Abstract
Alzheimer’s disease(AD) patients have a high risk of developing mesial temporal lobe epilepsy(MTLE) and subclinical epileptiform activity.MTLE in AD worsens outcomes.Therefore, we need to understand the overlap between these disease processes.We hypothesize that AD with MTLE represents a distinct subtype of AD, with the interplay between tau and epileptiform activity at its core.We discuss shared pathological features including histopathology,an initial mesial temporal lobe(MTL) hyperexcitability followed by MTL dysfunction and involvement of same networks in memory(AD) and seizures(MTLE).We provide evidence that tau accumulation linearly increases neuronal hyperexcitability,neuronal hyper-excitability increases tau secretion, tau can provoke seizures and tau reduction protects against seizures.We speculate that AD genetic mutations increase tau, which causes proportionate neuronal loss and/or hyperexcitability, leading to seizures.We discuss that tau burden in MTL predicts cognitive deficits among 1)AD and 2)MTLE without AD. Finally, we explore the possibility that anti-seizure medications improve cognition by reducing neuronal hyper-excitability, which reduces seizures and tau accumulation and spread.
Keywords: cognition, dementia, mild cognitive impairment (MCI), Alzheimer’s disease (AD), epilepsy, temporal lobe epilepsy, seizures, tau
BACKGROUND
Dementia is the most costly disease in the US1. Alzheimer’s disease (AD) is the most common type of dementia.AD is a public health crisis, the leading cause of disability, morbidity and mortality among older adults,1. AD pathology is characterized by histopathological evidence of senile or beta-amyloid plaques (Aβ) and neurofibrillary tangles (NFTs)2 characterized by accumulation of abnormally phosphorylated tau (pTau).
Epilepsy is the third most common neurological condition among adults over 653 and is also considered a public health imperative. Its incidence is highest among the elderly age group3. People with epilepsy (PwE) have a high degree of associated comorbidity, especially in the elderly4.
AD patients have a substantially higher risk of developing epilepsy than the general population especially mesial temporal lobe epilepsy (MTLE) and late-onset PwE have a substantially higher risk of developing AD5-8. Recent meta-analyses have confirmed a very high prevalence of epilepsy and epileptiform activity in AD9,10. There is a large body of evidence that MTLE -related seizures are very common in AD patients, and they worsen prognosis and may even be associated with shorter life expectancy11. Despite the high incidence, co-occurrence, and worse outcomes, we do not understand the clinical presentation, neuropathology, clinical course, and outcomes of patients with AD and co-morbid epilepsy. To understand these, the interaction between the two disease processes needs to be delineated.
Is AD with MTLE a distinct subtype of AD with pTau as the predominant driver behind these disease processes?
We propose that AD with MTLE represents a clinically distinct subtype of AD with distinct neuropathology, clinical features, and a more aggressive disease course with worse outcomes than AD patients without MTLE. Herein, we provide evidence that these two disease processes are characterized by excessive tau pathology, and speculate that tau may provide the link between these two diseases. Further, we explore the possibility that the interplay between tau, neuronal hyperexcitability and epileptiform activity is at the core of this distinct subtype of AD.
High co-occurrence and bi-directional association between MTLE and AD
Unprovoked seizures occur in 10-64% of AD patients at varying stages of the disease process12. Patients with AD ≥ 65 years of age have an 8-to-10-fold higher rate of unprovoked seizures compared to the general population6. In addition, AD patients have a considerably high risk of developing epilepsy especially MTLE7. Early onset AD (<65 years at onset) has an even higher association with epilepsy compared to late onset AD13.
Older PwEhave a relative risk of 1.5 of developing dementia over a span of 8 years8,14. Late-onset PwE have a substantially higher risk of developing dementia especially AD5. AD and APOε4 have been found to be among the leading risk factors for the development of late-onset epilepsy (epilepsy onset at or after the age of 65)15.
In addition to seizures and epilepsy, many AD patients show evidence of subclinical interictal epileptiform activity and/or electroencephalographic (EEG) seizures without a known history of epilepsy16-19. As many as 42% of AD patients demonstrate subclinical interictal epileptiform discharges, especially of the mesial temporal regions16-20. . In addition, silent hippocampal seizures occur in AD patients18. Up to 90% of epileptiform activity is seen with extended EEG recorded exclusively during sleep16.
These findings suggest that occult mesial temporal hyper-excitability may contribute to AD pathogenesis.
The possible shared pathology between late onset MTLE and AD:
In both animal and human studies, increasing evidence suggests overlap and shared pathological features between late-onset MTLE and AD. AD and MTLE have several intersecting neuroimaging and histopathological features21. Both diseases primarily affect the mesial temporal lobe and memory16-18. Amnestic MCI and early AD patients demonstrate an initial mesial temporal lobe hyperexcitability followed by mesial temporal lobe dysfunction22. This is similar to mesial temporal lobe hyperexcitability observed during seizures in MTLE and the mesial temporal lobe dysfunction observed in between seizures.
There is also evidence that the same cortical networks that are involved in memory and altered in AD, are also affected in MTLE23. Hippocampus and entorhinal cortex are both involved in memory storage and retrieval as well as in generating mesial temporal seizures23. Alterations in local limbic circuits including papez circuit are often observed in both AD and MTLE 23. Hippocampal coupling with association cortices has also been shown to be involved in memory consolidation especially long term memory, in both animal and human studies24,25. Seizures in the mesial temporal lobe epilepsy also follow the same cortical functional connections of the entorhinal cortex and hippocampus.
