Abstract
Objective:
Macrophages are abundantly detected at sites of disc herniation, however, their function in the disease progression is unclear. We aim to investigate the functions of macrophages in acute disc herniation using a macrophage Fas-induced apoptosis (MaFIA) transgenic mouse strain.
Method:
To transiently deplete macrophages, a dimerizer, AP20187, or vehicle solution was administered via intraperitoneal injection to MaFIA mice immediately, day 1 and 2 after annular puncture induced disc herniation. Local infiltrated tissues at disc hernia and DRGs at corresponding levels were harvested to analyze immune cells and neuroinflammation on postoperative day (POD) 6 by flow cytometry and/or immunostaining. Mouse spines were harvested to analyze structures of degenerated discs and adjacent vertebrae and to assess osteoclast activity by histology and tartrate-resistant acid phosphatase (TRAP) staining on POD 6, 13, and 20, respectively.
Results:
On POD 6, abundant macrophages were confirmed at disc hernia sites. Compared to vehicle control, AP20187 significantly reduced GFP+ cells in blood, spleen, and local inflammatory tissue. At disc hernia sites, AP20187 markedly reduced macrophages (CD11b+, F4/80+, GFP+CD11b+, CD11b+F4/80+) while increasing neutrophils and B cells. Transient macrophage depletion decreased ectopic bone formation and osteoclast activity in herniated discs and adjacent cortical bones for up to 20 days post herniation. Disc herniation elevated expressions of TNF-α, IL-6, substance P, calcitonin gene-related peptide, accompanied by increasing GFP+, CD11b+ and F4/80+ macrophages. Macrophage depletion did not attenuate these markers of neuroinflammation.
Conclusions:
Transient depletion of macrophage altered local inflammatory response at the site of disc herniation.
Keywords: Intervertebral disc herniation, back pain, macrophage, inflammation, macrophage Fas-induced apoptosis (MaFIA)
Introduction
Intervertebral discs are important for spine function, supporting both structure and locomotion. Due to wear and tear, discs develop pathological changes that account for a major cause of back/leg pain. Back/leg pain is a leading cause of disability in the U.S., leading to more than 264 million lost workdays annually.1,2 Current treatments focus on symptom relief with limited disease-modifying potentials, since a detailed pathological mechanism has not been fully elucidated.
Inflammation has been strongly associated with progression of degenerative disc diseases. When discs herniate, disc cells produce proinflammatory cytokines and recruit immune cells like macrophages and lymphocytes to hernia sites.3–5 Immune cells further exacerbate the inflammatory responses by producing mediators, such as IL-4, IL-6, IL-12, IFN-γ, and ROS, leading to disc cell senescence and deterioration of disc microenvironment, further promoting discogenic pain.5 In a subcutaneous transplantation of autologous nucleus pulposus (NP) pig model, Geiss et al. detected macrophages, activated T and B cells surrounding NP tissue.6 In a recent bioinformatics study, Wang et al. revealed significantly higher proportions of regulatory T cells and macrophages in human lumbar disc hernia tissues among 22 types of immune cells studied by microassay.7 These findings suggest that complex immune cell interactions are involved in the inflammatory responses after disc herniation.
Macrophages account for the predominant immune cells infiltrated at disc hernia sites in both human and animal models. More than 60% of surgically obtained human herniated discs showed macrophage infiltration.8–11 We previously found abundant macrophages at disc hernia sites as early as day 3 by immunohistochemistry staining, which was confirmed by molecular imaging using a specific peptide targeting the formyl peptide receptor 1 (FPR-1) on activated macrophages.12 Accumulating evidence suggests the presence of multiple macrophage phenotypes with distinct functions in various tissues.13,14 For example, pro-inflammatory macrophages mediate anti-bacterial and anti-tumor function and induce tissue damage, while anti-inflammatory macrophages promote tissue regeneration associated with risk of fibrosis. Indeed, we and others have reported mixed phenotypes of macrophages at various stages of disc degeneration. In specific, heterogeneous macrophage populations were detected in a mouse model of acute disc herniation. This included iNOS+ and MMR+ macrophages, traditionally classified as pro-inflammatory and anti-inflammatory macrophages, respectively.15,16 Another study identified CCR7+, CD163+, and CD206+ cells in degenerative human discs.17 An immunohistochemistry analysis of 79 lumbar disc herniation patients revealed differences in morphology and phenotypes of macrophages originating from both recruited and resident macrophages at different degenerative stages.18 A recent study on human cervical discs demonstrated that the polarity of macrophages around newly developed microvasculature might be altered in different stages of disc degeneration, in which a higher number of infiltrating CD16+ macrophages around the vessels was associated with chronic inflammation and were suppressed by the infiltration of CD206+ macrophages.19 One important study utilizing a disc needle puncture animal model illustrated that TNF-α+ macrophages significantly increased from 1 to 14 days after injury and then gradually decreased while TGF-β+ macrophages gradually increased up to 28 days after injury.20 These reports indicate that infiltrated macrophages may play indispensable roles in mediating the disc disease progression and may hold the key for advancing pathophysiological understanding and treatment. However, our understanding is still in its infancy.
In this study, we aim to investigate the roles of macrophages on local inflammatory response, neuroinflammation, and progression of disc degeneration in a mouse model of acute disc herniation, using the macrophage Fas-induced apoptosis (MaFIA) transgenic mouse strain. In MaFIA mice, colony-stimulating factor 1 receptor (CSF-1R) expressing macrophages are tagged with enhanced green fluorescence protein (eGFP) and these macrophages can be transiently depleted by administration of a dimerizer compound AP20187 that binds to the suicide protein FKBP1A and induces macrophages apoptosis,21,22 and thus allow us to elucidate the roles of macrophages in disc herniation.
Method
Ethics statement
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Virginia.
MaFIA transgenic mice
MAFIA transgenic mice (C57BL/6-Tg(Csf1r-EGFP-NGFR/FKBP1A/TNFRSF6)2Bck/J)) were purchased from The Jackson Laboratory (005070, Ann Harbor, Maine).
Mouse model of disc herniation, animal grouping and tissue harvest
Needle annular puncture-induced acute disc herniation was performed per our established protocol.15 Three consecutive lumbar discs (L3—L6) were punctured using a 28G needle to induce herniation. As in Fig. S1, animals (10 weeks old, male and female) were randomly assigned to the following groups via coin flipping: vehicle control (n=20) or AP20187 macrophage depletion (n=20) groups. For macrophage depletion, mice were intraperitoneal (i.p.) injected with AP20187 (Takara Bio, San Jose, CA) solution (1.5 mg/kg in 4% ethanol, 10% of polyethylene glycol 400, and 1.7% Tween 20 in water) for 3 days (immediately after surgery, POD 1, and 2). For vehicle control, mice were i.p. injected with the vehicle solution. An addition sham group (10—12 weeks old, male and female) served as baselines for histology and DRGs analysis (n=6, without disc puncture).
