Summary
Background
Attention deficit/hyperactivity disorder (ADHD) is usually conceptualized as a childhood-onset neurodevelopmental disorder, in which symptoms either decrease steadily into adulthood or remain stable. A recent study challenged this view, reporting that for most with ADHD, diagnostic status fluctuates with age. We ask if such a ‘fluctuating’ ADHD symptom trajectory subgroup is present in other population-based and clinic-based cohorts, centered on childhood and adolescence.
Methods
Cohorts were the population-based Adolescent Brain Cognitive Development (ABCD: N = 9735), Neurobehavioral Clinical Research (NCR: N = 258), and the Nathan Kline Institute-Rockland (NKI-Rockland: N = 149). All participants had three or more assessments spanning different age windows. Participants were categorized into developmental diagnostic subgroups: fluctuant ADHD (defined by two or more switches between meeting and not meeting ADHD criteria), remitting ADHD, persisting ADHD, emerging ADHD and never affected. Data were collected between 2011 and 2022. Analyses were performed between May 2022 and April 2023.
Findings
A subgroup with fluctuant child and adolescent ADHD diagnoses was found in all cohorts (29.3% of participants with ADHD in ABCD, 26.6% in NCR and 17% in NKI-Rockland). While the proportion of those with fluctuant ADHD increased with the number of assessments, it never constituted the dominant subgroup.
Interpretation
We provide further evidence in three cohorts for the existence of a fluctuant ADHD diagnostic subgroup during childhood and adolescence, albeit in a minority of cases. Such fluctuant child and adolescent ADHD diagnoses may suggest a natural history more akin to relapsing-remitting mood disorders and/or a marked sensitivity to environmental shifts that occur across development.
Funding
Intramural programs of the NHGRI and NIMH.
Keywords: ADHD, Developmental trajectories, Neurodevelopment
Research in context.
Evidence before this study
Implicit in current conceptualizations of ADHD as a neurodevelopmental disorder is the idea of early symptom onset followed by gradual symptom change into adulthood. However, most previous studies have either examined only two diagnostic timepoints (typically in childhood and then in late adolescence or early adulthood), or reduced change to linear fits without consideration of potential idiosyncratic fluctuations in diagnostic status at the level of individual participants. A recent paper by Sibley and colleagues showed that roughly two-thirds of young people with ADHD in the Multimodal Treatment of Attention Deficit Hyperactivity Disorder (MTA) cohort moved between periods of remission and relapse multiple times over the course of development.
Added value of this study
Building on this work, we examined the presence and prevalence of fluctuating ADHD subgroups in three longitudinal cohorts (ABCD, NCR and NKI-Rockland), including >10,000 youth, with differing and complementary study populations and assessment procedures. We find that a significant proportion of participants follow fluctuant diagnostic trajectories (between 17 and 29.3%), a lower proportion than reported by Sibley and colleagues. We also show that the fluctuant ADHD subgroup is similar to more traditionally recognized subgroups of ADHD remission or persistence on a range of key clinical, cognitive and demographic variables. These findings were robust to the consideration of comorbidities and medication status.
Implications of all the available evidence
The converging evidence from this study and the previous work by Sibley and colleagues raises important nosological issues: is ADHD a relapsing-remitting disorder for some, rather than a neurodevelopmental disorder in which symptoms change gradually or remain stable over a period of years? Such a scenario would suggest the need for continued clinical monitoring beyond possibly only transient periods of remission in youth with ADHD.
Introduction
In the DSM-5, attention deficit/hyperactivity disorder (ADHD) is viewed as a quintessential neurodevelopmental disorder: it has its onset in childhood and its symptoms can continue into adolescence and adulthood.1,2 Implicit in this conceptualization of ADHD is the idea of gradual, unidirectional change: some individuals improve gradually over years, some have worsening symptoms, whereas others show symptomatic stability.1, 2, 3, 4, 5 While limited by heterogeneity in prevalence estimates arising from different assessment methods,6 a meta-analysis of prospective studies found that 15%–20% of children with ADHD show persistence of the full syndrome into adulthood, with a further 50% showing persistence of subthreshold symptoms with impairment.3
The neurodevelopmental view of ADHD has already been challenged by evidence suggesting that ADHD can have its onset in adolescence or even adulthood.7 Here we consider a second challenge from a recent cohort study that found that few children with ADHD show either gradual improvement or stable symptoms into adulthood. Rather the majority showed ‘waxing and waning’ fluctuations in ADHD diagnostic status over time.6,8 This perhaps surprising finding of ‘fluctuant’ trajectories arose from the charting of ADHD diagnostic histories from a mean of age 8–25 years, in 558 participants from the Multimodal Treatment Study of Children with ADHD (MTA Study).6
If replicated, such diagnostic fluctuations in ADHD could have profound implications for the nosological, etiological and clinical understanding of ADHD.8 Nosologically, it might suggest that ADHD bear similarities to a recurrent mood disorder, in which there are phases of acute symptomatology followed by remission and often relapse, as much as a neurodevelopmental disorder in which symptoms change gradually or remain stable over a period of years. Etiologically, diagnostic fluctuations need to be explained and draw focus to changes within the individual that could alter symptoms, such as lifestyle choices, as well as shifts in the environment, such as changing familial and school contexts. Fluctuating ADHD diagnoses might also represent an exquisite sensitivity to environmental shifts, further emphasizing the need to parse gene by environment interplay. Finally, at a clinical level, the presence of fluctuant ADHD diagnoses would argue for continued follow-up of those who have remitted as they may relapse.
