Abstract
Objective:
There has been a sustained increase in the utilization of venovenous extracorporeal membrane oxygenation (VV ECMO) over the last decade, further exacerbated by the COVID-19 pandemic. We set out to describe our institutional experience with extremely prolonged (>50 days) VV ECMO support for recovery or bridge to lung transplant candidacy in patients with acute respiratory failure.
Design:
Retrospective cohort study.
Setting:
A large tertiary urban care center
Patients:
Patients 18 years or older receiving VV ECMO support for greater than 50 days, with initial cannulation between January 2018 and January 2022.
Interventions:
None
Measurements and Main Results:
130 patients were placed on VV ECMO during the study period. Of these, 12 received prolonged (> 50 days) VV ECMO support. 11 patients (92%) suffered from acute respiratory distress syndrome (ARDS) secondary to COVID-19 while 1 patient with prior bilateral lung transplant suffered from ARDS secondary to bacterial pneumonia. The median age of patients was 39 years (IQR: 35-51 years). The median duration of VV ECMO support was 94 days (IQR: 70-128 days), with a maximum of 180 days. Median time from intubation to cannulation was 5 days (IQR:2-14 days). 9 patients (75%) were successfully mobilized while on VV ECMO support. Successful weaning of VV ECMO support occurred in 8 patients (67%); 6 (50%) were bridged to lung transplantation and 2 (17%) were bridged to recovery. Of those successfully weaned, 7 patients (88%) were discharged from the hospital. All 7 patients discharged from the hospital were alive 6 months post-decannulation; 83% (5/6) with sufficient follow-up time were alive 1-year after decannulation.
Conclusions:
Our experience suggests that extremely prolonged VV ECMO support to allow native lung recovery or optimization for lung transplantation may be a feasible strategy in select critically ill patients, further supporting the expanded utilization of VV ECMO for refractory respiratory failure.
Keywords: respiratory failure, acute respiratory distress syndrome, extracorporeal membrane oxygenation
INTRODUCTION
Venovenous extracorporeal membrane oxygenation (VV ECMO) has been increasingly utilized for the management of refractory respiratory failure [1]. With experience gained during the recent COVID-19 pandemic, VV ECMO has been shown to be an effective management strategy to improve survival in those suffering from severe disease as compared to conventional mechanical ventilation [2].
Prior studies examining the effect of VV ECMO support >21 days have demonstrated acceptable outcomes in patients with acute respiratory failure as compared to shorter durations of support [3, 4]. Additionally, individual case reports have highlighted the feasibility of VV ECMO runs of >100 days [5, 6]. There is no consensus on the definition of prolonged VV ECMO therapy, although a recent survey of experienced European ECMO centers identified support times of at least 21 days to be considered prolonged [7]. Given changing clinical practice, this boundary will likely continue to evolve. As such, we sought to evaluate our institutional experience with extremely prolonged VV ECMO support, which we defined as a duration exceeding 50 days. To our knowledge, this is the largest case series of such an extended duration of VV ECMO support.
METHODS
We performed a retrospective cohort analysis of patients who received VV ECMO support at our institution between January 1, 2018 and January 31, 2022 utilizing a prospectively-collected single center database. All potential candidates for VV ECMO are evaluated by a multidisciplinary team prior to cannulation (eFigure 1). We identified 130 patients who underwent VV ECMO support during this period, 12 of whom were supported on VV ECMO for more than 50 days (eTable 1). Extremely prolonged duration of VV ECMO support was defined as > 50 days as this was the 90th percentile of duration at our center across the study period. This study was approved by the Institutional Review Board at Cedars-Sinai Medical Center (protocol ID: STUDY00001188, approval date 2/19/2021). Procedures were followed in accordance with the ethical standards of the institutional review board and with the Helsinki Declaration of 1975.
Baseline patient characteristics, VV ECMO configuration and duration, and greatest level of mobility utilizing standard Extracorporeal Life Support Organization (ELSO) definitions while on support were reported for each patient [8]. We categorized patients into the following groups to characterize outcomes of VV ECMO therapy: expired, bridged to recovery, and bridged to transplant. Additionally, we performed a chart review to determine discharge status, discharge location, and to describe the care-team’s approach to ELSO-defined complications, mobilization, and evaluation for transplantation. Six-month and 1-year post-decannulation survival was assessed for all patients with sufficient follow-up time.
RESULTS
eTable 2 displays characteristics and outcomes for the 12 patients who were supported with prolonged VV ECMO. The median age was 39 years (IQR: 35-51 years). 11 patients (92%) suffered from acute respiratory distress syndrome (ARDS) secondary to COVID-19, while 1 patient with a history of bilateral lung transplantation suffered from ARDS secondary to bacterial pneumonia. The median time from intubation to cannulation was 5 days (IQR: 2-14 days), with maximum length of 42 days. Cannulation strategy included venous return via the right internal jugular vein for all patients; venous drainage was obtained via one femoral vein in 11 patients (92%) while 1 patient (8%) required bifemoral cannulation.
