Abstract
Human adolescence is characterized by a suite of changes in decision-making and emotional regulation that promote risky and impulsive behavior. Accumulating evidence suggests that behavioral and physiological shifts seen in human adolescence are shared by some primates, yet it is unclear if the same cognitive mechanisms are recruited. We examined developmental changes in risky choice, inter-temporal choice, and emotional responses to decision outcomes in chimpanzees, our closest-living relatives. We found that adolescent chimpanzees were more risk-seeking than adults, as in humans. However, chimpanzees showed no developmental change in inter-temporal choice, unlike humans, although younger chimpanzees did exhibit elevated emotional reactivity to waiting compared to adults. Comparisons of cortisol and testosterone indicated robust age-related variation in these biomarkers, and patterns of individual differences in choices, emotional reactivity, and hormones also supported a developmental dissociation between risk and choice impulsivity. These results shows that some but not all core features of human adolescent decision-making are shared with chimpanzees.
Keywords: comparative cognition, decision-making, impulsivity, adolescence, evolution
Introduction
Human adolescence is a uniquely fraught developmental period. This distinct phase of human life, between childhood and adulthood, involves both striking changes in physiological growth and maturation as well as transitions to independence and maturity in cognition and behavior (Sawyer, Azzopardi, Wickremarathne, & Patton, 2018). It is also associated with a striking increase in both morbidity and mortality in industrialized countries—negative health outcomes that are driven, in large part, by a spike in high-risk behaviors including substance use, reckless driving, violence, and risky sexual behavior (Reyna & Farley, 2006; Steinberg, 2015). In turn, the onset of risk-taking behavior in this period accompanies profound changes in brain circuitry and cognitive capacities that support decision-making, cognitive control, and emotion regulation (Crone & Steinbeis, 2017; Durston et al., 2002; Hare et al., 2008; Steinberg, 2005), changes that are driven in part by dramatic shifts in sex steroid production (Schulz & Sisk, 2016; Vigil et al., 2016). Understanding the mechanisms that drive risky and impulsive behavior across the lifespan has significant implications for public health, and is thus a major current focus of attention across the brain and behavioral sciences.
Developmental changes in brain systems underlying value-based decision-making are emerging as phenomena of particular importance for understanding adolescent risk-taking and impulsivity (Blakemore & Robbins, 2012; Hartley & Sommerville, 2015; Paulsen, Platt, Huetell, & Brannon, 2011; Rosenbaum & Hartley, 2018; Steinberg, 2007). For example, there is continuous improvement in the ability to forgo tempting immediate rewards in favor of larger delayed ones (i.e., delay of gratification) from childhood through adulthood (Green, Myerson, & Ostaszewski, 1999; Scheres et al., 2006). Likewise, choices made under conditions of risk (i.e., when decision outcomes are probabilistic) also show major shifts in this period. There are two major theories of age-related change in risk preferences that make contrasting predictions: fuzzy trace theory predicts linear changes (a monotonic pattern of declining risk preference), whereas dual process models predict nonlinear changes (a nonmonotonic pattern that peaks in adolescence) (Defoe & Romer, 2022; Reyna & Ellis, 1994; Reyna & Farley, 2006). Results from meta-analyses indicate that risk preference show linear declines from childhood to adulthood (Defoe, Dubas, Figner, & Van Aken, 2015), although adolescents can show greater risk seeking behavior than younger children in some contexts in line with non-linear changes (e.g., Burnett, Bault, Coricelli, & Blakemore, 2010; Paulsen, et al., 2011; van den Bos & Hertwig, 2017).
Adolescents further show reduced capacities for emotional regulation compared to adults (Hare, et al., 2008; Silvers et al., 2012). As emotional processes are an important component of human decision-making (Lerner, Li, Valdesolo, & Kassam, 2015; Loewenstein & Lerner, 2001), shifts in adolescents’ emotional reactions to their choices may shape the development of emerging choice preferences (Burnett, Bault, Coricelli, & Blakemore, 2010). Finally, these shifts in brain, cognition, and behavior are in part due to rapidly changing physiology: the steroid hormones testosterone and cortisol increase sharply across adolescence (Khairullah et al., 2014; Kiess et al., 1995; Shirtcliff, Dahl, & Pollak, 2009; Wudy, Hartmann, & Remer, 2007) and are associated with structural and functional changes in fronto-striatal and cortico-limbic circuitry linked to value-based decision-making and emotion regulation (Herting et al., 2014; Nguyen et al., 2012; Perrin et al., 2008; Wierenga et al., 2018). Accordingly, levels of testosterone predict pubertal changes in decision-making, including increased risk-taking and impulsivity (Cardoos et al., 2017; Duke, Balzer, & Steinbeck, 2014; Laube, Lorenz, & van den Bos, 2020; Vermeersch, T’Sjoen, Kaufman, & Vincke, 2008). Cortisol also can impact risky and inter-temporal choice (Herbert, 2018; Kandasamy et al., 2014; Takagishi, 2004). In addition, cortisol can moderate the effects of testosterone on decision-making (Mehta, Welker, Zilioli, & Carre, 2015), indicating that looking at both hormones in tandem can be important to understand choice behavior.
While our understanding of the cognitive mechanisms that shape impulsivity and risk-taking behavior in human adolescence is coming into increasing focus, the evolutionary origins of this phenomenon remain unclear. Some proposals argue that adolescence as a distinct, extended life history phase is an exclusively human adaptation (Bogin, 1999; Bogin & Smith, 1996). Relative to other animals, our species has a slow developmental trajectory that is accompanied by a degree of cognitive and behavioral skill acquisition that far exceeds even other primates (Kaplan, Hill, Lancaster, & Hurtado, 2000; Leigh, 2004; Robson & Wood, 2008). The neurodevelopmental, psychological and accompanying behavioral changes exhibited during this period may facilitate an adaptive shift from a dependence on caregivers that characterizes childhood, to the independence required of adulthood. Understanding the biological basis and evolutionary significance of human adolescence requires disentangling the components of this developmental stage that are shared with other species versus those specific to humans.
Several pieces of evidence suggest that other apes, particularly chimpanzees (Pan troglodytes), may share many of the developmental processes central to human adolescence. First, nonhuman apes exhibit substantially slower life histories than in other primates, including a longer period of development (Hamada, Udono, Teramoto, & Sugawara, 1996; Wood, Watts, Mitani, & Langergraber, 2017). For example, chimpanzees typically do not reach social and physical maturity until 15 years in the wild (Emery Thompson & Sabbi, in press), unlike other commonly-studied primates like macaques who may reach maturity by four to six years of age. Second, subadult chimpanzees have an extended period characterized by gradual shifts in social behavior that mirror those seen in the human adolescent period. This includes increased time away from their mother, increased contact with other adults, and a shift from play to aggression (Enigk, Emery Thomson, Machanda, Wrangham, & Muller, 2020; Pusey, 1990; Reddy & Mitani, 2020; Sandel, Langergraber, & Mitani, 2020). Third, chimpanzees share many changes seen in human adolescence in terms of physical and physiological maturation, such as increases in testosterone and cortisol (Behringer, Hohmann, Stevens, Weltring, & Deschner, 2012; Enigk, et al., 2020; Sabbi et al., 2020; Wobber, Hare, Lipson, Wrangham, & Ellison, 2013). Of note, some of these changes are specific to humans and other apes amongst primates more generally. While human adolescence starts with the maturation of the adrenal gland, most nonhuman primates do not exhibit an identifiable pre-pubertal rise in adrenal hormones—with the exception of nonhuman apes, who also share this feature (Behringer, et al., 2012; Campell, 2006; Conley, Bernstein, & Nguyen, 2012; Sabbi, et al., 2020). Finally, as in humans, postnatal brain development is extended in other apes relative to other primates (Leigh, 2012; Teffler et al., 2013). For example, chimpanzees exhibit slower rates of white matter maturation and longer periods of synaptogenesis compared to macaques (Bianchi et al., 2013; Sakai et al., 2011).
Despite mounting evidence that chimpanzees share some of the biological and behavioral aspects human adolescence, it is currently unclear if they also exhibit the shifts in cognition that are central to human adolescence. This is crucial for determining whether and how developmental changes in cognitive mechanisms are related to broader shifts in behavior and physiology, as well disentangling shared versus novel features of human adolescence. We therefore studied cognitive development in chimpanzees to understand the origins of human-like adolescent psychology. To date, there have been very few studies of cognitive development in nonhuman primates, likely due to the difficulty in assessing samples large enough to capture age-related differences (Bjorklund & Green, 1992; Gomez, 2005; Matsuzawa, 2007; Rosati, Wobber, Hughes, & Santos, 2014). The little prior work that does exist focuses on early social development (Matsuzawa, Tomonaga, & Tanaka, 2006; Tomasello, Hare, & Fogleman, 2001; Wobber, Herrmann, Hare, Wrangham, & Tomasello, 2014). The later period of cognitive development comprising adolescence, as well as change in decision-making more generally, has been relatively unstudied in nonhuman primates.
Here, we examined patterns of decision-making, emotional reactivity, and hormonal development in our closet living relative, chimpanzees. We experimentally assessed sensitivity to probability and delay costs using two cognitive tasks in 40 semi-free-ranging chimpanzees. Our sample size, larger than prior work on ape decision-making, comprised a distribution of ages ranging from late juvenility through adolescence and young adulthood, a period when wild chimpanzees show marked increases in cortisol, testosterone, as well as other relevant hormones like dehydroepiandrosterone sulfate in tandem with critical shifts in the development of social behavior (Enigk, et al., 2020; Pusey, 1990; Sabbi, et al., 2020).
