Abstract
Much research focuses on increasing carbon storage in mineral-associated organic matter (MAOM), in which carbon may persist for centuries to millennia. However, MAOM-targeted management is insufficient because the formation pathways of persistent soil organic matter are diverse and vary with environmental conditions. Effective management must also consider particulate organic matter (POM). In many soils, there is potential for enlarging POM pools, POM can persist over long time scales, and POM can be a direct precursor of MAOM. We present a framework for context-dependent management strategies that recognizes soils as complex systems in which environmental conditions constrain POM and MAOM formation.
Subject terms: Carbon cycle, Ecosystem ecology, Carbon cycle
Increasing C storage in mineral-associated organic matter is insufficient due to diverse, environmentally specific persistent soil organic matter formation. Context-dependent management strategies highlighting the importance of particulate organic matter are necessary.
Introduction
Since the late 1980s1–3, many studies have stated the necessity to distinguish particulate organic matter (POM) from mineral-associated organic matter (MAOM) in order to better understand soil organic matter (SOM) dynamics and manage soils as a carbon (C) sink4. The logic is simple: although SOM includes diverse biomolecules that are positioned continuously along many biophysical and spatiotemporal gradients in soil5, POM and MAOM are two pools that are reasonable to separate (physically) and differ broadly in their ecological functioning, chemical composition, and turnover times6. Particulate OM is mostly derived from partly decomposed plant fragments6, and where it is not occluded within aggregates7, it has a relatively short residence time8 and can be easily decomposed under the right environmental conditions. Contrastingly, MAOM is tightly bound to minerals or occluded within small microaggregates (<50 µm9) and assumed to persist in soil for hundreds to thousands of years10, even though MAOM can be recycled on shorter timescales11 and is a potential nutrient pool for plants12. The lower bioavailability of MAOM and its large contribution to bulk soil C storage in many soils has stimulated many researchers to mainly focus on factors that influence its formation, chemical composition, and accumulation.
Recently, much SOM research and conceptual development of soil management strategies has emphasized the remains of microbiota (or microbial necromass)13–15, which can constitute a substantial portion of MAOM16. To build-up microbial biomass, and microbial necromass retained in MAOM, some authors suggest manipulating plant inputs, e.g., by introducing plants that supply types of organic substrates that microorganisms can more efficiently convert to microbial biomass13,14,17 and thus enhancing microbial necromass. However, the performance of such microbe-and-MAOM-centric strategies for research and soil management may suffer from persistent uncertainty about the composition of MAOM and the efficiency of its formation from different precursors. For example, in many soils, biotic and abiotic factors likely allow plant-derived biomolecules to account for a substantial fraction of MAOM16. Particulate OM itself can be a precursor to MAOM, but the importance of such a link between these two pools is likely sensitive to environmental constraints. Recent studies of agricultural soil microcosms suggest that formation of MAOM is unrelated to POM, but linked to inputs of dissolved compounds leached from plant litter18,19, which can easily sorb to mineral surfaces (“direct sorption”) or be metabolized by the microbial community. Yet, similar elemental, isotopic, and chemical characteristics among POM and MAOM across diverse ecosystems20–23 indicate intricate links between MAOM formation and microbial depolymerization and transformation of POM into simpler forms and microbial necromass. Thus, different SOM precursors and formation pathways appear to have varying importance in different contexts24, and generalizations about MAOM formation mechanisms seem problematic. Dissolved organic compounds may be less relevant in systems that lack a thick organic layer or in which plant biomass or litter is regularly removed, such as in certain croplands25,26. Decomposing POM can also be a direct source of dissolved organic compounds in mineral soils and thus link POM and MAOM22,27. Likewise, microbial depolymerization and transformation of litter, or POM, and the related build-up of MAOM may be more or less efficient depending on multiple environmental constraints that determine microbial proliferation and the stabilization of microbial remains. For example, “recalcitrant” POM (with high lignin to nitrogen (N) or C:N ratios) can hamper formation of MAOM as compared to “high-quality” POM (low lignin:N or C:N ratios28).
Focusing research or management exclusively or primarily on MAOM also obscures several key facts: (i) C stocks in organic horizons and in POM within mineral soils can be large, and persist for hundreds to thousands of years (even if the residence time of each biomolecule or C atom is not long), (ii) some types of POM, such as that occluded in aggregates, are relatively stable as indicated by residence times of hundreds of years29), and (iii) proportionally, POM-C may contribute as much or more to total C storage than MAOM-C, such as in soils with a limited capacity for mineral protection of SOM30,31 or grassland soils in alpine or semi-arid environments29,30. In our view, emphasis toward MAOM also coincides with a problematic reduction in the number of studies and management strategies that consider the organic horizon, which is primarily comprised of POM and is a large pool of C and nutrients in many ecosystems. While the importance of POM has certainly been recognized for some soil systems and in some conceptual and quantitative models4,32,33, we see a critical need to develop a more holistic and integrative view of POM and MAOM dynamics, including their interactions and sensitivity to management practices and environmental conditions that vary in space and time.
In summary, we propose that understanding of SOM dynamics and effective management for C sequestration are hindered by an over-emphasis on MAOM, inattention to interactions between MAOM and POM, and context-blind generalizations about SOM dynamics. In our view, a recalibration of SOM research to address these hindrances will result in more integrative conceptual and quantitative models of SOM dynamics, enabling more accurate and applicable knowledge of the sources of SOM (i.e., microbial versus plant-derived organic matter16,28,34) and the functions and locations of SOM (in organic versus mineral horizons, in top- versus subsoils, in POM versus MAOM fractions).
Developing a systems approach for SOM research and management
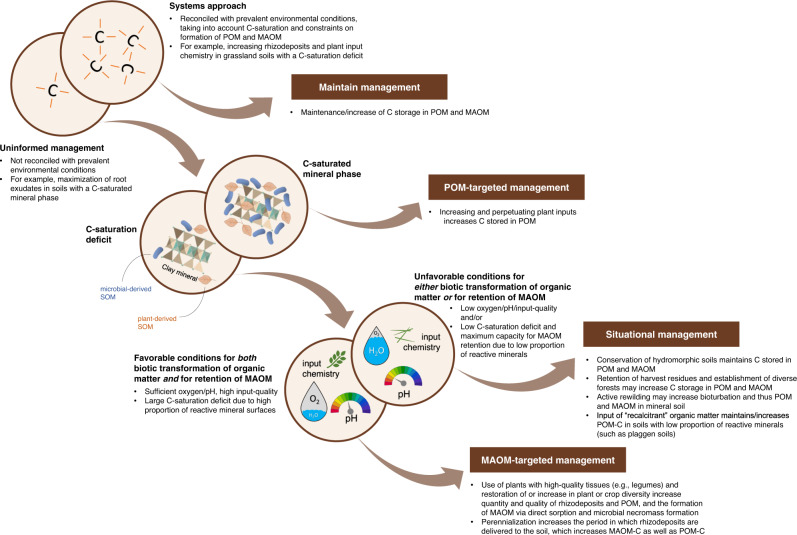
We advocate for a systems approach (Fig. 1) to studying and managing SOM that recognizes soils as complex systems in which POM and MAOM are distinctly important, but intertwined parts, and POM and MAOM formation, stocks, and stability determined by processes whose importance and outcomes are dependent on environmental factors and management practices that vary from site to site. Below, we outline this systems approach for maintaining and/or establishing soils as a C sink (Fig. 1). We specifically highlight how various constraints can affect the formation and interaction of MAOM and POM, with an emphasis on how optimal soil management might depend on C saturation, land use, or soil type.