Body weights were monitored daily, and spleen weights were measured upon harvest. Mice were euthanized in a CO2 chamber followed by cervical dislocation. Blood and spleen were harvested on POD 6 (n=4 per group) for flow cytometry. Local inflammatory tissues (n=5 per group, creamy granular tissue from the herniation sites with a gross size of ~2×2 mm) and DRGs (n=4 for vehicle control, n=5 for AP20187, n=3 for sham) were harvested under a surgical microscope, fixed in 2% p-formaldehyde-lysine-periodate (PLP) for 3 h, equilibrated in sucrose,21 and embedded in Optimal Cutting Temperature (O.C.T.) Compound (VWR, Radnor, PA). Lumbar spines were harvested on POD 6 (n=5 per group), POD 13 (n=3 per group), and POD 20 (n=3 per group), fixed in 4% paraformaldehyde (PFA) for 3 days, decalcified in 0.25 M ethylenediaminetetraacetic acid (EDTA) (pH 7.4) for 3 days, and embedded in paraffin. (see Supplementary Materials for additional information)
Flow cytometry
Single-cell suspension of inflammatory tissues was generated by enzyme digestion (33 μg/mL Liberase TM and 50 μg/ml DNase I) at 37 °C for 30 minutes. Cells were filtered through a 70 μm cell strainer and stained with antibodies [against CD45 (30-F11), CD11b (M170), F4/80 (BM8), and Ly6G (IA8) were purchased from Biolegend (San Diego, CA)23] for 1 hour at 4°C and analyzed on an Attune Nxt analyzer with FCS Express Software to confirm macrophage depletion in spleen and blood on POD 6 (Fig. S2).
Safranin-O/Fast Green staining, cell counting, and histomorphological analysis
Lumbar spines were sectioned at 5-μm-thick. Safranin-O (0.1 %) staining12 was performed on every 5—8 slides (3 sections per slide). Images were taken by the Nikon ECLIPSE E600 with Nikon Elements software. Number of infiltrated cells was counted by ImageJ (Fig. S3). Sham animals were not analyzed due to the absence of infiltration near intact discs.12,15 Histomorphological analysis of herniated discs was performed on 1 out of every 3 sections (~10 sections per animal, centered around needle puncture trajectory). Using ImageJ, areas of the whole disc, growth plate, ossification, and both cartilage endplates were measured according to the disc zoning guidance (Fig. S4).24
Tartrate-resistant acid phosphatase (TRAP) staining
TRAP (histochemical marker of osteoclasts) assay was performed according to the manufacturer’s protocol (Sigma-Aldrich, St. Louis, MO) to quantify osteoclasts (Supplementary Materials).
Immunohistochemistry to detect infiltrated immune cells at disc hernia
Per our protocols,25 endogenous peroxidase was blocked with 2 % H2O2/methanol for 30 min and antigen retrieval in sodium citrate buffer (10 mM, pH 6.0) at 85 °C for 30 min. Sections were incubated with blocking buffer followed by primary antibodies at 4 °C overnight, including Rat anti-neutrophil monoclonal antibody (Thermo Scientific, Waltham, MA), rabbit anti-Iba1 antibody (Abcam, Waltham, MA), or rabbit anti-B220 antibody (Abcam), then incubated with biotinylated secondary antibodies for 2 hrs. The signal was visualized with 3,3′-diaminobenzidine (DAB). IgG control staining was performed following identical procedures excluding primary antibodies, which showed no positive signal (Fig. S5). Images were taken by a Nikon ECLIPSE E600 microscope with Nikon Elements software. Numbers of neutrophils, macrophages, and B cells (brown signal) and total cells (blue-purple hematoxylin stained nuclei) per unit area were counted (Supplementary Materials).
Immunofluorescence staining
Cryo-tissue sections (5 μm) were permeablized with 0.3% Triton X-100 in DMEM (with 0.1% NaN3) for 10 minutes, blocked with anti-FcγRII/FcγRIII mAb (clone 2.4G2) and serum for 4 hr before staining with fluorochrome-conjugated antibodies.15,23,25 Antibodies from Biolegend were: rat anti-CD11b (M1/70), rat anti-F4/80 (BM8), mouse anti-NOS2 (5C1B52). Rabbit anti-GFP and mouse monoclonal calcitonin gene related peptide (CGRP) antibodies were from MilliporeSigma (Burlington, MA). Mouse monoclonal substance P (SP) and MMR Goat anti-mouse polyclonal antibodies were from R&D Systems (Minneapolis, MN). Polyclonal Rabbit anti-TNF-α and anti-IL-6 antibodies were from Peprotech (Princeton, NJ). For SP, CGRP, and GFP, secondary antibodies were used. AlexaFluor 647 donkey-anti-mouse NL637 IgG was from R&D Systems. AlexaFluor 532 goat anti-rabbit IgG was from Invitrogen (Waltham, MA). For the rest of the antibodies, direct conjugation with AlexaFluor (A) 555-, or A647- was performed using labeling kits (Invitrogen).25,26 Sections were mounted with Prolong Gold anti-fade reagent with DAPI (Life Technologies, Carlsbad, CA). Immunofluorescence images were taken with an Olympus IX81 Inverted Fluorescence Automated Live Cell Microscope with MetaMorph software (Molecular Devices, San Jose, CA) to quantify positive signals (Supplementary Materials). For direct labeling, fluorophore-labeled isotype control antibody was used per established protocols.25 When secondary antibodies were involved, IgG control staining was performed following identical procedures excluding the primary antibodies, which did not show positive signals in anterior inflamed tissue and DRG (Fig. S6, S7). Immunostaining of macrophage, neutrophil and B cells at disc hernia sites was performed on spine sections of POD 13 and POD 20 (Supplementary Materials)
Statistical analysis
All data are presented as mean ± 95% CI with p-values considered significant if below 0.05. Statistical analysis was performed using GraphPad Prism 7.0. Data comparing two groups was analyzed with unpaired t-test. Normality test was performed to confirm Gaussian Distribution. Unpaired t-test was used to compare two groups when appropriate. One-way ANOVA with multiple comparison was used to three or more groups. Two-way ANOVA with multiple comparisons test was performed to analyze time-dependent histomorphometric changes and infiltrated cell numbers.
Results
Macrophages were the predominant immune cells at disc hernia sites
To test the inflammatory response after disc herniation in MAFIA mice, we first performed flow cytometry to analyze the local macrophage population. CD11b+ and F4/80+ macrophages accounted for more than 60% of CD45+ cells in the inflammatory tissues at disc hernia in MaFIA mice (Fig. 1). We also detected ~7% of Ly6G+ neutrophils. This data confirmed the abundant macrophages in the MaFIA mice after acute disc herniation.
Figure 1.
