Abstract
INTRODUCTION:
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) confer risk for Alzheimer’s disease and related dementias (ADRD).
METHODS:
This study from the Million Veteran Program (MVP) evaluated the impact of APOE ε4, PTSD, and TBI on ADRD prevalence in veteran cohorts of European ancestry (EA; n=11,112 ADRD cases, 170,361 controls) and African ancestry (AA; n=1,443 ADRD cases, 16,191 controls). Additive-scale interactions were estimated using the Relative Excess Risk due to Interaction (RERI) statistic.
RESULTS:
PTSD, TBI and ε4 showed strong main-effect associations with ADRD. RERI analysis revealed significant additive ε4 interactions with PTSD and TBI in the EA cohort and TBI in the AA cohort. These additive interactions indicate that ADRD prevalence associated with PTSD and TBI increased with the number of inherited APOE ε4 alleles.
DISCUSSION:
PTSD and TBI history will be an important part of interpreting the results of ADRD genetic testing and doing accurate ADRD risk assessment.
Introduction
Late-onset Alzheimer’s disease (AD) is the most common form of dementia and is estimated to affect approximately 10% of the US population, with prevalence increasing with age (e.g., 3% between ages 65–74 to 32% for people 85 and older)[1]. The US Department of Veterans Affairs has placed a high priority on clinical research on Alzheimer’s disease and related dementias (ADRD, a category which includes AD and other age-related dementias such as vascular dementia) due to the aging population of veterans it serves: in 2019, the US Census Bureau estimated that 54% of veterans were 65 or older[2]. Military service may place veterans at elevated risk for ADRD. Large-scale longitudinal studies (Ns > 100,000) have estimated that veterans with posttraumatic stress disorder (PTSD) are at approximately 50–60% greater risk for the development of dementia compared to those without the disorder[3–5]. The risk for dementia conferred by a history of traumatic brain injury (TBI) may be even greater. For example, one study based on the medical records of over 350,000 veterans demonstrated a relationship between TBI and the likelihood of subsequent dementia, with adjusted hazard ratios of 2.36 for mild TBI without loss of consciousness to 3.77 for moderate to severe TBI[6].
Genetic factors also influence risk for the development of AD/ADRD. The APOE ε4 allele is the strongest genetic risk locus for AD, but the strength of the association varies by ancestry. In cohorts of European ancestry (EA), each APOE ε4 allele inherited approximately quadruples AD risk, while in African ancestry (AA) cohorts, each APOE ε4 allele approximately doubles AD risk[1]. Genome-wide association studies (GWASs) have identified additional single-nucleotide polymorphisms (SNPs) associated with AD, with relatively small effect sizes compared to the APOE locus. Recent large-scale AD GWASs in EA cohorts have identified as many as 70 distinct AD risk loci.[7, 8] Fewer AD loci have been identified in AA GWASs, primarily due to smaller sample sizes, but these studies suggest that the molecular pathways implicated in AD risk in EA and AA individuals overlap[9]. Finally, polygenic risk scores (PRS) have been developed from EA GWAS results that explain additional variance in AD risk independent of the APOE locus in EA cohorts[10].
Researchers have long suspected that AD-associated genetic variants may moderate the effects of adverse environmental factors that confer risk for the subsequent development of AD or vice versa. Although the available evidence is inconclusive, several studies have suggested that the APOE ε4 allele and AD polygenic risk scores may predict poorer outcomes post-TBI[11–14]. Similarly, other studies suggest that the APOE ε4 variant may confer risk for PTSD and poor cognitive functioning following exposure to psychological trauma[15–17].
The present study leveraged data from the US Department of Veterans Affairs’ Million Veteran Program (MVP) to examine the prevalence of ADRD in veterans aged 65 and older and estimated the effects of PTSD, TBI, and APOE ε4 on ADRD risk in EA and AA veterans. We hypothesized that PTSD and TBI will interact with genetic risk for AD and dementia such that the ADRD risk associated with PTSD and TBI exposure would be greater in subjects with a higher genetic risk for AD. First, we examined APOE ε4 for interactions with PTSD and TBI. Next, to examine whether interactive effects would be observed with AD genetic risk conferred by other loci, we examined the possibility of PTSD and TBI interactions with an AD PRS which excluded the APOE effect.
Methods
Million Veteran Program (MVP)
MVP launched in 2011. Methodological details for MVP have been described at length elsewhere[18]. Veteran users of the Veterans Health Administration services volunteer to provide a blood sample for genetic analysis, consent to access to their VA electronic medical record (EMR), and complete a baseline survey on a wide range of demographic and health factors including psychiatric conditions, health risks, and medical history. This study was based on data from the MVP 19.2 phenotype release (n=790,116), the Phase 3 genotype release (n=455,683) and the MVP Baseline Survey (n=485,599 at the time of the 19.2 data release). The analyses reported here included MVP participants with (a) genetic data and MVP Baseline Survey responses who were of either EA or AA as determined by the genotype-informed Harmonized Ancestry and Race/Ethnicity (HARE) method[19].
Measures
Alzheimer’s Disease and Related Dementias (ADRD):
Similar to prior VA EMR-related studies of dementia we focused our attention on ADRD rather than AD specifically[20–22]. This was done to allow for diagnostic uncertainty inherent in EMR studies and the high levels of comorbidity and symptomatic overlap between AD and related dementias such as vascular dementia[23, 24] and Lewy body dementia[25]. In this study, ADRD cases and controls were identified based on International Classification of Diseases (ICD) codes from the EMR. ADRD cases must have received two or more ICD-9 or ICD-10 codes from a list of dementia codes which included codes for AD, codes for other dementias such as vascular dementia and Lewy body dementia, and non-specific dementia codes (see Supplemental Table 1). Our diagnostic algorithm includes non-specific dementia codes (e.g. ICD-9 code 294.20: Unspecified dementia without behavioral disturbance) as these are widely used in the VA, even in cases where the symptom progression is consistent with AD[26]. This is similar to ICD-based ADRD algorithms used elsewhere[20–22]. Cases with ADRD onset (first ICD code) prior to age 65 were excluded. Controls were defined as MVP participants 65+ at the time of MVP enrolment who did not have ICD codes for any form of dementia in the EMR, who did not have any ICD codes for mild cognitive impairment (MCI), and who had not been prescribed an AD medication. Additionally, as a sensitivity analysis, we examined a stricter AD phenotype which only included cases that have received 2 or more ICD-9 and/or ICD-10 codes for AD. As these codes are underused in the VA system relative to the non-specific dementia codes, this greatly limited the number of cases relative to the ADRD diagnosis. See Table 1 for details.
