Abstract
Objective
The objective of this study was to evaluate the feasibility of minimally invasive surgery (MIS) and perioperative outcomes following neoadjuvant immunotherapy for resectable non-small-cell lung cancer (NSCLC).
Methods
Patients with stage I-III NSCLC treated with immunotherapy with or without chemotherapy or chemotherapy alone prior to lobectomy were identified in the National Cancer Data Base (2010–2018). The percentage of operations performed minimally invasively, conversion rates, and perioperative outcomes were evaluated using propensity-score matching. Propensity-score matching was also used to compare perioperative outcomes between patients who received an open vs. MIS lobectomy after neoadjuvant immunotherapy.
Results
Of the 4,229 patients identified, 218 (5%) received neoadjuvant immunotherapy and 4,011 (95%) received neoadjuvant chemotherapy alone. There was no difference in the rate of MIS lobectomy among patients who received immunotherapy vs. chemotherapy alone in propensity-score matched analysis (60.8% vs. 51.6%, p= 0.11). There were also no significant differences in the rate of conversion from MIS to open lobectomy (14% vs. 15%, p=0.83; OR 1.1 [95% CI: 0.51–2.24]) or in nodal downstaging, margin positivity, 30-day readmission, and 30- and 90-day mortality between the two groups. In a subgroup analysis of only patients treated with neoadjuvant immunotherapy, there were no differences in pathologic or perioperative outcomes between patients who received an open vs. MIS lobectomy.
Conclusions
In this national analysis, neoadjuvant immunotherapy for resectable NSCLC was not associated with an increased likelihood of requiring a thoracotomy, conversion from MIS to open lobectomy, or inferior perioperative outcomes.
Keywords: Non-small-cell lung cancer, minimally invasive surgery, immunotherapy, neoadjuvant
INTRODUCTION
Induction therapy offers the theoretical advantage of reducing tumor size and micrometastatic disease burden prior to surgery.1 Despite this, preoperative chemotherapy for stage IB-IIIA non-small-cell lung cancer (NSCLC) has been shown to only improve five-year survival by approximately 5%.2 Emerging data from randomized clinical trials, however, suggests that immunotherapy may significantly improve outcomes among patients with resectable NSCLC.3,4 When compared with chemotherapy alone, patients treated with combination immunotherapy and chemotherapy have exhibited increased rates of major and complete pathologic response, as well as improved event free and overall survival.3,4
Although the addition of immunotherapy to induction treatment regimens is promising from an oncologic perspective, there is concern among surgeons that preoperative immunotherapy may increase subsequent operative complexity, potentially leading to increased rates of open surgery and worse perioperative outcomes.5 Early case series have suggested that resection after immunotherapy is technically challenging due to treatment-related hilar and mediastinal fibrosis, resulting in more thoracotomies and higher rates of conversion from minimally invasive to open operations.6,7 In contrast, however, recent data from the phase III clinical trial Checkmate 816 found that patients treated with combination immunotherapy and chemotherapy prior to surgery had shorter operative times, required fewer thoracotomies, had less intraoperative conversion, and were less likely to require a pneumonectomy than patients treated with neoadjuvant chemotherapy alone.8 In light of this, the safety and feasibility of performing minimally invasive surgery after neoadjuvant immunotherapy remains unclear. The objective of this study was therefore to evaluate the feasibility of minimally invasive lobectomy and short-term outcomes following immunotherapy for clinical stage I-III NSCLC.
METHODS
Data Source
The Institutional Review Board (IRB) of Massachusetts General Hospital approved the study protocol and publication of data. Patient written consent for the publication of the study data was waived by the IRB given that all patient data was de-identified prior to analysis and publication (#2020P004110, 02/02/2021). Data used for this study were from the National Cancer Database (NCDB), a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The NCDB is estimated to capture at least 65% of all newly diagnosed cases of lung cancer in the United States and Puerto Rico across 1,500 Commission on Cancer (Co-C)- accredited institutions.9 Clinical and pathologic staging information is recorded in the NCDB using the 7th and 8th edition American Joint Committee on Cancer (AJCC) Tumor Nodes and Metastases (TNM) classifications. However, for the present study, we reclassified all staging information according to the AJCC 8th edition criteria with best available data.10
Study Design
Patients diagnosed with clinical stage I-III NSCLC who underwent lobectomy after neoadjuvant chemotherapy alone or immunotherapy with or without chemotherapy (hereafter referred to as “immunotherapy”) between 2010–2018 were identified for analysis. Patients who received preoperative radiation or underwent surgery more than seven months after the initiation of neoadjuvant treatment were excluded from this study.
Several analyses were performed to examine the feasibility of minimally invasive lobectomy (including video-assisted thoracoscopic and robotic approaches) after neoadjuvant immunotherapy. First, we compared the rates of open and minimally invasive lobectomy after neoadjuvant immunotherapy to the rates of open and minimally invasive lobectomy after neoadjuvant chemotherapy alone. In addition, we compared the perioperative outcomes, including nodal upstaging, nodal downstaging, margin positivity, 30-day readmission, and 30- and 90-day mortality, between patients who received neoadjuvant immunotherapy and neoadjuvant chemotherapy alone.
