Abstract
Background:
We sought to determine whether circulating modifiers of endothelial function are associated with cardiac structure and clinical outcomes in patients with HFrEF.
Methods:
We measured 25 proteins related to endothelial function in 99 patients from the GUIDE-IT study. Protein levels were evaluated for association with echocardiographic parameters and the incidence of all-cause death and hospitalization for heart failure (HHF).
Results:
Higher concentrations of ANGPT2, VEGFR1 and HGF were significantly associated with worse function and larger ventricular volumes. Over time, decreases in ANGPT2 and, to a lesser extent, VEGFR1 and HGF, were associated with improvements in cardiac size and function. Individuals with higher concentrations of ANGPT2, VEGFR1, or HGF concentrations had an increased risk for a composite of death and HHF in the following year (HR 2.76 (95% CI 1.73 to 4.40) per 2-fold change in ANGPT2; HR 1.76 (95% CI 1.11 to 2.79) for VEGFR1; and HR 4.04 (95% CI 2.19 to 7.44) for HGF).
Conclusions:
Proteins related to endothelial function associate with cardiac size, cardiac function, and clinical outcomes in patients with HFrEF. These results support the concept that endothelial function may be an important contributor to the progression and recovery from HFrEF.
Keywords: Angiopoietin 2, reverse remodeling, biomarker, Angiokine
Graphical Abstract
Take Home Visual Graphic: Changes in Circulating Angiokines are Associated with Reverse Remodeling and Outcomes in Chronic Heart Failure
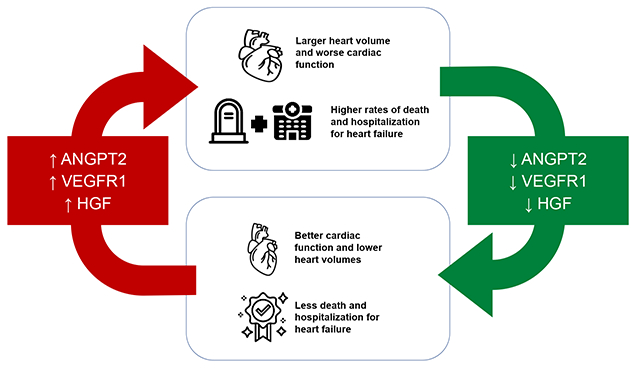
Lay Summary
Prior work has shown that failing hearts have a reduced density of blood vessels. We hypothesized that circulating proteins that regulate blood vessel growth and function might be altered in patients with heart failure. We measured protein levels in a group of patients from the GUIDE-IT study that had multiple assessments of heart size and function. We found that higher concentrations of ANGPT2, VEGFR1, and HGF in the blood are related to larger hearts with worse function. In hearts that recovered function over time, the concentrations of these three proteins decreased. In addition, patients with higher concentrations of ANGPT2, VEGFR1, and HGF were more likely to die or be hospitalized for heart failure. Our results suggest that changes in the blood vessels of the heart in response to the circulating factors ANGPT2, VEGFR1, and HGF could regulate the severity of heart failure.
Introduction
Heart failure with reduced ejection fraction (HFrEF) is associated with significant morbidity and mortality, but there is considerable variation in outcomes. Patients with significant reverse left ventricular (LV) remodeling, characterized by a reduction in LV volumes, have improved survival and fewer hospitalizations during follow-up.1–5 Treatment with guideline-directed medical therapies (GDMT) can improve the probability of reverse remodeling in HFrEF, but this response to GDMT remains variable.6,7 Furthermore, the mechanisms that mediate recovery of ventricular function are poorly defined. Better insight into the pathobiology of cardiac remodeling could allow for targeted interventions, could increase rates of reverse remodeling, and might improve outcomes among patients with HFrEF.
In normal hearts, an average cardiomyocyte is associated with approximately ~1-1.2 cardiac endothelial cells, such that nearly every cardiomyocyte is adjacent to a capillary.8 This close anatomic coupling suggests that myovascular cross-talk is a critical regulator of myocardial growth and adaptation to injury.9–12 In preclinical models, decreased microvascular density, known as vascular rarefaction, has been implicated in the transition from compensatory LV hypertrophy to HFrEF.13,14 Translational studies suggest a similar role in the human heart. Vascular rarefaction, with resultant decreases in myocardial blood flow, have been associated with advanced heart failure in patients with HFrEF15–19 and HFpEF.20 While preclinical studies to molecularly revascularize the heart have yet to be successfully translated into clinical success for HFrEF,21 several “pillars” of GDMT such as angiotensin-converting enzyme inhibitors, angiotensin receptor-neprolysin inhibitors, beta blockers, and sodium-glucose cotransporter-2 inhibitors have been associated with increases in myocardial capillary density.22–26 The precise mechanisms that drive vascular rarefaction, endothelial dysfunction, or recovery of the microvasculature are not well delineated but single center studies have linked alterations of systemic angiogenic factors to both ischemic and non-ischemic HFrEF.18,27,28 However, whether concentrations of angiogenic factors can be used to monitor treatment responses in HFrEF remains unknown.
We performed an exploratory analysis to test the hypothesis that concentrations of circulating modulators of endothelial growth and maintenance correlate with reverse remodeling and outcomes in patients with HFrEF. We utilized echocardiographic data and banked plasma samples from the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment (GUIDE-IT) trial for this work.29
Methods
Trial design and outcomes for the GUIDE-IT study have been previously reported.29,30 Briefly, GUIDE-IT was a multicenter trial that randomized 894 patients with a left ventricular ejection fraction (LVEF) ≤ 40% to NT-proBNP-guided management or usual care. Participants were planned for 24 months of follow-up after enrollment; however, GUIDE-IT was stopped early for futility. 268 participants from GUIDE-IT also participated in a pre-specified echocardiographic substudy.31 These participants underwent echocardiograms within 30 days of study enrollment and again at 12 months. Of these patients, 99 participants had both serial blood samples and underwent concurrent echocardiograms at 0 and 12 months (Figure 1).
Figure 1: Consort Diagram.

Patients from GUIDE-IT included in this analysis were limited to those with both echo and plasma samples at 0 and 12 months from enrollment.
All participants included in this analysis provided written informed consent to participate in the GUIDE-IT trial and the accompanying echo and biomarker sub-studies. GUIDE-IT and its sub-studies were approved by institutional review boards at each site.
Echocardiographic Analysis
Protocolized echocardiograms from the echocardiographic sub-study of GUIDE-IT were analyzed in an independent core laboratory at the Duke Clinical Research Institute (Durham, NC), as previously reported. 31 All echocardiographic interpretations were performed by blinded readers in accordance with current guidelines.32
Protein Profiling
All included participants provided EDTA-plasma samples at the time of their baseline and 12-month echocardiograms. Samples were stored at −80°C in a centralized biobank (Covance, Indianapolis, IN) until analysis. We employed a validated assay panel, termed the Angiome, to evaluate concentrations of key circulating protein biomarkers related to angiogenesis, inflammation, and extracellular matrix (ECM) remodeling. The Angiome panel has been validated and approved for use by the National Cancer Institute for use across a variety of cancer clinical trials.33–35 The panel consists of enzyme linked immunoassays (ELISAs) using the Quanterix SPX platform (Quanterix; Billerica, MA), the Meso Scale Discovery platform (Meso Scale Diagnostics; Rockville, MD), and the Ella platform (ProteinSimple; San Jose, CA). All samples were assessed using a single batch printed multiplex plates to minimize assay variability. Included on each plate was a standard sample of pooled plasma from healthy volunteers to ensure consistency across plates.34 Lab personnel were blinded to any demographic and clinical outcome data. An averaged value across two technical replicates was used for analysis. For this exploratory analysis, only proteins with a coefficient of variation greater than 0.75 across baseline samples were considered for additional analyses to minimize multiple testing. Proteins with incomplete data (>15% of samples below limit of detection) in the study cohort were also excluded. A full list of sample proteins is available in Supplemental Table 1.
