Abstract
Introduction
Efficacy of ponatinib-based treatment for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL) has not been compared to imatinib-based treatments in head-to-head clinical trials. We evaluated its efficacy versus imatinib-based regimens using a matching adjusted indirect comparison.
Methods
Two ponatinib studies were used: the phase 2 MDACC study of ponatinib + hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) in adult patients and the phase 2 GIMEMA LAL1811 study of ponatinib + steroids in patients > 60 years/unfit for intensive chemotherapy and stem cell transplant. Studies on imatinib as first-line treatment in adults with Ph + ALL were identified using a systematic literature search. Population adjustment was based on the prognostic factors and effect modifiers identified by clinical experts. Hazard ratios (HRs) were calculated for overall survival (OS) and odds ratios (ORs) for complete molecular response (CMR).
Results
The systematic literature search identified two studies (GRAAPH-2005 and NCT00038610) reporting the efficacy of first-line imatinib + hyper-CVAD and one study reporting the efficacy of first-line imatinib monotherapy induction + imatinib-based consolidation (CSI57ADE10). Ponatinib + hyper-CVAD prolonged OS and gave a higher CMR rate than imatinib + hyper-CVAD. The adjusted HR [95% confidence interval (CI)] for OS was 0.35 (0.17–0.74) for MDACC vs. GRAAPH-2005 and 0.35 (0.18–0.70) for MDACC vs. NCT00038610; the adjusted OR (95% CI) for CMR was 12.11 (3.77–38.87) for MDACC vs. GRAAPH-2005 and 5.65 (2.02–15.76) for MDACC vs. NCT00038610. Ponatinib + steroids prolonged OS and gave a higher CMR rate than imatinib monotherapy induction + imatinib-containing consolidation. The adjusted HR (95% CI) for OS was 0.24 (0.09–0.64) and the adjusted OR (95% CI) for CMR was 6.20 (1.60–24.00) for GIMEMA LAL1811 vs. CSI57ADE10.
Conclusion
In adults with newly diagnosed Ph + ALL, first-line treatment with ponatinib was associated with better outcomes than first-line treatment with imatinib.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02497-y.
Keywords: Acute lymphoblastic leukemia, Efficacy, Imatinib, Indirect treatment comparison, Matching adjusted indirect comparison, Philadelphia chromosome, Ponatinib, Tyrosine kinase inhibitor
Key Summary Points
| Ponatinib has been investigated as a first-line treatment for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL). |
| However, it has not been compared to standard first-line imatinib-based regimens in a head-to-head clinical trial. |
| Matching adjusted indirect comparison was used to compare the efficacy of ponatinib versus imatinib + hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and imatinib monotherapy induction + imatinib-containing consolidation. |
| Compared to imatinib, ponatinib prolonged overall survival and increased complete molecular response rates in patients with newly diagnosed Ph + ALL. |
| This study suggests that first-line treatment with ponatinib gives better outcomes than first-line treatment with imatinib-based regimens. |
Introduction
The Philadelphia chromosome (Ph) is a common cytogenetic abnormality associated with acute lymphoblastic leukemia (ALL) and accounts for approximately 20–30% of ALL cases in adults [1, 2]. The incidence of Ph + ALL increases with age and may be up to 50% in patients > 50 years [2–6]. Ph is formed from a reciprocal translocation that causes the fusion of the ABL1 tyrosine kinase gene on chromosome 9 to the BCR gene on chromosome 22 and results in the formation of the BCR::ABL1 oncogene [7, 8]. This oncogene encodes the p190- or p210-BCR::ABL1 isoforms detected in most adults with Ph + ALL [7, 9]. The BCR::ABL1 fusion protein has elevated tyrosine kinase activity that drives the transformation of immature lymphoid cells in Ph + ALL.
Prognosis for Ph + ALL has historically been poor, especially for old or unfit patients. Treatment options for ALL initially included chemotherapy-based regimens; however, recurrence rates were high [2, 10]. Stem cell transplantation improves outcomes but is not suitable for elderly patients. Tyrosine kinase inhibitor (TKI)-based treatments targeting the BCR::ABL1 oncoprotein combined with standard or reduced-intensity chemotherapy have revolutionized the treatment response and improved the long-term survival of Ph + ALL patients [1, 2, 8, 11–16].
TKI-based treatment strategies for patients with Ph + ALL include a TKI in combination with chemotherapy or steroids. Imatinib is a first-generation TKI approved by the European Medicines Agency in combination with chemotherapy for newly diagnosed Ph + ALL patients and as monotherapy in patients with relapsed or refractory Ph + ALL. In the US, imatinib is approved for adults with relapsed or refractory Ph + ALL [17, 18]. Dasatinib is a second-generation TKI approved in the EU and US for adult Ph + ALL patients with resistance or intolerance to prior therapy [19–21]. Ponatinib is a third-generation TKI with potent activity against unmutated and mutated BCR::ABL1, including the BCR::ABL1 T315I mutant [14, 22]. First-line treatment with ponatinib in combination with chemotherapy achieved long-term remission in patients with newly diagnosed Ph + ALL in a phase 2 study (NCT01424982) [23, 24]. Ponatinib was additionally effective against relapsed or refractory Ph + ALL [1, 8, 25–28]. Recent trials combining TKIs (dasatinib or ponatinib) and immunotherapy (blinatumomab) as front-line therapy for Ph + ALL yielded promising outcomes [29–31]. Ponatinib is approved in the EU for adults with Ph + ALL who are resistant to dasatinib; who are intolerant to dasatinib and for whom subsequent treatment with imatinib is not clinically appropriate; or who have the T315I mutation [32]. In the US, it is approved for adults with Ph + ALL for whom no other kinase inhibitors are indicated or who have T315I-positive Ph + ALL [33].
The efficacy of ponatinib as a first-line treatment for patients with Ph + ALL has not been compared to the standard first-line imatinib-based treatment in a head-to-head clinical trial. The aim of this study was to evaluate the relative efficacy of ponatinib versus imatinib-based treatments for Ph + ALL. The efficacy of ponatinib was evaluated based on two studies: (1) MDACC (NCT01424982), a phase 2, single-center, open-label, single-arm study of ponatinib in combination with hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) in adults with Ph + ALL; and (2) GIMEMA LAL1811 (NCT01641107), a phase 2, multicenter, open-label, single-arm study of ponatinib therapy with prednisone in adults > 60 years with newly diagnosed with Ph + ALL [24, 34]. Ponatinib showed a good efficacy profile in the MDACC study, with 3-year event-free survival (EFS) of 70% and a complete molecular response (CMR) in 83% of patients. Similarly, ponatinib showed good efficacy in the GIMEMA LAL1811 study, with over 86% of patients in complete hematologic response (CHR) after 6 months, over 95% of patients showing a CHR sometime during treatment, and more than 81% of patients achieving a CMR sometime during treatment.
