Abstract
Individuals with more complex jobs experience better cognitive function in old age and a lower risk of dementia, yet complexity has multiple dimensions. Drawing on the Social Networks in Alzheimer Disease (SNAD) study, we examine the association between occupational complexity and cognition in a sample of older adults (N=355). A standard deviation (SD) increase in complex work with people is associated with a 9–12 percent reduction in the probability of mild cognitive impairment or dementia, a .14–.19 SD increase in episodic memory, and a .18–.25 SD increase in brain reserve, defined as the gap (residual) between global cognitive function and MRI indicators of brain atrophy. In contrast, complexity with data or things is rarely associated with cognitive outcomes. We discuss the clinical and methodological implications of these findings, including the need to complement data-centered activities (e.g., Sudoku puzzles) with person-centered interventions that increase social complexity.
Keywords: cognitive aging, social enrichment, occupational complexity, cognitive reserve, brain reserve
1. NARRATIVE
Researchers are increasingly interested in identifying social and behavioral factors that slow the rate of aging-related cognitive decline and dementia, allowing older adults to maintain higher quality of life and preserve functional independence at advanced ages. In recent years, occupational complexity has received attention, both in its own right and as a mechanism of educational differences in cognitive outcomes (e.g., [1]). Because we spend the majority of our waking lives at work, occupations carry an outsized influence on cognitive processes across the life course, affecting trajectories of cognitive decline even well after retirement [2]. In general, studies find that the more complex one’s job, the greater the opportunity for cognitive stimulation and the better one’s cognitive resilience in late life. Occupational complexity is theorized to build cognitive reserve by increasing the volume and functional connectivity of the brain in ways that reduce the cognitive impact of Alzheimer’s disease (AD) neuropathology [3,4].
Social scientists first developed the concept of occupational complexity in the 1970s to explain inequalities in psychosocial outcomes such as job satisfaction [5], self-esteem [6], intellectual flexibility and self-direction [7], and feelings of powerlessness [8], yet more recent research has examined its role in cognition. Because the activities performed in any given occupation are fairly standard, they provide a convenient measure of the nature and magnitude of exposure to cognitive enrichment over the life course. Occupational complexity is a tripartite concept, defined as the level of challenge an employee faces when working with people, with data, and with things [9,10]. Jobs with high complexity in these areas might include a social worker, a data analyst, and a watch repairman, respectively [11]. Importantly, there are often tradeoffs between these forms of complexity: a social worker is not likely to engage in much stimulating activity when it comes to working with things, while a watch repairman is not likely to engage in much complex interaction with customers or coworkers. Yet both social workers and watch repairmen (as well as data analysts) have jobs with high complexity in at least one domain. Thus, examining how these forms of complexity and other work-related cognitive exposures are related to different domains of cognition and clinical cognitive outcomes can help determine which enrichment activities drive the association between occupation and cognitive resilience, and may even explain socioeconomic disparities in cognition among older adults.
In the present study, we examine the three types of occupational complexity and their relationship with global cognitive function (MoCA), six functionally specific domains, clinical diagnosis (MCI or dementia), and a neuroimaging measure of brain reserve. To our knowledge, this is the first study to examine the association between multiple forms of occupational complexity and a broad range of cognitive outcomes, including neuropsychological tests, clinician-assessed ADRD, and measures derived from neuroimaging biomarkers. We hypothesize, and indeed find, that complexity in working with people has the strongest association with later-life cognition and brain reserve due to the unique effects of social enrichment on the brain. In what follows, we discuss the unique properties of social complexity in occupations and beyond, highlighting its mechanistic role in cognitive reserve and dementia resilience.
Social complexity and the human brain.
Why might working with people matter more than other forms of occupational complexity? To begin to answer this question, we introduce “complexity in working with people” by a more familiar name: social complexity. Neuroscientists have long posited a connection between social and neural structures; indeed, from an evolutionary perspective, social complexity has been the driving force of selective advances in the human brain. Seeking to explain the large brain size of humans relative to other primates, the “social brain hypothesis” argued that the growing size of human social networks necessitated a change in brain structure and function [12,13]. While the increased size of social groups, and the concomitant need for brain structures capable of language processing, may not be the only contributor to the growth of the prefrontal cortex and the general increase in human brain size, this social process was likely a key contributor. In addition to the size of our networks, humans are nearly unique in the degree to which we have developed social skills that enable us to effectively communicate, empathize, and show altruism [14]. Strong selective pressures to develop the social brain and social cognitive skills have produced an organ with precisely the structural and functional requisites to achieve these aims.
Yet these advantages are not automatically conferred: they are conditional, to a degree not found in other organisms, on opportunities for “socially mediated learning” [15]. The parsimony of the human genome—containing far fewer genes than scientists originally predicted—means that our brains are uniquely sensitive to, and dependent upon, epigenetic changes induced by environmental stimuli [15]. Just as social complexity necessitated the development of the brain at an evolutionary time scale, so too does individual brain development occur in part as a response to social processes [16] At least since Hebb (1947) [17], scientists have noted the effect of environmental enrichment on brain structure and function [e.g., 20]. Unfortunately, the literature rarely distinguishes between enrichment in general (e.g., increased cage size, objects to play with, opportunities for exercise) and social enrichment (e.g., playmates). Environmental enrichment is defined as “a combination of complex inanimate and social stimulation” [18] (cited in [19, p. 233]), and some have argued that the interaction of inanimate and social factors matters most [19]. However, the more recent studies we cite distinguish between inanimate and social factors, finding distinct benefits of each.
In rodents, non-human primates, and humans, environmental enrichment and social complexity are associated with greater neural development and resilience, including increased dendritic branching and decreased apoptosis, respectively (see Ref. [19] for a review). The benefits of social enrichment in rodents include improved hippocampal function, social memory, and adult neurogenesis (Refs. 21–24, but see Refs. 18, 25). In primates, benefits include increased dendritic branching and synaptic protein levels in the prefrontal cortex and hippocampus [26] and a larger corpus callosum [27].
In humans, participation in a variety of social activities has been linked to improved cognitive and brain function via “neural resource enrichment” [28]. As Immordino-Yang and colleagues (2019) argue, “Research is revealing that the brain’s malleability … is triggered and organized largely via socially enabled, emotionally driven opportunities for cognitive development,” suggesting a need for “high-quality social interaction” [15, p. 186]. These processes begin early in life but continue well past the critical period of childhood [29, 30]. For example, adult socioeconomic status—a key interest here given our focus on occupations—is associated with brain function and anatomy even after conditioning on childhood SES, due in part to unequal opportunities for enrichment [15, 30–35]. As individuals grow older and their social networks contract, “interindividual differences in social interaction tendencies,” determined in part by demographics such as occupation, can have a profound influence on brain structure at midlife and beyond [30, p. 2].
