Abstract
Introduction/Aims:
In the Diabetes Control and Complications Trial (DCCT), the minimal nerve conduction (NC) criterion for diabetic sensorimotor polyneuropathy (DSPN) was abnormality of NC in >1 peripheral nerve without specifying the attributes of NCs to be evaluated. In the present study, we assess individual and composite scores of NCs meeting the DCCT criterion and signs for improved diagnosis and assessment of DSPN severity.
Methods:
Evaluated were 13 attributes and 6 composite NC scores and signs and symptoms in 395 healthy subjects (HS) and 388 persons with diabetes (DM).
Results:
Percent abnormality between subjects with DM and HS was remarkably different among individual attributes and the 6 composite NC scores. For diagnosis of DSPN using the DCCT criterion, assessment of conduction velocities (CVs) and distal latencies (DLs) provided sensitive diagnoses of DSPN. NC amplitudes provided stronger measures of severity. In studied cohorts, DSPN was staged: N0, no NC abnormality using NC score #2 (CVs and DLs), 60.0%; N1, NC abnormality only, 18.4%; N2, NC abnormality and signs of feet or legs, 16.3%; and N3, NC abnormality and signs of thighs, 5.3%.
Discussion:
For sensitive and standard diagnosis of DSPN using the DCCT NC criterion, specifically defined composite scores of CVs and DLs, e.g., score #2, is recommended. A composite score of amplitudes, e.g., score #4, provides a stronger measure of neuropathy severity. Also, provided are HS reference values of evaluated attributes of NCs and estimates of staged severity of DSPN of mid North American DM cohorts.
Keywords: composite nerve conduction scores, diagnosis of diabetic sensorimotor polyneuropathy, staged severity of diabetic sensorimotor polyneuropathy, validation of diagnosis and staged severity of diabetic sensorimotor polyneuropathy, diabetic complication in Northern Plains Indians and Latinos, epidemiology survey of diabetic sensorimotor polyneuropathy, nerve conduction assessment and reference values in health and in diabetes
INTRODUCTION
For the detection, characterization, and quantitation of diabetic sensorimotor polyneuropathy (DSPN), the following assessments have been introduced: nerve conductions (NCs),1–15 clinical signs and symptoms,16–30 quantitative sensation and autonomic tests,31–36 morphometric counts of nerve fibers of biopsied cutaneous nerves or of skin,37–39 and counts of corneal nerve fibers.40–43 Of these, referenced assessment of NCs and neuropathy signs are the most commonly used clinical endpoints for the diagnosis and quantitation of severity of DSPN.4,8,12
Not sufficiently addressed in previous research studies are which referenced individual or grouped attributes of NCs (composite NC scores) are most sensitive and reliable for the diagnosis and assessment of severity of DSPN.1,6,12 For the conduct of the Diabetes Control and Complications Trial (DCCT), a research study of glycemic control on development of DSPN and other complications in type 1 diabetes mellitus (DM), abnormality of NCs in >1 anatomical nerve, and polyneuropathy signs were selected as diagnostic criteria of DSPN.8,12 In the present study, we address how the DCCT criterion can be improved to more accurately assess prevalence of DSPN and its severity. Also, assessed is the prevalence and staged severity of DSPN in large mid North American cohorts of healthy subjects (HS) and persons with DM.
METHODS
Recruitment of mid North American ethnic cohorts of HS and persons with DM
Clinical and NC data of persons without DM or neurological diseases (HS) and persons with DM without and with DSPN were available from the three previously conducted epidemiology surveys, i.e., Rochester Diabetic Neuropathy Study (RDNS), a cohort mainly of northern European extraction, Northern Plains Indians (NPI), and Latino (Lat) cohorts. All cohorts studied had persons with type 1 and 2 DM without and with DSPN. For the RDNS cohort, the medical records of Mayo Clinic, Rochester and Olmsted County Medical Group (essentially the only medical care providers in Olmsted County, MN, USA) research subjects (RDNS) having an Olmsted County, MN, USA address were reviewed and sent letters inviting them to participate in research studies. Their HS or DM status was determined by reviewing their medical records, obtaining a medical history and neuropathy examination, and by measuring fasting plasma glucose and A1c values. In NPI and Lat studies, persons of these ethnicities were invited to participate in field epidemiology surveys to identify whether they had DM and whether they had polyneuropathy. The basis for the diagnosis of DM was a history of DM and/or an A1c value ≥6.5%. The NPI cohort came from Minnesota, North and South Dakota, and Wisconsin, and the Lat cohort was mainly persons of Mexican descent. All research subjects signed informed consent forms approved by appropriate institutional review boards. In the case of the NPI and Lat cohort studies, preliminary public meetings about the proposed studies were held describing the studies. The specific studies were performed at Mayo Clinic (RDNS), at a research trailer at Prairie Island, MN (NPI), at local Rochester, MN churches and at Mayo Clinic (Lat). Some of the obtained data of the RDNS cohorts has previously been evaluated and, to varying degrees, published.6,7,22,44,45
Assessment of polyneuropathy signs and symptoms
The clinical instruments used to assess neuropathy signs (Neuropathy Impairment Score, NIS) and symptoms (Neuropathy Symptoms and Change score, NSC) were described in previous publications.7,46–48 For judgment of abnormality of Lower Limb Function (LLF), one of us (PJD) made the assumption that healthy persons without DSPN should be able to walk on their toes and heels into their eighth decade of life unless they were excessively obese or physically unfit, whereas arising from a kneeled position might not be possible even in some healthy subjects in the seventh or later decades of life. The assessment of neuropathy signs of the NIS, the NSC score, and assessment of LLF score were performed by one of the authors (PJD) without knowledge of abnormality of NCs. The examinations were performed in the following order: LLF, NIS, and NSC and individual item assessment following the order listed in the printed standard Clinical Neuropathy Assessment form.46,48 For judgment of abnormality of these evaluations, the investigator (PJD) used “unequivocal abnormality” as the basis for scoring clinical abnormality, which judgment was later supported by Clinical versus Neurophysiology trials.49,50 Muscle weakness was graded using a combination of the Mayo Clinic and Medical Research Council criteria, i.e., weakness of 25% = 1, 50% = 2, 75% = 3, and 100% = 4 points and separately for each side of the body. For scores between 3 and 4, a score of 3.25 (minimal movement just possible against gravity), 3.5 (movement just possible with gravity eliminated), and 3.75 (muscle twitch only without movement at the joint) was assigned. A three-point score (0 = no abnormality; 1 = decrease; and 2 = absent) was used to assess decreased muscle stretch reflexes and four modalities of sensation decreases (touch pressure, vibration, joint motion, and pin prick) of fingers and toes. For assessment of individual symptoms as assessed by NSC, abnormality was based on the specific judgment of the examining physician (PJD) that the elicited abnormality was unequivocally present and attributable to DSPN.