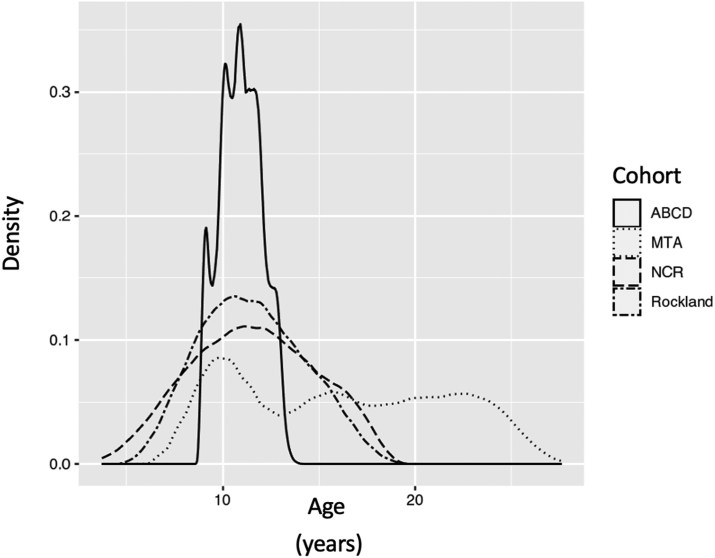
In the current study we ask if similar ‘fluctuant’ ADHD diagnostic trajectories are found in other cohorts. We examine clinic-based (Neurobehavioral Clinical Research; NCR) and community-based (enhanced Nathan Kline Institute-Rockland; NKI-Rockland) cohorts, enriched for ADHD,9,10 and a population based and demographically distributed cohort, the Adolescent Brain Cognitive Development (ABCD) cohort, to estimate the proportion of children showing ‘fluctuant’ ADHD diagnostic subtype.11,12 These cohorts have important differences from the MTA, summarized in Fig. 1. The MTA spanned childhood to adulthood (from a mean age of 8 to 25 years), with up to nine observations taken approximately 2 years apart. By contrast, the ABCD cohort had three observations, made annually, over a narrower late childhood window of between 9 and 12 years. The clinical cohorts of the NCR and NKI-Rockland both covered childhood and adolescence (around 5–18 years), but while the NKI-Rockland cohort had three observations with a median inter-observation interval of 1.1 years (IQR 0.29), the NCR cohort had between three to nine observations at a median interval of 1.46 (IQR 1.09) years. While in some ways a limitation, these diverse features might also serve as a strength if fluctuating diagnostic trajectories are found even in cohorts with fewer observations obtained mainly over childhood and adolescence.
Fig. 1.
Density plot showing the age-ranges covered for each cohort.
We also sought to rule out if a fluctuating ADHD diagnostic course might accentuate relatively minor shifts in underlying symptoms. For example, a fluctuating ADHD could arise when a child is just below the diagnostic threshold at baseline (e.g., 5 symptoms of inattention), just at threshold at the first follow-up (i.e., 6 symptoms) and then goes back to near threshold at the final follow-up (5 symptoms). This factor does not explain the MTA findings: for participants who fluctuated the inattention varied from an average high of 8 to average low of 2 symptoms.13 However, we seek to ensure that this possibility does not explain fluctuation in other cohorts, via a number of sensitivity analyses requiring more stringent criteria be met for ADHD remission. We also examine effects of mode of assessment. The MTA used rating scales obtained on multiple informants as they found this approach had better psychometric properties than a clinical interview.14 However, most other studies rely upon a structured DSM-based interview with the parent, which may impact on the detection of more stable underlying trends.8,15 Thus, we determine if diagnoses based on interview with the parent will return a similar proportion of individuals with fluctuant courses as those found in the multi-informant MTA study.
In this work, we examined three cohorts with differing design features, assessment methods, and study populations for evidence of a fluctuant diagnostic ADHD subgroup. We also aimed to examine whether the developmental subgroups differed according to demographic and neuropsychological variables.
Methods
Samples
The central inclusion criterion was the availability of three or more assessments of ADHD symptoms, as at least three assessments are needed to detect a fluctuant clinical course. A side-by-side comparison of the included cohorts is provided in Table 1. Details on excluded participants are provided in Supplementary Table S1.
Table 1.
Overview of three cohorts of children and adolescents with ADHD included in the primary analyses.
| ABCD | NCR | NKI-Rockland | |
|---|---|---|---|
| N ADHD | 1518 | 154 | 47 |
| Design | Large (21 sites) developmental longitudinal cohort approximating the diversity of the United States population on sex, race/ethnicity, and socioeconomic status. | Clinical cohort using accelerated longitudinal design. | Community sample based in Rockland County (15 miles northwest of New York City). |
| Mean age at baseline (sd) | 9.89 (0.62) | 8.76 (2.28) | 9.47 (2.23) |
| Mean age at final follow-up (sd) | 11.97 (0.66) | 14.64 (2.29) | 11.67 (2.24) |
| Age range | >9, <13 years | >4, <18 years | >6, <18 years |
| Median duration between assessments (IQR) | 1 years (0) | 1.46 years (1.09) | 1.1 years (0.29) |
| N assessments | 3 | Range 3–9, median = 4 (IQR = 2) | 3 |
| Sex (%) | |||
| Male | 1023 (67.4%) | 105 (68.2%) | 35 (74.5%) |
| Female | 495 (32.6%) | 49 (31.83%) | 12 (25.5%) |
| Race (%) | |||
| Black/African American | 218 (14.4%) | 20 (13%) | 10 (21.3%) |
| More than one race/declined/other | 315 (20.8%) | 24 (15.5%) | 6 (12.8%) |
| White | 985 (64.9%) | 110 (71.4%) | 31 (66%) |
| Ethnicity (%) | |||
| Non-Hispanic | 1244 (83.3%) | 140 (90.3%) | 35 (74.5%) |
| Hispanic | 249 (16.7%) | 15 (9.7%) | 12 (25.5%) |
Abbreviations. ABCD, Adolescent Brain Cognitive Development Study; IQR, Interquartile range; NCR, Neurobehavioral Clinical Research; NKI-Rockland, Enhanced Nathan Kline Institute-Rockland.
