Abstract
Frailty is a syndrome of older age that reflects an impaired physiologic reserve and decreased ability to recover from medical stressors. While the impact of frailty on mortality in cardiovascular disease has been well described, its impact on cardiovascular disease-specific health status—cardiac symptoms, physical functioning, and quality of life—has been less well-studied. In this review, we summarize the impact of frailty on health status outcomes across different cardiovascular conditions. In heart failure, frail patients have markedly impaired disease-specific health status and are at risk for subsequent health status deteriorations. However, frail patients have similar or even greater health status improvements with interventions for heart failure, such as cardiac rehabilitation or guideline-directed medical therapy. In valvular heart disease, the impact of frailty on disease-specific health status is of even greater concern, since management involves physiologically taxing procedures that can worsen health status. Frailty increases the risk of poor health status outcomes after transcatheter aortic valve intervention or surgical aortic valve replacement for aortic stenosis, but there is no evidence that frail patients benefit more from one procedure versus another. In both heart failure and valvular heart disease, health status improvements may reverse frailty, highlighting the overlap between cardiovascular disease and frailty and emphasizing that treatment should typically not be withheld based on the presence of frailty alone. Meanwhile, data are limited on the impact of frailty on health status outcomes in the treatment of coronary artery disease, peripheral artery disease, and atrial fibrillation and requires further research.
INTRODUCTION
Frailty is an aging-related syndrome of impaired reserve across multiple physiologic systems that results in increased vulnerability and an impaired ability to recover from acute medical stressors. A diagnosis of frailty increases the risk of morbidity and mortality after nearly any intervention or clinical insult, from acute illnesses to elective surgeries (cardiac and noncardiac). Frailty also increases the risk of failure to regain physical and mental function after an illness or surgery, which are vital to preventing dependence and social isolation.
While the impacts of frailty on physical function, generic quality of life, and mortality in persons with cardiovascular disease are well established, how frailty impacts cardiovascular disease-specific health status has been less studied. Disease-specific health status assesses the symptoms, functional limitations, and quality of life associated with a particular disease process. For example, a heart failure-specific health status measure asks the patient to quantify their burden of oedema and dyspnoea, how these specific symptoms impair their physical and social functioning, and how heart failure-related symptoms and functional limitations impact their quality of life. Disease-specific health status outcomes are the best indicators of the patient’s symptomatic response to cardiovascular treatments, are often the target of the intervention (e.g., coronary stenting for stable angina), and are prioritized by patients who are older and with comorbidities.1 It is therefore essential to understand the effect of frailty on disease-specific health status and, even more importantly, the impact of frailty on improvement in health status after cardiovascular treatments and interventions.
In this review, we discuss the cross-sectional and longitudinal associations of frailty with disease-specific health status in different forms of cardiovascular disease (Table 1, Supplemental Table) and highlight some of the gaps in knowledge. Notably, this review does not detail the logistics of assessing frailty (other than to highlight the overlap of frailty measures with some of the symptoms of cardiovascular diseases), the prevalence of frailty in patients with cardiovascular conditions,2–4 or the impact of frailty on clinical outcomes or mortality—each of these topics has been covered more than adequately in several prior studies, meta-analyses, and reviews. Instead, we focus on how frailty affects the specific symptoms of cardiovascular disease—e.g., chest pain, palpitations, oedema—and its impact on treatments designed to target these symptoms.
Table 1.
Summary of the impact of frailty on health status outcomes in cardiovascular disease.
| Cardiovascular condition | Common health status measures | Impact of frailty on cross-sectional health status | Impact of frailty on longitudinal health status | Impact of frailty on health status treatment outcomes |
|---|---|---|---|---|
| Heart failure | Kansas City Cardiomyopathy Questionnaire Minnesota Living with Heart Failure Questionnaire |
Worse health status19–25 | Greater likelihood of clinically significant health status deterioration20 |
LVAD: potentially reverses frailty. Patients no longer frail after LVAD derive larger health status benefits compared with those still frail26
Sacubitril-valsartan: similar health status improvements in frail versus non-frail patients with HFpEF.23 Not yet studied in HFrEF SGLT2 inhibitors: greater health status improvements as degree of frailty increases in HFrEF and HFpEF21,24 Exercise therapy: improves frailty markers and health status, 30 similar health status improvements in frail versus non-frail patients22 |
| Valvular heart disease | Kansas City Cardiomyopathy Questionnaire | Worse health status31, 32 | Persistently poor health status after TAVI34–36 | No differential treatment benefit with TAVI versus SAVR38,39
Greater health status benefit in frail versus non-frail patients with M-TEER40 |
| Coronary artery disease | Seattle Angina Questionnaire | Similar angina burden46
More related limitations Worse related quality of life |
Not yet studied | Not yet studied |
| Peripheral artery disease | Walking Impairment Questionnaire Peripheral Artery Questionnaire |
Worse physical function48 Unknown impact on symptoms and quality of life | Not yet studied | Not yet studied |
| Atrial fibrillation | Atrial Fibrillation Effect on Quality of Life Questionnaire | Possibly worse health status50,51 | Not yet studied | Not yet studied |
Abbreviations: LVAD, left ventricular assist device; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; SGLT2, sodium-glucose cotransporter 2; TAVI, transcatheter aortic valve implantation; SAVR, surgical aortic valve replacement; M-TEER, mitral transcatheter edge-to-edge repair.
