Abstract
Introduction
Pembrolizumab was approved in the US as adjuvant treatment of patients with stage IIB or IIC melanoma post-complete resection, based on prolonged recurrence-free survival vs. placebo in the Phase 3 KEYNOTE-716 trial. This study aimed to evaluate the cost-effectiveness of pembrolizumab vs. observation as adjuvant treatment of stage IIB or IIC melanoma from a US health sector perspective.
Methods
A Markov cohort model was constructed to simulate patient transitions among recurrence-free, locoregional recurrence, distant metastasis, and death. Transition probabilities from recurrence-free and locoregional recurrence were estimated via multistate parametric modeling based on patient-level data from an interim analysis (data cutoff date: 04-Jan-2022). Transition probabilities from distant metastasis were based on KEYNOTE-006 data and network meta-analysis. Costs were estimated in 2022 US dollars. Utilities were based on applying US value set to EQ-5D-5L data collected in trial and literature.
Results
Compared to observation, pembrolizumab increased total costs by $80,423 and provided gains of 1.17 quality-adjusted life years (QALYs) and 1.24 life years (LYs) over lifetime, resulting in incremental cost-effectiveness ratios of $68,736/QALY and $65,059/LY. The higher upfront costs of adjuvant treatment were largely offset by reductions in costs of subsequent treatment, downstream disease management, and terminal care, reflecting the lower risk of recurrence with pembrolizumab. Results were robust in one-way sensitivity and scenario analyses. At a $150,000/QALY threshold, pembrolizumab was cost-effective vs. observation in 73.9% of probabilistic simulations that considered parameter uncertainty.
Conclusion
As an adjuvant treatment of stage IIB or IIC melanoma, pembrolizumab was estimated to reduce recurrence, extend patients’ life and QALYs, and be cost-effective versus observation at a US willingness-to-pay threshold.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02525-x.
Keywords: Adjuvant treatment, Cost-effectiveness analysis, Pembrolizumab, Stage IIB–IIC melanoma, US health sector perspective
Key Summary Points
| Why carry out this study? |
| Surgical resection is the primary treatment for stage IIB–IIC melanoma but is often not curative because of high risk of disease recurrence post-surgery |
| This study evaluated the cost-effectiveness of adjuvant pembrolizumab versus routine observation alone (no adjuvant treatment) in patients with resected stage IIB–IIC melanoma from a US health sector perspective |
| What was learned from the study? |
| Over a lifetime horizon, pembrolizumab was estimated to extend life years and quality-adjusted life years relative to observation and was cost-effective based on a typical US-specific willingness-to-pay threshold |
| Results from this study can inform decision-making by payers, physicians, and patients regarding the optimal treatment strategy for resected stage IIB–IIC melanoma |
Introduction
Melanoma is a type of cancer that affects melanocytes, the melanin-producing cells in the skin. Melanoma is the most lethal form of skin cancer. In the USA, the incidence of melanoma has risen by 1.2% annually over the last decade, with an estimated 99,780 newly diagnosed cases and 7650 deaths attributed to melanoma in 2022 [1].
The primary treatment for stage II melanoma is surgery in the form of wide excision with a safety surgery margin of 2 cm (depending on anatomical location) [2]. Though surgery can be curative for some patients, 19–37% of patients died within 5 years of resection because of disease recurrence [3–5]. The risk of recurrence depends on several factors, including tumor thickness or depth, ulceration status, histology, and completeness of surgical resection [6]. Patients with stage IIB or IIC melanoma are at an increased risk of recurrence, higher than stage IIIA and similar to stage IIIB, after complete surgical resection [6]. Pathologic staging to define stage IIB or IIC includes a negative sentinel lymph node biopsy, per American Joint Committee on Cancer 8th edition criteria [4].
For patients at a high risk of recurrence post-surgery, there is a need for effective adjuvant therapies to prevent recurrence of melanoma and improve survival. Adjuvant interferon-alfa in melanoma has been shown to provide modest improvements in recurrence-free survival (RFS) (i.e., a hazard ratio [HR] of 0.86 vs. placebo) but is associated with significant toxicities that can negatively affect quality of life [7, 8]. With high-dose interferon-alfa, approximately 40% of patients have treatment-related adverse events (AEs) that lead to dose delays or reductions [8]. National Comprehensive Cancer Network (NCCN) guidelines therefore no longer recommend interferon-alfa as adjuvant therapy for cutaneous melanoma [2].
Pembrolizumab is a high-affinity monoclonal antibody which binds to the programmed cell death protein 1 (PD-1) receptor and blocks its interaction with the programmed cell-death ligands, PD-L1 and PD-L2. This reactivates the tumor-specific cytotoxic T-lymphocytes, which destroy tumor cells, and re-establishes anti-tumor immunity in affected patients [9]. Pembrolizumab was approved by the US Food & Drug Administration (FDA) as adjuvant treatment of patients with stage IIB or IIC melanoma post-complete resection, based on statistically significant longer RFS vs. placebo in the Phase 3 KEYNOTE-716 trial (NCT03553836) [10]. In the third interim analysis of KEYNOTE-716 (data cutoff date: 04-Jan-2022; median follow-up: 27.4 months [interquartile range 23.1–31.7]), pembrolizumab significantly improved time to distant metastases (HR 0.64; 95% confidence interval [CI] 0.47–0.88) relative to placebo [11].
A comprehensive economic evaluation is needed to guide insurance coverage and clinical treatment decisions regarding the use of pembrolizumab in this setting. The objective of this study was to evaluate the cost-effectiveness of pembrolizumab as an adjuvant treatment following complete resection of stage IIB or IIC melanoma from a US health sector perspective. Pembrolizumab was compared to the strategy of routine observation alone, represented by the placebo arm of the KEYNOTE-716 trial.
Methods
Model Overview
A Markov cohort model was implemented in Excel 2016 (Microsoft Corp., Redmond, WA) to evaluate the cost-effectiveness of pembrolizumab versus observation among patients (ages ≥ 12 years) who have undergone complete surgical resection and have a histologically/pathologically confirmed new diagnosis of stage IIB or IIC melanoma. Pembrolizumab was evaluated based on the trial-based, label-recommended dosing schedule in this setting (200 mg for ages 18 + years or 2 mg/kg [up to 200 mg] for ages 12–17 years, administered intravenously [IV] every 3 weeks for up to 17 cycles [~ 1 year]).
Patients in the Markov cohort transitioned among four mutually exclusive health states: recurrence-free (RF), locoregional recurrence (LR), distant metastases (DM), and death (Fig. 1). This model structure allowed for extrapolation of key survival endpoints in each treatment arm, including RFS (defined as time to LR, DM, or death, whichever occurs first), DMFS (defined as time to DM or death, whichever occurs first), and overall survival (OS) (defined as time to death). Based on these endpoint definitions, RFS depended on all transition probabilities starting from the RF state, DMFS depended on transition probabilities starting from RF and LR, and OS depended on all transition probabilities in the model.
Fig. 1.