We hypothesized that adolescent chimpanzees would, like humans, show increased choice impulsivity and increased risk-taking compared to adults. Given the specific age range we examined here (e.g., not including a sample of younger juveniles), we predicted that these changes would be linear decreases in risk-seeking and choice impulsivity. Second, we assessed emotional reactions in these tasks to test if chimpanzees show developmental changes in emotional reactivity in decision-making contexts. We predicted that younger chimpanzees would show stronger emotional reactions than adults, paralleling the increases in emotional regulation seen in human development. Third, we assessed baseline (i.e., pre-task) levels of salivary cortisol and testosterone in these semi free-ranging chimpanzees to confirm that, as in the wild, both hormones increase through this developmental transition. Importantly, testosterone and cortisol correspond reliably with physical indicators of puberty stage in humans; even for girls, who exhibit lower testosterone production than boys, testosterone is a more reliable index of relative pubertal development than other ovarian steroids (e.g., estradiol), due to their cyclic variation (Khairullah, et al., 2014; Kiess, et al., 1995; Shirtcliff, et al., 2009; Wudy, et al., 2007). Given that cognitive changes in human adolescence are linked to pubertal hormonal shifts, we sought to determine whether steroid concentrations predicted task outcomes. Finally, as both risk-seeking and impulsive choice preferences are sometimes thought to reflect distinct facets of a general behavioral domain of disinhibition (Dalley & Robbins, 2017; Lopez-Guzman, Konova, & Glimcher, 2018), we examined inter-individual differences in patterns of choice, emotional reactions, and physiology across individuals.
Methods
We tested 40 semi-free-ranging chimpanzees ranging from juvenility to young adulthood in a cross-sectional design. This sample size, exceeding those used in prior comparable work on chimpanzee decision-making, allowed us to examine developmental changes in responses. We examined performance on two tasks assessing core aspects of value-based decision-making: a risky choice task and an inter-temporal choice task. In both, we assessed choice preferences as well as emotional responses to decision outcomes. Finally, we collected voluntary saliva samples from each chimpanzee to index individual differences in levels of cortisol and testosterone over this age range.
Ethics statement
Behavioral tests and saliva collection procedures were approved by University of Michigan and Harvard University’s Institutional Animal Care and Use Committees, as well as by Republic of Congo’s Ministry of Scientific Research. Biological samples were exported with permits from the Convention on International Trade in Endangered Species from Republic of Congo and the United States. All work adhered to guidelines from Jane Goodall Institute-Congo and the Pan African Sanctuary Alliance.
Participants
We tested 40 semi-free-ranging, wild-born chimpanzees from Tchimpounga Chimpanzee Sanctuary in Republic of Congo (21 males and 19 females; mean age 15 years; range 6 to 25 years). Apes living in African sanctuaries are typically wild-born and arrive at the sanctuary between 1–3 years of age. These chimpanzees spent most of their time in large forest enclosures in species-appropriate social groups, and prior work shows normal patterns of cognition, behavior, and physiology in this population (Cole et al., 2020; Rosati et al., 2013; Wobber & Hare, 2011). All apes were socially housed, and the majority free-range in large tracts of tropical forest during the day (5–40 hectares across groups). In the evening, apes voluntarily enter indoor dormitories (12 m2-160 m2) to sleep and receive supplemental feedings; apes were tested individually in these familiar dormitory buildings and then were released back to their larger social groups. Apes had ad libitum access to water and were never food-deprived for testing. In addition to the food in the forest, they were fed a variety of fruits, vegetables, and species-appropriate foods. All tests were voluntary: if subjects stopped participating, the test was stopped.
General procedures
We analyzed two tasks from a decision-making battery assessing several components of cognition and cooperation in chimpanzees (see also Cantwell, Buckholtz, Atencia, & Rosati, 2022; Rosati, DiNicola, & Buckholtz, 2018). We specifically examined the two tasks assessing value-based decision-making: risky choice and inter-temporal choice. Each task was completed on a different day of the battery and comprised an approximately 30 min testing session. In sessions, apes sat across from a human experimenter at a table with a sliding top (80 cm wide, 40 cm deep, 50 cm tall), separated by bars or mesh of their familiar dormitory walls. The experimenter placed relevant options on the tabletop within view of the ape, and then pushed the table forward so chimpanzees could indicate their choice by pointing or touching one option by protruding their fingers. The experimenter looked down or along the midline of the table during the choice phase in order to avoid any potential social cueing.
Risky choice task
In this task, we examined chimpanzees’ responses to variation in reward payoffs using methods from prior studies (Rosati & Hare, 2011, 2012, 2013; Rosati & Hare, 2016). Here, apes made decisions between a safe option which reliably provided an intermediately-preferred food type (three peanuts), and a risky option which provided either a highly-preferred food (banana slice) or a non-preferred food (cucumber slice) with equal likelihood (see Figure 1a). Chimpanzees first completed a food preference pretest on an initial day of the battery, with 18 trials (involving 6 trials which each possible food type pairing) to ensure they exhibited appropriate preferences. Chimpanzees’ preferences in the food preference test showed that they exhibited appropriate choices (e.g., preferred the high-value food over the intermediate food, and the intermediate food over the non-preferred food). In the main session, they completed 10 exposure trials (only one option available at a time) to introduce the rewards and probability contingences. They finally completed 20 test trials, where they made choices between the risky and safe options. Thus, in this task chimpanzees faced a choice between taking a reliable option or gambling on receiving a more-preferred option.
Figure 1: Diagram of the risky and inter-temporal choice tasks.
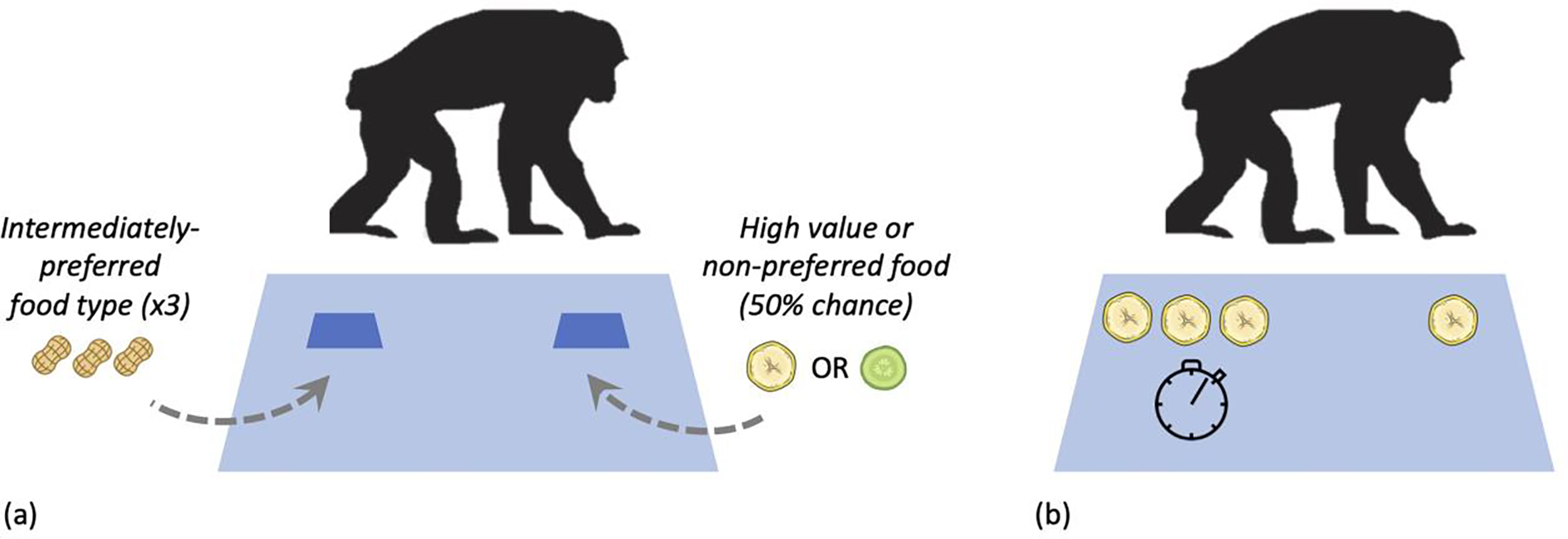
(a) In the risky choice task, chimpanzees chose between a safe option that reliably provided three pieces of an intermediately-preferred food (peanuts), and a risky option that provided either a preferred food (a slice of banana) or a non-preferred food (a slice of cucumber) with 50% probability. While the safe food option was baited under an overturned container while the chimpanzee watched (and thus the knew what was there with certainty), the risky reward was baited behind an occluder such that they did not know which of the two possible food items had been placed under the container. (b) In the inter-temporal choice task, chimpanzees chose between a larger, delayed reward (three banana slices available after a minute) and a smaller immediate reward (one slice). In both tasks, rewards were placed on a table in front of the chimpanzee, who could indicate their preference by pointing at one of the options. Side assignment of options was counterbalanced across trials in both tasks.
On each test trial, apes watched the experimenter place the safe food reward (peanuts) on the table and cover it with a bowl. Then she placed an identical but empty bowl on the other side of the table, and then covered that bowl with an occluder. Next the experimenter showed the ape the ‘risk outcome container’ (a bowl with both the good and bad risk outcomes in it), and behind the occluder placed just one of the items so the chimpanzee does not know which outcome they receive. Finally, the experimenter touched both the risk and safe option bowls, while lifting the safe bowl to remind the chimpanzee of its contents, and then pushed the table forward for choice (see supplement Figure S1 for photographs of these steps). The procedure for exposure trials was identical, but one only of the two options (risk or safe) was used. The side assignment of the risky and safe options is counterbalanced and quasi-randomized (no more than 3 trials in a row with the same assignment) across trials, and there was a fixed 20s inter-trial interval (ITI) between trials, starting when the chimpanzee placed the last piece of food in their mouth. Prior work has shown that chimpanzees exhibit ceiling-level performance on a variety of comprehension controls in this task (Rosati & Hare, 2011, 2012, 2013; Rosati & Hare, 2016), but in the context of this battery we implemented the standard exposure trials without the full complement of control trials.
Inter-temporal choice task
Apes made decisions between a smaller option (one banana slice) available immediately, and a larger option (three banana slices) available after a one-minute delay (see Figure 1b), following prior studies (Rosati & Hare, 2013; Warneken & Rosati, 2015) (see Rosati, et al., 2018 for prior analyses of this data). Here, in the earlier warmup session chimpanzees first completed a number pretest with four trials (involving a different food type and no delays) to ensure they could discriminate these quantities and preferred the larger amount when there were no time costs imposed. In the main session, they completed 8 exposure trials (only one option available at a time) to introduce the rewards and delay contingencies. They finally completed 14 test trials, where they made choices between the smaller, immediate and larger, delayed rewards. Thus, this task measured chimpanzee’s willingness to forgo an immediate reward in favor of waiting for a larger reward.
