infusion of KB in healthy volunteers for the dose range of 0.06–0.24 mg/kg was safe. These findings are comparable to those of Wang et al., who reported no major AEs associated with a 0.24 mg/kg dose regimen in patients with sepsis and so recommended it for further clinical trials [17].
Additionally, hypertriglyceridemia was the most frequently reported AE in both the KB group (4, 22.22%) and the placebo group (2, 33.33%) during the trial. The incidence of hypertriglyceridemia was slightly lower in the KB group than in the placebo group. Nevertheless, it is crucial to monitor plasma triglyceride levels in future clinical trials to address this concern.
After 18 healthy volunteers received multiple intravenous infusions of 0.06 mg/kg, 0.12 mg/kg, and 0.24 mg/kg KB (administration once every 8 h, infusion time 1 h), the plasma concentration of KB gradually increased after the start of intravenous infusion and decreased rapidly after drug withdrawal. The plasma concentration–time curve of KB in each dose group was similar, and the plasma concentration–time curve after the last administration was essentially the same as that after the first administration. After the first administration of 0.06 mg/kg, 0.12 mg/kg, and 0.24 mg/kg KB, the t1/2 values of KB were 3.72 ± 0.78 h, 4.88 ± 2.93 h, and 3.40 ± 0.50 h, respectively, while the t1/2 values of KB after the last administration were 6.22 ± 1.46 h, 8.48 ± 6.80 h, and 6.49 ± 1.41 h, respectively, indicating that the plasma t1/2 of each dose group was slightly prolonged after continuous administration. This may be related to the limited number of volunteers and individual variation. The body exposure of KB increased with the increase of the administration dose. The mean AUC0–8h and Cmax increased by 4.3-fold and 4.2-fold over the approximately 4-fold dose range, respectively, indicating a dose-proportional pharmacokinetics. Because the range of dose increment was limited and there were only six volunteers in each dose group, the power model was not used to calculate the dose proportion relationship. The half-life of KB was very short, only about 4 h, so three doses a day will not result in drug accumulation. During the infusion, one subject in the 0.12 mg/kg group experienced a pump failure. The investigators considered it a minor deviation that did not impact the results, and included the subject’s data in the PK analysis. A separate analysis was conducted without this participant, and the difference in PK parameters between the data sets with and without this volunteer was found to be less than 10.8%.
This is the report of the safety, tolerability, and PK characteristics of single and multiple doses of KB in healthy volunteers. Since healthy volunteers do not have biomarkers for sepsis efficacy evaluation, it is impossible to evaluate the preliminary efficacy in this study. In addition, because the drug clearance may be different between healthy volunteers and patients with sepsis, and the number of volunteers in this study is limited, it is necessary to further evaluate the pharmacokinetics in patients with sepsis. A phase I study (NCT03237728) and a phase II study (NCT04803955) to assess the safety and efficacy of KB in patients with sepsis have been conducted.
In the phase II study, the time of blood collection on day 1 of the first drug administration only lasted until 8 h after the drug administration, and sampling points of the elimination phase were insufficient; thus, some essential information about the elimination phase of the drug (such as t1/2 and elimination rate constant K) and data of Vd could not be obtained. The phase II study did not completely characterize the drug’s pharmacokinetics. In this study, we specifically designed the time points of blood collection to illustrate the drug’s pharmacokinetics. The time of blood collection on day 1 of the first administration lasted until 24 h after administration, which could reasonably describe the elimination phase of the drug. This study is an important complement to phase II studies, which can provide important data such as drug half-life.
Conclusion
Single and multiple intravenous infusions of KB at a dose range of 0.06–0.24 mg/kg are safe and tolerable for healthy individuals. The plasma concentration of KB increased gradually after the start of intravenous infusion and decreased rapidly after drug withdrawal. No accumulation was found after multiple intravenous infusion doses (8 h intervals) of KB.
Acknowledgements
The authors would like to thank staff in the study team and all volunteers.
Funding
This study was sponsored by Tianjin ChaseSun Pharmaceutical Co., Ltd, China for the cost of conducting the clinical trial and Rapid Service Fee for the publication of the article. The work was also supported by National High Level Hospital Clinical Research Funding (No. 2022-PUMCH-A-144), the Jinqiao Project of Beijing Association of Science and Technology (No. ZZ19005), National High Level Hospital Clinical Research Funding (No. 2022-PUMCH-B-033), Drug Development and Application of Chinese Pharmacological Society (No. 2019DL001). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Author Contributions
Pei Hu, Qian Zhao, Ji Jiang, Kai Dong, Shuai Chen wrote manuscript, designed experiments, performed data analysis and interpreted results. Hongzhong Liu and Chunyan Jin were responsible for subject dosing and clinical trial operations. Zhenlei Wang, Teng Wang, Cheng Cui, Huanhuan Wang and Lili Li performed sample testing. Wen Zhong performed statistical analysis. All authors read and approved the final manuscript.
Disclosures
Qian Zhao, Hongzhong Liu, Zhenlei Wang, Teng Wang, Cheng Cui, Lili Li, Ji Jiang and Pei Hu declare no conflict of interest. Kai Dong, Shuai Chen and Chunyan Jin are full-time employees of Tianjin Chasesun Pharmaceutical Co., Ltd.
