Abstract
Introduction
The primary aim is to estimate the cost-effectiveness of transjugular intrahepatic portosystemic stent shunt (TIPSS) in two indications from a Spanish perspective. Firstly, as pre-emptive treatment for patients with acute variceal bleeding (indication 1) compared with endoscopic band ligation plus drug therapy. Secondly, to treat refractory ascites (indication 2) compared with large volume paracentesis.
Methods
A two-state (alive and dead) Markov model was developed to capture the costs and health impact for the two indications over a 2-year time horizon with monthly cycles. In the alive state, patients could experience adverse event(s), associated with costs and disutility, such as recurrent variceal bleeding, ascites, and hepatic encephalopathy. Discount rates of 3% for utilities and costs and a cost-effectiveness threshold of €25,000 per QALY were applied.
Results
In the base case analysis, TIPSS was estimated to be cost-effective as a pre-emptive treatment for indication 1 (incremental cost and QALYs of − €230 and 0.211, respectively). TIPSS also remained cost-effective (€16,819/QALY) in a conservative scenario analysis, conducted with an alternate source for clinical parameters. The key drivers of the outcomes were survival for the comparator arm, mean band ligation outpatient procedures, and TIPSS treatment costs. TIPSS was estimated to dominate the comparator for indication 2 (incremental cost and QALYs of − €25,687 and 0.531, respectively). The key drivers of the outcomes were monthly paracentesis sessions and cost per inpatient stay for those undergoing paracentesis.
Conclusions
TIPSS is likely to be a cost-effective and a cost-saving treatment in patients with cirrhosis in indications 1 and 2, compared with standard treatments. The analyses estimate clinical benefits along with reduced healthcare costs from avoided downstream resource consumption.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02517-x.
Keywords: Variceal bleeding, Ascites, Cirrhosis, TIPSS, Endoscopic band ligation, Large volume paracentesis, Spain, Cost–utility, QALY
Key Summary Points
| Cirrhosis of the liver can be associated with complications, such as acute variceal bleeding and refractory ascites, which can be life-threatening and/or result in substantial healthcare costs. |
| The economic impact of the use of TIPSS within these two indications has been estimated previously for a UK setting; however, the cost and health impact within a Spanish setting are not well established. |
| The aims of this study were to estimate the health and cost impact of using TIPSS as a treatment for refractory ascites and as a pre-emptive treatment in people with acute variceal bleeding. |
| TIPSS is cost-effective for people with acute variceal bleeding and refractory ascites (incremental cost and QALYs of €230 and 0.21 QALYs in acute variceal bleeding; incremental costs and QALYs of €25,687 and 0.53 QALYs in refractory ascites). |
| The use of TIPSS is associated with health gains and cost savings for both indications considered. This is attributable to resource savings from events such as avoided hospital attendance and reduced adverse events. |
Introduction
Liver disease is a multistage, often chronic, condition that can give rise to various complications. Caused predominantly by alcohol consumption, obesity, and viral hepatitis, the largest burden is observed in Europe [1]. Prolonged damage to the liver often results in the development of cirrhosis, the hallmarks of which include variceal bleeding, ascites, and hepatic encephalopathy (HE) with substantial mortality implications associated with these complications [2]. In the decompensated cirrhotic state, where individuals can suffer from one or more of these hallmarks, median survival is estimated to be 2 years [2].
Acute variceal bleeding (AVB), a life-threatening complication of cirrhosis that leads to significant mortality risk, develops as a result of uncontrolled increased portal hypertension from progressive damage of hepatic tissue. Primary management of AVB aims to restore haemodynamic stability and curb the bleeding from varices [3]. As such, transfusion may be performed for volume replacement and administration of vasoactive agents such as terlipressin and somatostatin to reduce portal pressure. Once individuals are stabilised an endoscopic band ligation (EBL) is performed to confirm and manage the bleeding [3]. However, even with timely treatment, there is an estimated 15–21% risk of treatment failing or rebleeding occurring with mortality as high as 80% [2, 3]. Evidence gathered suggests that, in addition to using expanded polytetrafluoroethylene (ePTFE)-covered stents in transjugular intrahepatic portosystemic stent shunt (TIPSS) for symptom-based treatment, pre-emptive use of TIPSS may lead to improved clinical outcomes [3]. TIPSS is a procedure whereby a passage is created to connect the portal and hepatic veins [4]. One of the primary aims of the procedure is to reduce the portal vein pressure, due to formation of scar tissue within the liver [4], where complications can often occur as a consequence of increased pressure.
Ascites is another commonly occurring complication of decompensated liver cirrhosis and one that is refractory in 5–10% of people (i.e. it is unresponsive to dietary sodium restriction and administration of diuretics) [5]. Liver transplant remains the only curative treatment for refractory ascites (RA); however, interim treatment options include repeat large volume paracentesis (LVP) in combination with albumin, or the use of TIPSS [5]. Whilst the former has low procedural risk it is associated with significant burden, both in terms of patient health and the overall economic cost [6]. Clinical data for TIPSS suggest that it has an improved safety and efficacy profile when compared with LVP [5].
Key clinical trials that explored the effectiveness of TIPSS for RA and as pre-emptive treatment in AVB have been reported in detail in a previous paper by García-Pagán and colleagues [7]. Evidence from randomised control trials (RCTs) support the use of TIPSS for AVB based on improved clinical outcomes such as reduction in cases with uncontrolled bleeding, overall reduction in mortality, as well as a favourable safety profile due to the non-surgical nature of the intervention [8, 9]. Similarly, two RCTs examining individuals with RA have shown that the use of ePTFE-covered stents in TIPSS led to significantly reduced incidences of rebleeding [10]. There was also a demonstrated reduction in mortality compared to standard care [10].