Statistical Analysis
Continuous and categorical variables were compared between groups using the Wilcoxon rank sum test and the Fisher exact test, respectively. Biomarker concentrations are presented as median ± IQR unless otherwise noted; continuous variables are presented as mean ± SD; categorical variables are shown as counts and percentages. Prior to modeling, biomarker concentrations were log2 transformed. Mixed effect linear regression models were constructed to estimate protein effects on echocardiographic parameters, including protein concentrations as a fixed effect and subject number as a random effect to acknowledge repeated measurements from single participants over time.
For ANGPT2, VEGFR1, and HGF, we used linear regression to model changes in echocardiographic parameters by the change in biomarker concentrations over the same time frame. To determine effects on reverse remodeling, we examined patients with or without a relative decrease in LVESVi by ≥15% over 12 months, a threshold previously associated with better outcomes.31 The correlation between NT-proBNP concentrations and biomarker concentrations was evaluated by linear regression, with R values of <0.4 considered weak, ≥0.4 considered moderate, and those ≥0.6 considered strong. As participants had to survive to 12 months for participation in the echocardiographic sub-study, landmark analysis was set at 12 months, and clinical outcomes were evaluated from 12 through 24 months after study enrollment. For time-to-event analysis for the composite outcome of death and HHF and the outcome of death alone, Kaplan-Meier (KM) cumulative risk curves were used to estimate the event rate, and log-rank statistics were used for comparisons between angiokine tertiles. For time to event analyses using the HHF outcome, cumulative incidence curves were generated after adjusting for the competing risk of death and Gray’s method was used to compare angiokine tertiles.36 Cox proportional hazard modeling was used to evaluate the hazard ratio (HR) for outcomes based on ANGPT2, VEGFR1 and HGF and NT-proBNP concentrations at 12 months. HRs are presented as change per 2-fold change in each biomarker. Cause specific hazard ratios are reported for all-cause death and HFH.
The threshold for statistical significance was 2-sided with a type I error rate of 0.05. Due to the exploratory nature of this work, no adjustment for multiple comparisons was performed. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina) and R.37
Results
Patient Population
99 participants from the GUIDE-IT Echo sub-study had available plasma for Angiome profiling. Cohort characteristics are presented in Table 1. The average age was 60.9 ± 11.2 years (mean ± SD); 29.3% (29/99) of patients were female; and 48.5% (48/99) of participants had an ischemic cardiomyopathy. Mean left ventricular ejection fraction (LVEF) at the time of enrollment was 28.2 ± 10.0% with improvement in mean LVEF over 12 months to 34.5 ± 11.9%. Median follow-up was 18.7 months (IQR 15.0-23.8 months). Overall, 7.1% (7/99) of all patients died, 10.1% (10/99) were hospitalized for HF , and 12.1% (12/99) experienced either death or a hospitalization for HF over the 12 month follow-up.
Table 1: Baseline Characteristics of the Study Participants Baseline Characteristics of Patient Cohort.
Continuous variables are presented as mean (SD) and categorical variables are presented as counts (%), with the exception of NT-proBNP concentrations, which are presented as median (IQR). Blue denotes p <0.05; purple <0.01, and green <0.001, p value is difference in means between those with and without remodeling. Body mass index (BMI); chronic obstructive pulmonary disease (COPD); left ventricular ejection fraction (LVEF); heart failure (HF); myocardial infarction (MI); month (mo); obstructive sleep apnea (OSA); peripheral arterial disease (PAD); ventricular tachycardia (VT).
| Parameter | Overall (n=99) | <15% Reduction in LVESVi (n=48) | ≥15% Reduction in LVESVi (n=51) | P Value |
|---|---|---|---|---|
| Age, years | 60.9 (11.2) | 60.1 (10.2) | 61.6 (12.2) | 0.36 |
| Male | 70 (70.7%) | 33(68.8%) | 37(72.5%) | 0.85 |
| Race | ||||
| White | 57(57.6%) | 27(56.3%) | 30(58.8%) | 0.34 |
| Black | 38(38%) | 17(35.4%) | 21(41.2%) | 0.31 |
| Smoking History | 38(38.4%) | 16(33.3%) | 22(43.1%) | 0.43 |
| BMI (kg/m2) | 30.1(7.3) | 29.9(7.04) | 30.3(7.6) | 0.94 |
| Hypertension | 74(74.7%) | 39(81.3%) | 35(68.6%) | 0.23 |
| Hyperlipidemia | 58(58.6%) | 35(72.9%) | 23(45.1%) | <0.01 |
| Type 2 Diabetes | 44(44.4%) | 27(56.3%) | 17(33%) | 0.04 |
| Atrial Fibrillation | 39(39.4%) | 17(35.4%) | 22(43.1%) | 0.56 |
| COPD | 15(15.2%) | 8(16.7%) | 7(13.7%) | 0.9 |
| OSA | 25(25.3%) | 13(27.1%) | 12(23.5%) | 0.86 |
| PAD | 13(13.1%) | 10(20.8%) | 3(5.9%) | 0.06 |
| Chronic Kidney Disease | 32(32.3%) | 18(37.5%) | 14(27.5%) | 0.39 |
| Prior MI | 38(38.4%) | 26(54.2%) | 12(23.5%) | <0.01 |
| Prior Stroke | 8(8.1%) | 2(4.2%) | 6(11.8%) | 0.31 |
| Prior VT | 18(18.2%) | 10(20.8%) | 8(15.7%) | 0.69 |
| HF duration, mo | 64.4 (89.4) | 90.6 (97.4) | 42.1(76.3) | <0.01 |
| Ischemic Cardiomyopathy | 48(48.5%) | 31(64.6%) | 17(33.3%) | <0.01 |
| Baseline LVEF,% | 28.2(9.97) | 28(9.6) | 28.4(10.4) | 0.96 |
| 12 mo LVEF, mean | 34.5(11.9) | 28.8(9.47) | 39.8(11.6) | <0.001 |
| Baseline NT-proBNP, (pg/mL) | 2446(1394-4331) | 2556(1496-5668) | 2263(1321.5-3623) | 0.22 |
| 12 mo NTproBNP, (pg/mL) | 1305(507.8-2887) | 2306(1133.8-5201.2) | 666.3 (273.7-1438) | <0.001 |
Concentrations of Circulating Angiokines are Associated with Ventricular Size and Function
To determine whether systemic concentrations of endothelial modulators are related to ventricular structure, we assayed a panel of 25 proteins originally developed for monitoring responses to anti-angiogenic therapies in cancer patients.33–35 We assayed plasma samples obtained at the baseline visit and at the 12-month follow-up visit of the GUIDE-IT study. Proteins with incomplete data (i.e., with >15% below limit of detection) in the study cohort and/or with little variance across baseline samples were filtered to minimize multiple testing, leaving 10 candidate proteins (Supplemental Table 1, Supplemental Figure 1). Using these proteins, we next evaluated for associations between factor concentrations and contemporaneous echocardiographic parameters. We noted multiple significant associations of protein concentrations with echocardiographic parameters (Figure 2, Supplemental Figure 2, and Supplemental Table 2).