Second, in an effort to minimize selection bias confounding interpretation of outcomes among patients who underwent minimally invasive surgery, we conducted a subgroup analysis of only patients who underwent minimally invasive lobectomy after neoadjuvant immunotherapy or neoadjuvant chemotherapy alone. We compared the same perioperative outcomes detailed above between patients in this subgroup. Additionally, we assessed whether patients who underwent preoperative immunotherapy were more likely to undergo intraoperative conversion to an open lobectomy compared to patients who underwent preoperative chemotherapy alone.
Third, we performed another subgroup analysis including only patients who received neoadjuvant immunotherapy, again attempting to minimize selection bias attributed to a predominantly clinical trial based patient population. Among this cohort of patients who received neoadjuvant immunotherapy, we compared the same perioperative outcomes described above between patients who underwent an open vs. minimally invasive lobectomy.
Finally, we performed a fourth subgroup analysis comparing the rates of open and minimally invasive lobectomy, as well as perioperative outcomes, between patients with stage II NSCLC treated with immunotherapy prior to lobectomy vs. those treated with upfront surgery.
Statistical Analyses
For each analysis detailed above, baseline characteristics and pathologic and perioperative outcomes between patient groups were evaluated using the Wilcoxon rank-sum test for continuous variables and Pearson’s chi-square test, or Fisher’s Exact Test when appropriate, for discrete variables.
The association between the receipt of preoperative immunotherapy and undergoing a subsequent minimally invasive operation was examined using multivariable logistic regression. This model adjusted for covariates that were determined a priori to be clinically significant, including age, sex, Charlson Deyo Comorbidity (CDCC) score, insurance status, clinical T status, clinical N status, tumor location, tumor size, grade, histology, facility type , and volume of lobectomies performed at each institution. In addition, among a subgroup of patients who underwent minimally invasive lobectomy, the association between the receipt of preoperative immunotherapy and the odds of intraoperative conversion to open lobectomy was examined using multivariable logistic regression, adjusting for the aforementioned covariates. We also used restricted cubic splines to model the association between time from neoadjuvant immunotherapy to lobectomy and outcomes such as surgical margin positivity, conversion from minimally invasive to open surgery, 30-day readmission, and 90-day mortality.
Propensity-score matching was then used to assess the proportion of lobectomies performed via a minimally invasive approach and pathologic and perioperative outcomes among patients who received neoadjuvant immunotherapy vs. neoadjuvant chemotherapy alone followed by lobectomy. The covariates we matched by included sex, age, race, tumor location, histology, CDCC score, tumor size, clinical T status, clinical N status, grade, year of diagnosis, insurance status, facility type, and volume of lobectomies performed at each institution. Median census tract education, income, and the distance of the patient’s residence from the hospital were also included as covariates in an attempt to minimize confounding due to factors related to socioeconomic status and access to care. Matched groups were assigned using a greedy nearest neighbor algorithm without replacement with a caliper of 0.01 and balance was assessed using standardized differences.
Among a subgroup of patients who only underwent minimally invasive lobectomy, propensity-score matching, using the same methodology described above, was used to examine rates of intraoperative conversion and other perioperative outcomes between patients who received neoadjuvant immunotherapy vs. neoadjuvant chemotherapy alone.
Further subgroup analysis with propensity-score matching according to the same methodology was then used to compare perioperative outcomes between patients who underwent minimally invasive vs. open lobectomy after neoadjuvant immunotherapy.1
Finally, the rates of open surgery and perioperative outcomes were compared between similarly propensity score-matched groups belonging to a subcohort of patients with stage II NSCLC that were treated with immunotherapy followed by lobectomy vs. upfront surgery.
All statistical analyses were performed using Stata Statistical Software: Release 13.0 (StataCorp LP, College Station, TX).
RESULTS
Patient and treatment characteristics of the overall cohort
We initially identified 5,878 patients treated with chemotherapy alone or immunotherapy with or without chemotherapy (“immunotherapy”) followed by surgery. Among these patients, lobectomy was the most common operation performed (72% vs. 76% after chemotherapy or immunotherapy, respectively), followed by pneumonectomy (13% vs. 10%), and extended lobectomy or bilobectomy (9% vs.8%). Sublobar resection was performed in less than 6% of patients who received either induction therapy and, overall, there was no significant difference in the distribution of operations performed between patients in either treatment group (p=0.19).
Of the 4,229 patients who received a lobectomy and thus met study inclusion criteria, 4,011 (95%) patients received neoadjuvant chemotherapy alone and 218 (5%) patients received neoadjuvant immunotherapy.
Table I shows the baseline characteristics of patients who underwent neoadjuvant chemotherapy alone vs. neoadjuvant immunotherapy followed by lobectomy. In unadjusted analysis, patients who received neoadjuvant immunotherapy were significantly more likely to receive care at an academic center when compared to patients treated with neoadjuvant chemotherapy alone (76.6% vs. 48.5%, p<0.001). They were also more likely to undergo a minimally invasive lobectomy (60.5% vs. 37.5%, p<0.001). However, in multivariable-adjusted analysis, the odds of undergoing a minimally invasive lobectomy were similar between patients who had received neoadjuvant immunotherapy vs. neoadjuvant chemotherapy alone (multivariable-adjusted odds ratio [aOR]: 1.34, [95% CI: 0.85–2.12).