In addition to the extensive negative health impacts, cirrhosis of the liver and the consequent complications are estimated to pose a substantial economic burden to healthcare systems and population health [1]. A previous analysis reported that €142 million was spent on patients with cirrhosis in Catalonia in 2013 alone [11]. This was equivalent to an average spend of €4234 per patient with the cost estimated to vary depending on the morbidity risk categorisation of patients with cirrhosis [11]. Overall expenditure per patient ranged from €874 for low morbidity risk category to €15,876 for very high morbidity risk, where the largest component of the costs in the high-risk category was from hospitalisation [11]. Furthermore, the overall costs for ascites and oesophageal varices were both estimated to be above €9000 annually per patient [11]. A more recent cost-effectiveness study estimated the total per patient cost of treating RA to be €15,360 over 2 years [12]. In a related study, the authors also estimated standard treatment for AVB to cost €7600 per patient over 2 years [13].
Owing to the clinical benefits described previously, TIPSS is already in use within the Spanish healthcare system. However, whilst the use of TIPSS was found to be cost saving and cost-effective in the UK setting in patients with AVB as a pre-emptive treatment and in patients with RA, to the best of the authors’ knowledge, a formal cost-effectiveness analysis has not been completed from a Spanish perspective [14]. The only relevant study identified is from Pérez-Mitru and colleagues who published an abstract which reported cost savings from the use of TIPSS for RA, compared with LVP, within the Spanish setting [12]. Therefore, the aim of this work was to assess the cost-effectiveness of TIPSS with a covered stent, as opposed to the traditional metal stent, for use in two populations: those with AVB and RA, compared with standard care, from a Spanish perspective. The previously developed UK cost-effectiveness models for these two indications were adapted for this purpose [14].
Methods
Cost–utility analyses were conducted, based on the existing UK models [14], exploring the use of TIPSS in two indications:
Indication 1—patients with cirrhosis and haemodynamically stable AVB, following first-line treatment with EBL and drug therapy. The comparator was continued EBL and drug therapy.
Indication 2—patients with cirrhosis and RA and the comparator was LVP plus albumin.
The overall modelling approach that was adopted for the UK analyses, including model structure, population, key assumptions, and parameter values, was validated with three Spanish clinical experts via questionnaires and interviews. It was agreed that, in general, the modelling approach adopted for the UK was applicable to the Spanish setting. Therefore, no major changes were required to the model structure or population. However, updates were required to reflect differences in parameter values for the model to be applicable to a Spanish population and setting, either for the base case analysis or as scenario analyses. These updates included treatment efficacy data used (e.g. survival rates), resource use (e.g. healthcare staff involved and frequency of treatment sessions), and unit costs.
Health benefits were captured using the quality-adjusted life-year (QALY), which is a generic measurement of patient quality of life. The incremental cost-effectiveness ratio (ICER) was also estimated and compared to a willingness to pay threshold of €25,000 to determine whether TIPSS was cost-effective (i.e. an ICER of less than €25,000 per QALY indicates cost-effectiveness).
The willingness to pay value was based on a study by Vallejo-Torres and colleagues who reported €25,000 per QALY as the threshold used most commonly for economic evaluations within a Spanish setting [15]. A healthcare perspective was adopted for the model and a 2-year time horizon applied. A 3% discount rate was applied to both costs and QALYs [16].
Model Structure
A two-state Markov model was developed comprising alive and dead health states. Whilst in the alive state individuals could experience multiple adverse events (AEs), either as a consequence of the intervention or from the underlying disease. These included rebleeding, recurrent ascites, HE, and shunt dysfunction. The last of these was limited to the TIPSS intervention group. A full description of the model structure, including the relevant populations for each AE applicable, can be found in Mattock et al. [14]. For the TIPSS cohort, prior to entering the model, individuals were assigned to either the TIPSS or standard care downstream treatment pathway depending on if TIPSS resulted in technical success or failure, respectively.
Model Population
For indication 1 (AVB), clinical experts agreed that in those who were haemodynamically stable, following an initial management with EBL and beta blockers, pre-emptive TIPSS would be administered within 72 h of the index bleed. Additionally, TIPSS should be targeted at patients who are at high risk of variceal bleeding (i.e. Child C 10—13 points and Child B with active bleeding at the time of endoscopy). The standard of care with EBL in combination with beta-blockers was considered appropriate with multiple EBL sessions followed by monitoring appointments to check for reappearance of varices, although differences in frequency of resource use were noted by clinical experts.
For indication 2 (RA), clinical expert opinion agreed TIPSS would be administered following initial treatment with LVP that had failed to manage ascites. For the comparator arm individuals would continue to receive multiple LVPs. Whilst expert opinion stated that time between each LVP session can vary between individuals (e.g. once per week or two sessions every 3 weeks), it was agreed that a duration of 2 weeks was applicable for the Spanish setting.
Model Inputs
The inputs, and therefore the analysis, is informed by previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Clinical Parameters
The models were predominately informed by two RCTs that were judged to be the most robust source of relevant data on the effectiveness of TIPSS in the two indications of interest. A study by García-Pagán (2010) explored the clinical impact of earlier use of TIPSS in patients with cirrhosis and AVB when compared with patients who received EBL therapy alone [8]. The study reported 12-month survival for patients treated with TIPSS and EBL, estimated to be 86% and 61%, respectively, with no additional mortality reported from 12 to 24 months [8]. AE risks were also sourced from this study.