Assessment of attributes of NCs
NC reference values were assessed in the HS cohort. Thirteen individual attributes of NCs were assessed by one of us (WJL) using highly standard pre-study subject testing conditions, standard equipment, testing peripherals, and standard technique of testing. Thirteen individual attributes of NCs were assessed in the present study (Table 1). The specific techniques of assessment of NCs are outlined in a syllabus prepared by one of us (WJL) which can be obtained on written request. All NCs were assessed on the left side of the body unless there were medical reasons not to do so. If limb temperature was below 31°C, the limb was pre-warmed in a water bath (40°C for 10 minutes). It should be noted that none of these 13 individual attributes of NCs fulfilled DCCT NC criterion for DSPN; abnormality in >1 nerve is needed. Normality/abnormality of individual attributes of NCs were expressed as measured values and as points from percentile ranges, i.e., >5th/<95th = 0, ≤5th - >1st = 1, and ≤1/≥99th = 2 points. The six composite scores used are defined in Table 2.
Table 1 -.
Number and Percent of Healthy Subjects (HS) and Persons with Diabetes (DM) with Abnormality of Individual Attributes of Nerve Conduction
| 3 HS Cohorts Combined | 3 DM Cohorts Combined* | Difference in % | Fisher’s Exact Test | |||
|---|---|---|---|---|---|---|
| Total n | n (%) Abnormal | Total n | n (%) Abnormal | p | ||
| 1. Fibular MNCV ≤ 5th | 394 | 18 (4.57) | 379 | 179 (47.23) | 42.66 | <0.0001 |
| 2. Tibial MNCV ≤ 5th | 395 | 20 (5.06) | 386 | 132 (34.20) | 29.14 | <0.0001 |
| 3. Ulnar MNCV ≤ 5th | 395 | 24 (6.08) | 387 | 109 (28.17) | 22.09 | <0.0001 |
| 4. Tibial F-wave ≥ 95th | 392 | 16 (4.08) | 377 | 86 (22.81) | 18.73 | <0.0001 |
| 5. Sural SNDL ≥ 95th | 387 | 19 (4.91) | 329 | 68 (20.67) | 15.76 | <0.0001 |
| 6. Ulnar F-wave ≥ 95th | 393 | 18 (4.58) | 383 | 76 (19.84) | 15.26 | <0.0001 |
| 7. Sural SNAP ≤ 5th | 395 | 18 (4.56) | 388 | 71 (18.30) | 13.74 | <0.0001 |
| 8. Tibial CMAP ≤ 5th | 395 | 23 (5.82) | 388 | 69 (17.78) | 11.96 | <0.0001 |
| 9. Fibular CMAP ≤ 5th | 395 | 23 (5.82) | 388 | 67 (17.27) | 11.45 | <0.0001 |
| 10. Ulnar CMAP ≤ 5th | 395 | 18 (4.56) | 388 | 58 (14.95) | 10.39 | <0.0001 |
| 11. Fibular MNDL ≥ 95th | 394 | 19 (4.82) | 379 | 45 (11.87) | 7.05 | 0.0004 |
| 12. Ulnar MNDL ≥ 95th | 395 | 25 (6.33) | 388 | 31 (7.99) | 1.66 | 0.4066 |
| 13. Tibial MNDL ≥ 95th | 395 | 23 (5.82) | 386 | 20 (5.18) | −0.64 | 0.7548 |
MNCV = motor nerve conduction velocity, F-wave = F-wave latency, SNDL = sensory nerve distal latency, SNAP = sensory nerve action potential amplitude, CMAP = compound muscle action potential amplitude, MNDL = motor nerve distal latency
McNemar’s test (S; p) of differences of frequencies of abnormality between pairs of individual attributes of nerve conduction: 1 vs. 2 (32.11; <0.0001), 8 vs. 9 (0.06; 0.9007), 1 vs. 9 (108.45; <0.0001)
Table 2 -.
Number and Percent of Healthy Subjects (HS) and Persons with Diabetes (DM) with Abnormality of Composite Measures of Attributes of Nerve Conduction Meeting DCCT Criteria
| 3 HS Cohorts Combined | 3 DM Cohorts Combined† | Difference in % | Fisher’s Exact Test | |||
|---|---|---|---|---|---|---|
| Total n | n (%) Abnormal | Total n | n (%) Abnormal | p | ||
| 1. ≥ 2 pts* of any of > 1 Nerve of Fibular, Ulnar or Tibial CMAPs, MNCVs or MNDLs or Sural SNAP or SNDL† | 393 | 44 (11.20) | 379 | 189 (49.87) | 38.87 | <0.0001 |
| 2. ≥ 2 pts of any of > 1 of Fibular, Tibial or Ulnar MNCV or Sural SNDL | 394 | 15 (3.81) | 376 | 151 (40.16) | 36.35 | <0.0001 |
| 3. ≥ 2 pts of any of > 1 of Ulnar or Fibular MNCV | 395 | 8 (2.03) | 383 | 83 (21.67) | 19.64 | <0.0001 |
| 4. ≥ 2 pts of any of > 1 of Fibular, Ulnar or Tibial CMAPs or Sural SNAP | 395 | 9 (2.28) | 388 | 72 (18.56) | 16.28 | <0.0001 |
| 5. ≥ 2 pts of any of > 1 of Ulnar or Tibial F-wave | 394 | 10 (2.54) | 380 | 48 (12.63) | 10.09 | <0.0001 |
| 6. ≥ 2 pts of any of > 1 of Ulnar MNCV or Sural SNAP | 395 | 4 (1.01) | 388 | 28 (7.22) | 6.21 | <0.0001 |
MNCV = motor nerve conduction velocity, F-wave = F-wave latency, SNDL = sensory nerve distal latency, SNAP = sensory nerve action potential amplitude, CMAP = compound muscle action potential amplitude, MNDL = motor nerve distal latency
> 5th \ < 95th = 0 pts (points); > 1st to ≤ 5th \ ≥ 95th to < 99th = 1 pt; ≤ 1st \ ≥ 99th = 2 pts
McNemar’s test (S; p) of differences of frequencies of abnormality between pairs of composite measures of attributes of nerve conduction: 1 vs. 2 (29.00; <0.0001), 1 vs. 4 (117.00; <0.0001), 2 vs. 4 (64.88; <0.0001)
Six composite NC scores, each meeting the DCCT criterion for DSPN, were assessed both in HS and persons with DM. These composite NC scores are defined in the footnote to Table 2.