In this study, we used matching adjusted indirect comparison (MAIC) to compare the efficacies of ponatinib and imatinib in the treatment of Ph + ALL. Dasatinib was not considered for this MAIC analysis as it is currently not recommended as first-line therapy for Ph + ALL, but is instead used to treat patients who are not responsive to imatinib-based treatment regimens [21].. The MAIC is an approach that enables treatments in different trials to be compared by adjusting for between-trial differences in baseline characteristics [35]. An MAIC can be used when individual patient data are available only for one treatment and aggregate data are available for the other treatment. Given that both MDACC and GIMEMA LAL18115 are single-arm studies lacking a comparator arm that could be used as an anchor for the indirect treatment comparison, an unanchored MAIC was used to compare the relative efficacy between treatments.
Methods
Study Design
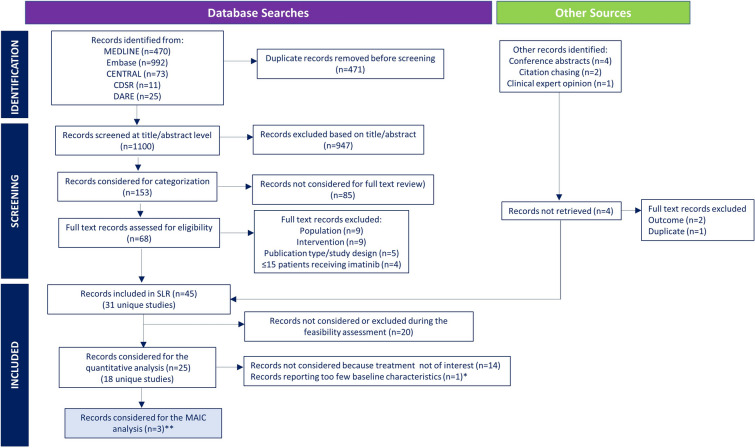
Individual patient data for ponatinib and aggregated data for imatinib from published sources were compared using MAIC. Individual patient data for ponatinib were obtained from the MDACC (high-dose eligible population, i.e., patients were eligible to receive high-dose therapy and stem cell transplantation) and GIMEMA LAL1811 studies (high-dose non-eligible population, i.e., patients were not eligible to receive high-dose therapy and stem cell transplantation) [24, 34]. A systematic literature search was conducted on 15 March 2021 to identify studies investigating the clinical efficacy of imatinib as a first-line treatment for adults with Ph + ALL (Fig. 1). Literature searches and abstract screening also included studies on ponatinib, but these were not considered for full-text review as the aim of the systematic literature search was to identify imatinib data for indirect treatment comparison. Systematic searches were conducted in MEDLINE, Embase, the Cochrane Library, and the Database of Abstracts of Reviews of Effectiveness to identify peer-reviewed studies (published between 2000 and 2021) and in conference proceedings to identify abstracts from recent scientific meetings (2015–2021). ClinicalTrials.gov was searched to identify clinical trials that were not detected in the electronic database searches. The systematic literature search included randomized controlled, single-arm, and non-randomized controlled trials (phase II–IV) and observational/real-world studies published in English that included ≥ 15 patients receiving imatinib therapy as first-line treatment. Studies evaluating other interventions or imatinib/ponatinib in combination with other targeted therapies or immunotherapy or evaluating imatinib as second- or later-line treatment were excluded, as were studies including only pediatric patients and studies of patients with Ph- ALL and other leukemias.
Fig. 1.
Selection of imatinib studies for the MAIC analysis. CDSR Cochrane Database of Systematic Reviews, CENTRAL Cochrane Central Register of Controlled Trials, DARE Database of Abstracts of Reviews of Effectiveness, MAIC matching adjusted indirect comparison, SLR systematic literature review. *Study only reported median age of patients. **The SLR identified 18 unique studies investigating imatinib-based regimens for patients with newly diagnosed Philadelphia chromosome-positive acute lymphocytic leukemia. Studies that did not follow a treatment of interest (i.e., induction without imatinib; not from a German multicenter study group for adult acute lymphoblastic leukemia protocol for hyper-CVAD; imatinib not given continuously) or that reported too few baseline characteristics to allow a meaningful population-adjustment were excluded from the MAIC analysis
Results from the literature search were documented using DistillerSR software (Evidence Partners, Canada) [36]. Titles and abstracts of all articles identified in the literature searches were reviewed independently by two researchers to determine whether they met the inclusion and exclusion criteria of the systematic literature search, and discrepancies were resolved by a third reviewer. Data were then extracted from full-text articles by one reviewer and validated by a second reviewer.
Studies identified in the systematic literature search were divided into two groups: studies that included patients who were eligible to receive intensive therapy and stem cell transplantation (intensive eligible population), and studies that included patients not eligible to receive intensive therapy and stem cell transplantation (non-intensive eligible population). Clinical experts were consulted to confirm imatinib-based treatment protocols reflecting current clinical practices.
Studies of imatinib meeting all the following criteria were included in the indirect treatment comparison: studies of imatinib in combination with hyper-CVAD or as part of the German multicenter study group for adult acute lymphoblastic leukemia; studies in which imatinib was started at the beginning of the induction therapy; studies in which imatinib was given throughout the study; and studies which reported data on critical prognostic factors. To be included, randomized controlled trials of imatinib had to pass a quality assessment performed using the Cochrane Risk of Bias tool for RCTs v2.0 [37].
Matching Adjusted Indirect Comparison
The outcomes evaluated in this study included overall survival (OS), CMR, disease-free survival (DFS), EFS, CHR, and major molecular response (MMolR). The definitions for efficacy outcomes are listed in Table S1.
The MAIC was conducted according to the methods described by Signorovitch et al. and the guidelines of the National Institute for Health and Care Excellence [35, 38]. The MAIC consisted of estimating balancing weights through propensity score-like regression to weight the ponatinib populations (index populations) so that they became comparable to the imatinib populations (comparator populations) on key demographic and clinical characteristics. All available prognostic factors were considered for the population adjustment, and those identified by clinical experts were used in the analysis (Table S2). The key prognostic factors and effect modifiers identified by clinical experts for population adjustment were age, Eastern Cooperative Oncology Group (ECOG) performance status, white blood cell (WBC) count, and type of BCR::ABL transcript at baseline. Cytogenetic factors could not be included in the population adjustment because they were not available in all studies.