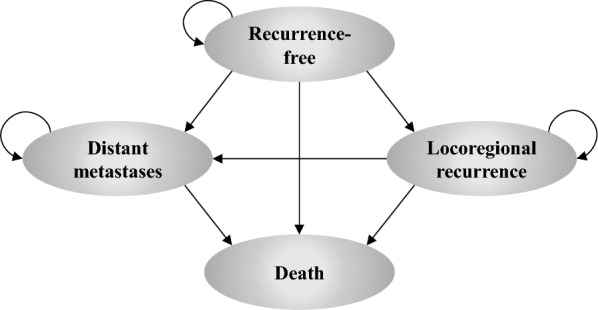
Model schematic. The model schematic illustrates the four mutually exclusive health states in the Markov model. Allowable transitions among the four states are represented by arrows. Patients enter the model in the recurrence-free state after having undergone complete resection of stage IIB–IIC melanoma and are at risk of transitioning to other health states at the end of each weekly model cycle
Patients entered the model in the RF state following surgical resection. Starting age (59.3 years) and percentage female (39.7%) were consistent with the KEYNOTE-716 population at baseline. From RF, patients’ risks of transitioning directly to LR, DM, or death differed between the two model arms (adjuvant pembrolizumab or observation), based on results from KEYNOTE-716. After developing LR, patients were assumed to be potentially eligible for an FDA-approved adjuvant systemic treatment of resected stage III melanoma. Transition probabilities from the LR state directly to DM or death were estimated for each treatment arm based on KEYNOTE-716 data. Upon developing DM, patients were expected to initiate subsequent systemic therapies for advanced/metastatic melanoma. Transition probabilities from DM to death were linked to the efficacy of first-line therapies received in the advanced/metastatic setting.
The analysis adopted a weekly cycle length with half-cycle correction. Costs from a US health sector perspective, life years (LYs), and quality-adjusted LYs (QALYs) were estimated over a lifetime based on patients’ health state distribution in each cycle and the costs and utilities assigned to each state. Costs and health effects were discounted by 3.0% annually. Cost parameters were inflation-adjusted to 2022 US dollars (USD) where applicable. Model parameters related to transition probabilities, safety, health state utilities, and costs are presented in Table S1 of the Supplement.
Transition Probabilities
Transition probabilities between states were estimated through parametric multi-state modeling [12–15]. All transition probabilities to death were constrained to be at least as high as mortality in the general US population, given the age and gender distribution of the model cohort at each weekly cycle [16].
Transitions Starting from RF
Transition probabilities from RF to other states were estimated for each treatment arm (pembrolizumab and observation) using patient-level data from KEYNOTE-716. Using R software (R Development Core Team, Vienna, Austria), parametric distributions were fitted to the cause-specific hazards of RF → LR, RF → DM, and RF → death, accounting for competing risks. For RF → death, exponential distributions were used because of the small number of events. For RF → LR and RF → DM, candidate distributions included: (1) six distributions separately fitted to each arm (exponential, Weibull, Gompertz, log-logistic, log-normal, generalized gamma); (2) three proportional hazards distributions (exponential, Weibull, Gompertz) jointly fitted to both arms with a time-constant HR for pembrolizumab vs. placebo; (3) three proportional hazards distributions (exponential, Weibull, Gompertz) jointly fitted to both arms with a time-varying HR that allowed the treatment effect to differ before versus after 1 year from randomization.
All transition probabilities from RF depended upon all three cause-specific hazard functions. Base case distributions were therefore selected from all 54 possible combinations of distributions for RF → LR and RF → DM, including 36 (i.e., 6 × 6) under approach 1, 9 (i.e., 3 × 3) under approach 2, and 9 (i.e., 3 × 3) under approach 3. Consistent with methodologic guidelines from the National Institute for Health and Care Excellence (NICE) Decision Support Unit [14, 17], the same distribution types were selected for both arms based on statistical fit with observed RFS and DMFS, visual fit, and external validity and clinical plausibility of long-term extrapolations. The process of selecting base case distributions for RF → LR and RF → DM is summarized in Table 1. Further details are included in the Supplemental Methods and Figures S1–S3.
Table 1.
Selection process for base case parametric distributions of RF → LR and RF → DM
| Step | Criteria applied at each step | Combinations of distributions that meet criteria |
|---|---|---|
| 0 | All candidate combinations of parametric functions | 54 |
| 1 |
Initial exclusions based on clinical plausibility 18 out of 54 combinations of parametric distributions resulted in implausible crossing of the survival curves for pembrolizumab and observation and were therefore excluded from base case consideration 6 of these 12 combinations were also excluded based on the use of 4-year RFS and DMFS in KEYNOTE-054 (adjuvant stage III melanoma setting) as lower bounds |
36 |
| 2 |
Statistical fit Mean squared errors (MSEs) relative to observed RFS and observed DMFS were ranked for all 54 combinations of distributions in each arm Because MSEs were generally lower in the pembrolizumab arm than the observation arm, statistical fit in the observation arm was prioritized. Therefore, 7 combinations ranked among the ten worst-fitting for both RFS and DMFS in the observation arm were excluded |
29 |
| 3 |
Visual assessment of fit Visual assessment of fit was consistent with statistical fit; thus, no further exclusions were applied based on visual inspection alone |
29 |
| 4a |
External validity of long-term extrapolations in the observation arm Predicted RFS and DMFS in the observation arm up to 7 years was required to fall within ± 5 percentage points of external RFS and DMFS data 14 combinations met this external validity assessment. All of these combinations used either log-normal (under approach 1) or exponential (under approaches 1, 2, or 3) for RF → DM |
14 |
| 4b | 3 of the 14 combinations were among the 10 best-fitting combinations with respect to RFS in the observation arm. Of these three, the selected base case (approach 1/log-normal/log-normal) was closest to the average of external RFS sources at 6 out of the 7 yearly time points | 1 |
| 5 |
Plausibility of predicted incremental benefit with pembrolizumab vs. observation Relative to other combinations of distributions that met the external validity requirement, the selected base case predicted moderate incremental RFS and DMFS benefits with pembrolizumab vs. observation |
1 Base case: Approach 1/log-normal/log-normal |
DM distant metastases, DMFS distant metastases-free survival, LR locoregional recurrence, MSE mean squared error, RF recurrence-free, RFS recurrence-free survival
A retrospective study among 738 patients with resected stage II melanoma showed that 91.2% of relapses occurred in the first 5 years, with the cumulative incidence of relapses increasing more slowly as time progresses [18]. Two further retrospective studies in stage I–II or I–III melanoma reported that 73% to 90.7% of recurrences were detected within 5 years [19, 20]. Based on this evidence, the current model applied a cure assumption among patients who achieve long-term RFS. Specifically, the per-cycle risks of transitions from the RF state (as estimated under the scenario with no cure assumption) were reduced by 95% for patients who achieve RFS ≥ 10 years. This percentage reduction in recurrence risk was assumed to linearly increase from 0% at 7 years to 95% by 10 years onward. Similar cure assumptions have been applied in past appraisals of early stage cancer treatments by NICE and the Canadian Agency for Drugs and Technologies in Health (CADTH) [21–25]. This assumption was also consistent with NCCN guideline recommendations to discontinue routine imaging to screen for recurrences beyond 3–5 years in patients with resected stage IIB–IIC melanoma [2].