Overlap between frailty measures and symptomatic cardiovascular disease.
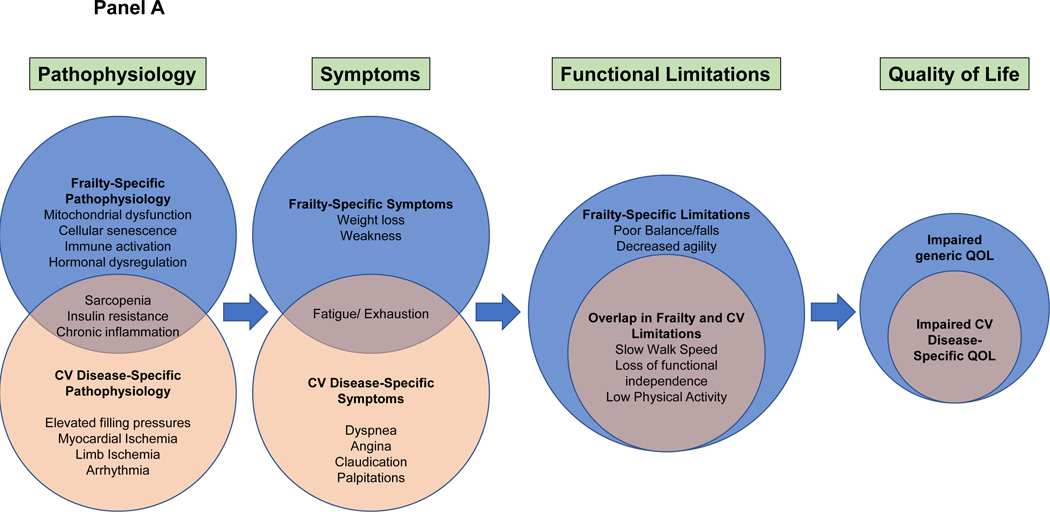
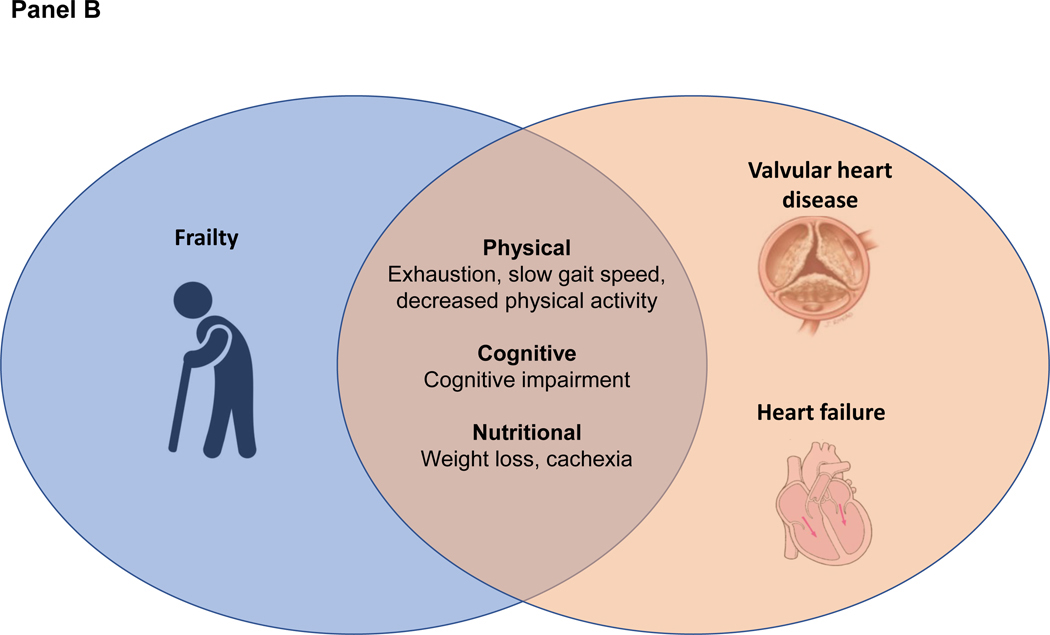
Frailty and cardiovascular disease share similar risk factors, such as older age, lack of exercise, poor diet, and smoking. Cardiovascular disease itself increases the risk for frailty due to physical deconditioning, polypharmacy, and other mechanisms. Similarly, a treatment that improves cardiovascular symptoms can reduce functional limitations and consequently prevent, improve, or even reverse frailty. When assessing frailty in patients with cardiovascular disease, it is important to recognize that there is substantial overlap between the measures of frailty and the symptoms and functional limitations of cardiovascular disease (Figure, panel A). For example, weakness, fatigue, and slow gait speed are all key markers of frailty; however, a person may have these symptoms due entirely to heart failure or valvular heart disease without underlying frailty (Figure, panel B). As such, it can be challenging to discern true frailty from symptomatic cardiovascular disease, which will improve with interventions to treat the underlying cardiovascular disease. For example, after transcatheter aortic valve implantation (TAVI), a patient often has less fatigue and a faster gait speed. This has been termed “reversible frailty” and is important to distinguish from irreversible frailty, where cardiovascular interventions may have limited benefit and potentially create an acute insult from which the patient does not recover.
