Abstract
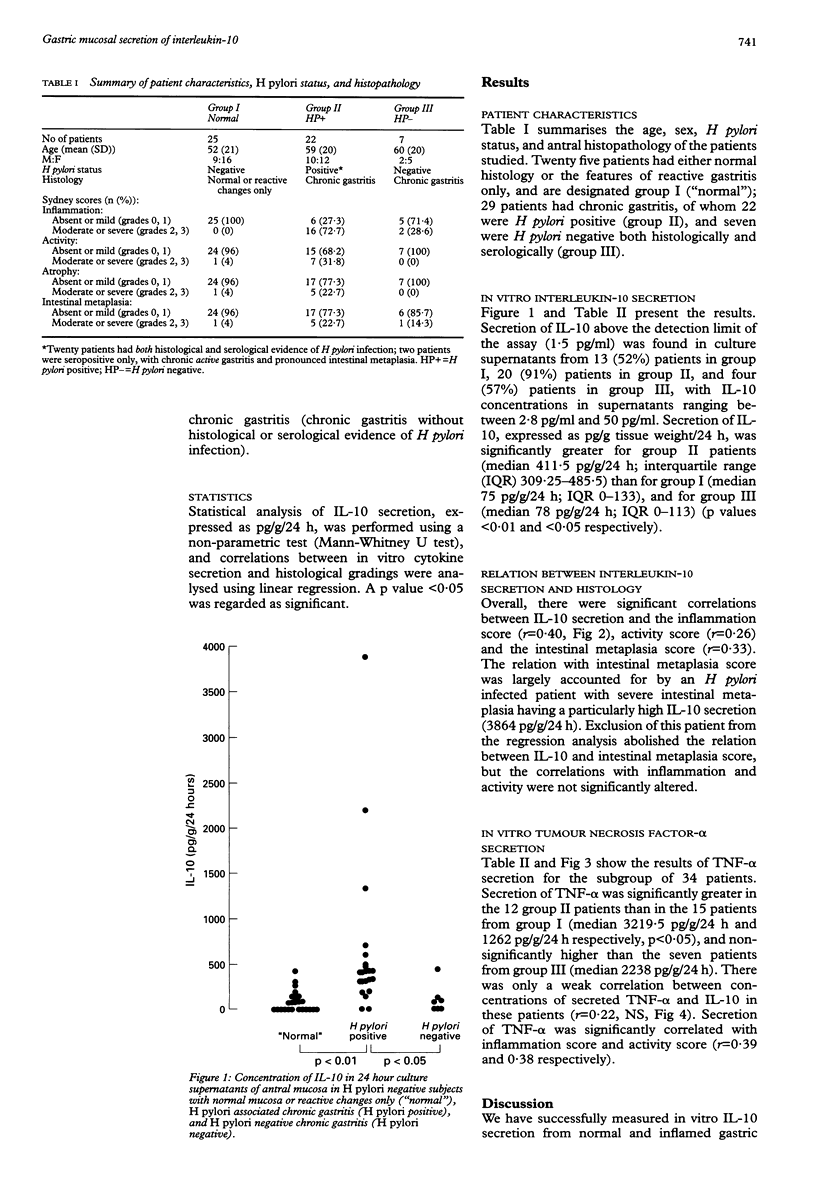
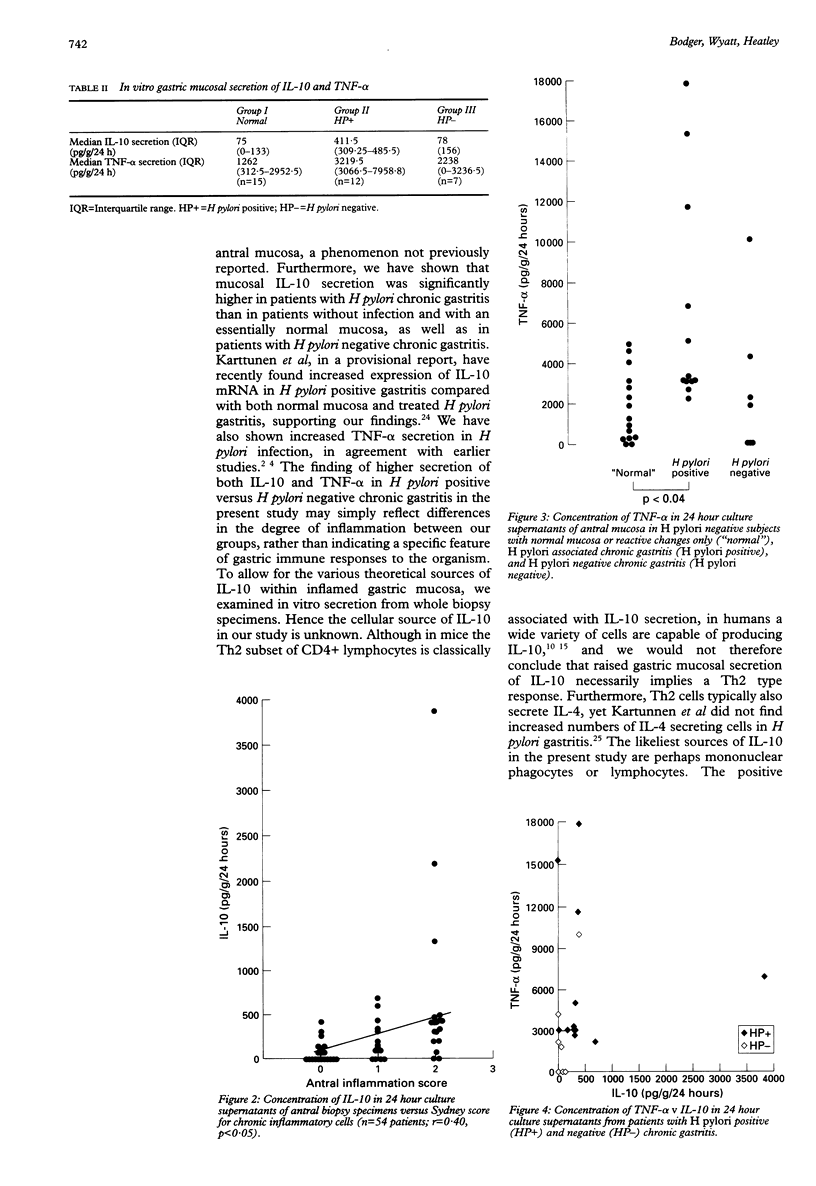
BACKGROUND: Interleukin-10 (IL-10) is an 18 kDa peptide with a range of anti-inflammatory and immunosuppressive properties. AIM: To determine whether this cytokine is involved in gastric mucosal inflammation in Helicobacter pylori infection. METHODS: The production of IL-10 by antral mucosal biopsy specimens during short term in vitro culture was determined by measuring IL-10 content of supernatants by enzyme linked immunosorbent assay (ELISA). H pylori status was determined by serology and histology, with gastritis scored using the Sydney system. Tumour necrosis factor-alpha (TNF-alpha) content of supernatants was also determined in a subgroup of patients. RESULTS: IL-10 secretion was significantly greater in patients with H pylori associated chronic gastritis than in patients who were H pylori negative with normal mucosa/reactive changes, and those with H pylori negative chronic gastritis (p < 0.01 and < 0.05 respectively). There was a significant correlation overall between IL-10 secretion and chronic inflammation score (r = 0.40). Secretion of TNF-alpha, which was significantly higher in H pylori infected patients than uninfected patients with a normal mucosa (p < 0.04), correlated with scores for chronic inflammation and activity (r = 0.39 and 0.38 respectively), but was only weakly correlated with IL-10 secretion (r = 0.22, NS). CONCLUSIONS: Gastric mucosal production of IL-10 and TNF-alpha are increased in chronic gastritis associated with H pylori infection, and mucosal cytokine secretion varies with important histopathological aspects of gastric inflammation. Whereas the secretion of IL-10 in H pylori infection may be protective, limiting tissue damage caused by inflammation, it may also contribute towards failure of the immune response to eliminate the organism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beissert S., Hosoi J., Grabbe S., Asahina A., Granstein R. D. IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells. J Immunol. 1995 Feb 1;154(3):1280–1286. [PubMed] [Google Scholar]
- Burdin N., Van Kooten C., Galibert L., Abrams J. S., Wijdenes J., Banchereau J., Rousset F. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J Immunol. 1995 Mar 15;154(6):2533–2544. [PubMed] [Google Scholar]