Base case distributions were log-normal for RF → LR and log-normal for RF → DM under approach 1. This combination of distributions yielded a more modest incremental RFS benefit of pembrolizumab vs. observation than most other distributions with good visual/statistical fit and external validity. Alternative distributions were tested in scenario analyses.
Transitions Starting from LR
Patient-level time-to-event data from the KEYNOTE-716 trial were used to estimate exponential rates and standard errors for the LR → DM and LR → death transitions. No direct transitions from LR → death were observed in KEYNOTE-716; the cause-specific hazard for LR → death was therefore approximated using the exponential rate of RF → death in the placebo arm of KEYNOTE-716 (i.e., the arm with the higher rate of RF → death) based on the expectation that the LR → death rate would be at least as high.
No adjustments were performed for rechallenge or crossover regimens within the LR state; thus, the resulting transition probabilities incorporate any effect of crossover/rechallenge on risk of DM or death. This approach was considered appropriate because, in real-world practice, patients experiencing LR would be eligible to receive adjuvant treatments of resected stage III melanoma (including pembrolizumab).
Transitions Starting from DM
Transition probabilities from DM → death were modeled based on market shares and efficacy of first-line treatments for advanced melanoma. First-line treatment options included pembrolizumab, ipilimumab, nivolumab, nivolumab + ipilimumab, dabrafenib + trametinib, and encorafenib + binimetinib.
For each advanced melanoma treatment option, exponential models of OS and progression-free survival (PFS) were estimated. For pembrolizumab as a first-line treatment of advanced melanoma, exponential models of OS and PFS were fitted to patient-level time-to-event data from the pembrolizumab arm of KEYNOTE-006, a multicenter, randomized, open-label phase III trial among ipilimumab-naïve unresectable or advanced melanoma patients [26]. For other advanced melanoma treatments, HRs for OS and PFS vs. pembrolizumab were obtained from a network meta-analysis (NMA) of trials conducted in advanced melanoma.
Market shares of first-line subsequent treatments in each arm were estimated based on unpublished market research data and results from a retrospective chart review [27]. In the adjuvant pembrolizumab arm, market shares were differentiated between patients who entered the DM state ≥ 3 months from adjuvant treatment initiation (who were assumed eligible for further anti-PD-1/PD-L1 treatment) vs. those who entered DM after < 3 months (who were assumed anti-PD-1/PD-L1-ineligible). Alternative time points (12 or 18 months from adjuvant treatment initiation) were explored in scenario analyses.
For each model arm, mean OS within the DM state was calculated as a market share-weighted average of mean OS associated with different first-line treatments of advanced melanoma. Mean OS was then converted into an exponential rate of DM → death in each arm. Mean PFS within the DM state was also calculated using a similar approach.
AEs
AE risks were considered for grade 3–5 all-cause AEs that affected ≥ 5.0% at any grade for pembrolizumab or placebo in KEYNOTE-716. Risks of diarrhea (grades 2 +) were also considered in the model based on the high expected cost of this AE even at grades 1–2. For each included AE, the mean duration (in weeks) per AE episode and mean number of episodes per affected patient were obtained from KEYNOTE-716, pooling across both treatment arms.
Quality of Life
Utility was linked to patients’ health state in each cycle. Health state utilities for RF (without toxicity), LR, and DM were estimated through linear mixed-effects regression of EQ-5D-5L data collected during KEYNOTE-716. The regression analyses were conducted using patient-visits in which both health state and EQ-5D-5L were assessed and included patient-level random effects. EQ-5D-5L index scores were calculated using the US algorithm [28].
Utility in the DM state was computed as a weighted average of the utilities associated with pre- and post-progression DM, based on the ratio of mean PFS to mean OS within the DM state (given the market shares of first-line treatments received in this state). The base case utility for pre-progression DM was estimated based on EQ-5D-5L data from KEYNOTE-716. The utility for post-progression DM was obtained from KEYNOTE-006, as the available follow-up in KEYNOTE-716 was too limited to capture average utility over the entire post-progression disease course until death.
AE-related disutility was applied as a one-time QALY decrement at model entry. This QALY decrement was calculated in each model arm as a function of treatment-specific AE risks, mean number of AE episodes per affected patient, mean duration of each AE episode, and disutility associated with an active grade 3 + AE, as estimated in the regression analysis of EQ-5D-5L data from KEYNOTE-716.
Costs
Drug costs for adjuvant pembrolizumab were calculated based on Wholesale Acquisition Cost (WAC) ($5237.04 per 100 mg) [29]. The relative dose intensity of pembrolizumab in KEYNOTE-716 was applied to account for potential dosage reductions. Unit costs of IV administration were obtained from the Centers for Medicare & Medicaid Services (CMS) Physician Fee Schedule [30]. The duration of adjuvant pembrolizumab treatment was based directly on the Kaplan-Meier curve for time to discontinuation in KEYNOTE-716.
Subsequent treatment in the LR state was expected to include one-time salvage surgery for a proportion of patients who enter this state. The percentages of patients undergoing different surgical procedures were calculated among patients who developed LR in KEYNOTE-716, pooling across both treatment arms. Unit costs of salvage surgeries were obtained from the CMS Hospital Outpatient Prospective Payment System [31].
Costs of subsequent adjuvant therapy for resected stage III melanoma were applied as a lump sum cost for a proportion of patients entering the LR state in each cycle. Costs of first- and second-line systemic therapies for advanced melanoma were similarly included as lump sum costs for patients entering the DM state. In each of these subsequent treatment lines, total costs of different treatment options were estimated over the duration of treatment using WAC, recommended dosing schedules, and (for IV-administered therapies) drug administration costs. For subsequent treatments in the LR state, mean ToT was estimated using exponential rates of discontinuation derived from reported ToT statistics in trials of adjuvant treatments for stage III melanoma (Table S2a). First-line treatment durations were approximated using exponential PFS distributions up to the label-recommended maximum if applicable (Table S2b). Second-line treatments in the DM state were assumed to be used for a mean duration of 21 weeks (or the label-recommended maximum, if < 21 weeks) (Table S2b). Market shares of subsequent treatments are shown in Table S1.
Per-episode AE costs were obtained from the Healthcare Cost and Utilization Project database [32]. AE costs were applied at model entry as a one-time cost, calculated as the sum-product of per-episode AE costs, AE risks in each arm, and mean number of AE episodes per affected patient.
Disease management costs were assigned to each health state based on literature and public sources. In the RF state, healthcare resource use included the recommended schedule of office visits and radiologic exams (i.e., MRIs, PET/CT scans, and nodal basin ultrasounds) for patients with stage IIB–IV no evidence of disease (NED), according to NCCN guidelines (v3.2022) in cutaneous melanoma [2]. Unit costs of these services were obtained from the CMS Physician Fee Schedule [30]. Disease management costs per week in LR were based on all-cause healthcare costs during LR as reported by Tarhini et al. [33], with an adjustment to avoid double-counting of salvage surgery costs. In each model arm, disease management costs per week in the DM state was computed as a weighted average of the disease management costs associated with pre- vs. post-progression DM based on the estimated proportion of time spent progression-free within the DM state. Weekly disease management costs for pre- and post-progression DM were based on the medical costs and non-melanoma-related pharmacy costs derived from Klink et al. [34] and Tarhini et al. [35].