The MAIC adjusted for imbalances in as many prognostic factors and effect modifiers as possible while maximizing the effective sample size resulting from the population adjustment. The effective sample size corresponded to the number of individual (unweighted) patients that would give the same level of uncertainty in the estimates as the weighted cohorts. Three scenarios were considered to determine the strategy for deriving MAIC weights: all available baseline characteristics (scenario 1); all clinically validated prognostic factors and effect modifiers (scenario 2); and factors prioritized by clinical experts (scenario 3).
Several matching models were tested and those that resulted in a pre-specified effective sample size of < 15 were excluded from the analysis. This threshold value was used as it represented the value below which the central limit theorem might not apply. The specifications for the matching models (base case and sensitivity) used in the study are outlined in the Supplemental Appendix (Tables S3, S4, and S5).
Statistical Analysis
For OS, DFS, and EFS, relative efficacy was quantified as the hazard ratio (HR) and 95% confidence interval (CI). HRs were obtained using a Cox regression analysis [39, 40]. For CMR and CHR, relative efficacy was quantified as the odds ratio (OR) and 95% CI. The MDACC and NCT00038610 studies included some patients who had previously received minimal treatment for Ph + ALL. For these patients, only those who were not in response at the start of the study were included in the efficacy analyses. The ORs were calculated using a logistic regression analysis. The Cox and logistic regression models were fitted to the ponatinib study data and reconstructed individual patient data from the comparator imatinib study used in the matching. To estimate the reduction in bias induced by the population adjustment, regression models were fitted using unweighted and weighted MDACC/GIMEMA LAL1811 data. Robust estimators were used to calculate standard errors. Reconstructed individual patient data for imatinib time-to-event outcomes were generated using the algorithm developed by Guyot et al. [41].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Studies Identified in the Systematic Literature Review
Two imatinib studies (GRAAPH-2005 and NCT00038610) including high-dose therapy eligible patients and one imatinib study (CSI57ADE10) including high-dose therapy non-eligible patients were selected for the MAIC analysis (Table 1) [11, 14, 15, 34, 42]. The GRAAPH-2005 (NCT00327678) trial was a phase 3, randomized, multicenter, open-label trial in adults with newly diagnosed Ph + ALL. The NCT00038610 trial was a phase 2, single-arm, single-center, open-label study of imatinib + hyper-CVAD in adults with newly diagnosed or minimally treated Ph + ALL (i.e., patients had received ≤ 2 courses of prior chemotherapy without TKIs). The CSTI571ADE10 trial was a multicenter, randomized, open-label, phase 2 study of single-agent imatinib or multi-agent chemotherapy induction in adults with Ph + ALL aged ≥ 55 years who were not eligible for stem cell transplantation. Study attrition for high-dose therapy eligible and non-eligible patients is presented in Table S6 and Table S7, respectively [11, 12, 15, 16, 43–59].
Table 1.
Summary of the ponatinib and imatinib studies included in the MAIC
| Characteristic | MDACC [14] | GIMEMA LAL1811 [34] | GRAAPH-2005 [15] | NCT00038610 [11] | CSI57ADE10 [42] |
|---|---|---|---|---|---|
| Treatment | Ponatinib + hyper-CVAD | Ponatinib + steroids | Imatinib + reduced intensity chemotherapy or Imatinib + hyper-CVADa | Imatinib + hyper-CVAD | Imatinib monotherapy followed by age-adapted/intrathecal chemotherapy consolidation with imatinib |
| Study design | Single-center, open-label, single-arm, phase 2 | Multicenter, open-label, single-arm, phase 2 | Multicenter, randomized, open-label, two-arms, phase 3 | Single-center, open-label, single-arm, phase 2 | Multicenter, randomized, open-label, single-arm, phase 2 |
| Population size | 87 | 44 | 133b/268c | 54 | 55 |
| Intervention(s) |
Oral ponatinib at a dose of 45 mg/day for the first 14 days of cycle 1, then daily dose of 45 mg/day for the next 7 cycles of consolidation therapy 8 cycles of 21 days, alternating between two drug combinations: hyper-CVAD and high-dose methotrexate + cytarabine Patients in complete remission received maintenance with ponatinib 45 mg/day with vincristine and prednisone monthly for 2 years followed by ponatinib for up to 3 years or indefinitely |
Oral ponatinib at a dose of 45 mg/day for 6 weeks (defined as one course) for 8 courses Prednisone at 60 mg/m2/day from days 1 to 21; the dose was then tapered, and treatment was stopped at day 29 |
Induction with imatinib + reduced-intensity chemotherapy vs. induction with hyper-CVAD in cycle 1 Induction in cycle 1 followed by imatinib + methotrexate + cytarabine cycle 2 to bridge to stem cell transplantation Up to 8 cycles of treatment alternating imatinib + hyper-CVAD with imatinib + methotrexate + cytarabine for patients not receiving transplant |
8 induction-consolidation courses of imatinib + hyper-CVAD alternated with imatinib + methotrexate + cytarabine |
Imatinib induction followed by imatinib + age-adapted/intrathecal chemotherapy consolidation After pre-phase chemotherapy, patients were randomly assigned to imatinib at a dose of 600 mg/day or multi-agent induction chemotherapy in a 4-week cycle After completing remission therapy, patients randomized to either treatment arm (imatinib vs. chemotherapy) received imatinib at a dose of 600 mg/day imatinib + successive cycles of consolidation and reinduction chemotherapy |
| Primary endpoint | EFS | Proportion of patients with a CHR | MMolR rate after 2 years | Response to induction therapy with imatinib + hyper-CVAD (from baseline to 6 months), CR, PR, induction death, DFS | Rate of hematological remission after induction therapy |
| Other endpoints | ORR, OS, DFS, complete remission, partial remission, molecular response | Molecular response, cytogenetic response, duration of molecular response, duration of cytogenetic response, EFS, OS | EFS, RFS, CIR, cumulative incidence of NRM, OS | OS | OS, DFS, complete remission, molecular response |
| Median follow-up duration | 45.1 months | 34.9 months | 4.8 years | 130 months among surviving patients | 11.2 months among surviving patients |
| Eligible for HDT-SCT | Yes | No | Yes | Yes | No |
| Comparator MDACC or GIMEMA LAL1811 population | MDACC patients < 60 years with active disease at baseline, not previously treated with TKIs | MDACC patients not previously treated with TKIs | GIMEMA LAL1811 patients > 54 years | ||
| Year of study completion | 2021 | Not reported | 2014 | 2014 | 2006 |
CHR complete hematologic response, CIR cumulative incidence of relapse, CVAD cyclophosphamide, vincristine, doxorubicin, and dexamethasone, CR complete remission, DFS disease-free survival, EFS event-free survival, HDT-SCT, high-dose therapy and stem cell transplant, MAIC matching adjusted treatment comparison, MMolR major molecular response, NRM non-relapse-related mortality, ORR overall response rate, OS overall survival, PR partial remission, RFS relapse-free survival, TKI tyrosine kinase inhibitor
aThe MAIC only used data for the imatinib + hyper-CVAD arm
bNumber of patients treated with imatinib + hyper-CVAD
cTotal number of patients in the study
Baseline Characteristics of MAIC Populations
The characteristics of the ponatinib study populations were successfully matched with the relevant imatinib study populations, i.e., MDACC vs. GRAAPH-2005 (Table S8) MDACC vs. NCT00038610 (Table S9), and GIMEMA LAL1811 vs. CSI57ADE10 (Table S10), at the treatment-arm level following population adjustment.