FIGURE:
A. Overlap between cardiovascular disease and frailty in pathophysiology, symptoms, functional limitations, and quality of life. Frailty can increase the burden of disease-specific symptoms, as in heart failure or valvular heart disease, or exacerbate the functional limitations due to a cardiovascular disease process, as in peripheral artery disease. Frailty widens the discrepancy between observed versus desired symptom burden and physical functioning, leading to an even greater impairment in quality of life.
B. Overlap of heart failure and valvular heart disease with frailty. Because of similar pathophysiologic mechanisms, there is substantial overlap between frailty and the symptoms of heart failure and valvular heart disease. Elevated filling pressures and pulmonary congestion result in exhaustion, slow gait speed due to dyspnoea, and decreased physical activity due to deconditioning. Decreased cerebral blood flow may also result in cognitive impairment. Gut oedema leads to early satiety and unintentional weight loss. Abbreviations: CV, cardiovascular; QOL, quality of life.
In situations where the disease process impacts the assessment of frailty, it may be useful to focus more on measures of physiological impairment that are less impacted by the disease process (e.g., unintentional weight loss, grip strength), measures that are not predominantly comprised of functional assessments, measures of chronic disease (e.g., low albumin or haemoglobin levels), or higher thresholds for measures (e.g., very slow gait speed or wheelchair bound, activities of daily living dependence) to identify irreversible frailty. Further work in these clinical settings is needed to identify those patients with positive markers for frailty but who will improve with treatment.
Frailty measures.
There has been increasing awareness of the concept of frailty in cardiology and cardiac surgery as a major risk factor for increased morbidity and mortality and the need to evaluate frailty prior to invasive procedures. This assessment has been traditionally based on an “eyeball” test from a single physician, which is limited by personal biases and low reproducibility.5 However, even with objective assessments of frailty, there is no consensus about specific clinical or laboratory measures that should be included.6,7 As such, numerous tools exist to quantify frailty (Table 2),8 and estimates of the prevalence of frailty among individuals with cardiovascular disease vary widely depending on the method used.
Table 2.
Commonly used frailty measures and their use in cardiovascular disease.
| Frailty Measure | Criteria | Advantages | Disadvantages |
|---|---|---|---|
| Fried Frailty Phenotype | 1. Unintentional weight loss ≥10 pounds or ≥5% body weight in prior year 2. Weak grip strength Men: ≤29.0 kg if BMI ≤24 kg/m2 ≤30.0 kg if BMI 24–26 kg/m2 ≤31.0 kg if BMI 26–28 kg/m2 ≤32.0 kg if BMI >28 kg/m2 Women: ≤17.0 kg if BMI ≤23 kg/m2 ≤17.3 kg if BMI 23–26 kg/m2 ≤18.0 kg if BMI 26–29 kg/m2 ≤21.0 kg if BMI >29 kg/m2 3. Low activity Men <128 kCal expended /week Women <90 kCal expended/ week 4. Exhaustion: self-reported tiredness or weakness “all of the time” or most of the time” 5. Slowness, based on 5-m walk speed Men: ≤0.65 m/s if ≤173 cm tall, ≤0.76 m/s if >173 cm tall. Women: ≤0.65 m/s if ≤159 cm tall, ≤0.76 m/s if >159 cm tall Presence of 1–2 markers indicates pre-frailty, ≥3 indicates frailty |
Most widely cited and commonly used in clinical research Easy to administer in clinical setting |
Comprised of functional assessments that overlap with CVD symptoms |
| Rockwood Frailty Index (FI) | Between 30–70 deficits, including comorbidities, anthropomorphic measures, labs, self-reported health, and functional status—are summed to create an index FI expressed as a ratio of number of deficits present divided by number of deficits evaluated Deficits can be expressed as binary (0 or 1) or multi-level (0, 0.25, 0.50, 0.75, 1.0) FI of ≥0.21 indicate frailty |
Can be used to estimate frailty without the need for functional measures Can be used to estimate frailty retrospectively (i.e., claims-based data) |
Requires multiple variables to construct Requires electronic health record infrastructure to collect in clinical setting FI developed in one population may not be generalizable to another More reflective of comorbidity burden than functional status Depending on number of patient-reported outcome measures used, may overlap with symptoms of CVD |
|
Short Physical
Performance Battery |
Points assigned for performance in balance (standing, semi-tandem, and tandem stance), chair raises, and 4-m walk speed 0–3 points: very low performance 4–6 points: low performance 7–9 points: moderate performance 10–12 points: high performance |
Objectively assesses multiple domains of physical functioning: balance, strength, agility, and speed | Only includes physical elements of frailty; may not fully represent frailty as a construct Impractical to administer during clinical visit |
| Timed Up-and-Go | Patient sits up from chair, walks 3m, turns around and walks back to chair, then sits. Time of ≥16s suggestive has 96% specificity for frailty |
Easy to administer during clinical visit Multiple dimensions of physical function incorporated into single test |
Only includes physical elements of frailty; may not fully represent frailty as a construct Overlaps with CVD symptoms |
|
Essential Frailty
Toolkit |
1. Complete 5 chair raises: ≥15s (1 point) Unable to complete (2 points) 2. MMSE <24 (1 point) 3. Low hemoglobin (1 point) Men: <13mg/dL Women: <12mg/dL 4. Albumin <3.5g/dL (1 point) Scoring: 0 points = robust 1–2 points = pre-frail ≥3 points = frail |
Outperforms other tests in predicting mortality and disability Easy to administer during clinical visit Multiple domains of frailty assessed Does not overlap significantly with CVD symptoms |
Recently developed and not as commonly used in cardiovascular research. |
| Clinical Frailty Scale | Clinician-assigned frailty assessment; ranges from 1 (“very fit”) to 9 (“terminally ill”). | Easiest measure to administer | Low reproducibility, high risk of bias since it is based on individual clinician’s subjective assessment. |