The unit cost of a BRAF V600E test was assumed to be incurred at the time of patients' first disease recurrence (LR or DM, whichever occurs first) to guide the selection of subsequent treatments [36].
One-time terminal care costs during the month preceding death were considered [35]. In the base case, this cost was assumed to be incurred only by those who transition to death from the DM state, as most deaths that occur directly from the RF or LR states were expected to be attributable to causes other than melanoma. In a scenario analysis, terminal care costs were instead applied to all deaths.
Sensitivity Analyses
One-way deterministic sensitivity analyses (DSAs) were performed by individually varying parameters above and below their base case values. Scenario analyses were conducted to examine the impact of alternative assumptions on the model results. A probabilistic sensitivity analysis (PSA) was also conducted in which parameters were simultaneously varied according to specified distributions (Table S1). Where available, the variance or variance-covariance matrix of a distribution was obtained from the same source as the base case value.
Model Validation
Internal validations were performed by plotting modeled RFS and DMFS in each arm against observed Kaplan-Meier curves from KEYNOTE-716. Modeled versus observed cumulative incidence curves for RF → LR, RF → DM, and RF → death were similarly compared.
External validations were performed by comparing modeled survival in the observation arm against digitized Kaplan-Meier curves from several real-world studies, including: long-term RFS, DMFS, and OS observed in a real-world study of patients with resected stage IIB or IIC melanoma within US Oncology Network electronic health records; long-term RFS and/or OS from three published studies conducted in real-world cohorts of patients diagnosed with AJCC 8th edition stage IIB or IIC melanoma [37–39].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Base Case Results
Under base case distributional assumptions, modeled RFS and DMFS for the pembrolizumab and observation arms aligned with RFS and DMFS Kaplan–Meier curves during the available follow-up period of KEYNOTE-716 (Fig. 2A, B). Modeled and observed cumulative incidences of RF → LR, RF → DM, and RF → death were similarly well aligned in each arm (Figure S3).
Fig. 2.
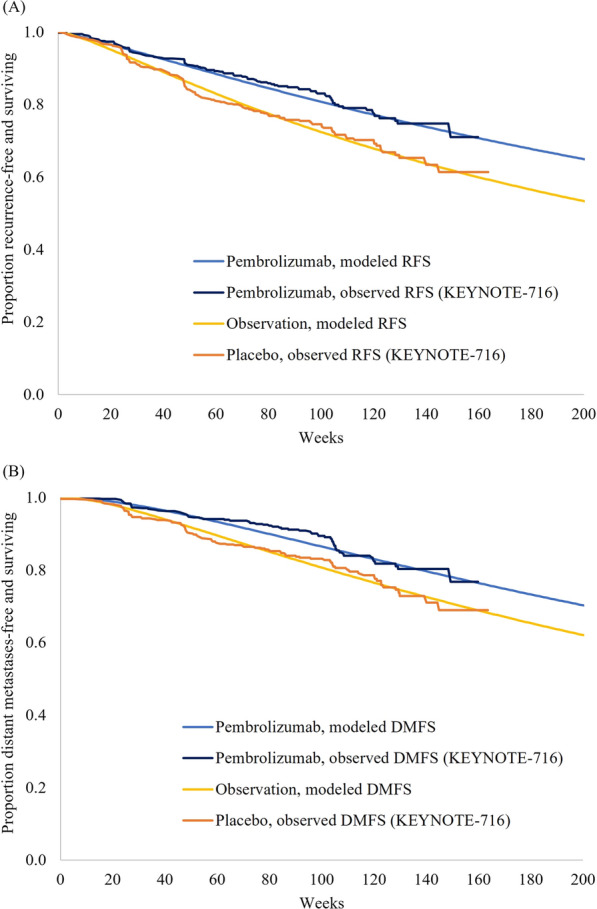
Modeled vs. observed (A) RFS and (B) DMFS in each arm. DMFS distant metastases-free survival, RFS recurrence-free survival
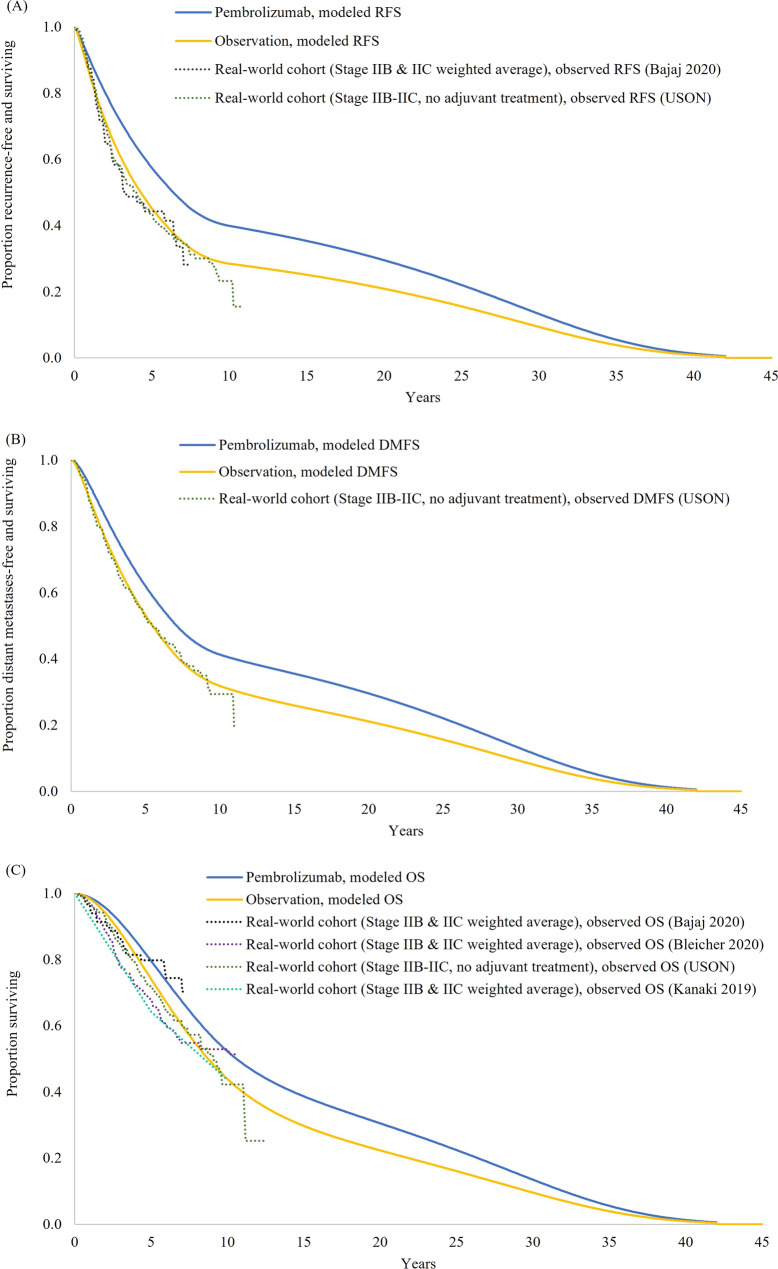
Longer term projections of RFS, DMFS, and OS in the observation arm were in line with external data from real-world cohort studies in stage IIB–IIC melanoma (Fig. 3A–C). Pembrolizumab was projected to extend RFS, DMFS, and OS relative to observation.