Comparative Efficacy in the MAIC Populations
OS and CMR data were available from all studies included in the MAIC analysis, while DFS and EFS were only reported in some of the studies. Results obtained using base case matching models are discussed here. Results from sensitivity models were aligned with the base case analyses and are presented in the Supplemental Appendix (Tables S3, S4, S5, S11, and S12).
Overall Survival
Ponatinib + Hyper-CVAD vs. Imatinib + Hyper-CVAD
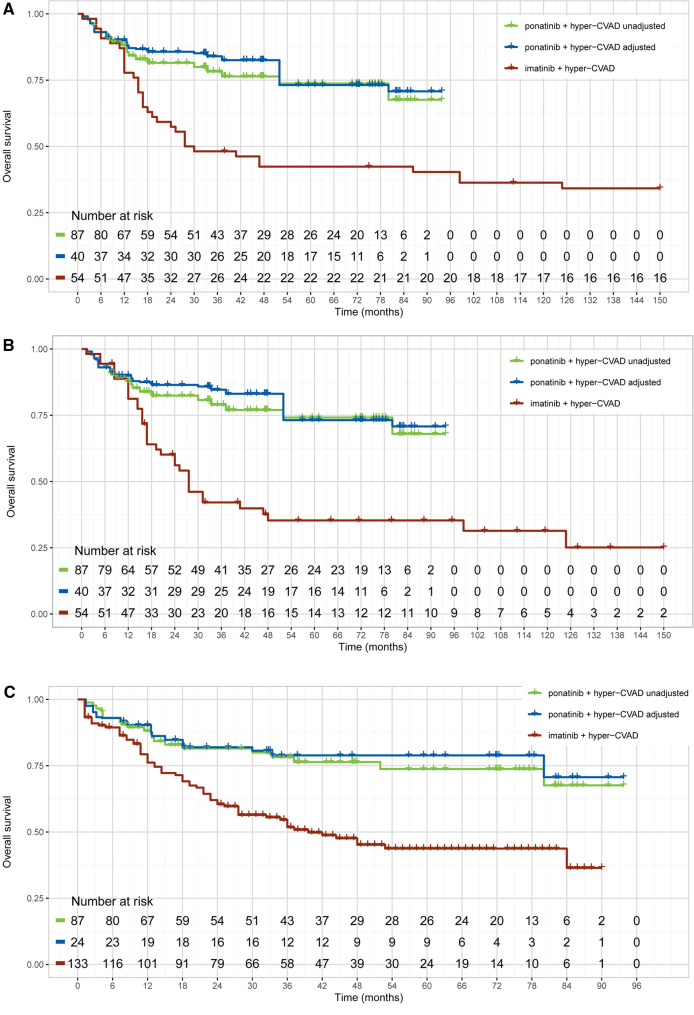
Overall, ponatinib + hyper-CVAD significantly prolonged OS compared to imatinib + hyper-CVAD. The HR (95% CI) for OS was 0.41 (0.25–0.68) before population adjustment and 0.35 (0.17–0.74) after population adjustment for MDACC (ponatinib) vs. GRAAPH-2005 (imatinib) (Table 2 and Fig. 2).
Table 2.
Relative efficacy of ponatinib vs. imatinib—overall survival
| OS | MDACC vs. GRAAPH-2005 | MDACC vs. NCT00038610 | GIMEMA LAL1811 vs. CSI57ADE10 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Unadjusted comparison | 0.41 (0.25, 0.68) | < 0.001 | 0.42 (0.24, 0.73)a | 0.002 | 0.40 (0.20, 0.81) | 0.011 |
| 0.36 (0.20, 0.63)b | < 0.001 | |||||
| Population-adjusted comparison | 0.35 (0.17, 0.74) | 0.006 | 0.35 (0.18, 0.70)a | 0.003 | 0.24 (0.09, 0.64) | 0.004 |
| 0.30 (0.15, 0.59)b | 0.001 | |||||
CI confidence interval, HR hazard ratio, OS overall survival
aHR with no censoring on stem cell transplantation
bHR with censoring on stem cell transplantation
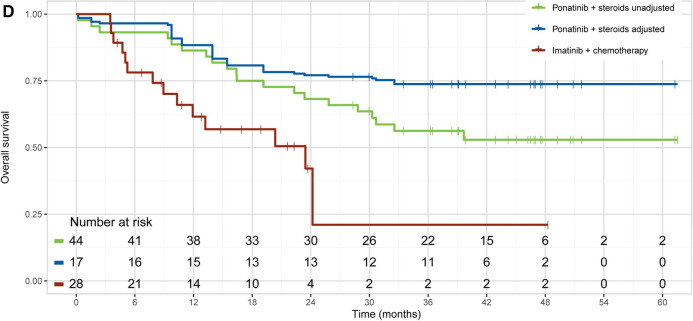
Fig. 2.