Fig. 1. Systems approach for the contextualization of carbon-focused management strategies.
Management strategies that are adapted to prevailing environmental conditions (i.e., following the systems approach) should be maintained, while strategies not well-adapted to site-specific conditions (i.e., uninformed) can be improved (first double circle from the upper left). The optimal management strategy depends on whether the soil has a carbon (C)-saturated mineral phase (second double circle) and favorable conditions for biotic transformation of organic matter and retention of mineral-associated organic matter (MAOM; third double circle). Recommended management strategies are indicated in boxes and examples provided in the bullet points below. POM particulate organic matter, SOM soil organic matter. Some elements in this figure adapted from Angst et al.58.
Targeting POM in systems with C-saturated mineral phase
We argue that whether C-focused management strategies should target POM or MAOM (or both) is principally determined by the C saturation of a soil. The C-saturation concept is based on the assumption that the amount of silt- and clay-sized minerals determine the overall capacity of a soil to store C35–37. The concept primarily focuses on MAOM as the major soil C pool and on the specific mineral surface area of fine-sized minerals as the main driver of MAOM stabilization. While MAOM-C indeed tends to “saturate” with increasing C contents, the capacity of a soil to store additional C is not reached at that point. Near and above the C-saturation threshold of a soil, formation of MAOM will be less efficient13,18,38, but not zero39–41. Likewise, there is ample evidence that additional (plant) inputs can continue to accumulate as labile (free) or stabilized (occluded) POM42–47. Thus, for soils (or soil horizons48,49) that are at or near their theoretical C-saturation limit, MAOM-centric strategies aimed at increasing C storage will be ineffective48 and may even be counterproductive50,51. For example, in C-saturated soils, promoting plants with ‘high quality’ litter or greater root exudation could diminish both POM pools (because of more complete decomposition of plant litter and mineralization of plant-derived C28) or MAOM pools (due to exudation-induced priming50). In our opinion, for soils with a C-saturated mineral phase, focus should rather be on increases in POM, e.g., via higher amounts of structural, perhaps more recalcitrant, plant inputs (Fig. 1). If these inputs are perpetuated, C storage in such soils can be increased in the long term via the rather labile POM pool, even if MAOM formation is low. For example, in forests managed for timber production, management practices that retain larger fractions of biomass residues (e.g., leaves and branches) could increase SOC stocks52,53, even if MAOM-C pools are close to saturation. In croplands, increased litter inputs combined with practices such as no or reduced tillage in agricultural systems may also increase the formation of aggregates54–56 and thus the persistence of POM occluded within these aggregates.
Increasing MAOM relies on favorable soil conditions for biotic transformation of organic matter and for retention of MAOM
Globally, the average C-saturation deficit of surface soils (≤30 cm depth) is estimated to be roughly at 50%, and soils further from C-saturation may accrue C more effectively48. For these soils, improved management could increase MAOM-C. However, whether MAOM-targeted management in such soils is effective primarily depends on both (i) environmental conditions such as pH, chemistry of organic matter inputs, or availability of oxygen, and (ii) the overall proportion of reactive mineral surfaces. These parameters determine the rates at which soil fauna and microorganisms depolymerize and transform organic matter into simpler forms and microbial necromass, which is central to the formation of MAOM14,20,23,57,58, and the capacity of a soil to store C as MAOM, respectively. We thus expect that formation of MAOM can be boosted by implementation of appropriate management in soils with conditions that favor transformation of organic matter (e.g., organic matter inputs with low C/N and lignin/N or sufficient oxygen) and that have abundant reactive minerals, such as soils rich in silt and clay (Fig. 1).
For example, aerobic grassland soils with a C-saturation deficit and high abundance of reactive minerals provide the potential to increase C stored in MAOM via increased root exudates59 and structural plant inputs60. Grasslands typically have a continuous input of plant material but no organic horizons61,62, low C:N ratios and low ratios of lignin to N in plants63, and near-neutral soil pH64, resulting in an efficient transformation of plant-derived organic matter into microbial products and subsequent formation of more persistent MAOM. Recent studies suggest that suitable management strategies, such as optimized grazing intensities, multitrophic rewilding, or restoration of plant diversity, can alter the quantity of rhizodeposits and the quantity and quality of POM in grasslands, boosting formation of MAOM-C (and POM as precursor pool) both via direct sorption of dissolved organic matter and biotic transformation of POM60,65–68.
Likewise, soils in an intermediate development stage on loess-rich parent materials, such as Chernozems, Cambisols, or Luvisols, are usually fertile, well-aerated, and have a high reactive mineral surface area69. These characteristics provide favorable conditions for microbial transformation and stabilization of SOM, as indicated by high amounts of microbial necromass in bulk soil and SOM fractions16,70. Many of these soils are under agricultural use and consequently low in POM due to biomass export via harvest and rapid decomposition of plant inputs in these systems69,71,72. Total SOM stocks in these circumstances may be increased by an improved management of plant inputs (e.g., use of high-quality cover crops [legumes], cultivars with higher or deeper root-derived inputs, and/or perennials; retention of crop residues) in combination with reduced tillage, optimized fertilization, or organic amendments56,68,73–75 (but also refer to Schlesinger76). These management practices may eventually boost MAOM-C in the medium- to long-term via root exudates and plant-derived POM as precursor pools48,59,77.
Reconsidering POM to meet specific soil and management conditions
Soils with adverse conditions for faunal and microbial activity have often hampered decomposition of plant residues that leads to the accumulation of POM both in mineral soils and in organic horizons, which can comprise high C stocks78. The conditions that favor POM accumulation (vs. MAOM formation) are likely caused by the interplay of various environmental factors (such as precipitation, topographic position, soil type, or vegetation)32 that cannot easily be shifted towards a state favorable for MAOM formation. Moreover, soils with low mineral surface area have a reduced capacity for retaining MAOM, so even if those soils have a C-saturation deficit and favorable conditions for biotic transformation of organic matter inputs, POM could be an important pathway for additional C sequestration. We argue that effective management of such soils as a C sink has to be situational and will require a renewed consideration of POM, especially where its formation and stabilization are more favorable (e.g., forests compared to most grasslands and some croplands; cold versus warm climates; acidic versus neutral soils; sandy soils; see below; Fig. 1).
