Abstract
Objective:
Abnormal tau, a hallmark Alzheimer’s Disease (AD) pathology, may appear in the locus coeruleus (LC) decades before AD symptom onset. Reports of subjective cognitive decline are also often present prior to formal diagnosis. Yet, the relationship between LC structural integrity and subjective cognitive decline has remained unexplored. Here, we aimed to explore these potential associations.
Methods:
We examined 381 community-dwelling men (mean age=67.58; SD=2.62) in the Vietnam Era Twin Study of Aging (VETSA) who underwent LC-sensitive MRI and completed the Everyday Cognition scale (ECOG) to measure subjective cognitive decline along with their selected informants. Mixed models examined the associations between rostral-middle and caudal LC integrity and subjective cognitive decline after adjusting for depressive symptoms, physical morbidities, and family. Models also adjusted for current objective cognitive performance and objective cognitive decline to explore attenuation.
Results:
For participant ratings, lower rostral-middle LC contrast to noise ratio (LCCNR) was associated with significantly greater subjective decline in memory, executive function, and visuospatial abilities. For informant ratings, lower rostral-middle LCCNR was associated with significantly greater subjective decline in memory only. Associations remained after adjusting for current objective cognition and objective cognitive decline in respective domains.
Conclusions:
Lower rostral-middle LC integrity is associated with greater subjective cognitive decline. Although not explained by objective cognitive performance, such a relationship may explain increased AD risk in people with subjective cognitive decline as the LC is an important neural substrate important for higher-order cognitive processing, attention, and arousal and one of the first sites of AD pathology.
Keywords: subcortical, cognitive complaints, cognitive impairment, brain stem, noradrenergic, norepinephrine
Introduction
Over 5 million older adults in the United States live with Alzheimer’s disease (AD) and related dementias (Matthews et al., 2019). One major goal is to discover noninvasive in-vivo brain imaging biomarkers related to early dementia risk factors decades before major impairment (Braak, Thal, Ghebremedhin, & Del Tredici, 2011). One early risk factor is subjective cognitive decline, defined as reporting worsening cognition (Jessen et al., 2014). In the AD pathway, subjective cognitive decline is thought to occur before objective cognitive impairment (Jessen et al., 2014; Rabin et al., 2015). Aligned with this idea, subjective cognitive decline in cognitively unimpaired older adults relates to a 2–4-fold increased risk of converting to mild cognitive impairment and dementia (Snitz et al., 2018; van Harten et al., 2018). Subjective cognitive decline is also linked to higher levels of amyloid and tau (Buckley et al., 2017; Miebach et al., 2019; Snitz et al., 2015). As such, neuroimaging biomarkers related to subjective cognitive decline may help find who is at risk for AD pathology in a non-invasive manner.
MRI studies have detected slightly smaller medial temporal, parietal, hippocampal, and prefrontal gray matter volumes in people with subjective cognitive decline compared to peers without (Jessen et al., 2006; Saykin et al., 2006). Such differences are consistent with areas affected in later AD stages (Csernansky et al., 2005; Morris et al., 2009). However, there is a need to go beyond examining allocortical and neocortical brain regions as substantial atrophy due to AD pathology may not occur until later in the disease. Neuroimaging of the brainstem, and the locus coeruleus (LC) in particular, is one promising target as it shows abnormal tau long before tau and amyloid pathology spread into the cortex (Braak et al., 2011). Neuroimaging of the LC may help explain subjective cognitive decline related to AD pathology.
The LC is located in the dorsal pons and is critical for higher-order cognitive processing, arousal, and attention through tonic and phasic release of norepinephrine/noradrenaline throughout the brain (Aston-Jones & Bloom, 2005; Aston-Jones & Bloom, 1981). Tonic norepinephrine/noradrenaline release from the LC keeps the brain in “readied” exploratory states of attention essential for bottom-up information processing, while phasic norepinephrine/noradrenaline releases aid the strategic use of attention for purposeful tasks, i.e., attentional control (Aston-Jones & Bloom, 2005). Injured LC neurons release tonic norepinephrine/noradrenaline, which may disrupt cognitive function by offsetting phasic norepinephrine/noradrenaline releases and increasing distractibility (Chiodo, Acheson, Zigmond, & Stricker, 1983). As a possible driver of early AD symptoms, people may report subjective cognitive decline as they face attentional difficulties due to LC damage. Studies show that LC damage occurs often due to early tau pathology as early as midlife (Braak et al., 2011).
Autopsy studies describe the LC as one of the first structures to show abnormal tau, even before the appearance of amyloid in the cortex (Braak et al., 2011). Accumulation of abnormal tau may damage the LC leading to the persistent release of tonic norepinephrine/noradrenaline (Janitsky, 2020), which may contribute to subjective cognitive decline in early AD stages. Furthermore, AD’s effects in the LC appear region specific. Abnormal tau mostly accumulates and damages the rostral-middle region of the LC, which is responsible for delivering norepinephrine/noradrenaline to the hippocampus and areas of the neocortex (Betts, Cardenas-Blanco, Kanowski, Jessen, & Duzel, 2017; Betts et al., 2019; German et al., 1992; Theofilas et al., 2017). Deterioration of the rostral-middle LC due to abnormal tau has also been linked to cognitive decline (Dahl et al., 2019; Hämmerer et al., 2018). By comparison, the caudal LC, which has most projections linked to the spinal cord, is less affected by AD pathology and its integrity has been unrelated to objective cognitive performance (Elman et al., 2021). As such, the rostral-middle LC may be more linked to subjective cognitive decline than the caudal region. Recent technology now allows us to investigate this link.
Brainstem regions are notoriously difficult to image in vivo, as their deep, small structures are not visible on commonly used structural MRI sequences. However, researchers have noticed that the LC shows hyperintensity compared to surrounding regions on certain imaging protocols. Although reasons for hyperintensity are still under investigation (Priovoulos et al., 2020; Watanabe et al., 2019), LC signal intensity can shine light onto its structural integrity. Researchers have used LC-sensitive MRI sequences to compare signal intensity from the LC region to surrounding brainstem structures, known as an LC contrast to noise ratio (LCCNR). LCCNR has been shown to not only relate to LC neuronal count shown in post-mortem autopsies but also has been linked to episodic memory performance in older adults (Dahl et al., 2019; Elman et al., 2021; Hämmerer et al., 2018) and tau accumulation (Jacobs et al., 2021). Using this in vivo assessment of LC integrity, our study aimed to provide the first in vivo examination of the LC and subjective cognitive decline.
Our study had a central hypothesis and two exploratory aims. Our central hypothesis was that lower rostral-middle LC integrity would be associated with greater overall subjective cognitive decline. This association is expected due to the demonstrated associations between rostral-middle LC integrity and objective cognitive performance (Elman et al., 2021; Dahl et al., 2019; Hämmerer et al., 2018) assuming subjective cognitive decline is an indirect measure of actual declines in cognitive function. Subjective cognitive decline may also capture subtle problems in higher-order cognitive processing, arousal, and attention when objective testing is normal, as suggested by studies showing disruption of neural networks (Smart, Segalowitz, Mulligan, & MacDonald, 2014; Tu et al., 2018) and lowered alertness to stimuli (Esmaeili et al., 2021). We did not hypothesize relationships between caudal LC region and subjective cognitive decline, although this was investigated to assess regional specificity of effects. For our first exploratory aim, we examined associations of LC integrity across individual subscales of subjective cognitive decline, including decline in subjective memory, executive function, language, and visuospatial ability. Our second exploratory aim assessed whether any significant associations between LC integrity and subjective cognitive decline were attenuated after controlling for objective cognitive performance, captured as current levels of performance or decline from about 12 years prior. This aim helped us directly test our assumption that associations between LC integrity and subjective cognitive decline primarily reflect LC-related decline in objective cognitive performance. In supplemental analyses, we assessed associations with hippocampal volume as a comparison region affected much in later stages of AD pathology as compared to the LC (Braak et al., 2011). Findings clarify the role of the LC in subjective cognitive decline experienced in early old age.
Methods
Participants
Participants were from the Wave 3 of the Vietnam Era Twin Study of Aging (VETSA) project when LC imaging was added to the protocol (Kremen et al., 2013; Kremen, Franz, & Lyons, 2019; Kremen et al., 2006). VETSA is a longitudinal aging project designed to investigate behavioral genetics of cognitive and brain aging. VETSA participants were from a random sample recruited from the Vietnam Era Twin Registry (VETR), a national registry of male-male adult twin pairs who served during the Vietnam War era (1965–1975), who also participated in the Harvard Drug Study (Tsuang, Bar, Harley, & Lyons, 2001). Nearly 80% did not report combat exposure. VETSA participants are comparable to community-dwelling men in the U.S. on demographics and lifestyle factors as well (Schoeneborn & Heyman, 2009). More details of this project have been reported elsewhere (Kremen, Franz, & Lyons, 2013; Kremen et al., 2019) and data remain available for external access (http://www.vetsatwins.org/for-researchers/).