Table I.
Characteristics of the overall cohort of patients with clinical stage I-III NSCLC undergoing lobectomy after neoadjuvant chemotherapy vs neoadjuvant immunotherapy.
| Characteristics | Chemotherapy (n=4011) | Immunotherapy (n=218) | p-value |
|---|---|---|---|
| Age at diagnosis, median (IQR) | 64.0 (57.0, 70.0) | 65.0 (57.0, 72.0) | 0.13 |
| Sex, no. (%, female) | 2037 (50.8%) | 119 (54.6%) | 0.27 |
| Race, no. (%) | |||
| White | 3401 (84.8%) | 190 (87.2%) | 0.71 |
| Black | 376 (9.4%) | 17 (7.8%) | |
| Native American | 13 (0.3%) | 0 (0.0%) | |
| Asian | 148 (3.7%) | 6 (2.8%) | |
| Unknown | 73 (1.8%) | 5 (2.3%) | |
| Charlson-Deyo Comorbidity Score, no. (%) | |||
| 0 | 2416 (60.2%) | 122 (56.0%) | 0.29 |
| 1 | 1135 (28.3%) | 67 (30.7%) | |
| 2 | 338 (8.4%) | 18 (8.3%) | |
| 3+ | 122 (3.0%) | 11 (5.0%) | |
| Clinical stage, no. (%) | |||
| Ia | 106 (2.6%) | 11 (5.0%) | <0.001 |
| Ib | 155 (3.9%) | 17 (7.8%) | |
| IIa | 105 (2.6%) | 12 (5.5%) | |
| IIb | 682 (17.0%) | 56 (25.7%) | |
| IIIa | 2087 (52.0%) | 91 (41.7%) | |
| IIIb | 824 (20.5%) | 29 (13.3%) | |
| IIIc | 52 (1.3%) | 2 (0.9%) | |
| Tumor size (mm; median, IQR) | 40.0 (26.0, 59.0) | 41.0 (25.0, 60.0) | 0.75 |
| Tumor location, no. (%) | |||
| Main bronchus | 25 (0.6%) | 2 (0.9%) | 0.09 |
| Right upper lobe | 1656 (41.3%) | 79 (36.2%) | |
| Right middle lobe | 163 (4.1%) | 5 (2.3%) | |
| Right lower lobe | 550 (13.7%) | 44 (20.2%) | |
| Left upper lobe | 1042 (26.0%) | 53 (24.3%) | |
| Left lower lobe | 440 (11.0%) | 30 (13.8%) | |
| Unknown/other | 160 (3.9%) | 7 (3.2%) | |
| Histology, no. (%) | |||
| Adenocarcinoma | 1996 (49.8%) | 119 (54.6%) | 0.18 |
| Squamous cell carcinoma | 1285 (32.0%) | 70 (32.1%) | |
| Other/Unknown | 730 (18.2%) | 29 (13.4%) | |
| Grade differentiation, no. (%) | |||
| Well differentiated | 135 (3.4%) | 11 (5.0%) | 0.002 |
| Moderately differentiated | 1012 (25.2%) | 43 (19.7%) | |
| Poorly differentiated | 1541 (38.4%) | 69 (31.7%) | |
| Undifferentiated | 41 (1.0%) | 0 (0.0) | |
| Cell type not determined | 1282 (32.0%) | 95 (43.6%) | |
| Primary payor at diagnosis, no. (%) | |||
| Not insured | 82 (2.0%) | 1 (0.5%) | 0.15 |
| Private insurance | 1673 (41.7%) | 96 (44.0%) | |
| Medicaid | 288 (7.2%) | 17 (7.8%) | |
| Medicare | 1846 (46.0%) | 98 (45.0%) | |
| Other government | 60 (1.5%) | 0 (0.0%) | |
| Unknown | 62 (1.5%) | 6 (2.8%) | |
| Facility type, no. (%) | |||