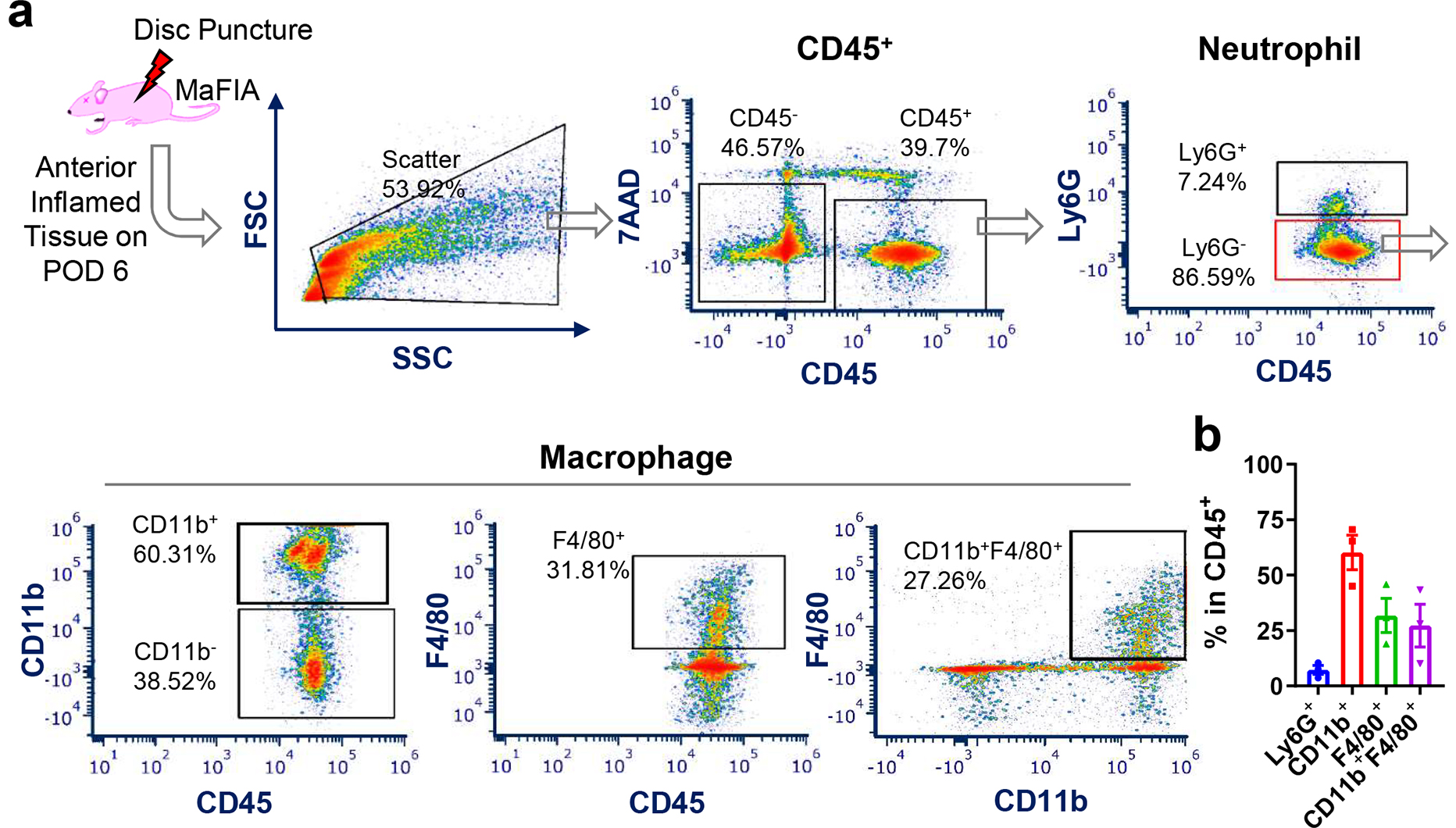
Acute disc herniation increased immune cells, especially macrophages, at disc hernia sites. (a) Representative flow cytometry plots of immune cell infiltrates at disc hernia, including neutrophils (CD45+Ly6G+), monocytes/macrophages (CD45+CD11b+, CD45+F4/80+, and CD45+F4/80+CD11b+) after 6 days of disc herniation in MaFIA mice. (b) Quantification of the monocytes/macrophages at disc hernia sites 6 days post disc herniation in MaFIA mice (n = 3).
Transient depletion of macrophages reduced total cell infiltration at disc hernia sites
As shown in Fig. 2 with histological staining and quantitative cell counting, total infiltrated cells in AP20187 group were reduced by more than 50% on POD 6 (822.38±158.48 in vehicle control, 418.38±213.81 in AP20187, p=0.001), remained stable on POD 13 (400±98.46 in vehicle control, 380.78±85.69 in AP20187, p=0.999), and had a slight downward trend despite no significance on POD 20 (242.06±78.91 in vehicle control, 203.67±50.35 in AP20187, p=0.999), compared to vehicle control. Cell counts in vehicle control dropped significantly from POD 6 to POD 13 (p=0.0094) and plateaued through POD 20 (p>0.999), while AP20187 group had stable cell numbers time (p=0.122, POD 6 vs.13; p=0.618, POD 13 vs. 20). Accordingly, body weights of AP20187 group temporarily decreased during POD 2—6 and gradually recovered up to POD 20 (Fig. S8a). Significant increases in spleen sizes also indicated that AP20187 possessed a systemic effect (Fig. S8b).
Figure 2.

Transient depletion of macrophages reduced total infiltrated cells at disc hernia sites. Safranin-O/Fast Green staining of sagittal lumbar spine sections at the anterior region of herniated discs on (a) POD 6, (b) POD 13, and (c) POD 20. On POD 6, disc herniation induced massive cell infiltration at disc hernia in vehicle control, which was attenuated in AP20187 group. Total infiltrates decreased in later time points. Images were taken at magnifications 40× (left column, grey box) and 200× (right column, red/black boxes). Scale bar=100 μm. (d) Quantification of cell counts per unit area (~0.34 mm2) exhibited inflammatory cells of vehicle control dropped continuously from POD 6 to 13 (p=0.0094), and then stabilized through POD 20, while AP20187 group had comparable cell numbers at various time points (p > 0.05) with the most significant cell count difference between the two groups on POD 6 (p=0.001). n=5 per group on POD 6, n = 3 per group on POD 13 and 20. Two-way ANOVA with multiple comparison was used.
Depletion of macrophages altered local immune cell profile
AP20187 significantly reduced total macrophages while increasing neutrophils and B cell infiltration at disc hernia (Fig. 3a). In AP20187 group, the decrease in macrophages was greater than 50% (182.5±38.36, p=0.0037) (Fig. 3b) while the increase in neutrophils was 4-fold (49.01±17.67, p=0.0031) (Fig. 3c), compared to vehicle control (76.69±14.79 for macrophages, 11.18±6.09 for neutrophils) per unit area. Macrophage depletion resulted in an increasing trend of B cell count per unit area despite no significance (46.1±13.24 in AP20187, 28.29±10.91 in vehicle control, p=0.168) (Fig. 3d). A small portion of macrophages (~1—5%) were detected at later time points without significant differences between groups, while B cell and neutrophils were scarcely detectable in both groups (Fig. S9, S10). This result suggested that systemic macrophage depletion might lead to increased recruitment of neutrophils and B cells to disc hernia sites at early stages.