Missing data ABCD: N = 25 missing ethnicity.
The first cohort was the ABCD,12,16,17 a population cohort of N = 11,876 youth, from which we used data on 9735 participants with diagnoses available from the parent-answered Computerized Schedule of Affective Disorders and Schizophrenia for School-Age Children (KSADS-COMP) for DSM-5, collected at three timepoints (ages 9–10, 10–11 and 11–12 years).18 All procedures were approved by a central Institutional Review Board (IRB) at the University of California, San Diego, and by individual site IRBs. Parents/guardians provided written informed consent and children assented before participation in the study.
The second cohort was the clinic-based NCR accelerated longitudinal cohort.9,10,19 ADHD diagnostic status was determined at each timepoint by one of two clinicians (with high diagnostic kappa > 0.9) following the diagnostic algorithm of the DICA-IV. The NCR study included multiple assessments (median 4, range 3–9) between 4 and 18 years of age (mean age at baseline = 8.76 [SD 2.4]).6,9 The study was approved by the IRB of the NIH and children and parents provided written assent or consent.
The NKI-Rockland cohort20,21 drew from the childhood population in a suburban/rural county northwest of New York City. While the study aimed to recruit a demographically representative cohort, the high proportion of participants with ADHD suggests that there may have been some participation bias. Like the ABCD cohort, NKI-Rockland had three observations per individual, and like the NCR cohort it used an accelerated longitudinal design (baseline age: 10.4 [SD 2.5], range 6.1–18 years) and a DSM-5 based, clinician administered semi-structured interview to assess ADHD. Institutional Review Board Approval was obtained for this project at the Nathan Kline Institute. Written informed consent was obtained from legal guardians and written assent was obtained from the participants.
Definition of developmental subgroups
Developmental subgroups were defined according to longitudinal patterns of ADHD diagnosis, as indicated by the KSADS-COMP algorithm (ABCD cohort) or clinician-administered interviews (NCR and NKI-Rockland cohorts; see Supplement). For all datasets, the fluctuant ADHD subgroup showed at least two switches between meeting full ADHD criteria and not meeting criteria (e.g., ADHD at baseline, no ADHD at first follow-up, ADHD at final assessment). The persistent ADHD subgroup met diagnostic criteria at all assessments. The emergent subgroup included participants who did not meet criteria for ADHD at baseline, but who met diagnostic criteria at a later timepoint and at all assessments thereafter. The remitting subgroup met ADHD diagnostic criteria at baseline but ceased meeting criteria at a later assessment and for all subsequent assessments.
For all cohorts, the proportions in each diagnostic developmental subgroup were expressed with reference to the number of participants who met ADHD criteria at any timepoint.
Associations with demographic, neuropsychological variables
We next examined whether the developmental subgroups differed according to demographic and neuropsychological variables, using the lmerTest (version 3.1.3) software package22 for R (version 4.0.2; http://www.r-project.org). For the ABCD cohort, we included nested random-terms for site and nuclear family. For the single site NCR, we included a random term for nuclear family. NKI-Rockland was a single site study and did not provide data on familial relatedness for participants. See Supplement.
Robustness analyses
Consideration of family relatedness in ABCD and NCR
There were some related individuals in the ABCD cohort and in the NCR cohort, and we thus repeated analyses randomly selecting one member per family in 1000 bootstraps, and estimated the proportions in each outcome group.
Is diagnostic fluctuation driven by medication or comorbid diagnoses?
We first examined whether similar subgroups were observable when including only participants free of all ADHD medications throughout the study. We also include analyses that require the absence of ADHD to be coded only if the participant was off all ADHD medications at that assessment (thus the subject would be coded as having ADHD if they were on any ADHD medication at that timepoint, regardless of symptom number).
We determined if differences between groups in comorbidities may be driving fluctuations in ADHD diagnostic status by repeating analyses removing all those who met diagnostic criteria for a comorbidity at any assessment.
Analyses using case definition similar to those in the MTA study
We repeated analyses in a manner similar to that used in the MTA study, defining the absence of ADHD as less than 4 symptoms of both inattention and hyperactivity/impulsivity, no impairment and taking no ADHD medication.
Analyses incorporating teacher reports
Teacher reports on symptoms using the Brief Problem Monitor at each timepoint were available for 118 participants with KSADS-COMP diagnosed ADHD in the ABCD cohort (7.7% of all participants with ADHD included in the main analyses).23 We conducted analyses in which a t-score of the attention scale of 65 or greater was taken to indicate a clinically significant level of symptoms. Thus, ADHD remission was defined as the absence of an ADHD diagnosis on the KSADS-COMP in conjunction with a teacher rating t-score of <65. We did not have sufficient longitudinal teacher data on the other two cohorts (NKI-Rockland and NCR) to consider teacher informants.