Compliance with Ethics Guidelines
This clinical study was designed, conducted, and reported per the Declaration of Helsinki. The study was approved by the Ethics Committee of Peking Union Medical College Hospital, and all volunteers signed an informed consent form.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
Footnotes
Qian Zhao and Hongzhong Liu contributed equally to this work.
References
- 1.Chakraborty RK, Burns B. Systemic inflammatory response syndrome. Treasure Island: StatPearls. 2022. [PubMed]
- 2.Lupu F. "Crossroads in sepsis research" review series overview of the pathophysiology of sepsis. J Cell Mol Med. 2008;12:1072–1073. doi: 10.1111/j.1582-4934.2008.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boller EM, Otto CM. Sepsis. In: Silverstein DC, Hopper K, editors. Small animal critical care medicine. Saint Louis: Saunders; 2009. pp. 454–458. [Google Scholar]
- 4.Gavins FNE. Sepsis. In: Gavins FNE, Stokes KY, editors. Vascular responses to pathogens. Boston: Academic; 2016. pp. 1–9. [Google Scholar]
- 5.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 6.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagata I, Abe T, Ogura H, Kushimoto S, Fujishima S, Gando S. Intensive care unit model and in-hospital mortality among patients with severe sepsis and septic shock: a secondary analysis of a multicenter prospective observational study. Medicine (Baltimore) 2021;100:e26132. doi: 10.1097/MD.0000000000026132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pravda J. Sepsis: evidence-based pathogenesis and treatment. World J Crit Care Med. 2021;10:66–80. doi: 10.5492/wjccm.v10.i4.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Levy MM. Treatment of patients with severe sepsis and septic shock: current evidence-based practices. R I Med J. 2013;2019(102):18–21. [PubMed] [Google Scholar]
- 10.Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. E-Book: Elsevier Health Sciences; 2019.
- 11.Matsuura M. Structural modifications of bacterial lipopolysaccharide that facilitate Gram-negative bacteria evasion of host innate immunity. Front Immunol. 2013;4:109. doi: 10.3389/fimmu.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briassouli E, Tzanoudaki M, Daikos G, et al. 0104. Modulatory effects of heat shock with or without glutamine compared to LPS on peripheral blood mononuclear cells heat-shock-protein 90α expression in severe sepsis and trauma. Intensive Care Med Exp. 2014;2(Suppl 1):14.
- 13.Kim TH, Kim D, Lee H, et al. CpG-DNA induces bacteria-reactive IgM enhancing phagocytic activity against Staphylococcus aureus infection. BMB Rep. 2019;52:635–640. doi: 10.5483/BMBRep.2019.52.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TH, Park J, Kim D, et al. Anti-bacterial effect of CpG-DNA involves enhancement of the complement systems. Int J Mol Sci. 2019;20:3397. doi: 10.3390/ijms20143397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hume DA, Underhill DM, Sweet MJ, Ozinsky AO, Liew FY, Aderem A. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001;2:11. doi: 10.1186/1471-2172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Nardo D, De Nardo CM, Nguyen T, Hamilton JA, Scholz GM. Signaling crosstalk during sequential TLR4 and TLR9 activation amplifies the inflammatory response of mouse macrophages. J Immunol. 2009;183:8110–8118. doi: 10.4049/jimmunol.0901031. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Hu X, Wang T, et al. Exposure-response modeling to support dosing selection for phase IIb development of kukoamine B in sepsis patients. Front Pharmacol. 2021;12:645130. doi: 10.3389/fphar.2021.645130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Q, Li L, Zhu Y, et al. Kukoamine B ameliorate insulin resistance, oxidative stress, inflammation and other metabolic abnormalities in high-fat/high-fructose-fed rats. Diabetes Metab Syndr Obes. 2020;13:1843–1853. doi: 10.2147/DMSO.S247844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Zheng X, Wang N, et al. Kukoamine B, a novel dual inhibitor of LPS and CpG DNA, is a potential candidate for sepsis treatment. Br J Pharmacol. 2011;162:1274–1290. doi: 10.1111/j.1476-5381.2010.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin WT, Wang X, Shen WC, Sun BW. A novel role of kukoamine B: inhibition of the inflammatory response in the livers of lipopolysaccharide-induced septic mice via its unique property of combining with lipopolysaccharide. Exp Ther Med. 2015;9:725–732. doi: 10.3892/etm.2015.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Qin W, Lyu W, Shen W, Wang X, Sun B. [Inhibitory effect of kukoamine B on lung inflammatory responses in mice with sepsis] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:493–497. doi: 10.3760/cma.j.issn.2095-4352.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Lyu W, Qin W, Zhang J, Shen W, Wang X, Sun B. [Inhibitory effects of Kukoamine B on the inflammatory response of small intestine in lipopolysaccharide-induced septic mice and its potential mechanisms] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27:121–126. doi: 10.3760/cma.j.issn.2095-4352.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Zhao Q, Li L, et al. Development and validation of a rapid and sensitive UPLC-MS/MS method for quantification of kukoamine B in human plasma: application to a clinical pharmacokinetic study. J Pharm Biomed Anal. 2017;132:1–6. doi: 10.1016/j.jpba.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Q, Li L, Wang Z, et al. Ultra performance liquid chromatography tandem mass spectrometry assay for determination of kukoamine B in human blood and urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1031:8–14. doi: 10.1016/j.jchromb.2016.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.