| Community cancer program | 88 (2.2%) | 1 (0.5%) | <0.001 |
| Comprehensive community cancer program | 1156 (28.8%) | 32 (14.7%) | |
| Academic research program | 1944 (48.5%) | 167 (76.6%) | |
| Integrated network cancer program | 784 (19.5%) | 15 (6.9%) | |
| Unknown | 39 (1.0%) | 3 (1.4%) | |
| Distance from hospital (median, IQR) | 13.6 (5.9, 34.0) | 18.8 (8.4, 56.7) | <0.001 |
| Median income (census tract), no. (%) | |||
| <$40,227 | 610 (15.2%) | 25 (11.5%) | 0.053 |
| $40,227-$50,353 | 752 (18.7%) | 39 (17.9%) | |
| $50,354-$63,332 | 815 (20.3%) | 37 (17.0%) | |
| ≥$63,333 | 1344 (33.5%) | 92 (42.2%) | |
| Unknown | 490 (12.2%) | 25 (11.5%) | |
| Median education (census tract), no. (%) | |||
| 17.6% without a high school degree | 683 (17.0%) | 37 (17.0%) | 0.38 |
| 10.9–17.5% without a high school degree | 943 (23.5%) | 54 (24.8%) | |
| 6.3–10.8% without a high school degree | 1092 (27.2%) | 50 (22.9%) | |
| <6.3% without a high school degree | 816 (20.3%) | 53 (24.3%) | |
| Unknown | 477 (11.9%) | 24 (11.0%) | |
| Year of diagnosis, median (IQR) | 2014.0 (2012.0, 2016.0) | 2017.0 (2017.0, 2018.0) | <0.001 |
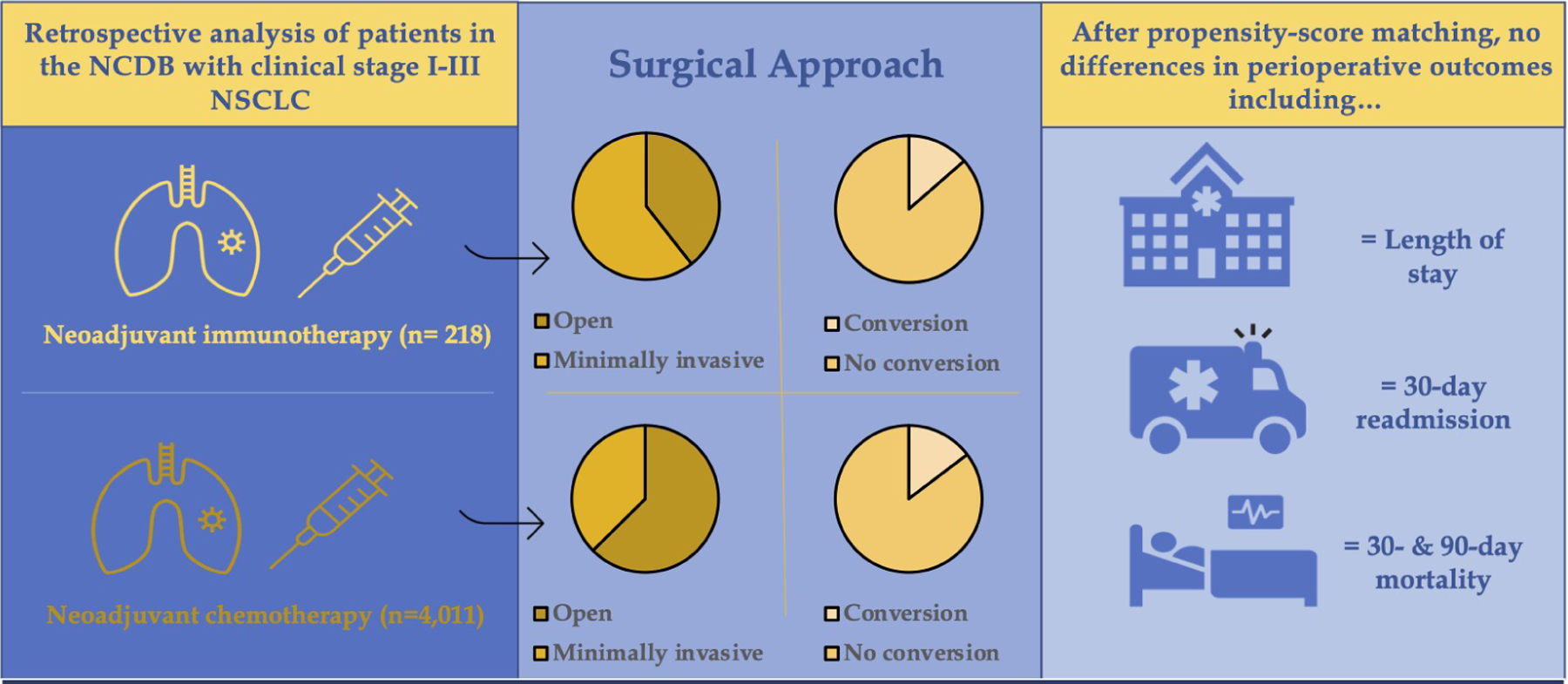
Perioperative outcomes of the overall cohort
The pathologic characteristics and perioperative outcomes of patients receiving neoadjuvant immunotherapy vs. neoadjuvant chemotherapy alone followed by lobectomy are shown in Table II. In unadjusted analysis, patients who received neoadjuvant immunotherapy had significantly more lymph nodes evaluated intraoperatively compared to patients who received neoadjuvant chemotherapy alone (17 (IQR: 11–28) vs. 13 (IQR: 7–21), p<0.001), however patients who received neoadjuvant chemotherapy alone were significantly more likely to experience nodal downstaging (52.7% vs. 35.1%, p<0.001). There were no significant differences in 30-day readmission, or 30- or 90-day mortality between patients treated with either neoadjuvant therapy. There were also no significant positive or negative associations between time from induction therapy to lobectomy and outcomes such as surgical margin positivity, conversion to an open lobectomy, 30-day readmission, or 90-day mortality. Of note, the average duration between induction therapy and surgery was shorter among patients treated with neoadjuvant immunotherapy vs. those treated with neoadjuvant chemotherapy alone (72 vs. 107 days).