Unadjusted and adjusted Kaplan–Meier curves of OS. MDACC vs. NCT00038610 without censoring for stem cell transplantation (A) and with censoring for stem cell transplantation (B); MDACC vs. GRAAPH-2005 (C); and GIMEMA LAL1811 vs. CSI57ADE10 (D). CVAD cyclophosphamide, vincristine, doxorubicin, and dexamethasone, OS overall survival
The NCT00038610 study (imatinib) reported OS censoring of patients who received stem cell transplantation therapy. This enabled an assessment of the effect of ponatinib + hyper-CVAD vs. imatinib + hyper-CVAD unconfounded by the proportion of patients who received a subsequent stem cell transplant. For MDACC vs. NCT00038610, the unadjusted HR (95% CI) for OS was 0.42 (0.24–0.73) before censoring for stem cell transplantation and 0.36 (0.20–0.63) after censoring for stem cell transplantation. The adjusted HR (95% CI) for OS was 0.35 (0.18–0.70) without censoring patients who received a subsequent stem cell transplant and 0.30 (0.15–0.59) when censoring patients who received a subsequent stem cell transplant. Notably, the OS estimates for imatinib + hyper-CVAD were worse after censoring patients who received a stem cell transplant in the NCT00038610 study, whereas the OS estimates for ponatinib + hyper-CVAD changed slightly as only a few patients received a subsequent stem cell transplant in MDACC. Overall, the HR for OS was better for ponatinib + hyper CVAD compared with imatinib + hyper-CVAD.
Median OS was not achieved at the data cut-off point in the MDACC study, despite a median follow-up of 45.1 months. For the GRAAPH-2005 study, the median OS for the imatinib + hyper-CVAD arm was 1.8 years after a median follow-up of 4.8 years. For the NCT00038610 study, the median OS was reached at 31 months (27 months with censoring of patients receiving stem cell transplants).
Ponatinib + Steroids vs. Imatinib Monotherapy Induction Followed by Age-adjusted Consolidation with Imatinib + Intrathecal Chemotherapy
Ponatinib prolonged OS compared to imatinib both before and after population adjustment. The unadjusted HR (95% CI) was 0.40 (0.20–0.81) and the adjusted HR was 0.24 (0.09–0.64) for GIMEMA LAL1811 (ponatinib) vs. CSI57ADE10 (imatinib) (Table 2; Fig. 2). However, the median OS was not achieved at the data cut-off point in the GIMEMA LAL1811 study, despite a median follow-up of 34.9 months. In the CSI57ADE10 study, the median follow-up was 11.2 months; the median OS was 23.5 months in patients who received imatinib induction and 12.3 months in patients who received chemotherapy induction.
Complete Molecular Response
Ponatinib + Hyper-CVAD vs. Imatinib + Hyper-CVAD
Patients who received ponatinib + hyper-CVAD achieved a CMR significantly more frequently than those who received imatinib + hyper-CVAD. The adjusted OR (95% CI) for CMR was 12.11 (3.77–38.87) for MDACC vs. GRAAPH-2005 and 5.65 (2.02–15.76) for MDACC vs. NCT00038610 (Table 3).
Table 3.
Relative efficacy of ponatinib vs. imatinib—complete molecular response
| CMR | MDACC vs. GRAAPH-2005 | MDACC vs. NCT00038610 | GIMEMA LAL1811 vs. CSI57ADE10 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Unadjusted comparison | 12.34 (5.77, 26.41) | < 0.001 | 4.93 (2.13, 11.39) | < 0.001 | 7.65 (2.48, 23.64) | < 0.001 |
| Population-adjusted comparison | 12.11 (3.77, 38.87) | < 0.001 | 5.65 (2.02, 15.76) | < 0.001 | 6.20 (1.60, 24.00) | 0.008 |
CI confidence interval, CMR complete molecular response, OR odds ratio
Ponatinib + Steroids vs. Imatinib Monotherapy Induction Followed by Age-adjusted Consolidation with Imatinib + Intrathecal Chemotherapy
Patients who received ponatinib + steroids were more likely to achieve a CMR than patients who received imatinib monotherapy. The adjusted OR (95% CI) for CMR was 6.20 (1.60–24.00) for GIMEMA LAL1811 vs. CSI57ADE10 (Table 3).
Complete Hematologic Response, Event-Free Survival, and Disease-Free Survival
As all the patients in the MDACC study achieved a CHR, no relative efficacy estimate could be calculated. Ninety-one percent of patients in the GRAAPH-2005 study and 93% of patients in the NCT00038610 study achieved a CHR. As 95.5% of the ponatinib-treated patients in the GIMEMA LAL1811 study and all imatinib-treated patients in the CSI57ADE10 study achieved a CHR, the two treatments were regarded as similar in the achievement of CHR.
In a comparison of MDACC vs. GRAAPH-2005, ponatinib + hyper-CVAD significantly prolonged EFS compared to imatinib + hyper-CVAD (Tables S11, S12; Fig. S1). The adjusted HR (95% CI) for EFS was 0.42 (0.21–0.83). The NCT00038610 and CSI57ADE10 studies did not report any data for EFS.
Ponatinib + hyper-CVAD also significantly prolonged DFS compared to imatinib + hyper-CVAD. The unadjusted HR (95% CI) for DFS was 0.55 (0.32–0.92) and the adjusted HR (95% CI) was 0.50 (0.27–0.93) in MDACC vs. NCT00038610 (Tables S11, S12; Fig. S2). The GIMEMA LAL1811 and GRAAPH-2005 studies did not report data for DFS.
Discussion
This is the first study to compare the efficacy of ponatinib and imatinib in the treatment of Ph + ALL. Treatment with ponatinib in combination with hyper-CVAD prolonged survival and increased molecular response rates in Ph + ALL patients compared to treatment with imatinib in combination with hyper-CVAD. Similarly, ponatinib in combination with steroids prolonged survival and increased the proportion of patients achieving a CMR compared to imatinib monotherapy induction + imatinib-containing consolidation. Compared to treatment with imatinib, OS was longer and the proportion of patients achieving a CMR was higher following treatment with ponatinib in both high-dose eligible and high-dose non-eligible patients with Ph + ALL. Sensitivity analyses conducted using alternative matching scenarios provided results consistent with the base case.
Imatinib, the first approved BCR::ABL1 inhibitor, revolutionized the treatment of Ph + ALL [1]. However, imatinib is not effective against tumors harboring emerging BCR::ABL mutants, including the BCR::ABL T315I “gatekeeper” mutation, which disrupts the interaction between the drug and its target ATP-binding site on the BCR::ABL tyrosine kinase. The BCR::ABL T315I mutation occurs in a subset of patients with Ph + ALL and tumors with this mutation are resistant to the first- and second-generation TKIs used to treat Ph + ALL [60]. Ponatinib is the only approved TKI for the BCR::ABL T315I mutation in Ph + ALL. A recent study showed that treatment with ponatinib (30 mg/day) with standard induction and consolidation chemotherapy followed by allogeneic hematopoietic stem cell transplant was well tolerated and showed promising EFS in adults with newly diagnosed Ph + ALL [61]. Further, a comparison between this trial and the ALLPh08 trial investigating the same schedule with imatinib showed significant improvement in OS for patients treated with ponatinib (3-year OS 96% vs. 53%, p = 0.002) [61]. Therefore, our findings add to existing evidence that ponatinib could be an effective first-line treatment for patients with Ph + ALL.