| FRAIL scale | Five-item patient-reported outcome assessing fatigue, difficulty walking stairs, difficulty walking on flat ground, comorbidity burden, weight loss. 0 points = robust 1–2 points= pre-frail ≥3 points = frail |
Patient-reported outcome Easy to administer during clinical visit |
No objective functional measures Predominantly comprised of physical function measures that overlap with CVD symptoms |
Abbreviations: CVD, cardiovascular disease.
Several commonly used frailty measures focus only on physical attributes, such as the Short Physical Performance Battery (SPPB)9 and the timed up-and-go test (TUG),10 or use single-item measures of physical functioning, such as the 5-meter walk11 and handgrip strength.12 The Fried frailty phenotype is the most commonly used frailty measure and combines slow gait speed, weakness, unintentional weight loss, low physical activity, and self-reported exhaustion.13 A different conceptual model for frailty is the accumulation of deficits Frailty Index14, where between 30 and 70 anthropomorphic measures, comorbidities, laboratory values, and health status data are integrated into an index, under the principle that the more deficits an individual has, the more likely they are to be frail.15 Although health status and survey measures increase the accuracy of the frailty index, it can be constructed using entirely claims-based data.6,7 While this diagnostic strategy has clear limitations, being more centred on comorbidity burden than physical function, it can be useful for estimating frailty in existing studies without explicit prospectively-collected frailty measurements.
Both strategies for assessing frailty in cardiovascular disease have limitations: frailty measures focused on physical functioning can be impacted by cardiovascular-specific symptoms and the accumulation of deficits measure is heavily impacted by comorbidity burden and the severity of the underlying cardiovascular disease. Understanding the overlap between symptomatic cardiovascular disease and the frailty assessment used in a particular study is essential for determining the impact of frailty on outcomes. Given this overlap, it is also imperative to recognize that meeting criteria for frailty should not solely determine a lack of eligibility for a cardiovascular treatment, particularly if this treatment is focused on improving cardiovascular symptoms. Patients with the highest burden of cardiovascular symptoms and limitations due to those symptoms often have the greatest potential to benefit from these treatments, although perhaps a less-invasive approach could be considered in these cases.
Impact of frailty on health status
Heart failure.
There is substantial overlap in the pathophysiology of the frailty syndrome with heart failure,16 where hemodynamic abnormalities lead to global tissue hypoxia, cellular apoptosis, skeletal muscle dysfunction, and systemic inflammation. Similarly, frailty is characterized by systemic inflammation and neurohormonal dysfunction (particularly insulin resistance), resulting in muscle infiltration with adipose tissue and mitochondrial dysfunction.17,18 Both pathophysiologic pathways result in sarcopenia, sustained declines in functional status, and diminished physiologic reserve. Cross-sectionally, frailty is associated with a greater burden and longer duration of heart failure symptoms and poorer health status in patients with ambulatory heart failure and reduced ejection fraction19–22, heart failure and preserved ejection fraction23,24, and recent heart failure decompensation.25 Longitudinally, frail patients are also at greater risk of experiencing health status declines than non-frail patients. A secondary analysis of two large trials of patients with heart failure and reduced ejection fraction (PARADIGM-HF and ATMOSPHERE) showed that frailty was associated with a 60% greater odds of experiencing a clinically significant deterioration in health status at 1 year.20
Given the substantial overlap in pathophysiology between heart failure and frailty, therapies that target the adverse hemodynamic sequelae of heart failure (and therefore improve disease-specific health status) may play an important role in reversing frailty. In a multicentre study of 29 frail patients with advanced heart failure, the average number of Fried frailty criteria decreased from 3.9 ± 0.9 at baseline to 2.8 ± 1.4 at 6 months after left ventricular assist device implantation. Moreover, patients who were no longer frail had substantially larger improvements in heart failure disease-specific health status compared with those who still met criteria for frailty, further highlighting the close relationship between these two conditions.26 The impact of frailty on the effects of guideline-directed medical therapy (GDMT) on health status can be more complicated. Titration of medical therapy to trial-proven doses can improve heart failure disease-specific health status27,28, but frail patients are less likely achieve optimal doses of GDMT due to increased susceptibility of adverse side effects.29 In patients with heart failure and reduced ejection fraction (PARADIGM-HF), sacubitril-valsartan showed similar benefits in frail and non-frail patients for heart failure hospitalization and cardiovascular death, although the effect of frailty on health status outcomes was not examined.20 In patients with preserved ejection fraction (PARAGON-HF), sacubitril-valsartan reduced heart failure hospitalizations to a greater degree as frailty increased, but there were no differential effects of sacubitril-valsartan on death or health status outcomes.23 Notably, in both trials, frail patients had a greater risk of falls and study drug discontinuation due to adverse events than non-frail patients. In contrast, SGLT-2 inhibitors appear to be well-tolerated in frail patients, without additional adverse effects. In patients with either reduced ejection fraction (DAPA-HF)21 or preserved ejection fraction (DELIVER),24 there was a greater differential treatment benefit with dapagliflozin versus placebo in improving heart failure symptoms, functional limitations, and quality of life as the degree of frailty increased.