In trials, the experimenter sat across from the chimpanzee at the sliding table and placed the rewards visibly on the table (always baiting the left side followed by right) and then pushed the table forward for the chimpanzee to choose. If they selected the smaller, immediate reward this was provided immediately, whereas if they chose the larger, delayed reward the experimenter removed the forgone option and waited the one-minute delay before the chimpanzee could access it (see supplement Figure S2 for photograph). In exposure trials, only one option was available. The side assignment of the immediate and delayed options was counterbalanced and quasi-randomized (no more than 3 trials in a row with the same assignment) across trials. Given that animals are generally insensitive to post-reward delays in inter-temporal choice tasks (Hayden, 2016), we implemented a fixed 20s ITI starting when the chimpanzee placed the last piece of food in their mouth.
Hormone sampling and assays
Concurrent with the period of behavioral data collection, we collected 3–8 saliva samples from each individual, across different days, to assess individual variation in cortisol and testosterone. Samples were always taken prior to any cognitive testing that day in the same time period (9:30–11AM) to account for any circadian changes in hormones. Samples were collected voluntarily by swabbing the chimpanzee’s mouth with a cotton pad and expressing the saliva, following procedures in previous work in this population (Wobber & Hare, 2011; Wobber et al., 2010). Samples were stored in the field at room temperature using sodium azide. Once returned to the US, the samples were immediately frozen and later analyzed using standard radioimmunoassay procedures for cortisol and testosterone (see supplement for detailed assay procedures). During the assays, some samples could only be assays for one hormone due to insufficient sample volume, and some assays were excluded based on high inter-assay coefficient of variation following standard procedures (see supplemental methods), so number of analyzed samples per hormone varied as reported below.
Data coding
Choices were coded live by the experimenter for both tasks, and a second coder (blind to the study’s hypotheses) coded 100% of sessions from videotape for choice reliability and emotional responses. The primary experimenter coded 20% of sessions from video for reliability on emotion metrics. In particular, we coded three signatures of emotional responses in the chimpanzees following prior work looking at emotional responses to decision-making in primates (De Petrillo, Tonachella, & Addessi, 2017; Rosati & Hare, 2013; Sánchez-Amaro, Tan, Kaufhold, Fernández-Navarro, & Rossano, 2021): (1) producing negative vocalizations specifically pout moans, whimpers, or screams; (2) scratching, a sign of stress in primates; and (3) banging the mesh, e.g., throwing a tantrum. As in prior work, the chimpanzee was then assigned an ‘affect score’ ranging from 0–3 on each trial, based on how many of these different reactions they exhibited on that trial. In the risky choice task, we assessed these responses in the 10s after the choice outcome was revealed, and in the inter-temporal choice task we assess these reactions in the 10s after the chimpanzee’s choice (as the options were directly visible). In the risk task, we further assessed if apes attempted to switch their choice by pointing at the alterative option immediately following the reveal of the outcome of their chosen option as in the prior work (note that this switching response was not possible in the inter-temporal choice task, as both outcomes were inherently visible at time animals made their choice). There was high reliability in the risky choice task for choices (100% agreement; Cohen’s Kappa Κ = 1.0), negative vocalizations (98.8% agreement; Κ = 0.94), scratching (97.1% agreement, Κ = 0.91), banging (99.6% agreement; Κ = 0.89), and switching (99.4% agreement; Κ = 0.97). There was also excellent reliability for these measures in the inter-temporal choice task for choices (100% agreement; Κ = 1.0), negative vocalizations (98.2% agreement; Κ = 0.96), scratching (97.7% agreement; Κ = 0.95), and banging (99.5% agreement; Κ = 0.94).
Statistical analyses
We first analyzed performance in the individual cognitive tasks and physiology measures to examine age-related change in each metric. We analyzed cognitive task data using mixed models implemented in the lme4 package (Bates, 2010) in R version 4.2.1 (R Development Core Team, 2022) to account for trial-by-trial responses. We compared model fit using likelihood ratio tests (Bolker et al., 2008). Post-hoc comparisons of factors were performed with the emmeans package (Lenth, 2018) using Tukey corrections. Figures depicting model output were created using the effects package (Fox, 2003). In some cases, we also report t-test comparisons of individuals’ mean performance, which are all two-sided.
We modeled choices as binomial outcomes using the glmer function; we used the same approach for binary emotional metrics (e.g., switching). We analyzed the affect scores from the decision tasks using the lmer function. All models included random subject intercepts to account for within-subject repeated measures; included sex a factor; and included trial number as a continuous predictor. As relevant, the base model included additional control variables (like food preference score in the risk task). We then added age in years as a continuous predictor to examine developmental change. Additional checks and some figures depict age cohorts in which we contrasted a younger cohort (less than 15 years; n = 18) with an adult cohort (15 years and up; n = 22) a common cutoff of adulthood in the wild.
Analysis of developmental changes in hormones took a similar approach, but accounted for positive skew in values by implementing GLMMs with a gamma distribution and a log link (equivalent of log-transforming values). As with cognitive task data, we then examined how age and sex predicted values. Since the collection of these samples from all subjects were constrained to a 1.5 hour time period each day, we did not model time of day in these analyses.
Finally, to examine relationships between decision-making, emotional responses, and hormone levels across individuals, we conducted exploratory analyses to examine whether performance on a given measure predicts other. We first used pairwise bivariate Pearson correlations between tasks by using the corr package (Kuhn, Jackson, & Cimentada, 2020). We then implemented a principal component analysis to detect whether performance in different tasks co-varies across individuals using the prcomp function. These analyses related task performance as well as an individual’s mean log-transformed hormone values.
Data and materials accessibility
Data and analysis scripts for this study are available at Dryad Digital Repository and can be accessed at https://doi.org/10.5061/dryad.kwh70rz7d (Rosati, Emery Thompson, Atencia, & Buckholtz, 2022). Research materials consist of task demonstration procedures; detailed diagrams of the tasks and setup are depicted in Figure 1 and supplemental Figures S1 and S2. This study was not preregistered.
Results
Risky choice task
We first examined chimpanzees’ preferences for risk (Figure 2a, b). An initial pretest confirmed appropriate preferences for the food options, strongly preferring the high-value food (e.g., the good risk outcome) over both the intermediate food (e.g., the safe outcome) and non-preferred food type (e.g., the bad risk outcome), and further strongly preferring the intermediate over the non-preferred type (see supplement). There was also no difference across ago cohorts in terms of quantitative choices for these options [high versus low: all chimpanzees choose the high-valued food 100% of trials; high versus intermediate: t38 = 1.37, p > 0.17, n.s., Cohen’s D = 0.43; intermediate versus low: t38 = −1.39, p > 0.17, n.s., Cohen’s D = 0.43]. This indicates that there were no major differences in how younger and older chimpanzees intrinsically valued the food types used in the task. In contrast, in the main task, younger chimpanzees chose the risky option on mean = 56.1 ± SE = 8.1% of trials, whereas adults chose it 36.8 ± 5.2% [t38 = 2.07, p = 0.046; Cohen’s D = 0.66].
Figure 2: Choice patterns and emotional responses in the risk task.
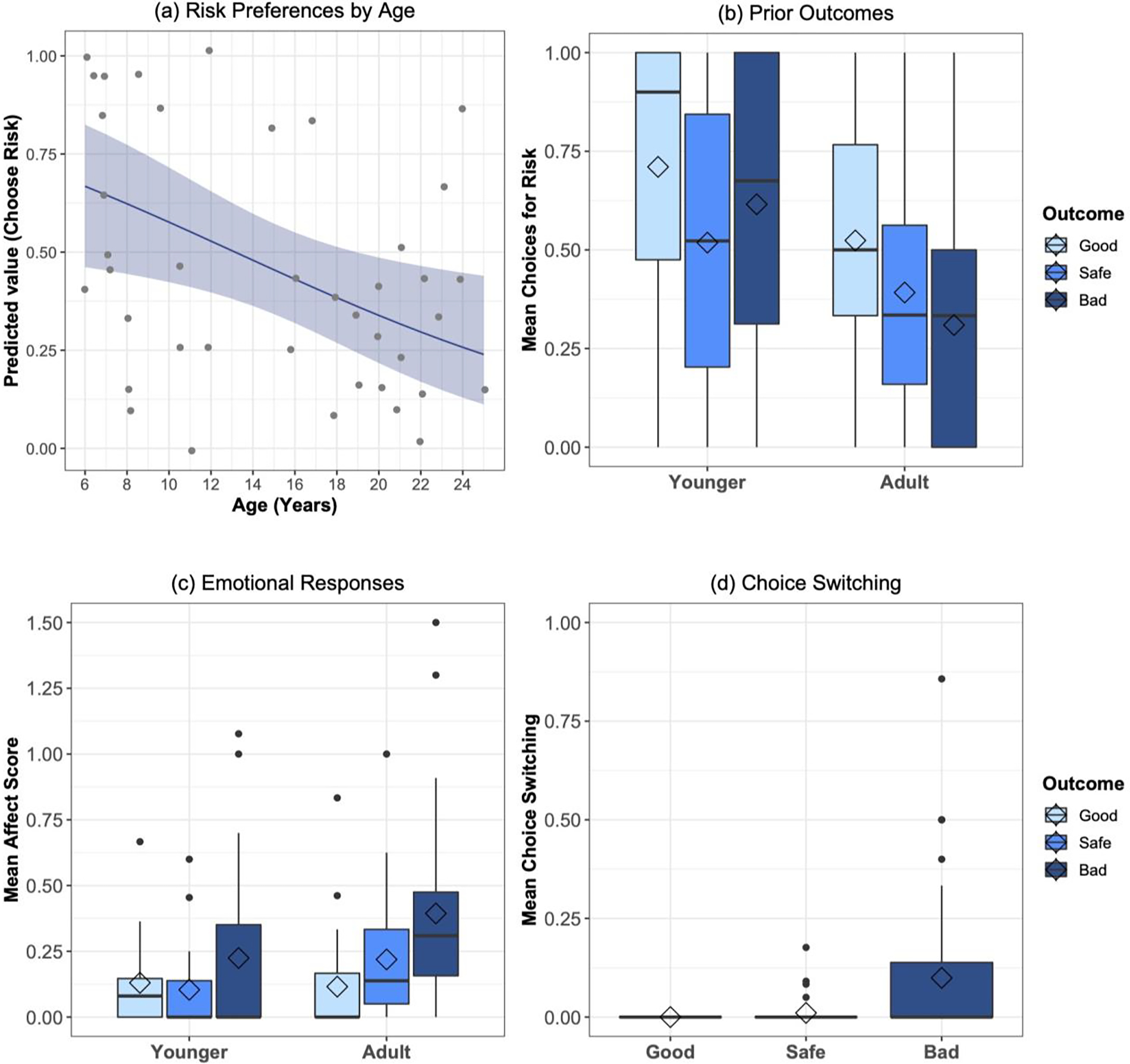
(a) Younger chimpanzees showed stronger preferences for risk than adults; ribbon indicates 95% CI from GLMM model estimates of trial-by-trial data accounting for age, sex, trial number, food preference score, and prior reward outcome; scatter plot indicates individuals’ mean proportion choice for the risky option. (b) Younger and adult chimpanzees showed weak proclivities to adjust their choice behavior in response to prior decision outcomes. (c) Both younger and older chimpanzees showed more negative affective responses to bad risk outcomes compared to good risk or safe outcomes. (d) Chimpanzees of all ages showed more attempts to switch their choice in response to bad risk outcomes. Boxplot hinges indicate the lower and upper quartile, the horizontal line represents the median, diamonds indicate the mean, and whiskers indicate the minimum and maximum range of the analyzed data. Outliers are plotted as individual points.