Table 1:
Clinical and demographic characteristics of European and African ancestry ADRD cases and controls
| European Ancestry |
African Ancestry |
|||||
|---|---|---|---|---|---|---|
| Controls (n=170,361) | Controls (n=170,361) | p-value | Controls (n=16,191) | Controls (n=1,443) | p-value | |
|
| ||||||
| Strict AD diagnosis | 0 (0) | 2,786 (25.07) | ---- | 0 (0) | 271 (18.78) | ---- |
|
| ||||||
| Age (mean/SD) | 77.77 (6.70) | 82.86 (7.57) | <2.2e-16 | 76.07 (5.91) | 80.70(7.76) | <2.2e-16 |
| Male | 166,274 (97.60) | 10,837 (97.53) | 0.43 | 15,604 (96.37) | 1,404 (97.51) | 0.034 |
| Lifetime PTSD diagnosis | 15,089 (8.86) | 1,175 (10.57) | <2.2e-16 | 3,028 (18.70) | 258 (17.88) | 0.62 |
| PTSD Onset Age (mean/SD) | 54.99 (14.23) | 53.15 (18.17) | 0.0024 | 53.49 (14.65) | 52.62(17.09) | 0.52 |
| History of TBI | 12,446 (7.31) | 1,294 (11.65) | <2.2e-16 | 692 (4.27) | 130 (9.01) | 5.041e-16 |
| Comorbid PTSD and TBI | 2,238 (1.31) | 250 (2.25) | <2.2e-16 | 221 (1.36) | 31 (2.15) | 0.0070 |
| Education (mean/SD) | 3.65 (1.57) | 3.40 (1.63) | <2.2e-16 | 3.29 (1.43) | 2.98(1.49) | 5.03e-14 |
| Smoking History | 126,914 (74.50) | 7,957 (71.61) | 0.00046 | 11,655 (71.98) | 977 (67.71) | 0.047 |
| Problematic Alcohol Use | 55,071 (32.33) | 2,263 (20.37) | <2.2e-16 | 3,739 (23.09) | 203 (14.07) | <2.2e-16 |
| APOE ε4 carrier | 39,138 (22.97) | 3,922 (35.30) | <2.2e-16 | 5,223 (32.26) | 601 (41.65) | <2.2e-16 |
Note: Numbers listed are counts (and percentages of cases or controls) unless noted otherwise. Age is based on the veteran’s age at last visit in the medical record from the MVP 19.2 phenotype release. Education was based on a 7-point scale.
Lifetime PTSD diagnosis:
PTSD case/control status was derived from a validated EMR-based algorithm described previously[27] which was recently used for an MVP PTSD GWAS[28]. As in the GWAS, we utilized the same threshold of 70% likelihood to classify MVP participants as PTSD cases and controls for our analyses.
Traumatic Brain Injury (TBI):
A history of TBI was based on self-report items from the MVP Baseline Survey. A TBI case was defined as someone who endorsed having been diagnosed with at least one of two conditions: “traumatic brain injury” and “concussion or loss of consciousness”. TBI controls were those who did not endorse either item. We focused our attention on self-report TBI as opposed to an ICD-code based algorithm, as we wished to include information about historical TBIs in aging veterans which could pre-date the VA EMR system.
Covariates & Potential Confounders.
We also evaluated associations with potential confounders including education, smoking history, and alcohol use based on items from the Baseline Survey. Education was ranked on a 7-point scale ranging from “Less than high school” (1) to “Professional or Doctorate degree” (7). Smoking history was coded based on their response to the dichotomous item: “Have you ever smoked daily or almost every day for one year?” Problematic alcohol use was based on the AUDIT-C which is a 13-point scale of alcohol consumption[29]. AUDIT-C scores were then dichotomized using an established clinical cut-off such that men with scores >=4 and women with scores >=3 were considered “positive” for problematic drinking.
Genotyping:
Genotype data generation and QC have been described in detail elsewhere[30]. Genotyping was based on the MVP 1.0 custom Axiom array which assesses 668,418 markers. Genotype data processing, cleaning, and imputation was performed by the MVP Bioinformatics core. APOE genotype was based on the two APOE isoform-defining SNPs (rs7412 and rs429358) which were well-imputed (r2=0.96 and 0.99 respectively in the EA cohort and r2= 0.87 and 0.99 in the AA cohort). We used “best guess” imputed genotypes with a 90% confidence threshold for these SNPs to derive the APOE genotype.
Alzheimer’s Disease Polygenic Risk Score (AD PRS) Calculation.
The AD PRS is a weighted average of imputed SNP dosages for each MVP participant with the GWAS log-odds ratios for each SNP used as the weights (as in e.g. [10]). AD PRSs were computed for EA MVP participants using the program PRSice-2[31] based on summary data from the Kunkle et al. 2019 EA AD GWAS[32, 33] as Kunkle et al. 2019 was the most recent large-scale GWAS performed without the use of “proxy” cases (survey responses about parental dementia), which can affect OR estimates[34]. We only examined the PRS in EA subjects, as PRSs tend to perform poorly when the GWAS population and the study population being scored are derived from different ancestries. We did not calculate a PRS for AA MVP participants using the recent AA GWAS from the Alzheimer’s Disease Genetics Consortium[9], as it had a relatively small sample size, hence it is expected to have poor performance. Rare SNPs (MAF <1%) and SNPs with high missingness for “best guess” genotypes (> 0.5%) were excluded from the PRS calculation. SNPs were trimmed for LD using PRSice’s default parameters. Here, we limited our PRS calculation to SNPs reaching genome-wide significance (p<5×10−8), based on the reported oligogenic genetic architecture of AD genetic risk[35]. As we were primarily interested in using the PRS as a measure of genetic risk which can be contrasted with the effect of APOE, we excluded variants in the region of strong linkage disequilibrium around the APOE gene on chromosome 19 from the PRS (GRCh37 Chr 19: 44,409,039–46,412,650). See Supplemental Table 2 for a listing of the SNPs used in PRS calculation.
Data Analysis
All analyses were performed in R (v3.6.1)[36]. We examined clinical and demographic differences between ADRD cases and controls using t-tests or chi-square tests as appropriate. We then we ran hierarchical logistic models that estimated the effects of age, APOE ε4, and PTSD on ADRD in one step, with the PTSD × APOE ε4 interaction added in a second step. Analyses involving APOE ε4 were performed in AA and EA subjects separately. Using the same approach in separate models, we also evaluated with effects of TBI (substituted for PTSD) and ran the models specified above with the PRS in place of APOE as an indicator of ADRD genetic risk in the EA cohort.