Table II.
Pathological characteristics and perioperative outcomes of the overall cohort of patients with clinical stage I-III NSCLC undergoing lobectomy after neoadjuvant chemotherapy vs neoadjuvant immunotherapy.
| Characteristics | Chemotherapy (n=4011) | Immunotherapy (n=218) | p-value |
|---|---|---|---|
| Resection approach, no. (%) | |||
| Thoracotomy | 2511 (62.6%) | 86 (39.4%) | <0.0012 |
| VATS | 1036 (25.8%) | 72 (33.0%) | 0.02 |
| Robotic | 464 (11.6%) | 60 (27.5%) | <0.001 |
|
| |||
| Pathologic T stage, no. (%) | |||
| T0/Tis | 514 (12.8%) | 15 (6.9%) | 0.15 |
| T1a | 103 (2.6%) | 8 (3.7%) | |
| T1b | 337 (8.4%) | 16 (7.3%) | |
| T1c | 456 (11.4%) | 29 (13.3%) | |
| T2a | 695 (17.3%) | 39 (17.9%) | |
| T2b | 449 (11.2%) | 33 (15.1%) | |
| T3 | 872 (21.7%) | 46 (21.1%) | |
| T4 | 573 (14.3%) | 30 (13.8%) | |
| Unknown | 12 (0.3%) | 2 (0.9%) | |
| Pathologic N stage, no. (%) | |||
| N0 | 2023 (58.0%) | 77 (60.2%) | 0.54 |
| N1 | 583 (16.7%) | 25 (19.5%) | |
| N2 | 870 (25.0%) | 26 (20.3%) | |
| N3 | 11 (0.3%) | 0 (0.0%) | |
| Unknown | 524 | 90 | |
| Pathologic M stage, no. (%) | |||
| M0 | 1049 (97.9%) | 101 (99.0%) | 0.43 |
| M1 | 23 (2.2%) | 1 (1.0%) | |
| Unknown | 2939 | 116 | |
| Nodal upstaging, no. (%) | 275 (7.0%) | 16 (7.5%) | 0.79 |
| Nodal downstaging, no. (%) | 1530 (52.7%) | 46 (35.1%) | <0.001 |
| Lymph nodes examined, no. (%) | 3896 (97.4%) | 211 (96.8%) | 0.6 |
| Number of lymph nodes examined (median, IQR) | 13.0 (7.0, 21.0) | 17.0 (11.0, 28.0) | <0.001 |
| Surgical margin, no. (%) | |||
| Negative | 3750 (94.2%) | 203 (94.4 %) | 0.90 |
| Positive | 230 (5.8%) | 12 (5.6%) | |
| Unknown | 31 | 3 | |
| Length of stay (median, IQR) | 5.0 (3.0, 7.0) | 4.0 (3.0, 6.0) | <0.001 |
| 30-day readmission, no. (%) | |||
| No | 3848 (96.5%) | 204 (94.0%) | 0.06 |
| Yes | 140 (3.5%) | 13 (6.0%) | |
| Unknown | 23 | 1 | |
| 30-day mortality, no. (%) | |||
| No | 3534 (98.2%) | 108 (98.2) | 0.99 |
| Yes | 66 (1.8%) | 2 (1.8%) | |
| Unknown | 411 | 108 | |
| 90-day mortality, no. (%) | |||
| No | 3434 (95.8%) | 107 (97.3%) | 0.45 |
| Yes | 150 (4.2%) | 3 (2.7%) | |
| Unknown | 427 | 108 | |
Propensity-matched analysis of patients undergoing lobectomy after neoadjuvant immunotherapy vs. neoadjuvant chemotherapy alone
Propensity-score matching was used to create two well-matched groups of 153 patients who underwent neoadjuvant immunotherapy or neoadjuvant chemotherapy alone prior to lobectomy. The median year of diagnosis was 2017 among patients in both groups. Using this matched analysis, we found that patients treated with neoadjuvant immunotherapy were just as likely to receive a minimally invasive lobectomy relative to patients treated with neoadjuvant chemotherapy alone (60.8% vs. 51.6%, p= 0.11). With regards to pathologic and perioperative outcomes, there were no significant difference in surgical margin positivity, nodal upstaging, nodal downstaging, hospital length of stay, 30-day readmission, or 30- or 90-day mortality between the two groups.
Subgroup analysis of patients who underwent minimally invasive lobectomy after neoadjuvant immunotherapy vs. neoadjuvant chemotherapy alone.