Figure 2: Association of Circulating Modulators of Endothelial Function with Echocardiographic Markers of Cardiac Size and Function.

Point estimates and 95% confidence intervals depicting the effect of a 2-fold increase in each protein with echocardiographic parameters. A. Association of each protein with left ventricular ejection fraction (LVEF). B. Association of each protein with global longitudinal strain (GLS). C. Association of each protein with left ventricular end systolic volume index (LVESVi). D. Association of each protein with left ventricular end diastolic volume index (LVEDVi).
Among the 10 candidates analyzed, concentrations of three protein modulators of angiogenesis, or angiokines, were most consistently correlated with measures of cardiac size and function: ANGPT2, VEGFR1, and HGF (Figure 2, Supplemental Figure 2, and Supplemental Table 2). ANGPT2 and HGF had significant correlations with 9 of 10 echocardiographic measures tested, while VEGFR1 was significantly associated with 8 of 10 parameters. Associations were stronger for ANGPT2 than for VEGFR1 or HGF. In general, increases in circulating ANGPT2, VEGFR1, and HGF were associated with worse cardiac function (decreased LVEF and higher GLS) and larger cardiac size (increased LVESVi, LVEDVi, LASVi, and RA area). All three markers were also associated with higher right-sided pressures (RVSP) and worse right ventricular function (lower TAPSE). However, only ANGPT2 and HGF were significantly associated with higher left-sided filling pressures (E/e’). In addition to identifying proteins that were associated with more advanced HFrEF, we also noted a positive association of VEGFA concentrations with higher LVEF and decreased ventricular size (LVESVi).
Association of ANGPT2, VEGFR1, and HGF Concentrations with Reverse Remodeling
Based on the close correlation of ANGPT2, VEGFR1, and HGF concentrations with multiple morphologic and functional features, we focused our subsequent analyses on these three biomarkers. To determine whether biomarker concentrations related to ventricular remodeling, we modeled change in echocardiographic parameters with the change in ANGPT2, VEGFR1, and HGF concentrations overtime (Supplemental Figure 3). A 2-fold increase in ANGPT2 over 12 months was associated with a 2.31% decrease in LVEF (95% CI, −3.90 to −0.71%, p=0.005). Similarly, a 2-fold increase in VEGFR1 was associated with a 2.86% decrease in LVEF (95% CI −4.75 to −0.97, p=0.003), and a 2-fold increase in HGF was associated with a 2.01% decrease in LVEF (95% CI −3.84 to −0.18, p=0.03). We also observed a significant relationship between ANGPT2 and LVESVi, such that increasing ANGPT2 concentrations over time were associated with increased LVESVi and LVEDVi, with similar though less robust trends for VEGFR1 and HGF (Supplemental Figure 3). Taken together, these findings indicate that increases in ANGPT2, VEGFR1, and HGF concentrations are related to adverse changes in cardiac size and function over time within a given patient (Take Home Visual Graphic).
We next sought to determine how ANGPT2, VEGFR1, and HGF concentrations change in patients with significant reverse remodeling, defined as a 15% relative decrease in LVESVi based on previous work.32 Among the 99 patients included, 51 (51.5%) participants in our cohort had at least a 15% decrease in LVESVi (Table 1). Patients with reverse remodeling were less likely to have an ischemic cardiomyopathy (33.3% vs 64.9%, p= 0.004), less likely to have had a prior myocardial infarction (23.5% vs 41.7%, p=0.0034), and had a shorter duration of HF (42.1 ± 76.3 months vs 90.6 ± 97.4 months, p=0.003). Importantly, there was no significant difference between the remodeling and non-remodeling groups for baseline mean LVEF (28.4 ± 10.4% vs 28% ± 9.6%, p=0.964) or baseline median NT-proBNP concentrations (2263 pg/mL, IQR 1322-3623 pg/mL vs 2556 pg/mL, IQR 1596-5668 pg/mL, p=0.22). When we considered the change in ANGPT2, VEGFR1, and HGF, we identified significant differences in the change in protein levels depending on whether the definition for reverse remodeling was met (Figure 3). Specifically, we found that concentrations for all three proteins decreased in participants with a ≥15% reduction in LVESVi, and there was a significant difference in these biomarkers over time between patients with and without reverse remodeling (unadjusted analyses). Interestingly, VEGFR1 concentrations tended to be higher at baseline in participants who went on to reverse remodeling compared to those without (median 149 pg/ml, IQR 115-236 pg/ml vs 134, IQR 102-182 pg/ml, p=0.06).
Figure 3: Changes in ANGPT2, VEGFR1, and HGF Concentrations Based on the Presence of Reverse Remodeling Over the Study Period.
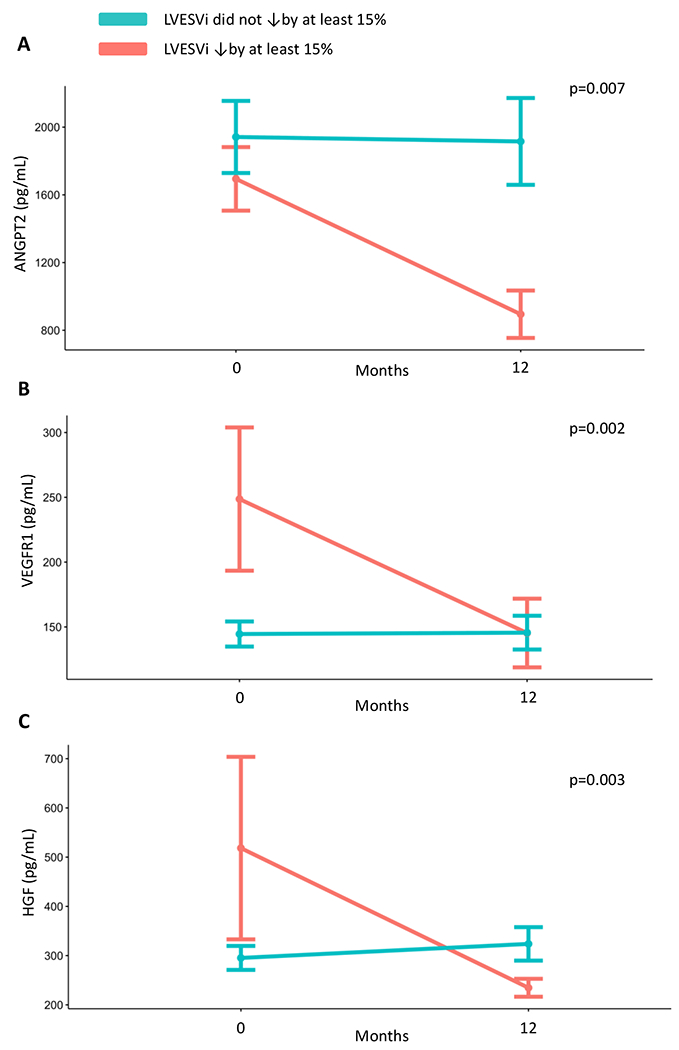
Mean biomarker concentrations across the study period are plotted based on the presence (orange line) or absence (blue line) of reverse modeling. Error bars indicate SEM. P values are the results of Wilcoxon rank-sum testing for a difference in change in biomarker concentrations between patients with and without reverse remodeling at time points 0 and 12 months. Panel A: ANGPT2 Panel B: VEGFR1 Panel C: HGF Left Ventricular end systolic volume index (LVESVi).