Statistical analysis
Nonparametric Fisher’s exact test was used to assess differences in percentage abnormality of individual or composite NC scores between HS and persons with DM. NIS and NSC abnormalities were compared by studied cohort for those without and with NC abnormality for HS and persons with DM using Fisher’s exact test. Medians and ranges for NIS total, NSC severity and NC as points were compared between stages of DSPN using nonparametric Wilcoxon rank sum tests. Ninety-five percent confidence intervals were provided using Hodges-Lehmann Estimation. Fifth and first percentile lines from simple linear regressions were plotted for NC of ulnar, tibial, and fibular motor nerve conduction velocities (MNCVs) and sural sensory nerve distal latencies (SNDLs) on height and for ulnar, tibial, and fibular compound muscle (CMAP) and sural sensory nerve (SNAP) action potential amplitudes on age. For assessment of conduction velocities (CVs) and distal latencies (DLs), more variables than age were required for setting reference limits (see Results). Frequencies of DSPN stages were determined for HS and DM patients. Receiver Operating Characteristic (ROC) curves were plotted for different measures of DSPN as compared to difference from threshold of NC score #2. Statistical analyses were conducted using the SAS System version 9.4 (Cary, NC) and R using haven and pROC packages.51–53
RESULTS
Distribution and percentile reference limits of individual attributes of NCs assessed in HS
In Figure 1, we provide a plot of individual HS values of ulnar, fibular, and tibial MNCVs and sural SNDLs (attributes of composite NC score #2) plotted on height in HS. The percentile range difference related to age is indicated by showing the difference in a person aged 25 and 75 years. Composite score #2 is judged to be abnormal when >1 of these 4 assessed attributes are abnormal, i.e., ≤5th/≥95th percentile value.
Figure 1:
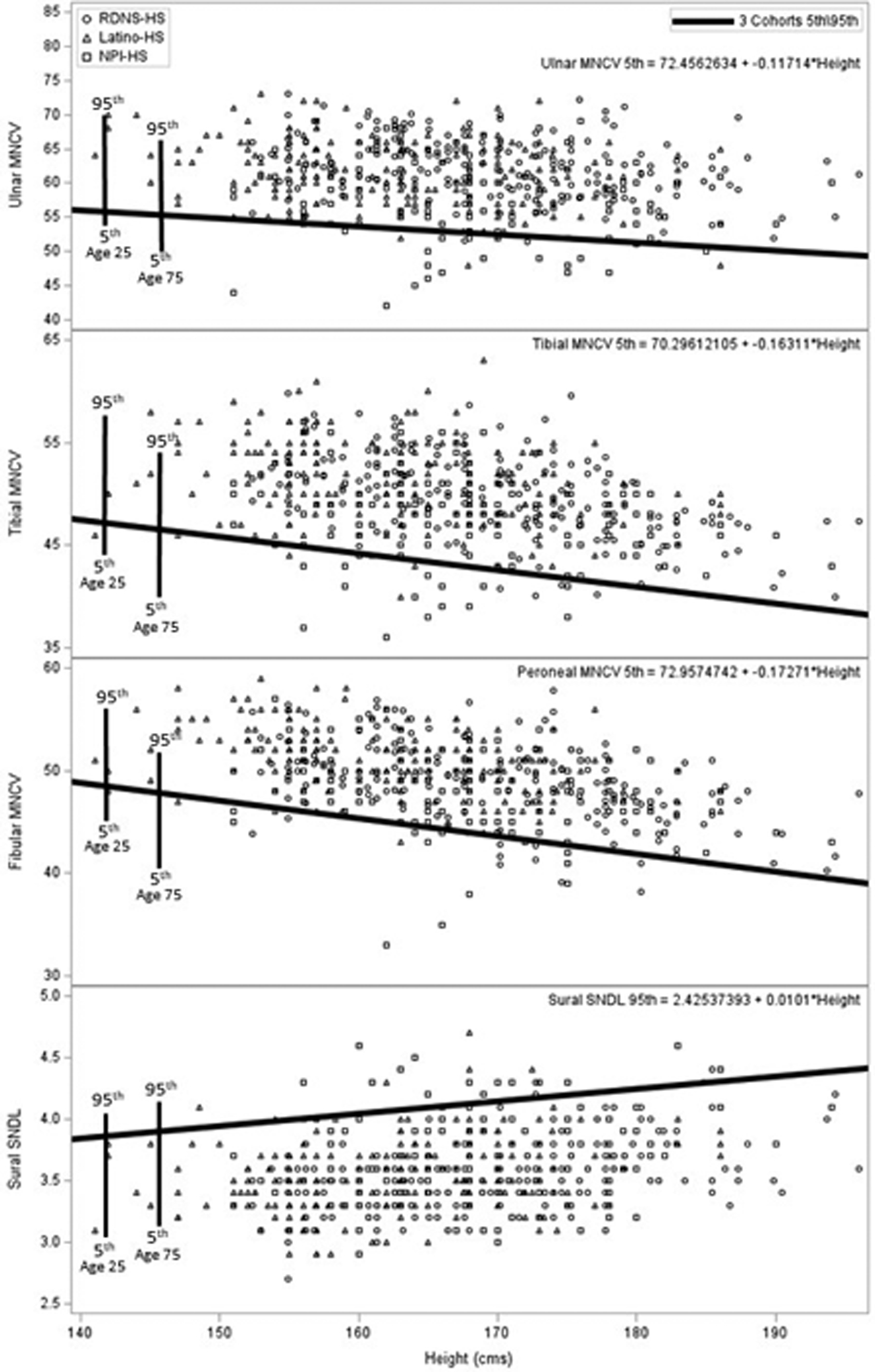
Shown are the plotted individual values of motor nerve conduction velocities of ulnar, fibular, and tibial nerves and of sural nerve distal latency of healthy subjects, without diabetes, drawn from studied ethnic cohorts: RDNS, NPI, and Lat cohorts. Shown also is the 5th percentile regression line on height for conduction velocities and the 95th regression line for sural nerve distal latency. The percentile range difference related to age is indicated by showing the difference in a person aged 25 and 75 years. These reference values were used by us in formulating composite NC score #2 highly sensitive for the NC diagnosis of DSPN.
In Figure 2, displayed are the HS values of ulnar, fibular, and tibial CMAP/sural SNAP amplitudes plotted on age. Fifth and first percentile lines are shown. These four attributes of NCs are the assessed NC attributes of composite NC score #4. This score is judged to be abnormal when >1 of the four assessed attributes are abnormal.
