Abstract
There is now increasing demand to improve the sensitivity of various immunoassays for fluoroquinolones (FQs) and other food hazards. In this study, different coating antigens were prepared by adjusting the content of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) to explore its influence on the immunoassay sensitivity of FQs. The results indicated that, unlike traditional assumptions, a reasonable EDC dosage should be addressed to reach the best analytical efficiency, and excessive EDC could enhance the hapten-carrier conjugation but significantly reduce the detection sensitivity. For the FQs investigated, the hapten:EDC:BSA proportion of 20:2.5:50 (Mole ratio:74:34:1) seemed the best for preparation of coating antigens, and the sensitivity could be improved more than 1000 times both for indirect competitive enzyme linked immunosorbent assay ELISA (ic-ELISA) and gold immunochromatography assay (GICA) due to two key factors including coupling-ratios and amide bond groups. Such an improved efficiency was also validated well with different food samples, which indicated the reasonable optimization of EDC in coating antigen synthesis may be widely used as a new, simple and more effective strategy to improve the immunoassay for low molecular targets in medical, environment and food detection filed.
Keywords: Fluoroquinolones, Coupling agent, Coating antigens, Sensitivity of immunoassay
1. Introduction
Fluoroquinolones (FQs) are synthetic antibiotics widely used in livestock and aquaculture throughout the world. Considering their significant risk to human health, most countries/areas have established strict maximum residue limits [[1], [2], [3]]. Various immunoassays, such as enzyme-linked immunosorbent assay (ELISA) [4], colloidal gold immunochromatographic assay (CGIA) [5] and chemiluminescence immunoassays (CLIA) [6], are now widely used in fast screening of drug residues and other food hazards [7], with the advantages of sensitivity, simplicity, low-cost, high throughput, and ease of automation [8]. To fulfill the increasing requirements to detect trace residue at extremely low concentrations, improvement in the sensitivity of traditional immunoassays still faces huge challenges and has become a research hotspot in this field [9].
Many studies have been carried out for this purpose, with most of them focused on generating high-quality antibodies [10] or developing new signal application tools such as nanoparticles; gas biosensors; or using oligonucleotides to achieve an exponential amplification effect [[11], [12], [13], [14], [15]]. In recent years, the influence of coating antigen on immunoassay sensitivity for small molecules has attracted increasing attention [[16], [17], [18]]. Some studies have reported that employment of heterologous haptens or carrier proteins in the preparation of coating antigens could improve sensitivity significantly [19,20]. A new derivative of enrofloxacin (ENR) was once used to prepare coating antigens with adjusted linker length between hapten-carrier proteins, and the IC50 value for ENR was decreased from 1.3 μg/L to 0.07 μg/L [21]. When moxifloxacin-BSA (with the cross-reactivity of 0.8% with norfloxacin) was utilized as the heterologous coating antigen, the sensitivity for norfloxacin was improved by 26-fold [19]. Meanwhile, regulating the dose ratio of haptens to carrier proteins during coating antigen preparation also could yield different sensitivities [22], and coating antigen with cationized proteins was demonstrated lower sensitivity than that with native proteins, which might be caused by the difference in spatial structures [23]. Unfortunately, till now there still lacks of comprehensive understanding on coating antigen synthesis, as well as its influence on the subsequent immunoassays [[24], [25], [26], [27]]. Overall, simple and effective strategies for the design and preparation of coating antigens are still very limited.
For synthesis of complete antigens, EDC or/and N-hydroxysuccinimide (NHS) induced-polycondensation is the most common method to facilitate amino-carboxyl group covalent bonding between haptens and carrier proteins. In our previous study, numerous amide groups were found to be formed during the process and demonstrated significant immunogenicity to generate corresponding antibodies, therefore greatly interfere to the production of hapten-specific antibodies as well as their affinity with target FQs (data unpublished). These results allowed us assume that the EDC mediated amino-carboxyl group conjugation may significantly affect the chemical structure of the coating antigens and the efficiency of corresponding immunoassays. Hence in this study, different amount of EDC was used to prepare FQs coating antigens, and its influence on ELISA and CGIA was investigated. Based on the results, a new and simple strategy to improve sensitivity was proposed and validated with food samples.