Wave 3 of VETSA occurred from 2016 to 2019, when the average age was 68 years. Of the sample, 487 met standard MRI inclusion criteria (e.g., no metal in the body). Of these, 442 had LC and cortical imaging data. From this, we removed people with MRI-based cerebral abnormalities (encephalomalacia, meningioma, large infarct, etc.; n=6) or who had low LC imaging quality due to excessive head motion in the scanner (n=4) as assessed by visual inspection. We also excluded people with a self-reported history of stroke (n=18), seizures (n=5), HIV (n=2), schizophrenia (n=1), and alcohol dependency (n=24). One more person was removed for having no data on subjective cognitive decline (n=1). In the final sample for this analysis (n=381), participants were an average of 67.58 years of age (SD=2.62, range=62.96 to 71.00), 88% Non-Hispanic White (n=336), and had an average education of 13.98 years (SD=2.07). No participants were diagnosed with dementia, but 57 participants had MCI (15%) as defined below.
All procedures were approved by the Institutional Review Board at the University of California San Diego and Boston University, and all participants gave written informed consent for the study. Procedures were in accordance with the Helsinki Declaration.
LC MRI Acquisition and Processing
Our analyses use MRI data from Wave 3 of VETSA. Description of our MRI imaging for the LC has been published in detail (Elman et al., 2017). Neuroimaging was conducted using two GE 3T Discover 750x scanners (GE Healthcare, Waukesha, WI, USA) equipped with eight-channel phased-array head coils. Imaging of the LC was completed using oblique axial FSE-T1-weighted images (TR=600 ms; TE=14 ms; flip angle=90°; matrix=512 × 320; FOV=220 mm; pixel size 0.42 × 0.68 mm; 10 slices; slice thickness=2.5 mm; interslice gap=1 mm, acquisition time=4 minutes and 44 seconds, online averaging).
LC-related hyperintensity was visible on three slices or four slices (about half of the participants each), with the three slices showing the most visible LC-related hyperintensity being used. Each image was marked by two out of four experienced raters using a modified version of the Clewett et al. method (Clewett et al., 2016). Signal intensities were derived from manually marked regions of interest (ROI) on three slices corresponding to the LC rostral-middle, middle, and caudal portions (shown in Figure 1). The middle slice was selected by taking the slice 7mm below the inferior edge of the inferior colliculus. Two 3mm2 voxel crosses were manually placed over the left and right sides of the middle LC region centered on the voxel of highest signal within the area of LC-related hyperintense signal. We controlled for overall signal intensity variability by taking the contrast-to-noise ratio of signal in the LC compared to a reference region. The reference region was marked with a 10mm2 ROI over the pontine tegmentum (PT) – located 6 voxels anterior to the central voxel of the LC ROI. The same processes were followed to then mark one slice superior (i.e., rostral LC) and one slice inferior (i.e., caudal LC) relative to the middle slice. Left and right LC values were averaged together on each slice. Next, an LC contrast to noise ratio (LCCNR) was calculated to get a single value of LC signal intensity (where higher scores indicate better integrity) for each slice with the following equation:
Figure 1.
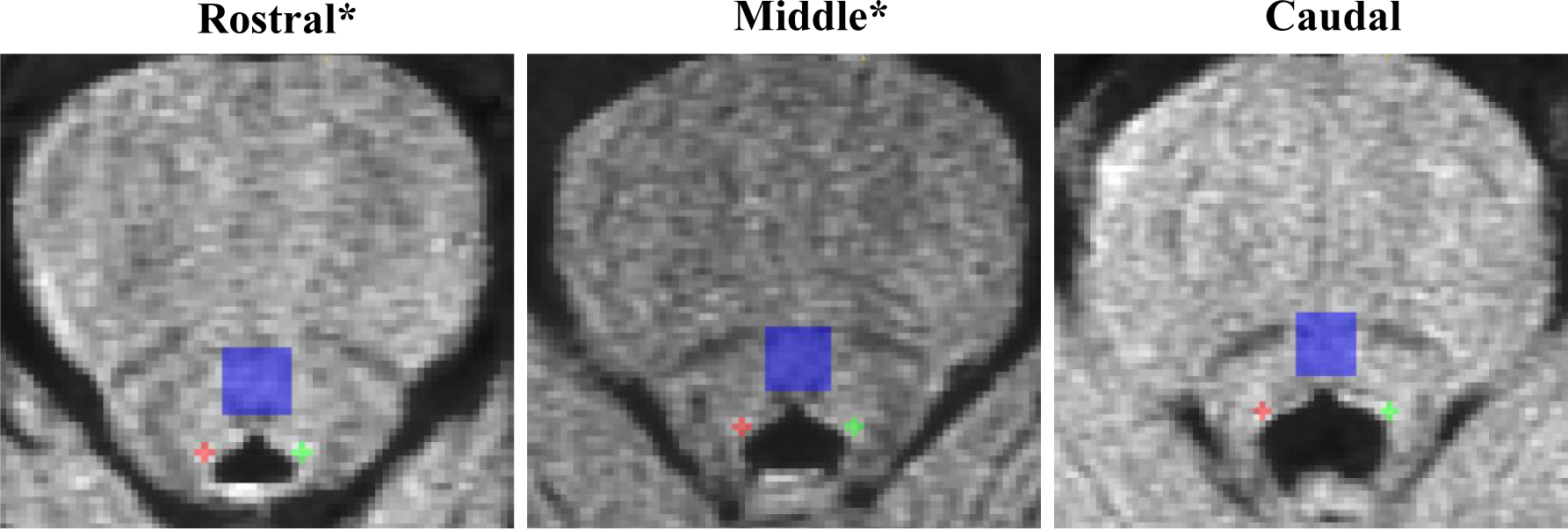
Summary of the manual marking method of the LC. Note. The middle slice is selected 7 mm below the inferior colliculus. Left and right portions of the locus coeruleus (LC) are marked on the rostral, middle, and caudal slices with a 3mm2 cross. Signal intensity is averaged from left and right regions to calculate rostral, middle, and caudal LC intensity. As a reference region, we placed a 10 mm2 square placed over the pontine tegmentum (PT). The same marking rules were used to calculate signal intensity for the rostral, middle, and caudal slice of the PT. A contrast to noise (CNR) is created for each region using LC signal intensity subtracted by PT signal intensity and divided by PT signal intensity for each region.
*For this study, we averaged rostral and middle LC CNR as both regions show similar age and disease-related effects. The caudal LC CNR was used as an exploratory aim and comparison region.
For this study, signals from the rostral and middle slices were averaged to create a rostral-middle LCCNR as they both show more prominent changes due to aging and Alzheimer’s disease (Betts et al., 2019; German et al., 1992). Caudal LCCNR was defined as the contrast-to-noise ratio in the most caudal slice. Regarding reliability, four raters showed 95% inter-rater reliability across the entire dataset (calculated from the results of a mixed model; Wald’s Z=15.14, p <.001). LCCNR was standardized (z-scored) for ease of interpretability.
Hippocampal volume.
Hippocampal volume was estimated using atlas-based volumetric segmentation (Fischl et al., 2002; Fischl et al., 2004) performed using FreeSurfer version 6.0 (http://surfer.nmr.mgh.harvard.edu). Details provided in the Supplemental Material.
Subjective cognitive decline
At Wave 3, subjective cognitive decline was measured using the participant- and informant-rated versions of the Everyday Scale of Cognition (ECOG). This scale has been previously validated (Farias et al., 2008) and higher scores correspond to an elevated risk of MCI and dementia pathology (Shokouhi et al., 2019; van Harten et al., 2018). Domains of everyday cognition are queried through four subscales: Memory (8 items), Executive Function (15 items), Language (9 items), and Visuospatial Abilities (7 items). For each item, participants and their informants separately rated current behavioral functioning with that of 10 years earlier. They rated items on a 4-point Likert-type scale: 1=better or no change, 2=questionable/occasionally worse, 3=consistently a little worse, and 4=consistently much worse. A total score was calculated by averaging the scores across all items of the ECOG, which can be thought to capture changes in global cognitive function. This has been done previously (Farias et al., 2008) and seemed appropriate as subscales were highly intercorrelated (range from .35 to .68, see Table S1 in the Supplementary Material). The ECOG and its subscales demonstrated high reliability across participant and informant ECOG scales (αs range from .81 to .86).
MCI Classification
MCI classification followed the Jak-Bondi approach, which defined MCI as performing >1.5 SDs worse on 2 or more tasks within a cognitive domain after adjusting for age and education (Bondi et al., 2014; Jak et al., 2009). These were pre-adjusted for practice effects, age, education, and young adult cognitive ability as described in the Supplemental Material.
Covariates
Covariates included age (years), young adult cognitive ability (Armed Forces Qualification Test; Lyons et al., 2017; Lyons et al., 2009), education (years), objective cognitive function (factor scores of episodic memory, executive function, fluency, and visuospatial ability), objective cognitive decline (change in factor scores from Wave 1), depressive symptoms (CESD; Radloff, 2016), and number of physical morbidities. Covariates are described in detail in the Supplemental Material.
Statistical Analysis
Descriptive statistics for major variables of interest and sample characteristics are shown in Table 1. Variables were assessed for normality before analyses using a cutoff of>|2| on metrics of skewness and kurtosis. Rostral-middle and caudal LCCNR were within acceptable bounds and did not require transformation. ECOG scores, however, showed a negative skew (between 2.25 and 4.25) and were hyper-kurtotic (range from 2.21 to 24.59), which normalized after a logarithmic transformation (skewness and kurtosis<|2|). Distributions of the major variables are provided in Figure S1 in the Supplementary Material.