We then evaluated a subgroup of only patients who underwent MIS lobectomy. In unadjusted analysis, patients who underwent a minimally invasive lobectomy after immunotherapy were more likely to receive a robotic (as opposed to thoracoscopic) lobectomy when compared with patients who received neoadjuvant chemotherapy alone (Supplemental Table I). While patients who received chemotherapy alone were more likely to have clinical N2 disease or higher, they were also more likely to experience nodal downstaging with surgery and there was no difference in the distribution in nodal pathologic staging between the two groups. Notably, there were no significant differences in the rate of intraoperative conversion from minimally invasive to open lobectomy between patients undergoing either neoadjuvant treatment regimen (13.6% for immunotherapy vs. 14.7% for chemotherapy, p=0.75).
Next, propensity-score matching was used to create two well-matched groups of 84 patients who underwent minimally invasive lobectomy after immunotherapy or chemotherapy alone. Again, the median year of diagnosis was 2017 among patients in. both groups. Patients who received neoadjuvant chemotherapy alone were significantly more likely to experience nodal downstaging relative to patients treated with neoadjuvant immunotherapy (48% vs. 29%, p=0.04). Otherwise, there were no significant differences in short-term outcomes between the two groups, including with regards to surgical margin positivity, hospital length of stay, 30-day readmission, or 30- or 90-day mortality between the two groups. There were also no significant differences in the rates of intraoperative conversion to an open operation between patients undergoing neoadjuvant immunotherapy vs. neoadjuvant chemotherapy alone (14% vs. 15%, p=0.83).
Subgroup analysis of patients who underwent open vs. minimally invasive lobectomy after immunotherapy
We then performed another subgroup analysis that directly compared patients who had received neoadjuvant immunotherapy followed by a minimally invasive or open lobectomy. Out of this cohort of 218 patients, there were no significant differences in the baseline characteristics of patients who received either operative approach except in that patients who received a minimally invasive lobectomy tended to be slightly older than the patients who underwent a thoracotomy (median age 67 vs. 61, p=0.008). There were no differences with regards to pathologic or short-term perioperative outcomes between the two groups (Supplemental Table II).
In order to corroborate these findings while minimizing baseline confounding, we used propensity-score matching to create two well-matched groups of 33 patients who received a minimally invasive or open lobectomy after neoadjuvant immunotherapy. Although the power of this analysis was limited due to sample size, there were no significant differences in rates of nodal stage migration, lymph node examination, surgical margin positivity, hospital length of stay, 30-day readmission, or 30- or 90-day mortality between these two groups.
Subgroup analysis of patients treated with neoadjuvant immunotherapy vs. upfront surgery for stage II NSCLC
In our final subgroup analysis, we directly examined patients who received a lobectomy after neoadjuvant immunotherapy or as upfront surgery for stage II NSCLC. Propensity-score matching was used within this subgroup to create well matched groups of 49 patients who did or did not receive induction immunotherapy. Among these matched patients, there were no significant differences in the rates of thoracotomy between patients who received neoadjuvant immunotherapy vs. upfront surgery (35% vs. 31%, p= 0.67). There were also no significant differences between pathologic or perioperative outcomes between these two groups.
DISCUSSION
In this national analysis, we found that patients with stage I-III NSCLC treated with neoadjuvant immunotherapy were not more likely to require a thoracotomy or have higher rates of conversion from minimally invasive to open surgery when compared to patients treated with neoadjuvant chemotherapy alone. There were also no significant differences in surgical margin positivity, nodal stage migration, hospital length of stay, 30-day readmission, or 30- or 90-day mortality between patients receiving neoadjuvant immunotherapy vs. neoadjuvant chemotherapy alone (Figure I). These findings were consistent among a subgroup of patients who only received minimally invasive surgery. Among a subgroup of patients who only received neoadjuvant immunotherapy, we found that there were no significant differences in perioperative outcomes between patients who received open vs. minimally invasive lobectomy. Finally, among a subgroup of patients with stage II NSCLC treated with neoadjuvant immunotherapy vs. upfront surgery, we found no differences in perioperative outcomes or the rate of MIS lobectomy.
Figure 1:
Neoadjuvant immunotherapy is not associated with increased rates of thoracotomy, intraoperative conversion, or perioperative morbidity relative to neoadjuvant chemotherapy.
The present study is the first national analysis of the safety and feasibility of minimally invasive lobectomy after neoadjuvant immunotherapy for patients with resectable NSCLC. Prior case series have raised concerns that neoadjuvant immunotherapy may increase the complexity of subsequent operations, ultimately requiring more operations to be performed via an open approach and more intraoperative conversions.6,7 Chaft and colleagues were among the first to observe that patients treated with neoadjuvant immunotherapy may develop dense adhesions secondary to intrathoracic fibrosis, thereby complicating future anatomic resection.5 In addition, Bott et al. reported that the majority of surgical resections performed after immunotherapy required thoracotomies; of the 13 patients in whom MIS invasive lobectomy was attempted, 54% were converted to open.6 Furthermore, initial reports from NEOSTAR, a randomized phase II trial, found that 40% of resections after immunotherapy were classified by surgeons as more technically challenging than usual.11 It is worth noting, however, that all of these prior studies evaluated relatively small patient cohorts.