Histopathological features of AD and MTLE:
Both disease processes demonstrate early involvement of the entorhinal cortex and hippocampus. AD associated NFTs are composed of abnormally phosphorylated tau protein. The pattern of NFT deposition in AD is relatively stereotyped and the degree, as well as areas of NFT involvement, tend to be associated with the degree of cognitive impairment and the disease duration2,26. Senile,neuritic or beta-amyloid (Aβ) plaques represent another cardinal histopathological findings among AD patients 2,27.
In AD, tau pathology involves entorhinal cortex early-on and loss of neurons in layer II and IV of entorhinal cortex is one of the earliest changes seen28,29. Hippocampal CA1 (cornu ammonis 1) receives its inputs from the entorhinal cortex and is also involved next in the pre-clinical stages of disease28. It spreads from cell to cell transneuronally and trans-synaptically secondary to neuronal activity. Tau deposits follow patterns of entorhinal functional connectivity. From the entorhinal cortex, tau spreads to involve limbic regions including hippocampus, parahippocampal gyrus, amgydala, perirhinal cortex and eventually to inferior temporal region and the basal forebrain28,30. During the later stages of disease, neocortex especially precuneus and lateral parietal regions become involved28,30.Other histopathological findings in AD tend to also occur in the hippocampus. These include granulovacuolar degeneration, hirano bodies and synaptic loss. 2.
Studies have shown that drug resistant MTLE patient undergoing temporal lobectomy show findings similar to AD histopathology and involve similar regions. Most commonly stigmata of high pTau in the mesial temporal structures on immunohistochemistry is observed31,32. Less commonly, amyloid plaques may be seen in MTLE patients undergoing temporal lobectomy33. Other less common findings that may be seen in both conditions include reduction of calbintin-D28k (calcium binding protein) in dentate gyrus34 and hippocampal sclerosis35.
Revised BRAAK staging shows that, during the pretangle stage of AD, pTau accumulates in subcortical locus coeruleus before any cortical regions are involved, including mesial temporal regions36. While locus coeruleus in autopsy specimens of MTLE patients has not been studied for evidence of pTau, it is interesting to note that neurostimulation of locus coeruleus as a target for the treatment of epilepsy has shown successful results in improvement of epilepsy37-39. Therefore, potential role of locus coeruleus in MTLE needs to be elucidated.
Hippocampal neuronal hyperexcitability and dysfunction is at the core of both disease processes:
Seizures are defined as abnormal, synchronous excitation of neurons in the brain. It is well-known that network and neuronal hyperexitability is the primary cause of seizures and hippocampal neuronal hyperexcitability is seen mesial temporal lobe seizures . While during ictal states, hippocampal hyperexcitability is seen, interictally hippocampal dysfunction is observed. Very similar to this, recent evidence has suggested that AD also begins with network hyperexcitability. In earlier stages of AD,,in patients with subjective cognitive impairment or MCI and some cognitively healthy adults with familial risk for AD40, hippocampal hyperactivation is observed41 just like the hippocampal hyperactivation seen in MTLE. Hippocampal hyperactivation is accompanied by cortical thinning in the regions of the brain classically implicated in AD40. Hippocampal hyperexcitability is also evident in AD patients from the increased amount of subclinical IEDs and silent seizures reported on EEG of these patients16-18.
It has been proposed that hippocampal activity follows an inverted U shape pattern and early hyperactivity is followed by hypoactivity in the later stages of AD22. Hippocampal hyperactivity seen in early stages of cognitive impairment can manifest as epileptiform discharges and seizures since ictal discharges commonly arise from the hippocampus in MTLE. Similarly, hippocampal hypoactivity41, seen in the later stages of cognitive impairment and overt AD is a manifestation of an increasingly diseased and dysfunctional hippocampus. This is similar to hippocampal dysfunction often seen in advanced MTLE.
Network dysfunction in AD has also been observed in functional magnetic resonance imaging (fMRI) studies of these patients during memory tasks and involves the regions typically implicated in MTLE. Network alterations are most prominent in mesial temporal lobe and neocortical regions involved in memory, including precuneus and posterior cingulate42.
Moreover, hippocampal atrophy and often visible sclerosis is a common neuro-imaging abnormality often observed in both conditions21 (Figure 1).
Figure 1: Similarities between neuro-imaging findings of AD and MTLE.
Note the atrophied hippocampi bilaterally, which may be sclerosed in either of the conditions.
This evidence of hippocampal hyper and hypoactivity at various stages of cognitive impairment and the presence of hippocampal sclerosis (Figure 1) can explain the increased co-occurrence of epileptiform activity and seizures in the mesial temporal regions in patients with AD.
Memory circuits in AD and seizure network in MTLE involve the same brain regions:
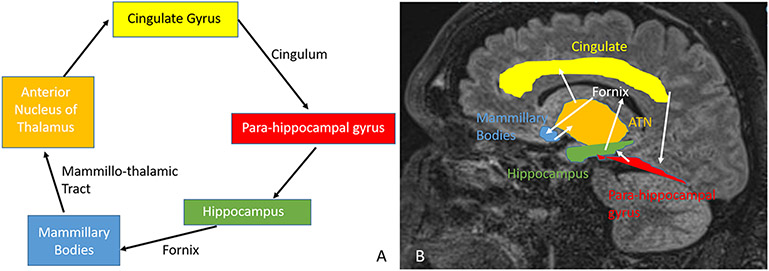
Papez circuit (Figure 2) connects hippocampus via fornix to mammillary body and anterior nucleus of thalamus which sends projections via cingulate gyrus to parahippocampal gyrus which connects back to the hippocampus, and plays a major role in memory consolidation23. Various degrees of alteration in the papez circuit is observed in AD. Similarly, alteration of the papez circuit is often implicated in many PwE especially those with MTLE, and various regions of the papez circuit are increasingly being studied and used as therapeutic targets for neuro-modulation in the management of drug-resistant epilepsy23.