Table 1.
Demographics of sample (n=381).
| % | n | M | SD | Range | |
|---|---|---|---|---|---|
|
| |||||
| Age | 67.58 | 2.62 | 61.96 to 71.00 | ||
| Race | |||||
| Non-Hispanic White | 88% | 336 | |||
| Education (years) | 13.98 | 2.07 | 8 to 20 | ||
| Physical Morbidities | |||||
| 0 | 19% | 73 | |||
| 1 | 34% | 130 | |||
| 2+ | 48% | 178 | |||
| Mild Cognitive Impairment | 15% | 57 | |||
| Depressive Symptoms (CESD) | 6.42 | 6.5 | 0 to 38.00 | ||
| ECOG Participant-Rated Cognitive | |||||
| Decline* | 1.55 | 0.45 | 1.00 to 3.75 | ||
| Memory | 1.85 | 0.63 | 1.00 to 3.75 | ||
| Executive Function | 1.49 | 0.58 | 1.00 to 3.73 | ||
| Language | 1.61 | 0.56 | 1.00 to 3.89 | ||
| Visuospatial Ability | 1.26 | 0.44 | 1.00 to 3.86 | ||
| ECOG Informant-Rated Cognitive Decline* | 1.49 | 0.59 | 1.00 to 3.97 | ||
| Memory | 1.57 | 0.59 | 1.00 to 3.75 | ||
| Executive function | 1.67 | 0.89 | 1.00 to 3.93 | ||
| Language | 1.35 | 0.62 | 1.00 to 3.78 | ||
| Visuospatial Ability | 1.36 | 0.88 | 1.00 to 3.71 | ||
Notes. CESD=Center for Epidemiological Studies Depression scale; ECOG=Everyday Cognition scale. Race was coded as Non-Hispanic White and non-White. The variable of physical morbidities was a summed index from a medical interview of the presence of heart attack, heart failure, peripheral vascular disease, thrombolysis, hypertension, angina, diabetes, bronchitis, asthma, cancer, osteoarthritis, rheumatoid arthritis, and cirrhosis. ECOG asked participants and informants to rate changes in behaviors in the last ten years.
ECOG overall average and subscales were later log-transformed for normality in analyses but shown untransformed in this table for clarity.
For our first analyses, we performed Spearman-rank correlations between major variables in our study, including LC integrity, participant and informant ECOG subscales, and current objective cognitive performance at Wave 3, shown in Table 2. The purpose of these initial analyses was to understand the correlation of the LCCNR with outcomes before covariate adjustment, examine the relationship between participant and informant ratings, and examine how much ECOG ratings related to current objective cognitive performance in respective domains.
Table 2.
Correlations between Locus Coeruleus Integrity, Subjective Cognitive Decline, and Objective Cognitive Performance (n=381).
| ECOG Informant-Rated Decline | Rostral--middle LCCNR | Caudal LCCNR | Objective Cognitive Performance |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global Cognition | Episodic Memory | Executive Function | Verbal Fluency | Visuospatial Ability | ||||||||||||
|
| ||||||||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
|
| ||||||||||||||||
| LCCNR | ||||||||||||||||
| Rostral-middle LCCNR | .14 | .006 | .16 | .001 | .04 | .416 | .12 | .024 | .08 | .123 | ||||||
| Caudal LCCNR | .09 | .096 | .05 | .293 | .03 | .610 | .09 | .089 | .03 | .516 | ||||||
| ECOG | ||||||||||||||||
| Participant-Rated Decline | ||||||||||||||||
| Cognition | .30 | <.001 | −.15 | .004 | −.06 | .285 | −.26 | <.001 | −.26 | <.001 | −.20 | <.001 | −0.20 | <.001 | −.11 | .035 |
| Memory | .33 | <.001 | −.10 | .048 | −.05 | .358 | −.21 | <.001 | −.26 | <.001 | −.13 | .010 | −.17 | .001 | −.07 | .197 |
| Executive Function | .30 | <.001 | −.19 | <.001 | −.08 | .104 | −.2 | <.001 | −.20 | <.001 | −.12 | .016 | −.18 | <.001 | −.02 | .637 |
| Language | .26 | <.001 | −.11 | .033 | −.03 | .550 | −.22 | <.001 | −.23 | <.001 | −.22 | <.001 | −.21 | <.001 | −.08 | .125 |
| Visuospatial Ability | .19 | <.001 | −.15 | .004 | −.06 | .212 | −.29 | <.001 | −.21 | <.001 | −.20 | <.001 | −.15 | .003 | −.22 | <.001 |
| Informant-Rated Decline | ||||||||||||||||
| Cognition | −.09 | .086 | −.09 | .087 | −.16 | .007 | −.19 | .001 | −.10 | .067 | −.03 | .553 | −.14 | .010 | ||
| Memory | −.08 | .145 | .06 | .257 | −.05 | .36 | −.11 | .035 | .01 | .889 | −.02 | .69 | −.05 | .354 | ||
| Executive Function | −.06 | .276 | −.07 | .178 | −.15 | .010 | −.15 | .005 | −.12 | .028 | −.04 | .438 | −.12 | .027 | ||
| Language | −.07 | .180 | −.07 | .180 | −.14 | .020 | −.19 | <.001 | −.12 | .021 | −.04 | .48 | −.18 | .001 | ||
| Visuospatial Ability | −.13 | .009 | −.13 | .012 | −.05 | .011 | −.11 | .050 | −.11 | .050 | −.05 | .333 | −.12 | .028 | ||
Note. ECOG=Everyday Cognition Scale. Correlations are derived from spearman-tau correlations between each ECOG domain shown in the row with its objective performance counterpart. Specifically, correlations for subjective cognitive decline, subjective memory decline, subjective executive function decline, subjective language decline, subjective visuospatial decline are analyzed with objective factors scores of global cognition, memory, executive function, verbal fluency, and visuospatial function, respectively. ECOG cognition scores are the average of all ECOG items.
For main analyses, mixed models were fitted in SPSS software Version 26 (MIXED; IBM Corp) to test associations between LCCNR and ECOG scales. In mixed models shown in Table 3 as Models 1a to 2, predictor variables included rostral-middle or caudal LCCNR and covariates of interest (age, objective cognitive scores, depressive symptoms, and physical morbidities). In our primary models, we adjusted for young-adult cognitive ability as a possible confounder of the relationship between LC integrity and subjective cognitive decline. Young-adult cognitive ability is a more precise measure than years of education, however, results did not change when controlling for years of education instead (see Table S3 in the Supplementary Material). Separate mixed models were run with each participant and informant-rated ECog scale as the outcome. Rostral-middle and caudal LCCNR were placed as predictors in the same model with low multicollinearity (r=.41, p<.001) (ECOG score ~ β0 + β1(rostral-middle LCCNR)t + β2(caudal LCCNR)t + [covariates] + eit; t=observations nested within twin). Mixed models assumed a Gaussian distribution and adjusted for family (being in the same twin pair). For these models, we interpret the standardized betas with 95% confidence intervals. A repeated measures model additionally nesting rostral-middle and caudal LC values within participant was used to examine whether one LCCNR region was more predictive than another (LCCNR ~ β0 + β1(region type [rostral-middle or caudal])it + β2(ECOG score) + β3(region type*ECOG score)it + [covariates] + eit; i=observations nested within individual; t=observations nested within twin). As a complementary analysis, we look at these results when adjusting for current objective cognitive performance (Table 4, Models 1b to 2b). As a complementary analysis, we examined associations when adjusting for objective cognitive decline in a subsample of participants who also completed tests at Wave 1 (Table 5, Models 1c to 2c). For supplemental analyses, we examined associations looking at hippocampal volume as a predictor of ECOG scores.
Table 3.
Associations between the Locus Coeruleus and Subjective Cognitive Decline (n=381).