2. Experimental section
2.1. Regents
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Enrofloxacin (ENR), ofloxacin (OFL), lomefloxacin (LOM), N,N-dimethylformamide (DMF), bovine serum albumin (BSA), HAuCl4·4H2O, citrate sodium, and ovalbumin (OVA) were obtained from Aladdin Reagent Co., Ltd. (Shanghai, China). Goat anti-rabbit IgG-HRP, goat anti-rabbit IgG, goat anti-mouse IgG-HRP, goat anti-mouse IgG, 3,3′,5,5′-tetramethylbenzidine solution (TMB), protein-A affinity column, and Tween- 20 were obtained from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Rabbit anti-ENR polyclonal antibody, mouse anti-OFL polyclonal antibody, mouse anti-LOM polyclonal antibody, and mouse anti-amide group polyclonal antibody were prepared by our laboratory in the previous studies (The immunogens of these antibodies were respectively ENR-cOVA, OFL-cOVA, LOM-cOVA, and cOVA, the titers of antisera were all over than 200,000). Monoclonal antibody specific to ENR was purchased from Wuhan Huamei Tech. Co., Ltd. (Wuhan, China). All other reagents were analytical or higher grade.
Detailed formulas of all solutions are given in Supporting Information.
2.2. Materials
96-well polystyrene microtiter plates were obtained from Thermo Fisher Scientific Inc. (No. 442404, Pittsburgh, PA, USA), and nitrocellulose membranes, sample pads, absorbent pads, and glass cellulose membrane were supplied by Shanghai Jieyi Biological Tech. Co., Ltd. (Shanghai, China). The milk, honey, and pork sausage were purchased from Qingdao LiQun supermarket (Qingdao, China). Turbot and Micropterus Salmoides were supplied by Chinese Academy of fishery Sciences Yellow Sea Fishery Research Institute (Qingdao, China). The samples were fluoroquinolone negative detected by LC-MS.
2.3. Preparation and characterization of coating antigens with different amount of EDC
The coating antigen (ENR-BSA) was prepared by a carbodiimide method, according to previously reported procedures [28]. Briefly, 20 mg of ENR (0.0556 mM), 1 mg of EDC (0.0101 mM), and 20 mg of NHS (0.174 mM) were dissolved in 2 mL of DMF with stirring 110 rpm at 20 °C for 12 h. Then, the solution was slowly added to 10 mL of PBS (0.1 M, pH 7.4) containing 50 mg of BSA (0.75 μM) and incubated at room temperature for another 6 h. The mixture was finally dialyzed in PBS (0.1 M, pH 7.4) for 3 d. Different amounts of EDC (2.5, 5, 10, 20, and 40 mg) (0.025 mM, 0.05 mM, 0.1 mM, 0.2 mM and 0.4 mM) and another two haptens (OFL and LOM) were used to prepare different coating antigens with the same procedure.
The protein concentration in coating antigens was measured by the BCA protein assay kits according to the instructions (Beijing Solarbio Science & Technology Co., Ltd.). The amount of FQs in prepared coating antigens was determined by UV–Vis spectroscopy (UV-2550, Shimadzu Corp., Kyoto, Japan). Different FQs standards were dissolved in 0.03% NaOH and then serially diluted with PBS to reach different concentrations (20, 15, 10, 7.5, 5, 3.75, 2.5, and 1.25 μg/mL). A standard curve was established between the absorbance at the characteristic wavelength and FQs concentration. The hapten-carrier protein coupling ratio was calculated as following:
| (1) |
where n represents the coupling ratios of hapten to BSA, CHapten on BSA represents the concentration of hapten-BSA, MwBSA is the average molecular weight of BSA (∼68,000), CHapten-BSA is the concentration of hapten-BSA, and Mw Hapten represents the MW of hapten.
The amide groups formed during the preparation of coating antigens were determined by indirect ELISA. The 96-well microtiter plates were coated with the prepared coating antigen (30 μL/well) and incubated at 4 °C overnight. The plates were washed three times with PBS containing Tween-20 (PBST), and then blocked with skim milk blocking buffer (300 μL/well) at 37 °C for 2 h. Antibodies against amide groups were added to the wells and incubated at 37 °C for 1.5 h. After washing three times with PBST, 100 μL of goat anti-mouse IgG-HRP (1/5000) were added and incubated at 37 °C for 1 h and then washed again three times. The TMB solution (100 μL/well) was added and incubated at 37 °C for 5 min before quenching by adding 2 M H2SO4 (50 μL/well). The absorbances at 450 nm (A450) were measured with a microplate reader (Synergy HT, BioTek Instruments, Inc., Winooski, VT, USA).