Relationship between ANGPT2, VEGFR1, and HGF with NT-proBNP Concentrations
The correlation of ANGPT2, VEGFR1, and HGF concentrations with cardiac structure and function is similar to associations observed with NT-proBNP.30 Thus, we assessed the relationship between each biomarker and NT-proBNP concentrations (Supplemental Figure 4). We found that ANGPT2 concentrations were weakly, but significantly, correlated with NT-proBNP at baseline (R=0.32, p=0.001), and moderately correlated at 12 months and when assessed as change from baseline in both proteins (R=0.59 and 0.42, respectively, p<0.001 for both). VEGFR1 concentrations were weakly, but significantly, correlated with baseline, 12-month, and the change in NT-proBNP concentrations. HGF was only associated with NT-proBNP level at 12 months (R=0.40, p<0.001).
Higher ANGPT2, VEGFR1, and HGF Concentrations are Associated with Poor Outcomes
Morphologic and functional cardiac parameters are highly prognostic of outcomes in patients with HFrEF, and we sought to determine whether ANGPT2, VEGFR1, and HGF concentrations might be similarly prognostic. After grouping patients into tertiles for each biomarker at 12 months, we found that participants in the highest tertile of each biomarker had a significantly increased incidence of a composite endpoint of all-cause death and need for HHF (Figure 4). When we analyzed each marker for associations with each component of the composite endpoint, those with the highest tertile of ANGPT2 or HGF concentrations had a significantly increased incidence of both death and HHF (Supplemental Figure 5. By contrast, adverse outcomes for individuals with the highest tertile of VEGFR1 were primarily the result an increased incidence of HHF (Supplemental Figure 5). Overall, elevated ANGPT2, VEGFR1, and HGF concentrations are highly associated with adverse outcomes in this cohort of patients with HFrEF.
Figure 4: Association of ANGPT2, VEGFR1, and HGF Concentrations with a Composite of Death and Hospitalization for Heart Failure.
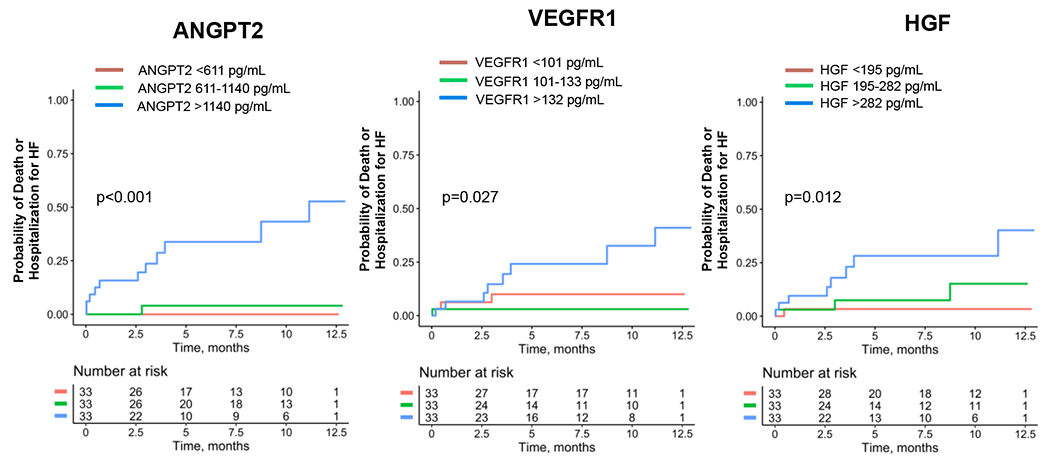
P value is the result of log-rank testing. Note that this was a landmark analysis, and all patients necessarily survived for the 12 months prior to these events; patients may have been hospitalized during this initial 12 month period).
When we assessed the cause-specific hazard ratios for all-cause death or HHF, and relative hazard ratio for a composite endpoint of all-cause death and HHF for ANGPT2, VEGFR1, HGF, and NT-proBNP, we found that 12-month concentrations for each of these biomarkers was highly associated with the composite outcome. When considering individual components of the composite, ANGPT2, HGF, and NT-proBNP concentrations were associated with both the hazard of death and HHF. However, VEGFR1 concentrations were primarily driven by an association with the incidence of HHF (Table 2).
Table 2. Hazard for Death and Hospitalization for Heart Failure by Biomarker Concentration.
Hazard ratios are shown with 95% confidence intervals and are per 2-fold change in each biomarker. Cause-specific HRs are provided for death and hospitalization for heart failure (HHF). Blue denotes p <0.05; purple <0.01, and green <0.001
| Composite | Death | HHF | ||||
|---|---|---|---|---|---|---|
| HR | p value | HR | p value | HR | p value | |
| ANGPT2 | 2.76 (1.73 to 4.40) | <0.001 | 2.98 (1.57 to 5.67) | <0.001 | 3.02 (1.77 to 5.14) | <0.001 |
| VEGFR1 | 1.76 (1.11 to 2.79) | 0.016 | 1.62 (0.86 to 2.04) | 0.14 | 1.98 (1.25 to 3.12) | 0.003 |
| HGF | 4.04 (2.19 to 7.44) | <0.001 | 3.02 (1.58 to 5.80) | < 0.001 | 5.28 (2.60 to 10.7) | <0.001 |
| NT-proBNP | 2.28 (1.55 to 3.35) | <0.001 | 2.11 (1.29 to 3.44) | 0.003 | 2.01 (1.35 to 3.00) | <0.001 |
Discussion
Much of the biology of reverse cardiac remodeling has focused on changes to the cardiomyocyte. For example, decreases in cardiomyocyte size, increased β-adrenergic receptor density, and improved calcium handling have all been reported with reverse remodeling.38 Importantly, the fetal gene expression program that is induced in failing myocytes is attenuated, likely reflected clinically by decreased NT-proBNP concentrations during reverse remodeling.31 Other work has suggested that restoration of microvascular density occurs with reverse remodeling.11 Thus, we hypothesized that systemic concentrations of angiogenic factors are associated with reverse remodeling.
In this study, we assessed concentrations of 25 modulators of endothelial homeostasis and function for their association with cardiac structure and function in a cohort of patients with HFrEF.39 While we found several of these proteins to be associated with different echocardiographic parameters, ANGPT2, VEGFR1, and HGF were most consistently correlated with a broad array of echocardiographic parameters, such that higher concentrations of these biomarkers corresponded to larger cardiac volumes and worse function (Figure 2, Supplemental Table 2). Furthermore, we found that changes in ANGPT2 and, to a lesser extent, VEGFR1 and HGF, over time paralleled changes in markers of cardiac function (Supplemental Figure 3), and that changes in ANGPT2, VEGFR1 and HGF over time were associated with a clinically significant measure of reverse remodeling (Figure 3). Finally, we observed that ANGPT2, VEGFR1, and HGF concentrations are highly associated with a composite outcome of death and HHF (Figure 4). Taken together, these findings strongly support the role of ANGPT2, VEGFR1, and HGF as important mediators of progressive HF, with prognostic significance.