| Outcomes: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjective Cognitive Decline | Subjective Memory Decline | Subjective Executive Function Decline | Subjective Language Decline | Subjective Visuospatial Decline | ||||||
|
|
||||||||||
| Predictors: | β (95%CI) | p * | β (95%CI) | p * | β (95%CI) | p * | β (95%CI) | p * | β (95%CI) | p * |
|
|
||||||||||
| Participant-Rated Decline | ||||||||||
| Model 1a. Rostral-middle LCCNR |
−.18 (−.29 to −.07) | .001 | −.15 (−.26 to −.04) | .007 | −.16 (−.27 to −.05) | .005 | −.14 (−.25 to −.03) | .012 | −.15 (−.26 to −.04) | .010 |
| Caudal LCCNR | .02 (−.08 to .12) | .860 | −.0003 (−.10 to .10) | .995 | −.004 (−.11 to .10) | .946 | .04 (−.07 to .14) | .618 | .03 (−.08 to .13) | .789 |
| Young-adult Cognitive Ability | −.01 (−.11 to .09) | .860 | .001 (−.10 to .10) | .995 | .03 (−.07 to .13) | .906 | .02 (−.08 to .12) | .638 | −.10 (−.20 to .0003) | .128 |
| Age (years) | .08 (−.02 to .18) | .268 | .08 (−.02 to .18) | .195 | .03 (−.07 to .13) | .906 | .13 (.03 to .23) | .033 | −.003 (−.10 to .10) | .949 |
| Depressive Symptoms | .26 (.16 to .36) | <.001 | .20 (.10 to .30) | <.001 | .23 (.13 to .33) | <.001 | .25 (.15 to .35) | <.001 | .18 (.08 to .28) | .005 |
| Physical Morbidities | .09 (−.01 to .18) | .860 | .13 (.03 to .22) | .033 | .02 (−.08 to .12) | .906 | .08 (−.02 to .18) | .208 | .05 (−.05 to .15) | .612 |
| Informant-Rated Decline | ||||||||||
| Model 2a. Rostral-middle LCCNR |
−.10 (−.21 to .003) | .057 | −.01 (−.13 to .11) | .865 | −.05 (−.14 to .05) | .356 | −.01 (−.18 to .17) | .938 | −.03 (−.15 to .08) | .577 |
| Caudal LCCNR | .05 (−.05 to .15) | .713 | .06 (−.04 to .17) | .593 | .05 (−.04 to .14) | .433 | .10 (−.04 to .25) | .796 | .08 (−.02 to .18) | .373 |
| Young-adult Cognitive Ability | −.02 (−.12 to .09) | .844 | −.11 (−.21 to −.01) | .145 | −.07 (−.16 to .02) | .310 | −.02 (−.18 to .14) | .796 | −.06 (−.18 to .06) | .485 |
| Age (years) | −.01 (−.12 to .10) | .844 | .03 (−.08 to .14) | .658 | .10 (.01 to .19) | .135 | −.05 (−.21 to .12) | .796 | .09 (−.03 to .20) | .373 |
| Depressive Symptoms | −.03 (−.12 to .07) | .844 | .02 (−.07 to .12) | .658 | −.04 (−.13 to .05) | .465 | .02 (−.12 to .16) | .796 | −.04 (−.15 to .08) | .533 |
| Physical Morbidities | .07 (−.04 to .17) | .713 | −.02 (−.12 to .08) | .658 | .02 (−.07 to .10) | .666 | −.02 (−.19 to .14) | .796 | −.05 (−.18 to .07) | .485 |
Notes. CNR=contrast-to-noise ratio; LC=Locus Coeruleus. Each column represents an ECOG subscale regressed on predictors shown in the rows. Rows under “Participant-Rated Decline” show effects when predicting respective ECOG subscales using participant ratings; rows under the “Informant-Rated Decline” show effects when predicting respective ECOG subscales using informant ratings. Models were assessed in a general estimating equation accounting for related observations between twins in the same pair. Models were adjusted for age, depressive symptoms measured by the Center of Epidemiological Studies – Depression Scale (CESD), and physical morbidities.
P-values for effects outside of the hypothesized relationship with rostral-middle LC have been corrected for multiple testing using FDR.
Table 4.
Associations between the Locus Coeruleus and Subjective Cognitive Decline after Adjusting for Objective Cognitive Performance (n=381).
| Outcomes: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjective Cognitive Decline | Subjective Memory Decline | Subjective Executive Function Decline | Subjective Language Decline | Subjective Visuospatial Decline | ||||||
|
|
||||||||||
| Predictors: | β (95%CI) | p * | β (95%CI) | p * | β (95%CI) | p * | β (95%CI) | p * | β (95%CI) | p * |
|
|
||||||||||
| Participant-Rated Decline | ||||||||||
| Model 1b. Rostral-middle LCCNR |
−.16 (−.28 to −.04) | .011 | −.12 (−.22 to −.01) | .030 | −.16 (−.28 to −.05) | .005 | −.13 (−.23 to −.02) | .022 | −.13 (−.24 to −.02) | .020 |
| Caudal LCCNR | .08 (−.04 to .19) | .390 | −.01 (−.11 to .09) | .862 | −.003 (−.11 to .10) | .953 | .04 (−.06 to .15) | .394 | .02 (−.08 to .13) | .689 |
| Objective Cognitive Performance | −.30 (−.45 to −.17) | <.001 | −.23 (−.33 to −.14) | <.001 | −.11 (−.22 to .001) | .298 | −.20 (−.30 to −.10) | <.001 | −.08 (−.19 to .04) | .376 |
| Informant-Rated Decline | ||||||||||
| Model 2b. Rostral-middle LCCNR |
−.07 (−.18 to .05) | .248 | −.01 (−.13 to .12) | .925 | −.04 (−.14 to .05) | .368 | .02 (−.16 to .20) | .834 | −.05 (−.17 to .07) | .406 |
| Caudal LCCNR | .04 (−.09 to .17) | .538 | .05 (−.05 to .16) | .466 | .05 (−.04 to .14) | .455 | .11 (−.04 to .25) | .302 | .08 (−.03 to .18) | .298 |
| Objective Cognitive Performance | −.004 (−.16 to .15) | .963 | −.05 (−.15 to .05) | .459 | .02 (−.07 to .12) | .630 | −.08 (−.26 to .10) | .355 | .07 (−.08 to .22) | .466 |
Notes. CNR=contrast-to-noise ratio; LC=Locus Coeruleus. Each column represents an ECOG subscale regressed on predictors shown in the rows. Rows under “Participant-Rated Decline” show effects when predicting respective ECOG subscales using participant ratings; rows under the “Informant-Rated Decline” show effects when predicting respective ECOG subscales using informant ratings. Models were assessed in a general estimating equation accounting for related observation between twins in the same pair. Models were adjusted for age, depressive symptoms measured by the Center of Epidemiological Studies – Depression Scale (CESD), and physical morbidities. For objective cognitive performance as a covariate, models with subjective cognitive decline, subjective memory decline, subjective executive function decline, subjective language decline, subjective visuospatial decline are analyzed with factors scores of global cognition, memory, executive function, verbal fluency, and visuospatial ability, respectively.
P-values for effects outside of the hypothesized relationship with rostral-middle LC have been corrected for multiple testing using FDR.
Table 5.
Associations between the Locus Coeruleus and Subjective Cognitive Decline after Adjusting for Objective Cognitive Decline (n=287).
| Outcomes |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predictors: | Subjective Cognitive Decline | Subjective Memory Decline | Subjective Executive Function Decline | Subjective Language Decline | Subjective Visuospatial Decline | |||||
|
|
||||||||||
| Participant-Rated Decline | β (95%CI) | p * | β (95%CI) | p * | β (95%CI) | p * | β (95%CI) | p * | β (95%CI) | p * |
|
|
||||||||||
| Model 1c. Rostral-middle LCCNR |
−.19 (−.32 to −.06) | .004 | −.13 (−.25 to −.002) | .046 | −.17 (−.30 to −.05) | .005 | −.15 (−.27 to −.02) | .021 | −.17 (−.30 to −.03) | .016 |
| Caudal LCCNR | .06 (−.06 to .18) | .419 | −.01 (−.13 to .11) | .870 | .03 (−.09 to .14) | .675 | .05 (−.07 to .17) | .633 | .07 (−.06 to .20) | .455 |
| Objective Cognitive Decline | −.10 (−.30 to .11) | .419 | .16 (.002 to .33) | .288 | −.10 (−.27 to .07) | .455 | .04 (−.15 to .21) | .705 | .02 (−.10 to .14) | .761 |
| Informant-Rated Decline | ||||||||||
| Model 2c. Rostral-middle LCCNR |
−.06 (−.18 to .05) | .279 | −.02 (−.18 to .15) | .849 | −.07 (−.19 to .05) | .246 | .03 (−.19 to .24) | .808 | −.13 (−.26 to −.002) | .047 |
| Caudal LCCNR | .03 (−.10 to .16) | .619 | .07 (−.09 to .23) | .449 | .04 (−.09 to .16) | .640 | .10 (−.09 to .29) | .455 | .22 (.09 to .35) | .006 |
| Objective Cognitive Decline | −.12 (−.32 to .08) | .455 | .12 (−.07 to .31) | .416 | .002 (−.15 to .15) | .977 | .01 (−.32 to .33) | .962 | −.03 (−.17 to .10) | .646 |
Notes. CNR=contrast-to-noise ratio; LC=Locus Coeruleus. Each column represents an ECOG domain regressed on predictors shown in the rows. Rows under “Participant-Rated Decline” show effects when predicting respective ECOG subscales using participant ratings; rows under the “Informant-Rated Decline” show effects when predicting respective ECOG subscales using informant ratings. Models were assessed in a general estimating equation accounting for related observations between twins in the same pair. For objective cognitive decline as a covariate, models with subjective cognitive decline, subjective memory decline, subjective executive function decline, subjective language decline, subjective visuospatial decline are analyzed with declines in objective factors scores of global cognition, memory, executive function, verbal fluency, and visuospatial ability from Wave 1 to Wave 3, respectively.
P-values for effects outside of the hypothesized relationship with rostral-middle LC have been corrected for multiple testing using FDR.
Finally, we conducted sensitivity analyses excluding people with MCI in the main analyses (mixed models) to provide results generalizable to people not cognitively impaired. Statistical significance was determined with an α at .05. FDR multiple testing correction was applied for all analyses outside the main hypothesis that rostral-middle LC integrity would be related to ECOG scores. Given what is known about rostral-middle versus caudal LC, we did not expect a significant association between subjective decline and caudal LC.