In addition to examining interaction terms estimated from logistic models (multiplicative interactions) we also examined the interactions on an additive scale. The additive scale interactions provide additional context for interpreting the multiplicative interaction estimates generated from a logistic model, and it has been suggested that both additive and multiplicative scale interactions be presented in epidemiological studies[37, 38]. It is perhaps underappreciated outside public-health contexts, that the interaction term from the logistic model is, by itself, not immediately interpretable in a way that can prioritize interventions. That is, you cannot infer from whether the interaction OR between two risk factors (A and B) is greater or less than 1, whether the increased probability of disease for those exposed to risk factor A is greater for those who are additionally exposed to risk factor B or less. Either is possible depending on the other parameters of the model. Measuring interactions on an additive scale helps remove this uncertainty[39]. We evaluated the presence of additive interactions by calculating the Relative Excess Risk due to Interaction (RERI) statistic as described in Knol et al. 2007[40]. As recommended[40], we computed 95% RERI confidence intervals (CIs) based on the 2.5th and 97.5th percentiles of a RERI distribution from a 10,000-replicate bootstrap simulation. RERI estimates with 95% CIs that do not include 0 were considered significant.
Next, to evaluate possible confounding between PTSD and TBI and to assess the effects of other relevant risk factors, we performed more comprehensive analyses with PTSD and TBI included in the same model along with age, sex, APOE ε4, education, smoking history, and alcohol use. As before, we then added a set of gene × environment (G × E) interaction terms evaluating the APOE ε4 × PTSD interactions and APOE ε4 × TBI interactions. It should be noted that both PTSD and TBI outcomes are themselves partially heritable[41–43]. Additionally, we then evaluated the possibility of PTSD × TBI and 3-way APOE ε4 × PTSD × TBI interactions. We also evaluated the impact of non-APOE related genetic interactions in the comprehensive model by substituting the PRS for APOE in the models. Additionally, we performed several sensitivity analyses to examine the robustness of our results. First, we examined our phenotype by analyzing our stricter AD phenotype. Second, we performed a sensitivity analysis where APOE ε4 carriers were excluded from the PRS analysis.
Results
MVP Population Characteristics and Differences between ADRD Cases and Controls
Table 1 lists the clinical and demographic characteristics of EA and AA ADRD cases and controls. ADRD cases were older, less educated, more likely to be APOE ε4 carriers, and more likely to have a history of TBI compared to controls. The prevalence of problematic alcohol use and smoking was lower in cases than in controls. Finally, while in EA participants the prevalence of PTSD was higher among cases than controls, there was no such difference among AA individuals, where the prevalence of PTSD was higher than the EA cohort for both ADRD cases and controls.
APOE ε4 Models
Table 2 lists the results from the simple models of ADRD risk in EA and AA individuals as a function of age, APOE ε4, TBI or PTSD and their interactions with APOE ε4. In the EA cohort, the PTSD model yielded significant main effects of age, PTSD, and APOE ε4 and a weak APOE ε4 × PTSD interaction suggesting a marginally lower ADRD odds ratios for ε4 carriers with PTSD. The TBI model showed similar parameter estimates but no interaction. In the AA cohort, we found similarly strong effects of age, PTSD, TBI, and APOE ε4 but no significant APOE ε4 × PTSD or APOE ε4 × TBI interactions. The odds ratios and confidence intervals show that the estimated effect of PTSD was similar to that conferred by inheriting a copy of ε4 in both the EA and AA groups. As expected, the APOE ε4 effect was weaker in in the AA than the EA cohort. The PTSD effect in the EA cohort was larger than the TBI effect and the PTSD effect in the AA cohort. The TBI effect in the AA cohort was larger than the PTSD effect and the TBI effect estimated in the EA cohort.
Table 2:
Simple logistic regression models of ADRD risk in individuals of European and African ancestry as a function of age, APOE ε4, TBI, or PTSD and their interactions with APOE ε4.
| European Ancestry |
African Ancestry |
||||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
|
| |||||
| PTSD Model | |||||
| Age | 1.12 (1.11, 1.12) | ~0 | 1.13 (1.12, 1.14) | 5.53e-119 | |
| PTSD | 2.65 (2.47, 2.85) | 1.90e-161 | 1.73 (1.46, 2.06) | 3.03e-10 | |
| APOE ε4 | 2.18 (2.09, 2.28) | 1.69e-272 | 1.69 (1.50, 1.90) | 2.20e-18 | |
| ε4 × PTSD | 0.84 (0.74, 0.95) | 0.0046 | 0.94 (0.72, 1.21) | 0.62 | |
| TBI Model | |||||
| Age | 1.10 (1.10, 1.10) | ~0 | 1.11 (1.10, 1.12) | 7.13e-144 | |
| TBI | 1.96 (1.84, 2.09) | 1.67e-95 | 2.69 (2.18, 3.32) | 1.70e-20 | |
| APOE ε4 | 2.05 (1.98, 2.13) | ~0 | 1.70 (1.54, 1.87) | 4.70e-27 | |
| ε4 × TBI | 0.95 (0.85, 1.07) | 0.40 | 1.08 (0.77, 1.52) | 0.65 | |
Note: The parameter estimates for the main effects listed were derived from main effects-only models. The GxE parameter estimates were from models with the main effects and interaction term included in the same model. Sample sizes for the European ancestry PTSD and TBI models were 140,613 and 177,928. Sample sizes for the African ancestry PTSD and TBI models were 11,626 and 15,928. Sample sizes differed due to patterns of item-level missing data from the baseline survey.
Our analysis of the additive-scale interactions yielded significant positive interactions for three of the four models. Specifically, in the EA group, the RERI analysis revealed a significant APOE ε4 × PTSD interaction (RERI = 1.28; 95% CI: 0.75, 1.81, significant as the CI does not include 0) and APOE ε4 × TBI interaction (RERI = 0.86; 95% CI: 0.48, 1.24). In the AA group, the RERI analysis showed a significant APOE ε4 interaction with TBI (RERI = 1.45; 95% CI: 0.15, 2.75) but no significant PTSD interaction (RERI = 0.37; 95% CI: −0.21, 0.94). To illustrate the impact of these additive interactions, Figure 1 depicts the relationship between the number of copies of APOE ε4, PTSD or TBI, and ADRD as a function of age and ancestry as determined by the estimated parameters of the logistic regression model. Each graph shows the prevalence of ADRD increasing from age 65 to 85 and the substantial association between ADRD and both PTSD and TBI in EA and AA MVP participants. The graphs also illustrate the positive additive scale interaction, that is, the increasing ADRD prevalence difference associated with PTSD and TBI status as a function of APOE ε4. For example, in EAs at age 80, the difference in the estimated prevalence of ADRD between PTSD cases and controls increased from 5.70% for individuals with 0 copies of ε4 to 8.55% and 11.19% for those with one or two copies, respectively. To demonstrate that this pattern is observed in the data and not an artifact of modeling, the observed ADRD prevalence as a function of PTSD × ε4 dosage and TBI × ε4 dosage in 5-year age bins spanning ages 65 to 80 can be found in Supplemental Tables 3–6. These raw proportions of ADRD cases across the age bins support the observation of an increasing effect of PTSD and TBI on ADRD prevalence as a function of ε4 dosage indicated by the logistic models and RERI estimates, although it is clear that much of this is driven by the difference between those with 0 copies of ε4 vs. those with 1 copy of ε4 due to the low frequency of ε4 homozygotes.