Our results are concordant with previous studies. For each factor, prior work has suggested higher concentrations are related to acute decompensation (ANGPT228 and HGF40) or to worse clinical outcomes of patients with HFrEF (ANGPT241,42 HGF43 and VEGFR144–47). However, to our knowledge, our work is the first to link these factors with reverse remodeling. ANGPT2, in particular, is an emerging biomarker for HFrEF. Peplinski et al recently reported that higher ANGPT2 concentrations are correlated with increases of pulmonary capillary wedge pressure and right atrial pressures in patients with left-sided heart failure,39 and Haddad et al have additionally reported an association between ANGPT2 and presence of pulmonary hypertension,48 findings that parallel our association of ANGPT2 with a higher RVSP and larger right atrial size. Additionally, previous reports have shown that patients with HFrEF have significantly higher concentrations of ANGPT2 than the general population,27 even suggestive of a potential screening role.
Mechanistically, our work is suggestive of a blunted angiogenic program in the failing heart. Depending on its context, ANGPT2 can either stimulate angiogenesis, as in tumors, or inhibit endothelial cell proliferation.49 In the heart, pre-clinical work indicates that ANGPT2 causally mediates microvascular regression after injury.50 Mice subjected to experimental myocardial infarction demonstrate increased expression of Angpt2 in the border zone by cardiac endothelial cells and macrophages. Inhibition of ANGPT2 by either genetic deletion or treatment with an anti-ANGPT2 antibody prevents capillary loss and mitigates ventricular dysfunction after infarction. As a receptor, VEGFR1 can mediate vascular growth. However, the sFLT1 isoform of VEGFR1 that was measured here, is secreted into circulation where it limits angiogenesis by binding and trapping VEGF. Interestingly, VEGFA was the only protein for which higher concentrations were associated with better ventricular function and smaller chamber sizes (Figure 2, Supplemental Table 2). We noted that VEGFR1 concentrations trended to a higher concentration in individuals that would experience reverse remodeling. Hence, VEGFR1 may be a marker of at-risk, but recoverable myocardium. HGF, by contrast, is generally considered to be a mitogen for multiple cell types, including endothelial cells, in vitro and in pre-clinical models. 51,52 HGF has been proposed previously as a potential therapeutic for ischemic heart failure.51,53,54 However, our work and others linking higher HGF concentrations with more advanced heart failure raises questions about this approach.
We acknowledge several limitations to this exploratory work. First, our study was based on a subset of the GUIDE-IT study and is subject to biases of secondary analyses. Second, the absolute size of our cohort was relatively small, with relatively few events and an unusually high rate of reverse remodeling. Thus, our work may have been underpowered to identify significant relationships of additional proteins, with echocardiographic parameters or HFrEF outcomes. For example, whether VEGFR1 concentrations can be used to identify individuals likely to remodel should be explored further. Sample size and event rates also limited further analyses on the relationship between angiogenic proteins and GDMT use and the use of multivariable models to adjust for NT-proBNP and other clinical parameters know to be prognostic in HFrEF. Third, our studies focused on a handful of modulators of endothelial growth and maintenance with well-developed assays, but additional proteins are likely to be active in HFrEF and may have similarly predictive roles.
Taken together, our work supports a role for circulating modulators of endothelial homeostasis as mediators of HFrEF. Future work is needed to verify the ability of ANGPT2, VEGFR1, and HGF to prognosticate outcomes in patients with HFrEF in a larger dataset, and to understand if and how these protein concentrations change in response to GDMT for HF. Mechanistically, we speculate that circulating angiokines could link progressive HFrEF with vascular rarefaction. In addition to testing a broader panel of proteins on additional patient populations, fundamental work is needed to define the mechanisms that link endothelial function to progressive HFrEF. In particular, the effects of circulating angiokines ANGPT2 and VEGFR1 on the coronary microvasculature needs to be established. Nevertheless, our findings suggest that angiokines may be an important class of biomarkers in HFrEF, and potential mediators of progressive heart failure.
Conclusion
We found that circulating regulators of endothelial homeostasis and function have varying concentrations among patients with HFrEF, with dynamics that are closely associated with differences in cardiac structure and function. Of the factors assayed, ANGPT2, VEGFR1, and HGF concentrations were most robustly related to cardiac morphologic and functional parameters and were highly associated with the need for hospitalization for heart failure (HHF) and death. Our work suggests that dynamic changes in circulating modulators of endothelial function could play a role in the regulation of cardiac function in HFrEF and adds to the growing body of literature supporting circulating angiokines as prognostic biomarkers in HFrEF.
Supplementary Material
Supplemental Figure 1: Heatmap Showing Clustering of Measured Factors. Baseline concentrations of factors are scaled and represented by red for increased concentrations and blue for decreased concentrations. Proteins are clustered by relative factor level (rows) and by patients (columns).
Supplemental Figure 2: Association of Endothelial Factors with Additional Echocardiographic Markers of Cardiac Size and Function. Point estimates and confidence intervals depicting the effect of a 2-fold increase in each protein with echocardiographic parameters. Association of each protein with A. E/e’ ratio, B. right ventricular systolic pressure (RVSP), C. tricuspid annular plane systolic excursion (TAPSE), D. cardiac index (CI), E. RA (right atrial) area and F. left atrial systolic volume index (LA SVi).
Supplemental Figure 3: Relationship Between Change from Baseline in ANGPT2, VEGFR1, and HGF with Change in Echocardiographic Markers of Cardiac Size and Function. Scatter plots showing changes in echocardiographic parameters as a function of changing ANGPT2, VEGFR1, and HGF concentrations within a given patient. The blue line indicates the best-fit regression line with the 95% confidence interval shown in gray. Dashed red lines indicate the 95% CI for predictions based on the regression line. P values indicated the significance of correlation testing. A. Change in left ventricular ejection fraction (LVEF) relative to change in ANGPT2. B. Change in left ventricular end systolic volume index (LVESVi) relative to change in ANGPT2. C. Change in left ventricular end diastolic volume index (LVEDVi) relative to change in ANGPT2. D. Change in LVEF relative to change in VEGFR1. E. Change in LVESVi relative to change in VEGFR1. F. Change in LVEDVi relative to change in VEGFR1 G. Change in LVEF relative to change in HGF. H. Change in LVESVi relative to change in HGF. I. Change in LVEDVi relative to change in HGF
Supplemental Figure 4: Relationship between ANGPT2, VEGFR1, and HGF with NT-proBNP. Scatterplots of ANGPT2 (Panels A, B, C), VEGFR1 (Panels D, E, F), HGF (Panels G, H, I), and NT-proBNP concentrations measured at the baseline study visit, the 12 month study visit, and as change from baseline. R value is the correlation coefficient and p value indicates significance of correlation. The blue line indicates the best-fit regression line with the 95% confidence interval shown in gray. Dashed red lines indicate the 95% CI for predictions based on the regression line.