Figure 2:
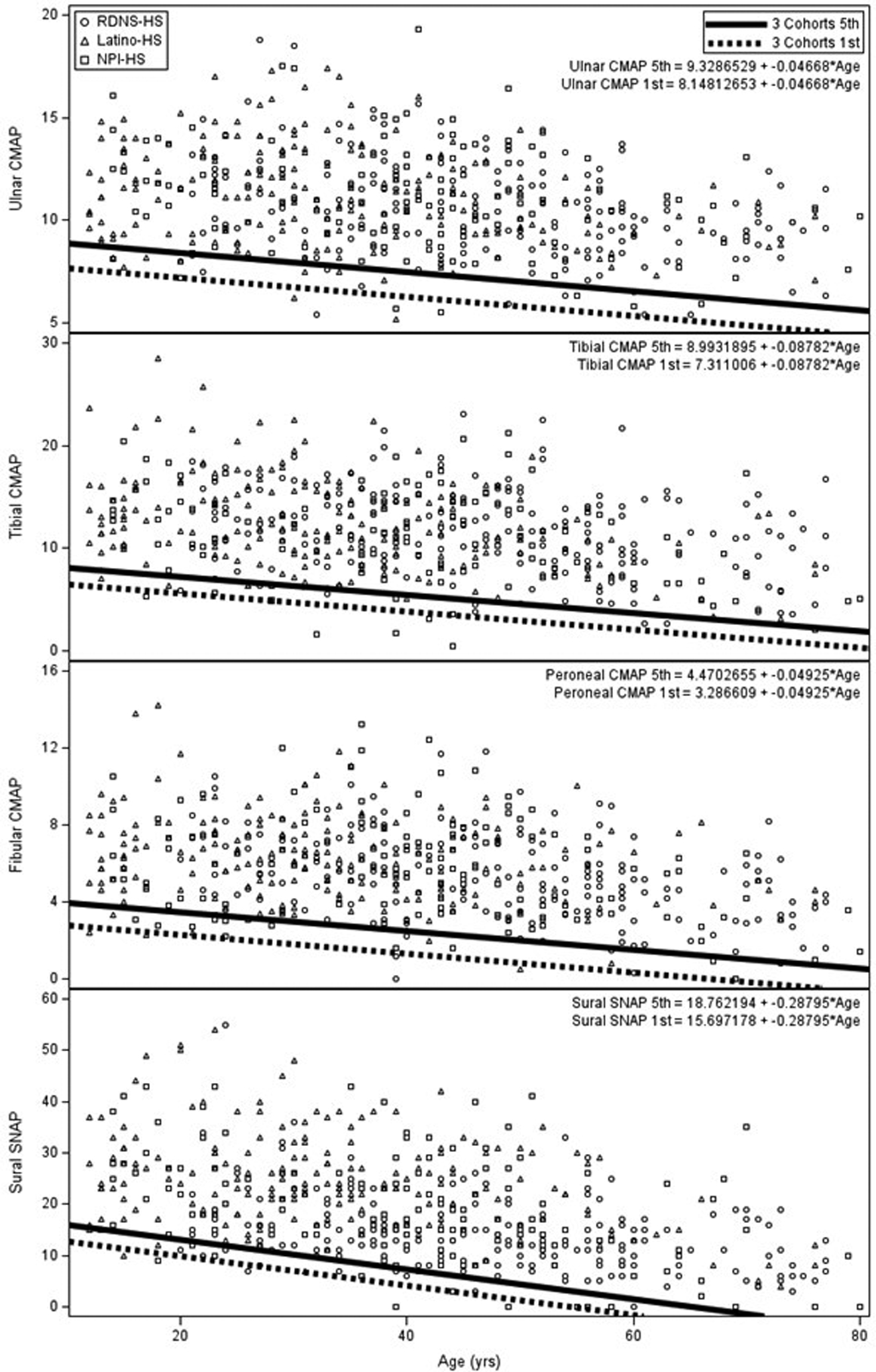
Shown are individual values of the CMAPs of ulnar, fibular, and tibial nerves and of SNAP amplitudes of sural nerve, attributes of NCs assessed in composite score #4. Shown are the 5th and 1st percentile regression lines for each of the assessed attributes of NCs. Composite score #4 is not as sensitive for diagnosis of DSPN as is composite score #2, but is a more useful measure of severity of DSPN as detailed in Discussion.
Number and percent of HS and persons with DM with abnormality of individual attributes and composite scores of NCs
Of these assessed individual attributes of NCs, fibular MNCV provided the highest percentage difference between DM and HS (42.7%). The next five most frequent individual attributes were CVs, F-waves, and DLs, ranging from differences in frequency from 29.1% to 15.3%. Lower percentage differences were observed, especially for CMAP/SNAP amplitudes, and motor nerve DLs. The data from Table 1 were used to choose composite attributes of NCs with abnormality in >1 nerve, meeting DCCT criterion for the diagnosis of DSPN.
Number and percent of HS and persons with DM with abnormality of composite measures of attributes of NCs meeting the DCCT criterion
The diagnostic performance of the six composite NC scores assessed meeting DCCT diagnostic criterion for DSPN is shown in Table 2. Composite score #1, assessing any of 11 individual attributes of NCs, provides the largest percentage difference between persons with DM from HS (38.9%). Score #2 of ulnar, fibular, and tibial MNCVs and sural SNDL provided the second highest difference between DM and HS (36.4%). Although score #1, as compared to #2, provides a slightly more sensitive measure of DSPN, it was considerably more complex and time-consuming to perform and calculate. Lower percentage differences were observed with use of the other four composite scores. Composite score #4 of NC amplitudes provided a considerably lower percentage difference between DM and HS of 16.3%. However, as we describe below, this score provides a better measure of neuropathy severity than does composite score #2.
Composite NC score #2 (CVs and DLs) as a more sensitive measure of DSPN than abnormality of neuropathy signs
Composite NC score #2 was the only abnormality of DSPN in 3.1% of HS and in 18.4% of DM subjects (Figure 3). By contrast, in HS and persons with DM, polyneuropathy signs or symptoms alone (without abnormality of NC score #2) occurred much less frequently and depended on what threshold signs, as measured by NIS, were set (Supplementary Table 1). Also, when signs occurred without abnormality of NCs, they typically were mild and of uncertain clinical significance, e.g., selective decrease of upper limb reflexes. When abnormality of NIS signs and NSC symptoms were each set at four points, frequency of DSPN in subjects without abnormality of NC score #2 abnormality occurred in only 0.3 and 3.7% of persons without and with DM (Supplementary Table 1). Symptoms without NC abnormality occurred in only 0 and 3.2% of HS and persons with DM (Supplementary Table 1).