Despite these reports, however, our results are consistent with the findings of CheckMate 816, a recent phase III clinical trial comparing neoadjuvant platinum doublet chemotherapy to neoadjuvant chemotherapy with nivolumab for 7th edition stage IB-IIIA NSCLC.4,8 In CheckMate 816, minimally invasive surgery was performed more frequently in the immunotherapy arm compared to the chemotherapy only arm (30% vs. 22%, respectively). There were also fewer intraoperative conversions and shorter operative durations among patients treated with neoadjuvant immunotherapy, with no associated increase in post-operative complications.8 Similarly, in the present study, we found that MIS lobectomy was more prevalent among patients treated with neoadjuvant immunotherapy vs. chemotherapy alone, with no differences in the rates of intraoperative conversions. We also found that minimally invasive surgery after neoadjuvant immunotherapy was associated with similar perioperative outcomes compared to minimally invasive surgery after neoadjuvant chemotherapy, as well as to open surgery performed after neoadjuvant immunotherapy. These findings, together with the findings of CheckMate 816, suggest that minimally invasive surgery does not increase perioperative morbidity or mortality when performed after neoadjuvant immunotherapy.
There are several limitations to this study. First, it is retrospective and therefore carries a risk of having unmeasured confounding by covariates that were not included in our multivariable-adjusted and propensity-score matched analyses. Second, there is no information in the NCDB regarding type of immunotherapy or chemotherapy administered or specific post-operative complications. There is also no detailed data regarding surgeon experience, however we attempted to account for this by including hospital lobectomy volume and facility type in our propensity-score matched analyses. Finally, while larger than prior studies, only 218 patients treated with neoadjuvant immunotherapy met inclusion criteria. Thus, the conclusions drawn from our results may be limited by this relatively small sample size.
Conclusion
In summary, in this national analysis, treatment with neoadjuvant immunotherapy for stage I-III NSCLC did not increase the likelihood of receiving an open vs. minimally invasive operation or contribute to worse perioperative outcomes when compared with neoadjuvant chemotherapy alone or upfront lobectomy. Moreover, while future studies are needed to further power these results, we found that patients who received a thoracoscopic or robotic lobectomy after neoadjuvant immunotherapy had favorable and unchanged perioperative outcomes when compared with those who received a thoracotomy, further supporting the safety and feasibility of minimally invasive lobectomy after neoadjuvant immunotherapy.
Supplementary Material
Table III.
Pathological characteristics and perioperative outcomes of the overall cohort of patients with clinical stage I-III NSCLC undergoing lobectomy after neoadjuvant chemotherapy vs neoadjuvant immunotherapy: propensity-score matched analysis.
| Characteristics | Chemotherapy (n=158) | Immunotherapy (n=158) | p-value |
|---|---|---|---|
| Resection approach, no. (%) | |||
| Open | 84 (53.2%) | 63 (39.9%) | 0.023 |
| VATS | 40 (25.3%) | 52 (32.9%) | 0.14 |
| Robot | 34 (21.5%) | 43 (27.2%) | 0.24 |
|
| |||
| Pathologic T stage, no. (%) | |||
| T0/Tis | 12 (7.6%) | 11 (7.0%) | 0.40 |
| T1a | 4 (2.5%) | 7 (4.4%) | |
| T1b | 5 (3.2%) | 13 (8.2%) | |
| T1c | 24 (15.2%) | 18 (11.4%) | |
| T2a | 27 (17.1%) | 30 (19.0%) | |
| T2b | 20 (12.7%) | 24 (15.2%) | |
| T3 | 46 (29.1%) | 35 (22.2%) | |
| T4 | 20 (12.7%) | 20 (12.7%) | |
| Pathologic N stage, no. (%) | |||
| N0 | 45 (60.0%) | 53 (63.1%) | 0.84 |
| N1 | 19 (25.3%) | 18 (21.4%) | |
| N2 | 11 (14.7%) | 13 (15.5%) | |
| Unknown | 83 | 74 | |
| Pathologic M stage, no. (%) | |||
| M0 | 56 (98.3%) | 64 (98.5%) | 1.00 |
| M1 | 1 (1.8%) | 1 (1.5%) | |
| Unknown | 101 | 93 | |
| Nodal upstaging, no. (%) | 15 (9.6%) | 7 (4.6%) | 0.08 |
| Nodal downstaging, no. (%) | 27 (30.3%) | 39 (39.8%) | 0.18 |
| Lymph nodes examined, no. (%) | 155 (98.1%) | 152 (96.2%) | 0.31 |
| Regional nodes examined, median (IQR) | 15.0 (10.0, 23.0) | 16.0 (11.0, 27.0) | 0.22 |
| Surgical margin, no. (%) | |||
| Negative | 146 (92.4%) | 147 (94.8%) | 0.78 |
| Positive | 12 (7.6%) | 8 (5.2%) | |
| Unknown | 0 | 3 | |
| Length of stay (median, IQR) | 4.0 (3.0, 7.0) | 4.0 (3.0, 6.0) | 0.17 |
| 30-Day readmission, no. (%) | |||
| No | 151 (96.8%) | 148 (93.7%) | 0.29 |
| Yes | 5 (3.2%) | 10 (6.3%) | |
| Unknown | 2 | 0 | |
| 30-day mortality, no. (%) | |||
| No | 76 (96.2%) | 83 (98.8%) | 0.36 |
| Yes | 3 (3.8%) | 1 (1.2%) | |
| Unknown | 79 | 74 | |
| 90-day mortality, no. (%) | |||
| No | 74 (94.9%) | 82 (97.6%) | 0.43 |
| Yes | 4 (5.1%) | 2 (2.4%) | |
| Unknown | 80 | 74 | |
CENTRAL MESSAGE.