Supplemental Figure 5: Association of ANGPT2, VEGFR1, and HGF Concentrations with Death and Hospitalization for Heart Failure. For death as an outcome, p value is the result of log-rank testing. For hospitalization for heart failure as an outcome, death was considered to be a competing risk and the p-value is the result of Gray’s method. Note that this was a landmark analysis, and all patients necessarily survived for the 12 months prior to these events; patients may have been hospitalized during this initial 12 month period). Please note that for panel A, cumulative incidence curves for the two lowest ANGPT2 tertiles are superimposed.
Clinical Perspective.
A decrease in cardiac capillary density has been associated with worsening heart failure. We identified associations between concentrations of proteins known to affect endothelial function with cardiac size and function in patients with HFrEF. Of measured factors, we found ANGPT2, VEGFR1, and HGF concentrations to be closely associated with ventricular remodeling and to be associated with future death and hospitalization for HFrEF. Our work suggests that endothelial remodeling is an important modifier of HFrEF and identifies new biomarkers with the potential to predict both clinical outcomes and reverse remodeling in HFrEF.
Acknowledgments
We thank all the patients and sites who generously participated in the GUIDE-IT study. We also thank Christopher Kontos and Michael Dee Gunn for helpful comments and discussion; Gayle Passmore for regulatory assistance; Andrew Shepple for assistance with sample transfer; and Mark Starr and Chris Brady for technical assistance.
Funding Sources
This work was funded by a grant from the Translating Duke Health Initiative (RK, GMF), a Duke University Strong Start Physician Scientist Award (RK), the Walker P. Inman Endowment (RK), and NHLBI R01 HL157277 (RK). GUIDE-IT was funded by the NHLBI, and the embedded biorepository and echo sub-studies used for these analyses were funded by Roche Diagnostics (Rotkreutz, Switzerland).
Disclosures
JH completed work for this manuscript while supported by grant T32HL069749
DJM serves as a consultant for CVRx, Cytokinetics, and Novonordisk, and receives grant funding from NovoNordisk and the NIH.
ABN receives funding from Genentech, HTG Molecular Diagnostics, MedImmune/AstraZeneca, Medpacto, Promega Corporation, and Seattle Genetics, and consulting or personal fees from Eli Lilly, GSK, Promega Corporation, Leap Therapeutics, and Adjuvolt Therapeutics.
ILP serves on the advisory board for Vifor and AstraZeneca
KFA has grant support from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb Company, Boehringer Ingelheim Pharmaceuticals Inc, Lilly USA, LivaNova USA Inc, Merck, Novartis and Otsuka, and has acted as a consultant to Amgen, Cytokinetics, Novartis, Roche Diagnostics, Relypsa, and Windtree Therapeutics.
JJ is a Trustee of the American College of Cardiology, a Board member of Imbria Pharmaceuticals, has received grant support from Applied Therapeutics, Innolife, Novartis Pharmaceuticals and Abbott Diagnostics, consulting income from Abbott, Janssen, Novartis, and Roche Diagnostics, and participates in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, MyoKardia and Takeda.
GMF has received research grants from NHLBI, American Heart Association, Amgen, Bayer, BMS, Merck, Cytokinetics; he has acted as a consultant to Novartis, Amgen, BMS, Cytokinetics, Medtronic, Cardionomic, Boehringer-Ingelheim, American Regent, Abbott, Astra-Zeneca, Reprieve, Myovant, Sequana, Windtree Therapuetics, and Whiteswell, and has served on clinical endpoint committees/data safety monitoring boards for Amgen, Merck, Medtronic, EBR Systems, V-Wave, LivaNova, Siemens, and Rocket Pharma
Abbreviations
- ANGPT2
Angiopoietin 2
- VEGFR1
Vascular endothelial growth factor 1
- HGF
Hepatocyte growth factor
- GLS
global longitudinal strain
- HHF
hospitalization for heart failure
- HFrEF
heart failure with reduced ejection fraction
- LVEF
left ventricular ejection fraction
- LVEDVi
left ventricular end diastolic index
- LVESVi
left ventricular end systolic index
- RVSP
right ventricular systolic pressure
- TAPSE
tricuspid annular plane systolic excursion
Biography

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CMO, EY, JE, MAD, MF and RK have no disclosures related to this work.
References
- 1.Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, Li S, Papadimitriou L, Butler J. Characteristics and Outcomes of Adult Outpatients With Heart Failure and Improved or Recovered Ejection Fraction. JAMA Cardiol. 2016;1:510–518. [DOI] [PubMed] [Google Scholar]
- 2.Tanabe K, Sakamoto T. Heart failure with recovered ejection fraction. J Echocardiogr. 2019;17:5–9. [DOI] [PubMed] [Google Scholar]
- 3.Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Kirk J, Fudim M, Green CL, Karra R. Heterogeneous Outcomes of Heart Failure with Better Ejection Fraction. J Cardiovasc Transl Res. 2020;13:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart Failure With Recovered Ejection Fraction: A Distinct Clinical Entity. Journal of Cardiac Failure. 2011;17:527–532. [DOI] [PubMed] [Google Scholar]
- 6.Florea VG, Rector TS, Anand IS, Cohn JN. Heart Failure With Improved Ejection Fraction: Clinical Characteristics, Correlates of Recovery, and Survival: Results From the Valsartan Heart Failure Trial. Circ Heart Fail. 2016;9:e003123. [DOI] [PubMed] [Google Scholar]
- 7.Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM, Solomon SD, PROVE-HF Investigators. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA. 2019;322:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisén J. Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161:1566–1575. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh PCH, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez CE, Bakovic M, Karra R. Endothelial Contributions to Zebrafish Heart Regeneration. J Cardiovasc Dev Dis. 2018;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talman V, Kivelä R. Cardiomyocyte—Endothelial Cell Interactions in Cardiac Remodeling and Regeneration. Frontiers in Cardiovascular Medicine. 2018;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBeneditis P, Karpurapu A, Henry A, Thomas MC, McCord T, Brezitski K, Prasad A, Baker CE, Kobayashi Y, Shah S, Kontos C, Tata PR, Lumbers RT, Karra R. Coupled myovascular expansion directs cardiac growth and regeneration. Development. 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrino C, Prasad SVN, Mao L, Noma T, Yan Z, Kim H-S, Smithies O, Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drakos SG, Kfoury AG, Hammond EH, Reid BB, Revelo MP, Rasmusson BY, Whitehead KJ, Salama ME, Selzman CH, Stehlik J, Clayson SE, Bristow MR, Renlund DG, Li DY. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J Am Coll Cardiol. 2010;56:382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsagalou EP, Anastasiou-Nana M, Agapitos E, Gika A, Drakos SG, Terrovitis JV, Ntalianis A, Nanas JN. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2008;52:1391–1398. [DOI] [PubMed] [Google Scholar]