Figure 3.

Depletion of macrophages decreased total macrophages, but increased neutrophil and B cell recruitment to disc hernia sites 6 days after disc herniation. (a) Representative images of immunohistochemistry detection of Iba1+ macrophages (top), neutrophils (middle), and B220+ B cells (bottom) at disc hernia sites on POD 6. AP20187 reduced macrophage but increased neutrophil and B cell infiltration. Images were taken at a magnification 200× in anterior regions of herniated discs. Arrows indicate positive immunostaining. Scale bar = 100 μm. (b-d) Quantification of immune cell count per unit area (0.34 mm2) at disc hernia sites confirmed that AP20187 decreased macrophages, increased neutrophils, while led to upward trending of B cell counts despite not significant (p = 0.168). Note: Tissue sections were selected from every 10—15 sections around the needle puncture trajectory with 2—3 sections per disc. For each disc per section, 3 images (with 0.34 mm2 area) were taken at the anterior region to obtain count of immune-positive cells. For each mouse, 2—3 herniated discs were analyzed. Each data point represents the mean value of one animal. n = 5 mice per group. Unpaired t-Test was used. * p < 0.05
Depletion of macrophages reduced CD11b+ and F4/80+ macrophages at disc hernia sites
On POD 6, DAPI staining showed that AP20187 reduced the total number of cells (p=0.002 vs. vehicle control) at disc hernia sites (Fig. S11). Abundant macrophages with both myeloid-derived (GFP+, CD11b+) and resident (F4/80+) phenotypes were detected at disc hernia in vehicle control, which were dramatically reduced in AP20187 group (Fig. 4a). GFP+, CD11b+, and F4/80+ macrophages decreased from 52.81±20.84, 22.23±6.74, and 51.41±3.84 cells per unit area in vehicle control to 5.05±4.70 (p=0.0005), 7.96±4.23 (p=0.0012), and 16.49±0.78 cells (p<0.0001) in AP20187 group, respectively (Fig. 4b–d). Percentages for GFP+ and F4/80+ dropped from 30.5±3.13% and 31.28±4.76% in vehicle control to 5.90±2.41% (p=0.0002) and 20.07±3.58% (p=0.0026) in AP20187, with similar percentages for CD11b+ (p=0.108) between the two groups (Fig. 4b–d). Among total cells, there were ~10% GFP+CD11b+ cells and ~8% CD11b+F4/80+ (arrowheads, Fig. 4e) in vehicle control, with both exhibiting a substantial reduction in the AP20187 group (p=0.002 and 0.0317, respectively) (Fig. 4f, g). This result suggested that depletion of CSF-1R+ macrophages reduced both myeloid-derived and resident macrophages at disc hernia on POD 6.
Figure 4.

Depletion of macrophages reduced both CD11b+ and F4/80+ macrophage infiltration at disc hernia sites after 6 days of disc herniation. Representative immunofluorescence images of (a) GFP+, CD11b+, F4/80+, and co-localized (e) GFP+ CD11b+, and CD11b+ F4/80+ macrophages at disc hernia sites on POD 6. Arrows indicate single marker, and arrowheads indicate co-localized dual marker staining. Scale bar = 50 μm. Quantitative cell count per unit area and percentage to total cells (DAPI count) confirmed reduced (b) GFP+, (c) CD11b+, (d) F4/80+, (f) GFP+ CD11b+ and (g) CD11b+ F4/80+ macrophages. Images were taken at a magnification of 600× with a calculated unit area of 0.028 mm2 (ROI). Around 15—20 ROIs were analyzed from 2—3 anterior infiltration tissues per mouse. Each data point represents mean value of one animal. n=5 mice per group. Unpaired t-Test was used. *p < 0.05
AP20187 decreased both pro-inflammatory and anti-inflammatory phenotypes of macrophages at disc hernia sites
On POD 6, both iNOS+ and MMR+ macrophages were detected in inflammatory tissue in vehicle control, and both phenotypes were markedly reduced in AP20187 group (Fig. 5a). Per unit area, AP20187 reduced iNOS+ cell count by ~63% (20.51±3.06 for vehicle control, 7.54±2.33 for AP20187, p=0.0035) and MMR+ cell count by ~40% (32.36±7.50 for vehicle control, 19.3±2.00 for AP20187, p=0.0031), respectively (Fig. 5b, 5c). Since AP20187 concurrently decreased total cell count (Fig. S11), percentage analysis showed no statistical difference between two groups, at ~9.67—12% for iNOS+ (p=0.532) and ~20.89—23.99% for MMR+ (p=0.099) (Fig. 5b, 5c). This data indicated that transient depletion of CSF-1R+ macrophages reduced both pro- and anti-inflammatory macrophages on POD 6 after acute herniation.
Figure 5.

Depletion of macrophages decreased both pro- and anti-inflammatory macrophages at disc hernia site after 6 days of disc herniation. (a) Representative immunofluorescence images of iNOS+ and MMR+ macrophages at disc hernia sites on POD6. Arrows indicate single marker, and arrowheads indicate co-localized dual marker staining. Scale bar = 50 μm. Quantitative analysis of (b) iNOS+ and (c) MMR+ counts per unit area (0.028 mm2) and percentage of iNOS+ and MMR+ to total cells (DAPI) between vehicle control and AP20187. Note: Images were taken at a magnification of 600× with a calculated unit area of 0.028 mm2 (ROI). Around 15—20 ROIs were analyzed from 2—3 anterior infiltration tissues per mouse. Each data point represents mean value of all ROIs in a single mouse. n = 5 mice per group. Unpaired t-Test was used. *p < 0.05
Transient depletion of macrophage decreased ectopic bone formation induced by disc herniation
Herniated discs showed typical degenerative changes, e.g. loss of extracellular matrix in NP, disordered AF structure, diminished AF and NP boundary, hypertrophic chondrocytes, ectopic bone in endplate (EP), and thickened growth plate (GP) (Fig. 6a), compared to native discs (Fig. 6b). On POD 6, EP area slightly increased in AP20187 (p=0.015 vs. vehicle control, p=0.014 vs. native) and decreased in vehicle control (p=0.007 vs. native), with no change in AP20187 on POD 13 (p=0.161 vs. vehicle control and p=0.442 vs. native) (Fig. 6c). EP area remained similar among all groups on POD 20 (p>0.427). GP area displayed no difference among groups on POD 6 (p>0.534), moderately increased in vehicle control on POD 13 (p=0.026 vs. native) and POD 20 (p=0.002 vs. native), and a slight decreasing trend in AP20187 despite no statistical significance at both time points (Fig. 6d). Percentages of ectopic-to-EP and ectopic-to-disc area also showed a time-dependent change. Ectopic bone area showed no difference among groups on POD 6, markedly increased in vehicle control by ~40% and ~45% on POD 13 and 20 (p<0.0001 vs. native), and this increase was partially reversed by AP20187 on POD 20 (p=0.0005 and 0.0011 vs. vehicle control, p=0.116 and 0.380 vs. native in Fig. 6e, 6f, respectively). Noticeably, there was a low incidence of ectopic bone present in native endplates in this strain (Fig. S12). On POD 6, herniated discs demonstrated a marked reduction of glycosaminoglycan (GAG) content, which was not restored by AP20187 (Fig. S13). The degeneration score remained similar between vehicle control and AP20187 groups at all three time points (Fig. S14). Relative disc height by X-ray showed a slight increasing trend in AP20187 groups compared to vehicle controls despite not statistically significant (Fig. S15). This data indicated transient macrophage depletion decreases the incidence of ectopic bone formation after acute herniation.