Figure 2: Papez Circuit:
The memory pathway and the pathway for seizures are the same. The interaction between different structures is demonstrated. A: Shows a simplified version of the Papez circuit, while B demonstrates the structures on a sagittal MRI scan. ATN: Anterior thalamic nucleus.
The connection between the hippocampus and cortex is required for memory formation in the brain25. Interictal epileptiform discharges (IEDs) in the hippocampus on depth recording have been shown to disrupt memory retrieval, consolidation and cognition in rat models25. It has been hypothesized that IEDs provide a way by which abnormal activity is transmitted to cortical brain regions and networks involved in memory consolidation. Thus, IEDs not only disrupt hippocampal networks, but also cortical networks and areas connected to hippocampus25. Depth electrode studies of MTLE PwEhave shown that hippocampal IEDs result in transient cognitive impairment and interrupt short-term memory retrieval and memory maintenance24. In humans, episodic memory formation involves the connections between hippocampus, other mesial temporal lobe structure including entorhinal cortex and the association neocortex including mesial prefrontal cortex, anterior cingulate and posterior parietal cortex such as precuneus24. Memory consolidation involves the transfer of memory from the hippocampus to these association cortices for long-term storage. The cortical-hippocampal network involved in memory consolidation includes the same regions that have been shown to synchronize in MTLE during the generation and progression of seizures on depth electrode recording23.
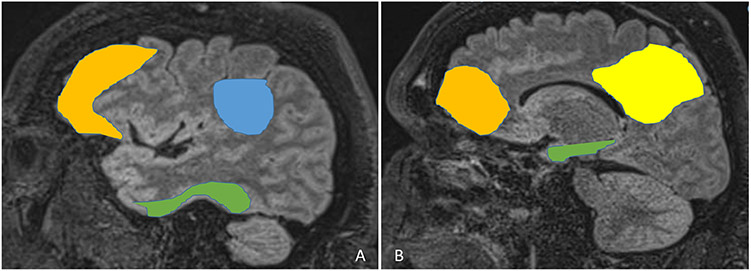
The default mode network (DMN) includes precuneus, posterior cingulate, lateral and inferior parietal lobes, and temporal and medial frontal lobes (Figure 3). It is well-established that DMN is impaired in AD22. In addition, recent evidence has demonstrated impaired DMN in MTLE as well33.
Figure 3: Default Mode Network:
The default mode network includes precuneus (B, yellow), posterior cingulate (B, yellow), lateral and inferior parietal lobes (A, blue), and temporal (A and B, green), and medial frontal lobes (A and B, mustard)
Thus it appears that both regional and cortical networks implicated in memory dysfunction in AD and in MTLE related seizures involve the same brain regions.
Tau pathology, network dysfunction, neuronal hyper-excitability and seizures:
Both animal and human studies have shown that AD pathology, especially pTau is associated with hippocampal-parahippocampal network dysfunction, aberrant neuronal firing, and neuronal hyperactivity22.
Animal studies of epilepsy and AD have shown that tau/A152T mutation, which confers a risk for AD and other taupathies can result in pTau accumulation, progressive neuronal loss in the hippocampi and excess tau aggregation which stimulates pre-synaptic glutamate release which in turn increases neuronal hyper-excitability43. Tau also has a linear correlation with neuronal hyperexcitability and network dysfunction34,44,45.
Neuronal activity and excitability regulate tau secretion and spread46. In a study, human pluripotent stem cell neurons were treated with picrotoxin, a non-competitive blocker of GABA receptor chloride channel, to induce neuronal hyper-excitability. Picrotoxin-induced neuronal activity was found to be associated with both tau release and propagation in vitro46.
Tau can provoke seizures34,44,47. Mice models have demonstrated that total tau levels parallel with seizure severity, and in one study, mice with the highest tau levels had the most severe seizures47. Thus epileptiform activity and seizures in AD can lead to further worsening and accumulation of Tau, worsening the disease process.
Several studies have demonstrated that reduction of tau reduces network hyper-excitability and is protective against seizures34,44,47. Genetic ablation of tau has been shown to reduce hyperexcitability in AD mice47. DeVos et al used anti-sense oligonucleotides to reduce endogenous tau expression and found that mice with less tau had less severe seizures than controls47.
Similar evidence of the association between tau pathology and neuronal dysfunction and hyper-excitability exists in human studies. EEG and magnetoencephalography (MEG) have been extensively studied for network dysfunction among AD patients. It has been shown that slow background activity and reduced alpha synchrony on EEG and MEG correlate with pTau burden and cognitive decline among AD patients48.
There are alternative hypotheses by which AD pathology leads to neuronal hyperexcitability and seizures. These include Aβ induced hyperexcitability, impaired glial transporters leading to accumulation of extrasynaptic glutamate, tyrisone kinase fyn over-expression, altered N-methyl-D-aspartate (NMDA) activity, dysfunction of GABAergic neurons especially in the hippocampus and enhanced synaptic transmission secondary to low amyloid beta among others19. The role of Aβ deposits in promoting seizures by altering neuronal membrane properties and destabilizing the synaptic cleft is well documented by animal models49,50.
There is no controversy that Aβ, pTau, and tyrosine kinase Fyn all have a role to play in seizure generation in AD. However, several mice models have shown that synaptic and network dysfunction and toxicity included by Aβ and fyn is dependent on tau levels34,44. The dendritic function of tau, has been implicated in mediating this toxicity44. Studies have also shown that reduction in tau has been shown to prevent synaptic transmission, enhance inhibitory current, reduce excitation/inhibition imbalance, reduce NMDA receptor mediated dysfunction, prevent cognitive deficit and prevent spontaneous epileptiform discharges that maybe induced by fyn and Aβ34. In mice with over-expression of fyn and Aβ , decreasing tau reduces the network hyper-excitability and severity of seizures34.