Figure 1:
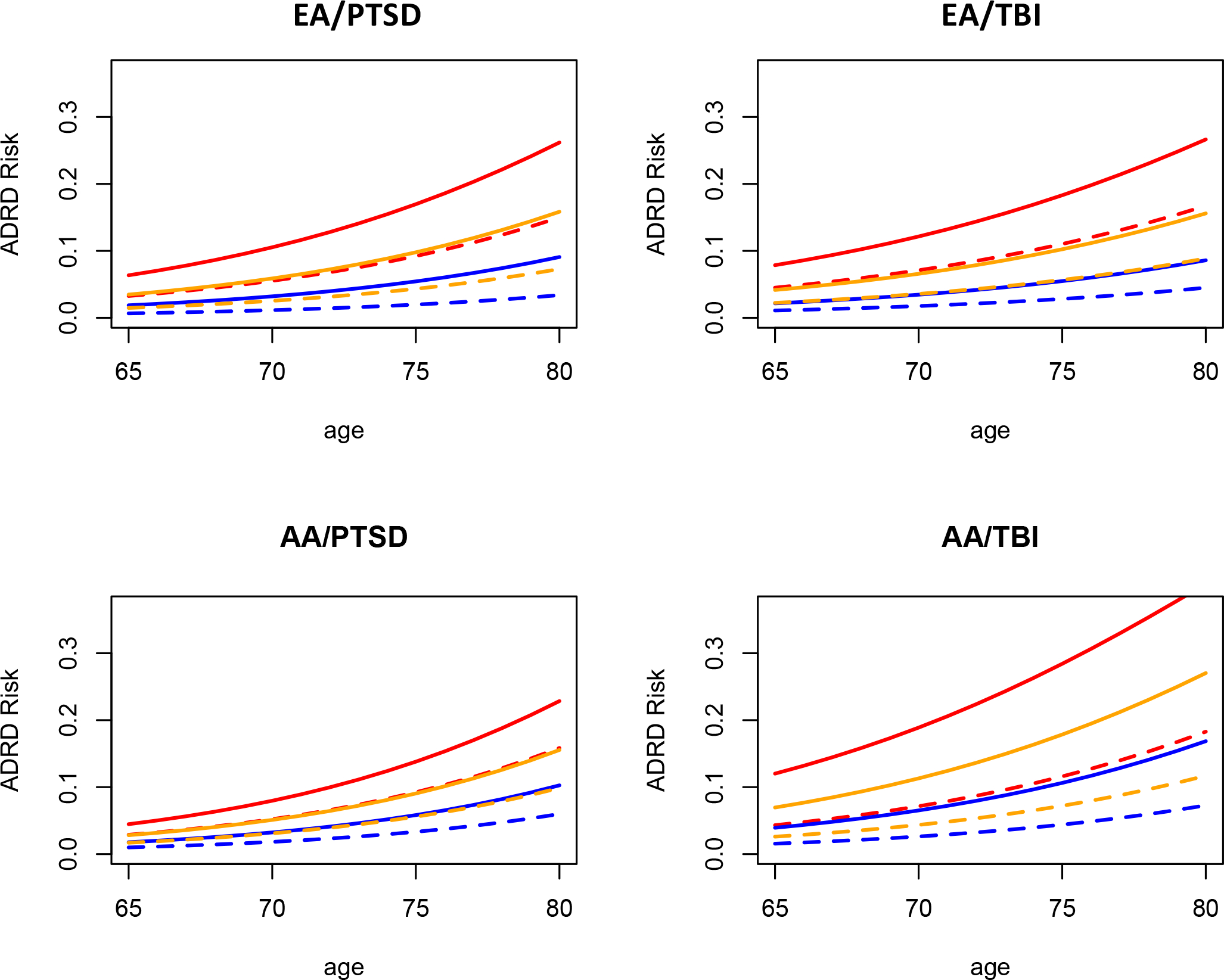
Estimated risk of ADRD as a function of age, APOE ε4 dosage, PTSD, and TBI, in EA and AA MVP veteran participants. Solid lines represent PTSD cases or TBI cases, while dashed lines represent controls. The color represents the predicted risk for those with 0 (blue), 1 (orange), or 2 (red) copies of the APOE ε4 variant. Distance between solid and dashed lines illustrates the greater ADRD risk associated with PTSD/TBI in EA participants and TBI in AA participants is higher for those with 1 or 2 copies of ε4 vs those with 0 copies, particularly in older veterans.
Results of our comprehensive models largely reiterated the results from the simple models in both EA and AA participants (Supplemental Table 7 and 8). Of the new covariates, alcohol and education were significantly associated with ADRD, with problematic alcohol use and higher education levels appearing protective. Smoking and sex were not significantly associated with ADRD. The APOE ε4, PTSD, and TBI main effects and, in EA participants, the PTSD × APOE ε4 interaction remained significant. TBI × PTSD, and 3-way PTSD × TBI × APOE ε4 interaction terms were nonsignificant.
AD Polygenic Risk Score Models
Next, we examined the possibility of G × E interactions with the AD PRS in the EA participants (Table 3). These models confirmed the main effect association of the AD PRS with ADRD in the EA participants. However, there were no significant PRS × PTSD or PRS × TBI associations in either the logistic model or the RERI analysis (PTSD × PRS RERI = 0.12, CI: −0.058, 0.30; TBI × PRS RERI = 0.10, CI: −0.018, 0.24). The comprehensive models with the expanded covariate set and higher order interactions yielded no significant new findings (Supplemental Table 9).
Table 3:
Simple logistic regression models of ADRD risk in individuals of European ancestry as a function of age, Alzheimer’s disease polygenic risk scores (PRS), PTSD or TBI and their interactions with the PRS.
| OR (95% CI) | p-value | ||
|---|---|---|---|
|
| |||
| PTSD Model | |||
| Age | 1.11 (1.11, 1.11) | ~0 | |
| PTSD | 2.61 (2.43, 2.79) | 2.30e-162 | |
| PRS | 1.15 (1.13, 1.18) | 1.11e-32 | |
| PRS × PTSD | 0.95 (0.89, 1.02) | 0.14 | |
| TBI Model | |||
| Age | 1.10 (1.09, 1.10) | ~0 | |
| TBI | 1.94 (1.82, 2.07) | 2.49e-96 | |
| PRS | 1.15 (1.13, 1.17) | 2.15e-44 | |
| PRS × TBI | 0.98 (0.92, 1.04) | 0.57 | |
Note: The parameter estimates for the main effects listed were derived from main effects-only models. The GxE parameter estimates were from models with the main effects and interaction term included in the same model.