These findings suggest that a complex social environment offers unique cognitive benefits. As we have suggested, such benefits may be especially outsized among humans, given that the prefrontal cortex (responsible for executive function and other key domains of cognition) developed in response to social-evolutionary pressures. Neuroimaging research suggests that social support and social network size are associated with differences in brain volume and functional brain activity [36–40], contributing to the social brain hypothesis that the large neocortex developed to support social environments that are cognitively taxing.
Social network complexity and social connectedness play an important role in cognitive function and building cognitive reserve in aging brains [29, 30, 41–45]. Several studies have confirmed this finding using proxy measures to capture different facets of social connectedness [42, 46–48]. Fewer studies have employed structural network measures [49, 50], yet the implications of these studies are significant. As shown by Perry and colleagues, older individuals with larger, more diverse, and less densely connected networks may experience greater social enrichment, leading to better global cognitive function even in the face of brain atrophy [45, 51]. Given that up to a third of individuals who meet the neuropathological criteria for ADRD do not show signs of cognitive impairment [52–54], the role of socially driven compensatory mechanisms (i.e., cognitive reserve) deserves further attention. Occupational complexity offers a powerful mechanism for exploration.
Occupational complexity and cognitive outcomes.
Because occupational complexity is generally treated as a measure of cognitive reserve, it is often included in statistical models as a mediator or moderator rather than an explanatory measure. For example, Fujishiro and colleagues [1] have shown that up to a fifth (22 percent) of the relationship between educational attainment and cognition is explained by differences in occupational complexity, with indirect effects varying by race and gender. While other studies have examined the direct effect of occupational complexity on cognitive outcomes, it remains unclear which kinds of enrichment drive cognitive resilience. As we will show, not all complexity is equal in its effects, and the differences have significant implications for intervention.
Results from a population-based twin study showed that higher complexity of work with people in a participant’s main occupation was associated with a lower risk of Alzheimer’s disease and all types of dementia, while complexity of work with data was associated with a lower risk of AD only [55]. Similarly, work with people and data were associated with better cognition as measured by the Mini-Mental State Examination [56]. In a follow-up to the first study [Ref. 55], researchers found that dementia and AD risk were predicted by occupational complexity, specifically higher complexity of work with people and data, though adjusting for education rendered these findings nonsignificant [57]. Curiously, another study found no effects of occupational complexity for participants who held their primary occupation for 23 years or less; for those who held it for more than 23 years, there was a protective effect against ADRD for those who worked with people and things, but a risk-inducing effect for those who worked with data [58].
These studies show an inconsistent pattern—sometimes finding a protective effect for working with data, sometimes finding an exacerbating effect; sometimes finding a protective effect for working with things, but often finding no effect (see Ref. 59 for a recent review). Importantly, in nearly every study reviewed, occupational complexity in working with people has a significant, protective effect—though the coefficients are reduced, sometimes to nonsignificance, when controlling for education and other predictors of occupational complexity. Only two studies found no significant relationship between complexity with people and cognitive outcomes in any model: a 2013 study from Brazil [60] and a 2015 study from Sweden [61]. While these results are not necessarily artefactual, it is worth noting that these studies (a) both draw on populations for which the Dictionary of Occupational Titles was not designed (that is, outside the U.S.), and (b) both collapse “working with people” into a few categories instead of the original eight.
A handful of studies have examined how occupational complexity relates to specific dimensions of cognition. These studies are similar to those above, finding consistent advantages for working with people (e.g., verbal, spatial, memory, processing speed, and task-switching), fewer benefits for working with data (processing speed, evaluating new information), and no significant advantages for working with things [11, 66, 67]. Finally, a parallel of research, not the main focus of our study, has tested the association between complexity and the rate of cognitive decline. Some studies have found that greater complexity of work with people predicts a faster rate of decline upon retirement [11, 62] some have found a slower rate [63], and some have found no significant association [64]. See Ref [65] for a recent review.
In the present study, we leverage variation in occupational complexity with people, data, and things to determine whether social enrichment deserves a privileged position in our understanding of cognitive resilience. Advancing prior research, we test a broad range of cognitive outcomes, including a measure of global cognitive function, the Montreal Cognitive Assessment, as well as six functionally specific cognitive domains drawn from the NACC Uniform Data Set (UDS): attention, processing speed, executive function, episodic memory, language, and visuospatial skills [68]. (We ultimately dropped attention from our analyses because it was not significantly associated with occupational complexity in any model.) We also include a diagnostic outcome indicating the presence of mild cognitive impairment (MCI) or dementia. Finally, we are the first known research team to examine the association between occupational complexity and a measure of brain reserve [69] derived from the residual of the association between structural neuroimaging biomarkers and global cognitive function [70].
We employ data from a unique source, the Social Networks in Alzheimer Disease (SNAD) study, a collaboration with the Indiana Alzheimer’s Disease Research Center (IADRC). The majority of study participants are retired (mean age: 70.8) and are no longer working in their longest-held job. Using structural equation modeling to utilize all available data, we fit a series of linear models that include each measure of occupational complexity—one at a time, then simultaneously in a final model. Covariates include age, gender, and race. In subsequent analyses, we also adjust for educational attainment, addressing concerns that these findings might be driven by cognitive stimuli that precede respondents’ occupation.
Our results are striking: complexity in working with people is linked to significantly better global cognitive function, better performance on five cognitive domains (speed, executive function, episodic memory, language, and visuospatial skills), greater brain reserve, and a lower risk of being diagnosed with MCI or dementia at baseline (that is, during the first IADRC clinical evaluation). When we include complexity measures in the same model, all of these associations remain significant, and effect sizes remain remarkably consistent. Adjusting for educational attainment does reduce the effect sizes somewhat, but the associations with episodic memory, MCI/dementia, and brain reserve remain significant in all models. On average, a standard deviation increase in complexity with people is associated with a .14–.19 SD increase in episodic memory, a 9–12 percent reduction in the probability of MCI or dementia, and a .18–.25 SD increase in brain reserve. Even after adjusting for parental education, a key confounder not often included in these studies, coefficients remain within those ranges.
In contrast, we find little evidence that working with data or things protects cognition: working with data is sometimes associated with MoCA score, but only before adjusting for education, while working with things is associated with visuospatial skills, but only after adjusting for data and people.
That complexity with people is associated not only with memory, but also with the risk of mild cognitive impairment or dementia—even after adjusting for educational attainment—is striking. What is perhaps more striking is that complexity with people is associated with greater brain reserve, the gap between brain atrophy (e.g., smaller hippocampal volume) and poor cognitive function, indicating that exposure to cognitive enrichment through social interaction may increase cognitive resilience to neuropathology. Though much research has posited occupational complexity as a mechanism of reserve, even using it as a proxy measure for this construct, our study may be the first to show its association with both cognitive function and a direct neuroimaging measure of brain reserve.