Bureau (2017) explored the impact of TIPSS compared with LVP in patients with cirrhosis and recurrent ascites. One-year survival curves were reported in which these data were extrapolated using a lognormal distribution to estimate survival probabilities at 24 months (see Mattock et al. [14] supplementary material for full details).
Based on these curves, 12-month survival was estimated to be 93% and 55% for TIPSS and LVP, respectively. The extrapolated 24-month survival was estimated to be 89% and 24% for TIPSS and LVP, respectively.
See Table 1 for the full list of clinical parameters, respective values, and sources for both indications.
Table 1.
Summary of the clinical parameters applied in the economic models
| Indication 1: AVB | Indication 2: RA | |||||
|---|---|---|---|---|---|---|
| TIPSS | EBL | Source | TIPSS | LVP | Source | |
| Survival at 12 months | 0.860 | 0.610 | García-Pagán et al. [8] | 0.928 | 0.553 | Bureau et al. [22] |
| Survival at 24 months | 0.860a | 0.610a | 0.890 | 0.241 | ||
| TIPSS technical success | 0.980 | NA | Perelló et al. [26] | 0.980 | NA | Perelló et al. [26] |
| Shunt dysfunction | 0.070 | NA | Lv et al. [27] | 0.030 | NA | Bureau et al. [22] |
| Recurrent VB at 12 months | 0.030 | 0.500 | García-Pagán et al. [8] | 0.044c | 0.182 | Shen et al. [28] |
| Recurrent VB at 24 months | 0.030b | 0.500b | 0.044 | 0.182 | Assumed same as 12 months | |
| Ascites at 24 months | 0.130 | 0.330 | 0.510 | 1.000 | Bai et al. [29] | |
| HE at 12 months | 0.280 | 0.400 | 0.345 | 0.333 | Bureau et al. [22] | |
| % HE severe | 0.250 | 0.250 | Lv et al. [27] | 0.400 | 0.636 | |
| SBP at 12 months | NA | NA | NA | NA | 0.060 | |
a24-month survival assumed to be same as 12 months based on survival curves in García-Pagán et al. [8]
b24-month recurrence assumed to be same as 12 months based on data extrapolated in García-Pagán et al. [8]
cBased on rate reported for variceal bleeding which was assumed to be applicable for RA, as used in Shen et al. [28]
The model structure and inputs were validated with three clinical experts currently practising in Spain. A recently published meta-analysis by Nicoară-Farcău [17] was referenced by one expert during this validation process. In this study, data from three RCTs and four observational studies were pooled to obtain estimates for survival and AEs such as rebleeding, HE, and recurrent ascites. The study estimated reduced survival with TIPSS (79%) and marginally increased survival with EBL (62%) compared with the García-Pagán (2010) study. The study also estimated a higher risk of HE and recurrent variceal bleeding with TIPSS than that reported by García-Pagán [8].
Healthcare Resource Use and Costs
As described by Mattock et al., the resource use for each indication was informed by UK clinical guidelines and interviews with three UK clinical experts. For the current analysis, these resource use data were presented to three Spanish clinical experts who validated the values from a Spanish healthcare perspective. It was agreed that resource use patterns are similar across the two settings and, therefore, it was appropriate to apply the resource use data in the Spanish models. However, there were certain deviations across the two settings with the key amendments listed below:
5% of initial implantations were assumed to be elective cases for indication 1 (AVB), compared with 0% in the UK models.
Before TIPSS implantation, 80% were assumed to have an abdominal computerised tomography (CT) scan, 50% underwent an echocardiogram (ECHO), and 0% were assumed to have an electrocardiogram (ECG).
Nadolol was assumed to not be used in Spain as part of pharmaceutical treatment with EBL.
Endoscopy was used to confirm bleeding rather than angiography in the Spanish setting.
Additional pharmacotherapies such as somatostatin and octreotide were included, alongside terlipressin acetate.
Table 2 summarises the resource use and costs applied for primary treatments in both indications. In addition to the initial cost of providing TIPSS, patients undergoing TIPSS procedure for indication 1, in usual practice, are monitored to evaluate TIPSS patency using Doppler ultrasound evaluation according to expert opinion. The cost of a Doppler ultrasound was estimated to be €67 [18]. No appointment costs were applied in the model as the ultrasound was assumed to be conducted as part of hepatocellular screening.
Table 2.
Summary of the primary treatment costs applied in the economic models
| Units | Total costs* | Source | |
|---|---|---|---|
| TIPSS procedure | |||
| Indication 1 (AVB) | |||
| Elective | 0.05 | €5851.99 | KOLs; [18, 30, 31] |
| Non-elective | 0.95 | €7615.99 | |
| Total | €7527.79 | ||
| Indication 2 (RA) | |||
| Elective | 1.00 | €5851.99 | KOLs; [18, 30, 31] |
| Non-elective | 0.00 | €7615.99 | |
| Total | €5851.99 | ||
| Standard care: indication 1 (AVB) | |||
| Outpatient EBL (months 1–2) | 4.00 | €4689.30 | KOLs; [32, 33] |
| Outpatient EBL (months 3–24) | 3.00 | ||
| Pharmaceuticals (per month) | Various** | €7.79 | KOLs; [33] |
| Total costs (24-month survival)*** | €4750.80 | ||
| Standard care: indication 2 (RA) | |||
| LVP per procedure | 2.17 | €2430.49 | KOLs; [18, 30, 33] |
| Total cost (24-month survival)*** | €58,331.68 | ||
KOL key opinion leaders
*See supplementary material for breakdown of AE costs
**Carvedilol (25% of population applied to) and propranolol (75% of population applied to)
***Undiscounted total 24-month cost estimates
The costs of AE management are presented in Table 3.
Table 3.