Sensitivity Analyses
Sensitivity Analyses examining the strict AD diagnosis largely recapitulate the results of the ADRD analyses (Supplemental Tables 10–14). In particular, logistic models of AD still yielded strong associations between AD and Age, PTSD, TBI, and APOE ε4 in EA (p<10−12) and AA (p<10−3) participants. In the EA participants, the multiplicative PTSD × ε4 interaction noted in ADRD analyses was not significant when analyzing AD (p>0.20). We did observe some nominally significant higher-order multiplicative interactions in the full AD PRS model in the EA cohort (p~0.04), but these would not be significant after any correction for multiple testing. The RERI analysis of AD yielded higher interaction estimates but wider confidence intervals. In the EA participants, there was a significant APOE ε4 × PTSD interaction (RERI = 1.63; 95% CI: 0.57, 2.73) and APOE ε4 × TBI interaction (RERI = 1.45; 95% CI: 0.66, 2.23). In the AA participants, the RERI analysis yielded a significant APOE ε4 × TBI interaction (RERI = 4.41; 95% CI: 0.42, 8.46) but no significant PTSD interaction (RERI = 1.24; 95% CI: −0.61, 3.33). The RERI for PRS interactions and AD in the EA cohort were not significant (PRS × PTSD RERI = 0.17, 95% CI: −0.16, 0.54; PRS × TBI RERI = 0.22, 95% CI: −0.02, 0.47).
Results for our logistic models of ADRD and the PRS in the APOE ε4 non-carriers were very similar to the results from the whole cohort except for reduced significance due to the smaller sample sizes (Supplemental Tables 15 and 16). No PRS interactions were indicated in the ε4 non-carriers from the logistic models or the RERI analysis.
Discussion
This was the first study on genetic and environmental contributions to risk for ADRD from the Million Veteran Program. The clinical and demographic characteristics of the EA and AA MVP cohorts used for this study generally reflected that of the total population of veterans over 65 as served by the VA. Participants were primarily male. ADRD prevalence estimates of 6.1% and 8.2% in EA and AA veterans, respectively, were slightly lower than the prevalence reported in a recent study of all veterans over age 65 receiving VA care between 1999 and 2016 (EA male veterans: 8.3%; AA male veterans: 11.5%)[20], potentially due to the elimination of early onset (onset before age 65) cases from the current cohort. Prevalence estimates of lifetime PTSD of 6% and 8% for EA and AA participants, respectively were similar to the pooled prevalence estimate of 8.4% (95% CI: 7.59–12.84%; N > 1.4M) from a recent meta-analysis of eight studies of older veterans[44]. MVP Baseline Survey items indicating a history of TBI were endorsed by 7.5% and 4.6% of EA and AA MVP participants, respectively. For EA veterans, this prevalence was similar to the 8.8% prevalence reported in white VHA users 55 and older based on medical record data[45]. However, TBI endorsement prevalence by AA MVP participants was substantially lower than the 11.5% prevalence for non-Hispanic black veterans in that study.
In this cohort, we observed strong associations between ADRD and APOE ε4 and, in the EA cohort where it was examined, an AD PRS constructed from genome-wide significant SNPs excluding loci in the APOE region. We observed strong evidence of main effect associations between ADRD and both PTSD and TBI. Sensitivity analyses indicated that the strong associations between ADRD and both PTSD and TBI were not due to confounding with alcohol use, smoking or education. When we evaluated the presence of additive interactions using the RERI statistic, we also found modest but significant APOE ε4 × PTSD and APOE ε4 × TBI interactions in EA veterans, indicating that the difference in ADRD prevalence associated with PTSD and TBI was greater in those of high genetic risk for AD as a function of the APOE ε4 locus. That differences in the direction of effect between additive and multiplicative interactions are quite possible has been noted elsewhere[39]. Examining the prevalence estimates generated from the logistic model as presented in Figure 1 demonstrates that the prevalences inferred by the logistic model parameter estimates are congruent with the conclusions suggested by the RERI statistic: the impact of PTSD is greater in ε4 carriers. In AA veterans, analyses showed a significant APOE ε4 × TBI additive interaction but no significant APOE ε4 × PTSD interaction. To our knowledge, this is the first well-powered study to find evidence for a significant APOE ε4 × adverse environmental factors on the prevalence of ADRD in a representative sample of VHA users. In contrast, we did not observe logistic or additive G × E interactions with the PRS which summarized non-APOE AD genetic risk. There are several possible explanations for this. The G × E interactions observed here may not be a function of AD genetic risk in general, but may be restricted to APOE ε4, or perhaps ε4 and other risk loci yield effects in similar molecular pathways. APOE plays an important role in neuron maintenance and repair by distributing lipids necessary for synaptogenesis, proliferation, and axon mylenation[46], while the variants included in the PRS play roles in a variety of pathways including inflammation, tau binding, and amyloid precursor protein (APP) regulation and metabolism[32, 33]. Further investigations of interactions between PTSD, TBI, and other AD risk variants may yield new insight into ADRD risk in veterans. However, it is also possible that the lower effect of the AD PRS compared to APOE ε4 has simply led to reduced power to detect the additive PRS interactions, and that these will be apparent in a larger sample.
We included sex, alcohol use, education, and smoking as covariates in our comprehensive models, as these factors have been associated with both PTSD[47–50] and dementia risk[51, 52]. While their inclusion did not appear to impact the associations between PTSD and TBI on ADRD, the associations observed with these covariates are interesting in their own right. First, our results support an association between education and lower prevalence of dementia, which has been observed in prior studies[53]. Smoking is generally thought to increase AD risk[51], although studies have been inconsistent (e.g.[54]), likely due to early mortality among smokers[55]. Here, we noted a lower prevalence of smokers in our ADRD cases (Table 1), but no significant effects of smoking in models that took age and other exposures into account (Supplemental Tables 7–9). This should not be taken as evidence against the association between smoking and dementia based on the noted biases which can affect case-control studies on this topic[56]. Although dementia risk is generally higher in women than men[52], no association was observed between sex and dementia in the EA or AA cohorts, presumably due to the small number of older women in MVP. Finally, we observed an association between problematic alcohol use and lower prevalence of ADRD, which has been reported in other studies (e.g. [57, 58]), although not consistently (e.g. [59, 60] [61, 62]). This could potentially reflect the somewhat controversial reported association between moderate alcohol use and cardiovascular health[63]. However, there are several potential biases which may account for this apparent protective effect. For example, it is possible that individuals with prodromal ADRD or averse health conditions associated with risk for ADRD (e.g., diabetes) have moderated their alcohol use (the “sick-quitter hypothesis”)[64] Another possibility is that aging veterans who died prematurely from alcohol-related diseases or accidents may have reduced the number of alcohol users who ultimately went on to develop AD[65]. Moderate alcohol use could also be confounded with other lifestyle factors that are potentially protective for AD, such as greater social engagement[66]. Although beyond the scope of this study, a careful analysis of MVP data examining alcohol use in detail, including information on duration, timing, and quantity of alcohol use, could provide valuable insight into this issue.