For example, accumulation of POM and reduced formation of MAOM are often related to low-quality plant inputs (e.g., high C to N or lignin to N ratios), low soil N availability, and low soil pH values. Such conditions are often found in coniferous forests69,79,80 and beneath some deciduous hardwood stands or species81. Plant inputs with high C:N ratios, rich in tannins, waxes, and lignin, can hamper microbial metabolization of POM derived from these plant tissues and thus the efficiency with which MAOM is formed82,83. The typically low soil pH under trees with low-quality tissues84,85 can have further detrimental effects on the microbial and faunal community (e.g., absence of earthworms and inhibition of bacteria), reduce the number of mineral surfaces available for the sorption of organic matter86, and favor the dissolution of minerals (typically in Podzols). Trees associated with ectomycorrhizal fungi may be more likely to create conditions unfavorable for efficient bacterial conversion of plant litter and POM to MAOM87–91. In systems with one or more of these ‘unfavorable’ conditions, formation of MAOM may proceed relatively slowly71 and be limited to, or dominated by, direct sorption of dissolved organic compounds92. These conditions result in the formation of a thick organic layer, a high contribution of POM to total SOM pools71, and an elevated contribution of plant compounds to MAOM (and reduced contribution of microbial necromass) as compared to other ecosystems16.
Notably, an overly MAOM-centric view of soil C sequestration and ecosystem management may undervalue forests with large organic horizons and higher contributions of POM to SOM in mineral soils71,78. Implementing MAOM-centric management practices in such forests without consideration of POM may result in little change in total SOM stocks and net C sequestration51. We argue that in many forests, soil C stocks may be maximized by maintaining or increasing the diversity of overstory tree species93, including species with both high and low litter quality, which may allow for relatively large pools of POM and MAOM at the ecosystem scale94. Such interventions will be most effective when combined with management strategies that minimize the export of organic matter, e.g., through retention of harvest residues52,95 or active rewilding (Fig. 1). For example, retention of harvest residues in a eucalyptus plantation for a period of three years measurably increased both POM- and MAOM-C (2.1- and 1.2-fold)96. Moreover, re-establishment of populations of large animals or introduction of earthworms may increase bioturbation rates, with potential positive effects on MAOM-C58,66,97, negative effects on C stored in the forest floor, but no substantial changes in combined C stocks of forest floors and mineral soil98.
Similarly, hydromorphic soils have reduced oxygen availability and anoxic zones within the soil profile that lead to low redox potentials99 and the dominance of K-strategists100. This decelerates the accrual of microbial necromass in MAOM101 and favors the accumulation of POM, which accounts for up to 100% of C in Histosols and a major proportion of C in Cryosols102–104, paddy soils105, and Gleysols99. Particulate OM in cryogenic and hydromorphic soils could also play an important role in their response to climate change. Thawing permafrost may accelerate mineralization of POM (and loss of C) previously protected from decomposition via water logging or low temperatures104. Likewise, in areas where precipitation and anoxia is increasing, POM may accumulate (with potential decreases in MAOM106,107). Conversely, if hydromorphic soils are subject to reduced precipitation and increased aeration, losses of unprotected POM may contribute to reduced SOM stocks and substantial CO2 emissions108,109, unless MAOM forms at the same rate as POM is lost. Conservation and restoration of wetlands110 and peatlands thus appear to be the most reasonable interventions to maintain and increase storage of POM-C in soil systems that naturally preserve large amounts of rather labile C over millennia (Fig. 1).
We further argue that soils with a low proportion of reactive minerals surfaces, even if not C-saturated, are inappropriate targets for MAOM-centric management. Increases in MAOM-C in such soils will have little effect on the total SOC stock because their capacity to accrue C in MAOM is strongly limited and they are commonly dominated by C stored in POM111. Heathland or plaggen soils in north-western Europe45,112, for example, are mostly sand-rich, the C content is uncorrelated to MAOM45,113, and they store exceptionally high C amounts in POM113,114. This POM likely persists due to high contents of lipids, aliphatic compounds, and sterols that mainly originate from heathland vegetation and sustained high inputs of organic matter112,113. In our view, maintenance of and/or increase in C stocks in such sandy soils should be based on sustained inputs of “recalcitrant” organic matter with high C:N ratios, such as biochar115, to maintain and increase POM-C, rather than on a MAOM-focused approach using high-quality plant inputs45 (Fig. 1).
Implications and outlook
We highlight that C sequestration in soil should not be a single-pool endeavor, and support our view by clarifying the varying relevance of POM and MAOM formation pathways across different environmental contexts, including levels of soil C saturation, climate, land use/cover, and soil type. Targeting MAOM alone will not optimize soils as a C sink in many contexts. To make full use of the C sequestration potential of soils, management strategies should recognize soils as complex systems and be tailored to the respective environmental conditions (Fig. 1). We advocate for the reconsideration of POM as a quantitatively and functionally important C pool and management target in a multitude of ecosystems. For example, POM is particularly important in ecosystems with a C-saturated mineral phase, with unfavorable conditions for biotic activity (and thus formation of MAOM), or with a low proportion of reactive minerals (such as in sand-rich soils) to accrue significant amounts of MAOM-C. Increasing and perpetuating organic matter inputs in such ecosystems is crucial to build and then maintain stocks of POM with a short mean-residence time compared to MAOM.
We further suggest that for many soils, C-sequestration can only be maximized by broadening the focus of MAOM-focused management strategies to build POM-C along with MAOM-C. Under suitable conditions, such as in fertile grassland soils with a C-saturation deficit and sufficient proportion of minerals, this could, for example, involve maintenance of diverse plant species with variable tissue quality (likely boosting POM- and MAOM-C116), instead of only promoting plant species with high-quality tissues (mainly boosting MAOM-C). In this context, we specifically see the need to more strongly address subsoils, whose large volume and C-saturation deficit enables additional storage of C in both POM and MAOM via rhizodeposits. We also believe a systems approach that aims to build both POM and MAOM is complementary to separate efforts to manage agricultural soils more holistically117, e.g., to boost soil health, biodiversity, nutrient-availability, and crop performance118–121.