Our primary analysis of risk task choices then modeled binary choices for risk using GLMMs accounting for subject, sex, trial number; and each individual’s preference score (derived from their performance in an initial food preference test involving the food rewards used in the task, to account for any individual variation in overall food preferences; see supplement). In the second model we added previous outcome (good, bad or safe) to assess if chimpanzees adjusted choices trial-by-trial in response to reward outcomes. This trended to improve fit [χ2 = 5.82, df = 1, p = 0.055]; post hoc tests indicated that chimpanzees trended to choose the risky option more after they choose the risk option and received a good outcome compared to when they received a bad outcome [p = 0.065], aligning with prior evidence indicating that chimpanzees do not show major trial-by-trial adjustments in response to prior outcomes (Rosati & Hare, 2013) To test if adolescent chimpanzees were more risk-seeking, we then added age (as a continuous predictor) which further improved model fit compared to the second model [χ2 = 5.71, df = 1, p = 0.017; see Table S1 for parameter estimates]: younger chimpanzees were more risk seeking. We found the same basic result when comparing adding cohort [χ2 = 3.97, df = 1, p = 0.046]. To finally test if adolescent versus adult chimpanzees show different rewards to previous outcomes, we then included the age X previous outcome interaction in a final model, which did not improve model fit compared to the age-only model [χ2 = 3.56, df = 2, p > 0.16; see Table S1 for parameters from this full model]. Overall, this shows that younger chimpanzees are less sensitive to probabilistic costs (i.e., more risk-taking) than adults.
We then examined patterns of affective responses to different outcomes (Figure 2c). To do so, we calculated a ‘negative affect score’ following prior work (Rosati & Hare, 2013), indexed the presence of negative emotional responses after chimpanzees observed the outcome of their choice (e.g., after the safe, good risk outcome, or bad risk outcome was revealed). Overall, chimpanzees exhibited higher affect scores after bad outcomes (mean score = 0.32± 0.06) compared to good or safe outcomes (0.12 ± 0.03 and 0.17 ± 0.04, respectively). We here used responses on both exposure and test trials since chimpanzees produced emotional responses in both, as well as to ensure we had measured responses to all possible trial outcomes (e.g., as some chimpanzees rarely or never freely choice one option in the test trials). We first constructed a base LMM accounting for subject, sex, trial type (test or exposure trial), and trial number. Replicating prior work, inclusion of trial outcome improved fit [χ2 = 56.2, df = 2, p < 0.0001; see Table S2 for parameter estimates]; post-hoc test indicated chimpanzees showed more negative responses specifically following bad risk outcomes [p < 0.0001 for significant comparisons]. However, neither the inclusion of age [χ2 = 0.35, df = 1, p > 0.55, n.s.] or an age X trial outcome interaction [χ2 = 4.63, df = 3, p > 0.20, n.s.] further improved fit compared to the second model. Thus, chimpanzees across this age range showed similar responses to bad outcomes.
We similarly examined patterns of switching behavior, where chimpanzees attempted to change their choice after observing the outcome, which is conceptualized as a ‘regret-like’ response (Rosati & Hare, 2013; Santos & Rosati, 2015). We here looked only at test trials, as it is not possible to produce a switching response on exposure trials where only one option was available. Overall, chimpanzees tried to switch their responses on 9.9 ± 3.1% of test trials after bad risk outcomes, never did so following good risk outcomes, and on only 1.1 ± 0.5% after safe outcomes; since switching responses were rare for both good and safe outcomes, we collapsed these in analyses. Using GLMMs to model the presence of a switch attempt, we found that chimpanzees attempted to switch their choice more often following bad outcomes compared to good or safe outcomes [χ2 = 35.71, df = 1, p < 0.0001; see Figure 2d, and Table S3 for parameter estimates]. However, neither the inclusion of age [χ2 = 0.19, df = 1, p > 0.65, n.s.] nor an age X trial outcome [χ2 = 1.82, df = 2, p > 0.40, n.s.] further improved fit compared to the second model. Together with the affect score comparisons, these results show that chimpanzees showed stronger emotional responses to bad risk outcomes as in prior work (Rosati & Hare, 2013), and further finds similar responses in both younger and adult chimpanzees.
Inter-temporal choice task
We next examined chimpanzees’ ability to delay gratification in the inter-temporal choice task. First, we confirmed that chimpanzees were sensitive to the delays imposed in the task by comparing their choices in the main test trials to choices the number preference pre-test where there were no delays imposed (see also results from Rosati, et al., 2018). Overall, chimpanzees choose the larger reward on 80.0 ± 3.3% of trials in the number pretest, but only on 65.2 ± 2.7% of trials in the choice task, a significant difference [t38 = 3.95, p < 0.0003, Cohen’s D = 0.62; see Figure 3a]. Importantly, there was no difference in how younger and older chimpanzees responded in the number pretest [t38 = 1.33, p = 0.19, Cohen’s D = 0.42], indicating that there were not major age differences in how they intrinsically valued the reward quantities used. Indeed, both younger [t17 = 2.97, p = 0.009, Cohen’s D = 0.70] and adult [t21 = 2.59, p = 0.017, Cohen’s D = 0.55] distinguished these trials. We further accounted for any potential differences in number discrimination abilities across individuals in the main analyses.
Figure 3: Choices and emotional responses in the inter-temporal choice task.
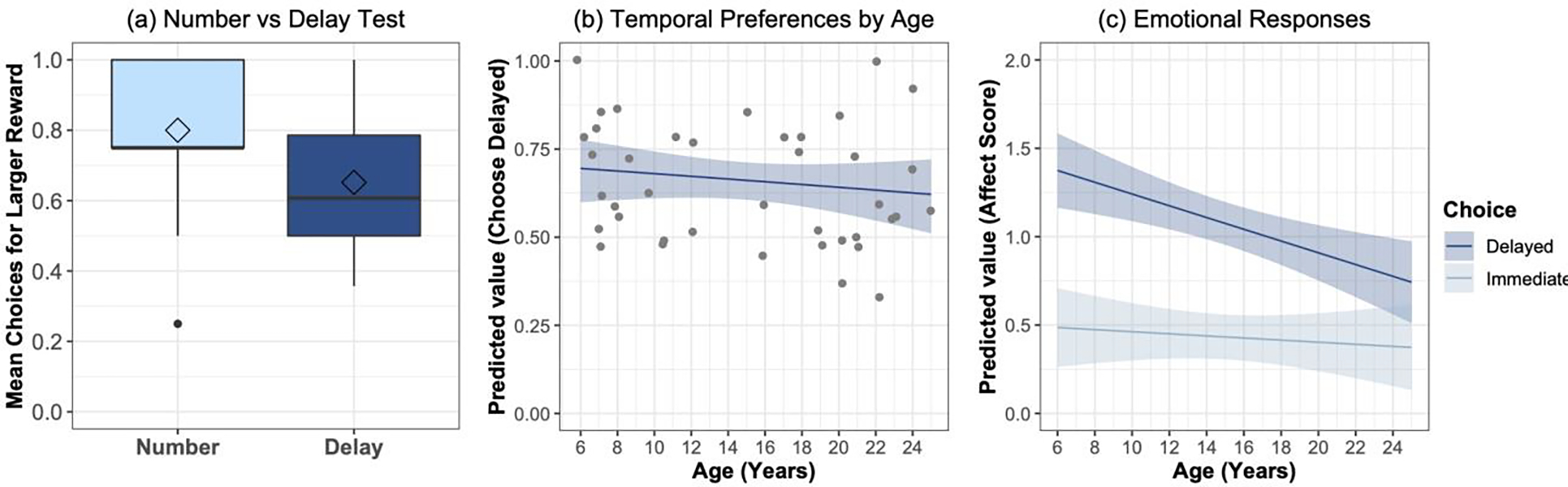
(a) Chimpanzees chose the larger reward more in the number pretest without delays, than in the main inter-temporal choice task where the larger reward was delayed. (b) Younger and older chimpanzees showed similar preferences for the larger, delayed reward in the inter-temporal choice task; ribbon indicates 95% CI from GLMM model estimates of trial-by-trial data accounting for age, sex, trial number, and number pretest performance; scatter plot indicates individuals’ mean proportion choice for the delayed option. (c) Emotional responses to immediate rewards were similar regardless of age, but younger chimpanzees showed more intense negative responses to waiting for delayed rewards; ribbons indicate 95% CI from LMM model estimates accounting for choice outcome (immediate or delayed), age, sex, trial number, trial type (exposure or choice trial).