The findings of this study should be evaluated in light of several limitations. Our ADRD diagnosis is ICD code-based and, by design, included codes for non-AD dementia diagnoses and non-specific dementia codes. It could be reasonably argued that “all-cause dementia” would be a more appropriate term for our primary analysis variable, as it is the broadest category that could conceivably be included in our case cohort. However, we used the term ADRD to stress the correspondence between our definition of ADRD and prior research. Our definition closely follows the NIA conceptualization of ADRD (https://www.nia.nih.gov/research/grants-funding/alzheimers-disease-administrative-supplements), which includes AD and other dementias such as vascular dementia that often co-occur and/or share diagnostic overlap with AD. Our study used a similar ICD algorithm as established in prior electronic medical record-based studies of ADRD (e.g. [20, 21]), including a recent publication in this journal[22]. Our definition also overlaps substantially with the Chronic Conditions Warehouse definition of “Alzheimer’s disease or related disorders or senile dementia” (https://www2.ccwdata.org/web/guest/condition-categories-chronic), which is used for monitoring Centers for Medicare & Medicaid Services (CMS) data. Like the definition employed for this study, the prior ADRD studies and the Chronic Conditions warehouse ADRD algorithms use non-specific dementia diagnosis codes, a similar set of related dementia codes, and exclude other dementias such as Huntington’s disease. Therefore, our use of the term ADRD here is appropriate, as long as the limitations of our approach are considered. For example, the inclusion of non-AD codes in our case definition likely contributed to differences in effect size estimates for APOE ε4 when compared to studies of a purely AD diagnosis[1]. However, when we performed our sensitivity analysis examining the strict AD phenotype, RERI estimates remained largely consistent between the analyses of AD and the analyses of ADRD.
We have focused our attention on the identification of “late-onset” ADRD cases, that is, cases with a first ICD code for dementia after age 65. We limited ourselves to late-onset based on a concern that early onset dementia cases may have a higher rate of false positives than late-onset cases, particularly in subjects with a TBI and/or psychopathology[67]. However, head injury may lower the onset rate of dementia [68], and hence, our study may not present the full impact of TBI-associated dementia.
Another study limitation was that history of TBI was based on self-report data from the MVP Baseline Survey. This was done to allow for the inclusion of TBIs which may predate the VA EMR. However, the survey data have not yet been evaluated relative to TBI ICD codes from the medical records or clinical interview-based assessments of TBI. With that said, much is known about the relative accuracy of self-report TBI data relative to EMR-based measures. For example, there is evidence that ICD code-based algorithms underreport the presence of head injury relative to self-report[69]. Furthermore, it has been well established that assessing the presence and severity of TBI can be challenging, as supporting information may be missing from the medical and service records[70, 71]. Although our survey data does not allow for a detailed assessment of injury severity, the overwhelming majority (~80%) of TBIs are likely to be mild [72], and even mild TBIs have long-term consequences including cognitive difficulties and psychiatric distress [73, 74]. Additionally, APOE might itself be associated with TBI by increasing susceptibility to injury and/or poor outcomes post-TBI[75–77]. For example, repeated mild head impacts associated with chronic traumatic encephalopathy (CTE) may have a greater impact in ε4 carriers[78]. Finally, history of smoking and problematic alcohol use were also based on self-report items. We do not have detailed information about the lifetime course of smoking and alcohol use which could yield stronger associations.
Other study limitations relate to sample size issues. While the AA subsample is large relative to other AA studies of AD risk, it is smaller than the EA cohort that was examined. The differences in findings between groups should be interpreted cautiously in light of the difference in sample sizes. That is, the lack of an additive scale interaction between APOE and PTSD in the AA cohort may be simply a product of sample size and the lower effect of APOE on ADRD risk in the AA population. Finally, we note that we have not yet incorporated information about current PTSD symptomatology and history of treatment. Although PTSD symptom course is often chronic, and treatments for PTSD are limited, there is a potential that adequate treatment for PTSD might alleviate some of this increased risk. However, this is beyond the scope of the current investigation.
To conclude, this study provides support for long-hypothesized G × E interactions associated with ADRD risk. Although APOE genetic testing is available in a direct-to-consumer fashion, current recommendations by the Alzheimer’s Association do not support their use[79]. This is for several reasons, which include possible discrimination on the basis of the results without an effective intervention. Once treatments are available that can delay or prevent the onset of AD, the APOE effects on AD risk may make it an important tool for identification of people at elevated risk. This study’s findings suggest that PTSD and TBI history can be an important component of genetic dementia risk assessment and targeting early intervention, particularly in the veteran population.
Supplementary Material
Systematic Review:
Prior work has demonstrated that posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) increased risk of dementia. Some work has suggested that APOE ε4, the strongest Alzheimer’s disease (AD) genetic risk factor might modify the dementia risk associated with PTSD and TBI.
Interpretation:
We observed strong associations between dementia prevalence and APOE ε4, posttraumatic stress disorder (PTSD), and TBI in a large US veteran biobank (the Million Veteran Project), and evidence that the impact of TBI and PTSD on dementia rates is greater in carriers of the high risk APOE ε4 variant.
Future Directions:
PTSD and TBI history can augment genetic information and identify individuals at risk of dementia for monitoring and intervention. When genetic testing for dementia risk is more widespread, these factors will likely play a role in interpreting test results in veterans and perhaps the broader population.
Funding:
This research is based on data from the Million Veteran Program, Office of Research and Development, Veterans Health Administration, and was supported by VA BLR&D grant 1 I01 BX004192 (MVP015). ZN was supported by National Institute of Mental Health award T32MH019836. JRF was supported by VA CSR&D grant 1IK2CX002192-01A2. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Footnotes
Conflicts: Nothing to disclose.