Induction immunotherapy for stage I-III non-small-cell lung cancer is not associated with increased rates of thoracotomy, increased rates of conversions or worse perioperative outcomes.
CENTRAL PICTURE.
Neoadjuvant immunotherapy does not lead to worse perioperative outcomes.
PERSPECTIVE STATEMENT.
While there is growing enthusiasm for induction immunotherapy in the treatment of resectable non-small-cell lung cancer, some fear that this may increase the difficulty and morbidity of subsequent surgical resection. Here, we find that induction immunotherapy is not associated with increased rates of thoracotomy, conversions, or worse perioperative outcomes relative to induction chemotherapy.
Acknowledgments
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
GLOSSARY OF ABBREVIATIONS
- NSCLC
Non-small-cell lung cancer
- MIS
Minimally invasive surgery
- NCDB
National Cancer Data Base
- AJCC
American Joint Committee on Cancer
- TNM
Tumor, Nodes and Metastases
- CDCC
Charlson Deyo Comorbidity (score)
Footnotes
Disclosures: The authors report no proprietary or commercial interest in any concept discussed in this article.
IRB approval number/date: IRB #2020P004110, 02/02/2021. Patient written consent for the publication of the study data was waived by the IRB given that all patient data was de-identified prior to analysis and publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Matching by hospital surgical volume was not performed in this subgroup alone in an effort to preserve sample size greater than thirty patients in each arm of the propensity-score matched analysis.
Bonferroni-corrected significant threshold of 0.017 was used to account for the three comparisons.
Bonferroni-corrected significant threshold of 0.017 was used to account for the three comparisons.
Citations
- 1.Qiu B, Cai K, Chen C, et al. Expert consensus on perioperative immunotherapy for local advanced non-small cell lung cancer. Transl Lung Cancer Res Sep 2021;10(9):3713–3736. doi: 10.21037/tlcr-21-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Group NM-aC. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet May 3 2014;383(9928):1561–71. doi: 10.1016/S0140-6736(13)62159-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedlaender A, Naidoo J, Banna GL, Metro G, Forde P, Addeo A. Role and impact of immune checkpoint inhibitors in neoadjuvant treatment for NSCLC. Cancer Treat Rev Jan 24 2022;104:102350. doi: 10.1016/j.ctrv.2022.102350 [DOI] [PubMed] [Google Scholar]
- 4.Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. New England Journal of Medicine 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaft JE, Hellmann MD, Velez MJ, Travis WD, Rusch VW. Initial Experience With Lung Cancer Resection After Treatment With T-Cell Checkpoint Inhibitors. Ann Thorac Surg Sep 2017;104(3):e217–e218. doi: 10.1016/j.athoracsur.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg Jul 2019;158(1):269–276. doi: 10.1016/j.jtcvs.2018.11.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bott MJ, Cools-Lartigue J, Tan KS, et al. Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors. Ann Thorac Surg Jul 2018;106(1):178–183. doi: 10.1016/j.athoracsur.2018.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spicer J Surgical outcomes from the phase 3 Checkmate 816 trial: nivolumab + platinum-doublet chemotherapy vs chemotherapy alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer. Oral presentation at: American Society of Clinical Oncology Annual Meeting; June 2021; virtual. [Google Scholar]
- 9.Mallin K, Browner A, Palis B, et al. Incident Cases Captured in the National Cancer Database Compared with Those in U.S. Population Based Central Cancer Registries in 2012–2014. Ann Surg Oncol Jun 2019;26(6):1604–1612. doi: 10.1245/s10434-019-07213-1 [DOI] [PubMed] [Google Scholar]
- 10.Amin MBE SG F; Byrd DR; Brookland RK; Washington MK;.Gershenwald JE; Compton CC; Hess KR, et al. (Eds.). AJCC Cancer Staging Manual (8th Edition). American Joint Commission on Cancer. 2017; [Google Scholar]
- 11.Sepesi BCT, William W, Lin H, Leung C, Weissferdt A, Walsh G, Rice D, Roth J, Mehran R, Hofstetter W, Antonoff M, Fossella F, Mott F, Le X, Skoulidis F, Zhang J, Byers L, Lam V, Glisson B, Kurie J, Blumenschein G, Tsao A, Lu C, Altan M, Elamin Y, Gibbons D, Papadimitrakopoulou V, Lee J, Heymach J, Vaporciyan A, Swisher S. OA13.06 Surgical Outcomes Following Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Non-Small Cell Lung Cancer - NEOSTAR Study. Journal of Thoracic Oncology 2019;14(10) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