2.4. Immunoassay of FQs with prepared coating antigens
For the indirect competitive ELISA (ic-ELISA), stock solutions of ENR, OFL, or LOM dissolved in 0.03% NaOH at the concentration of 1 mg/mL were serially diluted with PBS to 10 μg/mL, 2 μg/mL, 1 μg/mL, 500 ng/mL, 200 ng/mL, 100 ng/mL, 50 ng/mL, and 10 ng/mL. A 96-well microtiter plate was coated with the prepared coating antigen, incubated at 4 °C overnight, and then blocked with 5% skimmed milk solution in PBST at 37 °C for 2 h. The series dilutions of ENR, OFL, or LOM were premixed with an equal volume of corresponding antibody diluted with PBS to the working concentration [29] and incubated for 30 min at 37 °C. Then, 100 μL of the mixture was added to the plate wells and incubated at 37 °C for another 1.5 h. After washing three times with PBST, 100 μL of goat anti-rabbit IgG-HRP (1/5000) for ENR and goat anti-mouse IgG-HRP (1/5000) for OFL and LOM in PBS was added to each well and incubated at 37 °C for 1 h. TMB solution (100 μL/well) was added and incubated at 37 °C for 5 min before quenching by adding 2 M H2SO4 (50 μL/well). The OD A450 nm of each well was measured with the microplate reader. The inhibition ratio was calculated using the following forum:
| (2) |
where B0 is the absorbance of the blank group without FQs and B is the absorbances of groups with different FQs concentrations. The competitive curve was generated by 1-B/B0 versus drug level. The IC50 was calculated using four-parameter logistic curve fitting calculated by Graphpad Prism 9.
For CGIA of different FQs, gold-nanoparticle (GNP) and antibody-GNP were prepared according to a previous report, with some modifications [30]. The colloid gold test strips were assembled by a traditional procedure. Briefly, coating antigen (0.5 mg/mL) and corresponding secondary antibodies (0.5 mg/mL, anti-rabbit for ENR polyclonal antibody and anti-mouse for ENR monoclonal antibody) were coated on test and control zones, respectively. The test strips were dried at 37 °C for 30 min and then blocked by immersion in PBS containing 1% BSA at 37 °C for 30 min. The test strips were finally washed, dried and stored at 4 °C for further detection.
The CGIA procedures were according to usually exploited performance [30]. A positive result means that the test line (T line) does not develop color, and the quality control line (C line) develops color, a negative result means that the T line develops color, and the C line develops color, and a weak positive result means that the color development of the T line is weaker than that of the negative result. As determined by visualization, the result was negative (−), weakly positive (±), or positive (+) according to the color in detection line. The limit of detection (LOD) was the lowest concentration of ENR showed positive results.
2.5. Validation with food samples
The ic-ELISA with different food sample was performed as reported with some modifications [31]. In brief, each milk and honey aliquot (5 mL) were diluted with PBS to 10 times for ELISA analysis at the optimized coating antigen to calculate the recovery. The pork sausage, turbot, micropterus salmoides samples were homogenized and aliquot of 5 g was weighed for each analysis. Extraction solvent (5 mL) consisting of a 1:1 (v/v) mixture of methanol and PBS were mixed with above samples and then vortexed for 30 s, then all the samples were shaken for 30 min at 110 r/min. The supernatants were obtained after centrifugation at 4000 r/min for 20 min and then diluted 10 times for ic-ELISA as mentioned in 2.4 analysis to calculate recovery.
3. Results and discussions
3.1. The influences of EDC on coating antigen preparation and ELISA sensitivity
EDC is the most popular reagent in the synthesis of complete antigens as zero-valent cross-linker to bind carboxylic groups of the hapten to amino groups of carrier proteins [32]. For long term a high coupling ratio was emphasized to ensure the effective preparation of coating antigens, so that EDC was usually excessive added during the reaction [[33], [34], [35]]. Though some studies indicated the possible influence of the coupling ratio of coating antigens on the immunoassay efficiency, detailed information still remains unclear [22]. Here when the dosage of hapten and carrier protein remained constant, there was demonstrated overall positive correlation between the coupling ratio and EDC content, which verified the significant effect of EDC to enhance the hapten-carrier conjugation and consisted well with previous studies (Table .1, Figs. S1–S4). However, when the EDC content and coupling ratios were higher than certain values, the sensitivity of corresponding ic-ELISA (presented as IC50) was dramatically decreased, even 1000 times or more. Based on these results, the significant influence of EDC on coating antigens and therefore the following analytical efficiency could be clearly concluded, but unlike traditional performance, its dosage should be carefully controlled within certain range, otherwise would result in very poor sensitivity of immunoassays, especially when coating antigens with relatively high coupling ratio were used.
Table 1.