Disutilities and costs for the adverse events included in the economic models
| Total cost* | Utility | Source (utility) | |
|---|---|---|---|
| TIPSS procedure (initial implant) | Reported in Table 1 | 0.15 | Wechowski et al. [34] |
| Variceal bleeding | |||
| TIPSS (indications 1 and 2) | €4278.51 | 0.15 | Wechowski et al. [34] |
| Standard care (indication 1) | €8556.06 | 0.15 | |
| Standard care (indication 2) | €4278.51 | 0.15 | |
| Shunt dysfunction (indication 1) | €3192.29 | 0.15 | |
| Shunt dysfunction (indication 2) | €3888.26 | 0.15 | |
| Hepatic encephalopathy (mild) | €1253.54 | 0.07 | Kwan [35] |
| Hepatic encephalopathy (severe) | €1253.54 | 0.13 | |
| Ascites | |||
| TIPSS and standard care (indication 1) | €668.48 | 0.13 | Mosucci et al. [20] |
| TIPSS (indication 2) | €1120.04 | 0.13 | |
| Spontaneous bacteria peritonitis* | €2752.50 | 0.12 | Badia et al. [36] |
*See supplementary material for breakdown of AE costs
Utilities
Baseline utility was sourced for patients with cirrhosis. For indication 1, patients in the alive state were assigned a baseline utility of 0.62. This was calculated by taking the weighted average of the utility for patients with chronic liver disease and a Child–Pugh score of 2 (0.67) and Child–Pugh score of 3 (0.56) [19]. The weighting was based on the ratio of patients within the García-Pagán trial [7] (32:30 for Child–Pugh 2 to Child–Pugh 3).
For indication 2, patients in the alive state were assigned a baseline health utility of 0.65 [20], representing patients with cirrhosis without ascites. This was derived as the arithmetic mean across eight domains reported in Fig. 2 of the Moscucci et al. study [20].
Disutilities were applied on the basis of the treatment patients underwent and from the occurrence of any AEs. All disutilities were applied for the full cycle except for disutility relating to TIPSS and shunt dysfunction requiring re-intervention. In these cases, disutility was only applicable to the intraoperative and postoperative period (i.e. equal to the length of stay per procedure). See Table 3 for the AE-specific disutility scores applied in the model.
Model Analyses
As described previously, one Spanish expert suggested the Nicoară-Farcău analysis [17] may be a source of clinical inputs for indication 1. However, as a result of limitations in the analysis, this was considered to be less appropriate than the García-Pagán study [8]. One major limitation of the meta-analysis, within the context of this decision problem, relates to the inclusion of observational studies, in addition to the RCTs. Therefore, given the propensity for an increased risk of bias associated with observational studies (e.g. due to lack of blinding and randomisation across study arms) it was judged that the evidence from this meta-analysis may be less robust than RCT evidence alone. Additionally, within the analysis, outcomes were combined from studies comparing the use of covered and bare stents with EBL. There is strong evidence to support that bare stents are associated with lower effectiveness than covered stents [21]. Therefore, the inclusion of studies focusing on bare stents is likely to bias the outcomes for TIPSS. Nevertheless, given the benefits of pooling data (e.g. increased statistical power and improved estimates), the impact of utilising the more conservative estimates from the Nicoară-Farcău [17] study was explored through a scenario analysis for indication 1.
In addition to the scenario analysis, uncertainty in the model parameters was characterised through deterministic and probabilistic sensitivity analyses (DSA and PSA). All relevant parameters were included in the DSA with the upper and lower values informed by confidence intervals, as reported in the sources. Where this was not possible, 15% of the mean was used to estimate the upper and lower values. The results of the analysis were plotted as a tornado diagram.
PSA was also conducted whereby 10,000 model iterations were run to estimate the average costs and QALYs associated with the intervention and comparator for both indications. The alpha and beta values were estimated using 15% of the mean if standard error was not reported within the studies. The results were plotted on cost-effectiveness planes.
Results
The results of the base case analysis for both indications, and the scenario analysis for indication 1, are presented in Table 4. The cost-effectiveness plane for the PSA results is presented in the supplementary material.
Table 4.
Overview of the final results of the base case analysis for each indication
| Total discounted costs | Cost–utility analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TIPSS | VB | Ascites | HE | SBP | Costs | QALYs | Life years | ICER | |
| Indication 1 (AVB): base case | |||||||||
| TIPSS | €7866 | €179 | €165 | €708 | NA | €8918 | 1.034 | 1.728 | – |
| EBL | €1122* | €6815** | €358 | €853 | NA | €9148 | 0.823 | 1.360 | Dominant |
| Indication 1 (AVB): scenario—clinical parameters from meta-analysis | |||||||||
| TIPSS | €7855 | €379 | €139 | €859 | NA | €9232 | 0.971 | 1.627 | – |
| EBL | €597* | €5464** | €397 | €513 | NA | €6971 | 0.836 | 1.376 | €16,819 |
| Indication 2 (RA) | |||||||||
| TIPSS | €6144 | €361 | €2547 | €936 | €4 | €9991 | 1.141 | 1.797 | – |
| LVP | €0 | €991 | €33,910 | €580 | €198 | €35,679 | 0.611 | 1.163 | Dominant |
EBL endoscopic band ligation, HE hepatic encephalopathy, ICER incremental cost-effectiveness ratio, LVP large volume paracentesis, QALY quality-adjusted life-year, SBP spontaneous bacterial peritonitis, TIPSS transjugular intrahepatic portosystemic stent shunt, VB variceal bleeding
*Associated with TIPSS rescue in a proportion of patients who experienced further bleeding episode(s)
**Includes treatment for initial AVB and recurrent AVB minus use of TIPSS in a proportion of individuals who experience further bleeding events. Note: calculated totals and ICERs from the values reported in the table may not match reported totals and ICERs in the table as a result of rounding
Indication 1 (AVB)
For indication 1, the use of pre-emptive TIPSS was estimated to be cost saving when compared with EBL and pharmaceutical therapy over a 2-year time horizon, with cost savings of €230 per patient. It was also associated with additional health benefits of 0.21 QALYs per patient.