Figure 6.
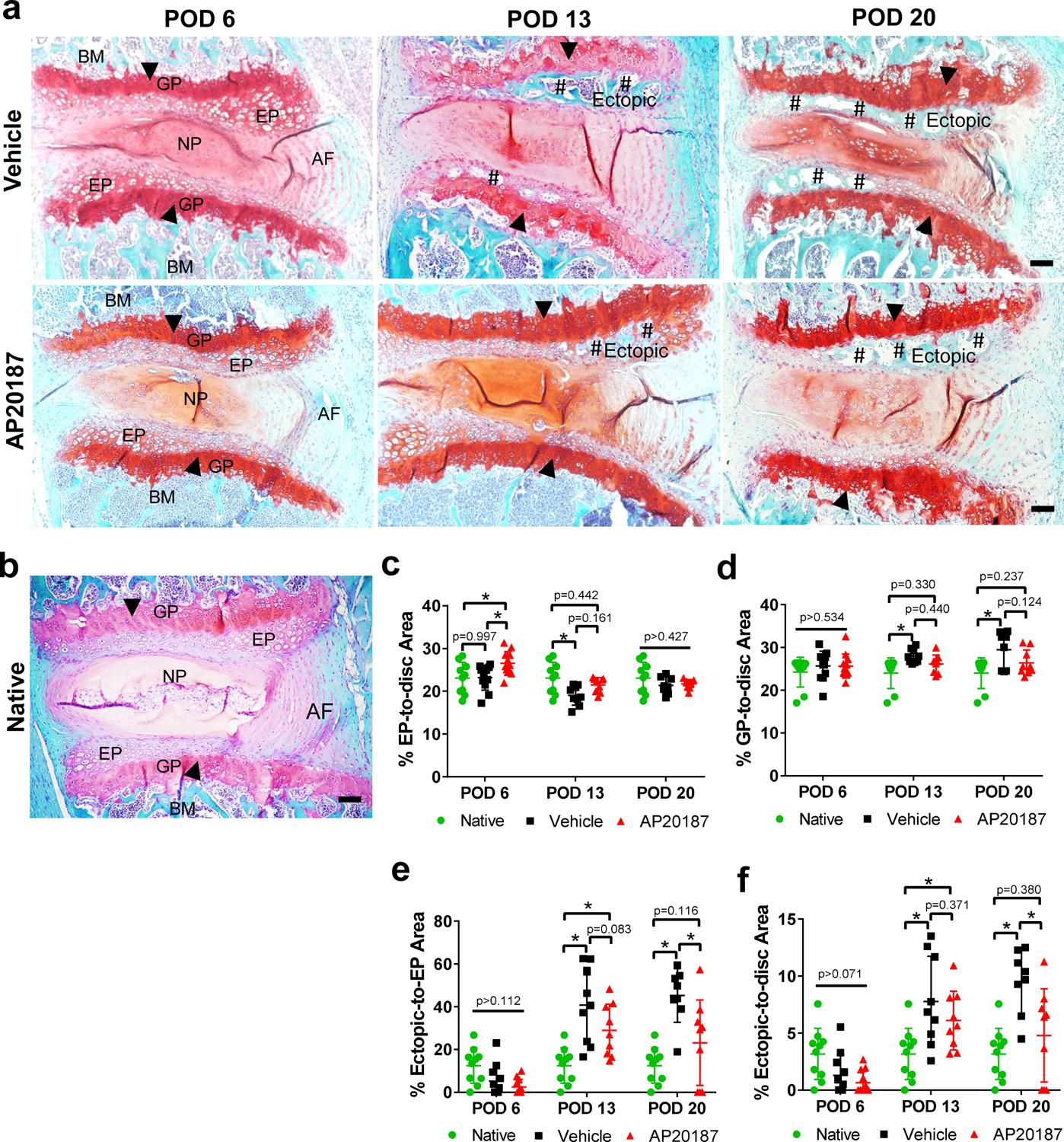
Transient depletion of macrophages decreased the incidence of ectopic bone formation for up to 20 days of disc herniation. Safranin-O/Fast Green staining of mid-sagittal sections of (a) herniated discs of vehicle control and AP20187 groups on POD 6, 13 and 20 and (b) native disc. Images taken at 100× were oriented by superior aspect of endplate (top), inferior endplate (bottom), anterior of disc (right) and posterior disc (left). Typical disc degeneration was observed in both groups, exhibited by widened GP (arrowheads), diminished AF organization and NP/AF boundaries, and increased incidence of ossification (pound sign) in endplates on POD 13 and POD 20 (arrows). Scale bar=100 μm. Histomorphometric analysis on percentages of (c) EP-to-disc area (d) GP-to-disc area, (e) ectopic-to-EP area, and (f) ectopic-to-disc area of native, vehicle control and AP20187 discs on POD 6, 13 and 20. Each data point represents mean value of a single disc. For each mouse, 2—3 herniated discs were analyzed. n=5 mice per group on POD 6, n = 3 mice per group on POD 13 and 20. Two-way ANOVA with Tukey’s multiple comparisons test (simple effects within rows); *p < 0.05
Macrophage depletion attenuated disc herniation induced osteoclast activity in endplates and anterior cortical bones
On POD 20, we observed progressive pathological changes, exemplified by ectopic bone formation (red dotted area) and increased osteoclasts (arrows) in herniated discs and adjacent anterior cortical bone in vehicle control, whereas these abnormalities were greatly delayed in AP20187 group (Fig. 7a—c). Osteoclast counts in cortical, GP and EP of vehicle control increased by ~16-, 15-, and 11- fold (p<0.0001 vs. native), all of which reduced markedly in AP20187 group (p<0.0001 vs. vehicle control) (Fig. 7d—f). At early time points (POD 6 and 13), increased surface roughness and osteoclast activity were also detectable in anterior cortical bone and herniated disc, in which AP20187 group exhibited similar inhibitory effects (Fig. S16, S17). This result indicated that acute disc herniation-induced inflammatory response promotes osteoclast activity and ectopic bone formation in endplates and adjacent cortical bone, while transient macrophage depletion may slow down this pathological change possibly via dampening osteoclast activity.
Figure 7.