Analyses using stricter definitions of impairment
For the ABCD cohort, we also examined the impact of requiring impairment to be present in two or more settings (rather than just one or more settings as is applied in DSM-5). The data required for this analysis (i.e., the number of settings in which ADHD symptoms caused impairment) were available on 9376 of the 9735 participants. We also defined ADHD as requiring impairment in two settings for the NCR cohort (data were available on all participants).
Role of the funding source
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation or review of the manuscript; and decision to submit the manuscript for publication but had a role in the approval of the manuscript.
Results
Characteristics of each cohort
The ABCD sample comprised 9735 individuals (5091 males (52.3%), 4644 females (47.7%)) with three clinical assessments. At the first assessment, 858 (8.8%) met criteria for ADHD, at the second assessment 961 (9.9%) met criteria and at the final assessment 688 (7.1%) met criteria. The NCR cohort had 258 participants (170 males (65.9%), 88 females (34.1%)), with a mean age of 8.8 (SD = 2.3) years at study entry and 14.5 (SD = 2.5) at the final observation. Of these, 154 (59.7%) met criteria for ADHD at one timepoint at least. The NKI-Rockland cohort had 149 individuals (83 (55.7%) males, 66 (44.3%) females) with a mean age of 10.4 (SD = 2.4) at entry, 11.5 (SD = 2.4) at second and 12.7 (SD = 2.5) at the third and final assessment. Forty-seven (31.5%) met criteria for ADHD at one timepoint at least. Table 1 and Supplementary Table S2.
Is there a fluctuant subtype of ADHD?
All cohorts had a subgroup that showed ‘fluctuant’ ADHD diagnoses—Fig. 2. For the ABCD population cohort, the fluctuant group comprised 445 individuals- 29.3% of those who met ADHD criteria at any point during the study. Of these, 96 (6.3%) had ADHD at the first assessment, did not meet criteria at the second assessment, but met criteria again at the third and final assessment. Conversely, 350 (23.1%) did not meet criteria at baseline, but met criteria at the second but not third assessment.
Fig. 2.
Shows the proportion of those with ADHD who had a fluctuant trajectory in the primary analysis and across various sensitivity analyses. (a) Shows the results of the primary analysis. (b) Shows the results of analyses that coded for ADHD when the child was on an ADHD medication (regardless of symptom count). (c) Shows the results when analyses restricted to those with no comorbid disorders throughout. (d) Shows the results when confining analyses to those who remained free of ADHD medication throughout. (e) Shows the results for ABCD and NCR when applying a definition of ADHD similar to that in the MTA report by Sibley and colleagues (absence of ADHD only when less than 4 symptoms of both inattention and hyperactivity/impulsivity, no impairment and no ADHD medication). (f) Shows the results for ABCD and NCR when two domains of impairment were required.
For NKI-Rockland, which also had three observations, 8 of the 47 (17%) and for the NCR cohort, when using all available data, 41 (26.6%) had a fluctuant ADHD course, switching at least twice between meeting and not meeting DSM criteria. Examples of individual level trajectories from individuals in the NCR cohort are given in Supplementary Figure S1. Despite the differences in cohort design, the proportion of those with a fluctuant diagnostic trajectory did not differ significantly across the three cohorts (χ2 = 3.8, p = 0.15).
Using data from NCR, we found that the proportion of ADHD participants in the fluctuant group rose in tandem with the number of assessments (Supplementary Table S3). When considering the 57 participants with three assessments, 8 (14%) fell into the fluctuant group; the proportion in the fluctuant group rose to 20% when considering the 35 participants with four assessments and to 41.9% for the 62 participants with five or more assessments- Supplementary Figure S2. Thus, the fluctuant subtype was more likely to be detected in participants with more assessments and may be underestimated by the ABCD and Rockland cohorts, which both had a maximum of three assessments.
Demographic and neuropsychological correlates
The ADHD developmental subgroups mostly did not differ in demographic features. See Table 2.
Table 2.
Demographic and clinical characteristics of N = 1719 children and adolescents with ADHD included in the primary analyses.