Beyond heart failure-specific interventions, physical rehabilitation can be of particular benefit to patients with heart failure and frailty, improving heart failure disease-specific health status, exercise tolerance, and functional mobility. In a secondary analysis of HF-ACTION, frail patients with heart failure and reduced ejection fraction had similar improvements in diseasespecific health status with aerobic exercise program compared with non-frail patients.22 In the REHAB-HF trial of patients with recent heart failure decompensation, a tailored physical therapy program that targeted strength, balance, mobility, and endurance improved both frailty markers and health status at 3 months, as compared with usual post-hospital care.30
Valvular heart disease.
Like heart failure, valvular heart disease has substantial overlap with frailty due to the cascade of abnormal cardiac filling pressures leading to systemic inflammation, musculoskeletal dysfunction, and sarcopenia. However, due to the higher average age of valve patients, the prevalence of frailty is generally higher in the valve population versus those with heart failure. Furthermore, concerns about any interaction of frailty with treatment benefit in valvular heart disease is even greater, as the treatment options in valvular heart disease are often invasive and thus could have a greater negative impact in patients with frailty. In the setting of severe aortic stenosis, frail patients have poorer health status at baseline31,32 and follow-up with TAVI33 or surgical aortic valve replacement (SAVR)32 compared with non-frail or pre-frail patients. Importantly, frailty is an important predictor of death and persistently poor health status after TAVI.34–36 However, frailty may also be potentially reversible after valve replacement, and improvements in frailty may be strongly driven by improvements in health status.37 Due to increased vulnerability to physiologic stressors and slower recovery, it has been postulated that patients who are frail may benefit more from a less invasive/transcatheter approach to valve replacement. However, in separate analyses from the PARTNER and CoreValve trials of patients with severe aortic stenosis, frailty was not associated with a differential treatment benefit of TAVI versus SAVR for death, heart failure hospitalization, or health status outcomes.38,39 Therefore, while frailty is an important predictor of poor outcomes, including persistently poor health status after valve replacement, there is no evidence that frail patients benefit more from TAVI versus SAVR.
The impact of frailty on health status after mitral valve intervention has been less studied. In a single centre study of patients undergoing mitral valve transcatheter edge-to-edge repair who were deemed prohibitive risk for valve surgery, surviving patients who were frail had greater improvement in health status over follow-up compared with non-frail patients.40 However, much of this was driven by the fact that frail patients were more symptomatic and had substantially worse quality of life before the procedure and therefore had more to gain from mitral valve repair.41
Coronary artery disease.
Although not as intricately linked as with heart failure or valve disease, the relationship between frailty and coronary artery disease (CAD) is also likely bidirectional and may be explained in part by an overlapping risk factor milieu (e.g., renal insufficiency, obesity, and elevated inflammatory biomarkers).42–44 The functional impairments caused by the frailty syndrome may increase sedentary behaviour, further increasing risk of development or progression of CAD.45
Among patients with CAD, frailty is associated with worse CAD disease-specific health status as compared with non-frail patients. In a cross-sectional study of 629 patients who underwent percutaneous coronary intervention (PCI), patients who were frail had a similar burden of angina symptoms but markedly worse CAD-related physical functioning and quality of life, as compared with non-frail and pre-frail patients.46 This disparity between angina burden and degree of angina-related physical impairment suggests that frail patients may be required to limit their physical activity more to maintain acceptable levels of angina. Given the existing mobility impairment in patients who are frail, any insult to functional ability, such as angina, will have greater negative impact on quality of life. The impact of frailty on the benefits of treatments, such as anti-anginal drugs, PCI, or coronary artery bypass grafting, on health status outcomes has not been studied but warrants further investigation.
Peripheral artery disease.