Transient depletion of macrophages decreased osteoclast activity for up to 20 days following disc herniation. (a) Safranin-O/Fast Green staining depicted increased ectopic bone formation in anterior cortical bone of the vertebrae between two herniated discs (red dotted area) in vehicle control, which was attenuated in AP20187 group. (b) TRAP staining of sagittal spine sections showed elevated osteoclasts (black arrows) in ectopic bone (#) in anterior cortical (black dotted area) in vehicle control, which was decreased in the AP20187 group. (c) TRAP staining of herniated discs showed increased osteoclasts (black arrows) at the boundary of GP and bone marrow (BM) and in the ectopic bone (#) of EP, which was decreased in AP20187 group. Scale bar=100 μm in all images. Osteoclast counts in (d) anterior cortical, (e) GP, and (f) EP confirmed image observations. Note: For (e) and (f), each data point represents mean value of each EP or GP, with 2 EP and 2 GP in each herniated disc. For (d), each data point represents mean value of each cortical bone adjacent to herniated discs. n = 5 mice per group on POD 6, n = 3 mice per group on POD 13 and 20. The same group of sham animals was used for all time points. One-way ANOVA with multiple comparisons; *p < 0.05
Macrophage depletion promoted neuroinflammation in dorsal root ganglia
Expressions of TNF-α and IL-6 cytokines (red), SP and CGRP neuropeptide (green) were elevated in DRGs of vehicle control and AP20187 groups, compared to native DRGs (Fig. 8a). Although the elevation of these markers in vehicle control DRGs was not significant, AP20187 promoted percentages of TNF-α+ (p=0.002 vs. vehicle control, p<0.0001 vs. native), IL-6+ (p=0.0125 vs. native), SP+ (p=0.012 vs. vehicle control, p=0.0045 vs. native) and CGRP+ (p=0.042 vs. vehicle control, p=0.0022 vs. native) (Fig 8b—e). Vehicle control DRGs exhibited higher percentage of GFP+ (p<0.0001 vs. native) and F4/80+ (p=0.010 vs. native) and a stable percentage of CD11b+ cells (p=0.967 vs. native) compared to native DRGs, while AP20187 decreased GFP+ (p=0.019 vs. vehicle control) and CD11b+ (p=0.021 vs. vehicle control) but increased F4/80+ cells (p<0.0001 vs. native, p=0.035 vs. vehicle control) (Fig. 8f—i). MMR+ and iNOS+ macrophages showed an increasing trend in herniated groups with higher percentage in AP20187 group (Fig. S18). Thus, depletion of CSF-1R+ macrophages reduced the infiltration of myeloid-derived macrophages in DRGs due to acute disc herniation, but augmented resident macrophages, neuroinflammation, and neuropeptide markers on POD 6.
Figure 8.

Depletion of macrophages reduced CD11b+ cells but increased F4/80+ cells and neuroinflammation in DRGs after 6 days of disc herniation. (a) Immunofluorescence staining of TNF-α (red), IL-6 (red), SP and CGRP (green) in DRGs of native, vehicle control and AP20187 groups on POD 6. Quantitative analysis showed increased percentages of (b) TNF-α+, (c) IL-6+, (d) SP+ and (e) CGRP+ cells in both vehicle control and AP20187 groups, with the latter group being further increased in all markers. (f) Immunofluorescence staining of GFP (green), CD11b (red), and F4/80 (red) in DRGs of native, vehicle control and AP20187. (g) Percentage of GFP+ cells in DRGs showed an increase in vehicle control and a decrease in AP20187 group. (h) Percentage of CD11b+ showed upward trending in vehicle control (vs. native), which was reduced in AP20187 group. (i) Percentage of F4/80+ cells showed a moderate increase in vehicle control and marked abundance in AP20187 group. Images were taken at a magnification 600× with a calculated unit area of 0.028 mm2 (ROI). Each data point represents the mean value of a DRG sample. For each marker, around 7–10 ROIs were analyzed per DRG with 2—3 DRGs per mouse (n = 4 mice for vehicle control, n = 5 mice for AP20187 group, n=3 mice for native). One-way ANOVA with multiple comparisons; *p < 0.05; Scale bar = 50 μm.
Discussion
Inflammation is highly associated with disc degeneration and back/leg pain.27 Macrophages might be crucial targets for therapeutic development as indicated in both animal models and human samples of disc herniation.12,15,28–30 Here, we employed a MaFIA mouse model to study impacts of macrophages on disc degeneration, neuroinflammation, and local immune cell profiles. In this mouse strain, genes encoding eGFP and mutant human FK-506 binding protein (FKBP)-Fas suicide construct were engineered under the macrophage-specific mouse CSF-1R promoter. Macrophages can thus be conditionally programmed to undergo apoptosis through administration of AP20187 that binds to mutant FKBP.21 As expected, we confirmed abundant macrophages at local disc hernia sites in MaFIA strain (Fig. 1) similar to other strains,15 and reduced macrophages in blood and spleen on POD 6 (Fig. S2), consistent with literature.21 Compared to POD 6, not only do total infiltrating cells show a declining trend (Fig. 2), but also macrophages accounted for just a small portion at disc hernia sites on POD 13 and 20 (Fig. S9). Therefore, we focused on POD 6 for cell infiltration analysis (Supplementary Materials).
Various types of immune cells, including neutrophils, macrophages, and lymphocytes, have been detected in animal models and patients’ samples of disc herniation.12,15,28,31 In an acute disc herniation model, we detected neutrophils from POD 1 to 3 and macrophages from POD 3 to 7.12 In this study, the increasing trend of neutrophils and B cells in AP20187 group (Fig. 3) suggested altered immune reaction upon transient macrophage depletion. Wu et al. observed a massive infiltration of CD3+ T cells and neutrophils upon macrophage depletion, but not B cells at 9 weeks post-surgery in an osteoarthritis and high-fat diet model using MaFIA mice.32 Differences in cell type (B vs. T cell) might be attributed to different animal models and disease stages.
Diverse phenotypes of macrophages are regulated by developmental origin, tissue of residence, and environmental cues. CD11b is highly expressed on monocytes and macrophages, critical for their migration and function.33 F4/80 is highly and constitutively expressed on most resident tissue macrophages.34 We detected CD11b+, F4/80+, iNOS+ and MMR+ macrophages in the acute stage of disc herniation (Fig. 4, Fig. 5), and transient depletion of CSF-1R expressing macrophages35 reduced total numbers of infiltrated cells and these subsets of macrophages, which corroborates with others. Wu et al. reported macrophage depletion in MaFIA resulted in fewer iNOS+ and CD206+ macrophages in osteoarthritis knee joints (surgically induced by destabilization of the medial meniscus) and did not mitigate cartilage degeneration in obese mice.32 Bailey et al. reported that depletion 2 days prior to bone fracture in MaFIA mice showed increased synovitis, reduced bone mineral density, more iNOS+ and less CD206+ macrophages compared to control.36 Further understanding these phenotypes can be a key step in determining the specific population of macrophages involved in the disease progression of disc herniation.