- 17.Parodi O, De Maria R, Oltrona L, Testa R, Sambuceti G, Roghi A, Merli M, Belingheri L, Accinni R, Spinelli F. Myocardial blood flow distribution in patients with ischemic heart disease or dilated cardiomyopathy undergoing heart transplantation. Circulation. 1993;88:509–522. [DOI] [PubMed] [Google Scholar]
- 18.Abraham D, Hofbauer R, Schäfer R, Blumer R, Paulus P, Miksovsky A, Traxler H, Kocher A, Aharinejad S. Selective Downregulation of VEGF-A165, VEGF-R1, and Decreased Capillary Density in Patients With Dilative but Not Ischemic Cardiomyopathy. Circulation Research. 2000;87:644–647. [DOI] [PubMed] [Google Scholar]
- 19.Mosseri M, Schaper J, Admon D, Hasin Y, Gotsman MS, Sapoznikov D, Pickering JG, Yarom R. Coronary capillaries in patients with congestive cardiomyopathy or angina pectoris with patent main coronary arteries. Ultrastructural morphometry of endomyocardial biopsy samples. Circulation. 1991;84:203–210. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taimeh Z, Loughran J, Birks EJ, Bolli R. Vascular endothelial growth factor in heart failure. Nat Rev Cardiol. 2013;10:519–530. [DOI] [PubMed] [Google Scholar]
- 22.Rengo G, Cannavo A, Liccardo D, Zincarelli C, de Lucia C, Pagano G, Komici K, Parisi V, Scala O, Agresta A, Rapacciuolo A, Perrone Filardi P, Ferrara N, Koch WJ, Trimarco B, Femminella GD, Leosco D. Vascular endothelial growth factor blockade prevents the beneficial effects of β-blocker therapy on cardiac function, angiogenesis, and remodeling in heart failure. Circ Heart Fail. 2013;6:1259–1267. [DOI] [PubMed] [Google Scholar]
- 23.Miura S-I, Nishikawa H, Zhang B, Matsuo Y, Kawamura A, Tsuchiya Y, Matsuo K, Saku K. Angiotensin-converting enzyme inhibitor promotes coronary collateral circulation in patients with coronary artery disease. Circ J. 2003;67:535–538. [DOI] [PubMed] [Google Scholar]
- 24.Yazawa H, Miyachi M, Furukawa M, Takahashi K, Takatsu M, Tsuboi K, Ohtake M, Murase T, Hattori T, Kato Y, Murohara T, Nagata K. Angiotensin-converting enzyme inhibition promotes coronary angiogenesis in the failing heart of Dahl salt-sensitive hypertensive rats. J Card Fail. 2011;17:1041–1050. [DOI] [PubMed] [Google Scholar]
- 25.Pfau D, Thorn SL, Zhang J, Mikush N, Renaud JM, Klein R, deKemp RA, Wu X, Hu X, Sinusas AJ, Young LH, Tirziu D. Angiotensin Receptor Neprilysin Inhibitor Attenuates Myocardial Remodeling and Improves Infarct Perfusion in Experimental Heart Failure. Sci Rep. 2019;9:5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varricchi G, Loffredo S, Bencivenga L, Ferrara AL, Gambino G, Ferrara N, de Paulis A, Marone G, Rengo G. Angiopoietins, Vascular Endothelial Growth Factors and Secretory Phospholipase A2 in Ischemic and Non-Ischemic Heart Failure. J Clin Med. 2020;9:E1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong AY, Caine GJ, Freestone B, Blann AD, Lip GYH. Plasma angiopoietin-1, angiopoietin-2, and angiopoietin receptor tie-2 levels in congestive heart failure. J Am Coll Cardiol. 2004;43:423–428. [DOI] [PubMed] [Google Scholar]
- 29.Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, Januzzi JL, Mark DB, Piña IL, Passmore G, Whellan DJ, Yang H, Cooper LS, Leifer ES, Desvigne-Nickens P, O’Connor CM. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2017;318:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felker GM, Ahmad T, Anstrom KJ, Adams KF, Cooper LS, Ezekowitz J, Fiuzat M, Houston-Miller N, Januzzi JL, Leifer E, Mark D, Desvigne-Nickens P, Paynter G, Piña IL, Whellan DJ, O’Connor CM. Rationale and Design of the GUIDing Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT) Study. JACC Heart Fail. 2014;2:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daubert MA, Adams K, Yow E, Barnhart HX, Douglas PS, Rimmer S, Norris C, Cooper L, Leifer E, Desvigne-Nickens P, Anstrom K, Fiuzat M, Ezekowitz J, Mark DB, O’Connor CM, Januzzi J, Felker GM. NT-proBNP Goal Achievement Is Associated With Significant Reverse Remodeling and Improved Clinical Outcomes in HFrEF. JACC: Heart Failure. 2019;7:158–168. [DOI] [PubMed] [Google Scholar]
- 32.Gold MR, Daubert C, Abraham WT, Ghio S, Sutton MStJ, Hudnall JH, Cerkvenik JJ, Linde C. The effect of reverse remodeling on long-term survival in mildly symptomatic patients with heart failure receiving cardiac resynchronization therapy: Results of the REVERSE study. Heart Rhythm. 2015;12:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Lyu J, Bell Burdett K, Sibley AB, Hatch AJ, Starr MD, Brady JC, Hammond K, Marmorino F, Rossini D, Goldberg RM, Falcone A, Cremolini C, Owzar K, Ivanova A, Moore DT, Lee MS, Sanoff HK, Innocenti F, Nixon AB. Prognostic and Predictive Biomarkers in Patients with Metastatic Colorectal Cancer Receiving Regorafenib. Mol Cancer Ther. 2020;19:2146–2154. [DOI] [PubMed] [Google Scholar]
- 34.Nixon AB, Pang H, Starr MD, Friedman PN, Bertagnolli MM, Kindler HL, Goldberg RM, Venook AP, Hurwitz HI, Alliance for Clinical Trials In Oncology. Prognostic and predictive blood-based biomarkers in patients with advanced pancreatic cancer: results from CALGB80303 (Alliance). Clin Cancer Res. 2013;19:6957–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armstrong AJ, Nixon AB, Carmack A, Yang Q, Eisen T, Stadler WM, Jones RJ, Garcia JA, Vaishampayan UN, Picus J, Hawkins RE, Hainsworth JD, Kollmannsberger CK, Logan TF, Puzanov I, Pickering LM, Ryan CW, Protheroe A, George DJ, Halabi S. Angiokines Associated with Targeted Therapy Outcomes in Patients with Non-Clear Cell Renal Cell Carcinoma. Clin Cancer Res. 2021;27:3317–3328. [DOI] [PubMed] [Google Scholar]
- 36.The Annals of Statistics. 1988. p. 1141–1154. [Google Scholar]
- 37.R: The R Project for Statistical Computing [Internet]. [cited 2021 Oct 27];Available from: https://www.r-project.org/
- 38.Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol. 2018;15:83–96. [DOI] [PubMed] [Google Scholar]
- 39.Peplinski BS, Houston BA, Bluemke DA, Kawut SM, Kolb TM, Kronmal RA, Lima JAC, Ralph DD, Rayner SG, Steinberg ZL, Tedford RJ, Leary PJ. Associations of Angiopoietins With Heart Failure Incidence and Severity. J Card Fail. 2021;27:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueno S, Ikeda U, Hojo Y, Arakawa H, Nonaka M, Yamamoto K, Shimada K. Serum hepatocyte growth factor levels are increased in patients with congestive heart failure. J Card Fail. 2001;7:329–334. [DOI] [PubMed] [Google Scholar]
- 41.Eleuteri E, Di Stefano A, Giordano A, Corrà U, Tarro Genta F, Gnemmi I, Giannuzzi P. Prognostic value of angiopoietin-2 in patients with chronic heart failure. Int J Cardiol. 2016;212:364–368. [DOI] [PubMed] [Google Scholar]