Fig. 3.
Long-term extrapolations of (A) RFS, (B) DMFS, and (C) OS, with validations against external studies. DMFS distant metastases-free survival, OS overall survival, RFS recurrence-free survival, USON US Oncology Network. With the exception of the real-world USON study, all of the external studies shown in the RFS and OS graphs separately reported these survival endpoints for stage IIB and IIC melanoma. Therefore, to allow for interpretable comparisons against modeled RFS and OS in the modeled target population, the stage IIB and IIC Kaplan-Meier curves from each study were pooled as a weighted average based on the percentage of patients with stage IIB melanoma in KEYNOTE-716 (i.e., 64.8%)
In the base case analysis (Table 2), total QALYs over a lifetime horizon were estimated to be 9.26 for pembrolizumab and 8.09 for observation. Total LYs were estimated as 10.35 years for pembrolizumab and 9.12 years for observation. The proportion of LYs spent in the RF state was 83.3% with pembrolizumab and 74.9% with observation. Fewer recurrences per patient were expected with pembrolizumab than observation in terms of both LR events (0.20 vs. 0.23; Δ = − 0.03) and DM events (0.54 vs. 0.66; Δ = − 0.12).
Table 2.
Base case cost-effectiveness results
| Outcomes | Pembrolizumab | Observation | Δ |
|---|---|---|---|
| Costs (2022 USD) | |||
| Total costs | 492,237 | 411,813 | 80,423 |
| Adjuvant treatment costs (RF state) | 142,264 | 0 | 142,264 |
| Salvage surgery and subsequent adjuvant treatment costs (LR state) | 10,476 | 22,371 | − 11,895 |
| Subsequent treatment costs (DM state) | 244,835 | 276,355 | − 31,521 |
| AE costs | 1513 | 841 | 672 |
| BRAF testing costs | 222 | 277 | − 55 |
| Disease management costs | 90,486 | 108,960 | − 18,474 |
| RF state | 8898 | 7887 | 1011 |
| LR state | 4662 | 8158 | − 3495 |
| DM state | 76,925 | 92,915 | − 15,990 |
| Terminal care costs | 2441 | 3008 | − 567 |
| Effectiveness | |||
| Total QALYs | 9.26 | 8.09 | 1.17 |
| RF state | 7.87 | 6.24 | 1.64 |
| LR state | 0.32 | 0.56 | − 0.24 |
| DM state | 1.07 | 1.29 | − 0.22 |
| AE-related disutility | − 0.0036 | − 0.0021 | − 0.0014 |
| Total LYs | 10.35 | 9.12 | 1.24 |
| RF state | 8.62 | 6.83 | 1.79 |
| LR state | 0.36 | 0.63 | − 0.27 |
| DM state | 1.37 | 1.65 | − 0.28 |
| Number of LR events per patient | 0.20 | 0.23 | − 0.03 |
| Number of DM events per patient | 0.54 | 0.66 | − 0.12 |
| ICERs (2022 USD) of pembrolizumab vs. observation | |||
| Incremental cost per QALY gained | – | – | 68,736 |
| Incremental cost per LY gained | – | – | 65,059 |
DM distant metastases, ICER incremental cost-effectiveness ratio, LR locoregional recurrence, LY life year, QALY quality-adjusted life year, RF recurrence-free, USD US dollars
Total lifetime costs were $492,237 for pembrolizumab and $411,813 for observation. Compared with observation, the upfront costs of adjuvant pembrolizumab treatment in year 1 were partly offset by reduced costs of subsequent treatment, LR- and DM-related disease management, and terminal care. The resulting incremental cost-effectiveness ratios (ICERs) of pembrolizumab versus observation were $68,736/QALY and $65,059/LY.
DSA and Scenario Analysis Results
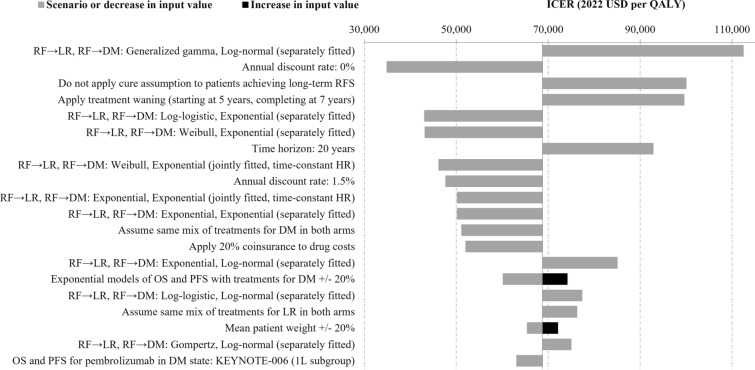
Across all one-way DSAs and scenario analyses, the ICER of pembrolizumab vs. observation ranged from $34,931/QALY to $112,422/QALY. The tornado diagram in Fig. 4 illustrates the 20 one-way sensitivity analyses with the largest impact on the ICER. Results from all DSAs and scenario analyses are included in Table S3.
Fig. 4.
Tornado diagram based on one-way DSAs and scenario analyses of adjuvant pembrolizumab versus observation. DM distant metastases, DSA deterministic sensitivity analysis, HR hazard ratio, ICER incremental cost-effectiveness ratio, LR locoregional recurrence, OS overall survival, PFS progression-free survival, QALY quality-adjusted life year, RF recurrence-free, USD US dollars
The ICER was most sensitive to the distributional assumptions affecting transition probabilities from the RF state as well as the annual discount rate, time horizon, efficacy, and market shares of subsequent treatments in the DM state and scenario that reduced drug costs by 20% to account for patient coinsurance. Other moderately influential parameters and assumptions included market shares of subsequent adjuvant treatments in LR, approach for estimating transition probabilities from LR, mean patient weight, and data source for pembrolizumab PFS and OS in DM state.
The results were not sensitive to high/low variation in drug administration costs, disease management costs, terminal care costs, health state utilities, or AE-related costs and disutilities.