References
- [1].2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020. [DOI] [PubMed]
- [2].United States Census Bureau. Veterans Day 2020: Nov. 11, RELEASE NUMBER CB20-FF.10. Facts for Features; 2020. [Google Scholar]
- [3].Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10:S236–41. [DOI] [PubMed] [Google Scholar]
- [5].Yaffe K, Lwi SJ, Hoang TD, Xia F, Barnes DE, Maguen S, et al. Military-related risk factors in female veterans and risk of dementia. Neurology. 2019;92:e205–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Barnes DE, Byers AL, Gardner RC, Seal KH, Boscardin WJ, Yaffe K. Association of Mild Traumatic Brain Injury With and Without Loss of Consciousness With Dementia in US Military Veterans. JAMA Neurol. 2018;75:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bellenguez C, Küçükali F, Jansen I, Andrade V, Moreno-Grau S, Amin N, et al. New insights on the genetic etiology of Alzheimer’s and related dementia. medRxiv. 2020:2020.10.01.20200659. [Google Scholar]
- [8].Wightman DP, Jansen IE, Savage JE, Shadrin AA, Bahrami S, Rongve A, et al. Largest GWAS (N=1,126,563) of Alzheimer’s Disease Implicates Microglia and Immune Cells. medRxiv. 2020:2020.11.20.20235275. [Google Scholar]
- [9].Kunkle BW, Schmidt M, Klein HU, Naj AC, Hamilton-Nelson KL, Larson EB, et al. Novel Alzheimer Disease Risk Loci and Pathways in African American Individuals Using the African Genome Resources Panel: A Meta-analysis. JAMA Neurol. 2021;78:102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Escott-Price V, Sims R, Bannister C, Harold D, Vronskaya M, Majounie E, et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain. 2015;138:3673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hayes JP, Logue MW, Sadeh N, Spielberg JM, Verfaellie M, Hayes SM, et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Samatovicz RA. Genetics and brain injury: apolipoprotein E. J Head Trauma Rehabil. 2000;15:869–74. [DOI] [PubMed] [Google Scholar]
- [13].Friedman G, Froom P, Sazbon L, Grinblatt I, Shochina M, Tsenter J, et al. Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology. 1999;52:244–8. [DOI] [PubMed] [Google Scholar]
- [14].Merritt VC, Lange RT, Lippa SM, Brickell TA, Soltis AR, Dalgard CL, et al. Apolipoprotein e (APOE) epsilon4 genotype influences memory performance following remote traumatic brain injury in U.S. military service members and veterans. Brain Cogn. 2021;154:105790. [DOI] [PubMed] [Google Scholar]
- [15].Averill LA, Abdallah CG, Levey DF, Han S, Harpaz-Rotem I, Kranzler HR, et al. Apolipoprotein E gene polymorphism, posttraumatic stress disorder, and cognitive function in older U.S. veterans: Results from the National Health and Resilience in Veterans Study. Depress Anxiety. 2019;36:834–45. [DOI] [PubMed] [Google Scholar]
- [16].Mota NP, Han S, Harpaz-Rotem I, Maruff P, Krystal JH, Southwick SM, et al. Apolipoprotein E gene polymorphism, trauma burden, and posttraumatic stress symptoms in U.S. military veterans: Results from the National Health and Resilience in Veterans Study. Depress Anxiety. 2018;35:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lyons MJ, Genderson M, Grant MD, Logue M, Zink T, McKenzie R, et al. Gene-environment interaction of ApoE genotype and combat exposure on PTSD. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, et al. Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214–23. [DOI] [PubMed] [Google Scholar]
- [19].Fang H, Hui Q, Lynch J, Honerlaw J, Assimes TL, Huang J, et al. Harmonizing Genetic Ancestry and Self-identified Race/Ethnicity in Genome-wide Association Studies. Am J Hum Genet. 2019;105:763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cheng Y, Ahmed A, Zamrini E, Tsuang DW, Sheriff HM, Zeng-Treitler Q. Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias in Older African American and White Veterans. J Alzheimers Dis. 2020;75:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Andreev A, Erdinc B, Shivaraj K, Schmutz J, Levochkina O, Bhowmik D, et al. The Association Between Anemia of Chronic Inflammation and Alzheimer’s Disease and Related Dementias. J Alzheimers Dis Rep. 2020;4:379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jutkowitz E, Halladay C, Tsai J, Hooshyar D, Quach L, O’Toole T, et al. Prevalence of Alzheimer’s disease and related dementias among veterans experiencing housing insecurity. Alzheimers Dement. 2022;18:1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease--lessons from pathology. BMC Med. 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE. Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimers Dement. 2013;9:76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Foguem C, Manckoundia P. Lewy Body Disease: Clinical and Pathological “Overlap Syndrome” Between Synucleinopathies (Parkinson Disease) and Tauopathies (Alzheimer Disease). Curr Neurol Neurosci Rep. 2018;18:24. [DOI] [PubMed] [Google Scholar]
- [26].Cho K, Gagnon DR, Driver JA, Altincatal A, Kosik N, Lanes S, et al. Dementia Coding, Workup, and Treatment in the VA New England Healthcare System. Int J Alzheimers Dis. 2014;2014:821894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Harrington KM, Quaden R, Stein MB, Honerlaw JP, Cissell S, Pietrzak RH, et al. Validation of an Electronic Medical Record-Based Algorithm for Identifying Posttraumatic Stress Disorder in U.S. Veterans. J Trauma Stress. 2019;32:226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stein MB, Levey DF, Cheng Z, Wendt FR, Harrington K, Pathak GA, et al. Genome-wide association analyses of post-traumatic stress disorder and its symptom subdomains in the Million Veteran Program. Nat Genet. 2021;53:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–95. [DOI] [PubMed] [Google Scholar]
- [30].Hunter-Zinck H, Shi Y, Li M, Gorman BR, Ji SG, Sun N, et al. Genotyping Array Design and Data Quality Control in the Million Veteran Program. Am, J Hum Genet.106:535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Choi SW, O’Reilly PF. PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Author Correction: Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet. 2019;51:1423–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet. 2019;51:414–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu JZ, Erlich Y, Pickrell JK. Case-control association mapping by proxy using family history of disease. Nat Genet. 2017;49:325–31. [DOI] [PubMed] [Google Scholar]
- [35].Zhang Q, Sidorenko J, Couvy-Duchesne B, Marioni RE, Wright MJ, Goate AM, et al. Risk prediction of late-onset Alzheimer’s disease implies an oligogenic architecture. Nat Commun. 2020;11:4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Team RDC. R: A language and environment for statistical computing, V3.6.1. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- [37].Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–24. [DOI] [PubMed] [Google Scholar]
- [38].Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41:514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].VanderWeele TJ, Knol MJ. A Tutorial on Interaction. Epidemiologic Methods. 2014;3:33–72. [Google Scholar]
- [40].Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36:1111–8. [DOI] [PubMed] [Google Scholar]
- [41].True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–64. [DOI] [PubMed] [Google Scholar]