The coupling ratios and IC50 value of different coating antigens.
| The system of coating antigen | ENR |
OFL |
LOM |
|||
|---|---|---|---|---|---|---|
| CR* | IC50 (ng/mL) | CR | IC50 (ng/mL) | CR | IC50 (ng/mL) | |
| Hapten:EDC:BSA 20:1:50 (Mole ratio:74:13.6:1) | 3.86 | 3.64 | 0.04 | 7.84 | 0.04 | 34.67 |
| Hapten:EDC:BSA 20:2.5:50 (Mole ratio:74:34:1) | 5.84 | 3.27 | 0.39 | 14.65 | 0.2 | 15.72 |
| Hapten:EDC:BSA 20:5:50 (Mole ratio:74:68:1) | 6.61 | 17.4 | 0.46 | ∼38 | 0.96 | 38.81 |
| Hapten:EDC:BSA 20:10:50 (Mole ratio:74:136:1) | 7.76 | ∼122 | 1.85 | >1000 | 1.46 | 125.23 |
| Hapten:EDC:BSA 20:20:50 (Mole ratio:74:272:1) | 7.73 | >500 | 5.15 | >1000 | 6.97 | >1000 |
| Hapten:EDC:BSA 20:40:50 (Mole ratio:74:544:1) | 9.8 | >1000 | 8.03 | >5000 | 5.4 | >5000 |
CR* Coupling Ratios.
3.2. The mechanism of key roles of EDC on immunoassays
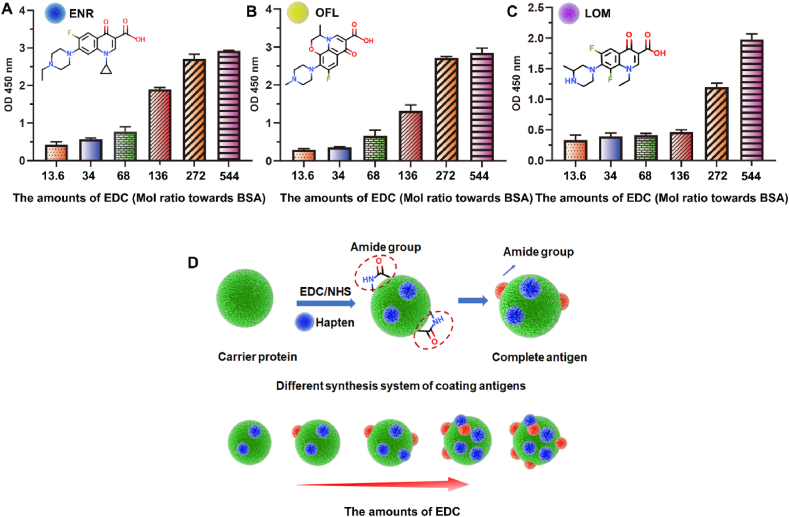
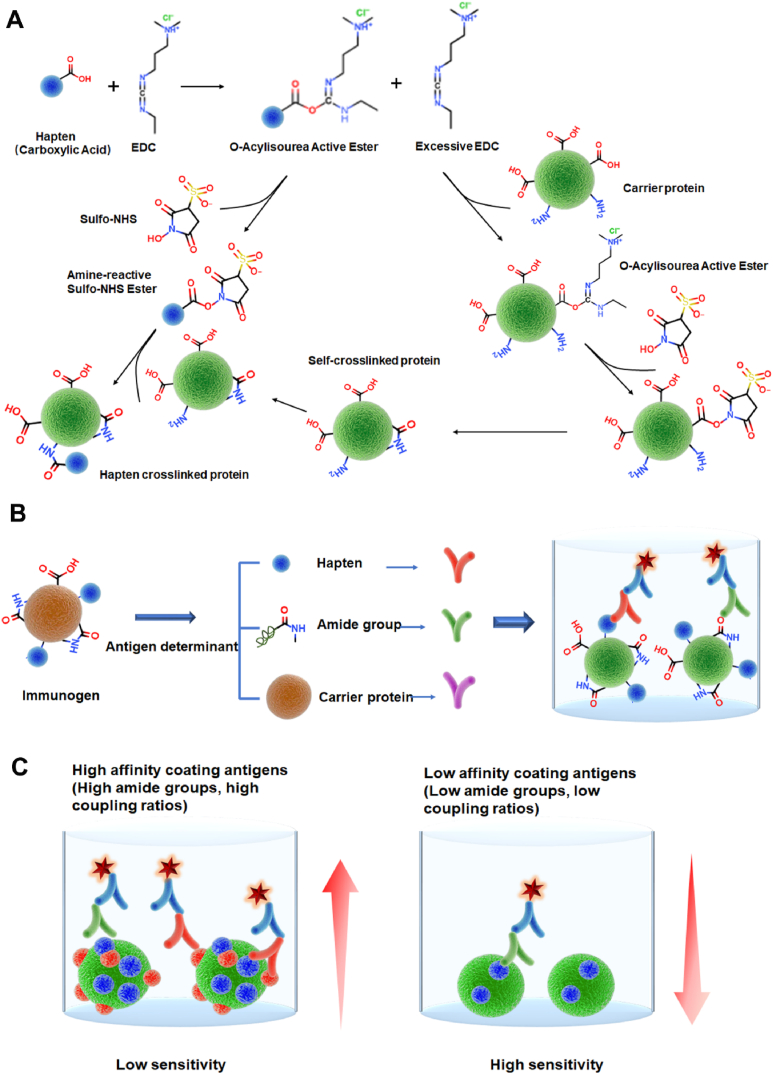
Our previous research has demonstrated that numerous amide groups could be produced in the preparation of FQs immunogens, with positive correlation with the EDC dosage during amino - carboxyl group conjugation. Similar results were also observed here in FQs coating antigens. With increased EDC content, the binding ability of the coating antigens to the mouse anti-amide groups polyclonal antibody (generated against cationized OVA) was significantly enhanced (Fig. 1A-C), indicating corresponding increase of amide group in the coating antigens (Fig. 1D). Considering that the antiserum used for ic-ELISA of FQs was prepared with EDC-mediated immunogens (Fig. 2A) and contained large number of anti-amide group antibodies, their interaction with increased amide group of coating antigens will certainly lead to poor competition of target FQs and therefore dramatically reduce the sensitivity (Fig. 2B and C).