| Stat |
p |
|||||
|---|---|---|---|---|---|---|
| ABCDa | ||||||
| Emergent | Fluctuant | Persistent | Remitted | |||
| N (%) | 312 (20.6%) | 445 (29.3%) | 279 (18.4%) | 482 (31.8%) | ||
| Age at baseline | 9.9 (0.6) | 9.9 (0.6) | 9.9 (0.6) | 9.9 (0.6) | F (3, 1506) = 0.1 | 0.96 |
| Age at final follow-up | 12 (0.6) | 12 (0.67) | 12 (0.7) | 12 (0.7) | (3, 1506) = 0.14 | 0.94 |
| Sex | χ2 (3) = 7.79 | 0.05 | ||||
| Male | 196 (62.8%) | 298 (67%) | 205 (73.5%) | 324 (67.2%) | ||
| Female | 116 (37.2%) | 147 (33%) | 74 (26.5%) | 158 (32.8%) | ||
| WISC-V Matrix Reasoning Total Scaled Score | 9.6 (3) | 9.4 (3.1) | 9.6 (2.9) | 9.3 (3.2) | F (3, 1450) = 0.77 | 0.51 |
| Socioeconomic status mean household income (SD) in thousands of dollars | 107 (77) | 102 (82) | 111 (85) | 102 (80) | F (3, 65) = 0.43 | 0.73 |
| Raceb | χ2 (3) = 19.71 | <0.001 | ||||
| Asian | – | 10 (2.3%) | 1 (0.4%) | 6 (1.3%) | ||
| Black/African American | 23 (7.4%) | 69 (15.6%) | 37 (13.3%) | 89 (18.7%) | ||
| More than one race | 52 (16.8%) | 62 (14%) | 38 (13.7%) | 71 (14.9%) | ||
| Other | 13 (4.2%) | 22 (5%) | 10 (3.6%) | 19 (4%) | ||
| White | 222 (71%) | 279 (63.1%) | 192 (69.1%) | 292 (61.2%) | ||
| Ethnicity | χ2 (3) = 1.91 | 0.59 | ||||
| Non-Hispanic | 250 (81.7%) | 362 (82.7%) | 233 (85%) | 399 (84%) | ||
| Hispanic | 56 (18.3%) | 76 (17.4%) | 41 (15%) | 76 (16%) | ||
| NCR | ||||||
| N (%) | 18 (11.7%) | 41 (26.6%) | 65 (42.2%) | 30 (19.5%) | – | – |
| Age at baseline (mean, SD) | 8.1 (1.1) | 9.1 (2.1) | 8.7 (2.6) | 8.9 (2.3) | F (3, 148) = 1.5 | 0.22 |
| Age at final follow-up (mean, SD) | 14.4 (1.8) | 15.8 (1.6) | 14 (2.5) | 14.6 (2.3) | F (3, 146) = 4.87 | 0.003c |
| Number of observations (median, range) | 4.5 (3–7) | 5 (3–9) | 4 (3–8) | 3 (3–8) | χ2 (3) = 7.3 | 0.06 |
| Median years follow-up time (IQR) | 1.4 (1.2) | 1.4 (0.9) | 1.51 (1.1) | 1.73 (1.4) | F (3, 148) = 1.09 | 0.35 |
| Sex | χ2 (3) = 0.91 | 0.82 | ||||
| Male | 11 (61.1%) | 27 (65.9%) | 45 (69.2) | 22 (73.3%) | ||
| Female | 7 (38.9%) | 14 (34.2%) | 20 (30.8) | 8 (26.7%) | ||
| Full-scale IQ (mean, SD) | 110.2 (17.7) | 108.6 (14.4) | 104.9 (14.1) | 108.8 (14.8) | F (3, 148) = 0.31 | 0.82 |
| Socioeconomic status (Hollingshead; smaller values indicate higher SES) | 32.37 (15.3) | 28.12 (12.6) | 37.59 (17.2) | 32.03 (11.8) | F (3, 59) = 3.18 | 0.03d |
| Raceb | χ2 (3) = 1.03 | 0.79 | ||||
| American Indian/Alaska Native | 0 | 0 | 1 (1.5%) | 0 | – | – |
| Asian | 1 (0.6%) | 2 (4.9%) | 2 (3.1%) | 0 | ||
| Black/African American | 1 (0.6%) | 6 (14.6%) | 11 (16.9%) | 2 (6.7%) | – | – |
| More than one race | 3 (16.6%) | 3 (7.3%) | 7 (9.2%) | 6 (20%) | ||
| White | 13 (72.2%) | 30 (73.2%) | 45 (69.2%) | 22 (73.3%) | – | – |
| Ethnicity | χ2 (3) = 5.95 | 0.11 | ||||
| Not Hispanic | 18 (100%) | 38 (92.7%) | 57 (87.7%) | 25 (83.3%) | ||
| Hispanic | 0 | 3 (7.3%) | 8 (12.3%) | 5 (16.7%) | ||
| NKI-Rockland | ||||||
| N (%) | 10 (21.3%) | 8 (17%) | 19 (40.4%) | 10 (21.3%) | ||
| Age at baseline | 9.2 (2.4) | 9.6 (2.1) | 9.7 (2.6) | 9.2 (1.6) | F (3, 43) = 0.14 | 0.93 |
| Age at final follow-up | 11.4 (2.3) | 11.8 (2) | 11.8 (2.6) | 11.5 (1.7) | F (3, 43) = 0.1 | 0.96 |
| Median years follow-up time (IQR) | 1.1 (0.4) | 1 (0.2) | 1.1 (0.3) | 1.1 (0.3) | F (3, 43) = 0.5 | 0.68 |
| Sex | χ2 (3) = 2.12 | 0.55 | ||||
| Male | 6 (60%) | 7 (87.5%) | 15 (78.9%) | 7 (70%) | ||
| Female | 4 (40%) | 1(12.5%) | 4 (21.1%) | 3 (30%) | ||
| Full-scale IQ | 103.3 (11.9) | 92.6 (7.9) | 102.8 (14.6) | 97.1 (8.1) | F (3, 43) = 1.81 | 0.16 |
| Socioeconomic status mean household income (SD) in thousands of dollars | 129 (107) | 121 (88) | 85 (95) | 62 (40) | F (3, 43) = 1.16 | 0.34 |
| Raceb | χ2 (3) = 8.3 | 0.22 | ||||
| American Indian or Native Alaskan | 1 (10%) | 0 | 0 | 0 | ||
| Asian | 1 (10%) | 0 | 1 (5.3%) | 0 | ||
| Black/African American | 1 (10%) | 3 (37.5%) | 5 (26.3%) | 1 (10%) | – | – |
| More than one race | 1 (10%) | 0 | 2 (10.5%) | 0 | – | – |
| White | 6 (60%) | 5 (62.5%) | 11 (57.9%) | 9 (90%) | – | – |
| Ethnicity | χ2 (3) = 2.12 | 0.55 | ||||
| Non-Hispanic | 7 (70%) | 7 (87.5%) | 15 (78.9%) | 6 (60%) | ||
| Hispanic | 3 (30%) | 1 (12.5%) | 4 (21.1%) | 4 (40%) | ||
Abbreviations. ABCD, Adolescent Brain Cognitive Development Study; IQ, Intelligence quotient; IQR, Interquartile range; NCR, Neurobehavioral Clinical Research; NKI-Rockland, enhanced Nathan Kline Institute-Rockland; WISC-V, Wechsler Intelligence Scale for Children® Fifth Edition.