As we stated at the outset, the relationship between occupational complexity and cognition is not just a matter of intellectual curiosity. As rates of Alzheimer’s disease and related dementias continue to grow in the United States and abroad, there is a pressing need to develop effective research-driven interventions [71]. Yet existing interventions, whether pharmaceutical or psychosocial, have largely failed to make significant improvements in the prognosis or course of cognitive decline [72].
Our research suggests that academics, health practitioners, and even journalists who write about cognition for a lay audience may wish to place less emphasis on popular exercises such as Sudoku and Sudoku puzzles [73–75] and more emphasis on interventions that offer social enrichment. Not only are the dominant interventions data-driven rather than people-driven, they also encourage older adults to spend more time by themselves. This is problematic given that older adults are already at high risk for social isolation, and this condition has adverse consequences not only for cognition, but also for mental and physical health [76]. Previous research suggests that older adults with broader, more diverse social networks may have more opportunities for complex social interactions that are protective of cognitive health, and it is precisely these opportunities that we should be encouraging to maximize cognitive resilience [45, 76]. Humans are fundamentally social animals with brains that have adapted to expect and depend on social complexity. In the absence of such complexity, our cognitive capacity is likely to suffer.
2. CONSOLIDATED RESULTS AND STUDY DESIGN
The analyses presented here draw on the Social Networks in Alzheimer Disease (SNAD) study, a unique collaboration with the Indiana Alzheimer’s Disease Research Center (IADRC). The study is designed to assess the social characteristics of an aging population and their relationship to cognitive function and decline. We used the first wave of interviews (N=383) for each respondent, dropping outliers to retain an analytic sample of N=355. Of these respondents, 38 percent were diagnosed with mild cognitive impairment or dementia, while the rest were cognitively normal. This statistic excludes the 23 respondents for whom IADRC diagnoses were unavailable.
To code occupational complexity, we used the job held longest by each respondent. We then matched each occupation with complexity scores developed by Roos and Treiman (1980) [77] based on the Dictionary of Occupational Titles (DOT) [78]. The DOT draws on occupations listed in the 1970 U.S. Census. Our matches were facilitated by an O*NET Crosswalk available at https://www.onetonline.org/crosswalk/DOT/. (For details, see Refs 10,55.)
Global cognitive function was measured via the Montreal Cognitive Assessment (MoCA). Mild cognitive impairment and dementia were diagnosed by a consensus of IADRC clinicians and study personnel. Brain reserve was computed using the residualization method described in Peng et al. 2022 [70]; see details below. All other cognitive outcomes were derived from the NACC Uniform Data Set (UDS) Neuropsychological Battery. We consulted with the IADRC on the cognitive tests used to operationalize cognitive domains (attention, processing speed, executive function, episodic memory, language, and visuospatial skills); see Table 1 for details.
Table 1.
Cognitive Outcomes
| Outcome | Measure(s) |
|---|---|
| Global Cognitive Function | Montreal Cognitive Assessment (MoCA) |
| Cognitively Normal vs. MCI/Dementia | IADRC Clinical Consensus (based on neuroimaging, cognitive testing, etc.) |
| Attention (dropped) | Weschler Adult Intelligence Scale (WAIS) Digit Span Forward Weschler Adult Intelligence Scale (WAIS) Digit Span Backward |
| Processing Speed | Trail Making Test Part A Weschler Adult Intelligence Scale–Revised (WAIS–R) Digit Symbol Substitution Test |
| Executive Function | Trail Making Test Part B (Task-Switching) |
| Episodic Memory | Craft Story 21 Recall (Delayed) Paraphrase Scoring Rey Auditory Verbal Learning Test (AVLT) Delayed Recall Benson Complex Figure Recall |
| Language | Animal Naming Vegetable Naming Multilingual Naming Test (MINT) |
| Visuospatial Skills | Benson Complex Figure Copy |
| Brain Reserve | Standardized residual after regressing MoCA on intracranial volume, total hippocampal volume, total amygdala volume, white matter hyperintensities volume (logged), and thickness of the frontal, parietal, temporal, and occipital lobe |
We also controlled for gender, age, race (white vs. nonwhite) and, in some models, educational attainment (high school degree or less, some college or associate’s degree, college graduate, or post-college). We used full-information maximum likelihood (FIML) to fit a series of linear models examining the relationship between occupational complexity and cognition, conditional on covariates. Our first set of models includes each occupational complexity measure separately, and then adds educational attainment as a potential confounder. Our second set of models includes the occupational complexity measures simultaneously, again adding educational attainment. Supplementary models adjust for parental education. Our results offer strong evidence that complexity in working with people is associated with better global and domain-specific cognitive function as well as a lower risk of mild cognitive impairment or dementia and greater brain reserve. In contrast, we find little evidence that complexity in working with data or things has a protective effect. These findings highlight the unique cognitive health benefits of social enrichment during one’s working years, extending long after retirement.
3. DETAILED METHODS AND RESULTS
3.1. Measures:
We operationalized occupational complexity by matching each respondent’s longest-held job to the 9-digit code listed in the Dictionary of Occupational Titles (DOT) [78]. The DOT draws on occupations listed in the 1970 U.S. Census; each digit in the nine-digit code corresponds to an aspect of that occupation, as rated by professional observers. Three key “worker functions” are indicated by the fourth, fifth, and sixth digit: namely, complexity in working with data (range: 0–6), with people (0–8), and with things (0–7). We reverse-coded these items so that higher scores reflect greater occupational complexity in each domain. We then standardized the items to more easily compare effect sizes. DOT codes are publicly available via the O*NET Crosswalk: https://www.onetonline.org/crosswalk/DOT/. For more details on the complexity scores, see Refs 10,55.
We operationalized cognition via nine measures: global cognitive function (MoCA score), a binary indicator of cognitively normal vs. MCI/dementia (the latter are combined to maximize statistical power), and six subdomains of cognitive function: attention, processing speed, executive function, episodic memory, language, and visuospatial skills. We dropped attention given its nonsignificance in all models. For those subdomains that comprise multiple items, each item was standardized before the group mean (average z-score) was computed. For ease of comparison, the MoCA score was also standardized. Items were coded so that higher scores indicate better cognitive function. See Table 1 for a complete list of cognitive outcomes and how they are measured.
Finally, we included a measure of brain reserve derived from Peng et al. (2022) [70]. We regressed MoCA score on eight MRI measures: intracranial volume, total hippocampal volume, total amygdala volume, white matter hyperintensities volume (logged), and thickness of the frontal, parietal, temporal, and occipital lobe. Brain reserve is simply the standardized residual derived from this OLS regression. All scans were performed on a research dedicated Siemens Prisma 3T scanner using the ADNI protocol (http://adni.loni.usc.edu) by the Indiana ADRC Neuroimaging Core. Structural scan data analyzed here included T1-weighted MPRAGE scans that were postprocessed using FreeSurfer, version 6.0.
Covariates included gender (0 = male, 1 = female), age during the first SNAD interview, race (white vs. nonwhite due to small cell sizes; all but four nonwhite respondents are Black) and, in some models, educational attainment (high school or less, some college or associate’s degree, college, and post-college). In supplementary models, we included parents’ educational attainment, operationalized as the respondent’s most educated parent.