Therefore, the constellation of human and animal studies provides evidence that is overall most compelling for the correlation between excess tau, neuronal hyper-excitability, dysfunction, and seizures. Based on the evidence detailed above, we hypothesize that in AD patients with MTLE, tau leads to neuronal excitability and provokes epileptiform activity and seizures, and neuronal hyperexcitability, epileptiform activity and seizures increase tau accumulation and spread, thereby creating a vicious cycle (Figure 4A). This vicious cycle accelerates the progression of disease, leading to worse cognitive and clinical outcomes. The possible mechanisms by which tau causes hyperexcitability in AD and MTLEs are summarized in Table 1. We speculate that these mechanisms are the same in both pathologies. Likewise, we speculate that tau targeting therapies reduce tau build-up and propagation, which in turn reduce neuronal hyperexcitability, epileptiform activity and seizures, and anti-seizure medications reduce neuronal hyper-excitability and seizures and, as a consequence, accumulation, and spread of tau (Figure 4B).
Figure 4: The interplay between neuronal hyper-excitability, epileptiform activity, seizures and tau in Alzheimer’s disease and mesial temporal lobe epilepsy.
A: Tau accumulation leads to neuronal excitability which manifests as epileptiform activity and seizures. This neuronal hyperexcitability, epileptiform activity and seizures in turn increase tau accumulation and spread thereby creating a vicious cycle resulting in accelerating progression of disease, cognitive decline and worse outcomes. B: Tau targeting therapies reduce tau buildup and propagation which in turn reduces neuronal hyperexcitability and seizures. Anti-seizure medications reduce neuronal hyper-excitability, epileptiform activity and seizures which in turn reduce accumulation and spread of tau.
Table 1:
| The dendritic function of tau mediates toxicity induced by Aβ and tyrosine kinase Fyn |
| Tau is associated with the depletion of Kv4.2, a dendritic potassium channel which in turn leads to dendritic hyperexcitability |
| Tau facilitates the enhancement of Aβ-induced defects in axonal transport. |
| Excess tau releases pre-synaptic glutamate. |
| Tau leads to enhanced NMDA receptor-mediated dysfunction |
| Tau is associated with increased excitation/inhibition imbalance |
| Tau leads to increased synaptic transmission |
| Tau reduces inhibitory current, |
AD: Alzheimer’s disease, MTLE: Mesial temporal lobe epilepsy, Aβ: Amyloid beta
Genetic mutations in AD, increased tau burden, and increased epileptogenicity:
Apolipoprotein ε4 (APOε4) allele is a well-established risk factor for developing AD and is associated with early age of disease onset. Increased occurrence of spontaneous seizures has been reported among mice models of APOε451. More recently, APOε4 has been identified as a risk factors of late onset epilepsy15. This has been hypothesized to be secondary to decreased CNS inhibitory tone and/or increased excitatory tone leading to increased neuronal network hyperexcitability51. In mice models of AD, APOε4 has been shown to contribute to the tau-dependent reduction in GABAergic interneurons in the dentate with resultant hippocampal hyperexictability52. In mice models, APOε4 has also been shown to worsen tau-mediated neurodegeneration significantly 53. APOε4 mice have significantly higher levels of tau and a significantly greater degree of somatodendritic tau redistribution,resulting in more significant brain atrophy and neuro-inflammation53. These findings suggest that APOε4 leads to higher tau levels in the AD brain. We hypothesize this higher accumulation of tau, in turn, may contribute to seizures.
Mutations in amyloid precursor protein (APP), including in Down’s syndrome patients, and in presenilin I (PSEN1) and presenilin 2 (PSEN2) are not only linked to the familial autosomal dominant form of AD but also confer a high risk of myoclonus and seizures54. The underlying neuropathology contributing to seizures in PSEN mutations is yet to be determined, but some cases have been reported of underlying pathological changes of ectopic neurons with NFTs and tau deposits in white matter as well as hippocampal sclerosis55. There, it is likely that even in PSEN mutation, alterations in tau level and NFTs may play a role in seizure occurrence.
Overall, these findings support the possibility that genetic mutations in AD lead to an increased tau burden, which may lead to seizures.
Tau pathology is the predominant predictor of cognitive deficits among AD patients:
The best predictors of global cognitive scores on AD neuropathological studies are total neurofibrillary tangle count in area 9 in the frontal lobe and entorhinal cortex, and the neuronal count in CA1 of the hippocampus56. In addition, the regional burden of NFT strongly correlates with clinical manifestation and region/domain-specific cognitive scores among AD patients57. NFTs appear to parallel both the disease duration and severity of AD26. In contrast, amyloid volume is not significantly associated with the disease duration, degree of cognitive decline, or disease severity56.
Tau PET using tracer 18F-AV1451 shows the regional distribution of tau within the human brain. Recent studies have shown that tau pathology, as demonstrated by Tau PET, has a robust correlation with anatomical, clinical manifestation, and cognitive decline among AD patients58,59.
Thus it appears that pTau burden is the predominant predictor of global and region-specific cognitive deficits among AD patients.
Tau pathology is the predominant predictor of cognitive deficits among MTLE patients WITHOUT AD:
In a study of 138 deceased patients with drug-resistant epilepsy, postmortem Braak staging was done. The study found a correlation between epilepsy-related trauma to have an association with tau accumulation. Also, asymmetric patterns of tau deposits were noted within the sclerosed hippocampus of these PwE32.
Another study of 33 drug-resistant MTLE patients with hippocampal sclerosis who underwent temporal lobectomy after age 50 demonstrated that 31 (94%) patients had evidence of abnormally phosphorylated tau pathology on histopathological analysis. In addition, the degree of tau pathology correlated with the degree of verbal learning and naming scores one year after the surgery, and the patient with the highest tau burden developed AD 9 years after the resection31 . A significant association between a history of secondary generalized seizures and tau burden was also found31.