Results
Descriptives and bivariate correlations
As shown in Table 1, most people and their informants reported subjective cognitive decline in the range from “better to no change (1)” to “questionably/occasionally worse (2)” with mean scores ranging from 1.35 to 1.61. Regarding bivariate correlations shown in Table 2, lower rostral-middle LCCNR was related to worse participant-rated ECOG scores (r’s range from −.15 to −.10, ps <.05) while the caudal LC was not related to any participant-rated ECOG score (ps>.05). Lower rostral-middle LCCNR was related to the worse informant-rated ECOG score of subjective visuospatial ability (r=−.13, p=.009) while the caudal LCCNR was not related to any informant-rated ECOG score (ps>.05). Lower rostral-middle LCCNR and higher ECOG scores were related to worse objective cognitive function as shown in Table 2. Rostral-middle LCCNR, caudal LCCNR, and most ECOG scores were not associated with objective cognitive decline as shown in Supplementary Table S2.
Relationship of LCCNR with participant-rated decline
Lower rostral-middle LCCNR was related to greater decline in participant-rated subjective cognition (β=−.18, 95% CI [−.29, −.07], p=.001, see Figure 2). For our first exploratory analyses, we examined associations between LCCNR and ECOG subscales. As shown in Table 3 Model 1a, lower rostral-middle LCCNR related to greater decline in subjective memory (β=−.15, 95% CI [−.26, −.04], p=.007), subjective executive function (β=−.16, 95% CI [−.27, −.05], p=.005), subjective language (β=−.14, 95% CI [−.25, −.03], p=.012), and subjective visuospatial ability (β=−.15, 95% CI [−.26, −.04], p=.010). Associations are visualized in Figure 2. Shown in Table 3, no significant associations appeared when looking at the caudal LCCNR as a predictor (ps>.05). Non-significant association of caudal LCCNR is illustrated in Figure 2. To test differences in effect size, we ran a repeated measures model nesting LCCNR within participant testing an interaction of participant-rated subjective cognitive decline with region type. Overall, the interaction term was significant (p<.011), showing that the association of subjective cognitive decline and LCCNR was more significant for rostral-middle LCCNR (β=−.12, 95% CI [−.21 to −.03], p=.011) than caudal LCCNR (β=−.05; 95%CI [95%CI: −.14 to .03]; p=.217).
Figure 2.
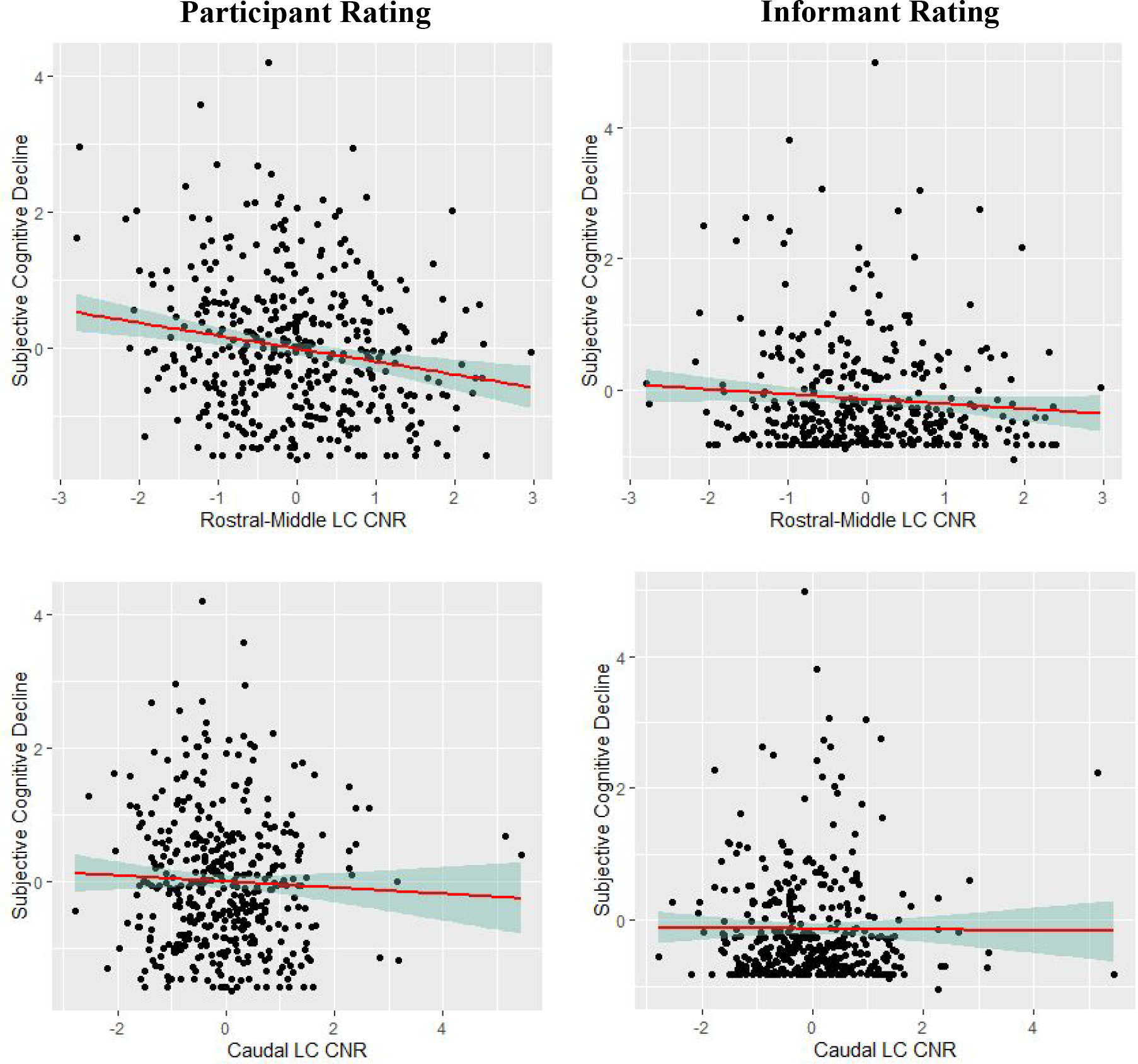
Scatterplots of the associations of rostral-middle and caudal locus coeruleus integrity and participant-rated and informant-rated subjective cognitive decline. Note. Associations are adjusted for age, young-adult cognitive ability, depressive symptoms, and morbidities. Participant-rated and informant-rated subjective cognitive decline was log transformed from its original scale. CNR = contrast to noise ratio; LC = locus coeruleus. All variables are standardized to z-scores.
For our complementary analyses, we adjusted for objective cognitive performance and objective cognitive decline. Significant findings remained when adjusting for objective cognitive performance (βs range from −.16 to −.12, ps<.05, see Table 4) or objective cognitive decline (βs range from −.19 to −.13, ps<.05, see Table 5).
Relationship of LCCNR with informant-rated decline
Rostral-middle and caudal LCCNR were unrelated to decline in informant-rated subjective cognition and other ECOG subscales (ps>.05, see Table 3). Non-significant associations of rostral-middle and caudal LCCNR with informant-rated subjective cognitive decline are illustrated in Figure 2.
Sensitivity analyses excluding participants with MCI.
As shown in Table S4 in the Supplementary Material, the pattern of associations between LCCNR and participant-rated ECOG scales was similar when excluding people with MCI. Lower rostral-middle LCCNR was related to greater decline in participant-rated subjective cognition (β=−.16, 95% CI [−.27, −.04], p=.007), subjective executive function (β=−.14, 95% CI [−.26, −.03, p=.018]), subjective language (β=−.12, 95% CI [−.24, −.004, p=.043]), and subjective visuospatial ability (β=−.14, 95% CI [−.24, −.03], p=.013). The association between lower rostral-middle LCCNR and greater subjective memory decline was now marginal with a similar effect size (β=−.23, 95% CI [−.27, .0001], p=.050). Neither rostral-middle nor caudal LCCNR were significantly associated with informant-rated subjective cognitive decline or ECOG subscales when excluding people with MCI (ps>.05).
Supplemental analyses looking at hippocampal volume.
Rostral and caudal LCCNR were unrelated to hippocampal volume (p=.957). As shown in Table S3 in the Supplementary Material, there were no significant effects of hippocampal volume on subjective cognitive decline or ECOG subscales after adjusting for age, young-adult cognitive ability, depressive symptoms, and physical morbidities in mixed models (ps>.05).
Discussion
Subjective cognitive decline is one of the earliest symptoms of AD (Snitz et al., 2018; van Harten et al., 2018). We found an inverse relationship between rostral-middle LC integrity, a brain stem region affected early in AD pathology, and participant-rated subjective cognitive decline. Below we integrate these findings into existing research on recent studies of the LCCNR, discuss possible explanatory factors, and summarize implications for AD risk research.