Figure 3:
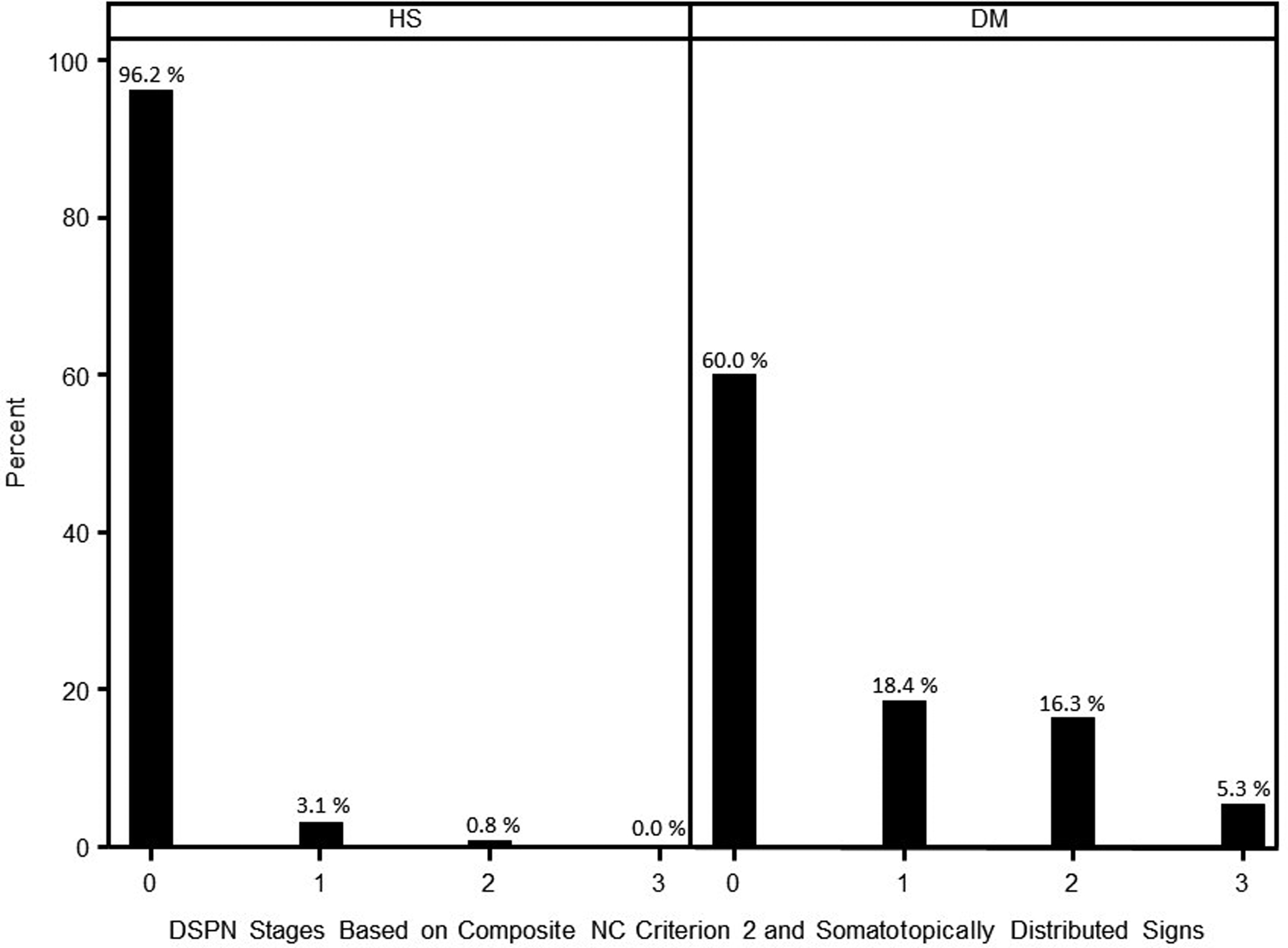
A plot of staged severity of DSPN in HS and persons with DM in studied mid North American ethnic cohorts. Stage 0 is without abnormality of composite NC score #2, of four sensitive CVs and DLs of limb nerves. Stage 1 persons have abnormality of composite NC score #2 without neuropathy signs. Stage 2 has NC abnormality and neuropathy signs of feet or legs. Stage 3 has NC abnormality and signs extending to the thighs.
The ROC curves of assessed measures of DSPN
The ROC curves and area under the curve (AUC) percentage performance of different measures of DSPN as compared to composite NC score #2 are shown in Figure 4. Fibular MNCV abnormality alone provides a high value, i.e., 0.98. This finding is not unexpected since the assessed attribute of NC is a major sensitive component of composite NC score #2. By comparison, composite score #4 (of 3 CMAPs and 1 SNAP amplitude) and neuropathy signs provided lower AUC values of 0.81 and 0.84.
Figure 4:
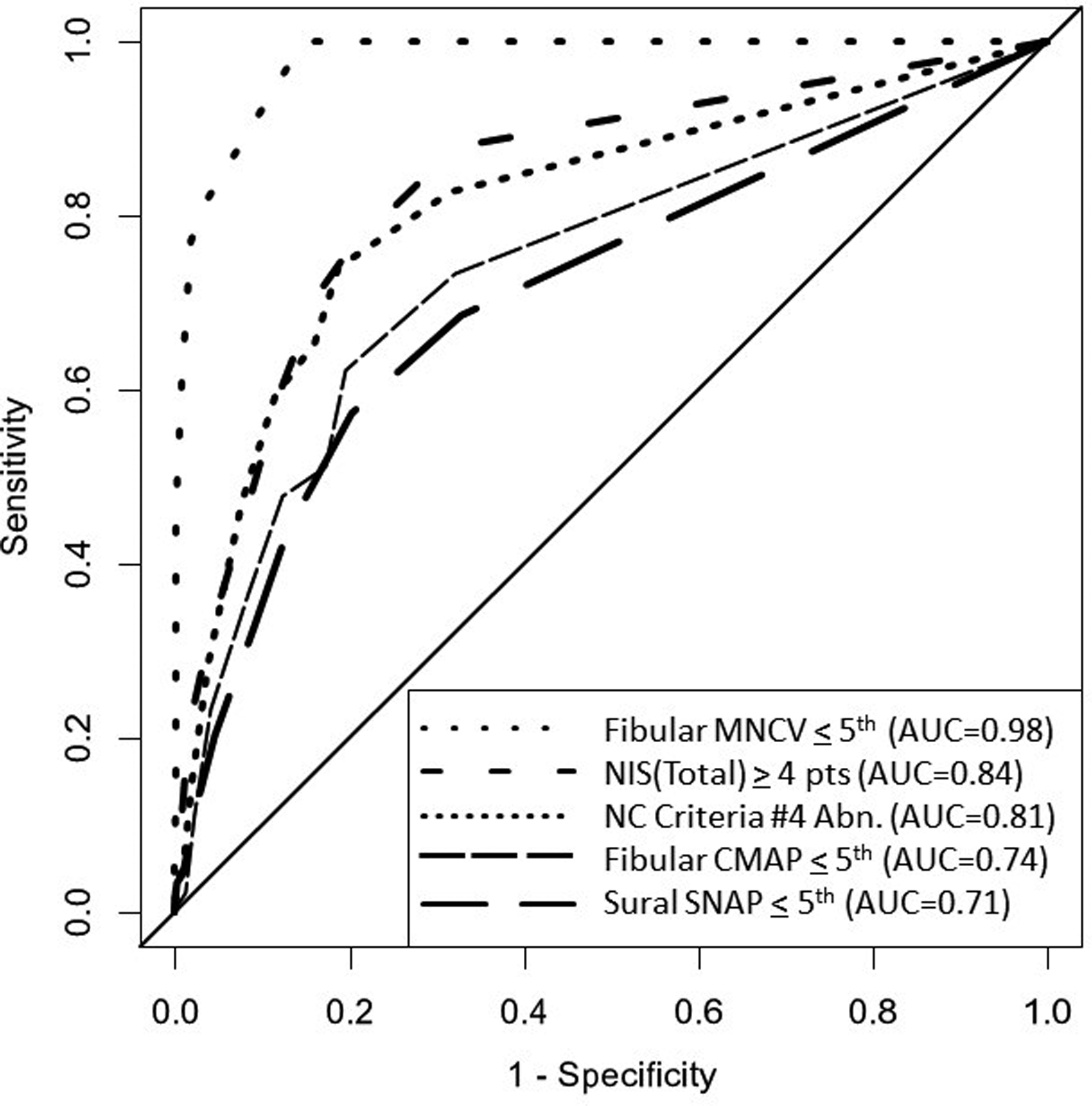
The ROC/AUC plots of different measures of DSPN as compared to composite NC score #2 of sensitive CVs and DLs of limb nerves. Individual sensitive MNCVs, as expected, have large AUCs. NIS and composite NC score #4 had intermediate AUC values and fibular CMAPs and sural SNAPs had lower values. This data indicates that composite score #2 of CVs and DLs are sensitive measures of DSPN especially useful for diagnosis. Composite score #4 of CMAP/SNAP amplitudes are less sensitive for the diagnosis of DSPN than composite score #2 but are valid measures of staged severity proposed in this report.
Staging severity of DSPN
Using composite NC score #2 for the diagnosis of DSPN and the lower limb distribution of neuropathy signs, we staged severity of DSPN in studied cohorts by the following criteria: N0, no abnormality of NCs as assessed by composite NC score #2; N1, abnormality of NC without neuropathy signs; N2, NC abnormality and polyneuropathy signs of feet or legs; and N3, abnormality of NCs and signs of thigh involvement. The percentage frequency of staged abnormality in HS and persons with DM is shown in Figure 3. In HS and DM, staged abnormality 1 occurred in 3.1 and 18.4%, stage 2 in 0.8 and 16.3%, and stage 3 in 0 and 5.3%.
Validation of staged severity of DSPN
In Supplementary Table 2, we assess for significant differences of NIS, NSC, and NC scores between stage 1 vs 0, 2 vs 1, and 3 vs 2. Of those with significant differences, scores were greater with higher stages of severity, with two exceptions, NIS and NIS-reflex, which scores were significantly higher in stage 0 than in stage 1. Obviously, this result is not credible or consistent with expectations nor with the observed results of other scores: NSC severity, NSC autonomic, composite NC score #2, and composite NC score #4; all were significantly more abnormal in stage 1 as compared to stage 0 (Supplementary Table 2). Significant differences between stage 2 vs 1 were observed for: NIS, NIS-reflex, NIS-sensation, NSC-sensation, and the composite NC score of CMAP/SNAP amplitudes (composite score #4). Stage 3, as compared to stage 2, was significantly different and in the correct direction for: NIS, NIS-weakness, NIS-reflex, NSC-weakness, and the composite NC score of CMAP/SNAP amplitudes, i.e., composite NC score #4.
DISCUSSION