Thus, it appears that, tau pathology burden in the mesial temporal structures is the predominant predictor of cognitive deficits among PwE31,32.
Does tau burden cause neuronal loss, which in turn causes seizures in AD?
Neuropathology analysis has shown that tau burden correlates with neuronal loss in AD patients28. Neurofibrillary tangles have been shown to have an inverse relation with the neuronal number in AD28. In addition, there is evidence to suggest that AD patients with generalized motor seizures have more significant brain atrophy and neuronal loss than AD patients without seizures, specifically in large pyramidal cells in the parietal cortex (area 7) and parahippocampal gyrus (area 28)60,61.
These findings raise the possibility that tau pathology leads to neuronal loss, which in turn causes seizures in AD patients.
MTLE is associated with faster cognitive and clinical decline among AD patients:
Both animal and human studies have shown that epileptiform activity and seizures, especially those involving the mesial temporal lobe, can impact memory and cognition adversely in both acute and chronic stages of AD.
While epileptiform abnormalities may be a consequence of neurodegeneration, there is evidence to suggest that their presence is associated with faster cognitive impairment. Epileptiform activity results in temporary cognitive impairment and interrupts with short-term memory formation and retrieval in intracranial studies of mesial temporal regions in both animals and humans23,25. In addition to acute effects, epileptiform discharges and seizures can have a chronic impact on cognition as well. Hippocampal network remodeling is a long-term sequela of epileptiform activity, which can adversely affect memory and cognition19.
In a case-control study, AD patients with seizures have been shown to have a more rapid language and cognitive decline over a 12-month period compared to AD patients without seizures62. A recent prospective study showed that AD patients with subclinical epileptiform activity on 24 hour video EEG without overt clinical seizures also experienced a faster decline in global cognition when compared to AD patients without subclinical epileptiform activity16. Both executive function and mini-mental state examination (MMSE) were found to decline faster among these patients. In a study of Down syndrome patients with AD, patients who experienced seizures were found to have more profound cognitive decline than those without seizures19.
Recent evidence also points towards the neurodegenerative effect of long-lasting epilepsies on the cortex. Recent longitudinal studies have shown that patients with uncontrolled focal epilepsy can experience widespread cortical thinning, especially in the frontal and temporal lobes. This effect was reduced for patients who underwent epilepsy surgery to control seizures63,64.
Overall, these findings suggest that subclinical epileptiform activity and clinical seizures especially in the temporal region, are associated with accelerated cognitive decline among AD patients.
Anti-seizure medication reduce neuronal hyperexcitability and seizures, and can potentially slow cognitive decline among AD patients:
It has been shown that N-methyl-D-aspartate (NMDA) receptor mediates tau-induced toxicity leading to cell death65. It is also well-established that glutamate-mediated neuronal hyperexcitability via NMDA receptors can cause seizures. Memantine, a non-competitive NMDA receptor antagonist, has been shown to treat both AD and seizures. It is widely used for the management of dementia because it improves cognition. At low doses, memantine has been shown to reduce hyper-excitability and seizures in animal models66. These findings raise the possibility that memantine reduces cognitive decline and seizures by reducing hyper-excitabiltiy and tau-induced toxicity.
There is evidence to suggest that certain anti-seizure medications that stop seizure may also have a positive impact on the cognition of AD patients19. A case-control study of levetiracetam, lamotrigine, and phenobarbital utilization in AD patients with epilepsy over a 12-month period showed 72% seizure remission at one year, improved cognitive outcomes, and better MMSE performance, which was comparable to AD patients without epilepsy19.
In transgenic mice models of AD, suppression of epileptiform activity with anti-seizure medications (ASMs) improved network hyper-excitability in the hippocampus and cognitive deficits67,68. An 8-week trial of lamotrigine among 11 AD patients WITHOUT history of epilepsy showed improved naming and recognition tasks at the end of the study period19. In a small-scale randomized controlled trial, treatment with low dose levetiracetam (250 mg/day) for 4 weeks improved executive function and spatial memory in AD patients who had subclinical epileptiform activity on extended EEG19,69. However, these findings need to be confirmed in larger prospective and rigorous studies of longer duration.
We hypothesize that this impact of anti-seizure medications on improved cognition is likely because of the reduction of neuronal hyper-excitability, which in turn reduces epileptiform activity and seizures and leads to reduced accumulation and further spread of tau pathology.
Conclusion:
Evidence suggests several shared pathological features and a strong association between AD and MTLE. AD patients with MTLE or subclinical epileptiform activity appear to have accelerated and worse disease course with more significant neuronal loss and rapid cognitive and clinical decline16,19,60 and shorter lifespans than AD patients without MTLE or subclinical epileptiform activity4,11.
There is also evidence that these two disease processes may be characterized by excessive tau. Phosphorylated tau is frequently observed in epileptogenic tissue of post-surgical MTLE patients and in postmortem examination of deceased PwE31,32. In addition, phosphorylated tau burden correlates with post-operative cognitive deficits among MTLE patients31,32. Similarly among AD patients, it is the abnormally phosphorylated tau and NFT burden, rather than the Aβ load, especially in the entorhinal cortex and CA1 of the hippocampus, that is the predominant predictor of AD-related cognitive decline, cortical atrophy, and domain-specific cognitive changes56-59. Phosphorylated tau is also associated with network dysfunction and neuronal hyperexcitability in a dose-dependent manner22.
Understanding the interplay between tau pathology, neuronal hyperexcitability, high co-occurrence of MTLE among AD patients, and factors that contribute to the development of epileptiform activity among AD patients is a crucial step toward understanding the overlap between the two diseases.