During intervertebral disc degeneration and aging, aberrant mechanical loading-induced hypertrophy of chondrocytes and calcification of endplates lead to an osteoclast-initiated remodeling sclerotic process.37,38 Here, we showed progression of disc degeneration is accompanied by ectopic bone formation and elevated osteoclast activity possibly due to inflammatory response at disc hernia sites and adjacent tissues, whereas transient macrophage depletion might help lessen these pathological changes (Fig. 6, Fig. 7). However, macrophage functions may be very distinct in different disease models. Howell et al reported that macrophage depletion in MaFIA mice led to impaired functional healing, reduced cell proliferation, reduced GFP+ neo-tendon formation, and altered tendon gene expression.39 In this vain, further studies like the present one are needed to characterize this complex relationship.
Elevated inflammatory cytokines and neuropeptides have been associated with neuroinflammation and pain.28,40,41 We previously reported anti-inflammatory nanoparticles alleviated lumbar radiculopathy in mice via down-regulating SP and CGRP production in DRGs.28,42 In DRG tissue, we showed disc herniation induced expressions of TNF-α, IL-6, substance P, CGRP, along with increased GFP+ and F4/80+ macrophages, and AP20187 decreased GFP+ and CD11b+ cell infiltration, but not neuroinflammation markers (Fig. 8). The increasing trending of MMR+ and iNOS+ macrophages in AP20187 group (Fig. S17) may be associated with increased F4/80+ cell count in DRGs (Fig. 8). It is also possible that the drug AP20187 may not be able to reach DRG tissues, as application of clodronate liposomes depleted CD11b/CX3CR1 blood monocytes but not DRG macrophages or spinal microglia.43 The increased neutrophils in AP20187 group (Fig. 3) may also play a role in local inflammatory environment as well as DRG sensitization. In addition, macrophage depletion may disrupt the interactions between neurons and microglia/macrophages.44 In a brain ischemia study, CSF-1R inhibition increased the expression of inflammatory mediators in neurons and triggered neuronal death and astrocyte proliferation.45 Wu et al. also reported that macrophage depletion led to a markedly elevated proinflammatory cytokines, such as granulocyte colony‐stimulating factor, IL‐1β, IL‐6, IL‐8, and TNF-α in both serum and joint synovial fluid.32
Our study renders several limitations. We focused on the impact of macrophages on acute disc herniation. A depletion protocol in sub-acute and chronic stages of disc herniation may be employed to investigate disease stage-specific macrophage functions. Additionally, we did not measure animal behavior since the significant reduction in body weight upon AP20187 administration was likely to confound behavioral results. Systemic depletion of macrophages from the entire body may result in side effects and warrants detailed investigation. Meanwhile, molecular regulators of macrophage-disc crosstalk remain largely unknown. Immunostaining of F4/80+ and CD11b+ markers may not completely distinguish myeloid-derived and tissue-resident macrophages. Our future studies may focus on transcriptional profiling by single-cell RNA sequencing of surgically harvested infiltration tissues at various stages of disc herniation.
In summary, transient depletion of macrophages altered local inflammatory responses at the site of disc herniation in a transgenic mouse model. This study characterizes the inflammatory profile and contribution of macrophages in acute disc degeneration using the MaFIA mouse model, which deepens our understanding in macrophage functions during acute disc herniation.
Supplementary Material
Acknowledgements
This work is supported by research grants from U.S. NIH NIAMS R01AR064792, R01AR078888, R21AR072334, R21AR078547, NHLBI P03-074940, and Commonwealth Health Research Board (CHRB) 207-10-18.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest statement
The authors have no competing interests to declare.
Data statement
Data supporting the findings of this manuscript are available from the supplementary materials.
Other data request shall be made to the corresponding author.
References
- 1.Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–974. doi: 10.1136/annrheumdis-2013-204428 [DOI] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88 Suppl 2:21–24. doi: 10.2106/JBJS.E.01273 [DOI] [PubMed] [Google Scholar]
- 3.Wuertz K, Haglund L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Global Spine J. 2013;3(3):175–184. doi: 10.1055/s-0033-1347299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha C, Silva AJ, Pereira P, Vaz R, Gonçalves RM, Barbosa MA. The inflammatory response in the regression of lumbar disc herniation. Arthritis Res Ther. 2018;20(1):251. doi: 10.1186/s13075-018-1743-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiss A, Larsson K, Rydevik B, Takahashi I, Olmarker K. Autoimmune properties of nucleus pulposus: an experimental study in pigs. Spine. 2007;32(2):168–173. doi: 10.1097/01.brs.0000251651.61844.2d [DOI] [PubMed] [Google Scholar]
- 7.Wang L, He T, Liu J, Tai J, Wang B, Zhang L, et al. Revealing the immune infiltration landscape and identifying diagnostic biomarkers for lumbar disc herniation. Front Immunol. 2021;12:666355. doi: 10.3389/fimmu.2021.666355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baba H, Maezawa Y, Furusawa N, Fukuda M, Uchida K, Kokubo Y, et al. Herniated cervical intervertebral discs: histological and immunohistochemical characteristics. Eur J Histochem. 1997;41(4):261–270. [PubMed] [Google Scholar]
- 9.Kokubo Y, Uchida K, Kobayashi S, Yayama T, Sato R, Nakajima H, et al. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J Neurosurg Spine. 2008;9(3):285–295. doi: 10.3171/SPI/2008/9/9/285 [DOI] [PubMed] [Google Scholar]
- 10.Koike Y, Uzuki M, Kokubun S, Sawai T. Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine. 2003;28(17):1928–1933. doi: 10.1097/01.BRS.0000083324.65405.AE [DOI] [PubMed] [Google Scholar]
- 11.Arai Y, Yasuma T, Shitoto K, Yamauchi Y, Suzuki F. Immunohistological study of intervertebral disc herniation of lumbar spine. J Orthop Sci. 2000;5(3):229–231. doi: 10.1007/s007760050156 [DOI] [PubMed] [Google Scholar]
- 12.Xiao L, Ding M, Zhang Y, Chordia M, Pan D, Shimer A, et al. A novel modality for functional imaging in acute intervertebral disk herniation via tracking leukocyte infiltration. Mol Imaging Biol. 2017;19(5):703–713. doi: 10.1007/s11307-016-1038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dou L, Shi X, He X, Gao Y. Macrophage phenotype and function in liver disorder. Front Immunol. 2019;10:3112. doi: 10.3389/fimmu.2019.03112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178(1):19–25. doi: 10.1016/j.ajpath.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin L, Xiao L, Ding M, Pan A, Balian G, Sung SJ, et al. Heterogeneous macrophages contribute to the pathology of disc herniation induced radiculopathy. Spine J. 2022;22(4):677–689. doi: 10.1016/j.spinee.2021.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto Y, Kokubo Y, Nakajima H, Honjoh K, Watanabe S, Matsumine A. Distribution and Polarization of Hematogenous Macrophages Associated with the Progression of Intervertebral Disc Degeneration. Spine. 2022;47(4):E149–E158. doi: 10.1097/BRS.0000000000004222 [DOI] [PubMed] [Google Scholar]
- 17.Nakazawa KR, Walter BA, Laudier DM, Krishnamoorthy D, Mosley GE, Spiller KL, et al. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. 2018;18(2):343–356. doi: 10.1016/j.spinee.2017.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XC, Huang CM, Luo SJ, Fan W, Zhou TL, Chen W. Expression and Distribution of M1 and M2 Macrophages in the Degeneration Process of Human Lumbar Intervertebral Disc Herniation: A Histological and …. 2020.