This study has some limitations. The ponatinib and imatinib populations may have been imbalanced because of unobserved factors that were not included in the population adjustment. Notably, few baseline characteristics were available for adjustment in the comparison conducted in the high-dose therapy-non-eligible population. Moreover, population adjustment for cytogenetic and additional molecular factors was not possible as data were not reported comparably across studies. Additionally, baseline ECOG performance status was not reported in the CSI57ADE10 study and so could not be included in the population adjustment. Therefore, the analyses of ponatinib versus imatinib in the high-dose non-eligible population may have been affected by unobserved imbalances.
Unanchored MAICs can mitigate bias in relative efficacy estimates due to imbalances in baseline characteristics between studies. However, they cannot mitigate bias that is caused by differences in study designs and definitions of outcomes. In this MAIC analysis, the definitions used for some of the efficacy outcomes were different in the MDACC and comparator studies. The definitions for molecular responses differed slightly across studies but were regarded as comparable. The definitions of CHR in the GIMEMA LAL1811 and CSI57ADE10 studies were different; therefore, the CHR could not be compared.
The ORs for CMR differed between the GRAAPH-2005 and NCT00038610 studies. This might be due to the different monitoring of patients for response in the GRAAPH-2005 study, which investigated imatinib + hyper-CVAD as a bridging therapy to stem cell transplantation. Specifically, CMR was assessed only during the initial two cycles of treatment; patients who continued to receive imatinib + hyper-CVAD without undergoing stem cell transplantation were not monitored further. By contrast, CMR was monitored throughout the NCT00038610 study. This might explain why more CMRs were captured in the NCT00038610 study compared to the GRAAPH-2005 study. As patients were monitored throughout the MDACC study, it is expected that the OR values obtained in the comparison against the NCT00038610 study are more representative of the true treatment effect of ponatinib versus imatinib than the ORs obtained in the comparison against the GRAAPH-2005 trial.
The comparison of ponatinib versus imatinib in high-dose therapy non-eligible patients was subject to considerable uncertainty because of the small effective sample size achieved following MAIC. Moreover, the OS data in ponatinib studies was immature and the median OS was not reached in the MDACC or GIMEMA LAL1811 studies. As a result, the true picture of OS is unknown for ponatinib and the relative efficacy of ponatinib versus imatinib for OS might change if the analysis was repeated using OS data for ponatinib with longer follow-up. Finally, MAICs do not provide the same level of statistical evidence as randomized controlled trials [35].
Conclusions
Our findings warrant further research comparing ponatinib-based and imatinib-based regimens. Several studies investigating ponatinib as a first-line treatment for Ph + ALL are currently ongoing. These include a phase 3 clinical trial (NCT03589326) comparing ponatinib versus imatinib (each in combination with reduced-intensity chemotherapy) and a phase 3 study (NCT04722848) investigating treatment with ponatinib and blinatumomab (sequence) versus imatinib and chemotherapy (combined) in patients with newly diagnosed Ph + ALL [62, 63]. Another phase 2 study is investigating treatment with ponatinib in combination with blinatumomab in patients with Ph + and/BCR::ABL + ALL. This MAIC analysis of ponatinib versus imatinib suggests that ponatinib is more effective than imatinib in both high-dose therapy eligible and non-eligible patients with newly diagnosed or minimally treated Ph + ALL, and that ponatinib could be a potential first-line treatment for these patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was funded by Incyte. The journal’s Rapid Service and Open Access fees were funded by Incyte.
Medical Writing Assistance
Medical writing was provided by Surayya Taranum, PhD (Evidera) and was paid for by Incyte.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of its research and content, and have given their approval for this version to be published.
Author Contributions
Josep-Maria Ribera provided clinical expert feedback on the analysis of the systematic literature review results. Thibaud Prawitz, Lorenzo Sabatelli, and Petros Patos contributed to the design of the study and the analysis and interpretation of the study results. Andreas Freitag contributed to the design and execution of the systematic literature review. Anuj Sharma and Balázs Dobi contributed to the analysis and interpretation of the study results. Federica Rizzo contributed to the review of the analysis and interpretation of the study results. All authors contributed to drafting the manuscript or revising it critically for important intellectual content and approve the final submitted version. Other: Giovanni Martinelli (I.R.C.C.S., Istituto Romagnolo per lo Studio dei Tumori “Dino Amadori,” Meldola, Italy) provided clinical expert feedback on the analysis of the systematic literature review results and has given permission to be named in the manuscript.
Disclosures
Josep-Maria Ribera was the principal investigator for clinical trials conducted by Pfizer and Ariad and has received honoraria for serving on advisory boards and as a speaker for Pfizer, Amgen, Shire, and Ariad. Thibaud Prawitz is an employee of Evidera Inc., which was paid by Incyte for work relating to this study and owns shares in Thermo Fisher Scientific. Andreas Freitag and Anuj Sharma are former employees of Evidera Inc. Balazs Dobi is an employee of Evidera Inc. Federica Rizzo and Petros Patos are employees of Incyte, which funded this study. Lorenzo Sabatelli was an employee and shareholder at Incyte during the time this study was conducted.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in the main article or as supplementary files.