Fig. 1.
(A) The amide group antibody binding ability with ENR-BSA with different amounts of EDC. (B) The amide group antibody binding ability with OFL-BSA with different amounts of EDC. (C) The amide group antibody binding ability with LOM-BSA with different amounts of EDC. (D) The effect of EDC on coupling ratios and amide groups of coating antigens.
Fig. 2.
(A) Scheme of polycondensation in synthesis of immunogen and coating antigens induced by the EDC/NHS method. (B) The mechanism of amide group in complete antigens interference immunoassay. (C) The mechanism of different sensitivities for different EDC contents.
Besides the interference of amide groups, the coupling ratio itself may also affect the analytical efficiency of coating antigens. In principle a high coupling ratio usually indicates enhanced competition ability of haptens in coating antigens, therefore more free targets will be required to achieve certain inhibition (Fig. 2C). Especially for immunoassay exploiting monoclonal antibodies, which should exhibit no significant reactivity against amide groups, this may be the main factor to influence the sensitivity of immunoassays. Chen et al. once demonstrated significantly improved ic-ELISA sensitivity for ceftiofur with relatively higher carrier protein concentration in coating antigens [22], and it from the side supported well the proposal mentioned here.
3.3. Improvement of immunoassays based on adjustment of EDC during preparation of coating antigens
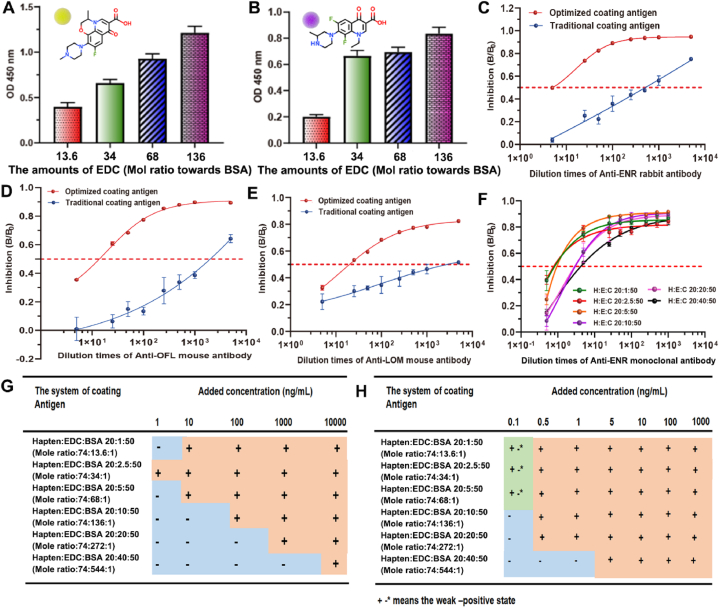
The effect of EDC content in the preparation of coating antigens on the immunoassay was further investigated with different FQs. As mentioned above, excessive usage of EDC could reach a high hapten-carrier conjugation efficiency but result in very poor sensitivity of ic-ELISA. Too low content of EDC was also demonstrated not reasonable: in this case the binding ability of coating antigens with antibodies seemed very weak and not enough to give stable results, thus could not reach satisfactory precision of the analysis (Fig. 3A and Fig. 3B); moreover, to meet the requirement of usually exploited chessboard titration, large amounts of antibodies and coating antigens will be added, which might increase the cost and enhance unspecific interactions among different reagents. Overall a hapten:EDC:BSA ratio of 20:2.5:50 (Mole ratio:74:34:1) was observed the best for coating antigen preparation within the range investigated. With the optimized coating antigens, the ic-ELISA sensitivity (presented as IC50 value) performed with polyclonal antibodies was increased more than 200–1000 times for ENR, OFL and LOM, respectively, in comparison to that using traditional coating antigens (EDC was far excess without precise control during preparation of coating antigens) (Fig. 3D-F). When monoclonal antibody was used in the analysis, the performance with the optimized coating antigen also demonstrated significantly enhanced sensitivity compared with other coating antigens, but the difference seemed not so large as that using polyclonal antibodies (Fig. 3G). As we proposed before, under this condition the influence of amide groups would be ignored and the sensitivity is mainly affected by the coupling ratio.