3.2. Analysis
Our analytic approach was as follows: we fit a series of linear models to examine the association between occupational complexity and cognitive function, adjusting for gender, age, race and, in some models, educational attainment. We excluded outliers, defined as respondents with at least two cognitive outcomes that fall ±2 SD outside the mean (N=28). Because brain scans at the IADRC are conducted biennially, only a subsample of respondents (148) had neuroimaging data from which we could calculate brain reserve. This challenge, along with high rates of missingness on some measures, necessitated careful consideration of our modeling strategy. To avoid losing cases due to missingness, we used full information maximum likelihood (FIML), a technique that recovers parameter estimates by using all available data [79, 80]. In practice, this means that linear models were estimated through maximum likelihood rather than through ordinary least squares (OLS) regression, and the one binary outcome in this study (cognitively normal vs. MCI/dementia) is assessed with a linear probability model (LPM) since FIML does not allow logistic regression. Sensitivity analyses using listwise deletion instead of FIML and using logistic regression instead of a linear probability model for MCI/dementia showed that the overall pattern of findings is very similar, and in most cases the FIML approach produces more conservative estimates (available upon request). Our estimates, along with robust standard errors, were computed via structural equation modeling in Stata 17.
We began by examining the effects of each occupational complexity measure (people, data, and things) on each cognitive outcome, net of covariates, before adding educational attainment as a potential confounder (Table 2). We then repeated these analyses with all three measures of occupational complexity entered simultaneously (Table 3). In supplementary analyses, we added parental education as another confounder, though high rates of missingness on this item demand caution in interpretation (Tables S1–S2 in the appendix).
Table 2.
Effect of Occupational Complexity on Cognition, OC Entered Individually (N = 355)
| MoCA | MCI or Dementia | ProcessingSpeed | Executive Function | Episodic Memory | Language | Visuospatial | Brain Reserve | ||
|---|---|---|---|---|---|---|---|---|---|
| Panel A: Not Adjusting for Educational Attainment | |||||||||
| People | .20*** (.05) |
−.09** (.03) |
.09* (.04) |
.18*** (.05) |
.19*** (.05) |
.14*** (.04) |
.13** (.04) |
.21** (.07) |
|
| Data | .17** (.05) |
.01 (.03) |
.03 (.04) |
.02 (.06) |
.08 (.06) |
.03 (.04) |
.02 (.05) |
.10 (.08) |
|
| Things | −.08 (.05) |
.04 (.03) |
−.04 (.04) |
−.07 (.06) |
−.11 (.06) |
−.07 (.05) |
.03 (.04) |
.01 (.08) |
|
| Panel B: Adjusting for Educational Attainment | |||||||||
| People | .13* (.06) |
−.09** (.03) |
.03 (.05) |
.11 (.07) |
.15* (.07) |
.07 (.05) |
.09 (.05) |
.18* (.08) |
|
| Data | .11 (.06) |
.03 (.03) |
−.01 (.04) |
−.04 (.06) |
.03 (.06) |
−.03 (.04) |
−.01 (.05) |
.03 (.09) |
|
| Things | −.05 (.05) |
.03 (.03) |
−.02 (.04) |
−.05 (.06) |
−.10 (.06) |
−.05 (.04) |
.05 (.04) |
.00 (.09) |
|
p<0.05;
p<0.01;
p<0.001 (Two-tailed tests)
Notes: (1) Estimates calculated via full information maximum likelihood (FIML) (2) Robust standard errors in parentheses (3) Covariates include race, gender, age, and (Panel B only) educational attainment. (4) Occupational complexity measures are entered individually, not collectively, in each model. (5) All measures are standardized except “MCI or Dementia.”
Table 3.
Effect of Occupational Complexity on Cognition, OC Entered Simultaneously (N = 355)
| MoCA | MCI or Dementia | Processing Speed | Executive Function | Episodic Memory | Language | Visuospatial | Brain Reserve | ||
|---|---|---|---|---|---|---|---|---|---|
| Panel A: Not Adjusting for Educational Attainment | |||||||||
| People | .17** (.05) |
−.12*** (.03) |
.09* (.04) |
.20*** (.05) |
.18** (.06) |
.14** (.05) |
.18*** (.05) |
.25** (.08) |
|
| Data | .09 (.06) |
.06 (.03) |
−.01 (.04) |
−.06 (.06) |
−.01 (.06) |
−.04 (.05) |
−.04 (.05) |
−.02 (.09) |
|
| Things | .00 (.06) |
.00 (.03) |
−.00 (.04) |
−.00 (.06) |
−.04 (.06) |
−.02 (.05) |
.10* (.04) |
.08 (.09) |
|
| Panel B: Adjusting for Educational Attainment | |||||||||
| People | .10 (.06) |
−.11** (.04) |
.03 (.05) |
.13 (.07) |
.14* (.07) |
.08 (.05) |
.15** (.05) |
.22* (.09) |
|
| Data | .07 (.06) |
.06 (.03) |
−.02 (.04) |
−.08 (.06) |
−.02 (.06) |
−.06 (.04) |
−.05 (.06) |
−.05 (.10) |
|
| Things | −.01 (.06) |
.00 (.03) |
−.02 (.04) |
−.02 (.06) |
−.06 (.06) |
−.04 (.05) |
.09* (.04) |
.06 (.09) |
|
p<0.05;
p<0.01;
p<0.001 (Two-tailed tests)
Notes: (1) Estimates calculated via full information maximum likelihood (FIML) (2) Robust standard errors in parentheses (3) Covariates include race, gender, age, and (Panel B only) educational attainment. (4) Occupational complexity measures are entered simultaneously in each model. (5) All measures are standardized except “MCI or Dementia.”
3.3. Detailed Results
When the three measures of occupational complexity are entered individually, complexity with people is significantly associated with all cognitive outcomes, net of covariates (Table 2, Panel A). After adjusting for educational attainment (Panel B), some of these effects are reduced to nonsignificance, though a one-SD increase in complexity with people is still associated with a .18 SD increase in brain reserve, a .15 SD increase in episodic memory, and a .13 SD increase in MoCA score. Notably, a one-SD increase in complexity predicts a 9 percent decrease in likelihood of MCI or dementia, a substantial effect size that does not change when we control for educational attainment. In contrast, complexity with data has a significant association only for MoCA score, and the coefficient is reduced to nonsignificance after adjusting for educational attainment. Complexity with things is not significant in any Table 2 model.