A systems approach could also help develop sounder policies and standards for C-farming, which to date have not considered the fact that multiple C pools exist in soil122. More specifically, monitoring of C accrual (or maintenance) in both POM and MAOM is clearly needed to evaluate the “permanence” of soil C pools in response to C-farming schemes. We also advocate for allocating C-credits for accrual of C in POM, despite its potential lability, particularly in certain environmental contexts (Fig. 1); proper management could maintain large POM pools, like thick organic horizons, for decades to centuries, and even short-term C sinks entail measurable climate benefits123. We also believe that C-farming schemes will be more effectively implemented and subsequently improved by widespread and standardized assessment of POM and MAOM stocks, and environmental factors, as detailed in our systems approach124, both before and after implementation of C-farming strategies. Such data could, for example, reduce the risk of C loss and financial uncertainty for farmers, and help researchers test and improve newer models of SOM dynamics that explicitly incorporate POM and MAOM33,125–128 (and are thus more useful for informing soil management that is POM-and-MAOM-centric). To this end, fast and cost-effective approaches to quantify POM and MAOM, particularly simplified fractionation schemes129, are required for optimizing C-farming frameworks.
Collectively, we advocate for a more holistic view on SOM pools and their context-dependent formation and interactions; such a view will help to improve the efficiency of soil management for C sequestration and help recalibrate SOM research to be less narrowly focused on MAOM. Our systems approach (Fig. 1) enables the alignment of management strategies with the complexity of soils, which will be key to unlock and maintain them as a sustainable C sink.
Acknowledgements
G.A. gratefully acknowledges the support by the Deutsche Forschungsgemeinschaft (DFG—German Research Foundation)—grant no. AN 1706/2-1. C.W.M. thanks the Independent Research Fund Denmark for the funding received for the project: “Limiting N2O emission from hot spots in Danish agricultural soils – linking crop roots and nitrate dynamics to develop new strategies to mitigate trace gasses” (# 0217-00322B) in the frame of the Danish green transition program. C.V. was supported by the DFG, under grant VO738 2111/4-1. K.E.M. and M.J.C. were supported by USDA AFRI grant number 2019-67019-29404. We thank Gabriele Rada for support in revising Fig. 1.
Author contributions
G.A. and C.W.M. conceived of the idea of the perspective, which was critically discussed among G.A., K.E.M., M.J.C., C.V., M.W., and C.W.M; G.A. drafted the manuscript, which was, including all subsequent versions, jointly revised by G.A., K.E.M., M.J.C., C.V., M.W., and C.W.M. under the lead of G.A. and K.E.M.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Golchin A, Oades JM, Skjemstad JO, Clarke P. Soil structure and carbon cycling. Aust. J. Soil Res. 1994;32:1043. doi: 10.1071/SR9941043. [DOI] [Google Scholar]
- 2.Elliott ET, Cambardella CA. Physical separation of soil organic matter. Agric. Ecosyst. Environ. 1991;34:407–419. doi: 10.1016/0167-8809(91)90124-G. [DOI] [Google Scholar]
- 3.Beudert G, Kögel-Knabner I, Zech W. Micromorphological, wet-chemical and 13C NMR spectroscopic characterization of density fractionated forest soils. Sci. Total Environ. 1989;81–82:401–408. doi: 10.1016/0048-9697(89)90148-4. [DOI] [Google Scholar]
- 4.Lavallee JM, Soong JL, Cotrufo MF. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020;26:261–273. doi: 10.1111/gcb.14859. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann J, Kleber M. The contentious nature of soil organic matter. Nature. 2015;528:60–68. doi: 10.1038/nature16069. [DOI] [PubMed] [Google Scholar]
- 6.von Lützow M, et al. SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007;39:2183–2207. doi: 10.1016/j.soilbio.2007.03.007. [DOI] [Google Scholar]
- 7.Mueller CW, et al. Initial differentiation of vertical soil organic matter distribution and composition under juvenile beech (Fagus sylvatica L.) trees. Plant Soil. 2009;323:111–123. doi: 10.1007/s11104-009-9932-1. [DOI] [Google Scholar]
- 8.Bol R, Poirier N, Balesdent J, Gleixner G. Molecular turnover time of soil organic matter in particle-size fractions of an arable soil. Rapid Commun. Mass Spectrom. 2009;23:2551–2558. doi: 10.1002/rcm.4124. [DOI] [PubMed] [Google Scholar]
- 9.Totsche, K. U. et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 1–33. 10.1002/jpln.201600451 (2018).
- 10.Lehmann J, et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020;13:529–534. doi: 10.1038/s41561-020-0612-3. [DOI] [Google Scholar]
- 11.Kleber, M. et al. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 10.1038/s43017-021-00162-y (2021).
- 12.Jilling A, et al. Minerals in the rhizosphere: overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry. 2018;139:103–122. doi: 10.1007/s10533-018-0459-5. [DOI] [Google Scholar]
- 13.Castellano MJ, Mueller KE, Olk DC, Sawyer JE, Six J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 2015;21:3200–3209. doi: 10.1111/gcb.12982. [DOI] [PubMed] [Google Scholar]
- 14.Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013;19:988–995. doi: 10.1111/gcb.12113. [DOI] [PubMed] [Google Scholar]
- 15.Miltner A, Kindler R, Knicker H, Richnow HH, Kästner M. Fate of microbial biomass-derived amino acids in soil and their contribution to soil organic matter. Org. Geochem. 2009;40:978–985. doi: 10.1016/j.orggeochem.2009.06.008. [DOI] [Google Scholar]
- 16.Angst G, Mueller KE, Nierop KGJ, Simpson MJ. Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol. Biochem. 2021;156:108189. doi: 10.1016/j.soilbio.2021.108189. [DOI] [Google Scholar]
- 17.Kallenbach CM, Grandy AS, Frey SD, Diefendorf AF. Microbial physiology and necromass regulate agricultural soil carbon accumulation. Soil Biol. Biochem. 2015;91:279–290. doi: 10.1016/j.soilbio.2015.09.005. [DOI] [Google Scholar]
- 18.Cotrufo MF, Haddix ML, Kroeger ME, Stewart CE. The role of plant input physical-chemical properties, and microbial and soil chemical diversity on the formation of particulate and mineral-associated organic matter. Soil Biol. Biochem. 2022;168:108648. doi: 10.1016/j.soilbio.2022.108648. [DOI] [Google Scholar]