The findings above raise the possibility that AD patients with subclinical epileptiform activity or MTLE may represent a distinct disease process with the interplay between tau, neuronal hyperexcitablity and epileptiform activity at its core. It is imperative to understand this distinct disease process further to improve its clinical characterization, timely identification, and treatment. Another important unanswered question that needs to be explored in future studies is whether the more severe symptoms of AD with MTLE are due to the summation of two diseases or if it is a more distinct feature of AD beyond epilepsy.
Improved clinical characterization and a better understanding of how these disease overlap will not only help us reduce epileptogenicity among AD patients but also help us halt accelerated cognitive decline among these conditions and could potentially improve outcomes. Once the factors that contribute to epileptogenesis among AD patients have been identified, appropriate management strategies can be developed to reduce AD, more specifically, tau pathology as well as epileptiform activity among AD patients.
Future Directions:
Future studies are needed to establish a direct link between these two phenomena. Research is also needed to identify risk factors for developing MTLE among AD patients and identify predictors of cognitive decline and AD among MTLE patients. In addition, the link between lateral temporal lobe epilepsies and dementia remains unexplored and needs to be studied more thoroughly in future studies.
There is also a need to identify valid, reliable, and scalable biomarkers to predict better which AD patients are at the highest risk of developing epilepsy so that we can modify this risk with more personalized treatment approaches. Finally, there is a need to develop treatment strategies that will help reduce AD pathology and epileptogenesis among AD patients and PwE in order to halt the progression of cognitive decline and improve mortality outcomes.
Supplementary Material
AKNOWLEDGEMENTS
The following internal and external funding of investigators offers no potential conflicts of interest. Dr Zawar receives funding from NIH-NINDS (NeuroNEXT U24NS107182) and Dr Kapur acknowledges funding from NIH.
FUNDING:
Dr Zawar receives funding from Alzheimer’s Association (Grant: AACSFD-22-974008) for this and related work.
Footnotes
CONFLICT OF INTERESTS
All authors declare no conflicts of interest relevant to this study. We confirm that we have read the Journal’s position on issues involved in ethical publications and affirm that this report is consistent with those guidelines.
All authors declaration of interest: None
All authos conflicts of interest: None
COMPETING INTERESTS
The authors have no competing interests to declare
REFERENCES
- 1.Prince M, Wimo A, Guerchet M, Gemma-Claire A, Wu Y-T, Prina M. World Alzheimer Report 2015: The Global Impact of Dementia - An analysis of prevalence, incidence, cost and trends. Alzheimer’s Dis Int. Published online 2015. doi: 10.1111/j.0963-7214.2004.00293.x [DOI] [Google Scholar]
- 2.Perl DP. Neuropathology of Alzheimer’s disease. Mt Sinai J Med. 2010;77(1):32–42. doi: 10.1002/MSJ.20157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen A, Jette N, Husain M, Sander JW. Epilepsy in older people. Lancet (London, England). 2020;395(10225):735–748. doi: 10.1016/S0140-6736(19)33064-8 [DOI] [PubMed] [Google Scholar]
- 4.Degiorgio CM, Curtis A, Carapetian A, Hovsepian D, Krishnadasan A, Markovic D. Why are epilepsy mortality rates rising in the United States? A population-based multiple cause-of-death study. BMJ Open. Published online 2020. doi: 10.1136/bmjopen-2019-035767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson EL, Krauss GL, Kucharska-Newton A, et al. Dementia in late-onset epilepsy. Neurology. Published online 2020. doi: 10.1212/wnl.0000000000011080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.N. S, L.S. H, H. C, et al. Seizures in Alzheimer disease: Who, when, and how common? Arch Neurol. Published online 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imfeld P, Bodmer M, Schuerch M, Jick SS, Meier CR. Seizures in patients with Alzheimer’s disease or vascular dementia: A population-based nested case-control analysis. Epilepsia. 2013;54(4):700–707. doi: 10.1111/EPI.12045 [DOI] [PubMed] [Google Scholar]
- 8.Breteler MM, van Duijn CM, Chandra V, et al. Medical history and the risk of Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. Published online 1991. [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, Shen LX, Ou YN, et al. Risk of seizures and subclinical epileptiform activity in patients with dementia: A systematic review and meta-analysis. Ageing Res Rev. 2021;72. doi: 10.1016/J.ARR.2021.101478 [DOI] [PubMed] [Google Scholar]
- 10.Subota A, Pham T, Jetté N, Sauro K, Lorenzetti D, Holroyd-Leduc J. The association between dementia and epilepsy: A systematic review and meta-analysis. Epilepsia. Published online 2017. doi: 10.1111/epi.13744 [DOI] [PubMed] [Google Scholar]
- 11.Subota A, Jetté N, Josephson CB, et al. Risk factors for dementia development, frailty, and mortality in older adults with epilepsy – A population-based analysis. Epilepsy Behav. Published online 2021. doi: 10.1016/j.yebeh.2021.108006 [DOI] [PubMed] [Google Scholar]
- 12.Friedman D, Honig LS, Scarmeas N. Seizures and Epilepsy in Alzheimer’s Disease. CNS Neurosci Ther. Published online 2012. doi: 10.1111/j.1755-5949.2011.00251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samson WN, van Duijn CM, Hop WCJ, Hofman A. Clinical features and mortality in patients with early-onset alzheimer’s disease. Eur Neurol. Published online 1996. doi: 10.1159/000117218 [DOI] [PubMed] [Google Scholar]
- 14.Breteler MMB, De Groot RRM, Van Romunde LKJ, Hofman A. Risk of dementia in patients with Parkinson’s disease, epilepsy, and severe head trauma: a register-based follow-up study. Am J Epidemiol. 1995;142(12):1300–1305. doi: 10.1093/OXFORDJOURNALS.AJE.A117597 [DOI] [PubMed] [Google Scholar]