- [42].Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159:1675–81. [DOI] [PubMed] [Google Scholar]
- [43].Kals M, Kunzmann K, Parodi L, Radmanesh F, Wilson L, Izzy S, et al. A genome-wide association study of outcome from traumatic brain injury. EBioMedicine. 2022;77:103933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Williamson V, Stevelink SAM, Greenberg K, Greenberg N. Prevalence of Mental Health Disorders in Elderly U.S. Military Veterans: A Meta-Analysis and Systematic Review. Am J Geriatr Psychiatry. 2018;26:534–45. [DOI] [PubMed] [Google Scholar]
- [45].Kornblith E, Peltz CB, Xia F, Plassman B, Novakovic-Apopain T, Yaffe K. Sex, race, and risk of dementia diagnosis after traumatic brain injury among older veterans. Neurology. 2020;95:e1768–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bales KR. Brain lipid metabolism, apolipoprotein E and the pathophysiology of Alzheimer’s disease. Neuropharmacology. 2010;59:295–302. [DOI] [PubMed] [Google Scholar]
- [47].Vilaplana-Perez A, Sidorchuk A, Perez-Vigil A, Brander G, Isoumura K, Hesselmark E, et al. Assessment of Posttraumatic Stress Disorder and Educational Achievement in Sweden. JAMA Netw Open. 2020;3:e2028477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132:959–92. [DOI] [PubMed] [Google Scholar]
- [49].Smith NDL, Cottler LB. The Epidemiology of Post-Traumatic Stress Disorder and Alcohol Use Disorder. Alcohol Res. 2018;39:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kearns NT, Carl E, Stein AT, Vujanovic AA, Zvolensky MJ, Smits JAJ, et al. Posttraumatic stress disorder and cigarette smoking: A systematic review. Depress Anxiety. 2018;35:1056–72. [DOI] [PubMed] [Google Scholar]
- [51].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England). 2020;396:413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Beam CR, Kaneshiro C, Jang JY, Reynolds CA, Pedersen NL, Gatz M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J Alzheimers Dis. 2018;64:1077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].van Duijn CM, Hofman A. Relation between nicotine intake and Alzheimer’s disease. BMJ. 1991;302:1491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chang CC, Zhao Y, Lee CW, Ganguli M. Smoking, death, and Alzheimer disease: a case of competing risks. Alzheimer Dis Assoc Disord. 2012;26:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kukull WA. The association between smoking and Alzheimer’s disease: effects of study design and bias. Biol Psychiatry. 2001;49:194–9. [DOI] [PubMed] [Google Scholar]
- [57].Orgogozo JM, Dartigues JF, Lafont S, Letenneur L, Commenges D, Salamon R, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153:185–92. [PubMed] [Google Scholar]
- [58].Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–6. [DOI] [PubMed] [Google Scholar]
- [59].Harwood DG, Kalechstein A, Barker WW, Strauman S, St George-Hyslop P, Iglesias C, et al. The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer’s disease. Int J Geriatr Psychiatry. 2010;25:511–8. [DOI] [PubMed] [Google Scholar]
- [60].Fratiglioni L, Ahlbom A, Viitanen M, Winblad B. Risk factors for late-onset Alzheimer’s disease: a population-based, case-control study. Ann Neurol. 1993;33:258–66. [DOI] [PubMed] [Google Scholar]
- [61].Sabia S, Fayosse A, Dumurgier J, Dugravot A, Akbaraly T, Britton A, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ. 2018;362:k2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Schwarzinger M, Pollock BG, Hasan OSM, Dufouil C, Rehm J, QalyDays Study G. Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study. Lancet Public Health. 2018;3:e124–e32. [DOI] [PubMed] [Google Scholar]
- [63].O’Keefe JH, Bhatti SK, Bajwa A, DiNicolantonio JJ, Lavie CJ. Alcohol and cardiovascular health: the dose makes the poison...or the remedy. Mayo Clin Proc. 2014;89:382–93. [DOI] [PubMed] [Google Scholar]
- [64].Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT Jr., Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289:1405–13. [DOI] [PubMed] [Google Scholar]
- [65].Hartz SM, Oehlert M, Horton AC, Grucza RA, Fisher SL, Culverhouse RC, et al. Daily Drinking Is Associated with Increased Mortality. Alcohol Clin Exp Res. 2018;42:2246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Evans IEM, Martyr A, Collins R, Brayne C, Clare L. Social Isolation and Cognitive Function in Later Life: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2019;70:S119–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Marceaux JC, Soble JR, O’Rourke JJF, Swan AA, Wells M, Amuan M, et al. Validity of early-onset dementia diagnoses in VA electronic medical record administrative data. Clin Neuropsychol. 2020;34:1175–89. [DOI] [PubMed] [Google Scholar]
- [68].Li W, Risacher SL, McAllister TW, Saykin AJ. Traumatic brain injury and age at onset of cognitive impairment in older adults. J Neurol. 2016;263:1280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Whiteneck GG, Cuthbert JP, Corrigan JD, Bogner JA. Prevalence of Self-Reported Lifetime History of Traumatic Brain Injury and Associated Disability: A Statewide Population-Based Survey. J Head Trauma Rehabil. 2016;31:E55–62. [DOI] [PubMed] [Google Scholar]
- [70].Radigan LJ, McGlinchey RE, Milberg WP, Fortier CB. Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime and the VA Comprehensive TBI Evaluation. J Head Trauma Rehabil. 2018;33:E51–E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kim S, Currao A, Fonda JR, Beck B, Kenna A, Fortier CB. Diagnostic Accuracy of the Boston Assessment of Traumatic Brain Injury-Lifetime Clinical Interview Compared to Department of Defense Medical Records. Mil Med. 2022. [DOI] [PubMed] [Google Scholar]
- [72].Traumatic Brain Injury Center of Excellence. DoD Numbers for Traumatic Brain Injury Worldwide - Totals. In: Branch AFHS, editor. Falls Church, VA: Defense Health Agency; 2021. [Google Scholar]
- [73].Barnes DE, Byers AL, Gardner RC, Seal KH, Boscardin WJ, Yaffe K. Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurology. 2018;75:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Perry DC, Sturm VE, Peterson MJ, Pieper CF, Bullock T, Boeve BF, et al. Traumatic brain injury is associated with subsequent neurologic and psychiatric disease: A meta-analysis. Journal of Neurosurgery 2016;124:511–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ariza M, Pueyo R, Matarin Mdel M, Junque C, Mataro M, Clemente I, et al. Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2006;77:1191–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Crawford FC, Vanderploeg RD, Freeman MJ, Singh S, Waisman M, Michaels L, et al. APOE genotype influences acquisition and recall following traumatic brain injury. Neurology. 2002;58:1115–8. [DOI] [PubMed] [Google Scholar]
- [77].Sundstrom A, Marklund P, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, et al. APOE influences on neuropsychological function after mild head injury: within-person comparisons. Neurology. 2004;62:1963–6. [DOI] [PubMed] [Google Scholar]
- [78].Atherton K, Han X, Chung J, Cherry JD, Baucom Z, Saltiel N, et al. Association of APOE Genotypes and Chronic Traumatic Encephalopathy. JAMA Neurol. 2022;79:787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Alzheimer’s Association Medical and Scientific Advisory Council. Genetic Testing Statement. 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