When the three occupational complexity measures are entered simultaneously (Table 3, Panel A), the relationship between complexity with people and cognition remains significant for all outcomes. Importantly, the effect sizes generally remain stable, or even increase, as complexity with data and things are held constant (compare to Table 2, Panel A). Previously, a one-SD increase in complexity with people was associated with a 9 percent decreased risk of MCI or dementia in Table 2; now, the advantage expands to a 12 percent decreased risk. As before, adjusting for educational attainment reduces the effect sizes for many of these items (Table 3, Panel B), though significant associations persist for brain reserve (.22 SD), visuospatial skills (.15 SD) episodic memory (.14 SD), and MCI/dementia (11% decreased risk). Complexity with data has no significant effects in any Table 3 models, but complexity with things is significantly associated with visuospatial skills even after adjusting for education, suggesting that complex manual labor (like that of a watchmaker) might help preserve visuospatial skills later in life. For a graph of this final set of models, see Figure 1.
Figure 1. Effect of Occupational Complexity on Cognition (Results Correspond to Table 3 Panel B).
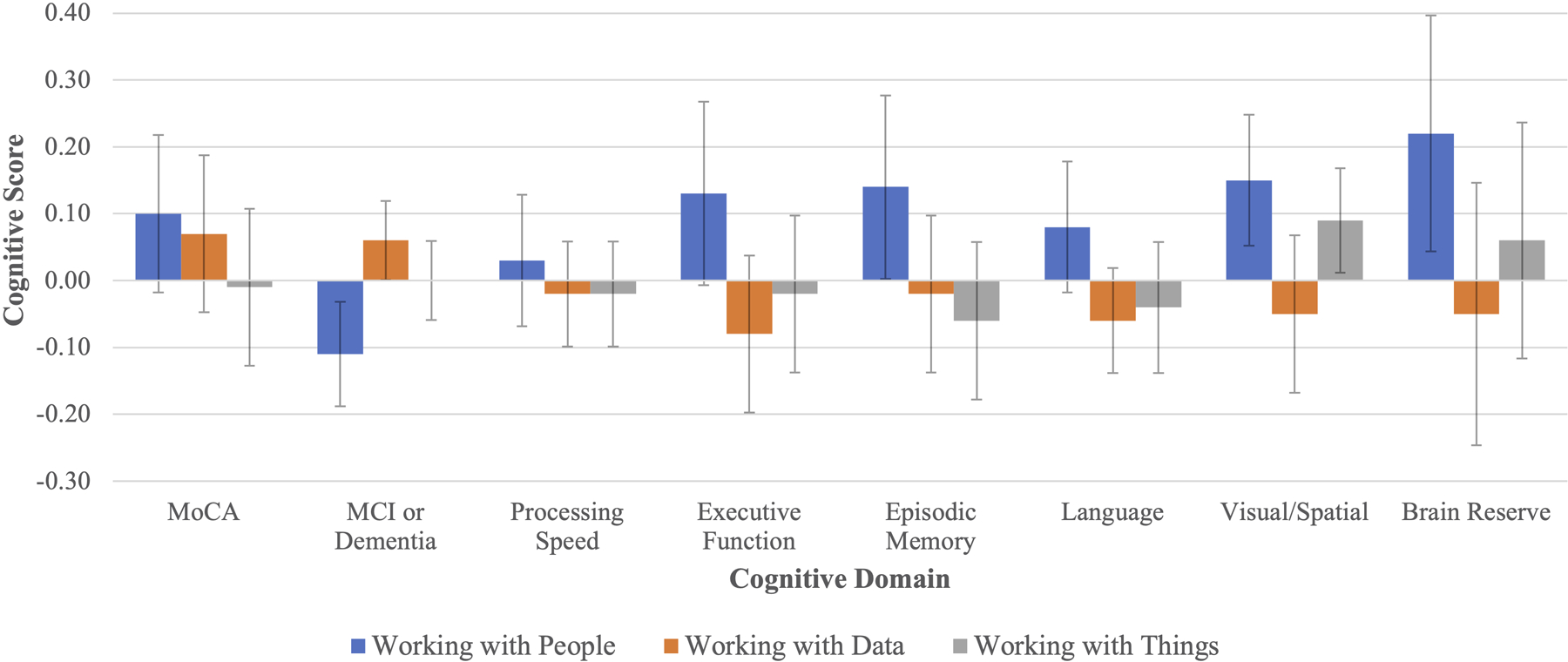
Notes: (1) For all items except MCI/dementia, a one-SD increase in occupational complexity is associated with an SD increase in a particular cognitive outcome. For MCI/dementia, the interpretation is that of a change in the probability of having MCI or dementia. (2) Error bars show 95% confidence intervals.
These results provide strong evidence for an association between complexity in working with people and a variety of cognitive outcomes. In all models, including supplementary analyses that control for parental education (Tables S1–S2 in the appendix), complex work with people is linked to greater brain reserve, improved episodic memory, and a decreased risk of MCI or dementia. In some models, complexity with people is also associated with higher scores on global cognitive function, processing speed, executive function, language, and visuospatial skills, though most of these effects appear to be explained by differences in educational attainment, which presumably predate one’s longest-held job.
In contrast, we do not find much evidence for the protective effects of working with data or things, although some other research reviewed earlier does. Future research should attempt to specify under what conditions complexity with data and things might be significantly associated with cognition, with special attention to the cognitive outcomes tested and the covariates adjusted for.
3.4. Limitations
Many of the limitations of this study are endemic to the literature on occupational complexity. As several researchers note (e.g., Refs 59,66), these occupational complexity codes are based on the Dictionary of Occupational Titles (1977) [78], which in turn draws on the 1970 U.S. Census. It has now been over fifty years since that Census was conducted, and a number of social and technological forces have reshaped employment in the United States and abroad. Many jobs have increased in technical complexity, while others have become more routine as a result of automation. Still, many of our respondents were at their prime working age in the 1970s, so this may be a greater concern for future research.
Second, occupational complexity is often a proxy, or stand-in, for a variety of unmeasured factors, including childhood IQ, educational attainment, and parental education. As Smart and colleagues (2014:2289) warn, “Studies not accounting for prior ability may overestimate the beneficial effect of complex occupational environments on later cognition” [66]. To address this issue, we conducted supplementary analyses controlling for the attainment of the more-educated parent (see Tables S1–S2 in the supplementary materials). Although high rates of missingness leave us cautious in interpreting these findings, we did observe the same overall pattern as presented in the main results. Unfortunately, we do not have measures of childhood IQ or related early-life measures.
Other limitations are specific to the SNAD study. First, our sampling frame covers only individuals from the state of Indiana. Our data may not capture the breadth of occupations found throughout the United States. It is difficult to know whether this would cause any systematic bias in the results. Second, these data contain high rates of missingness on occupation (impacting occupational complexity), and only a subset of participants have received MRI scans (impacting brain reserve). However, sensitivity analyses (not shown here) indicate that the overall pattern of findings is consistent even when individuals missing occupation or neuroimaging measures are dropped. Finally, the individuals in this study do not represent the nation as a whole: they are better-educated than most Americans (about two-thirds have at least 16 years of education), and only 20 percent are nonwhite (while African Americans are well-represented, Latinos, Asian Americans, and other groups are underrepresented). Future studies should make every effort to recruit a more diverse sample, especially since the relationship between education, occupational complexity, and cognition is known to differ by race due to the effects of structural racism on the job market [1, 81].