References
- 1.Forghieri F, Luppi M, Potenza L. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology. 2015;20(10):618–619. doi: 10.1179/1024533215Z.000000000402. [DOI] [PubMed] [Google Scholar]
- 2.Ravandi F, Kebriaei P. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematol Oncol Clin N Am. 2009;23(5):1043–63 (vi). [DOI] [PMC free article] [PubMed]
- 3.Chiaretti S, Vitale A, Cazzaniga G, Orlando SM, Silvestri D, Fazi P, et al. Clinico-biological features of 5202 patients with acute lymphoblastic leukemia enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica. 2013;98(11):1702–1710. doi: 10.3324/haematol.2012.080432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gleissner B, Gökbuget N, Bartram CR, Janssen B, Rieder H, Janssen JW, et al. Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99(5):1536–1543. doi: 10.1182/blood.V99.5.1536. [DOI] [PubMed] [Google Scholar]
- 5.Faderl S, Kantarjian HM, Talpaz M, Estrov Z. Clinical significance of cytogenetic abnormalities in adult acute lymphoblastic leukemia. Blood. 1998;91(11):3995–4019. doi: 10.1182/blood.V91.11.3995. [DOI] [PubMed] [Google Scholar]
- 6.Faderl S, Kantarjian HM, Thomas DA, Cortes J, Giles F, Pierce S, et al. Outcome of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Leuk Lymphoma. 2000;36(3–4):263–273. doi: 10.3109/10428190009148847. [DOI] [PubMed] [Google Scholar]
- 7.de Klein A, van Kessel AG, Grosveld G, Bartram CR, Hagemeijer A, Bootsma D, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300(5894):765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- 8.(NCCN) NCCN. NCCN Clinial practice guidelines in oncology—acute lymphoblastic leukemia—version 1.2021 2021. https://www.nccn.org/patients/guidelines/content/PDF/all-patient.pdf. Accessed 6 Dec 2022.
- 9.Qiu LL, Lu YJ, Jing Y, Yu L, Liu DH, Wang LL. Comparison of clinical outcomes between P190 and P210 trans-cripts in adult Ph chromosome positive acute lymphoblastic leukemia in the new era of TKI. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24(2):369–374. doi: 10.7534/j.issn.1009-2137.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Liu-Dumlao T, Kantarjian H, Thomas DA, O'Brien S, Ravandi F. Philadelphia-positive acute lymphoblastic leukemia: current treatment options. Curr Oncol Rep. 2012;14(5):387–394. doi: 10.1007/s11912-012-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daver N, Thomas D, Ravandi F, Cortes J, Garris R, Jabbour E, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100(5):653–661. doi: 10.3324/haematol.2014.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim SN, Joo YD, Lee KH, Kim DY, Lee JH, Lee JH, et al. Long-term follow-up of imatinib plus combination chemotherapy in patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Am J Hematol. 2015;90(11):1013–1020. doi: 10.1002/ajh.24137. [DOI] [PubMed] [Google Scholar]
- 13.Kim DY, Joo YD, Lim SN, Kim SD, Lee JH, Lee JH, et al. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood. 2015;126(6):746–756. doi: 10.1182/blood-2015-03-636548. [DOI] [PubMed] [Google Scholar]
- 14.Jabbour E, Kantarjian H, Ravandi F, Thomas D, Huang X, Faderl S, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. Lancet Oncol. 2015;16(15):1547–1555. doi: 10.1016/S1470-2045(15)00207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalandon Y, Thomas X, Hayette S, Cayuela JM, Abbal C, Huguet F, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711–3719. doi: 10.1182/blood-2015-02-627935. [DOI] [PubMed] [Google Scholar]
- 16.Chiaretti S, Vitale A, Vignetti M, Piciocchi A, Fazi P, Elia L, et al. A sequential approach with imatinib, chemotherapy and transplant for adult Ph+ acute lymphoblastic leukemia: final results of the GIMEMA LAL 0904 study. Haematologica. 2016;101(12):1544–1552. doi: 10.3324/haematol.2016.144535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. Information on Gleevec (Imatinib Mesylate). 2003. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-gleevec-imatinib-mesylate. Accessed 6 Dec 2022.
- 18.European Medicines Agency. Imatinib accord 2017. https://www.ema.europa.eu/en/documents/overview/imatinib-accord-epar-summary-public_en.pdf. Accessed 6 Dec 2022.
- 19.European Medicines Agency. Dasatinib Accordpharma. https://www.ema.europa.eu/en/medicines/human/EPAR/dasatinib-accordpharma. Accessed 6 Dec 2022.
- 20.FDA. SPRYCEL® (dasatinib) tablet for oral use. Full prescribing information 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021986s7s8lbl.pdf. Accessed 6 Dec 2022.
- 21.Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v69–v82. doi: 10.1093/annonc/mdw025. [DOI] [PubMed] [Google Scholar]
- 22.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki K, Jabbour EJ, Ravandi F, Short NJ, Thomas DA, Garcia-Manero G, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a propensity score analysis. Cancer. 2016;122(23):3650–3656. doi: 10.1002/cncr.30231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabbour E, Short NJ, Ravandi F, Huang X, Daver N, DiNardo CD, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. 2018;5(12):e618–e627. doi: 10.1016/S2352-3026(18)30176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367(22):2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah NP, Talpaz M, Deininger MW, Mauro MJ, Flinn IW, Bixby D, et al. Ponatinib in patients with refractory acute myeloid leukaemia: findings from a phase 1 study. Br J Haematol. 2013;162(4):548–552. doi: 10.1111/bjh.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Incyte Biosciences Distribution BV. Iclusig (ponatinib). Summary of product characteristics. https://www.ema.europa.eu/documents/product-information/iclusig-epar-product-information_en.pdf. Accessed 6 Dec 2022.
- 28.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393–404. doi: 10.1182/blood-2016-09-739086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foà R, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo MC, et al. Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383(17):1613–1623. doi: 10.1056/NEJMoa2016272. [DOI] [PubMed] [Google Scholar]
- 30.Couturier MA, Thomas X, Raffoux E, Huguet F, Berthon C, Simand C, et al. Blinatumomab + ponatinib for relapsed/refractory Philadelphia chromosome-positive acute lymphoblastic leukemia in adults. Leuk Lymphoma. 2021;62(3):620–629. doi: 10.1080/10428194.2020.1844198. [DOI] [PubMed] [Google Scholar]
- 31.Advani AS, Moseley A, O'Dwyer KM, Wood BL, Park JH, Wieduwilt MJ, et al. dasatinib/prednisone induction followed by blinatumomab/dasatinib in Ph+ acute lymphoblastic leukemia. Blood Adv. 2023;7(7):1279–1285. doi: 10.1182/bloodadvances.2022008216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Medicines Agency. Iclusig. https://www.ema.europa.eu/en/medicines/human/EPAR/iclusig. Accessed 6 Dec 2022.
- 33.FDA. ICLUSIG® (ponatinib) tablets for oral use. Full prescribing information 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203469lbl.pdf. Accessed 6 Dec 2022.