Fig. 3.
(A) The binding ability of antibody against OFL with different coating antigens. (B) The binding ability of antibody against LOM with different coating antigens. (C) The sensitivity of antibody against ENR on the optimized coating antigen and traditional coating antigen. (D) The sensitivity of antibody against OFL on the optimized coating antigen and traditional coating antigen. (E) The sensitivity of antibody against LOM on the optimized coating antigen and traditional coating antigen. (F) The ic-ELISA results of monoclonal antibody against ENR on different coating antigen systems. (G) Sensitivities of the CGIA strips assembled with different coating antigens and polyclonal antibodies. (H) Sensitivities of the CGIA strips assembled with different coating antigens and monoclonal antibodies.
The idea was also supported by the results of colloidal gold immunochromatography assay (CGIA), another typical immunoassay widely used for on-site screening in farms and supermarkets [36,37]. The lowest detection limit (LOD) for ENR using polyclonal antibodies was decreased about 1000 times with the optimized coating antigen (from 10 μg/mL to 1 ng/mL), and for that using monoclonal antibodies, the LOD decreased about 10 times (from 1 ng/mL to 0.1 ng/mL). (Fig. 3G and Fig. 3H, the naked-eye results were showed in Supporting Information Fig. S5 and Fig. S6).
To validate the feasibility of the method in real samples, the ic-ELISA with the optimized coating antigens was performed with different foodstuffs including milk, honey, pork sausage, turbot and micropterus salmoides. At the ENR spiking level of 25 μg/kg, 50 μg/kg and 250 μg/kg, the average recovery was calculated as 62.04%–130.7%, with the coefficient of variation (CV) below 18.0%, which indicated satisfactory accuracy, sensitivity and precision of the performance (Table 3). All these results confirmed the correctness of the proposals, and allowed us to suggest the reasonable controlling of EDC content in synthesis of coating antigen as a simple, new, and efficient strategy to improve the sensitivity of immunoassays. Compared to other methods reported before for the same purpose, the approach developed in this study seemed much easier, simpler and more efficient, and could be hopefully applied for FQs and other low molecular hazards (Table 2).
Table 3.
Ic-ELISA results of enrofloxacin in different food samples (n = 3).
| Sample | Spiked level (ng/mL or μg/kga) | Measured value (ng/mL or μg/kga) | Recovery (%) | CV (%) |
|---|---|---|---|---|
| Milk | 250 | 291.77 | 116.70% | 17.60% |
| 50 | 65.37 | 130.70% | 9.60% | |
| 25 | 28.35 | 113.40% | 9.50% | |
| Honey | 250 | 230.98 | 92.40% | 17.80% |
| 50 | 36.61 | 73.22% | 8.37% | |
| 25 | 15.94 | 63.80% | 4.80% | |
| Pork Sausage | 250 | 277.18 | 110.90% | 13.10% |
| 50 | 44.8 | 89.60% | 5.30% | |
| 25 | 19.98 | 79.93% | 9.82% | |
| Turbot | 250 | 182.27 | 72.91% | 3.70% |
| 50 | 41.14 | 82.30% | 15.30% | |
| 25 | 29.49 | 117.91% | 12.77% | |
| Micropterus Salmoides | 250 | 189.28 | 75.71% | 5.30% |
| 50 | 31.02 | 62.04% | 4.61% | |
| 25 | 19.04 | 76.16% | 2.95% |
The enrofloxacin concentration was presented as ng/mL for milk and honey samples and μg/kg for other samples.
Table 2.