- Crabtree J. E., Farmery S. M., Lindley I. J., Figura N., Peichl P., Tompkins D. S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994 Oct;47(10):945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Shallcross T. M., Heatley R. V., Wyatt J. I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991 Dec;32(12):1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Fan X. J., Chua A., O'Connell M. A., Kelleher D., Keeling P. W. Interferon-gamma and tumour necrosis factor production in patients with Helicobacter pylori infection. Ir J Med Sci. 1993 Oct;162(10):408–411. doi: 10.1007/BF02996319. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., James S. L., Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992 Mar 15;148(6):1792–1796. [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., James S. L., Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992 Mar 15;148(6):1792–1796. [PubMed] [Google Scholar]
- Greenberger M. J., Strieter R. M., Kunkel S. L., Danforth J. M., Goodman R. E., Standiford T. J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995 Jul 15;155(2):722–729. [PubMed] [Google Scholar]
- Greenberger M. J., Strieter R. M., Kunkel S. L., Danforth J. M., Goodman R. E., Standiford T. J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995 Jul 15;155(2):722–729. [PubMed] [Google Scholar]
- Kambayashi T., Alexander H. R., Fong M., Strassmann G. Potential involvement of IL-10 in suppressing tumor-associated macrophages. Colon-26-derived prostaglandin E2 inhibits TNF-alpha release via a mechanism involving IL-10. J Immunol. 1995 Apr 1;154(7):3383–3390. [PubMed] [Google Scholar]
- Kambayashi T., Alexander H. R., Fong M., Strassmann G. Potential involvement of IL-10 in suppressing tumor-associated macrophages. Colon-26-derived prostaglandin E2 inhibits TNF-alpha release via a mechanism involving IL-10. J Immunol. 1995 Apr 1;154(7):3383–3390. [PubMed] [Google Scholar]
- Karttunen R., Andersson G., Poikonen K., Kosunen T. U., Karttunen T., Juutinen K., Niemelä S. Helicobacter pylori induces lymphocyte activation in peripheral blood cultures. Clin Exp Immunol. 1990 Dec;82(3):485–488. doi: 10.1111/j.1365-2249.1990.tb05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karttunen R., Andersson G., Poikonen K., Kosunen T. U., Karttunen T., Juutinen K., Niemelä S. Helicobacter pylori induces lymphocyte activation in peripheral blood cultures. Clin Exp Immunol. 1990 Dec;82(3):485–488. doi: 10.1111/j.1365-2249.1990.tb05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karttunen R., Karttunen T., Ekre H. P., MacDonald T. T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995 Mar;36(3):341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievits F., Ivanyi P. A subpopulation of mouse cytotoxic T lymphocytes recognizes allogeneic H-2 class I antigens in the context of other H-2 class I molecules. J Exp Med. 1991 Jul 1;174(1):15–19. doi: 10.1084/jem.174.