Whilst higher costs were incurred from primary treatment provision (estimated to be €7866 for TIPSS), this was compensated for by large cost savings from reduced AEs and avoided downstream EBL sessions. Therefore, TIPSS was found to be the dominant treatment strategy being both more effective and leading to overall cost savings.
A scenario was conducted using the alternative clinical data from Nicoară-Farcău [17], the results of which are also presented in Table 4. The key differences in inputs compared with the base case relate to reduced overall survival and increased AE risk with TIPSS. Given these scenario inputs, the use of TIPSS was associated with increased costs to the healthcare system (excess cost of €2261 per patient). Additionally, the incremental QALYs were lower compared to the base case analysis but TIPSS was still associated with clinical benefits (incremental gain of 0.13 QALYs per patient). TIPSS was no longer cost saving in this scenario primarily because of reduced cost savings from preventing recurrent variceal bleeding. Furthermore, additional costs were incurred from managing other consequences such as HE (€346) per patient instead of savings as previously estimated. Overall, TIPSS remained cost-effective at a threshold of €25,000 per QALY gained with an ICER of €16,819.
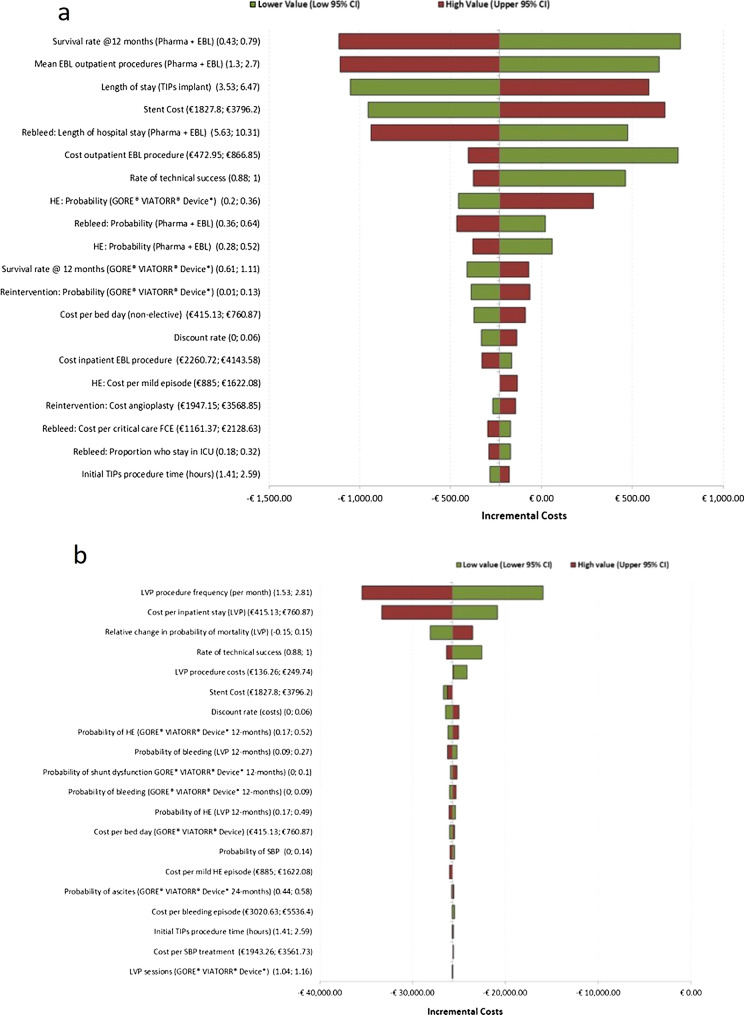
The key drivers of the base case results, as determined by the DSA, were survival at 12 months (for the EBL arm), mean EBL outpatient procedures, and TIPSS treatment-related costs such as length of stay for implantation and stent cost (Fig. 1a). The comparator arm was associated with ongoing costs through treatment sessions and AE management. Therefore, increased survival at 12 months was associated with increased medical costs thereby increasing cost savings with TIPSS. Similarly, the mean number of EBL procedures per month would be applicable for the full duration of the time horizon. Therefore, increased monthly sessions resulted in increased costs for EBL treatment.
Fig. 1.
Tornado plots for AVB and RA analyses. a Tornado plot presenting results of the deterministic sensitivity analysis for indication 1 (AVB). b Tornado plot presenting results of the deterministic sensitivity analysis for indication 2 (RA)
On the basis of the PSA, TIPSS was estimated to be cost saving 47% of the time given the base case inputs, with an average incremental cost of €66 estimated from 10,000 iterations. It has, however, a substantially higher probability of being cost-effective (93%) at a threshold of €25,000 per QALY gained (see supplementary material for the cost-effectiveness plane).
Indication 2 (RA)
The use of TIPSS for treating RA when compared with LVP resulted in substantial cost savings of €25,687 per patient over a 2-year time horizon. It was also associated with health benefits with an incremental gain in QALYs of 0.53 per patient, thereby dominating LVP. The largest cost savings were observed from avoiding multiple LVP sessions, as required for the comparator arm, with estimated savings of €33,910 per patient over 2 years (equivalent to preventing 29 hospital-based LVP sessions).