We next examined developmental changes in chimpanzees’ preferences in the main intertemporal choice task. Overall, younger chimpanzees chose the delayed option on 67.9 ± 3.6% of test trials, and adults chose it on 63.0 ± 3.9%, not statistically different [t38 = 0.90, p > 0.37, n.s., Cohen’s D = 0.29; see Figure 3b]. In GLMMs accounting for subject, sex, trial number, and that individual’s number pretest performance (e.g., accounting for individual’s number discrimination capacities), inclusion of age also did not improve fit [χ2 = 0.75, df = 1, p > 0.38, n.s.; see Table S4 for parameter estimates]. We found the same basic result when comparing across cohort (as a categorical predictor) [χ2 = 0.40, df = 1, p > 0.52, n.s]. Overall, this indicates that both younger and older chimpanzees showed delay sensitivity during cost-benefit decision-making.
We then examined patterns of affective responses to waiting (see Figure 3c) using the negative affect score, here measured after the chimpanzees made their choice as in prior work (Rosati & Hare, 2013). Chimpanzees overall showed more negative affect to waiting (mean score = 1.10 ± 0.08) than choosing the immediate reward (mean score = 0.40 ± 0.05). We first constructed a base LMM model accounting for subject, sex, trial type (test or exposure), and trial number. Inclusion of choice (immediate versus delayed option) improved fit [χ2 = 193.5, df = 1, p < 0.0001]; chimpanzees showed more negative responses towards waiting for the delayed option compared to choosing the immediate option, as expected. The inclusion of age [χ2 = 5.61, df = 1, p < 0.05] and the age X choice interaction both improved fit [χ2 = 15.93, df = 3, p < 0.0001; see Table S5 for parameter estimates]. Post-hoc comparisons indicated that while younger and older chimpanzees had similar baseline levels of affect when they choose the immediate reward, younger chimpanzees exhibited elevated negative affect scores compared to adults they choose the delayed reward [p < 0.0001 for differences in age slopes]. Thus, despite showing a similar capacity to delay gratification compared to adults, younger chimpanzees showed more intense aversive reactions to waiting.
Physiological changes in chimpanzee adolescence
We next examined changes in cortisol and testosterone, two hormones that shift dramatically both in humans and in apes in this period, to confirm that our sample of chimpanzees showed patterns of change expected from prior work. Analyzing 169 samples assayed for cortisol revealed positive correlation between a chimpanzees’ age and their mean logged-transformed cortisol value [rp = 0.79, n = 40, p < 0.0001]. GLMMs accounting for repeated measurements and sex similarly showed that inclusion of age improved fit [χ2 = 27.89, df = 1, p < 0.0001; see Figure 4a]. There were similar age-related differences in males and females, as the interaction between age X sex did not further improve fit [χ2 = 1.01, df = 1, p > 0.31, n.s.; see Table S6 for model parameters]. We found similar results when using age cohorts rather than continuous age.
Figure 4: Physiological changes over development.
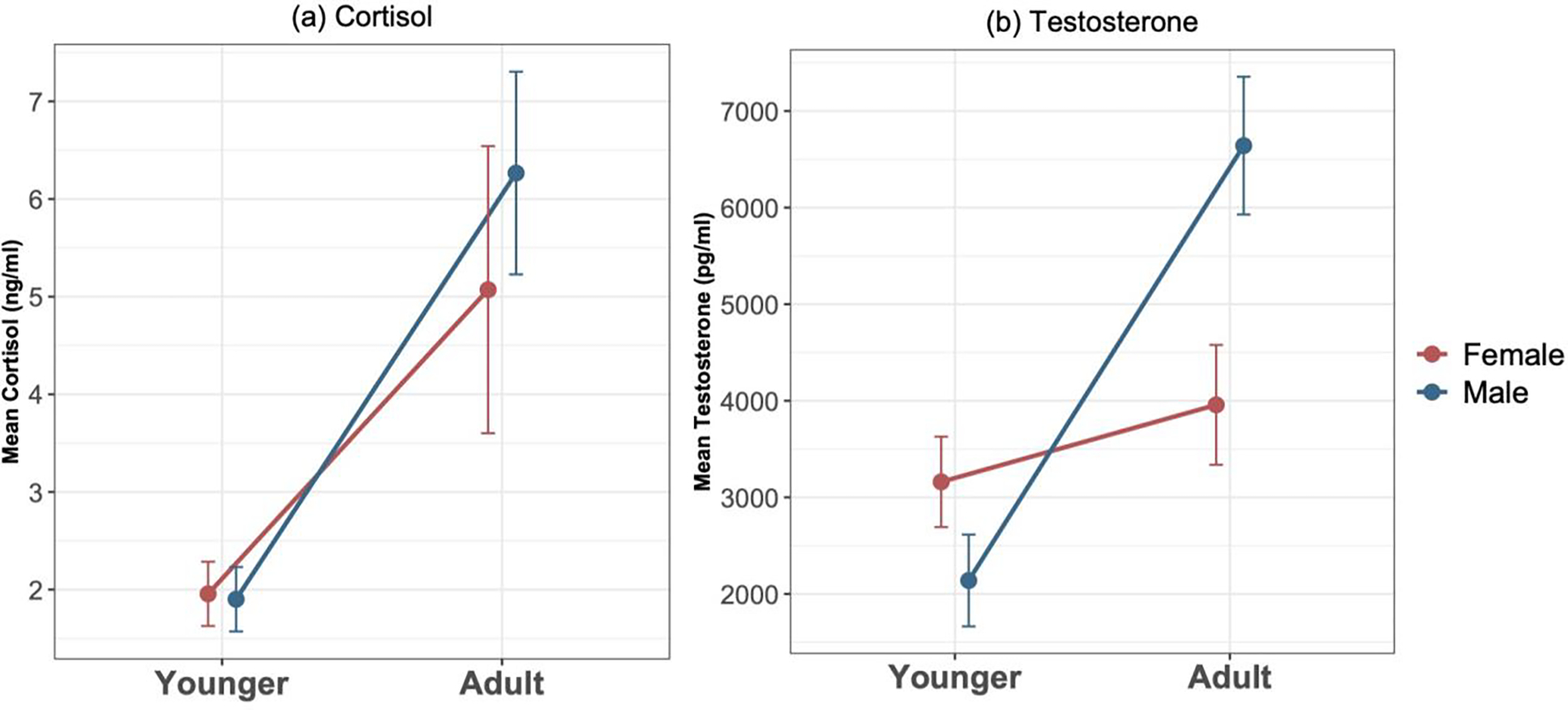
(a) Chimpanzees of both sexes showed increasing cortisol levels over the sampled age range. (b) Chimpanzees showed increasing testosterone levels over this age range, a shift exacerbated in males. Error bars indicate SE.
Analyses of 160 samples assayed for testosterone similarly showed a positive relationship between an individual’s age and their mean log-transformed testosterone value [rp = 0.62, n = 40, p < 0.0001]. Using GLMMs, we found that inclusion of age improved fit compared to a base model only accounting for sex [χ2 = 9.88, df = 1, p = 0.0017]. There was also a trend for the interaction between age X sex to further improve fit [χ2 = 3.73, df = 1, p = 0.054; see Figure 4b and Table S7 for model parameters], and post-hoc comparisons of slope indicated a greater increase with age in males [p < 0.05]. We found similar results when using age cohorts, with the main difference that the cohort X sex interaction reached significance [χ2 = 5.91, df = 1, p = 0.015]. Importantly, salivary measures like those used here tend to underestimate the difference in circulating testosterone between males and females (Khan-Dawood, Choe, & Dawood, 1984), so these patterns of sex differences are in line with this. Overall, this confirmed that our sample exhibited appropriate physiological signatures of development, aligning with prior work from wild and free-ranging chimpanzees (Behringer, et al., 2012; Sabbi, et al., 2020; Wobber, et al., 2013; Wobber, et al., 2010).
Individual differences in choices, emotions, and hormones
In our final set of analyses, we examined patterns of covariation in physiological, affective and behavioral responses across individuals. Our first question was whether senstivity to probability and delay costs covaried in this sample. To assess this, we looked at the correlation between time and risk preferences, and found no relationship across the entire sample [rp = 0.16, p = 0.33, n.s.]. We further examined the possibility that the relationship between risk-taking and choice impulsivity depends on developmental stage, which would align with the earlier results indicating age-related changes for risk but not time preferences [e.g., correlation with risk preference and age: rp = −0.36, p = 0.021; correlation with time preference and age: rp = −0.17, p = 0.28, n.s.]. In particular, we tested if age cohort moderated the relationship between sensitivity to probability and delay during decision-making. In fact, it did [χ2 = 4.59, df = 1, p = 0.032]; post-hoc tests indicated a stronger relationship between risk and time preferences in in adults than in adolescents [p < 0.05 for comparison of slopes across these cohorts]. This suggests that there is an important developmental dissociation between these facets of cognition in chimpanzees.
We also looked more generally at relationships between choices, emotional responses, and physiology (see Figure 5a and Table S8). First, bivariate correlations between these measures and age revealed that risk preferences, the inter-temporal choice affect score, and the hormone measures were correlated with age, aligning with trial-by-trial analyses reported above. Second, relationships with physiology measures revealed that testosterone was positively correlated with the risk affect score [rp = 0.37, p = 0.017] but not other behavioral measures; this relationship held when age was also accounted for (see supplement for details). Cortisol, in contrast, was negatively related to inter-temporal choice affect score [rp = −0.35, p = 0.028], and there was a trend for a negative relationship with risk-taking [rp = −0.30, p = 0.057]. Together, these findings suggest that higher cortisol is associated attenuated negative affect when faced with delay costs, whereas higher testosterone is linked to potentiated negative affect when making risky choices.
Figure 5: Relationships between cognitive, affective, and hormonal measures.