In the present study, we show that assessment of NC abnormality is a more sensitive measure of DSPN than is assessment of neuropathy signs. We have further shown, using six different composite NC scores, that the NC judgment of DSPN is markedly influenced by which attributes of nerve conduction are evaluated. Composite scores of CVs and DLs are sensitive for diagnoses whereas composite scores of CMAP/SNAP amplitudes are better measures of polyneuropathy severity. We use composite score #2 (of CVs and DLs) to demonstrate the prevalence of DSPN and composite score #4 (of CMAP/SNAP amplitudes) for assessment of severity of DSPN.
The pathologic basis underlying abnormality of CVs and DLs from CMAP/SNAP amplitudes has been studied in uremic, DSPN, and other varieties of peripheral neuropathy.54–56 In teased nerve fiber assessments at mid-calf level, axonal atrophy and secondary segmented demyelination predominated, whereas at ankle level, axonal degeneration predominated. Assuming that these length dependent pathologic alterations are typical of DSPN would explain why abnormalities of CVs and DLs would develop prior to decrease of CMAP/SNAP amplitudes. These functional/structural differences between use of CVs and DLs for detection and CMAP/SNAP amplitudes for assessment of severity have increasingly been used in epidemiology surveys and therapeutic trials of sensorimotor polyneuropathy.22,45,57–61 These composite measures of NCs could increasingly also find a place in high quality medical practice.8
The present study also provides information, albeit imperfect, on the prevalence and staged severity of DSPN in mid North America. This information has special importance because detection of DSPN was based on specific referenced diagnostic NC criteria and severity of DSPN was based on highly defined and referenced NC criteria and the somatotopic distribution of neuropathy signs. Although our survey of HS and persons with DM included persons of different ethnicities, we recognize that recruitment to our studies was not as disease or population based as desirable. To illustrate, we judge that NPI from outlying reservations and persons with DM and end-stage kidney disease typically failed selectively to attend our clinics. Also, selection of Lat patients probably was biased toward selection of young and healthy persons actively recruited to southern Minnesota for agricultural work. Selection bias therefore could have reduced prevalence and staged severity of DSPN.58,62,63 However, the large number of HS and persons with DM, with and without DSPN of varying severity, provided a wide range of DSPN severity useful for setting minimal criteria for DSPN and for staging its severity, major objectives of present studies.
Supplementary Material
Acknowledgements:
Financial support for the epidemiology surveys of three ethnic cohorts was obtained from NIH grants NS14304 and NS 36797. Healthy subjects and persons with diabetes were recruited from inhabitants of Olmsted County, MN, USA (Rochester Diabetic Neuropathy Study, RDNS), persons mainly of northern European extraction, Northern Plains Indians from Minnesota, North and South Dakota, and Wisconsin (NPI), and Latinos (Lat) mainly from southern Minnesota, USA. Jenny L. Davies, Karen A. Lodermeier, William J. Litchy, Michelle L. Mauermann, P. James B. Dyck, and Peter J. Dyck have also received financial support from Ionis, Inc., Carlsbad, CA; Alnylam, Inc., Cambridge, MA; Eidos, Inc., San Francisco, CA; and Intellia, Inc., Cambridge, MA for their training of investigators, advice in the design and use of polyneuropathy endpoints, instruction of investigators, and surveillance of trial endpoints in oligonucleotide and other therapeutic trials of transthyretin amyloidosis polyneuropathy.