- 19.Haddix ML, et al. Climate, carbon content, and soil texture control the independent formation and persistence of particulate and mineral-associated organic matter in soil. Geoderma. 2020;363:114160. doi: 10.1016/j.geoderma.2019.114160. [DOI] [Google Scholar]
- 20.Witzgall K, et al. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021;12:4115. doi: 10.1038/s41467-021-24192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu W, Huang W, Weintraub-Leff SR, Hall SJ. Where and why do particulate organic matter (POM) and mineral-associated organic matter (MAOM) differ among diverse soils? Soil Biol. Biochem. 2022;172:108756. doi: 10.1016/j.soilbio.2022.108756. [DOI] [Google Scholar]
- 22.Schlüter S, et al. Microscale carbon distribution around pores and particulate organic matter varies with soil moisture regime. Nat. Commun. 2022;13:2098. doi: 10.1038/s41467-022-29605-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal A, et al. Visualizing the transfer of organic matter from decaying plant residues to soil mineral surfaces controlled by microorganisms. Soil Biol. Biochem. 2021;160:108347. doi: 10.1016/j.soilbio.2021.108347. [DOI] [Google Scholar]
- 24.Derrien D, et al. Current controversies on mechanisms controlling soil carbon storage: implications for interactions with practitioners and policy-makers. A review. Agron. Sustain. Dev. 2023;43:21. doi: 10.1007/s13593-023-00876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong H, Simpson AJ, Paul EA, Simpson MJ. Land-use change and environmental properties alter the quantity and molecular composition of soil-derived dissolved organic matter. ACS Earth Sp. Chem. 2021;5:1395–1406. doi: 10.1021/acsearthspacechem.1c00033. [DOI] [Google Scholar]
- 26.Guo Z, et al. Soil dissolved organic carbon in terrestrial ecosystems: global budget, spatial distribution and controls. Glob. Ecol. Biogeogr. 2020;29:2159–2175. doi: 10.1111/geb.13186. [DOI] [Google Scholar]
- 27.Filep T, Zacháry D, Jakab G, Szalai Z. Chemical composition of labile carbon fractions in Hungarian forest soils: Insight into biogeochemical coupling between DOM and POM. Geoderma. 2022;419:115867. doi: 10.1016/j.geoderma.2022.115867. [DOI] [Google Scholar]
- 28.Córdova SC, et al. Plant litter quality affects the accumulation rate, composition, and stability of mineral-associated soil organic matter. Soil Biol. Biochem. 2018;125:115–124. doi: 10.1016/j.soilbio.2018.07.010. [DOI] [Google Scholar]
- 29.Mueller CW, Koegel-Knabner I. Soil organic carbon stocks, distribution, and composition affected by historic land use changes on adjacent sites. Biol. Fertil. Soils. 2009;45:347–359. doi: 10.1007/s00374-008-0336-9. [DOI] [Google Scholar]
- 30.Angst G, et al. Soil organic carbon stocks in topsoil and subsoil controlled by parent material, carbon input in the rhizosphere, and microbial-derived compounds. Soil Biol. Biochem. 2018;122:19–30. doi: 10.1016/j.soilbio.2018.03.026. [DOI] [Google Scholar]
- 31.Filley TR, Boutton TW, Liao JD, Jastrow JD, Gamblin DE. Chemical changes to nonaggregated particulate soil organic matter following grassland-to-woodland transition in a subtropical savanna. J. Geophys. Res. Biogeosciences. 2008;113:G03009. doi: 10.1029/2007JG000564. [DOI] [Google Scholar]
- 32.Cotrufo, M. F. & Lavallee, J. M. Chapter One - Soil organic matter formation, persistence, and functioning: a synthesis of current understanding to inform its conservation and regeneration. in (ed. Sparks, D. L.) vol. 172 1–66 (Academic Press, 2022).
- 33.Robertson, A. D. et al. Unifying soil organic matter formation and persistence frameworks: the MEMS model. Biogeosciences Discuss. 1–36. 10.5194/bg-2018-430 (2018).
- 34.Man, M. et al. Twenty years of litter manipulation reveals that above-ground litter quantity and quality controls soil organic matter molecular composition. Biogeochemistry10.1007/s10533-022-00934-8 (2022).
- 35.Hassink J. The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil. 1997;191:77–87. doi: 10.1023/A:1004213929699. [DOI] [Google Scholar]
- 36.Hassink J, Whitmore AP. A model of the physical protection of organic matter in soils. Soil Sci. Soc. Am. J. 1997;61:131–139. doi: 10.2136/sssaj1997.03615995006100010020x. [DOI] [Google Scholar]
- 37.Six J, Conant RT, Paul EA, Paustian K. Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil. 2002;241:155–176. doi: 10.1023/A:1016125726789. [DOI] [Google Scholar]
- 38.Samson M-É, Chantigny MH, Vanasse A, Menasseri-Aubry S, Angers DA. Coarse mineral-associated organic matter is a pivotal fraction for SOM formation and is sensitive to the quality of organic inputs. Soil Biol. Biochem. 2020;149:107935. doi: 10.1016/j.soilbio.2020.107935. [DOI] [Google Scholar]
- 39.Li M, Meador T, Sauheitl L, Guggenberger G, Angst G. Geoderma Substrate quality effects on stabilized soil carbon reverse with depth. Geoderma. 2022;406:115511. doi: 10.1016/j.geoderma.2021.115511. [DOI] [Google Scholar]
- 40.Vogel C, et al. Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils. Nat. Commun. 2014;5:2947. doi: 10.1038/ncomms3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart C, Paustian K, Conant R, Plante A, Six J. Soil carbon saturation: concept, evidence and evaluation. Biogeochemistry. 2007;86:19–31. doi: 10.1007/s10533-007-9140-0. [DOI] [Google Scholar]
- 42.Rodrigues LAT, et al. Carbon sequestration capacity in no-till soil decreases in the long-term due to saturation of fine silt plus clay-size fraction. Geoderma. 2022;412:115711. doi: 10.1016/j.geoderma.2022.115711. [DOI] [Google Scholar]
- 43.Schweizer SA, Mueller CW, Höschen C, Ivanov P, Kögel-Knabner I. The role of clay content and mineral surface area for soil organic carbon storage in an arable toposequence. Biogeochemistry. 2021;156:401–420. doi: 10.1007/s10533-021-00850-3. [DOI] [Google Scholar]
- 44.Mazzilli SR, Kemanian AR, Ernst OR, Jackson RB, Piñeiro G. Priming of soil organic carbon decomposition induced by corn compared to soybean crops. Soil Biol. Biochem. 2014;75:273–281. doi: 10.1016/j.soilbio.2014.04.005. [DOI] [Google Scholar]
- 45.Vos C, Jaconi A, Jacobs A, Don A. Hot regions of labile and stable soil organic carbon in Germany–Spatial variability and driving factors. Soil. 2018;4:153–167. doi: 10.5194/soil-4-153-2018. [DOI] [Google Scholar]
- 46.Steffens M, Kölbl A, Kögel-Knabner I. Alteration of soil organic matter pools and aggregation in semi-arid steppe topsoils as driven by organic matter input. Eur. J. Soil Sci. 2009;60:198–212. doi: 10.1111/j.1365-2389.2008.01104.x. [DOI] [Google Scholar]