Comparation of different methods to improve the immunoassay sensitivity by coating antigen.
| Targets | Antibodies | Methods | Sensitivity | Efficient | References |
|---|---|---|---|---|---|
| Melamine | polyclonal | heterogeneous coating antigen | IC50 1.7 ng/mL | 5–10 times | [38] |
| Melamine | monoclonal | heterogeneous coated antigen | IC50 35.4 ng/mL | 5.9–17.8 times | [39] |
| Norfloxacin | polyclonal | using hapten cross-reactivity to choose coating antigen | IC50 0.2 ng/mL | 26 times | [19] |
| Enrofloxacin | polyclonal | optimizing the concentration of EDC | IC50 3.27 ng/mL | >1000 times | this study |
| Enrofloxacin | monoclonal | optimizing the concentration of EDC | LOD 0.1 ng/mL | >10 times | this study |
| Ofloxacin | polyclonal | optimizing the concentration of EDC | IC50 14.65 ng/mL | >1000 times | this study |
| Lomefloxacin | polyclonal | optimizing the concentration of EDC | IC50 15.72 ng/mL | >1000 times | this study |
Thus, according to all conclusions we got, we could give some general recommendations about synthesis of coating antigens in immunoassay. The cross-linking agent must be optimized when synthesizing coating antigens. As a general rule, the hapten should be far in excess of the cross-linking agent and, at the same time, the amount of cross-linking agent should be reduced as much as possible while maintaining the coupling ratio.
4. Conclusions
Evidence has indicated that EDC plays a crucial function in the production of coating antigens, and therefore has a considerable impact on the sensitivity of immunoassays. An overabundance of EDC may lead to a significant increase in the hapten-carrier coupling ratio, yet this can considerably diminish the sensitivity of the system due to heightened interference from amide groups and the increased competition ability of the coating antigens. For FQs investigated, the optimal EDC dosage in the preparation of coating antigens was found as Hapten:EDC:BSA 20:2.5:50 (Mole ratio: 74:34:1). In comparison to coating antigens prepared according to traditional methods (the mole ratios of EDC towards hapten and BSA are far excessive), the sensitivity of immunoassays (both ELISA and CIGA) could be improved more than 1000 times with the new approach, and its real efficiency was validated well with different food samples. Based on these findings, we propose that the optimization of the EDC concentration in coating antigen preparation represents a novel, streamlined, and efficient approach for enhancing immunoassay sensitivity.
Author contribution statement
Xiangning Han: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Chang Liu, Xinping Guo: Analyzed and interpreted the data.
Jianxin Sui: Conceived and designed the experiments.
Hong Lin, Xiangfeng Chen: Contributed reagents, materials, analysis tools or data.
Limin Cao: Conceived and designed the experiments; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the earmarked fund for China Agriculture Research System (CARS)-47 and National Natural Science Foundation of China (No. 32072308).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16821.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hu Z.-H., Wang Y.F., Omer A.M., Ouyang X.k. Fabrication of ofloxacin imprinted polymer on the surface of magnetic carboxylated cellulose nanocrystals for highly selective adsorption of fluoroquinolones from water. Int. J. Biol. Macromol. 2018;107:453–462. doi: 10.1016/j.ijbiomac.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Madikizela L.M., Nomngongo P.N., Pakade V.E. Synthesis of molecularly imprinted polymers for extraction of fluoroquinolones in environmental, food and biological samples. J. Pharmaceut. Biomed. Anal. 2022;208 doi: 10.1016/j.jpba.2021.114447. [DOI] [PubMed] [Google Scholar]
- 3.Meng Z., Shi Z., Liang S., Dong X., Li H., Sun H. Residues investigation of fluoroquinolones and sulphonamides and their metabolites in bovine milk by quantification and confirmation using ultra-performance liquid chromatography–tandem mass spectrometry. Food Chem. 2015;174:597–605. doi: 10.1016/j.foodchem.2014.11.067. [DOI] [PubMed] [Google Scholar]
- 4.Taron W., Phooplub K., Sanchimplee S., Piyanamvanich K., Jamnongkan W., Techasen A., Phetcharaburanin J., Klanrit P., Namwat N., Khuntikeo N., Boonmars T., Sithithaworn P., Ouiganon S., Kanatharana P., Thavarungkul P., Buranachai C., Loilome W., Ngeontae W. Smartphone-based fluorescent ELISA with simple fluorescent enhancement strategy for Opisthorchis viverrini (Ov) antigen detection in urine samples. Sensor. Actuator. B Chem. 2021;348 [Google Scholar]