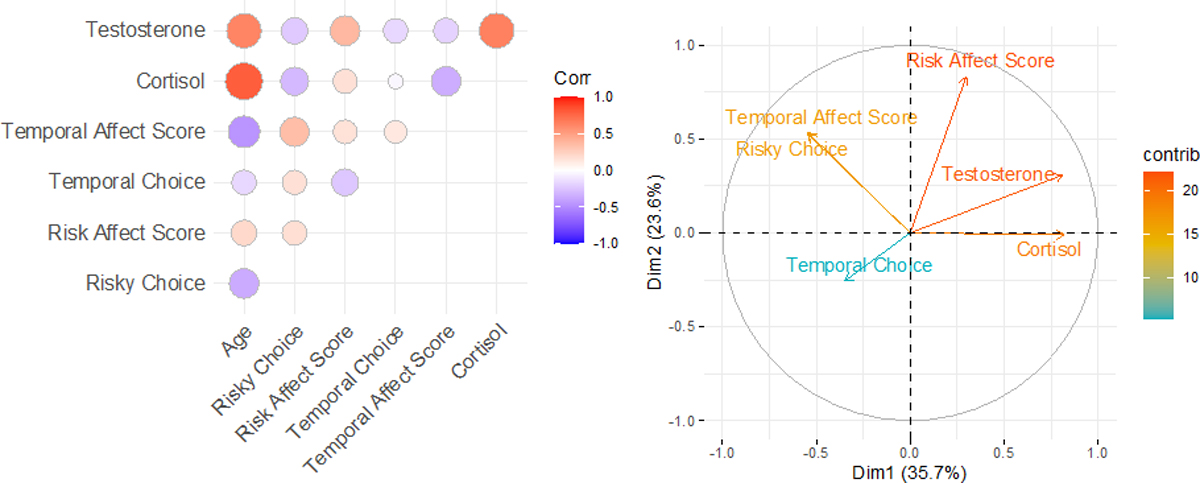
(a) Pairwise correlations between choice measures (mean risky choice, mean inter-temporal choice), emotional measures (mean risk affect score, mean inter-temporal affect score), physiological measures (mean log-transformed cortisol, mean log-transformed testosterone), and age; strength of correlation is indicated by color on plot. (b) Contribution of each of the measures to the two distinct dimensions extracted from the principal components analysis.
As a complimentary analysis aimed at assessing patterns of responses more holistically across all measured variables, and then to test how age and sex impacts these responses, we then implemented a principal component analysis (PCA) using the summary scores of each individuals’ responses across the choice, emotion, and hormonal measures (see Figure 5b). We first assessed the adequacy of our correlation matrix by implementing a Bartlett’s test for sphericity [χ26 = 44.61, p < 0.0001], which indicated that the correlations between measures were sufficient for PCA. The main analysis yielded two principal components (with adjusted eigenvalue >1: PC1 = 1.58 and PC2 = 1.15); parallel analysis confirmed retention of both components (see Figure 4b). The first principal component explained 35.7% of the variance; testosterone and cortisol loaded positively (> |0.3|) whereas risk-taking behavior and the inter-temporal choice affect score had negative contributions to this score. The second component explained 23.6% of the variance; risk, risk affect score, and inter-temporal choice affect score contributed positively to this dimension (see Table S9).
To understand developmental change, we then used linear regressions to compare these summary scores by age and sex. We found that dimension 1 captured age-related variation: inclusion of age in the model improved fit [χ2 = 41.34, df = 1, p < 0.0001], whereas the additional inclusion of sex [χ2 = 0.25, df = 1, p = 0.62, n.s.] or age X sex [χ2 = 2.30, df = 2, p = 0.32, n.s.] did not further improve fit. In contrast, dimension 2 captured sex differences: inclusion of age in the model did not improve fit [χ2 = 0.04, df = 1, p = 0.83, n.s.], whereas the additional inclusion of sex did [χ2 = 6.19, df = 1, p = 0.012]; there was no addition improvement from including age X sex [χ2 = 0.02, df = 2, p = 0.87, n.s.]. Overall, this supports the conclusion that risk-taking, emotional reactions to waiting, and hormones changed in tandem with age during this developmental period as indicated by the age-related changes in dimension 1, whereas other measures were dissociated in development.
Discussion
We examined patterns of value-based decision-making, affective responses, and physiology in a large sample of chimpanzees spanning the adolescent and early adult period. We first examined patterns of risky and inter-temporal choice in a series of confirmatory analyses, and found that adolescent chimpanzees exhibited more risk-taking behavior, but not more impulsive choice behavior, compared to adults. However, despite a mature capacity to delay gratification, younger chimpanzees demonstrated more intense negative responses when they chose to wait for a delayed reward than did adults. Assessments of age-related differences in cortisol and testosterone further confirmed that these chimpanzees exhibited expected endocrinological signatures of adolescence, in line with prior work with this species from captivity and the wild. Finally, exploratory correlations between measures and a principal components analysis supported the observed behavioral dissociation between risk-taking and impulsivity, as well as our conclusion that age and hormone levels selectively influence distinct and dissociable aspects of value-based decision-making and associated affective responding. Taken together, these results show that some, but not all, core psychological features of human adolescence are shared with chimpanzees.
Our findings extend emerging evidence that several aspects of physiological and behavioral development are shared between humans and nonhuman apes to disentangle shared underlying cognitive mechanisms for these changes. During their life history period that is analogous to human adolescence, chimpanzees show rapid changes in sex and stress hormone levels, start forming new bonds with peers, show characteristic increases in aggression, and begin to overtly compete for social status (Enigk, et al., 2020; Pusey, 1990; Reddy & Mitani, 2020; Sabbi et al., 2021; Sabbi, et al., 2020; Sandel, et al., 2020). These changes are particularly noteworthy because similar shifts are thought to contribute to elevated risk-taking and impulsive behavior during the human adolescent period. The present work shows that chimpanzee adolescence is likewise characterized by increased risk-taking relative to adulthood. However, unlike human adolescents, chimpanzees do not appear to show a more impulsive pattern of choice behavior. Importantly, our correlational analyses and principle component analysis provide comparative developmental support for the notion that value-based decisions involving probabilistic and delay costs are driven by distinct underlying cognitive mechanisms (Lopez-Guzman, et al., 2018), and that this dissociation is conserved in our closest living evolutionary relative.
We further found that while hormones did change dramatically with age in this sample of chimpanzees, neither testosterone nor cortisol were strongly related to an individual’s propensities for risk-taking or impulsive choice behavior. In fact, there is also mixed evidence concerning the relationships between hormones and economic choice in both adolescent and adult humans (Laube, et al., 2020; Mehta, et al., 2015; Peper, Cédric, Koolschijn, & Crone, 2013; Stanton et al., 2011). However, there were stronger relations between hormone levels and affective reactivity: testosterone was positively related to magnitude of aversive responses to realized risky choice outcomes, while cortisol was negatively related to emotional reactions to waiting after choosing the delayed reward. Some work does link increased testosterone to greater emotional reactivity to poor risk outcomes in adult men (Wu et al., 2018), partially in line with these results. Notably, there are similar relationships between hormones, behavior, and age in wild chimpanzees: while chimpanzees exhibit a striking increase in testosterone during adolescence that can predict patterns of aggression, testosterone does not predict aggression when age is simultaneously accounted for (Enigk, et al., 2020). This highlights that when developmental trajectories are so strongly correlated, it is difficult to disentangle causal relationships between age, physiology, and behavioral patterns. Taken together, this suggests that further study of how hormones may shape decision-making in chimpanzees is needed.
Our work also suggests several avenues for future work looking at the adolescence development of cognitive and emotional traits in nonhuman apes. First, one important question concerns patterns of cognitive development in males versus females. Developing chimpanzees show important sex differences in many relevant behaviors, such as emerging increases in male aggression (Sabbi, et al., 2021), yet we did not find major differences by sex in the cognitive traits measured here, mirroring prior work on primate value-based decision-making (De Petrillo & Rosati, 2021) (but see Cantwell, et al., 2022 for sex differences in chimpanzees in other cognitive domains). However, one important question is whether sample size limitations preclude the detection of subtle sex biases, or whether these sex biases are specific to particular relevant socioecological contexts (such as aggression). A related question concerns the influence of social context on these choice preferences. In humans, adolescent risk-seeking behavior is exacerbated especially in emotionally-charged or ‘hot’ contexts involving peers. Social context might also be important for chimpanzees—and especially male chimpanzees, who face challenges associated with building new relationships and acquiring dominance status during adolescence that are attenuated in less-social females (Enigk, et al., 2020; Rosati et al., 2020; Sabbi, et al., 2021; Sandel, et al., 2020). Indeed, competitive interactions drive anticipatory increases in testosterone as well as increases in economic risk-taking in chimpanzees (Rosati & Hare, 2012; Wobber, et al., 2010), so one future question is whether this is social context effect is also exacerbated in adolescent chimpanzees.
Finally, an important question concerns the continuity of chimpanzee adolescence with earlier periods of cognitive development. For example, there are alternative theories about the patterns of developmental change in human risk-taking. In meta-analyses, adolescents do not show a characteristic peak compared to both adults and younger children (i.e., no evidence for a quadratic change with age; Defoe, et al., 2015; Paulsen, et al., 2011; van den Bos & Hertwig, 2017). However, other theories predict that this change is linear from childhood to adulthood when accounting for task confounds (Reyna et al., 2011; Reyna & Farley, 2006). Our work suggests a linear change in chimpanzees in the age range we examined, but did not include the younger sample of apes needed to best adjudicate these views. Conversely, while the development of delay of gratification in humans follows a linear pattern (Green, et al., 1999; Scheres, et al., 2006), we did not detect any age-related changes in chimpanzees. One possibility is that their development in this domain proceeds at a faster pace than in humans, and even adult chimpanzees show greater impulsivity than do adult humans. An alternative approach could be to examine time preferences using a task designed to infer individual discount rates (Blanchard, Pearson, & Hayden, 2013; Hayden, 2016), such as a titration procedure identifying ‘indifference points’. Some evidence indicates adult chimpanzees are more patient than expected according to rate maximization models when tested in this more complex procedure (Rosati, Stevens, Hare, & Hauser, 2007), so this approach may be more sensitive to detect developmental change as well.
The current study has several implications for understanding human cognitive development in biological context. First, these results extend emerging data that chimpanzees and humans show many homologous changes in behavior during their long developmental period. Our results provide novel evidence that these homologies also extend to some of the cognitive and affective processes that shape value-based decision-making in humans. Importantly, while adolescence per se may not be uniquely-human, our finding that chimpanzees—unlike humans—do not exhibit developmental changes in impulsive choice behavior suggests that some aspects of human adolescence may be specific to our species. Finally, our findings highlight how comparative studies can be used to understand both the structure of cognition and its evolution. Prior work suggests that risk-taking and impulsive choice are subserved by distinct facets of cognition, and our data provides evidence for evolutionary conservation of this dissociation across species. Overall, this highlights the how looking at extended processes of development in long-lived apes is crucial for understanding unique features of human cognition.