The key drivers of the model outcomes were monthly frequencies in LVP procedures and the cost per inpatient stay for the LVP arm (Fig. 1b). Both of these parameters determined the total cost of LVP treatment incurred for the comparator arm. Given the lower and higher values for the parameters considered, TIPSS was estimated to be cost saving across all the scenarios considered. Similarly, the base case results were considered to be robust on the basis of the PSA outputs. The use of TIPSS in those with RA was estimated to be both cost saving and cost-effective 100% of the time (see supplementary material for cost-effectiveness plane).
Comparison to UK Findings
For the UK analysis, the use of TIPSS was estimated to result in cost savings of £600 and 0.20 QALY gains as a pre-emptive treatment for AVB. For the RA population, TIPSS was estimated to result in savings of £17,983 and health benefits of 0.53 QALYs per patient over 2 years. Therefore, the QALY gains were almost equivalent in the Spanish and UK analyses for both populations, which is to be expected given the consistency in efficacy and utility data that were applied in both settings. In terms of costs, TIPSS led to a smaller savings in the AVB indication in Spain but substantially larger savings in RA. Overall, the cost-effectiveness estimates were largely consistent across the two settings despite differences in the structure of each healthcare system.
Discussion
Whilst previous studies have shown the economic benefits of TIPSS for RA and AVB indications, this study establishes the cost impact, potential health benefits, and cost-effectiveness of TIPSS within a Spanish healthcare setting. Results from the analysis conducted show that TIPSS is estimated to be a dominant treatment option in patients with RA (cost savings and incremental QALYs of €25,687 and 0.531 per person, respectively) and as pre-emptive treatment in those with AVB (cost savings and incremental QALYs of €230 and 0.211 per person, respectively). Therefore, it is estimated to be cost saving to the system for both indications. Though associated with increased costs from primary treatment in the first instance this is offset by decreased costs from avoided AE occurrences and other treatment-related healthcare resource use. The results also appear to be robust on the basis of sensitivity analyses conducted for the two indications.
An additional scenario analysis for the AVB population highlights similar outcomes although the extent of cost savings is reduced because of an increased risk of AEs and a conservative estimation of survival benefit with TIPSS when compared with EBL. These values were not used in the base case because the outcomes of the meta-analysis are likely to be biased as a result of inclusion of observational studies and RCTs incorporating bare stents. However, even with the use of conservative values, the model outputs support health benefits from using TIPSS within this patient population owing to QALY gains that result in an ICER of 16,819 €/QALY below the willingness to pay threshold of €25,000 per QALY.
Whilst there is no reimbursement system in place in Spain for medical devices, the findings of a cost-effectiveness analysis may still be of interest for payers and hospital managers. This analysis shows the clinical benefits both in terms of quality of life and life years lived for patients experiencing potentially life-threatening complications from liver disease such as AVB and RA. In addition to the clinical benefits, the uptake of TIPSS has the potential to reduce burden on healthcare practitioners (HCPs). The associated cost savings from this can offset the initial increased cost of providing treatment when managing treatment for these patients within a Spanish setting.
As with the UK models, there were data gaps, which increase the uncertainty in the results estimated for both indications. Namely, the dependence on individual RCTs rather than pooled data to inform key clinical parameters. Primary studies used to inform the AVB and RA models were García-Pagán et al. [8] and Bureau et al. [22], respectively. A limitation of the García-Pagán et al. [8] study in particular is the small sample size of the study. The uncertainty in central estimates is reflected in the wide confidence intervals associated with clinical parameters such as risk of ascites and rebleeding.
This was, however, still considered to be more appropriate than the meta-analysis for reasons previously discussed and various sensitivity analyses were conducted to explore the impact of these values on the results. A larger RCT would be required to provide more conclusive evidence on the true cost impact of TIPSS in patients with AVB.
Similarly for the ascites population, with 62 patients included in the cohort, Bureau et al.’s study [22] is also associated with a small sample size that is likely to impact the results of this analysis. For example, reduced risk of HE is reported for the TIPSS arm in the study; however, the values are non-significant with 10 (out of 29) and 11 (out of 33) patients experiencing HE for TIPSS and LVP, respectively. This was also the case for SBP AE risk. However, these were not key drivers of the results for the RA indication and therefore any variability is unlikely to have a substantial impact on the results.
A strength of this analysis is that it is based on a UK model that has previously undergone a peer-review process and expert validation. Furthermore, the applicability of the model structure, inputs used, and adaptations made were guided by clinical expert opinion familiar with the management of these indications within the Spanish population. Where there was contention in any data points used these were explored through scenario analyses to capture any additional uncertainties. From such analyses the benefits of TIPSS are still evident whilst explicitly quantifying the extent of uncertainty associated with cost savings.
Given the inputs used, the model outcomes are largely in line with results for the UK setting even with adjustments made to reflect Spanish clinical practice based on expert feedback. Additionally, the findings of this analysis are broadly consistent with previous cost-effectiveness analysis conducted by Pérez-Mitru and colleagues within a Spanish setting for both indications [12, 13]. For AVB, the early use of TIPSS was associated with a total incremental cost of €57 over 2 years (€7657 for TIPSS and €7600 for EBL) according to Pérez-Mitru et al. [13]. The study also reported that fewer recurrent bleeding episodes and related EBL procedures were the main points of cost savings [13]. Whilst the current base case deterministic result in our analysis estimated higher cost savings for TIPSS (incremental savings of €230), the uncertainty in the extent of cost savings was captured through the PSA with an estimated average incremental cost of €66 over 10,000 iterations. Therefore, the PSA results are in line with the findings from Pérez-Mitru et al. [13]. Whilst TIPSS had a 47% likelihood of being cost saving on the basis of the current PSA, the extent of additional costs incurred per patient over the 2 years may be considered small in light of the resource use saved (from avoided downstream EBL sessions) and clinical benefits. However, a full comparison of results was not possible because the results from Pérez-Mitru et al. were reported in an abstract only.