We thank Sharlene Sorensen, B.S. for preparation and handling of the manuscript and of correspondence.
Abbreviations:
- AUC
Area under the curve
- CMAP
Compound muscle action potential
- CVs
Conduction velocities
- DCCT
Diabetes Control and Complications Trial
- DLs
Distal latencies
- DM
Diabetes mellitus
- DSPN
Diabetic sensorimotor polyneuropathy
- HS
Healthy subjects
- Lat
Latinos
- LLF
Lower Limb Function
- MNCV
Motor nerve conduction velocity
- NCs
Nerve conductions
- NIS
Neuropathy Impairment Score
- NPI
Northern Plains Indians
- NSC
Neuropathy Symptoms and Change
- RDNS
Rochester Diabetic Neuropathy Study
- ROC
Receiver Operating Characteristic
- SNAP
Sensory nerve action potential
- SNDL
Sensory nerve distal latency
Footnotes
Ethical publication statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of conflicts of interest: None of the authors has any conflict of interest to disclose.
REFERENCES
- 1.Mulder DW, Lambert EH, Bastron JA, Sprague RG. The neuropathies associated with diabetes mellitus: a clinical and electromyographic study of 103 unselected diabetic patients. Neurology 1961;11:275–284. [DOI] [PubMed] [Google Scholar]
- 2.Skillman TG, Johnson EW, Hamwi GJ, Driskill HJ. Motor nerve conduction velocity in diabetes mellitus. Diabetes 1961;10:46–51. [Google Scholar]
- 3.Dyck PJ, Karnes JL, Daube J, O’Brien P, Service FJ. Clinical and neuropathological criteria for the diagnosis and staging of diabetic neuropathy. Brain 1985;108(Pt 4):861–880. [DOI] [PubMed] [Google Scholar]
- 4.Dahl-Jorgensen K, Brinchmann-Hansen O, Hanssen KF, et al. Effect of near normoglycemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Br Med J 1986;293:1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehle AL, Raskin P. Increased nerve conduction in diabetics after a year of improved glucoregulation. . J of the Neurological Sciences 1986;74:191–197. [DOI] [PubMed] [Google Scholar]
- 6.Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology 1993;43(4):817–824. [DOI] [PubMed] [Google Scholar]
- 7.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O’Brien PC. Variables influencing neuropathic endpoints: the Rochester Diabetic Neuropathy Study of healthy subjects (RDNS-HS). Neurology 1995;45:1115–1121. [DOI] [PubMed] [Google Scholar]
- 8.Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes treatment on nerve conduction in the diabetes control and complications trial. Ann Neurol 1995;38:869–880. [DOI] [PubMed] [Google Scholar]
- 9.Baba J, Ozaki I. Electrophysiological changes in diabetic neuropathy: from subclinical alterations to disabling abnormalities. Arch Physiol Biochem 2001;109:234–240. [DOI] [PubMed] [Google Scholar]
- 10.Perkins BA, Ngo M, Bril V. Symmetry of nerve conduction studies in different stages of diabetic polyneuropathy. Muscle Nerve 2002;25(2):212–217. [DOI] [PubMed] [Google Scholar]
- 11.Martin CL, Albers JW, Herman WH, et al. Neuropathy among the Diabetes Control and Complicaitons Trial cohort 8 years after trial completion. Diabetes care 2006;29:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albers JW, Herman WH, Pop-Busui R, Martin CL, Cleary PA, Waberski B, for the diabetes control and complications trial (DCCT)/epidemiology of diabetes intervention and complications (EDIC) research group. Subclinical neuropathy among diabetes control and complications trial participants without diagnosable neuropathy at trial completion. Possible predictors of incident neuropathy? Diabetes care 2007;30:2613–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charles M, Soedamah-Muthu SS, Tesfaye S, et al. Low peripheral nerve conduction velocities and amplitudes are strongly related to diabetic microvascular complications in type 1 diabetes. Diabetes care 2010;33:2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litchy WJ, Albers JW, Wolfe J, et al. Proficiency of nerve conduction using standard methods and reference values. Muscle & nerve 2014;50:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Andary MB, Buschbacher R, et al. Electrodiagnostic reference values for upper and lower limb nerve conduction studies in adult populations. Muscle & nerve 2016;54:371–377. [DOI] [PubMed] [Google Scholar]
- 16.Diabetes Control and Complications Trial Research Group. Full-scale clinical trial-phase III protocol. 1988.