Peripheral artery disease (PAD) results in skeletal muscle apoptosis and atrophy through ischemia-mediated tissue injury,47 leading to exercise intolerance, exertional limb symptoms, and progressive functional decline. Frailty likely further exacerbates this functional decline, though the impact of frailty on disease-specific health status in patients PAD has been only minimally studied. In a cross-sectional study of 216 patients with symptomatic PAD, frail patients reported more health status impairment than non-frail patients.48 However, health status was examined in this study with the Walking Impairment Questionnaire, which is a single-domain instrument that captures health status related to physical functioning and thus has substantial overlap with frailty itself. The effect of frailty on PAD disease-specific health status measures, such as the Peripheral Artery Questionnaire49, has not been examined. As such, the impact of claudication on functional impairment and quality of life in frail versus non-frail patients is unknown. Moreover, the impact of frailty on health status outcomes with guideline-recommended therapies, such as supervised exercise therapy and surgical or endovascular revascularization, is also unknown. These unanswered questions should be prioritized given the substantial overlap in functional limitations between PAD and frailty (perhaps even more so than those seen in heart failure or valve disease) and the potential to improve frailty with PAD-specific treatments.
Atrial fibrillation.
The relationship between frailty and health status in patients with atrial fibrillation is poorly defined. This is partly due to the more diverse cohort of patients with atrial fibrillation, while some is also due to a lack of well-validated disease-specific health status measures. In a cross-sectional analysis of 1165 patients in the SAGE-AF registry50, frail patients were 1.3-times more likely to have impaired disease-specific health status as compared with non-frail patients, but this difference was no longer significant after adjusting for comorbidity burden. Another cross-sectional study of 158 patients showed that frailty was associated with a greater burden of atrial fibrillation symptoms and poorer quality of life.51 Whether frailty alters the health status outcomes of different treatments for atrial fibrillation has not been studied.
Conclusion and future directions.
Frailty is common in patients with cardiovascular disease and can have a substantial impact on patients’ functional capacity and quality of life. Importantly, frailty and cardiovascular disease-specific health status are intricately linked, with shared risk factors and pathophysiology. Treatments that reduce cardiovascular symptoms and the resultant functional limitations can also improve frailty status or even prevent frailty among those with marginal reserve. Thus, we need to be cautious not to empirically withhold these types of treatments solely based on an assessment of frailty. However, cardiovascular interventions that are more invasive or have a higher risk of complications could be potentially harmful in these patients, who have an impaired ability to recover acute stressors. Therefore, understanding the role of frailty in the outcomes after cardiovascular interventions is critically important for knowing which treatments to target to which patients. Given the importance of health status outcomes in maintaining autonomy and functional independence in older patients with frailty, examining the differential impact of frailty on disease-specific health status outcomes with one treatment versus another must be a priority in future studies. This knowledge, in addition to better understanding how to readily identify vulnerable patients in routine clinical care, will be critical for shared decision-making, informing patient selection, and improving the health and well-being of older patients with cardiovascular diseases.
Supplementary Material
ACKNOWLEDGEMENTS
Funding:
Dr. Nguyen is supported by the National Heart, Lung, and Blood Institutes of Health Under Award Number T32HL110837; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributorship: Both authors contributed to all parts of manuscript development and editing.
Competing Interests: Dr. Arnold is on the editorial board of BMJ Heart.
REFERENCES
- 1.Tsevat J, Dawson NV, Wu AW, et al. Health values of hospitalized patients 80 years or older. HELP Investigators. Hospitalized Elderly Longitudinal Project. JAMA. 1998;279(5):371–375. doi: 10.1001/jama.279.5.371 [DOI] [PubMed] [Google Scholar]