The median total duration of VV ECMO support was 94 days (IQR: 70-128 days), with a maximum of 180 days. 9 patients (75%) were mobilized to some degree while cannulated for VV ECMO, and 6 of these patients (50%) were able to ambulate daily. 7 patients (58%) were able to be fully weaned off mechanical ventilation while on VV ECMO support.
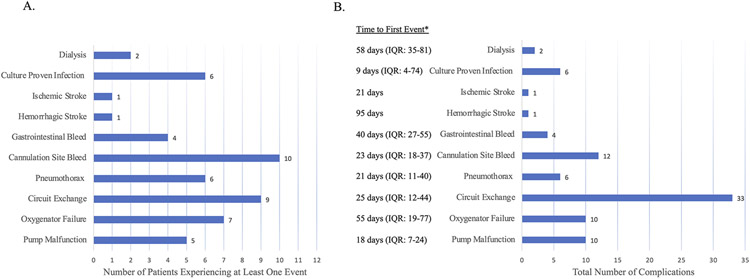
Complications related directly to the VV ECMO circuit included the following: 10 (83%) had cannulation site bleeding, 5 (42%) experienced pump malfunction secondary to centrifugal pump cone decoupling from thrombosis, and 9 (75%) required circuit exchanges. The median number of circuit exchanges required per patient was 1 (IQR: 1-5). All other complications while on VV ECMO support are highlighted in Figure 1.
Figure 1:
Complications while on VV ECMO support represented as A. Number of patients experiencing at least 1 event and B. Total number of events, with median time to first event type for each patient.
* Median (IQR) time from VV-ECMO cannulation to first event of each type
8 patients (67%) were successfully weaned from VV ECMO support: 6 patients (50%) underwent lung transplantation and 2 patients (17%) recovered sufficient native lung function. Of the patients successfully weaned, 7 (88%) were discharged from the hospital – 4 patients (50%) were discharged to a rehabilitation facility while 3 patients (38%) were discharged directly to home. The one in-hospital death following separation from VV ECMO was in a patient bridged to lung transplantation who suffered from severe right ventricular dysfunction postoperatively and ultimately succumbed to multi-system organ failure. Median ICU and hospital lengths of stay were 97 days (IQR: 75-142) and 97 days (IQR: 89-150), respectively. All 7 patients discharged from the hospital remained alive 6-months after decannulation, while 83% (5/6) with sufficient follow-up time remained alive 1-year after decannulation.
DISCUSSION
In this brief report we describe our institutional experience utilizing very prolonged VV ECMO support as a bridge to lung recovery or transplantation in patients suffering from acute respiratory failure. We utilized VV ECMO as a rescue therapy and report an overall successful decannulation rate of 67% and an overall discharge survival rate of 58%, including 88% in patients who were weaned from ECMO. Our analysis provides evidence that extended VV ECMO support can be utilized successfully in carefully selected patients as a bridge to recovery or transplantation.
Our cohort is unique, not only for the extremely long duration of support, but also the large proportion of irreversible lung pathology. Most patients in our series suffered from pneumonia secondary to COVID-19. At the time of VV ECMO cannulation, patients were managed with the expectation that they would recover native lung function. However, after an extended period (typically 8 weeks from cannulation) with no clinical evidence of lung recovery and radiographic evidence of significant parenchymal destruction, patients were considered for transplantation. Lung transplantation has been shown to be a viable therapeutic option for patients suffering from irreversible lung damage from COVID-19 [9]. Our experience indicates that extremely prolonged VV ECMO can be utilized to preserve candidacy and optimize patients for lung transplantation.
Growing evidence supports the safety and importance of early mobilization for patients on VV ECMO [10]. Improvements in physical conditioning are critical to optimizing a patient’s status for transplantation [11]. When patients were determined to have non-reversible lung injury (usually at 8 weeks), daily sedation weaning was performed to assess rehabilitation potential. Once appropriate, physical therapy protocols were initiated including in-bed passive and active ranges-of-motion exercises, use of gradual weight-bearing tilting beds, and assisted ambulation on VV ECMO. Ideally, patients would be ambulating prior to transplantation to mitigate postoperative debilitation; however, for the purpose of lung transplantation listing in our institution the ability to bear weight was considered sufficient.
Complications in the setting of extended VV ECMO runs are unavoidable [12] . Our case series highlights some of the morbidities associated with prolonged VV ECMO support. At our institution, we have developed protocols for circuit exchanges across a multidisciplinary group including perfusion, critical care providers, and nursing staff to ensure such exchanges are done safely and ideally in a non-emergent setting. Additionally, our anticoagulation protocol (eFigure 2) incorporates daily thromboelastography to guide anticoagulation and targeted transfusion management to reduce the risk of catastrophic bleeding or thrombotic complications. However, as evidenced by relatively high rates of pump malfunction and circuit exchanges secondary to thrombosis, these patients remain at heightened risk requiring close monitoring and prompt intervention.