Supplementary Material
Research in Context.
1. Systematic Review:
The authors reviewed the literature by using Google Scholar and searching the citations of key publications. While several studies have examined the relationship between occupational complexity and cognition, few have included both clinical diagnosis of ADRD and a variety of neuropsychological tests tapping into multiple cognitive domains. Even fewer have included measures of brain atrophy derived from MRI. These publications are appropriately citated.
2. Interpretation:
Our findings highlight the role of social enrichment (occupational complexity in working with people), with less evidence for other forms of complexity (working with data or things).
3. Future Directions:
This article outlines a framework for conceptualizing the protective role of social enrichment, drawing on evolutionary arguments about the development of the social brain. It advocates for novel interventions that facilitate social enrichment among older adults, suggesting that existing efforts focusing on cognitively stimulating activities (e.g., Sudoku puzzles) may be less effective.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health through the National Institute on Aging (grant numbers R01AG057739, R01AG070931, and P30AG010133), and by an Indiana University Collaborative Research Award through the Vice President for Research. This project also received support from a Clinical and Translational Science Award from the National Center for Advancing Translational Sciences (grant number UL1TR002529). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We would like to thank faculty and staff at the Indiana Alzheimer Disease Research Center, the Indiana University Department of Sociology, the Indiana University Sociomedical Sciences Institute, the Indiana Consortium for Mental Health Services Research, the Indiana University Department of Psychological and Brain Sciences, and the Indiana University Network Science Institute for their contributions to project conceptualization and data collection. Thanks, especially, to Hope Sheean, Meagan Brant, Mohit Manchella, Heather Francis, Evan Finley, Haosen Sun, Lucas Hamilton, Anne Krendl, Bernice Pescosolido, Erin Pullen, Kate Eddens, Alex Capshew, Tugce Duran, Mary Austrom, Sujuan Gao, and Frederick Unverzagt.
Funding
This work was funded by the National Institute on Aging (R01 AG057739, PI Brea Perry; P30 AG010133, PI Liana Apostolova), an Indiana University Collaborative Research Grant through the Vice President of Research, and a Proposal Development Grant from the Indiana Clinical Translational Sciences Institute (UL1TR002529). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Indiana University. Max E. Coleman: As research assistant to Perry, obtained funding for this research and for conferences from NIA grant R01 AG057739. Meghann E. H. Roessler: As research assistant to Perry, obtained funding related to this manuscript from NIA grant R01 AG057739. Siyun Peng: Obtained funding through NIA grants R01 AG057739; R01 AG070931; R01 AG078247; P30 AG010133, as well as R01 AG076032 unrelated to this project. Adam R. Roth: Obtained funding as PI of NIH grant R01 AG078247 to Oklahoma State University and was PI on an NIH pilot grant S000784-DHHS (2020-2021) that was sub-awarded from NIH parent grant R24 AG065159 to Indiana University. Shannon L. Risacher: Obtained funding through NIA grants (K01 AG049050; R01 AG061788; P30 AG010133, P30 AG072976) as well as a New Vision Award through Donors Cure Foundation. Andrew J. Saykin: Obtained funding related this manuscript from NIH grants including P30 AG072976, R01 AG019771, U19 AG024904, and U01 AG068057; also receives funding from P30 AG010133, P30 AG072976, R01 AG019771, R01 AG057739, U19 AG024904, R01 LM013463, R01 AG068193, T32 AG071444, and U01 AG068057 and U01 AG072177. Saykin serves on the external advisory boards for 6 NIH funded programs (4 ADRCs and 2 U19s), and on the MESA Observational Study Monitoring Board (NIH NHLBI). Avid Radiopharmaceuticals, a subsidiary of Eli Lilly, provided Saykin with the in-kind contribution of a PET tracer precursor to Indiana University. Saykin is also involved in Springer-Nature Publishing (Editorial Office Support as Editor-in-Chief, Brain Imaging and Behavior). Liana G. Apostolova: Received consulting fees from Biogen, NIH Biobank, Two Labs, Eli Lilly, IQVIA, GE Healthcare, Florida Department of Health, Eisai, Genentech, and Roche Diagnostics; honoraria from the American Academy of Neurology, Purdue University, MillerMed, the Mayo Clinic, NACC CME, MJH Physician Education Resource, CME Institute, Ohio State University, APhA, PeerView, and ASIM; and travel support from the Alzheimer Association. Apostolova has participated on data safety monitoring boards or advisory boards via IQVIA, New Mexico Exploratory ADRC, NIA R01 AG061111, the FDA, and the UAB Nathan Shock Center; has a leadership or fiduciary role in the Med Sci Council Alzheimer’s Association Greater Indiana Chapter, the Beeson Program Committee, the Alzheimer’s Association Science Program Committee, and the FDA PCNS Advisory Committee; holds stock or stock options in Cassava Neurosciences and Golden Seeds; and has received equipment from Avid Radiopharmaceuticals, Life Molecular Imaging, and Roche Diagnostics. Brea L. Perry: Obtained additional funding not related to this manuscript from NIH (R01 AG070931; MPIs Brea Perry and Anne Krendl) and a Trustee Grant from the Russell Sage Foundation. All grants were to the institution. Perry also received funding from Duke University, University of South Carolina, and University of Texas at Austin for speaking engagements.
Footnotes
Conflicts of Interest
Andrew J. Saykin: Avid Radiopharmaceuticals, a subsidiary of Eli Lilly, provided Saykin with the in-kind contribution of a PET tracer precursor to Indiana University. Liana G. Apostolova: Received consulting fees from Biogen, NIH Biobank, Two Labs, Eli Lilly, IQVIA, GE Healthcare, Florida Department of Health, Eisai, Genentech, and Roche Diagnostics; holds stock or stock options in Cassava Neurosciences and Golden Seeds; and has received equipment from Avid Radiopharmaceuticals, Life Molecular Imaging, and Roche Diagnostics. All other authors have no conflict of interest to disclose.