- 2.McNallan SM, Singh M, Chamberlain AM, et al. Frailty and healthcare utilization among patients with heart failure in the community. JACC Heart Fail. 2013;1(2):135–141. doi: 10.1016/J.JCHF.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polidoro A, Stefanelli F, Ciacciarelli M, Pacelli A, Di Sanzo D, Alessandri C. Frailty in patients affected by atrial fibrillation. Arch Gerontol Geriatr. 2013;57(3):325–327. doi: 10.1016/J.ARCHGER.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 4.Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J. 2014;35(26). doi: 10.1093/EURHEARTJ/EHU197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed A, Sorajja P, Pai A, et al. Prospective Evaluation of the Eyeball Test for Assessing Frailty in Patients With Valvular Heart Disease. J Am Coll Cardiol. 2016;68(25):29112912. doi: 10.1016/J.JACC.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Mañas L, Féart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62–67. doi: 10.1093/GERONA/GLS119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afilalo J. Frailty in Patients with Cardiovascular Disease: Why, When, and How to Measure. Curr Cardiovasc Risk Rep. 2011;5(5):467–472. doi: 10.1007/S12170-011-0186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ijaz N, Buta B, Xue QL, et al. Interventions for Frailty Among Older Adults With Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79(5):482–503. doi: 10.1016/J.JACC.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2). doi: 10.1093/GERONJ/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 10.Savva GM, Donoghue OA, Horgan F, O’Regan C, Cronin H, Kenny RA. Using Timed Up-and-Go to Identify Frail Members of the Older Population. Journals Gerontol Ser A. 2013;68(4):441–446. doi: 10.1093/GERONA/GLS190 [DOI] [PubMed] [Google Scholar]
- 11.Abellan Van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Heal aging 2009 1310. 2010;13(10):881–889. doi: 10.1007/S12603-009-0246-Z [DOI] [PubMed] [Google Scholar]
- 12.Ling CHY, Taekema D, De Craen AJM, Gussekloo J, Westendorp RGJ, Maier AB. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ. 2010;182(5):429–435. doi: 10.1503/CMAJ.091278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. Journals Gerontol - Ser A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 14.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/GERONA/62.7.722 [DOI] [PubMed] [Google Scholar]
- 15.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):1–10. doi: 10.1186/1471-2318-8-24/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonagh J, Martin L, Ferguson C, et al. Frailty assessment instruments in heart failure: A systematic review. Eur J Cardiovasc Nurs. 2018;17(1):23–35. doi: 10.1177/1474515117708888 [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Cohen AA, Xue QL, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging 2021 11. 2021;1(1):36–46. doi: 10.1038/s43587-020-00017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey A, Kitzman D, Reeves G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Hear Fail. 2019;7(12):1001–1011. doi: 10.1016/J.JCHF.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denfeld QE, Winters-Stone K, Mudd JO, Hiatt SO, Lee CS. Identifying a Relationship Between Physical Frailty and Heart Failure Symptoms. J Cardiovasc Nurs. 2018;33(1):E1–E7. doi: 10.1097/JCN.0000000000000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewan P, Jackson A, Jhund PS, et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction – an analysis of PARADIGM-HF and ATMOSPHERE. Eur J Heart Fail. 2020;22(11):2123–2133. doi: 10.1002/EJHF.1832 [DOI] [PubMed] [Google Scholar]
- 21.Butt JH, Dewan P, Merkely B, et al. Efficacy and Safety of Dapagliflozin According to Frailty in Heart Failure With Reduced Ejection Fraction : A Post Hoc Analysis of the DAPA-HF Trial. Ann Intern Med. 2022;175(6):820–830. doi: 10.7326/M21-4776 [DOI] [PubMed] [Google Scholar]
- 22.Pandey A, Segar MW, Singh S, et al. Frailty Status Modifies the Efficacy of Exercise Training Among Patients With Chronic Heart Failure and Reduced Ejection Fraction: An Analysis From the HF-ACTION Trial. Circulation. May 2022:101161CIRCULATIONAHA122059983. doi: 10.1161/CIRCULATIONAHA.122.059983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butt JH, Dewan P, Jhund PS, et al. Sacubitril/Valsartan and Frailty in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2022;80(12). doi: 10.1016/J.JACC.2022.06.037 [DOI] [PubMed] [Google Scholar]
- 24.Butt JH, Jhund PS, Belohlávek J, et al. Efficacy and Safety of Dapagliflozin According to Frailty in Patients with Heart Failure: A Prespecified Analysis of the DELIVER Trial. Circulation. August 2022. doi: 10.1161/CIRCULATIONAHA.122.061754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandey A, Kitzman D, Whellan DJ, et al. Frailty Among Older Decompensated Heart Failure Patients: Prevalence, Association With Patient-Centered Outcomes, and Efficient Detection Methods. JACC Hear Fail. 2019;7(12):1079–1088. doi: 10.1016/J.JCHF.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer MS, Horn E, Reyentovich A, et al. Can a Left Ventricular Assist Device in Advanced Systolic Heart Failure Improve or Reverse the Frailty Phenotype? J Am Geriatr Soc. 2017;65(11):2383. doi: 10.1111/JGS.15124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas M, Khariton Y, Fonarow GC, et al. Association of Changes in Heart Failure Treatment With Patients’ Health Status: Real-World Evidence From CHAMP-HF. JACC Heart Fail. 2019;7(7):615–625. doi: 10.1016/J.JCHF.2019.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khariton Y, Fonarow GC, Arnold SV., et al. Association Between Sacubitril/Valsartan Initiation and Health Status Outcomes in Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2019;7(11):933–941. doi: 10.1016/J.JCHF.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-theArt Review. J Am Coll Cardiol. 2020;76(20):2379–2390. doi: 10.1016/J.JACC.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 30.Kitzman DW, Whellan DJ, Duncan P, et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N Engl J Med. 2021;385(3):203–216. doi: 10.1056/NEJMOA2026141/SUPPL_FILE/NEJMOA2026141_DATASHARING.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold S V, Zhao Y, Leon MB, et al. Impact of Frailty and Prefrailty on Outcomes of Transcatheter or Surgical Aortic Valve Replacement. Circ Cardiovasc Interv. 2022;15(1):e011375. doi: 10.1161/CIRCINTERVENTIONS.121.011375 [DOI] [PubMed] [Google Scholar]