In this study, we used an in-vivo measure of LC integrity to explore associations with subjective cognitive decline, one of the earliest presenting AD symptoms (Jessen et al., 2014). Our work builds on recent studies linking LC integrity to related AD risk factors including depression and objective cognitive performance (Dahl et al., 2019; Elman et al., 2021; Guinea-Izquierdo et al., 2021), but we are unaware of any studies linking LC integrity to subjective cognitive decline. A previous study found that people with late-life major depression had lower LCCNR compared to healthy controls (Guinea-Izquierdo et al., 2021). In our sample, subjective cognitive decline remained related to LCCNR even after adjusting for depressive symptoms, which suggests that subjective cognitive decline captures something unique. In our second exploratory aim, we sought to determine if this was due to capturing LC-related differences in cognitive performance. Recent work has shown that people with higher rostral-middle LCCNR have better episodic memory and verbal fluency than people with lower rostral-middle LC integrity (Dahl et al., 2019; Elman et al., 2021). Counter to our expectations, however, associations remained after accounting for current objective cognitive performance and objective cognitive decline. Reasons for reporting LC-related subjective cognitive decline could involve the need for compensatory cognitive effort or the LC’s contribution to personality.
The Adaptive Gain Model postulates that the LC is a key neural substrate of higher-order cognitive processing, arousal, and attention through its tonic and phasic release of norepinephrine/noradrenaline throughout the cortex (Aston-Jones & Cohen, 2005). Greater ratings of subjective cognitive decline may arise as people exert greater cognitive effort to complete tasks in the face of dysregulation of higher-order cognitive processing, arousal, and attention. Engagement of cognitive effort would explain why objective cognitive function did not fully explain the relationship between rostral-middle LC integrity and subjective cognitive decline. Engagement of cognitive effort may also explain why rostral-middle LC integrity was related to participant-rated subjective cognitive decline rather than informant-rated subjective cognitive decline. If people with lower rostral-middle LC integrity engage in greater cognitive effort for compensation, then a portion of difficulties in higher-order cognitive processing and attention would go undetected by neuropsychological testing or informant observation at first. This is supported by research showing that cognitive decline is first noted by the participant before informants before MCI diagnosis (5 versus 2 years before; Caselli et al., 2014). As a note, researchers typically consider informant ratings to be more accurate in capturing objective cognitive decline than participant ratings, especially after MCI (Rabin et al., 2017). However, this boundary is not always so sharp as evidenced by considerable reversion of MCI to cognitively normal on follow-up (18% of cases; Canevelli et al., 2016) and higher participant ratings of subjective cognitive decline than controls in people with MCI (Jessen et al., 2022). Furthermore, participant and informant ratings are weakly related to objective cognitive decline in people with and without MCI (Gustavson et al., 2022; Ryu et al., 2016), emphasizing the larger role of other factors.
In support of the role of compensatory cognitive effort, studies have linked the LC system to objectively-measured cognitive effort and MCI risk. Activity of the LC system has been related to pupil dilation, an objective measure of cognitive effort (e.g., Alnæs et al., 2014; Joshi et al., 2016). We showed this recently in our sample as well. A subsample of VETSA participants completed functional imaging of the LC system as well as pupil dilation during a memory task at Wave 2. Overall, participants who had lower network efficiency in the LC system had greater pupil dilation, suggesting the need for greater cognitive effort (Elman et al., 2017). Furthermore, lower LC network efficiency and greater cognitive effort related to increased MCI risk (Granholm et al., 2017). Although further study is needed, these studies suggest that participant-rated subjective cognitive decline may arise from lower rostral LC integrity that requires the engagement of cognitive effort for compensation.
It is also possible that the relationship between rostral-middle LC integrity and subjective cognitive decline is not due to changes in later life due to aging or AD pathology, but instead related to long-standing differences in personality. Previous studies have shown that subjective cognitive decline is less related to objective cognitive performance (rs ~ .10) (Crumley, Stetler, & Horhota, 2014) and much more related to trait levels of neuroticism (rs>.40) (Bell, Hill, & Stavrinos, 2020; Merema, Speelman, Foster, & Kaczmarek, 2013) and is stable over time (Johansson, Björk, & Thorvaldsson, 2020). We also found in the VETSA sample that subjective cognitive decline corresponded more with levels of concurrent depressive symptoms than objective cognitive decline (Gustavson et al., 2022). The LC system may play an important role in neuroticism, explaining why lower rostral-middle LC is related to subjective cognitive decline over and beyond objective cognitive decline. Neuroticism is defined as the tendency to experience negative emotions due to greater physiological arousal and stress reactivity (Costa & McCrae, 1992; Eysenck, 1983). As mentioned, the LC regulates arousal through norepinephrine/noradrenaline release to the anterior cingulate cortex, prefrontal cortex, hippocampus, amygdala, and thalamus (Samuels & Szabadi, 2008). People with weaker rostral-middle LC structures may be more arousable leading to higher levels of trait neuroticism resulting in greater subjective cognitive decline. A significant role of neuroticism may also explain lower LC integrity found in major depressive disorder as well (Guinea-Izquierdo et al., 2021). Studies incorporating measures of personality and personality-related patterns of physiological arousal would help clarify this possibility. The role of the LC may be important in explaining why neuroticism and related outcomes like depressive symptoms and subjective cognitive decline are predictive of increased AD risk (Ownby et al., 2006; Terracciano et al., 2021).
In supplemental analyses, we found that hippocampal volume was not predictive of participant- or informant-rated subjective cognitive decline. Rostral-middle LC integrity remained associated with participant-rated subjective cognitive decline in these analyses. Tau appears in the hippocampus at later stages of spreading compared to the LC (Braak et al., 2011), so therefore tau in the hippocampus may not be involved in the early stage of subjective cognitive decline. Additional analyses will be needed to assess how relationships change as AD progresses. Hippocampal volume may be more predictive of subjective cognitive decline in later AD stages, and informant ratings may become more reliable as self-awareness decreases (Rabin et al., 2017).
Findings from this study should be considered alongside limitations. First, subjective cognitive decline and the LC were only assessed at a single timepoint, leaving temporal links unclear. VETSA is currently conducting a fourth wave of data collection which will provide prospective measures of subjective cognitive decline, cognitive function, and LC imaging; and these data will allow us to examine temporal relationships. Second, our measures were also unable to specify pathology. Damage to the LC could have been due to AD or other disease processes. Third, our measure of the LC was also based on manual marking in subject space. This approach avoids inaccuracies introduced by registration and interpolation, and shows high inter-rater reliability (Elman et al., 2017). The location of our slices can be compared to other studies based on the location of the middle and rostral slices relative to the inferior colliculus (e.g., the middle slices in 7mm below the inferior edge of the inferior colliculus). However, automated protocols for LC assessment are a key goal of ongoing research to provide further standardization across studies (Dünnwald et al., 2021). Another limitation of our LC measure is that the caudal LC is more diffuse in structure and is more difficult to visualize in acquisitions such as the one used here (Tona et al., 2017). Therefore, we are likely not capturing the caudal-most extent of the LC. However, the pattern of results seen here do still suggest a rostral-caudal gradient of effects. Fourth, our cognitive factor scores did not demonstrate strong invariance, which is expected due to developmental change in means and variances (Haberstumpf et al., 2022; Pentz et al., 1994, Tyrell et al., 2019), but could possibly be due to some measurement bias. Fourth, our sample was entirely male and largely white, non-Hispanic, making generalizations to women and other racial/ethnic groups uncertain. Nevertheless, men represent a group at high risk for MCI (Petersen et al., 2010), from which our findings can be extended.
In conclusion, lower rostral-middle LC integrity was significantly associated with greater participant-rated subjective cognitive decline, even after adjusting for other possible explanatory factors. As a goal for further study and translation, findings might differentiate which individuals with subjective cognitive decline are more likely to develop AD. Individuals with subjective cognitive decline might have trouble with higher-order cognitive processing, arousal, and attention due to lower LC integrity, which may reflect early AD tau deposition in this region (Jacobs et al., 2021; Wilson et al., 2013). Longitudinal studies with amyloid and tau biomarker collection will be worthwhile in examining this hypothesis further. Regardless, the LC appears a crucial factor in explaining why some individuals rate higher subjective cognitive decline than their peers, even in the absence of cognitive impairment.
Supplementary Material
Figure 3.