For RA, Pérez-Mitru and colleagues [12] reported costs with TIPSS over a 2-year time horizon of €14,728 and €15,360 for TIPSS and LVP, respectively. The estimated cost of TIPSS was lower in the current analysis at €9991 per patient over 2 years whilst the LVP cost per patient was substantially higher (€35,679). However, the authors note that the results may be an underestimation because of the meta-analysis used to inform key clinical parameters in the model, which was based on TIPSS with uncovered stents [12]. Evidence from RCTs suggests that clinical outcomes with uncovered stents are poorer when compared with covered stents [23–25]. In particular, the findings of a meta-analysis of RCTs suggest that covered stents provided improved shunt patency and survival with decreased risk of TIPSS-associated AEs, such as HE onset compared with uncovered stents [23, 25]. This is likely to have overestimated the costs associated with TIPSS treatment.
Conclusion
This analysis estimates the clinical and cost benefits associated with TIPSS use for those with RA or AVB, secondary to cirrhosis. For both indications, TIPSS is estimated to be both cost saving and cost-effective when compared with current standard of care in a Spanish setting. In the RA indication, TIPSS is associated with freeing up resource use from avoided hospital-based LVP sessions. In terms of AVB, whilst there are increased upfront costs for provision of treatment with TIPSS over EBL, it is expected to result in cost savings in the long term from a variety of pathways. These include savings from reduced AEs, such as additional variceal bleeds and HE, and avoided subsequent follow-up treatment-related appointments, resulting in a net reduction in healthcare resource use. Additionally, the freeing up of resources such as HCP time may improve system efficiencies and thereby support provision of care for more patients within the Spanish healthcare system.
Whilst there is uncertainty in the true costs associated with the treatment and comparators, particularly for the AVB indication, there are still benefits to be reaped through improved clinical outcomes within these patient populations as highlighted by the improved survival and reduced AEs. However, future analyses may benefit from gathering additional clinical evidence on the short- and long-term impact of TIPSS compared with EBL and LVP treatment for AVB and RA indications, respectively. This may include conducting a larger RCT, potentially combined with real-world evidence gathering, to increase the robustness of the evidence base utilised within the cost-effectiveness analysis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Financial support for this study was provided by W.L. Gore & Associates for undertaking the analysis and the development of this manuscript. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. W. L. Gore & Associates also funded the rapid service fee to the journal.
Authors Contributions
Rafael Bañares—model conceptualisation and design, writing (reviewing and editing). Agustín Albillos—model conceptualisation and design, writing (reviewing and editing). Will Green—model conceptualisation and design, model development, writing (original draft). Angel Varghese—writing (original draft). Mitesh Nakum—model conceptualisation and design, writing (reviewing and editing). Salvador Gea—writing (reviewing and editing).
Disclosures
William Green and Angel Varghese (YHEC) received consulting fees to undertake the economic analysis discussed in the manuscript as well as fees to develop the manuscript from GORE. Salvador Gea-Sanchez and Mitesh Nakum are currently an employees of W L Gore, manufacturer of GORE® VIATORR® TIPSS. Agustin Albillos is an advisor and lecturer for GORE. Rafael Bañares has presented lectures sponsored by GORE in the context of a teaching program regarding use of TIPS in cirrhosis, and received a travel bursary.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.
References
- 1.Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735. doi: 10.1016/j.jhep.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Asrani SK, Kamath PS. Natural history of cirrhosis. Curr Gastroenterol Rep. 2013;15(2):1–6. doi: 10.1007/s11894-012-0308-y. [DOI] [PubMed] [Google Scholar]
- 3.Hernández-Gea V, Berbel C, Baiges A, Garcia-Pagan JC. Acute variceal bleeding: risk stratification and management (including TIPS) Hep Intl. 2018;12(1):81–90. doi: 10.1007/s12072-017-9804-3. [DOI] [PubMed] [Google Scholar]
- 4.Cardiovascular and Interventional Radiological Society of Europe. Transjugular intrahepatic portosystemic shunt (TIPS). https://www.cirse.org/patients/ir-procedures/transjugular-intrahepatic-portosystemic-shunt-tips/.