REFERENCES
- [1].Fujishiro K, MacDonald LA, Crowe M, McClure LA, Howard VJ, Wadley VG. The role of occupation in explaining cognitive functioning in later life: Education and occupational complexity in a US national sample of black and white men and women. J Gerontol B. 2019;74(7):1189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Parker SK. Beyond motivation: Job and work design for development, health, ambidexterity, and more. Annu Rev Psychol. 2014;65:661–91. [DOI] [PubMed] [Google Scholar]
- [3].Boots EA, Schultz SA, Almeida RP, Oh JM, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, Asthana S. Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Arch Clin Neuropsychol 2015;30(7):634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25(5):625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Miller J. Individual and occupational determinants of job satisfaction: A focus on gender differences. Sociol Work Occup. 1980;7(3):337–66. [Google Scholar]
- [6].Gecas V, Seff MA. Social class, occupational conditions, and self-esteem. Sociol Perspect. 1989;32(3):353–64. [Google Scholar]
- [7].Kohn Melvin. Change and Stability: A Cross-National Analysis of Social Structure and Personality. Boulder, CO: Paradigm Publishers; 2006. [Google Scholar]
- [8].Tudor B. A specification of relationships between job complexity and powerlessness. Am Sociol Rev. 1972;37(5):596–604. [PubMed] [Google Scholar]
- [9].Kohn ML, Schooler C. Occupational experience and psychological functioning: An assessment of reciprocal effects. Am Sociol Rev. 1973;38(1):97–118. [Google Scholar]
- [10].Miller AR, Treiman DJ, Cain PS, Roos PA. Work, Jobs, and Occupations: A Critical Review of Occupational Titles. Washington, DC: National Academy Press; 1980. [Google Scholar]
- [11].Finkel D, Andel R, Gatz M, Pedersen NL. The role of occupational complexity in trajectories of cognitive aging before and after retirement. Psychol Aging 2009;24(3):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aiello LC, Dunbar RI. Neocortex size, group size, and the evolution of language. Curr Anthropol 1993;34(2):184–93. [Google Scholar]
- [13].Dunbar RI. The social brain hypothesis. Evol Anthropol. 1998;6(5):178–90. [Google Scholar]
- [14].Christakis N. Blueprint: The Evolutionary Origins of a Good Society. New York: Little, Brown; 2019. [Google Scholar]
- [15].Immordino-Yang MH, Darling-Hammond L, Krone CR. Nurturing nature: How brain development is inherently social and emotional, and what this means for education. Educ Psychol. 2019;54(3):185–204. [Google Scholar]
- [16].Wexler B. Brain and Culture: Neurobiology, Ideology, and Social Change. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- [17].Hebb DO. The effects of early experience on problem-solving at maturity. Am Psychol. 1947;2:306–7. [Google Scholar]
- [18].Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res 1978;153(3):563–76. [DOI] [PubMed] [Google Scholar]
- [19].Sale A, Berardi N, Maffei L. Enrich the environment to empower the brain. Trends Neurosci. 2009;32(4):233–239. [DOI] [PubMed] [Google Scholar]
- [20].Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78(1):57–65. [DOI] [PubMed] [Google Scholar]
- [21].Van Praag H, Kempermann G, Gage FH. Neural consequences of enviromental enrichment. Nat Rev Neurosci. 2000;1(3):191–8. [DOI] [PubMed] [Google Scholar]
- [22].Smith BM, Yao X, Chen KS, Kirby ED. A larger social network enhances novel object location memory and reduces hippocampal microgliosis in aged mice. Front Aging Neurosci. 2018;10:142. 10.3389/fnagi.2018.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Toyoshima M, Yamada K, Sugita M, Ichitani Y. Social enrichment improves social recognition memory in male rats. Anim Cogn. 2018;21:345–51. [DOI] [PubMed] [Google Scholar]
- [24].Moreno-Jiménez EP, Jurado-Arjona J, Ávila J, Llorens-Martín M. The social component of environmental enrichment is a pro-neurogenic stimulus in adult c57BL6 female mice. Front Cell Dev Biol. 2019;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brenes JC, Lackinger M, Höglinger GU, Schratt G, Schwarting RK, Wöhr M. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J Comp Neurol. 2016;524(8):1586–607. [DOI] [PubMed] [Google Scholar]
- [26].Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci. USA 2005;102(48):17478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998; 812(1–2):38–49. [DOI] [PubMed] [Google Scholar]
- [28].Reuter-Lorenz PA, Park DC. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev. 2014;24(3):355–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Charles ST, Carstensen LL. Social and emotional aging. Annu Rev Psychol. 2010;61:383–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kiesow H, Uddin LQ, Bernhardt BC, Kable J, Bzdok D. Dissecting the midlife crisis: disentangling social, personality and demographic determinants in social brain anatomy. Commun Biol. 2021;4(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chan MY, Na J, Agres PF, Savalia NK, Park DC, Wig GS. Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proc Natl Acad Sci. USA 2018;115(22):E5144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Farah MJ. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron. 2017;96(1):56–71. [DOI] [PubMed] [Google Scholar]
- [34].Yaple ZA, Yu R. Functional and structural brain correlates of socioeconomic status. Cereb Cortex. 2020;30(1):181–96. [DOI] [PubMed] [Google Scholar]
- [35].Tooley UA, Bassett DS, Mackey AP. Environmental influences on the pace of brain development. Nat Rev Neurosci. 2021;22(6):372–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Salinas J, O’Donnell A, Kojis DJ, Pase MP, DeCarli C, Rentz DM, Berkman LF, Beiser A, Seshadri S. Association of social support with brain volume and cognition. JAMA Netw Open. 2021;4(8):e2121122–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kwak S, Joo WT, Youm Y, Chey J. Social brain volume is associated with in-degree social network size among older adults. Proc R Soc B. 285:20172708. 10.1098/rspb.2017.2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Von Der Heide R, Vyas G, Olson IR. The social network-network: size is predicted by brain structure and function in the amygdala and paralimbic regions. Soc Cogn Affect Neurosci. 2014;9(12):1962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Powell J, Lewis PA, Roberts N, Garcia-Finana M, Dunbar RI. Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proc R Soc. B 279:2157–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dunbar RI. The social brain meets neuroimaging. Trends Cogn Sci. 2012;16(2):101–2. [DOI] [PubMed] [Google Scholar]
- [41].Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. [DOI] [PubMed] [Google Scholar]
- [42].Gow AJ, Corley J, Starr JM, Deary IJ. Which social network or support factors are associated with cognitive abilities in old age? Gerontol. 2013;59(5):454–63. [DOI] [PubMed] [Google Scholar]
- [43].Kelly ME, Duff H, Kelly S, et al. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Syst Rev. 2017;6(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kuiper JS, Zuidersma M, Zuidema SU, et al. Social relationships and cognitive decline: a systematic review and meta-analysis of longitudinal cohort studies. Int J Epidemiol. 2016;45(4):1169–1206. [DOI] [PubMed] [Google Scholar]