- 47.Stewart CE, Plante AF, Paustian K, Conant RT, Six J. Soil carbon saturation: linking concept and measurable carbon pools. Soil Sci. Soc. Am. J. 2008;72:379–392. doi: 10.2136/sssaj2007.0104. [DOI] [Google Scholar]
- 48.Georgiou K, et al. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 2022;13:3797. doi: 10.1038/s41467-022-31540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen S, et al. Fine resolution map of top- and subsoil carbon sequestration potential in France. Sci. Total Environ. 2018;630:389–400. doi: 10.1016/j.scitotenv.2018.02.209. [DOI] [PubMed] [Google Scholar]
- 50.Keiluweit M, et al. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 2015;5:588–595. doi: 10.1038/nclimate2580. [DOI] [Google Scholar]
- 51.Chari NR, Taylor BN. Soil organic matter formation and loss are mediated by root exudates in a temperate forest. Nat. Geosci. 2022;15:1011–1016. doi: 10.1038/s41561-022-01079-x. [DOI] [Google Scholar]
- 52.Mayer M, et al. Influence of forest management activities on soil organic carbon stocks: a knowledge synthesis. Ecol. Manag. 2020;466:118127. doi: 10.1016/j.foreco.2020.118127. [DOI] [Google Scholar]
- 53.Ortiz CA, Lundblad M, Lundström A, Stendahl J. The effect of increased extraction of forest harvest residues on soil organic carbon accumulation in Sweden. Biomass-. Bioenergy. 2014;70:230–238. doi: 10.1016/j.biombioe.2014.08.030. [DOI] [Google Scholar]
- 54.Singh S, et al. Soil organic carbon and aggregation in response to thirty-nine years of tillage management in the southeastern US. Soil Tillage Res. 2020;197:104523. doi: 10.1016/j.still.2019.104523. [DOI] [Google Scholar]
- 55.Almagro M, Garcia-Franco N, Martínez-Mena M. The potential of reducing tillage frequency and incorporating plant residues as a strategy for climate change mitigation in semiarid Mediterranean agroecosystems. Agric. Ecosyst. Environ. 2017;246:210–220. doi: 10.1016/j.agee.2017.05.016. [DOI] [Google Scholar]
- 56.Cates AM, Ruark MD, Hedtcke JL, Posner JL. Long-term tillage, rotation and perennialization effects on particulate and aggregate soil organic matter. Soil Tillage Res. 2016;155:371–380. doi: 10.1016/j.still.2015.09.008. [DOI] [Google Scholar]
- 57.Liang C, Schimel JP, Jastrow JD. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017;2:1–6. doi: 10.1038/nmicrobiol.2017.105. [DOI] [PubMed] [Google Scholar]
- 58.Angst G, et al. Earthworms as catalysts in the formation and stabilization of soil microbial necromass. Glob. Chang. Biol. 2022;28:4775–4782. doi: 10.1111/gcb.16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prescott CE, Rui Y, Cotrufo MF, Grayston SJ. Managing plant surplus carbon to generate soil organic matter in regenerative agriculture. J. Soil Water Conserv. 2021;76:99A–104A. doi: 10.2489/jswc.2021.0920A. [DOI] [Google Scholar]
- 60.Lange M, et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015;6:6707. doi: 10.1038/ncomms7707. [DOI] [PubMed] [Google Scholar]
- 61.Smith J, et al. Projected changes in mineral soil carbon of European croplands and grasslands, 1990–2080. Glob. Chang. Biol. 2005;11:2141–2152. doi: 10.1111/j.1365-2486.2005.001075.x. [DOI] [PubMed] [Google Scholar]
- 62.Thoms C, Gleixner G. Seasonal differences in tree species’ influence on soil microbial communities. Soil Biol. Biochem. 2013;66:239–248. doi: 10.1016/j.soilbio.2013.05.018. [DOI] [Google Scholar]
- 63.Schimel DS, et al. Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils. Glob. Biogeochem. Cycles. 1994;8:279. doi: 10.1029/94GB00993. [DOI] [Google Scholar]
- 64.Kaiser K, et al. Driving forces of soil bacterial community structure, diversity, and function in temperate grasslands and forests. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep33696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosier S, et al. Adaptive multi-paddock grazing enhances soil carbon and nitrogen stocks and stabilization through mineral association in southeastern U.S. grazing lands. J. Environ. Manag. 2021;288:112409. doi: 10.1016/j.jenvman.2021.112409. [DOI] [PubMed] [Google Scholar]
- 66.Kristensen JA, Svenning J-C, Georgiou K, Malhi Y. Can large herbivores enhance ecosystem carbon persistence? Trends Ecol. Evol. 2022;37:117–128. doi: 10.1016/j.tree.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Eisenhauer, N. et al. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass OPEN. Nat. Publ. Gr. 10.1038/srep44641 (2017). [DOI] [PMC free article] [PubMed]
- 68.Rui Y, et al. Persistent soil carbon enhanced in Mollisols by well-managed grasslands but not annual grain or dairy forage cropping systems. Proc. Natl Acad. Sci. 2022;119:e2118931119. doi: 10.1073/pnas.2118931119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kögel-Knabner I, Amelung W. Soil organic matter in major pedogenic soil groups. Geoderma. 2021;384:114785. doi: 10.1016/j.geoderma.2020.114785. [DOI] [Google Scholar]
- 70.Liang C, Amelung W, Lehmann J, Kästner M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019;25:3578–3590. doi: 10.1111/gcb.14781. [DOI] [PubMed] [Google Scholar]
- 71.Lugato E, Lavallee JM, Haddix ML, Panagos P, Cotrufo MF. Different climate sensitivity of particulate and mineral-associated soil organic matter. Nat. Geo. 2021;14:295–300. doi: 10.1038/s41561-021-00744-x. [DOI] [Google Scholar]
- 72.Sokol NW, et al. Global distribution, formation and fate of mineral-associated soil organic matter under a changing climate: a trait-based perspective. Funct. Ecol. 2022;36:1411–1429. doi: 10.1111/1365-2435.14040. [DOI] [Google Scholar]
- 73.van der Pol LK, et al. Addressing the soil carbon dilemma: Legumes in intensified rotations regenerate soil carbon while maintaining yields in semi-arid dryland wheat farms. Agric. Ecosyst. Environ. 2022;330:107906. doi: 10.1016/j.agee.2022.107906. [DOI] [Google Scholar]
- 74.Aulakh R, Bueno C, Kreuzwieser J, Rennenberg, H MW. Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol. 2001;3:139–148. doi: 10.1055/s-2001-12905. [DOI] [Google Scholar]
- 75.Whitbread A, Blair G, Konboon Y, Lefroy R, Naklang K. Managing crop residues, fertilizers and leaf litters to improve soil C, nutrient balances, and the grain yield of rice and wheat cropping systems in Thailand and Australia. Agric. Ecosyst. Environ. 2003;100:251–263. doi: 10.1016/S0167-8809(03)00189-0. [DOI] [Google Scholar]
- 76.Schlesinger, W. H. Biogeochemical constraints on climate change mitigation through regenerative farming. Biogeochemistry10.1007/s10533-022-00942-8 (2022).