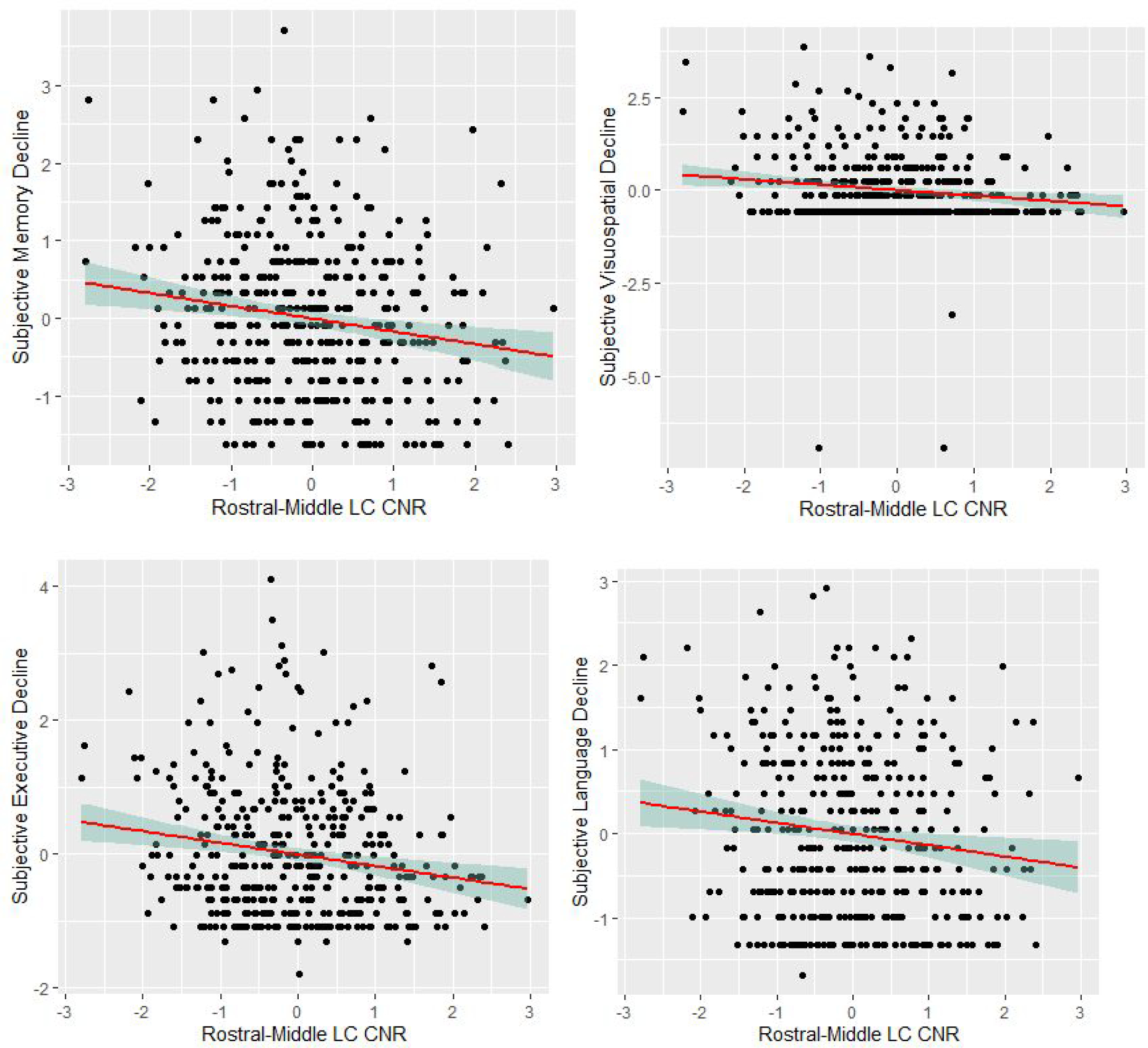
Scatterplots of the associations of rostral-middle locus coeruleus integrity and participant-rated ECog subscales. Note. Associations are adjusted for age, young-adult cognitive ability, depressive symptoms, and morbidities. ECog subscales were log transformed from its original scale. CNR = contrast to noise ratio; LC = locus coeruleus. All variables are standardized to z-scores.
Acknowledgments:
The content is the responsibility of the authors and does not necessarily represent official views of the NIA, NIH, or VA. The U.S. Department of Veterans Affairs, Department of Defense; National Personnel Records Center, National Archives and Records Administration; National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University provided invaluable assistance in the creation of the VET Registry. The Cooperative Studies Program of the U.S. Department of Veterans Affairs provided financial support for development and maintenance of the Vietnam Era Twin Registry. We would also like to acknowledge the continued cooperation and participation of the members of the VET Registry and their families.
Financial Support:
This work was supported by the National Institute on Aging at the National Institutes of Health grant numbers R01s AG050595 (WSK MJL CEF), AG022381 (WSK), AG037985, AG062483, and P01 AG055367, and K01AG063805.
Footnotes
Conflict of Interest Declaration: The authors declare the absence of known competing financial or personal relationships that could have influenced the work reported in this paper.
References
- Alnæs D, Sneve MH, Espeseth T, Endestad T, van de Pavert SHP, & Laeng B (2014). Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. Journal of Vision, 14(4), 1–20. doi: 10.1167/14.4.1 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, & Bloom FE (1981). Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci, 1(8), 876–886. doi: 10.1523/jneurosci.01-08-00876.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, & Cohen JD (2005). An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci, 28, 403–450. [DOI] [PubMed] [Google Scholar]
- Bell T, Hill N, & Stavrinos D (2020). Personality determinants of subjective executive function in older adults. Aging & Mental Health, 24(11), 1935–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, & Brown GG (2005). fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology, 64(3), 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, & Snyder AZ (2004). A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage, 23(2), 724–738. doi: 10.1016/j.neuroimage.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FI, Duarte A, Grady CL, . . . Reuter-Lorenz PA (2018). Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience, 19(11), 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canevelli M, Grande G, Lacorte E, Quarchioni E, Cesari M, Mariani C, … & Vanacore N (2016). Spontaneous reversion of mild cognitive impairment to normal cognition: A systematic review of literature and meta-analysis. Journal of the American Medical Directors Association, 17(10), 943–948. doi: 10.1016/j.jamda.2016.06.020 [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Chen K, Locke DE, Lee W, Roontiva A, Bandy D, … & Reiman EM (2014). Subjective cognitive decline: Self and informant comparisons. Alzheimer’s & Dementia, 10(1), 93–98. doi: 10.1016/j.jalz.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LA, Acheson AL, Zigmond MJ, & Stricker EM (1983). Subtotal destruction of central noradrenergic projections increases the firing rate of locus coeruleus cells. Brain Research, 264(1), 123–126. [DOI] [PubMed] [Google Scholar]
- Clement F, & Belleville S (2010). Compensation and disease severity on the memory-related activations in mild cognitive impairment. Biol Psychiatry, 68(10), 894–902. doi: 10.1016/j.biopsych.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Clewett DV, Lee TH, Greening S, Ponzio A, Margalit E, & Mather M (2016). Neuromelanin marks the spot: Identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiology of Aging, 37, 117–126. doi: 10.1016/j.neurobiolaging.2015.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, & McCrae RR (1992). Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychological Assessment, 4(1), 5. [Google Scholar]
- Crumley JJ, Stetler CA, & Horhota M (2014). Examining the relationship between subjective and objective memory performance in older adults: A meta-analysis. Psychology and Aging, 29(2), 250. [DOI] [PubMed] [Google Scholar]
- Dahl MJ, Mather M, Duzel S, Bodammer NC, Lindenberger U, Kuhn S, & Werkle-Bergner M (2019). Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat Hum Behav, 3(11), 1203–1214. doi: 10.1038/s41562-019-0715-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dünnwald M, Ernst P, Düzel E, Tönnies K, Betts MJ, & Oeltze-Jafra S (2021). Fully automated deep learning-based localization and segmentation of the locus coeruleus in aging and Parkinson’s disease using neuromelanin-sensitive MRI. International Journal of Computer Assisted Radiology and Surgery, 16(12), 2129–2135. doi: 10.1007/s11548-021-02528-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Oh H, Madison CM, Baker SL, Vogel JW, Marks SM, . . . Jagust WJ (2014). Neural compensation in older people with brain amyloid-β deposition. Nature Neuroscience, 17(10), 1316–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Panizzon MS, Hagler DJ Jr., Eyler LT, Granholm EL, Fennema-Notestine C, . . . Kremen WS (2017). Task-evoked pupil dilation and BOLD variance as indicators of locus coeruleus dysfunction. Cortex, 97, 60–69. doi: 10.1016/j.cortex.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Puckett OK, Beck A, Fennema-Notestine C, Cross LK, Dale AM, . . . Kremen WS (2021). MRI-assessed locus coeruleus integrity is heritable and associated with multiple cognitive domains, mild cognitive impairment, and daytime dysfunction. Alzheimers & Dementia, 17(6), 1017–1025. doi: 10.1002/alz.12261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Puckett OK, Hagler DJ, Pearce RC, Fennema-Notestine C, Hatton SN, … & Kremen WS (2021). Associations between mri-assessed locus coeruleus integrity and cortical gray matter microstructure. Cerebral Cortex. doi: 10.1093/cercor/bhab475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili M, Nejati V, Shati M, Vatan RF, Chehrehnegar N, & Foroughan M (2021). Attentional network changes in subjective cognitive decline. Aging Clinical and Experimental Research, 1–9. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ (1983). Psychophysiology and personality: Extraversion, neuroticism and psychoticism. In Individual differences and psychopathology (pp. 13–30): Elsevier. [Google Scholar]
- Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, & Decarli C (2008). The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology, 22(4), 531–544. doi: 10.1037/0894-4105.22.4.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … & Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. doi: 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM (2002). The Stroop Color and Word Test: A manual for clinical and experimental uses;[adult Version]. Stoelting. [Google Scholar]
- Granholm EL, Panizzon MS, Elman JA, Jak AJ, Hauger RL, Bondi MW, . . . Kremen WS (2017). Pupillary responses as a biomarker of early risk for Alzheimer’s disease. J Alzheimers Dis, 56(4), 1419–1428. doi: 10.3233/JAD-161078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinea-Izquierdo A, Giménez M, Martínez-Zalacaín I, Del Cerro I, Canal-Noguer P, Blasco G, . . . Camins A (2021). Lower locus coeruleus MRI intensity in patients with late-life major depression. PeerJ, 9, e10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstumpf S, Forster A, Leinweber J, Rauskolb S, Hewig J, Sendtner M, … & Herrmann MJ (2022). Measurement invariance testing of longitudinal neuropsychiatric test scores distinguishes pathological from normative cognitive decline and highlights its potential in early detection research. Journal of Neuropsychology, 16(2), 324–352. doi: 10.1111/jnp.12269 [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Callaghan MF, Hopkins A, Kosciessa J, Betts M, Cardenas-Blanco A, . . . Dolan RJ (2018). Locus coeruleus integrity in old age is selectively related to memories linked with salient negative events. Proceedings of the National Academy of Sciences, 115(9), 2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp. Source: https://www-01.ibm. [Google Scholar]