- 19.Yamagishi A, Nakajima H, Kokubo Y, Yamamoto Y, Matsumine A. Polarization of infiltrating macrophages in the outer annulus fibrosus layer associated with the process of intervertebral disc degeneration and neural ingrowth in the human cervical spine. Spine J. December 2021. doi: 10.1016/j.spinee.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 20.Nakawaki M, Uchida K, Miyagi M, Inoue G, Kawakubo A, Satoh M, et al. Changes in nerve growth factor expression and macrophage phenotype following intervertebral disc injury in mice. J Orthop Res. 2019;37(8):1798–1804. doi: 10.1002/jor.24308 [DOI] [PubMed] [Google Scholar]
- 21.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, et al. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75(4):612–623. doi: 10.1189/jlb.0903442 [DOI] [PubMed] [Google Scholar]
- 22.Hua L, Shi J, Shultz LD, Ren G. Genetic models of macrophage depletion. Methods Mol Biol. 2018;1784:243–258. doi: 10.1007/978-1-4939-7837-3_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung SJ, Ge Y, Dai C, Wang H, Fu SM, Sharma R, et al. Dependence of Glomerulonephritis Induction on Novel Intraglomerular Alternatively Activated Bone Marrow-Derived Macrophages and Mac-1 and PD-L1 in Lupus-Prone NZM2328 Mice. J Immunol. 2017;198(7):2589–2601. doi: 10.4049/jimmunol.1601565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Lenart BA, Lee JK, Chen D, Shi P, Ren J, et al. Histological features of endplates of the mammalian spine: from mice to men. Spine. 2014;39(5):E312–7. doi: 10.1097/BRS.0000000000000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung S-SJ, Fu SM. Interactions among glomerulus infiltrating macrophages and intrinsic cells via cytokines in chronic lupus glomerulonephritis. J Autoimmun. 2020;106:102331. doi: 10.1016/j.jaut.2019.102331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung SJ, Fu SM. A novel immunofluorescence detection method for renal cell-type specific in situ cytokine production by confocal microscopy. MethodsX. 2020;7:100935. doi: 10.1016/j.mex.2020.100935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molinos M, Almeida CR, Caldeira J, Cunha C, Gonçalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12(104):20141191. doi: 10.1098/rsif.2014.1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin L, Ding M, Oklopcic A, Aghdasi B, Xiao L, Li Z, et al. Nanoparticle fullerol alleviates radiculopathy via NLRP3 inflammasome and neuropeptides. Nanomedicine. 2017;13(6):2049–2059. doi: 10.1016/j.nano.2017.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao L, Ding M, Fernandez A, Zhao P, Jin L, Li X. Curcumin alleviates lumbar radiculopathy by reducing neuroinflammation, oxidative stress and nociceptive factors. Eur Cell Mater. 2017;33:279–293. doi: 10.22203/eCM.v033a21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao L, Huang R, Zhang Y, Li T, Dai J, Nannapuneni N, et al. A New Formyl Peptide Receptor-1 Antagonist Conjugated Fullerene Nanoparticle for Targeted Treatment of Degenerative Disc Diseases. ACS Appl Mater Interfaces. 2019;11(42):38405–38416. doi: 10.1021/acsami.9b11783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shamji MF, Setton LA, Jarvis W, So S, Chen J, Jing L, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974–1982. doi: 10.1002/art.27444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu CL, McNeill J, Goon K, Little D, Kimmerling K, Huebner J, et al. Conditional Macrophage Depletion Increases Inflammation and Does Not Inhibit the Development of Osteoarthritis in Obese Macrophage Fas-Induced Apoptosis-Transgenic Mice. Arthritis Rheumatol. 2017;69(9):1772–1783. doi: 10.1002/art.40161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng C, Yang Q, Xu C, Shou P, Cao J, Jiang M, et al. CD11b regulates obesity-induced insulin resistance via limiting alternative activation and proliferation of adipose tissue macrophages. Proc Natl Acad Sci USA. 2015;112(52):E7239–48. doi: 10.1073/pnas.1500396113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKnight AJ, Gordon S. The EGF-TM7 family: unusual structures at the leukocyte surface. J Leukoc Biol. 1998;63(3):271–280. doi: 10.1002/jlb.63.3.271 [DOI] [PubMed] [Google Scholar]
- 35.van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193(1):93–99. doi: 10.1016/0022-1759(96)00056-7 [DOI] [PubMed] [Google Scholar]
- 36.Bailey KN, Furman BD, Zeitlin J, Kimmerling KA, Wu CL, Guilak F, et al. Intra-articular depletion of macrophages increases acute synovitis and alters macrophage polarity in the injured mouse knee. Osteoarthr Cartil. 2020;28(5):626–638. doi: 10.1016/j.joca.2020.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bian Q, Jain A, Xu X, Kebaish K, Crane JL, Zhang Z, et al. Excessive activation of tgfβ by spinal instability causes vertebral endplate sclerosis. Sci Rep. 2016;6:27093. doi: 10.1038/srep27093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ni S, Ling Z, Wang X, Cao Y, Wu T, Deng R, et al. Sensory innervation in porous endplates by Netrin-1 from osteoclasts mediates PGE2-induced spinal hypersensitivity in mice. Nat Commun. 2019;10(1):5643. doi: 10.1038/s41467-019-13476-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howell KL, Kaji DA, Li TM, Montero A, Yeoh K, Nasser P, et al. Macrophage depletion impairs neonatal tendon regeneration. FASEB J. 2021;35(6):e21618. doi: 10.1096/fj.202100049R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. J Neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y-Q, Liu Z, Liu Z-H, Chen S-P, Li M, Shahveranov A, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 2016;13(1):141. doi: 10.1186/s12974-016-0607-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao L, Hong K, Roberson C, Ding M, Fernandez A, Shen F, et al. Hydroxylated Fullerene: A Stellar Nanomedicine to Treat Lumbar Radiculopathy via Antagonizing TNF-α-Induced Ion Channel Activation, Calcium Signaling, and Neuropeptide Production. ACS Biomater Sci Eng. 2018;4(1):266–277. doi: 10.1021/acsbiomaterials.7b00735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng J, Gu N, Zhou L, B Eyo U, Murugan M, Gan WB, et al. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun. 2016;7:12029. doi: 10.1038/ncomms12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front Cell Neurosci. 2018;12:323. doi: 10.3389/fncel.2018.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin WN, Shi SX, Li Z, Li M, Wood K, Gonzales RJ, et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J Cereb Blood Flow Metab. 2017;37(6):2224–2236. doi: 10.1177/0271678X17694185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this manuscript are available from the supplementary materials.
Other data request shall be made to the corresponding author.