Supplementary Material
Acknowledgments
We thank Lauren DiNicola for assistance with coding. At Tchimpounga, we thank Debby Cox, the chimpanzee caretakers, Jane Goodall Institute USA, and the Congolese Ministry of Research for supporting out work. This research was supported by NSF grant 1944881, NSF grant 1926653, NIH grant R01AG049395, and Sloan Foundation Fellowship FG-2019–12054 to AR. JWB. was supported by the Sloan Foundation, the Brain and Behavior Research Foundation, the Harvard Foundations of Human Behavior initiative, and the MGH Center for Law, Brain and Behavior.
References
- Bates D (2010). The LME4 package: linear mixed-effects models using S4 classes. See http://www.R-project.org.
- Behringer V, Hohmann G, Stevens JMG, Weltring A, & Deschner T (2012). Adrenarche in bonobos (Pan paniscus): evidence from ontogenetic changes in urinary dehydroepiandrosterone-sulfate levels. Journal of Endocrinology, 214, 55–65. [DOI] [PubMed] [Google Scholar]
- Bianchi S, Stimpson CD, Duka T, Larsen MD, Janssen WGM, Collins Z, . . . Sherwood CC (2013). Synaptogenesis and development of pyramidal neuron dendritic morphology in the chimpanzee neocortex resembles humans. Proceedings of the National Academy of Sciences, 110(Supplement 2), 10395–10401. doi: 10.1073/pnas.1301224110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund D, & Green B (1992). The adaptive nature of cognitive immaturity. American Psychologist, 47, 46–54. [Google Scholar]
- Blakemore SJ, & Robbins TW (2012). Decision-making in the adolescent brain. Nature Neuroscience, 15, 1184–1191. [DOI] [PubMed] [Google Scholar]
- Blanchard TC, Pearson JM, & Hayden BY (2013). Postreward delays and systematic biases in measures of animal temporal discounting. Proceedings of the National Academy of Sciences, 110, 15491–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin B (1999). Evolutionary perspective on human growth. Annual Review of Anthropology, 28, 109–153. [DOI] [PubMed] [Google Scholar]
- Bogin B, & Smith BH (1996). Evolution of the human life cycle. American Journal of Human Biology, 8, 703–716. [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, & White JSS (2008). Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution, 24, 127–135. [DOI] [PubMed] [Google Scholar]
- Burnett S, Bault N, Coricelli G, & Blakemore SJ (2010). Adolescents’ heightened risk-seeking in a probabilistic gambling task. Cognitive Development, 25, 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campell B (2006). Adrenarche and the evolution of human life history. American Journal of Human Biology, 18, 569–589. [DOI] [PubMed] [Google Scholar]
- Cantwell A, Buckholtz JW, Atencia R, & Rosati AG (2022). The origins of cognitive flexibility in chimpanzees. Developmental Science, 25, e13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoos SL, Ballonoff Suleiman A, Johnson M, van den Bos W, Hinshaw SP, & Dahl RE (2017). Social status strategy in early adolescent girls: Testosterone and value- based decision making. Psychoneuroendocrinology, 81, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MF, Cantwell A, Rukundo J, Ajarova L, Fernandez-Navarro S, Atencia R, & Rosati AG (2020). Healthy cardiovascular biomarkers across the lifespan in wild-born chimpanzees (Pan troglodytes). Philosophical Transactions of the Royal Society B, 375, 20190609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley AJ, Bernstein RM, & Nguyen AD (2012). Adrenarche in nonhuman primates: the evidence for it and the need to redefine it. Journal of Endocrinology, 214, 121–131. [DOI] [PubMed] [Google Scholar]
- Crone EA, & Steinbeis N (2017). Neural perspective on cognitive control development during childhood and adolescence. Trends in Cognitive Sciences, 21, 205–215. [DOI] [PubMed] [Google Scholar]
- Dalley JW, & Robbins TW (2017). Fractionating impulsivity: neuropsychiatric implications. Nature Reviews Neuroscience, 18, 158–171. [DOI] [PubMed] [Google Scholar]
- De Petrillo F, & Rosati AG (2021). Variation in primate decision-making under uncertainty and the roots of human economic behaviour. Philosophical Transactions of the Royal Society B 376, 20190671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrillo F, Tonachella G, & Addessi E (2017). Emotional correlates of probabilistic decision making in tufted capuchin monkeys (Sapajus spp.). Animal Behaviour, 129, 249–256. [Google Scholar]
- Defoe IN, Dubas JS, Figner B, & Van Aken MAG (2015). A meta-analysis on age differences in risky decision making: adolescents versus children and adults. Psychological Bulletin, 141, 29. [DOI] [PubMed] [Google Scholar]
- Defoe IN, & Romer D (2022). Theoretical advances in research on the development of risk taking. Developmental Review, 63, 101001. [Google Scholar]
- Duke SA, Balzer BWR, & Steinbeck KS (2014). Testosterone and its effects on human male adolescent mood and behavior: A systematic review. Journal of Adolescent Health, 55, 315–322. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, & Casey BJ (2002). A neural basis for the development of inhibitory control. Developmental Science, 5, F9–F16. [Google Scholar]
- Emery Thompson M, & Sabbi K (in press). Evolutionary demography of the great apes. In Burger O, Lee R & Sear R (Eds.), Human Evolutionary Demography. [Google Scholar]
- Enigk DK, Emery Thomson M, Machanda ZP, Wrangham RW, & Muller MN (2020). Competitive ability determines coalition participation and partner selection during maturation in wild male chimpanzees (Pan troglodytes schweinfurthii). Behavioral Ecology and Sociobiology, 74, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J (2003). Effect displays in R for generalised linear models. Journal of Statistical Software, 8, 1–27. [Google Scholar]
- Gomez JC (2005). Species comparative studies and cognitive development. Trends in Cognitive Sciences, 9, 118–125. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, & Ostaszewski P (1999). Discounting of delayed rewards across the life span: age differences in individual discounting functions. Behavioural Processes, 46, 89–96. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Udono T, Teramoto M, & Sugawara T (1996). The growth pattern of chimpanzees: Somatic growth and reproductive maturation inPan troglodytes. Primates, 37, 279–295. [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, & Casey BJ (2008). Biological substrates of emotional reactivity and regulation in adolescnece during an emotional go-nogo task. Biological Psychiatry, 63, 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, & Sommerville LH (2015). The neuroscience of adolescence decision-making. Current Opinion in Behavioral Science, 5, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY (2016). Time discounting and time preference in animals: A critical review. Psychonomic Bulletin & Review, 23, 39–53. [DOI] [PubMed] [Google Scholar]
- Herbert J (2018). Testosterone, cortisol and financial risk-taking. Frontiers in Behavioral Neuroscience, 12, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, & Sowell ER (2014). The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study. Human Brain Mapping, 5633–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy N, Hardy B, Page L, Schaffner M, Graggaber J, Powlson AS, . . . Coates J (2014). Cortisol shifts financial risk preferences. Proceedings of the National Academy of Sciences, 111, 3608–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H, Hill K, Lancaster J, & Hurtado M (2000). A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology, 9, 156–185. [Google Scholar]
- Khairullah A, Cousino Klein L, Ingle SM, May MT, Whetzel CA, Susman EJ, & Paus T (2014). Testosterone trajectories and reference ranges in a large longitudinal sample of male adolescents. PLoS One, 9, e108838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan-Dawood FS, Choe JK, & Dawood MY (1984). Salivary and plasma bound and “free” testosterone in men and women. American Journal of Obstetrics and Gynecology, 148, 442–445. [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendörfer R, Schriever K, Koeunig A, Schwarz HP, & Strasburger CJ (1995). Salivary cortisol levels throughout childhood and adolescence: Relation with age, pubertal stage, and eeight. Pediatric Research, 1995, 502–506. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Jackson S, & Cimentada J (2020). Package ‘corr’: Correlations in R. https://cran.r-project.org/web/packages/corrr/.