- 77.Romero CM, et al. Tillage-residues affect mineral-associated organic matter on Vertisols in northern Mexico. Geoderma Reg. 2021;27:e00430. doi: 10.1016/j.geodrs.2021.e00430. [DOI] [Google Scholar]
- 78.Grüneberg E, Ziche D, Wellbrock N. Organic carbon stocks and sequestration rates of forest soils in Germany. Glob. Chang. Biol. 2014;20:2644–2662. doi: 10.1111/gcb.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frey SD. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 2019;50:237–259. doi: 10.1146/annurev-ecolsys-110617-062331. [DOI] [Google Scholar]
- 80.Wu Y, et al. Global patterns in mycorrhizal mediation of soil carbon storage, stability, and nitrogen demand: a meta-analysis. Soil Biol. Biochem. 2022;166:108578. doi: 10.1016/j.soilbio.2022.108578. [DOI] [Google Scholar]
- 81.Reich PB, et al. Linking litter calcium, earthworms and soil properties: a common garden test with 14 tree species. Ecol. Lett. 2005;8:811–818. doi: 10.1111/j.1461-0248.2005.00779.x. [DOI] [Google Scholar]
- 82.Almeida LFJ, et al. Forest litter constraints on the pathways controlling soil organic matter formation. Soil Biol. Biochem. 2021;163:108447. doi: 10.1016/j.soilbio.2021.108447. [DOI] [Google Scholar]
- 83.Cyle KT, et al. Substrate quality influences organic matter accumulation in the soil silt and clay fraction. Soil Biol. Biochem. 2016;103:138–148. doi: 10.1016/j.soilbio.2016.08.014. [DOI] [Google Scholar]
- 84.Angst, G. et al. Soil organic carbon stability in forests: distinct effects of tree species identity and traits. Glob. Chang. Biol. 14548. 10.1111/gcb.14548 (2019). [DOI] [PubMed]
- 85.Mueller KE, et al. Tree species effects on coupled cycles of carbon, nitrogen, and acidity in mineral soils at a common garden experiment. Biogeochemistry. 2012;111:601–614. doi: 10.1007/s10533-011-9695-7. [DOI] [Google Scholar]
- 86.Blume, H.-P. et al. Soil Science. (Springer Berlin Heidelberg, 2015).
- 87.Steffens C, Beer C, Schelfhout S, Vesterdal L. Tree species affect the vertical distribution of soil organic carbon and total nitrogen. J. Plant Nutr. Soil Sci. 2018;185:864–875. doi: 10.1002/jpln.202200165. [DOI] [Google Scholar]
- 88.Craig ME, et al. Tree mycorrhizal type predicts within-site variability in the storage and distribution of soil organic matter. Glob. Chang. Biol. 2018;24:3317–3330. doi: 10.1111/gcb.14132. [DOI] [PubMed] [Google Scholar]
- 89.Lin G, et al. Mycorrhizal associations of tree species influence soil nitrogen dynamics via effects on soil acid–base chemistry. Glob. Ecol. Biogeogr. 2022;31:168–182. doi: 10.1111/geb.13418. [DOI] [Google Scholar]
- 90.Keller AB, Brzostek ER, Craig ME, Fisher JB, Phillips RP. Root-derived inputs are major contributors to soil carbon in temperate forests, but vary by mycorrhizal type. Ecol. Lett. 2021;24:626–635. doi: 10.1111/ele.13651. [DOI] [PubMed] [Google Scholar]
- 91.Cheeke TE, et al. Dominant mycorrhizal association of trees alters carbon and nutrient cycling by selecting for microbial groups with distinct enzyme function. N. Phytol. 2017;214:432–442. doi: 10.1111/nph.14343. [DOI] [PubMed] [Google Scholar]
- 92.Kalbitz K, Kaiser K. Contribution of dissolved organic matter to carbon storage in forest mineral soils. J. Plant Nutr. Soil Sci. 2008;171:52–60. doi: 10.1002/jpln.200700043. [DOI] [Google Scholar]
- 93.Augusto L, Boča A. Tree functional traits, forest biomass, and tree species diversity interact with site properties to drive forest soil carbon. Nat. Commun. 2022;13:1097. doi: 10.1038/s41467-022-28748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jia Y, et al. Plant and microbial pathways driving plant diversity effects on soil carbon accumulation in subtropical forest. Soil Biol. Biochem. 2021;161:108375. doi: 10.1016/j.soilbio.2021.108375. [DOI] [Google Scholar]
- 95.Pierson D, et al. Mineral stabilization of soil carbon is suppressed by live roots, outweighing influences from litter quality or quantity. Biogeochemistry. 2021;154:433–449. doi: 10.1007/s10533-021-00804-9. [DOI] [Google Scholar]
- 96.Ferreira GWD, et al. Retaining eucalyptus harvest residues promotes different pathways for particulate and mineral-associated organic matter. Ecosphere. 2021;12:e03439. doi: 10.1002/ecs2.3439. [DOI] [Google Scholar]
- 97.Don A, Hagen C, Grüneberg E, Vos C. Simulated wild boar bioturbation increases the stability of forest soil carbon. Biogeosciences. 2019;16:4145–4155. doi: 10.5194/bg-16-4145-2019. [DOI] [Google Scholar]
- 98.Ferlian O, et al. Soil chemistry turned upside down: a meta-analysis of invasive earthworm effects on soil chemical properties. Ecology. 2020;101:1–12. doi: 10.1002/ecy.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Szalai Z, et al. Accelerated soil development due to seasonal water-saturation under hydric conditions. Geoderma. 2021;401:115328. doi: 10.1016/j.geoderma.2021.115328. [DOI] [Google Scholar]
- 100.Noll M, Matthies D, Frenzel P, Derakshani M, Liesack W. Succession of bacterial community structure and diversity in a paddy soil oxygen gradient. Environ. Microbiol. 2005;7:382–395. doi: 10.1111/j.1462-2920.2005.00700.x. [DOI] [PubMed] [Google Scholar]
- 101.Sokol, N. W. et al. Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nat. Rev. Microbiol.10.1038/s41579-022-00695-z (2022). [DOI] [PubMed]
- 102.Mueller CW, et al. Large amounts of labile organic carbon in permafrost soils of northern Alaska. Glob. Chang. Biol. 2015;21:2804–2817. doi: 10.1111/gcb.12876. [DOI] [PubMed] [Google Scholar]
- 103.Hugelius G, et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences. 2014;11:6573–6593. doi: 10.5194/bg-11-6573-2014. [DOI] [Google Scholar]
- 104.Prater I, et al. From fibrous plant residues to mineral-associated organic carbon–the fate of organic matter in Arctic permafrost soils. Biogeosciences. 2020;17:3367–3383. doi: 10.5194/bg-17-3367-2020. [DOI] [Google Scholar]