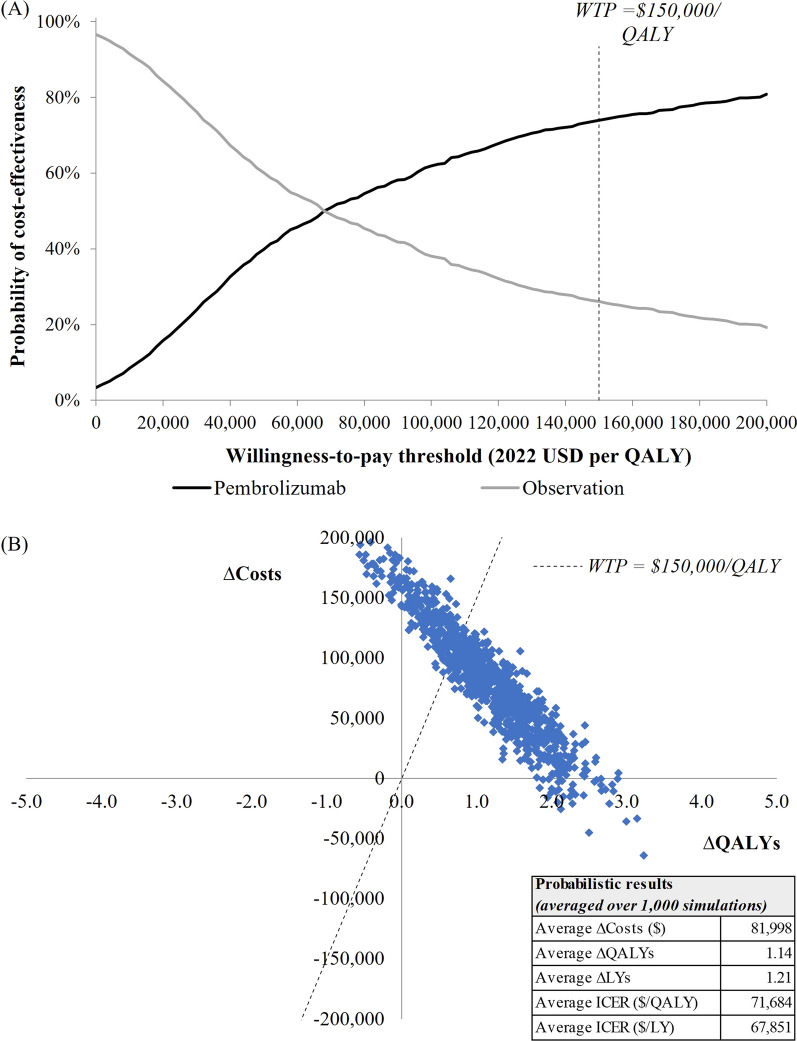
PSA Results
Based on 1000 PSA iterations, cost-effectiveness acceptability curves in Fig. 5a show the probability of each treatment being the more cost-effective strategy at different willingness-to-pay thresholds. At a $150,000/QALY threshold, pembrolizumab had a 73.9% probability of being cost-effective vs. observation. Probabilistic ICERs of pembrolizumab vs. observation (calculated as incremental costs averaged over 1000 iterations divided by incremental QALYs or LYs averaged over 1000 iterations) were $71,684/QALY and $67,851/LY (Fig. 5b), similar to the base case ICERs.
Fig. 5.
Results from 1000 probabilistic simulations: (A) cost-effectiveness acceptability curves; (B) scatterplot of incremental costs and effectiveness. LY life year, QALY quality-adjusted life year, USD United States dollars, WTP willingness-to-pay
Discussion
In this economic evaluation, adjuvant pembrolizumab therapy was expected to prolong QALYs and LYs relative to the strategy of observation alone in patients with resected stage IIB–IIC melanoma. Under base case assumptions, the incremental cost per QALY was $68,736, implying that pembrolizumab is cost-effective at the commonly cited willingness-to-pay threshold of $150,000/QALY. The ICER ranged from $34,931/QALY to $112,422/QALY in one-way DSAs and scenario analyses, with most variation observed when changing parameters that affect RFS predictions in each arm. In probabilistic simulations that considered uncertainty in the model parameters, pembrolizumab had a 73.9% probability of cost-effectiveness at the $150,000/QALY threshold.
The key transition probabilities driving the cost-effectiveness results are the three transitions starting from the RF state (i.e., RF → LR, RF → DM, and RF → death), which collectively determine RFS in each treatment arm. These transition probabilities were directly estimated using head-to-head comparative data from the randomized controlled KEYNOTE-716 trial, in which pembrolizumab has demonstrated a significant RFS benefit vs. placebo (HR = 0.64 as of the 04-Jan-2022 data cutoff date) [11]. There is strong published evidence supporting that an improvement in RFS, such as that observed in KEYNOTE-716, will translate into an OS benefit [40–43]. In particular, the EORTC 18071 trial in stage III melanoma found that the RFS and OS benefit of adjuvant treatment with an immune checkpoint inhibitor (ipilimumab) was sustained over the long term (median follow-up: 7 years) [40]. In a meta-analysis of 13 clinical studies (n > 5000 patients) involving adjuvant interferon for resected stage II–III melanoma, RFS was shown to be a good predictor and valid surrogate endpoint for OS [41]. Findings from this meta-analysis have since been supplemented by inclusion of data from EORTC 18071 which demonstrated that the association between RFS and OS is maintained when data specific to checkpoint inhibitors (in this case ipilimumab) in the resected stage III population are considered [42]. Most recently, a retrospective analysis of the National Cancer Database found that adjuvant immunotherapy was associated with significantly longer OS (HR = 0.66; p < 0.01) in patients with stage IIB–IIC melanoma [44].
At the time of model development, no cost-effectiveness analysis had been conducted to evaluate pembrolizumab as adjuvant treatment of resected stage IIB–IIC in the US. Several older cost-effectiveness studies, published between 1997 and 2000, evaluated interferon-based regimens versus placebo in a combined stage II–III melanoma population [45–47] but did not examine the stage II population separately. Prior economic evaluations have examined pembrolizumab as a treatment for advanced melanoma [48, 49] and as an adjuvant treatment of resected stage III melanoma [50, 51].
Consistent with methodologic guidance [14, 17], the selection of parametric functions to model transitions starting from the RF state was based on goodness of fit with observed trial data and validations of long-term survival predictions against external data. External validation results provide empirical support for the model’s long-term survival projections in the observation arm. Long-term RFS and DMFS predictions in the observation arm closely aligned with Kaplan-Meier curves from real-world cohorts with stage IIB or IIC melanoma; for example, at 7 years, modeled RFS was 35.0% compared with 33.6% in a US-based cohort study by Bajaj et al. (2020) or 35.0% in the US Oncology Network study. Long-term OS predictions in the observation arm were similar to observed OS data from the same two studies while being slightly high relative to two other external studies [38, 39]. This finding may reflect the advent of novel treatment options for advanced melanoma in recent years as the economic model accounts for the efficacy of first-line treatments in the DM state based on current market shares.
Other strengths of this study include the Markov cohort structure, a well-established modeling approach that has been commonly used in prior health technology appraisals of neoadjuvant/adjuvant cancer treatments. Given the 1-year maximum duration of adjuvant pembrolizumab, time on treatment with adjuvant pembrolizumab was precisely estimated based on observed Kaplan-Meier data from KEYNOTE-716 that did not require extrapolation. AE-related disutility and most health state utility inputs were directly obtained from the KEYNOTE-716 trial and were measured using a validated instrument (EQ-5D-5L).