- 5.Siqueira F, Kelly T, Saab S. Refractory ascites: pathogenesis, clinical impact, and management. Gastroenterol Hepatol. 2009;5(9):647. [PMC free article] [PubMed] [Google Scholar]
- 6.Stirnimann G, Berg T, Spahr L, et al. Treatment of refractory ascites with an automated low-flow ascites pump in patients with cirrhosis. Aliment Pharmacol Ther. 2017;46(10):981–991. doi: 10.1111/apt.14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Pagán JC, Saffo S, Mandorfer M, Garcia-Tsao G. Where does TIPS fit in the management of patients with cirrhosis? JHEP Reports. 2020;2(4):100122. doi: 10.1016/j.jhepr.2020.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 9.Holster IL, Tjwa ET, Moelker A, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + β-blocker for prevention of variceal rebleeding. Hepatology. 2016;63(2):581–589. doi: 10.1002/hep.28318. [DOI] [PubMed] [Google Scholar]
- 10.Sauerbruch T, Mengel M, Dollinger M, et al. Prevention of rebleeding from esophageal varices in patients with cirrhosis receiving small-diameter stents versus hemodynamically controlled medical therapy. Gastroenterology. 2015;149(3):660–668. doi: 10.1053/j.gastro.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Miquel M, Cleries M, Vergara M, Vela E. Economic burden of cirrhosis in Catalonia: a population-based analysis. BMJ Open. 2018;8(3):e018012. doi: 10.1136/bmjopen-2017-018012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pérez-Mitru A, Lordan AV, Scarpa F. Cost-effectiveness evaluation of tips procedures with expanded polytetrafluoroethylene (EPTFE) covered stent-grafts compared to large volume paracentesis in patients with refractory ascites–a Spanish scenario. Value Health. 2016;19(7):A693. doi: 10.1016/j.jval.2016.09.1988. [DOI] [Google Scholar]
- 13.Pérez-Mitru A, Lordan AV, Scarpa F. Cost-effectiveness of early tips with expanded polytetrafluoroethylene (EPTFE) covered stent-grafts compared to endoscopic procedures to manage acute variceal bleeding—a Spanish scenario. Value Health. 2016;19(7):A692–A693. doi: 10.1016/j.jval.2016.09.1987. [DOI] [Google Scholar]
- 14.Mattock R, Tripathi D, O'Neill F, et al. Economic evaluation of covered stents for transjugular intrahepatic portosystemic stent shunt in patients with variceal bleeding and refractory ascites secondary to cirrhosis. BMJ Open Gastroenterol. 2021;8(1):e000641. doi: 10.1136/bmjgast-2021-000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallejo-Torres L, Garcia-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27(4):746–761. doi: 10.1002/hec.3633. [DOI] [PubMed] [Google Scholar]
- 16.Attema AE, Brouwer WB, Claxton K. Discounting in economic evaluations. Pharmacoeconomics. 2018;36(7):745–758. doi: 10.1007/s40273-018-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicoară-Farcău O, Han G, Rudler M, et al. Effects of early placement of transjugular portosystemic shunts in patients with high-risk acute variceal bleeding: a meta-analysis of individual patient data. Gastroenterology. 2021;160(1):193–205. doi: 10.1053/j.gastro.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Tarifas para la facturación de servicios sanitarios y docentes de Osakidetza para el año 2018. Osakidetza; 2018.
- 19.Younossi ZM, Boparai N, McCormick M, Price LL, Guyatt G. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol. 2001;96(2):579–583. doi: 10.1111/j.1572-0241.2001.03537.x. [DOI] [PubMed] [Google Scholar]
- 20.Moscucci F, Nardelli S, Pentassuglio I, et al. Previous overt hepatic encephalopathy rather than minimal hepatic encephalopathy impairs health-related quality of life in cirrhotic patients. Liver Int. 2011;31(10):1505–1510. doi: 10.1111/j.1478-3231.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 21.Tripathi D, Stanley AJ, Hayes PC, et al. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69(7):1173–1192. doi: 10.1136/gutjnl-2019-320221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152(1):157–163. doi: 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Perarnau JMLGA, Nicolas C, d'Alteroche L, et al. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Xiao Z, Yue Z. Efficacy of covered and bare stent in TIPS for cirrhotic portal hypertension: a single-center randomized trial. Sci Reports. 2016 doi: 10.1038/srep21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xingshun Qi YT, Zhang W, Yang Z, Guo X. Covered versus bare stents for transjugular intrahepatic portosystemic shunt: an updated meta-analysis of randomized controlled trials. Ther Adv Gastroenterol. 2017;10(1):32–41. [DOI] [PMC free article] [PubMed]
- 26.Perelló MP, Mur JP, Vives MS, et al. Long-term follow-up of transjugular intrahepatic portosystemic shunt (TIPS) with stent-graft. Diagn Interv Radiol. 2019;25(5):346. doi: 10.5152/dir.2019.18416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv Y, Yang Z, Liu L, et al. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4(8):587–598. doi: 10.1016/S2468-1253(19)30090-1. [DOI] [PubMed] [Google Scholar]
- 28.Shen NT, Schneider Y, Congly SE, et al. Cost effectiveness of early insertion of transjugular intrahepatic portosystemic shunts for recurrent ascites. Clin Gastroenterol Hepatol. 2018;16(9):1503–1510. doi: 10.1016/j.cgh.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 29.Bai M, Qi X-S, Yang Z-P, Yang M, Fan D-M, Han G-H. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014;20(10):2704. doi: 10.3748/wjg.v20.i10.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catalan Institute of Health (ICS). Remuneration book. 2020.
- 31.GORE. GORE 2021.
- 32.Condiciones económicas aplicables a la prestación de determinados servicios de asistencia sanitaria a través de medios ajenos, en el ámbito de gestión del Sescam 2017.
- 33.Agencia Española de Medicamentos y Productos Sanitarios. 2021. https://www.aemps.gob.es/?lang=ca.
- 34.Wechowski J, Connolly M, Woehl A, et al. An economic evaluation of vasoactive agents used in the United Kingdom for acute bleeding oesophageal varices in patients with liver cirrhosis. Curr Med Res Opin. 2007;23(7):1481–1491. doi: 10.1185/030079907X199736. [DOI] [PubMed] [Google Scholar]
- 35.Kwan SW, Allison SK, Gold LS, Shin DS. Cost-effectiveness of transjugular intrahepatic portosystemic shunt versus large-volume paracentesis in refractory ascites: results of a Markov model incorporating individual patient-level meta-analysis and nationally representative cost data. J Vasc Interv Radiol. 2018;29(12):1705–1712. doi: 10.1016/j.jvir.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badia J, Casey A, Petrosillo N, Hudson P, Mitchell S, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96(1):1–15. doi: 10.1016/j.jhin.2017.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.