Missing data ABCD: 34 missing WISC-V Matrix Reasoning Total Scaled Score. 11 missing race. 25 missing ethnicity. Missing data NCR: 2 missing socioeconomic status. NKI-Rockland: 5 missing socioeconomic status.
All ABCD participants had 12 month intervals between assessments.
Statistical test compares African American vs White vs all other groupings.
Fluctuant > emergent.
Persistent > fluctuant.
Is diagnostic fluctuation driven by medication or comorbidity?
The fluctuant group was also detected when analyses were restricted to participants who were medication-free throughout. For the ABCD cohort, 257 (31.3%) of 820 medication-free ADHD participants showed diagnostic fluctuation; for NCR it was 9 (30%) out of the 30 medication-free; and 6 (18.1%) of the 33 medication-free participants in the NKI-Rockland cohort—Fig. 2. Further analyses required the absence of ADHD to be coded only if the participant was off all ADHD medications at that assessment. These analyses decreased the proportion of those with fluctuant trajectories in the NCR cohort (from 26.6% to 12.1%) but had a more modest impact in the ABCD (29.3%–19.4%) and the Rockland NKI cohorts (17–12.2%).
In analyses restricted to participants without any comorbid disorders, fluctuating ADHD was present at similar or higher rates among participants with ADHD as in the full cohorts–(ABCD: 168 out of 458 [36.7%]; NCR: 35 out of 120 [29.1%]; NKI-Rockland: 5 out of 25 [20%]) Fig. 2.
Analyses using case definitions similar to those in the MTA study
We repeated analyses defining the absence of ADHD in a manner similar to that used in the MTA study. For ABCD the proportion with fluctuant trajectories fell from 29.3% to 19.7%, with more marked decreases for the NCR cohort (26.6%–9.8%) Fig. 2.
Does the fluctuant subgroup survive when incorporating other data sources or a stricter definition of impairment?
Validity of a fluctuant group would be supported if the group remains when incorporating teacher data available longitudinally on 118 participants with ADHD during at least one timepoint based on KSADS-COMP in the ABCD cohort. Using a definition of remission that required both an absence of an ADHD diagnosis according to the K-SADS-COMP and a congruent absence of elevated attention problems on the teacher questionnaire, the fluctuant group remained present in 28 of the 118 participants (23.7%).
For the ABCD cohort, the proportion of those with fluctuant ADHD remained stable at 30.9% when requiring impairment in two settings, compared to 29.3% for the original analyses that required impairment in one or more setting-see Fig. 2. Using a stricter definition of impairment also had little impact on the proportion of those with fluctuant ADHD in the NCR cohort (moving from 26.6% to 25.1%).
The proportion in each outcome group also changed minimally when analyses retained only one child in each family in both the ABCD and the NCR cohort- See Supplemental Table S3.
Discussion
Across three cohorts of children and adolescents, we detected a diagnostically fluctuant group, best characterized as switching or ‘zig zagging’ between syndromic and non-syndromic states. Analyses in a cohort that spanned early childhood to late adolescence, found that a ‘fluctuant’ subgroup became more prominent as the number of assessments increased, but was not the dominant developmental course. Rather, it was present in similar proportions to more established courses of diagnostic persistence, remission or the emergence of ADHD. The fluctuant group was not composed of participants who alternated between being just below and just at or above diagnostic thresholds, and remained in analyses that incorporated the teacher as an informant, used more stringent definitions of impairment, and considered ADHD medication use and comorbidity.
We confirm in both population and clinical cohorts covering childhood and adolescence the presence of a group who showed diagnostic fluctuations, in keeping with findings of the recent study of the MTA.6 The fluctuant courses detected in the present work are surprising in light of several design features that should promote stable diagnoses in the cohorts studied, such as the use of the same assessment tool within each cohort over the entire study. It is important to note that there has been increasing recognition of instability in ADHD symptoms. For example, while DSM-IV referred to subtypes of ADHD, DSM-5 refers to presentations, recognizing that dominant symptoms are not fixed entities but change within individuals over time.24 Additionally, a recent study of adults with ADHD, assessed at three time points from an average of 34 to 47 years, found a quarter followed unstable ADHD diagnostic trajectories, many of whom showed the fluctuant ADHD diagnostic trajectory we consider-namely moving from presence to absence and then presence again of DSM-5 diagnosed ADHD.25 The mid-life adults who showed unstable trajectories were more likely to be male and had fewer comorbidities. However, fluctuating diagnostic trajectories are generally not well recognized, perhaps as most prospective studies of youth with ADHD have acquired, or only considered, two observations: a childhood assessment and a later endpoint assessment in adolescence or early adulthood.26 By definition, two assessments cannot detect a fluctuant trajectory which requires three or more observations.