- [45].Perry BL, McConnell WR, Coleman ME, Roth AR, Peng S, Apostolova LG. Why the cognitive “fountain of youth” may be upstream: Pathways to dementia risk and resilience through social connectedness. Alzheimer’s Dement. 2022;18(5):934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Barnes LL, De Leon CM, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurol. 2004;63(12):2322–6. [DOI] [PubMed] [Google Scholar]
- [47].Holtzman RE, Rebok GW, Saczynski JS, Kouzis AC, Wilcox Doyle K, Eaton WW. Social network characteristics and cognition in middle-aged and older adults. J Gerontol B Psychol Sci Soc Sci. 2004;59(6):P278–84. [DOI] [PubMed] [Google Scholar]
- [48].Zunzunegui MV, Alvarado BE, Del Ser T, Otero A. Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. J Gerontol B Psychol Sci Soc Sci 2003;58(2):S93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cornwell B. Network bridging potential in later life: Life-course experiences and social network position. J Aging Health. 2009;21(1):129–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li M, Dong X. Is social network a protective factor for cognitive impairment in US Chinese older adults? Findings from the PINE study. Gerontol. 2018;64(3):246–56. [DOI] [PubMed] [Google Scholar]
- [51].Perry BL, McConnell WR, Peng S, Roth AR, Coleman M, Manchella M, Roessler M, Francis H, Sheean H, Apostolova LA. Social networks and cognitive function: An evaluation of social bridging and bonding mechanisms. The Gerontol. 2022. Aug;62(6):865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Esiri MM, Matthews F, Brayne C, et al. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357(9251). [DOI] [PubMed] [Google Scholar]
- [53].Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev. 2004;3(4):369–382. [DOI] [PubMed] [Google Scholar]
- [54].Esiri MM, Chance SA. Cognitive reserve, cortical plasticity and resistance to Alzheimer’s disease. Alzheimer’s Res Ther. 2012;4(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Andel R, Crowe M, Pedersen NL, Mortimer J, Crimmins E, Johansson B, Gatz M. Complexity of work and risk of Alzheimer’s disease: a population-based study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2005;60(5):P251–8. [DOI] [PubMed] [Google Scholar]
- [56].Andel R, Kåreholt I, Parker MG, Thorslund M, Gatz M. Complexity of primary lifetime occupation and cognition in advanced old age. J Aging Health. 2007;19(3):397–415. [DOI] [PubMed] [Google Scholar]
- [57].Karp A, Andel R, Parker MG, Wang HX, Winblad B, Fratiglioni L. Mentally stimulating activities at work during midlife and dementia risk after age 75: follow-up study from the Kungsholmen Project. Am J Geriatr Psychiatr. 2009;17(3):227–236. [DOI] [PubMed] [Google Scholar]
- [58].Kröger E, Andel R, Lindsay J, Benounissa Z, Verreault R, Laurin D. Is complexity of work associated with risk of dementia? The Canadian Study of Health and Aging. Am J Epidemiol. 2008;167(7):820–30. [DOI] [PubMed] [Google Scholar]
- [59].Hussenoeder FS, Riedel-Heller SG, Conrad I, Rodriguez FS. Concepts of mental demands at work that protect against cognitive decline and dementia: A systematic review. Am J Health Promotion. 2019;33(8):1200–8. [DOI] [PubMed] [Google Scholar]
- [60].Correa Ribeiro PC, Lopes CS, Lourenço RA. Complexity of lifetime occupation and cognitive performance in old age. Occup Med. 2013;63(8):556–62. [DOI] [PubMed] [Google Scholar]
- [61].Dekhtyar S, Wang HX, Scott K, Goodman A, Koupil I, Herlitz A. A life-course study of cognitive reserve in dementia—from childhood to old age. Am J Geriatr Psychiatr. 2015;23(9):885–96. [DOI] [PubMed] [Google Scholar]
- [62].Andel R, Vigen C, Mack WJ, Clark LJ, Gatz M. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer’s patients. J Int Neuropsychol Soc. 2006;12(1):147–52. [DOI] [PubMed] [Google Scholar]
- [63].Vélez-Coto M, Andel R, Pérez-García M, Caracuel A. Complexity of work with people: Associations with cognitive functioning and change after retirement. Psychol Aging. 2021;36(2):143. [DOI] [PubMed] [Google Scholar]
- [64].Lane AP, Windsor TD, Andel R, Luszcz MA. Is occupational complexity associated with cognitive performance or decline? Results from the Australian Longitudinal Study of Ageing. Gerontol. 2017;63(6):550–9. [DOI] [PubMed] [Google Scholar]
- [65].Nexø MA, Meng A, Borg V. Can psychosocial work conditions protect against age-related cognitive decline? Results from a systematic review. Occup Environ Med. 2016;73(7):487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Smart EL, Gow AJ, Deary IJ. Occupational complexity and lifetime cognitive abilities. Neurol. 2014;83(24):2285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sörman DE, Stenling A, Sundström A, Rönnlund M, Vega-Mendoza M, Hansson P, Ljungberg JK. Occupational cognitive complexity and episodic memory in old age. Intell. 2021;89:101598. [Google Scholar]
- [68].Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E. The Alzheimer’s disease centers’ uniform data set (UDS): The neuropsychological test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Stern Y, Arenaza‐Urquijo EM, Bartrés‐Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2020;16(9):1305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Peng S, Roth AR, Apostolova LG, Saykin AJ, Perry BL. Cognitively stimulating environments and cognitive reserve: the case of personal social networks. Neurobiol Aging. 2022;112:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiol. 2007;29(1–2):125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cummings J, Ritter A, Zhong K. Clinical trials for disease-modifying therapies in Alzheimer’s disease: a primer, lessons learned, and a blueprint for the future. J Alzheimer’s Dis. 2018;64(s1):S3–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Geda YE, Topazian HM, Lewis RA, Roberts RO, Knopman DS, Pankratz VS, Christianson TJ, Boeve BF, Tangalos EG, Ivnik RJ, Petersen RC. Engaging in cognitive activities, aging, and mild cognitive impairment: a population-based study. J Neuropsychiatr Clin Neurosci. 2011;23(2):149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lachman ME, Agrigoroaei S, Murphy C, Tun PA. Frequent cognitive activity compensates for education differences in episodic memory. Am J Geriatr Psychiatr. 2010;18(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ridding M. What is ‘cognitive reserve’? How we can protect our brains from memory loss and dementia. Conversat 2017. https://theconversation.com/what-is-cognitive-reserve-how-we-can-protect-our-brains-from-memory-loss-and-dementia-76591.
- [76].Roth AR. Social networks and health in later life: A state of the literature. Sociol Health Illn. 2020;42(7):1642–56. [DOI] [PubMed] [Google Scholar]
- [77].Roos PA, Treiman DJ. DOT scales for the 1970 Census classification. In Miller AR, Treiman DJ, Cain PS, Roos PA (Eds.), Work, Jobs, and Occupations: A Critical Review of Occupational Titles. Washington, DC: National Academy Press; 1980, p. 336–389. [Google Scholar]
- [78].U.S. Department of Labor. Dictionary of Occupational Titles, 4th ed. Washington, DC: U.S. Government Printing Office; 1977. [Google Scholar]
- [79].Allison PD. Missing Data. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- [80].Enders C. Applied Missing Data Analysis. New York: Guilford Press; 2010. [Google Scholar]
- [81].Gonzales E, Whetung C, Lee YJ, Kruchten R. Work demands and cognitive health inequities by race and ethnicity: A scoping review. The Gerontol. 2022;62(5):e282–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