- Laube C, Lorenz R, & van den Bos W (2020). Pubertal testosterone correlates with adolescent impatience and dorsal striatal activity. Developmental Cognitive Neuroscience, 42, 100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh SR (2004). Brain growth, life history, and cognition in primate and human evolution. American Journal of Primatology, 62, 139–164. [DOI] [PubMed] [Google Scholar]
- Leigh SR (2012). Brain size growth and life history in human evolution. Evolutionary Biology, 39, 587–599. [Google Scholar]
- Lenth R (2018). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.2.3. https://CRAN.R-project.org/package=emmeans. [Google Scholar]
- Lerner JS, Li Y, Valdesolo P, & Kassam KS (2015). Emotion and decision making. Annual Review of Psychology, 66, 799–823. [DOI] [PubMed] [Google Scholar]
- Loewenstein GF, & Lerner JS (2001). The role of affect in decision making. In Davidson RJ & Goldsmit HH (Eds.), Handbook of Affective Science (pp. 619–642). Oxford: Oxford University Press. [Google Scholar]
- Lopez-Guzman S, Konova AB, & Glimcher PW (2018). Computational psychiatry of impulsivity and risk: how risk and time preferences interact in health and disease. Philosophical Transactions of the Royal Society B, 374, 20180135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa T (2007). Comparative cognitive development. Developmental Science, 10, 97–103. [DOI] [PubMed] [Google Scholar]
- Matsuzawa T, Tomonaga M, & Tanaka M (Eds.). (2006). Cognitive development in chimpanzees. Tokyo: Springer-Verlag. [Google Scholar]
- Mehta PH, Welker KM, Zilioli S, & Carre JM (2015). Testosterone and cortisol jointly modulate risk-taking. Psychoneuroendocrinology, 56, 88–99. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, . . . Group t. B. D. C. (2012). Testosterone-related cortical maturation across childhood and adolescence. Cerebral Cortex, 23, 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen DJ, Platt ML, Huetell SA, & Brannon EM (2011). Decision-making under risk in children, adolescents, and young adults. Frontiers in Psychology, 72, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Cédric P, Koolschijn MP, & Crone EA (2013). Development of risk taking: Contribution of adolescent testosterone and the orbito-frontal cortex. Journal of Cognitive Neuroscience, 25, 2141–2150. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Hervé P-Y, L. G, Perron M, Pike GB, Pitiot A, Richer L, . . . Paus T (2008). Growth of white matter in the adolescent brain: Role of testosterone and androgen receptor. The Journal of Neuroscience, 28, 9519–9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey AE (1990). Behavioural chnages at adolescence in chimpanzees. Behaviour, 115(203–246). [Google Scholar]
- R Development Core Team. (2022). A language and environment for statistical computing. Vienna, Austria. Retrieved from http://www.R-project.org [Google Scholar]
- Reddy RB, & Mitani JC (2020). Adolescent and young adult male chimpanzees form affiliative, yet aggressive, relationships with females. Journal of Human Evolution, 144, 102813. [DOI] [PubMed] [Google Scholar]
- Reyna VF, & Ellis SC (1994). Fuzzy-trace theory and framing effects in children’s risky decision making. Psychological Science, 5, 275–279. [Google Scholar]
- Reyna VF, Estrada SM, DeMarinis JA, Myers RM, Stanisz JM, & Mills BA (2011). Neurobiological and memory models of risky decision making in adolescents versus young adults. Journal of Experimental Psychology: Learning, Memory, and Cognition, 37, 1125–1142. [DOI] [PubMed] [Google Scholar]
- Reyna VF, & Farley F (2006). Risk and rationality in adolescent decision making: Implications for theory, practice, and public policy. Psychological Science in the Public Interes, 7, 1–44. [DOI] [PubMed] [Google Scholar]
- Robson SL, & Wood B (2008). Hominin life history: reconstruction and evolution. Journal of Anatomy, 212, 394–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, DiNicola L, & Buckholtz JW (2018). Chimpanzee cooperation is fast and independent from self-control. Psychological Science, 29, 1832–1845. [DOI] [PubMed] [Google Scholar]
- Rosati AG, Emery Thompson M, Atencia R, & Buckholtz JW (2022). Data from: Distinct developmental trajectories for risky and impulsive decision-making in chimpanzees. Dryad Digital Repository: 10.5061/dryad.kwh70rz7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Hagberg L, Enigk DK, Otali W, Emery Thomson M, Muller MN, . . . Machanda ZP (2020). Social selectivity in aging wild chimpanzees. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, & Hare B (2011). Chimpanzees and bonobos distinguish between risk and ambiguity. Biology Letters, 7, 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, & Hare B (2012). Decision-making across social contexts: Competition increases preferences for risk in chimpanzees and bonobos. Animal Behaviour, 84, 869–879. [Google Scholar]
- Rosati AG, & Hare B (2013). Chimpanzees and bonobos exhibit emotional respones to decision outcomes. PLoS One, 8, e63058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, & Hare B (2016). Reward type modulates human risk preferences. Evolution and Human Behavior, 37, 159–168. [Google Scholar]
- Rosati AG, Herrmann E, Kaminski J, Krupenye C, Melis AP, Schroepfer K, . . . Hare B (2013). Assessing the psychological health of captive and wild apes: A response to Ferdowsian et al. (2011). Journal of Comparative Psychology, 127, 329–336. [DOI] [PubMed] [Google Scholar]
- Rosati AG, Stevens JR, Hare B, & Hauser MD (2007). The evolutionary origins of human patience: Temporal preferences in chimpanzees, bonobos, and human adults. Current Biology, 17, 1663–1668. [DOI] [PubMed] [Google Scholar]
- Rosati AG, Wobber V, Hughes K, & Santos LR (2014). Comparative developmental psychology: How is human cognitive development unique? Evolutionary Psychology, 12, 448–473. [PubMed] [Google Scholar]
- Rosenbaum GM, & Hartley CA (2018). Developmental perspectives on risky and impulsive choice. Philosophical Transactions of the Royal Society B, 374, 20180133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbi KH, Emery Thompson M, Machanda ZP, Otali E, Wrangham RW, & Martin MN (2021). Sex differences in early experience and the development of aggression in wild chimpanzees. Proceedings of the National Academy of Sciences, 118, e2017144118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbi KH, Muller MN, Machanda ZP, Otali E, Fox SA, Wrangam RW, & Emery Thomson M (2020). Human-like adrenal development in wild chimpanzees: A longitudinal study of urinary dehydroepiandrosterone-sulfate and cortisol. American Journal of Primatology, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Mikami A, Tomonaga M, Matsui M, Suzuki J, Hamada Y, . . . Matsuzawa T (2011). Differential prefrontal white matter development in chimpanzees and humans. Current Biology, 1397–1402. [DOI] [PubMed] [Google Scholar]
- Sánchez-Amaro A, Tan J, Kaufhold SP, Fernández-Navarro S, & Rossano F (2021). How environmental unpredictability and harshness affect chimpanzees (Pan troglodytes) in risk-choice and temporal discounting tasks. Journal of Comparative Psychology. [DOI] [PubMed] [Google Scholar]
- Sandel AA, Langergraber KE, & Mitani JC (2020). Adolescent male chimpanzees (Pan troglodytes) form social bonds with their brothers and others during the transition to adulthood American Journal of Primatology, 82, e23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos LR, & Rosati AG (2015). The evolutionary roots of human decision making. Annual Review of Psychology, 66, 3221–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer SM, Azzopardi PS, Wickremarathne D, & Patton GC (2018). The age of adolescence. Lancet Child & Adolescent Health, 2, 223–228. [DOI] [PubMed] [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, & Castellanos FX (2006). Temporal and probabilistic discounting of rewards in children and adolescents: Effects of age and ADHD symptoms. Neuropsychologia, 44, 2092–2103. [DOI] [PubMed] [Google Scholar]
- Schulz KM, & Sisk CL (2016). The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neuroscience & Biobehavioral Reviews, 70, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, & Pollak SD (2009). Pubertal development: Correspondence between hormonal and physical Development. Child Development, 80, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JDE, Gross JJ, Remy KA, & Pschner KN (2012). Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion, 12, 1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Mullette-Gillman OA, McLaurin RE, Kuhn CM, LaBar KS, Platt ML, & Huettel SA (2011). Low- and high-testosterone individuals exhibit decreased aversion to economic risk. Psychological Science, 22, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2005). Cognitive and affective development in adolescence. Trends in Cognitive Sciences, 9, 69–74. [DOI] [PubMed] [Google Scholar]
- Steinberg L (2007). Risk-taking in adolescence: New perspectives from brain and behavioral science. Current Directions in Psychological Science, 16, 55–59. [Google Scholar]
- Steinberg L (2015). How to improve the health of American adolescents. Perspectives on Psychological Science, 10, 711–715. [DOI] [PubMed] [Google Scholar]
- Takagishi H (2004). Cortisol levels and time-discounting of monetary gaines in humans. NeuroReport, 15, 2145–2146. [DOI] [PubMed] [Google Scholar]
- Teffler K, Buxhoeveden DP, Stimpson CD, Fobbs AJ, Schapiro SJ, Baze WB, . . . Semendeferi K (2013). Developmental changes in the spatial organization of neurons in the neocortex of humans and common chimpanzees. The Journal of Comparative Neurology, 521, 4249–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, Hare B, & Fogleman T (2001). The ontogeny of gaze following in chimpanzee, Pan troglodytes, and rhesus macaques, Macaca mulatta. Animal Behaviour, 61, 335–343. [Google Scholar]
- van den Bos W, & Hertwig R (2017). Adolescents display distinctive tolerance to ambiguity and to uncertainty during risky decision making. Scientific Reports, 7, 40962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeersch H, T’Sjoen G, Kaufman JM, & Vincke J (2008). The role of testosterone in aggressive and non-aggressive risk-taking in adolescent boys. Hormones and Behavior, 53, 463–471. [DOI] [PubMed] [Google Scholar]
- Vigil P, Del Rio JP, Carrera B, Aranguiz FC, Rioseco H, & Cortés ME (2016). Influence of sex steroid hormones on the adolescent brain and behavior: An update. The Linacre Quarterly, 83, 308–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warneken F, & Rosati AG (2015). Cognitive capacities for cooking in chimpanzees. Proceeding of the Royal Society of London B, 282, 20150229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Bos MG, Schreuders E, von Kamp F, Peper JS, Tamnes CK, & Crone EA (2018). Unraveling age, puberty and testosterone effects on subcortical brain development across adolescence. Psychoneuroendocrinology, 91, 105–114. [DOI] [PubMed] [Google Scholar]
- Wobber V, & Hare B (2011). Psychological health of orphan bonobos and chimpanzees in African sanctuaries. Plos One, 6, e17147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, Hare B, Lipson S, Wrangham R, & Ellison P (2013). Different ontogenetic patterns of testosterone production reflect divergent male reproductive strategies in chimpanzees and bonobos. Physiology & Behavior, 116–117, 44–53. [DOI] [PubMed] [Google Scholar]
- Wobber V, Hare B, Maboto J, Lipson S, Wrangham R, & Ellison PT (2010). Differential changes in steroid hormones before competition in bonobos and chimpanzees. Proceedings of the National Academy of Sciences, 107, 12457–12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V, Herrmann E, Hare B, Wrangham R, & Tomasello M (2014). Differences in the early cognitive development of children and great apes. Developmental Psychobiology, 56, 547–573. [DOI] [PubMed] [Google Scholar]
- Wood BM, Watts DP, Mitani JC, & Langergraber KE (2017). Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. Journal of Human Evolution, 105, 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Clark L, Zilioli S, Eisenegger C, Gillan CM, Deng H, & Li H (2018). Single dose testosterone administration modulates emotional reactivity and counterfactual choice in healthy males. Psychoneuroendocrinology, 90, 127–133. [DOI] [PubMed] [Google Scholar]
- Wudy SA, Hartmann MF, & Remer T (2007). The sexual dimorphism in cortisol secretion starts after age 10 in healthy children: Urinary cortisol metabolite excretion rates during growth. American Journal of Physiology-Endocrinology and Metabolism, 293, E970–E976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