- Jacobs HI, Becker JA, Kwong K, Engels-Domínguez N, Prokopiou PC, Papp KV, . . . Sanchez JS (2021). In vivo and neuropathology data support locus coeruleus integrity as indicator of Alzheimer’s disease pathology and cognitive decline. Science Translational Medicine, 13(612), eabj2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Panizzon MS, Spoon KM, Fennema-Notestine C, Franz CE, Thompson WK, . . . Vuoksimaa E (2015). Hippocampal atrophy varies by neuropsychologically defined MCI among men in their 50s. The American Journal of Geriatric Psychiatry, 23(5), 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janitzky K (2020). Impaired phasic discharge of locus coeruleus neurons based on persistent high tonic discharge—A new hypothesis with potential implications for neurodegenerative diseases. Frontiers in Neurology, 11, 1–15. doi: 10.3389/fneur.2020.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, . . . Subjective Cognitive Decline Initiative Working, G. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement, 10(6), 844–852. doi: 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, . . . Scheef L (2006). Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiology of Aging, 27(12), 1751–1756. [DOI] [PubMed] [Google Scholar]
- Jessen F, Wolfsgruber S, Kleineindam L, Spottke A, Altenstein S, Bartels C, … & Düzel E (2022). Subjective cognitive decline and stage 2 of Alzheimer disease in patients from memory centers. Alzheimer’s & Dementia, online first, 1–11. doi: 10.1002/alz.12674 [DOI] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani RM, & Gold JI (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron, 89(1), 221–234. doi: 10.1016/j.neuron.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Björk MP, & Thorvaldsson V (2020). I rate my memory quite similar at age 40 and at age 70. The Journal of Gerontopsychology and Geriatric Psychiatry, 33(4), 235–244. doi: 10.1024/1662-9647/a000239 [DOI] [Google Scholar]
- Keren NI, Taheri S, Vazey EM, Morgan PS, Granholm ACE, Aston-Jones GS, & Eckert MA (2015). Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. Neuroimage, 113, 235–245. doi: 10.1016/j.neuroimage.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Panizzon MS, Franz CE, Spoon KM, Vuoksimaa E, Jacobson KC, … & Lyons MJ (2014). Genetic complexity of episodic memory: A twin approach to studies of aging. Psychology and aging, 29(2), 404–417. doi: 10.1037/a0035962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Franz CE, & Lyons MJ (2019). Current status of the Vietnam Era Twin Study of Aging (VETSA). Twin Research and Human Genetics, 22(6), 783–787. doi: doi: 10.1017/thg.2019.125 [DOI] [PubMed] [Google Scholar]
- Lezak MD (2004). Neuropsychological assessment. New York, New York: Oxford University Press. [Google Scholar]
- Lyons MJ, Panizzon MS, Liu W, McKenzie R, Bluestone NJ, Grant MD, . . . Jacobson KC (2017). A longitudinal twin study of general cognitive ability over four decades. Developmental Psychology, 53(6), 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, . . . Xian H (2009). Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychological science, 20(9), 1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merema MR, Speelman CP, Foster JK, & Kaczmarek EA (2013). Neuroticism (not depressive symptoms) predicts memory complaints in some community-dwelling older adults. The American Journal of Geriatric Psychiatry, 21(8), 729–736. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2007). Mplus. Statistical analysis with latent variables. Version, 3. [Google Scholar]
- Nyberg L, Maitland SB, Rönnlund M, Bäckman L, Dixon RA, Wahlin Å, & Nilsson LG (2003). Selective adult age differences in an age-invariant multifactor model of declarative memory. Psychology and Aging, 18(1), 149–160. doi: 10.1037/0882-7974.18.1.149 [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, & Loewenstein D (2006). Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry, 63(5), 530–538. doi: 10.1001/archpsyc.63.5.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz V, . . . Rocca W (2010). Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology, 75(10), 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentz MA, Chou CP (2019). Measurement invariance in longitudinal clinical research assuming change from development and intervention. J Consult Clin Psychol. 62(3), 450–62. doi: 10.1037//0022-006x.62.3.450. [DOI] [PubMed] [Google Scholar]
- Priovoulos N, van Boxel SCJ, Jacobs HIL, Poser BA, Uludag K, Verhey FRJ, & Ivanov D (2020). Unraveling the contributions to the neuromelanin-MRI contrast. Brain Structure & Function, 225(9), 2757–2774. doi: 10.1007/s00429-020-02153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, . . . Ellis KA (2015). Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. Journal of Alzheimer’s Disease, 48(s1), S63–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Smart CM, & Amariglio RE (2017). Subjective cognitive decline in preclinical Alzheimer’s disease. Annual Review of Clinical Psychology, 13, 369–396. 10.1146/annurev-clinpsy-032816-045136 [DOI] [PubMed] [Google Scholar]
- Radloff LS (2016). The CES-D Scale. Applied Psychological Measurement, 1(3), 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Ryu SY, Lee SB, Kim TW, & Lee TJ (2016). Memory complaints in subjective cognitive impairment, amnestic mild cognitive impairment and mild Alzheimer’s disease. Acta Neurologica Belgica, 116(4), 535–541. doi: 10.1007/s13760-016-0604-7 [DOI] [PubMed] [Google Scholar]
- Samuels ER, & Szabadi E (2008). Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol, 6(3), 235–253. doi: 10.2174/157015908785777229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin A, Wishart H, Rabin L, Santulli R, Flashman L, West J, . . . Mamourian A (2006). Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology, 67(5), 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW, Maitland SB, Willis SL, & Intrieri RC (1998). Longitudinal invariance of adult psychometric ability factor structures across 7 years. Psychology and Aging, 13(1), 8–20. doi: 10.1037/0882-7974.13.1.8 [DOI] [PubMed] [Google Scholar]
- Schmand B, Jonker C, Hooijer C, & Lindeboom J (1996). Subjective memory complaints may announce dementia. Neurology, 46(1), 121–125. [DOI] [PubMed] [Google Scholar]
- Schoeneborn CA, & Heyman KM (2009). Health characteristics of adults aged 55 years and over: United States, 2004–2007. National Health Statistics Reports; no. 16. Hyattsville, MD: National Center for Health Statistics; Retrieved from http://www.cdc.gov/nchs/nhis/nhis_nhsr.htm [PubMed] [Google Scholar]
- Smart CM, Segalowitz SJ, Mulligan BP, & MacDonald SW (2014). Attention capacity and self-report of subjective cognitive decline: A P3 ERP study. Biological Psychology, 103, 144–151. doi: 10.1016/j.biopsycho.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Terracciano A, Aschwanden D, Passamonti L, Toschi N, Stephan Y, Luchetti M, … & Sutin AR (2021). Is neuroticism differentially associated with risk of Alzheimer’s disease, vascular dementia, and frontotemporal dementia? Journal of Psychiatric Research, 138, 34–40. doi: 10.1016/j.jpsychires.2021.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona KD, Keuken MC, de Rover M, Lakke E, Forstmann BU, Nieuwenhuis S, & van Osch MJ (2017). In vivo visualization of the locus coeruleus in humans: quantifying the test–retest reliability. Brain Structure and Function, 222(9), 4203–4217. doi: 10.1007/s00429-017-1464-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, & Lyons MJ (2001). The Harvard Twin Study of Substance Abuse: What we have learned. Harvard Review of Psychiatry, 9, 267–279. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11600486 [PubMed] [Google Scholar]
- Tu M-C, Lo C-P, Huang C-F, Huang W-H, Deng JF, & Hsu Y-H (2018). Visual attention performances and related cerebral microstructural integrity among subjects with subjective cognitive decline and mild cognitive impairment. Frontiers in Aging Neuroscience, 10, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrell FA, Yates TM, Widaman KF, Reynolds CA, & Fabricius WV (2019). Data harmonization: Establishing measurement invariance across different assessments of the same construct across adolescence. Journal of Clinical Child & Adolescent Psychology, 48(4), 555–567. 10.1080/15374416.2019.1622124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoksimaa E, Panizzon MS, Chen CH, Eyler LT, Fennema-Notestine C, Fiecas MJA, … & Kremen WS (2013). Cognitive reserve moderates the association between hippocampal volume and episodic memory in middle age. Neuropsychologia, 51(6), 1124–1131. doi: 10.1016/j.neuropsychologia.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T et al. (2019) Magnetic resonance imaging of noradrenergic neurons. Brain Struct. Funct. 224, 1609–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, . . . Bennett DA (2013). Neural reserve, neuronal density in the locus coeruleus, and cognitive decline. Neurology, 80(13), 1202–1208. doi: 10.1212/WNL.0b013e3182897103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca FA, Bellei C, Giannelli S, Terreni MR, Gallorini M, Rizzio E, … & Zecca L (2006). Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: consequences for neuronal vulnerability. Journal of Neural Transmission, 113(6), 757–767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