- 15.Liang Y, Zhou Z, Wang H, Cheng X, Zhong S, Zhao C. Association of apolipoprotein E genotypes with epilepsy risk: A systematic review and meta-analysis. Epilepsy Behav. Published online 2019. doi: 10.1016/j.yebeh.2019.06.015 [DOI] [PubMed] [Google Scholar]
- 16.Vossel KA, Ranasinghe KG, Beagle AJ, et al. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann Neurol. 2016;80(6):858–870. doi: 10.1002/ANA.24794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam AD, Sarkis RA, Pellerin KR, et al. Association of epileptiform abnormalities and seizures in Alzheimer disease. Neurology. Published online 2020. doi: 10.1212/WNL.0000000000010612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam AD, Deck G, Goldman A, Eskandar EN, Noebels J, Cole AJ. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat Med. Published online 2017. doi: 10.1038/nm.4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol. Published online 2017. doi: 10.1016/S1474-4422(17)30044–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkis RA, Dickerson BC, Cole AJ, Chemali ZN. Clinical and neurophysiologic characteristics of unprovoked seizures in patients diagnosed with dementia. J Neuropsychiatry Clin Neurosci. Published online 2016. doi: 10.1176/appi.neuropsych.15060143 [DOI] [PubMed] [Google Scholar]
- 21.Duan Y, Lin Y, Rosen D, Du J, He L, Wang Y. Identifying Morphological Patterns of Hippocampal Atrophy in Patients With Mesial Temporal Lobe Epilepsy and Alzheimer Disease. Front Neurol. Published online 2020. doi: 10.3389/fneur.2020.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zott B, Busche MA, Sperling RA, Konnerth A. What happens with the circuit in Alzheimer’s disease in mice and humans? Annu Rev Neurosci. Published online 2018. doi: 10.1146/annurev-neuro-080317-061725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zangiabadi N, Ladino LDi, Sina F, Orozco-Hernández JP, Carter A, Téllez-Zenteno JF. Deep Brain Stimulation and Drug-Resistant Epilepsy: A Review of the Literature. Front Neurol. 2019;10(JUN). doi: 10.3389/FNEUR.2019.00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaz AP, Inati SK, Brunel N, Zaghloul KA. Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science. 2019;363(6430):975–978. doi: 10.1126/SCIENCE.AAU8956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelinas JN, Khodagholy D, Thesen T, Devinsky O, Buzsáki G. Interictal epileptiform discharges induce hippocampal-cortical coupling in temporal lobe epilepsy. Nat Med. 2016;22(6):641–648. doi: 10.1038/NM.4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. Published online 1992. doi: 10.1212/wnl.42.3.631 [DOI] [PubMed] [Google Scholar]
- 27.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/J.JALZ.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. Published online 1996. doi: 10.1523/jneurosci.16-14-04491.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. Published online 1991. doi: 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 30.Vogel JW, Iturria-Medina Y, Strandberg OT, et al. Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat Commun. Published online 2020. doi: 10.1038/s41467-020-15701-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tai XY, Koepp M, Duncan JS, et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: A study of temporal lobe resections. Brain. Published online 2016. doi: 10.1093/brain/aww187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thom M, Liu JYW, Thompson P, et al. Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain. 2011;134(Pt 10):2969–2981. doi: 10.1093/BRAIN/AWR209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen A, Capelli V, Husain M. Cognition and dementia in older patients with epilepsy. Brain. Published online 2018. doi: 10.1093/brain/awy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberson ED, Halabisky B, Yoo JW, et al. Amyloid-β/fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of alzheimer’s disease. J Neurosci. Published online 2011. doi: 10.1523/JNEUROSCI.4152-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidson YS, Raby S, Foulds PG, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s Syndrome: Association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. Published online 2011. doi: 10.1007/s00401-011-0879-y [DOI] [PubMed] [Google Scholar]
- 36.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in alzheimer disease: Age categories from 1 to 100 years. J Neuropathol Exp Neurol. Published online 2011. doi: 10.1097/NEN.0b013e318232a379 [DOI] [PubMed] [Google Scholar]
- 37.Min B, Guoming L, Jian Z. Treatment of mesial temporal lobe epilepsy with amygdalohippocampal stimulation: A case series and review of the literature. Exp Ther Med. Published online 2013. doi: 10.3892/etm.2013.968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faber J, Vladyka V. Antiepileptic effect of electric stimulation of the locus coeruleus in man. Act Nerv Super (Praha). Published online 1983. [PubMed] [Google Scholar]
- 39.Feinstein B, Gleason CA, Libet B. Stimulation of locus coeruleus in man: Preliminary trails for spasticity and epilespy. Stereotact Funct Neurosurg. Published online 1989. doi: 10.1159/000099484 [DOI] [PubMed] [Google Scholar]
- 40.Putcha D, Brickhouse M, O’Keefe K, et al. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J Neurosci. Published online 2011. doi: 10.1523/JNEUROSCI.4740-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zott B, Busche MA, Sperling RA, Konnerth A. What Happens with the Circuit in Alzheimer’s Disease in Mice and Humans? Annu Rev Neurosci. 2018;41:277–297. doi: 10.1146/ANNUREV-NEURO-080317-061725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional alterations in memory networks in early alzheimer’s disease. NeuroMolecular Med. Published online 2010. doi: 10.1007/s12017-009-8109-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Decker JM, Krüger L, Sydow A, et al. The Tau/A152T mutation, a risk factor for frontotemporal-spectrum disorders, leads to NR 2B receptor-mediated excitotoxicity . EMBO Rep. Published online 2016. doi: 10.15252/embr.201541439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-β toxicity in alzheimer’s disease mouse models. Cell. Published online 2010. doi: 10.1016/j.cell.2010.06.036 [DOI] [PubMed] [Google Scholar]