Nevertheless, this study is subject to several limitations. Because OS was not included as part of the pre-specified third interim analyses of KEYNOTE-716, KEYNOTE-716 data were not used to model transition probabilities starting from the DM state. In the absence of such data, the model used evidence from clinical trials in the advanced melanoma setting to inform transition probabilities from DM to death. OS predictions from the model should be validated against OS results from KEYNOTE-716 as these data become available.
There is inherent uncertainty in extrapolating lifetime RFS based on data from the available follow-up period of a clinical trial. Therefore, multiple scenario analyses were undertaken using alternative distributional assumptions, including conservative scenarios that assumed a smaller incremental RFS benefit of pembrolizumab vs. observation than that implied by the base case parametric functions. Results of these scenario analyses supported the robustness of the base case ICER.
Due to limited follow-up of patients after recurrence in KEYNOTE-716 as of the current data cutoff date, trial-based estimates of utility in the DM state may not accurately reflect health-related quality of life during the entire period from DM until death. Consequently, the base case analysis used results from a separate trial in advanced melanoma (KEYNOTE-006) to inform utility in the post-progression DM state. Scenario analyses were also undertaken using several alternative sources for health state utilities and yielded similar results.
Conclusion
In conclusion, adjuvant pembrolizumab was projected to extend QALYs by 1.17 and LYs by 1.24 relative to its within-trial comparator observation (i.e., placebo) among patients with completely resected stage IIB–IIC melanoma. From a US health sector perspective, pembrolizumab was estimated to be cost-effective over a lifetime horizon compared with observation based on a common willingness-to-pay threshold. One-way and probabilistic sensitivity analyses supported the robustness of the cost-effectiveness conclusions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Funding for this study, the Rapid Service fee, and the Open Access fee was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Medical Writing, Editorial, and Other Assistance
The authors would like to acknowledge Vasiliki Kalampoki and Shahrul Mt-Isa, employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, for providing statistical analysis support for this study. Funding for medical writing was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author Contributions
All co-authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this study. Shujing Zhang and Ruifeng Xu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Shujing Zhang, Arielle G. Bensimon, and Ali Greatsinger. Acquisition and analysis of data: Shujing Zhang, Ruifeng Xu, and Arielle G. Bensimon. Interpretation of data: All authors. Drafting of the manuscript: Arielle G. Bensimon, Ali Greatsinger, and Adina Zhang. Critical revision of the manuscript for important intellectual content: All authors. All authors contributed to the article and approved the submitted version.
Prior Presentation
A summary of these results has previously been presented at the 19th International Congress of the Society for Melanoma Research, Edinburgh, UK, October 17–22, 2022.
Disclosures
Shujing Zhang, Ruifeng Xu, Ruixuan Jiang, Mizuho Fukunaga-Kalabis, and Clemens Krepler are full-time employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Arielle G. Bensimon, Ali Greatsinger, and Adina Zhang are employees of Analysis Group, Inc., which received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA for the conduct of this research.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.National Cancer Institute Surveillance Epidemiology and End Results Program. Cancer Stat Facts: Melanoma of the Skin, 2022. https://seer.cancer.gov/statfacts/html/melan.html.
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Melanoma: Cutaneous. Version 3.2022-April 11, 2022. https://www.nccn.org/professionals/physician_gls/PDF/melanoma.pdf.
- 3.Miller R, Walker S, Shui I, Brandtmuller A, Cadwell K, Scherrer E. Epidemiology and survival outcomes in stages II and III cutaneous melanoma: a systematic review. Melanoma Manage. 2020;7(1):MMT39. doi: 10.2217/mmt-2019-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garbe C, Keim U, Amaral T, et al. Prognosis of patients with primary melanoma stage I and II according to American Joint Committee on Cancer Version 8 Validated in two independent cohorts: implications for adjuvant treatment. J Clin Oncol. 2022;20:1. doi: 10.1200/JCO.22.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poklepovic AS, Luke JJ. Considering adjuvant therapy for stage II melanoma. Cancer. 2019;2020(126):1166–1174. doi: 10.1002/cncr.32585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ives NJ, Suciu S, Eggermont AMM, et al. Adjuvant interferon-alpha for the treatment of high-risk melanoma: an individual patient data meta-analysis. Eur J Cancer. 2017;82:171–183. doi: 10.1016/j.ejca.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Trinh VA, Zobniw C, Hwu WJ. The efficacy and safety of adjuvant interferon-alfa therapy in the evolving treatment landscape for resected high-risk melanoma. Expert Opin Drug Saf. 2017;16(8):933–940. doi: 10.1080/14740338.2017.1343301. [DOI] [PubMed] [Google Scholar]
- 9.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luke JJ, Rutkowski P, Queirolo P, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399(10336):1718–1729. doi: 10.1016/S0140-6736(22)00562-1. [DOI] [PubMed] [Google Scholar]
- 11.Long GV, Luke JJ, Khattak MA, et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2022;23(11):1378–1388. doi: 10.1016/S1470-2045(22)00559-9. [DOI] [PubMed] [Google Scholar]
- 12.Williams C, Lewsey JD, Briggs AH, Mackay DF. Cost-effectiveness analysis in R using a multi-state modeling survival analysis framework: a tutorial. Med Decis Mak. 2017;37(4):340–352. doi: 10.1177/0272989X16651869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams C, Lewsey JD, Mackay DF, Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses: a comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Med Decis Mak. 2017;37(4):427–439. doi: 10.1177/0272989X16670617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence. DSU Technical Support Document 19: partitioned survival analysis for decision modelling in health care: a critical review; 2017.
- 15.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 16.Arias E, Xu J. United States Life Tables, 2019. Natl Vital Stat Rep. 2022;70(19):1–59. [PubMed] [Google Scholar]
- 17.National Institute for Health and Care Excellence. DSU Technical Support Document 14: survival analysis for economic evaluations alongside clinical trials—extrapolation with patient-level data 2013. http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf. [PubMed]
- 18.Lee AY, droppelmann N, panageas ks, et al. Patterns and timing of initial relapse in pathologic stage II melanoma patients. Ann Surg Oncol. 2017;24(4):939–946. doi: 10.1245/s10434-016-5642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mooney MM, Kulas M, McKinley B, Michalek AM, Kraybill WG. Impact on survival by method of recurrence detection in stage I and II cutaneous melanoma. Ann Surg Oncol. 1998;5(1):54–63. doi: 10.1007/BF02303765. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann U, Szedlak M, Rittgen W, Jung EG, Schadendorf D. Primary staging and follow-up in melanoma patients–monocenter evaluation of methods, costs and patient survival. Br J Cancer. 2002;87(2):151–157. doi: 10.1038/sj.bjc.6600428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence. Pertuzumab for adjuvant treatment of HER2-positive early stage breast cancer [TA569] 2019. https://www.nice.org.uk/guidance/ta569.
- 22.National Institute for Health and Care Excellence. Trastuzumab emtansine for adjuvant treatment of HER2-positive early breast cancer [TA632] 2020. https://www.nice.org.uk/guidance/ta632.