- 42.Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, Sterling DG, Williams SA. Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients With Stable Coronary Heart Disease. JAMA. 2016;315:2532–2541. [DOI] [PubMed] [Google Scholar]
- 43.Rychli K, Richter B, Hohensinner PJ, Kariem Mahdy A, Neuhold S, Zorn G, Berger R, Mörtl D, Huber K, Pacher R, Wojta J, Niessner A, Hülsmann M. Hepatocyte growth factor is a strong predictor of mortality in patients with advanced heart failure. Heart. 2011;97:1158–1163. [DOI] [PubMed] [Google Scholar]
- 44.Ky B, French B, Ruparel K, Sweitzer NK, Fang JC, Levy WC, Sawyer DB, Cappola TP. The vascular marker soluble fms-like tyrosine kinase 1 is associated with disease severity and adverse outcomes in chronic heart failure. J Am Coll Cardiol. 2011;58:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demissei BG, Valente MAE, Cleland JG, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Givertz MM, Bloomfield DM, Dittrich H, van der Meer P, van Veldhuisen DJ, Hillege HL, Voors AA. Optimizing clinical use of biomarkers in high-risk acute heart failure patients. Eur J Heart Fail. 2016;18:269–280. [DOI] [PubMed] [Google Scholar]
- 46.Hammadah M, Georgiopoulou VV, Kalogeropoulos AP, Weber M, Wang X, Samara MA, Wu Y, Butler J, Tang WHW. Elevated Soluble Fms-Like Tyrosine Kinase-1 and Placental-Like Growth Factor Levels Are Associated With Development and Mortality Risk in Heart Failure. Circ Heart Fail. 2016;9:e002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vorovich E, French B, Ky B, Goldberg L, Fang JC, Sweitzer NK, Cappola TP. Biomarker predictors of cardiac hospitalization in chronic heart failure: a recurrent event analysis. J Card Fail. 2014;20:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haddad F, Ataam JA, Amsallem M, Cauwenberghs N, Kuznetsova T, Rosenberg-Hasson Y, Zamanian RT, Karakikes I, Horne BD, Muhlestein JB, Kwee L, Shah S, Maecker H, Knight S, Knowlton K. Insulin Growth Factor Phenotypes in Heart Failure with Preserved Ejection Fraction, an INSPIRE Registry and CATHGEN Study: IGF axis in HFpEF. Journal of Cardiac Failure [Internet]. 2021. [cited 2022 Jan 7];0. Available from: https://www.onlinejcf.com/article/S1071-9164(21)00526-1/fulltext [DOI] [PubMed]
- 49.Souma T, Thomson BR, Heinen S, Carota IA, Yamaguchi S, Onay T, Liu P, Ghosh AK, Li C, Eremina V, Hong Y-K, Economides AN, Vestweber D, Peters KG, Jin J, Quaggin SE. Context-dependent functions of angiopoietin 2 are determined by the endothelial phosphatase VEPTP. Proc Natl Acad Sci U S A. 2018;115:1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S-J, Lee C-K, Kang S, Park I, Kim YH, Kim SK, Hong SP, Bae H, He Y, Kubota Y, Koh GY. Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction. J Clin Invest. 2018;128:5018–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morishita R, Aoki M, Hashiya N, Yamasaki K, Kurinami H, Shimizu S, Makino H, Takesya Y, Azuma J, Ogihara T. Therapeutic angiogenesis using hepatocyte growth factor (HGF). Curr Gene Ther. 2004;4:199–206. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura T. Hepatocyte growth factor as mitogen, motogen and morphogen, and its roles in organ regeneration. Princess Takamatsu Symp. 1994;24:195–213. [PubMed] [Google Scholar]
- 53.Jin H, Wyss JM, Yang R, Schwall R. The therapeutic potential of hepatocyte growth factor for myocardial infarction and heart failure. Curr Pharm Des. 2004;10:2525–2533. [DOI] [PubMed] [Google Scholar]
- 54.Meng H, Chen B, Tao Z, Xu Z, Wang L, Weizhu J, Hong Y, Liu X, Wang H, Wang L, Wu Z, Yang Z. Safety and Efficacy of Adenovirus Carrying Hepatocyte Growth Factor Gene by Percutaneous Endocardial Injection for Treating Post-infarct Heart Failure: A Phase IIa Clinical Trial. Curr Gene Ther. 2018;18:125–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Heatmap Showing Clustering of Measured Factors. Baseline concentrations of factors are scaled and represented by red for increased concentrations and blue for decreased concentrations. Proteins are clustered by relative factor level (rows) and by patients (columns).
Supplemental Figure 2: Association of Endothelial Factors with Additional Echocardiographic Markers of Cardiac Size and Function. Point estimates and confidence intervals depicting the effect of a 2-fold increase in each protein with echocardiographic parameters. Association of each protein with A. E/e’ ratio, B. right ventricular systolic pressure (RVSP), C. tricuspid annular plane systolic excursion (TAPSE), D. cardiac index (CI), E. RA (right atrial) area and F. left atrial systolic volume index (LA SVi).
Supplemental Figure 3: Relationship Between Change from Baseline in ANGPT2, VEGFR1, and HGF with Change in Echocardiographic Markers of Cardiac Size and Function. Scatter plots showing changes in echocardiographic parameters as a function of changing ANGPT2, VEGFR1, and HGF concentrations within a given patient. The blue line indicates the best-fit regression line with the 95% confidence interval shown in gray. Dashed red lines indicate the 95% CI for predictions based on the regression line. P values indicated the significance of correlation testing. A. Change in left ventricular ejection fraction (LVEF) relative to change in ANGPT2. B. Change in left ventricular end systolic volume index (LVESVi) relative to change in ANGPT2. C. Change in left ventricular end diastolic volume index (LVEDVi) relative to change in ANGPT2. D. Change in LVEF relative to change in VEGFR1. E. Change in LVESVi relative to change in VEGFR1. F. Change in LVEDVi relative to change in VEGFR1 G. Change in LVEF relative to change in HGF. H. Change in LVESVi relative to change in HGF. I. Change in LVEDVi relative to change in HGF
Supplemental Figure 4: Relationship between ANGPT2, VEGFR1, and HGF with NT-proBNP. Scatterplots of ANGPT2 (Panels A, B, C), VEGFR1 (Panels D, E, F), HGF (Panels G, H, I), and NT-proBNP concentrations measured at the baseline study visit, the 12 month study visit, and as change from baseline. R value is the correlation coefficient and p value indicates significance of correlation. The blue line indicates the best-fit regression line with the 95% confidence interval shown in gray. Dashed red lines indicate the 95% CI for predictions based on the regression line.
Supplemental Figure 5: Association of ANGPT2, VEGFR1, and HGF Concentrations with Death and Hospitalization for Heart Failure. For death as an outcome, p value is the result of log-rank testing. For hospitalization for heart failure as an outcome, death was considered to be a competing risk and the p-value is the result of Gray’s method. Note that this was a landmark analysis, and all patients necessarily survived for the 12 months prior to these events; patients may have been hospitalized during this initial 12 month period). Please note that for panel A, cumulative incidence curves for the two lowest ANGPT2 tertiles are superimposed.