- 45.Holth JK, Bomben VC, Graham Reed J, et al. Tau loss attenuates neuronal network hyperexcitability in mouse and drosophila genetic models of epilepsy. J Neurosci. Published online 2013. doi: 10.1523/JNEUROSCI.3191-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu JW, Hussaini SA, Bastille IM, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. Published online 2016. doi: 10.1038/nn.4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeVos SL, Goncharoff DK, Chen G, et al. Antisense reduction of tau in adult mice protects against seizures. J Neurosci. Published online 2013. doi: 10.1523/JNEUROSCI.2107-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ranasinghe KG, Petersen C, Kudo K, et al. Reduced synchrony in alpha oscillations during life predicts post mortem neurofibrillary tangle density in early-onset and atypical Alzheimer’s disease. Alzheimer’s Dement. Published online 2021. doi: 10.1002/alz.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palop JJ and ML. Amyloid-β Induced Neuronal Dysfunction in Alzheimer’s Disease : From Synapses toward Neural Networks. Nat Neurosci. Published online 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minkeviciene R, Rheims S, Dobszay MB, et al. Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. Published online 2009. doi: 10.1523/JNEUROSCI.5215-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter JM, Cirrito JR, Restivo JL, et al. Emergence of a seizure phenotype in aged apolipoprotein epsilon 4 targeted replacement mice. Brain Res. Published online 2012. doi: 10.1016/j.brainres.2012.05.048 [DOI] [PubMed] [Google Scholar]
- 52.Andrews-Zwilling Y, Bien-Ly N, Xu Q, et al. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. Published online 2010. doi: 10.1523/JNEUROSCI.4040-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y, Yamada K, Liddelow SA, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. Published online 2017. doi: 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shea YF, Chu LW, Chan AOK, Ha J, Li Y, Song YQ. A systematic review of familial Alzheimer’s disease: Differences in presentation of clinical features among three mutated genes and potential ethnic differences. J Formos Med Assoc. Published online 2016. doi: 10.1016/j.jfma.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 55.Larner AJ. Presenilin-1 mutation Alzheimer’s disease: A genetic epilepsy syndrome? Epilepsy Behav. Published online 2011. doi: 10.1016/j.yebeh.2011.03.022 [DOI] [PubMed] [Google Scholar]
- 56.Giannakopoulos P, Herrmann FR, Bussière T, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. Published online 2003. doi: 10.1212/01.WNL.0000063311.58879.01 [DOI] [PubMed] [Google Scholar]
- 57.Petersen C, Nolan AL, de Paula França Resende E, et al. Alzheimer’s disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta Neuropathol. Published online 2019. doi: 10.1007/s00401-019-02036-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. Published online 2016. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joie R La, Visani AV, Baker SL, et al. Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med. Published online 2020. doi: 10.1126/scitranslmed.aau5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Förstl H, Burns A, Levy R, Cairns N, Luthert P, Lantos P. Neurologic Signs in Alzheimer’s Disease: Results of a Prospective Clinical and Neuropathologic Study. Arch Neurol. Published online 1992. doi: 10.1001/archneur.1992.00530340054018 [DOI] [PubMed] [Google Scholar]
- 61.Gourmaud S, Stewart DA, Irwin DJ, et al. The role of mTORC1 activation in seizure-induced exacerbation of Alzheimer’s disease. Brain. 2022;145(1):324–339. doi: 10.1093/BRAIN/AWAB268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volicer L, Smith S, Volicer BJ. Effect of seizures on progression of dementia of the alzheimer type. Dement Geriatr Cogn Disord. Published online 1995. doi: 10.1159/000106956 [DOI] [PubMed] [Google Scholar]
- 63.Galovic M, de Tisi J, McEvoy AW, et al. Resective surgery prevents progressive cortical thinning in temporal lobe epilepsy. Brain. Published online 2021. doi: 10.1093/BRAIN/AWAA284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galovic M, Van Dooren VQH, Postma TS, et al. Progressive Cortical Thinning in Patients with Focal Epilepsy. JAMA Neurol. Published online 2019. doi: 10.1001/jamaneurol.2019.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. Published online 2006. doi: 10.1073/pnas.0511065103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meldrum BS, Turski L, Schwarz M, Czuczwar SJ, Sontag KH. Anticonvulsant action of 1,3-dimethyl-5-aminoadamantane - Pharmacological studies in rodents and baboon, Papio Papio. Naunyn Schmiedebergs Arch Pharmacol. Published online 1986. doi: 10.1007/BF00633204 [DOI] [PubMed] [Google Scholar]
- 67.Sanchez PE, Zhu L, Verret L, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci U S A. 2012;109(42). doi: 10.1073/PNAS.1121081109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corbett BF, You JC, Zhang X, et al. ΔFosB Regulates Gene Expression and Cognitive Dysfunction in a Mouse Model of Alzheimer’s Disease. Cell Rep. 2017;20(2):344–355. doi: 10.1016/J.CELREP.2017.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vossel K, Ranasinghe KG, Beagle AJ, et al. Effect of Levetiracetam on Cognition in Patients with Alzheimer Disease with and without Epileptiform Activity: A Randomized Clinical Trial. JAMA Neurol. Published online 2021. doi: 10.1001/jamaneurol.2021.3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall AM, Throesch BT, Buckingham SC, et al. Tau-dependent Kv4.2 depletion and dendritic hyperexcitability in a mouse model of Alzheimer’s disease. J Neurosci. Published online 2015. doi: 10.1523/JNEUROSCI.2552-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.