- 23.National Institute for Health and Care Excellence. Osimertinib for adjuvant treatment of EGFR mutation-positive non-small-cell lung cancer after complete tumour resection [TA761] 2022. https://www.nice.org.uk/guidance/ta761.
- 24.National Institute for Health and Care Excellence. Nivolumab for adjuvant treatment of resected oesophageal or gastro-oesophageal junction cancer [TA746] 2021. https://www.nice.org.uk/guidance/ta746.
- 25.Canadian Agency for Drugs and Technologies in Health. CADTH Reimbursement Recommendation: Nivolumab (Opdivo) for the adjuvant treatment of completely resected esophageal or gastroesophageal junction cancer in patients who have residual pathologic disease following prior neoadjuvant chemoradiotherapy [PC0253] 2022. https://cadth.ca/sites/default/files/DRR/2022/PC0253%20Opdivo%20-%20CADTH%20Final%20Rec%20Final.pdf. [PubMed]
- 26.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390(10105):1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 27.Owen CN, Shoushtari AN, Chauhan D, et al. Management of early melanoma recurrence despite adjuvant anti-PD-1 antibody therapy. Ann Oncol. 2020;31(8):1075–1082. doi: 10.1016/j.annonc.2020.04.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickard AS, Law EH, Jiang R, et al. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22(8):931–941. doi: 10.1016/j.jval.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 29.AnalySource. First Databank drug pricing database. https://www.analysource.com/.
- 30.Centers for Medicare & Medicaid Services. Physician Fee Schedule 2022. https://www.cms.gov/medicare/physician-fee-schedule/search/license-agreement?destination=/medicare/physician-fee-schedule/search%3F.
- 31.Centers for Medicare & Medicaid Services. Hospital outpatient prospective payment system 2022. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS.
- 32.Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project: 2015 Hospital Inpatient National Statistics. http://www.hcup-us.ahrq.gov/.
- 33.Tarhini A, Ghate SR, Ionescu-Ittu R, et al. Postsurgical treatment landscape and economic burden of locoregional and distant recurrence in patients with operable nonmetastatic melanoma. Melanoma Res. 2018;28(6):618–628. doi: 10.1097/CMR.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klink AJ, Chmielowski B, Feinberg B, Ahsan S, Nero D, Liu FX. Health care resource utilization and costs in first-line treatments for patients with metastatic melanoma in the United States. J Manage Care Spec Pharm. 2019;25(8):869–877. doi: 10.18553/jmcp.2019.18442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarhini A, Corman SL, Rao S, et al. Healthcare resource utilization and associated costs in patients with advanced melanoma receiving first-line ipilimumab. J Cancer Ther. 2015;6:833–840. doi: 10.4236/jct.2015.610091. [DOI] [Google Scholar]
- 36.Dalal AA, Guerin A, Mutebi A, Culver KW. Economic analysis of BRAF gene mutation testing in real world practice using claims data: costs of single gene versus panel tests in patients with lung cancer. J Med Econ. 2018;21(7):649–655. doi: 10.1080/13696998.2018.1450261. [DOI] [PubMed] [Google Scholar]
- 37.Bajaj S, Donnelly D, Call M, et al. Melanoma prognosis: accuracy of the American Joint Committee on Cancer staging manual eighth edition. J Natl Cancer Inst. 2020;112(9):921–928. doi: 10.1093/jnci/djaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bleicher J, Swords DS, Mali ME, et al. Recurrence patterns in patients with Stage II melanoma: the evolving role of routine imaging for surveillance. J Surg Oncol. 2020;122(8):1770–1777. doi: 10.1002/jso.26214. [DOI] [PubMed] [Google Scholar]
- 39.Kanaki T, Stang A, Gutzmer R, et al. Impact of American Joint Committee on Cancer 8th edition classification on staging and survival of patients with melanoma. Eur J Cancer. 2019;119:18–29. doi: 10.1016/j.ejca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer. 2019;119:1–10. doi: 10.1016/j.ejca.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Suciu S, Eggermont AMM, Lorigan P, et al. Relapse-free survival as a surrogate for overall survival in the evaluation of stage II–III melanoma adjuvant therapy. J Natl Cancer Inst. 2018;110:1. doi: 10.1093/jnci/djx133. [DOI] [PubMed] [Google Scholar]
- 42.Coart E, Suciu S, Squifflet P, et al. Evaluating the potential of relapse-free survival as a surrogate for overall survival in the adjuvant therapy of melanoma with checkpoint inhibitors. Eur J Cancer. 2020;137:171–174. doi: 10.1016/j.ejca.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Koruth RM, Sharma R, Kanters S, Druyts E, Kirkwood JM, editors. Establishing the relationship between relapse-free survival and overall survival in adjuvant high-risk radically resected cutaneous melanoma. The Society for Melanoma Research, Fifteenth International Congress; 2018; Manchester, England.
- 44.Wong WG, Perez Holguin RA, Stahl KA, Olecki EJ, Pameijer C, Shen C. Utilization and survival benefit of adjuvant immunotherapy in resected high-risk stage II melanoma. Surg Pract Sci. 2022;8:25. [Google Scholar]
- 45.Hillner BE, et al. Economic analysis of adjuvant interferon alfa-2b in high-risk melanoma based on projections from Eastern Cooperative Oncology Group 1684. J Clin Oncol. 1997;15(6):2351–2358. doi: 10.1200/JCO.1997.15.6.2351. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Larriba JL, et al. Cost-effectiveness analysis of interferon as adjuvant therapy in high-risk melanoma patients in Spain. Eur J Cancer. 2000;36(18):2344–2352. doi: 10.1016/S0959-8049(00)00304-X. [DOI] [PubMed] [Google Scholar]
- 47.Messori A, Becagli P, Trippoli S, Tendi E. A retrospective cost-effectiveness analysis of interferon as adjuvant therapy in high-risk resected cutaneous melanoma. Eur J Cancer. 1997;33(9):1373–1379. doi: 10.1016/S0959-8049(96)00413-3. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Chmielowski B, Pellissier J, Xu R, Stevinson K, Liu FX. Cost-effectiveness of pembrolizumab versus ipilimumab in ipilimumab-naive patients with advanced melanoma in the United States. J Manage Care Spec Pharm. 2017;23(2):184–194. doi: 10.18553/jmcp.2017.23.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miguel LS, Lopes FV, Pinheiro B, et al. Cost effectiveness of pembrolizumab for advanced melanoma treatment in Portugal. Value Health. 2017;20(8):1065–1073. doi: 10.1016/j.jval.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Bensimon AG, Zhou ZY, Jenkins M, et al. Cost-effectiveness of pembrolizumab for the adjuvant treatment of resected high-risk stage III melanoma in the United States. J Med Econ. 2019;22(10):981–993. doi: 10.1080/13696998.2019.1609485. [DOI] [PubMed] [Google Scholar]
- 51.Bensimon AG, Zhou ZY, Jenkins M, et al. An Economic evaluation of pembrolizumab versus other adjuvant treatment strategies for resected high-risk stage III melanoma in the USA. Clin Drug Investig. 2020;40(7):629–643. doi: 10.1007/s40261-020-00922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.