In keeping with the MTA, this is a study of diagnostic shifts, classed into different trajectory subgroups. The study is grounded in clinical practice, which requires diagnostic decisions to be made over time. Of course, just as the act of diagnosis is a dichotomous decision that entails losing some information, so classifying individuals into groups based on shifts in diagnostic categories over time is also inherently reductionistic. The goal of the study is not to reify diagnostic categories nor their shifting nature, but to emphasize that even within a reductive diagnostic framework, fluctuant trajectories have gone to some extent unrecognized. It remains an unanswered question whether at the individual symptom level, rather than diagnostic level, a linear or non-linear fit is more appropriate for some individuals. Such individual-level trajectory analyses are complex and can require a large number of observations, but as data become available, such analyses will provide a rich direction for future work.
While we detected a fluctuant diagnostic group across all cohorts, we note that the proportion differed from that reported in the MTA. This might reflect the high number of assessments made in the MTA study (median of seven) which gave more opportunities for diagnostic fluctuations to occur, compared to the ABCD and NKI-Rockland cohorts, which both had only three assessments. In this regard, we note that in the NCR cohort the proportion of those with fluctuant subtype rose with the number of assessments. It is likely that the proportion of those demonstrating a fluctuant course will increase with more assessment points taken over longer time windows, giving more complete assessments of the different symptom trajectories. A second factor that might explain the lower proportion of fluctuant trajectories in the current study is the age span covered by each cohort. The MTA followed participants into adulthood, with the last observation at a mean age of 25 years, whereas the ABCD cohort was confined to childhood and the NKI-Rockland and NCR cohorts both stopped around age 18. ADHD may be more fixed when people are younger but start to become less fixed in early adulthood. Indeed, it is possible that shifts that occur entering adult life, such as moving into the workplace, increased demands on executive functions and a loss of parental/academic structure could accentuate both symptoms and impairments. A third factor for different rates for the fluctuant group may be the use of multi-informant data for determining remission from ADHD in the MTA study, whereas the NCR and NKI-Rockland cohorts had only parental interview data available longitudinally. For children in the ABCD cohort, the proportion of those with fluctuant ADHD decreased only slightly from 29.3% to 27.7% when remission status required confirmation via teacher reports, although longitudinal teacher data were available only on a small proportion of those in the study.
In conclusion, we examined three cohorts spanning childhood into adolescence with varying designs, assessment methods, and study populations and find evidence across all cohorts for a fluctuant ADHD subgroup. Along with the work of Sibley and colleagues, these results provide converging evidence for the notion that a substantial number of patients with ADHD follow fluctuating diagnostic trajectories. Conceptually, the finding raises nosological issues: is ADHD a relapsing-remitting disorder for some? At a clinical level, the ‘fluctuant trajectories’ detailed by the MTA study suggest that remission from ADHD is usually temporary and does not equate with recovery, and there is thus a need for continued clinical monitoring beyond possibly transient periods of remission.6,8
Contributors
Luke Norman (data curation, formal analysis, writing—original draft, and writing—review & editing, visualisation); Jolie Price (data curation, writing—review & editing); Kwangmi Ahn (formal analysis, writing—review & editing); Gustavo Sudre (writing—review & editing); Wendy Sharp (data curation, project administration); Philip Shaw (conceptualisation, funding acquisition, data curation, formal analysis, writing—original draft, and writing—review & editing, visualisation). Luke Norman and Philip Shaw had full access to the raw data in the study.
Data sharing statements
The Adolescent Brain and Cognitive Development Study dataset, release 4.0, is freely available from the National Institute of Mental Health Data Archive at https://nda.nih.gov/. The NKI-Rockland dataset is freely available from http://fcon_1000.projects.nitrc.org/indi/enhanced/. For the NCR cohort, we will share de-identified data where the individual or parent has given consent for sharing. We will share all summary data. We aim to make the data available starting end of 2023 by depositing data in either Genotypes and Phenotypes (dbGaP; https://www.ncbi.nlm.nih.gov/gap/) and/or NIMH Data Archive (NDA; https://nda.nih.gov/).
Declaration of interests
The authors report no competing financial interests in relation to the work described.
Acknowledgements
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) study (https://abcdstudy.org), held in the NIMH Data Archive. This is a multisite longitudinal study designed to recruit more than 10,000 children ages 9–10 years and follow them over 10 years into early adulthood. The ABCD study is supported by NIH and additional federal partners (under awards U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025). A full list of supporters is available online (https://abcdstudy.org/federal-partners.html). A listing of participating sites and a complete listing of the study investigators are also available online (https://abcdstudy.org/scientists/workgroups). ABCD consortium investigators designed and implemented this study and/or provided data but did not necessarily participate in the analysis or writing of this article. This study reflects the views of the authors and may not reflect the opinions or views of NIH or the ABCD consortium investigators.
The Longitudinal Discovery of Brain Development Trajectories was principally supported by NIH U01MH099059. A subset of participants had baseline characterizations from the core enhanced NKI-RS protocol NIMH BRAINS R01MH094639-01. Additional support was provided by NIH R01MH101555, NIH R01AG047596, and the Child Mind Institute (1FDN2012-1). Funding for key personnel also provided in part by the New York State Office of Mental Health, NIH R01MH120601, and Research Foundation for Mental Hygiene.