The results of our study would suggest that it is feasible to extend the use of VV ECMO for very long durations as a bridge to lung recovery or transplantation unless the burden of complications overcomes the perceived benefits of ECMO. However, management of patients on VV ECMO is incredibly resource- and time-intensive, diverting resources during times in which hospital capacity is already maximized. Additionally, no consensus amongst providers exists regarding definitions of futile care and the timeline to withdraw support [7]. At our institution, palliative care teams are involved early during mechanical circulatory support to facilitate goals of care discussions.
This institutional case series describing outcomes of extremely prolonged VV ECMO has several limitations. This cohort represents a carefully selected group of patients at a high-volume transplant center treated with institution-specific policies which may limit generalizability. Second, we did not provide a comparison group and thus no conclusions can be made regarding differential outcomes of long versus short-term VV ECMO support. Furthermore, we did not examine long-term outcomes such as quality of life, functional status, and mental health (e.g. post-traumatic stress disorder, anxiety, depression) and thus we cannot comment on the global burden of extremely prolonged VV ECMO support.
Our experience suggests that extremely prolonged VV ECMO support (> 50 days) as a bridge to recovery or transplantation is a feasible strategy in a select group of critically ill patients who would have no other option for survival. As experience with VV ECMO grows, future studies are warranted to determine optimal patient selection, criteria for continuing VV ECMO support regardless of duration, and direct comparisons of outcomes in patients with prolonged and typical VV ECMO run durations.
Supplementary Material
KEY POINTS.
Objective –
Describe institutional experience with extremely prolonged (> 50 days) VV ECMO support.
Findings –
67% (8/12) of patients survived to decannulation after > 50 days of VV ECMO: 50% (6/12) underwent lung transplantation while 17% (2/12) recovered sufficient native lung function.
Meaning –
Our institutional experience suggests that extremely prolonged VV ECMO support to allow native lung recovery or optimization for lung transplantation may be a feasible strategy in select critically ill patients.
Copyright Form Disclosure:
Drs. Malas and Kumaresan’s institutions received funding from the National Institutes of Health (NIH). Drs. Malas, Chen, Ebinger, and Kumaresan received support for article research from the NIH. Dr. Chen received funding from the NIH (T32HL116273). Dr. Nurok received funding from Avant-Garde Health Inc; he disclosed the off-label product use of ECMO. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Disclosures:
Jad Malas and Qiudong Chen are supported by grants from the National Institutes of Health for advanced heart disease research (T32HL116273). The remaining authors have no relevant disclosures
REFERENCES
- 1.Tonna JE, Abrams D, Brodie D et al. : Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). Asaio j 2021, 67(6):601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urner M, Barnett AG, Bassi GL et al. : Venovenous extracorporeal membrane oxygenation in patients with acute covid-19 associated respiratory failure: comparative effectiveness study. Bmj 2022, 377:e068723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camboni D, Philipp A, Lubnow M et al. : Support time-dependent outcome analysis for veno-venous extracorporeal membrane oxygenation. Eur J Cardiothorac Surg 2011, 40(6):1341–1346;discussion 1346–1347. [DOI] [PubMed] [Google Scholar]
- 4.Kon ZN, Dahi S, Evans CF et al. : Long-Term Venovenous Extracorporeal Membrane Oxygenation Support for Acute Respiratory Distress Syndrome. Ann Thorac Surg 2015, 100(6):2059–2063. [DOI] [PubMed] [Google Scholar]
- 5.Wiktor AJ, Haft JW, Bartlett RH et al. : Prolonged VV ECMO (265 Days) for ARDS without Technical Complications. ASAIO Journal 2015, 61(2):205–206. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Xu Y, Liu D et al. : Case Report: Prolonged VV-ECMO (111 Days) Support in a Patient With Severe COVID-19. Front Med (Lausanne) 2021, 8:681548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepper PM, Barrett NA, Swol J et al. : Perception of prolonged extracorporeal membrane oxygenation in Europe: an EuroELSO survey. Perfusion 2020, 35(1_suppl):81–85. [DOI] [PubMed] [Google Scholar]
- 8.Extracorporeal Life Support Organization (ELSO): ELSO Registry Data Definitions [https://www.elso.org/portals/0/files/pdf/elso%20registry%20data%20definitions%2005_17_22.pdf]
- 9.Roach A, Chikwe J, Catarino P et al. : Lung Transplantation for Covid-19-Related Respiratory Failure in the United States. N Engl J Med 2022, 386(12):1187–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko Y, Cho YH, Park YH et al. : Feasibility and Safety of Early Physical Therapy and Active Mobilization for Patients on Extracorporeal Membrane Oxygenation. Asaio j 2015, 61(5):564–568. [DOI] [PubMed] [Google Scholar]
- 11.Wickerson L, Rozenberg D, Janaudis-Ferreira T et al. : Physical rehabilitation for lung transplant candidates and recipients: An evidence-informed clinical approach. World J Transplant 2016, 6(3):517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teijeiro-Paradis R, Gannon WD, Fan E: Complications Associated With Venovenous Extracorporeal Membrane Oxygenation-What Can Go Wrong? Crit Care Med 2022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.