- 34.Martinelli G, Papayannidis C, Piciocchi A, Robustelli V, Soverini S, Terragna C, et al. INCB84344-201: ponatinib and steroids in frontline therapy for unfit patients with Ph+ acute lymphoblastic leukemia. Blood Adv. 2022;6(6):1742–1753. doi: 10.1182/bloodadvances.2021004821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Signorovitch JE, Sikirica V, Erder MH, Xie J, Lu M, Hodgkins PS, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947. doi: 10.1016/j.jval.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Evidence Matters. DistillerSR: literature review software. https://www.evidencepartners.com/. Accessed 6 Dec 2022.
- 37.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 38.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Mak. 2018;38(2):200–211. doi: 10.1177/0272989X17725740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell MMB, Winchen T, van Schaik BAW. Engauge digitizer software.
- 40.AR. WebPlotDigitizer user manual version 3.4. 2014.
- 41.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ottmann OG, Wassmann B, Pfeifer H, Giagounidis A, Stelljes M, Dührsen U, et al. Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) Cancer. 2007;109(10):2068–2076. doi: 10.1002/cncr.22631. [DOI] [PubMed] [Google Scholar]
- 43.Thomas DA, Faderl S, Cortes J, O'Brien S, Giles FJ, Kornblau SM, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 44.(EMA) EMA. Imatinib summary of product characteristics. 2011. https://www.ema.europa.eu/en/documents/product-information/glivec-epar-product-information_en.pdf. Accessed 6 Dec 2022.
- 45.Wassmann B, Pfeifer H, Goekbuget N, Beelen DW, Beck J, Stelljes M, et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2006;108(5):1469–1477. doi: 10.1182/blood-2005-11-4386. [DOI] [PubMed] [Google Scholar]
- 46.Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F, et al. Karyotype at diagnosis is the major prognostic factor predicting relapse-free survival for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia treated with imatinib-combined chemotherapy. Haematologica. 2008;93(2):287–290. doi: 10.3324/haematol.11891. [DOI] [PubMed] [Google Scholar]
- 47.de Labarthe A, Rousselot P, Huguet-Rigal F, Delabesse E, Witz F, Maury S, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood. 2007;109(4):1408–1413. doi: 10.1182/blood-2006-03-011908. [DOI] [PubMed] [Google Scholar]
- 48.Tanguy-Schmidt A, Rousselot P, Chalandon Y, Cayuela JM, Hayette S, Vekemans MC, et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant. 2013;19(1):150–155. doi: 10.1016/j.bbmt.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 49.Lee S, Kim YJ, Chung NG, Lim J, Lee DG, Kim HJ, et al. The extent of minimal residual disease reduction after the first 4-week imatinib therapy determines outcome of allogeneic stem cell transplantation in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2009;115(3):561–570. doi: 10.1002/cncr.24026. [DOI] [PubMed] [Google Scholar]
- 50.Fielding AK, Rowe JM, Buck G, Foroni L, Gerrard G, Litzow MR, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123(6):843–850. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bassan R, Rossi G, Pogliani EM, Di Bona E, Angelucci E, Cavattoni I, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28(22):3644–3652. doi: 10.1200/JCO.2010.28.1287. [DOI] [PubMed] [Google Scholar]
- 52.Lee KH, Lee JH, Choi SJ, Lee JH, Seol M, Lee YS, et al. Clinical effect of imatinib added to intensive combination chemotherapy for newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2005;19(9):1509–1516. doi: 10.1038/sj.leu.2403886. [DOI] [PubMed] [Google Scholar]
- 53.Hatta Y, Mizuta S, Matsuo K, Ohtake S, Iwanaga M, Sugiura I, et al. Final analysis of the JALSG Ph+ALL202 study: tyrosine kinase inhibitor-combined chemotherapy for Ph+ALL. Ann Hematol. 2018;97(9):1535–1545. doi: 10.1007/s00277-018-3323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Towatari M, Yanada M, Usui N, Takeuchi J, Sugiura I, Takeuchi M, et al. Combination of intensive chemotherapy and imatinib can rapidly induce high-quality complete remission for a majority of patients with newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Blood. 2004;104(12):3507–3512. doi: 10.1182/blood-2004-04-1389. [DOI] [PubMed] [Google Scholar]
- 55.Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24(3):460–466. doi: 10.1200/JCO.2005.03.2177. [DOI] [PubMed] [Google Scholar]
- 56.Motlló C, Ribera JM, Morgades M, Granada I, Montesinos P, Mercadal S, et al. Frequency and prognostic significance of additional cytogenetic abnormalities to the Philadelphia chromosome in young and older adults with acute lymphoblastic leukemia. Leuk Lymphoma. 2018;59(1):146–154. doi: 10.1080/10428194.2017.1326596. [DOI] [PubMed] [Google Scholar]
- 57.Ribera JM, García O, Moreno MJ, Barba P, García-Cadenas I, Mercadal S, et al. Incidence and outcome after first molecular versus overt recurrence in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia included in the ALL Ph08 trial from the Spanish PETHEMA Group. Cancer. 2019;125(16):2810–2817. doi: 10.1002/cncr.32156. [DOI] [PubMed] [Google Scholar]
- 58.Delannoy A, Delabesse E, Lhéritier V, Castaigne S, Rigal-Huguet F, Raffoux E, et al. Imatinib and methylprednisolone alternated with chemotherapy improve the outcome of elderly patients with Philadelphia-positive acute lymphoblastic leukemia: results of the GRAALL AFR09 study. Leukemia. 2006;20(9):1526–1532. doi: 10.1038/sj.leu.2404320. [DOI] [PubMed] [Google Scholar]
- 59.Vignetti M, Fazi P, Cimino G, Martinelli G, Di Raimondo F, Ferrara F, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109(9):3676–3678. doi: 10.1182/blood-2006-10-052746. [DOI] [PubMed] [Google Scholar]
- 60.Cohen P, Cross D, Jänne PA. Kinase drug discovery 20 years after imatinib: progress and future directions. Nat Rev Drug Discov. 2021;20(7):551–569. doi: 10.1038/s41573-021-00195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ribera JM, García-Calduch O, Ribera J, Montesinos P, Cano-Ferri I, Martínez P, et al. Ponatinib, chemotherapy, and transplant in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022. [DOI] [PMC free article] [PubMed]
- 62.A study of ponatinib versus imatinib in adults with acute lymphoblastic leukemia.https://www.clinicaltrials.gov/ct2/show/NCT03589326. Accessed 6 Dec 2022.
- 63.Sequential treatment with ponatinib and blinatumomab vs chemotherapy and imatinib in newly diagnosed adult Ph+ ALL.https://clinicaltrials.gov/ct2/show/NCT04722848. Accessed 6 Dec 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the main article or as supplementary files.