- 17.Valk GD, Nauta JJP, Strijers RLM, Bertelsmann FW. Clinical examination versus neurophysiological examination in the diagnosis of diabetic polyneuropathy. Diabet Med 1992;9:716–721. [DOI] [PubMed] [Google Scholar]
- 18.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36(2):150–154. [DOI] [PubMed] [Google Scholar]
- 19.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes care 1994;17(11):1281–1289. [DOI] [PubMed] [Google Scholar]
- 20.Peripheral Nerve Society. Diabetic polyneuropathy in controlled clinical trials: consensus report of the peripheral nerve society. Annals of neurology 1995;38:478–482. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicenter randomized controlled trial (ALADIN Study). Diabetologia 1995;38:1425–1433. [DOI] [PubMed] [Google Scholar]
- 22.Dyck PJ, Davies JL, Litchy WJ, O’Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology 1997;49:229–239. [DOI] [PubMed] [Google Scholar]
- 23.Melzack R, Katz J. Pain measurement in persons in pain. In: Wall PD, Melzack R, editors. Textbook of Pain. Fourth ed. London: Harcourt Publishers Limited; 1999. p 409–426. [Google Scholar]
- 24.Apfel SC, Asbury AK, Bril V, et al. Positive neuropathic sensory symptoms as endpoints in diabetic neuropathy trials. Journal of the neurological sciences 2001;189(1–2):3–5. [DOI] [PubMed] [Google Scholar]
- 25.Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes care 2001;24:250–256. [DOI] [PubMed] [Google Scholar]
- 26.Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes care 2002;25(11):2048–2052. [DOI] [PubMed] [Google Scholar]
- 27.Yasuda H, Sanada M, Kitada K, et al. Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Research & Clinical Practice 2007;77S:S178–S183. [DOI] [PubMed] [Google Scholar]
- 28.Perkins BA, Orszag A, Grewal J, Ng E, Ngorri M, Bril V. Multi-site testing with a point-of-care nerve conduction device can be used in an algorithm to diagnose diabetic sensorimotor polyneuropathy. Diabetes care 2008;31:522–524. [DOI] [PubMed] [Google Scholar]
- 29.Bril V, Tomioka S, Buchanan RA, Perkins BA. Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med 2009;26(3):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pop-Busui R, Martin CL. Neuropathy in the DCCT/EDIC--what was done then and what we would do better now. Int Rev Neurobiol 2016;127:9–25. [DOI] [PubMed] [Google Scholar]
- 31.Dyck PJ, O’Brien PC, Johnson DM, Klein CJ, Dyck PJB. Quantitative Sensation Testing. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy, Fourth Edition. Philadelphia: Elsevier; 2005. p 1063–1094. [Google Scholar]
- 32.Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle & nerve 2006;34(1):57–61. [DOI] [PubMed] [Google Scholar]
- 33.Low PA, Benarroch EE. Clinical Autonomic Disorders, Third Edition. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 34.Backonja MM, Attal N, Baron R, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 2013:doi: 10.1016/j.pain.2013.1005.1047. [DOI] [PubMed] [Google Scholar]
- 35.Dyck PJ, Argyros B, Russell JW, et al. Multicenter trial of the proficiency of smart quantitative sensation tests. Muscle & nerve 2014;49(5):645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinto MV, Dyck PJB, Gove LE, et al. Kind and distribution of cutaneous sensation loss in hereditary transthyretin amyloidosis with polyneuropathy. J Neurol Sci 2018;394:78–83. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy BG, Hsieh ST, Stocks A, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology 1995;45:1848–1855. [DOI] [PubMed] [Google Scholar]
- 38.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Archives of neurology 1998;55(12):1513–1520. [DOI] [PubMed] [Google Scholar]
- 39.Engelstad JK, Taylor SW, Witt LV, et al. Epidermal nerve fibers: Confidence intervals and continuous measures with nerve conduction. Neurology 2012;79(22):2187–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg ME, Tervo TM, Immonen IJ, Muller LJ, Gronhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci 2000;41:2915–2921. [PubMed] [Google Scholar]
- 41.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a noninvasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003;46:683–688. [DOI] [PubMed] [Google Scholar]
- 42.Hossain P, Sachdev A, Malik RA. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet 2005;366:1340–1343. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed A, Bril V, Orszag A, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes. Diabetes care 2012;35:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dyck PJ, Davies JL, Wilson DM, Service FJ, Melton LJ, O’Brien PC. Risk factors for severity of diabetic polyneuropathy. Intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study cohort. Diabetes care 1999;22(9):1479–1486. [DOI] [PubMed] [Google Scholar]
- 45.Dyck PJ, Litchy WJ, Daube JR, et al. Individual attributes versus composite scores of nerve conduction abnormality: sensitivity, reproducibility, and concordance with impairment. Muscle & nerve 2003;27(2):202–210. [DOI] [PubMed] [Google Scholar]
- 46.Dyck PJ, Turner DW, Davies JL, O’Brien PC, Rask CA. Electronic case-report forms of symptoms and impairments of peripheral neuropathy. Can J Neurol Sci 2002;29(3):258–266. [DOI] [PubMed] [Google Scholar]
- 47.Dyck PJ, Kratz KM, Lehman KA, et al. The Rochester Diabetic Neuropathy Study: design, criteria for types of neuropathy, selection bias, and reproducibility of neuropathic tests. Neurology 1991;41:799–807. [DOI] [PubMed] [Google Scholar]
- 48.Dyck PJ, Hughes RAC, O’Brien PC. Quantitating Overall Neuropathic Symptoms, Impairments, and Outcomes. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy, Fourth Edition. Philadelphia: Elsevier; 2005. p 1031–1052. [Google Scholar]
- 49.Dyck PJ, Overland CJ, Low PA, et al. Signs and Symptoms Versus Nerve Conduction Studies to Diagnose Diabetic Sensorimotor Polyneuropathy: Cl vs. NPhys Trial. Muscle & nerve 2010;42:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dyck PJ, Overland CJ, Low PA, et al. “Unequivocally Abnormal” vs. “Usual” Signs and Symptoms for Proficient Diagnosis of Diabetic Polyneuropathy Cl vs. N Phys Trial. Archives of neurology 2012;69(12):1609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing., https://www.R-project.org/. Vienna, Austria: 2020. [Google Scholar]
- 52.Wickham H, Miller e. haven: import and export ‘SPSS,’ ‘Stata’ and ‘SAS’ files. R package version 231, https://CRAN.R-project.org/package=haven2020. [Google Scholar]
- 53.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dyck PJ, Johnson WJ, Lambert EH, O’Brien PC. Segmental demyelination secondary to axonal degeneration in uremic neuropathy. Mayo ClinProc 1971;46:400–431. [PubMed] [Google Scholar]
- 55.Dyck PJ, Lais AC, Karnes JL, et al. Permanent axotomy, a model of axonal atrophy and secondary segmental demyelination and remyelination. Ann Neurol 1981;9:575–583. [DOI] [PubMed] [Google Scholar]
- 56.Sugimura K, Dyck PJ. Sural nerve myelin thickness and axis cylinder caliber in human diabetes. Neurology 1981;31:1087–1091. [DOI] [PubMed] [Google Scholar]
- 57.Alhammad R, Davies J, Litchy WJ, Carter R, Dyck PJB, Dyck PJ. Variable differences of nerve conduction amplitudes versus velocities and distal latencies of healthy subjects assessed in ethnic cohorts. Muscle & nerve 2022;65:162–170. [DOI] [PubMed] [Google Scholar]
- 58.Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 2018;379:22–31. [DOI] [PubMed] [Google Scholar]
- 59.Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 2018;279:11–21. [DOI] [PubMed] [Google Scholar]
- 60.Dyck PJB, Gonzalez-Duarte A, Obici L, et al. Development of measures of polyneuropathy impairment in hATTR amyloidosis: from NIS to mNIS+7. Journal of the neurological sciences 2019;405:116424. [DOI] [PubMed] [Google Scholar]
- 61.Dyck PJ, Kincaid JC, Dyck PJB, et al. Assesing mNIS+7Ionis and international neurologists’ proficiency in a FAP trial. Muscle Nerve 2017;56:901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Massie R, Mauermann ML, Staff NP, et al. Diabetic cervical radiculoplexus neuropathy: a distinct syndrome expanding the spectrum of diabetic radiculoplexus neuropathies. Brain 2012;135(Pt 10): 3074–3088. [DOI] [PubMed] [Google Scholar]
- 63.Taylor SW, Laughlin RS, Kumar N, et al. Clinical, physiological and pathological characterization of the sensory predominant peripheral neuropathy in copper deficiency. J Neurol Neurosurg Psychiatry 2017;88:839–845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