- 105.Yan D, Wang D, Yang L. Long-term effect of chemical fertilizer, straw, and manure on labile organic matter fractions in a paddy soil. Biol. Fertil. Soils. 2007;44:93–101. doi: 10.1007/s00374-007-0183-0. [DOI] [Google Scholar]
- 106.Buettner SW, Kramer MG, Chadwick OA, Thompson A. Mobilization of colloidal carbon during iron reduction in basaltic soils. Geoderma. 2014;221–222:139–145. doi: 10.1016/j.geoderma.2014.01.012. [DOI] [Google Scholar]
- 107.Thompson A, Chadwick OA, Boman S, Chorover J. Colloid mobilization during soil iron redox oscillations. Environ. Sci. Technol. 2006;40:5743–5749. doi: 10.1021/es061203b. [DOI] [PubMed] [Google Scholar]
- 108.Peplau T, Schroeder J, Gregorich E, Poeplau C. Subarctic soil carbon losses after deforestation for agriculture depend on permafrost abundance. Glob. Chang. Biol. 2022;28:5227–5242. doi: 10.1111/gcb.16307. [DOI] [PubMed] [Google Scholar]
- 109.Huang Y, et al. Tradeoff of CO2 and CH4 emissions from global peatlands under water-table drawdown. Nat. Clim. Chang. 2021;11:618–622. doi: 10.1038/s41558-021-01059-w. [DOI] [Google Scholar]
- 110.Amelung W, et al. Towards a global-scale soil climate mitigation strategy. Nat. Commun. 2020;11:1–10. doi: 10.1038/s41467-020-18887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Angst G, Kögel-Knabner I, Kirfel K, Hertel D, Mueller CW. Spatial distribution and chemical composition of soil organic matter fractions in rhizosphere and non-rhizosphere soil under European beech (Fagus sylvatica L.) Geoderma. 2016;264:179–187. doi: 10.1016/j.geoderma.2015.10.016. [DOI] [Google Scholar]
- 112.Urbanski L, et al. Legacy of plaggen agriculture: high soil organic carbon stocks as result from high carbon input and volume increase. Geoderma. 2022;406:115513. doi: 10.1016/j.geoderma.2021.115513. [DOI] [Google Scholar]
- 113.Ae, S. S. et al. Composition of organic matter in sandy relict and cultivated heathlands as examined by pyrolysis-field ionization MS Abbreviations CLSM Confocal laser scanning microscopy MOC and MN Mineral protected organic C and N OC Organic carbon Py-FIMS Pyrolysis Field Ionization Mass Spectroscopy ROC and RN Recalcitrant organic C and N SOM Soil organic matter. 89, 253–271 (2008).
- 114.Springob G, Kirchmann H. C-rich sandy Ap horizons of specific historical land-use contain large fractions of refractory organic matter. Soil Biol. Biochem. 2002;34:1571–1581. doi: 10.1016/S0038-0717(02)00127-X. [DOI] [Google Scholar]
- 115.Kern J, Giani L, Teixeira W, Lanza G, Glaser B. What can we learn from ancient fertile anthropic soil (Amazonian Dark Earths, shell mounds, Plaggen soil) for soil carbon sequestration? CATENA. 2019;172:104–112. doi: 10.1016/j.catena.2018.08.008. [DOI] [Google Scholar]
- 116.Zhang Z, Kaye JP, Bradley BA, Amsili JP, Suseela V. Cover crop functional types differentially alter the content and composition of soil organic carbon in particulate and mineral-associated fractions. Glob. Chang. Biol. 2022;28:5831–5848. doi: 10.1111/gcb.16296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moinet GYK, Hijbeek R, van Vuuren DP, Giller KE. Carbon for soils, not soils for carbon. Glob. Chang. Biol. 2023;29:2384–2398. doi: 10.1111/gcb.16570. [DOI] [PubMed] [Google Scholar]
- 118.Ha KV, Marschner P, Bünemann EK. Dynamics of C, N, P and microbial community composition in particulate soil organic matter during residue decomposition. Plant Soil. 2008;303:253–264. doi: 10.1007/s11104-007-9504-1. [DOI] [Google Scholar]
- 119.Lehmann J, Bossio DA, Kögel-Knabner I, Rillig MC. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020;1:544–553. doi: 10.1038/s43017-020-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eisenhauer N. Plant diversity effects on soil microorganisms: spatial and temporal heterogeneity of plant inputs increase soil biodiversity. Pedobiologia (Jena.). 2016;59:175–177. doi: 10.1016/j.pedobi.2016.04.004. [DOI] [Google Scholar]
- 121.Jilling A, et al. Rapid and distinct responses of particulate and mineral-associated organic nitrogen to conservation tillage and cover crops. Geoderma. 2020;359:114001. doi: 10.1016/j.geoderma.2019.114001. [DOI] [Google Scholar]
- 122.Commission, E. et al. Setting up and implementing result-based carbon farming mechanisms in the EU: technical guidance handbook. (Publications Office of the European Union, 2021). 10.2834/056153.
- 123.Leifeld J. Carbon farming: Climate change mitigation via non-permanent carbon sinks. J. Environ. Manag. 2023;339:117893. doi: 10.1016/j.jenvman.2023.117893. [DOI] [PubMed] [Google Scholar]
- 124.Oldfield EE, et al. Crediting agricultural soil carbon sequestration. Sci. (80-.). 2022;375:1222–1225. doi: 10.1126/science.abl7991. [DOI] [PubMed] [Google Scholar]
- 125.Pierson D, et al. Optimizing process-based models to predict current and future soil organic carbon stocks at high-resolution. Sci. Rep. 2022;12:10824. doi: 10.1038/s41598-022-14224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abramoff RZ, et al. Improved global-scale predictions of soil carbon stocks with millennial version 2. Soil Biol. Biochem. 2022;164:108466. doi: 10.1016/j.soilbio.2021.108466. [DOI] [Google Scholar]
- 127.Abramoff R, et al. The Millennial model: in search of measurable pools and transformations for modeling soil carbon in the new century. Biogeochemistry. 2018;137:51–71. doi: 10.1007/s10533-017-0409-7. [DOI] [Google Scholar]
- 128.Zhang Y, et al. Simulating measurable ecosystem carbon and nitrogen dynamics with the mechanistically defined MEMS 2.0 model. Biogeosciences. 2021;18:3147–3171. doi: 10.5194/bg-18-3147-2021. [DOI] [Google Scholar]
- 129.Just, C. et al. A simple approach to isolate slow and fast cycling organic carbon fractions in central European soils—importance of dispersion method. Front. Soil Sci. 1, 692583 (2021).