- 32.Borregaard B, Dahl JS, Lauck SB, et al. Association between frailty and self-reported health following heart valve surgery. Int J Cardiol Hear Vasc. 2020;31. doi: 10.1016/J.IJCHA.2020.100671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okoh AK, Chauhan D, Kang N, et al. The impact of frailty status on clinical and functional outcomes after transcatheter aortic valve replacement in nonagenarians with severe aortic stenosis. Catheter Cardiovasc Interv. 2017;90(6):1000–1006. doi: 10.1002/CCD.27083 [DOI] [PubMed] [Google Scholar]
- 34.Green P, Arnold SV., Cohen DJ, et al. Relation of Frailty to Outcomes After Transcatheter Aortic Valve Replacement (from the PARTNER Trial). Am J Cardiol. 2015;116(2):264. doi: 10.1016/J.AMJCARD.2015.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold S V, Afilalo J, Spertus JA, et al. Prediction of Poor Outcome After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2016;68(17):1868–1877. doi: 10.1016/J.JACC.2016.07.762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osnabrugge RL, Arnold SV., Reynolds MR, et al. Health Status after Transcatheter Aortic Valve Replacement in Patients at Extreme Surgical Risk: Results from the CoreValve U.S. Trial. JACC Cardiovasc Interv. 2015;8(2):315. doi: 10.1016/J.JCIN.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones DR, Chew DP, Horsfall MJ, et al. Impact of Surgical and Transcatheter Aortic Valve Replacement on Frailty Score. Heart Lung Circ. 2022;31(4):566–574. doi: 10.1016/J.HLC.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 38.Arnold S V, Zhao Y, Leon MB, et al. Impact of Frailty and Prefrailty on Outcomes of Transcatheter or Surgical Aortic Valve Replacement. Circ Cardiovasc Interv. 2022;15(1):e011375. doi: 10.1161/CIRCINTERVENTIONS.121.011375 [DOI] [PubMed] [Google Scholar]
- 39.Strom JB, Xu J, Orkaby AR, et al. Role of Frailty in Identifying Benefit From Transcatheter Versus Surgical Aortic Valve Replacement. Circ Cardiovasc Qual Outcomes. 2021;14(12):e008566. doi: 10.1161/CIRCOUTCOMES.121.008566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metze C, Matzik AS, Scherner M, et al. Impact of Frailty on Outcomes in Patients Undergoing Percutaneous Mitral Valve Repair. JACC Cardiovasc Interv. 2017;10(19):1920–1929. doi: 10.1016/J.JCIN.2017.07.042 [DOI] [PubMed] [Google Scholar]
- 41.Arnold S V. Frail Elderly—the Ideal Patients For MitraClip. JACC Cardiovasc Interv. 2017;10(19):1930. doi: 10.1016/J.JCIN.2017.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsay SE, Arianayagam DS, Whincup PH, et al. Cardiovascular risk profile and frailty in a population-based study of older British men. Heart. 2015;101(8):616–622. doi: 10.1136/HEARTJNL-2014-306472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/J.ARR.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 44.Bouillon K, Batty GD, Hamer M, et al. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart. 2013;99(10):737–742. doi: 10.1136/HEARTJNL-2012-302922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart R. Cardiovascular Disease and Frailty: What Are the Mechanistic Links? Clin Chem. 2019;65(1):80–86. doi: 10.1373/CLINCHEM.2018.287318 [DOI] [PubMed] [Google Scholar]
- 46.Kanwar A, Roger VL, Lennon RJ, Gharacholou SM, Singh M. Poor quality of life in patients with and without frailty: co-prevalence and prognostic implications in patients undergoing percutaneous coronary interventions and cardiac catheterization. Eur Hear J - Qual Care Clin Outcomes. 2021;7(6):591–600. doi: 10.1093/EHJQCCO/QCAA065 [DOI] [PubMed] [Google Scholar]
- 47.Hiatt WR, Armstrong EJ, Larson CJ, Brass EP. Pathogenesis of the Limb Manifestations and Exercise Limitations in Peripheral Artery Disease. Circ Res. 2015;116(9):1527–1539. doi: 10.1161/CIRCRESAHA.116.303566 [DOI] [PubMed] [Google Scholar]
- 48.Farah BQ, Santos MF, Cucato GG, et al. Effect of frailty on physical activity levels and walking capacity in patients with peripheral artery disease: A cross-sectional study. J Vasc Nurs. 2021;39(3):84–88. doi: 10.1016/J.JVN.2021.07.001 [DOI] [PubMed] [Google Scholar]
- 49.Spertus J, Jones P, Poler S, Rocha-Singh K. The Peripheral Artery Questionnaire: A new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147(2):301–308. doi: 10.1016/j.ahj.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 50.Marino FR, Lessard DM, Saczynski JS, et al. Gait Speed and Mood, Cognition, and Quality of Life in Older Adults With Atrial Fibrillation. J Am Heart Assoc. 2019;8(22). doi: 10.1161/JAHA.119.013212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sławuta A, Jacek P, Mazur G, Jankowska-Polańska B. Quality of Life and Frailty Syndrome in Patients with Atrial Fibrillation. Clin Interv Aging. 2020;15:783–795. doi: 10.2147/CIA.S248170 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.