This work was funded by the intramural research program of the National Institute of Mental Health and the National Human Genome Research Institute (ZIAHG200378 to Dr Shaw). This funding supported data collection for the NCR cohort (ClinicalTrials.gov identifier: NCT01721720).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102021.
Appendix A. Supplementary data
References
- 1.Biederman J., Mick E., Faraone S.V. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- 2.Biederman J. Impact of comorbidity in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2004;65:3–7. [PubMed] [Google Scholar]
- 3.Faraone S.V., Biederman J., Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 4.Pingault J.-B., Viding E., Galéra C., et al. Genetic and environmental influences on the developmental course of attention-deficit/hyperactivity disorder symptoms from childhood to adolescence. JAMA Psychiatry. 2015;72:651–658. doi: 10.1001/jamapsychiatry.2015.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw P., Sudre G. Adolescent attention-deficit/hyperactivity disorder: understanding teenage symptom trajectories. Biol Psychiatry. 2021;89:152–161. doi: 10.1016/j.biopsych.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibley M.H., Arnold L.E., Swanson J.M., et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2021;179 doi: 10.1176/appi.ajp.2021.21010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asherson P., Agnew-Blais J. Annual Research Review: does late-onset attention-deficit/hyperactivity disorder exist? J Child Psychol Psychiatry. 2019;60:333–352. doi: 10.1111/jcpp.13020. [DOI] [PubMed] [Google Scholar]
- 8.Shaw P. Growing up with ADHD symptoms: smooth transitions or a bumpy course? Am J Psychiatry. 2022;179:88. doi: 10.1176/appi.ajp.2021.21121197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudre G., Sharp W., Kundzicz P., et al. Predicting the course of ADHD symptoms through the integration of childhood genomic, neural, and cognitive features. Mol Psychiatry. 2021;26:4046–4054. doi: 10.1038/s41380-020-00941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norman L.J., Sudre G., Bouyssi-Kobar M., et al. A longitudinal study of resting-state connectivity and response to psychostimulant treatment in ADHD. Am J Psychiatry. 2021;178:744–751. doi: 10.1176/appi.ajp.2021.20091342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heeringa S.G., Berglund P.A. A guide for population-based analysis of the Adolescent Brain Cognitive Development (ABCD) Study baseline data. bioRxiv. 2020 doi: 10.1101/2020.02.10.942011. [DOI] [Google Scholar]
- 12.Dick A.S., Lopez D.A., Watts A.L., et al. Meaningful associations in the adolescent brain cognitive development study. Neuroimage. 2021;239 doi: 10.1016/j.neuroimage.2021.118262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibley M.H., Arnold L.E., Swanson J.M., et al. 2021. For the MTA cooperative group: variable patterns of remission from ADHD in the multimodal treatment study of ADHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibley M.H., Swanson J.M., Arnold L.E., et al. Defining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. J Child Psychol Psychiatry. 2017;58:655–662. doi: 10.1111/jcpp.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caye A., Swanson J., Thapar A., et al. Life span studies of ADHD—conceptual challenges and predictors of persistence and outcome. Curr Psychiatry Rep. 2016;18:1–11. doi: 10.1007/s11920-016-0750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey B.J., Cannonier T., Conley M.I., et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garavan H., Bartsch H., Conway K., et al. Recruiting the ABCD sample: design considerations and procedures. Dev Cogn Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townsend L., Kobak K., Kearney C., et al. Development of three web-based computerized versions of the Kiddie Schedule for affective disorders and schizophrenia child psychiatric diagnostic interview: preliminary validity data. J Am Acad Child Adolesc Psychiatry. 2020;59:309–325. doi: 10.1016/j.jaac.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Norman L.J., Sudre G., Bouyssi-Kobar M., et al. An examination of the relationships between attention/deficit hyperactivity disorder symptoms and functional connectivity over time. Neuropsychopharmacology. 2021;1–7 doi: 10.1038/s41386-021-00958-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nooner K.B., Colcombe S.J., Tobe R.H., et al. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Front Neurosci. 2012;6:152. doi: 10.3389/fnins.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobe R.H., MacKay-Brandt A., Lim R., et al. A longitudinal resource for studying connectome development and its psychiatric associations during childhood. Sci Data. 2022;9:300. doi: 10.1038/s41597-022-01329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuznetsova A., Brockhoff P.B., Christensen R.H. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82:1–26. [Google Scholar]
- 23.Achenbach T., McConaughy S., Ivanova M., et al. University of Vermont Research Center for Children, Youth, and Families; Burlington: 2017. Manual for the ASEBA Brief problem monitor for ages 6–18 (BPM/6–18) [Google Scholar]
- 24.Lahey B.B., Pelham W.E., Loney J., et al. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- 25.Grevet E.H., Bandeira C.E., Vitola E.S., et al. The course of attention-deficit/hyperactivity disorder through midlife. Eur Arch Psychiatry Clin Neurosci. 2022;1–12 doi: 10.1007/s00406-022-01531-4. [DOI] [PubMed] [Google Scholar]
- 26.Mannuzza S., Klein R.G., Moulton J.L., III Persistence of attention-deficit/hyperactivity disorder into adulthood: what have we learned from the prospective follow-up studies? J Atten Disord. 2003;7:93–100. doi: 10.1177/108705470300700203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.