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipp U., Birkholz S., Kaup W., Opferkuch W. Immune suppressive effects of Helicobacter pylori on human peripheral blood mononuclear cells. Med Microbiol Immunol. 1993 May;182(2):63–76. doi: 10.1007/BF00189374. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Moss S. F., Legon S., Davies J., Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994 Nov;35(11):1567–1570. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noach L. A., Bosma N. B., Jansen J., Hoek F. J., van Deventer S. J., Tytgat G. N. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994 May;29(5):425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- Pang G., Couch L., Batey R., Clancy R., Cripps A. GM-CSF, IL-1 alpha, IL-1 beta, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1 alpha and TNF-alpha. Clin Exp Immunol. 1994 Jun;96(3):437–443. doi: 10.1111/j.1365-2249.1994.tb06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone B. J., Wyatt J. I., Worsley B. W., Shires S. E., Trejdosiewicz L. K., Heatley R. V., Losowsky M. S. Systemic and local antibody responses to gastric Campylobacter pyloridis in non-ulcer dyspepsia. Gut. 1986 Jun;27(6):642–647. doi: 10.1136/gut.27.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick D., Berg D., Holland G. Interleukin 10: an overview. Prog Growth Factor Res. 1992;4(3):207–227. doi: 10.1016/0955-2235(92)90020-i. [DOI] [PubMed] [Google Scholar]
- Sobala G. M., Crabtree J. E., Pentith J. A., Rathbone B. J., Shallcross T. M., Wyatt J. I., Dixon M. F., Heatley R. V., Axon A. T. Screening dyspepsia by serology to Helicobacter pylori. Lancet. 1991 Jul 13;338(8759):94–96. doi: 10.1016/0140-6736(91)90085-4. [DOI] [PubMed] [Google Scholar]
- Wardle T. D., Hall L., Turnberg L. A. Platelet activating factor: release from colonic mucosa in patients with ulcerative colitis and its effect on colonic secretion. Gut. 1996 Mar;38(3):355–361. doi: 10.1136/gut.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P. M., Forman D. Helicobacter pylori as a risk factor for cancer. Baillieres Clin Gastroenterol. 1995 Sep;9(3):563–582. doi: 10.1016/0950-3528(95)90049-7. [DOI] [PubMed] [Google Scholar]
- de Vries J. E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995 Oct;27(5):537–541. doi: 10.3109/07853899509002465. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H., de Vries J. E. Interleukin-10. Curr Opin Immunol. 1992 Jun;4(3):314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H., de Vries J. E. Interleukin-10. Curr Opin Immunol. 1992 Jun;4(3):314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]