- 5.Wang J., Jiang C., Jin J., Huang L., Yu W., Su B., Hu J. Ratiometric fluorescent lateral flow immunoassay for point-of-care testing of acute myocardial infarction. Angew. Chem. Int. Ed. 2021;60(23):13042–13049. doi: 10.1002/anie.202103458. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y.-K., Yan Y.-X., Ji W.-H., Wang H.-a., Zou Q., Sun J.-H. Novel chemiluminescence immunoassay for the determination of zearalenone in food samples using gold nanoparticles labeled with streptavidin–horseradish peroxidase. J. Agric. Food Chem. 2013;61(18):4250–4256. doi: 10.1021/jf400731j. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z., Zong S., Wu L., Zhu D., Cui Y. SERS-activated platforms for immunoassay: probes, encoding methods, and applications. Chem. Rev. 2017;117(12):7910–7963. doi: 10.1021/acs.chemrev.7b00027. [DOI] [PubMed] [Google Scholar]
- 8.García-Fernández J., Trapiella-Alfonso L., Costa-Fernández J.M., Pereiro R., Sanz-Medel A. A quantum dot-based immunoassay for screening of tetracyclines in bovine muscle. J. Agric. Food Chem. 2014;62(7):1733–1740. doi: 10.1021/jf500118x. [DOI] [PubMed] [Google Scholar]
- 9.Sun G., Yang H., Zhang Y., Yu J., Ge S., Yan M., Song X. Branched zinc oxide nanorods arrays modified paper electrode for electrochemical immunosensing by combining biocatalytic precipitation reaction and competitive immunoassay mode. Biosens. Bioelectron. 2015;74:823–829. doi: 10.1016/j.bios.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 10.Duan Y., Liu C., Dou X., Wu L., Liu H., Shan L., Yang S., Luo J., Yang M. Preparation of sensitive monoclonal antibodies against triptolide and establishment of a rapid and cost-effective icELISA method for authentication of Tripterygium wilfordii and related products. Microchem. J. 2020;158 [Google Scholar]
- 11.Guo J., Chen S., Guo J., Ma X. Nanomaterial labels in lateral flow immunoassays for point-of-care-testing. J. Mater. Sci. Technol. 2021;60:90–104. [Google Scholar]
- 12.Li H., Yan X., Kong D., Su D., Liu F., Sun P., Liu X., Wang C., Jia X., Lu G. Self-assembled multiprotein nanostructures with enhanced stability and signal amplification capability for sensitive fluorogenic immunoassays. Biosens. Bioelectron. 2022;206 doi: 10.1016/j.bios.2022.114132. [DOI] [PubMed] [Google Scholar]
- 13.Zeng R., Luo Z., Zhang L., Tang D. Platinum nanozyme-catalyzed gas generation for pressure-based bioassay using polyaniline nanowires-functionalized graphene oxide framework. Anal. Chem. 2018;90(20):12299–12306. doi: 10.1021/acs.analchem.8b03889. [DOI] [PubMed] [Google Scholar]
- 14.Zeng R., Luo Z., Su L., Zhang L., Tang D., Niessner R., Knopp D. Palindromic molecular beacon based Z-scheme BiOCl-Au-CdS photoelectrochemical biodetection. Anal. Chem. 2019;91(3):2447–2454. doi: 10.1021/acs.analchem.8b05265. [DOI] [PubMed] [Google Scholar]
- 15.Zeng R., Zhang L., Luo Z., Tang D. Palindromic fragment-mediated single-chain amplification: an innovative mode for photoelectrochemical bioassay. Anal. Chem. 2019;91(12):7835–7841. doi: 10.1021/acs.analchem.9b01557. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y., Zhou Q., Tang D., Niessner R., Knopp D. Signal-on photoelectrochemical immunoassay for aflatoxin B1 based on enzymatic product-etching MnO2 nanosheets for dissociation of carbon dots. Anal. Chem. 2017;89(10):5637–5645. doi: 10.1021/acs.analchem.7b00942. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y., Zhou Q., Tang D. Dopamine-loaded liposomes for in-situ amplified photoelectrochemical immunoassay of AFB1 to enhance photocurrent of Mn2+-doped Zn3(OH)2V2O7 nanobelts. Anal. Chem. 2017;89(21):11803–11810. doi: 10.1021/acs.analchem.7b03451. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y., Zhou Q., Tang D., Niessner R., Yang H., Knopp D. Silver nanolabels-assisted ion-exchange reaction with CdTe quantum dots mediated exciton trapping for signal-on photoelectrochemical immunoassay of mycotoxins. Anal. Chem. 2016;88(15):7858–7866. doi: 10.1021/acs.analchem.6b02124. [DOI] [PubMed] [Google Scholar]
- 19.Hu S., Huang Z., Wang C., Peng J., Lai W.-H. Using hapten cross-reactivity to screen heterologous competitive antigens for improving the sensitivity of ELISA. Food Chem. 2020;303 doi: 10.1016/j.foodchem.2019.125379. [DOI] [PubMed] [Google Scholar]
- 20.Yao J., Wang Z., Guo L., Xu X., Liu L., Kuang H., Xu C. Lateral flow immunoassay for the simultaneous detection of fipronil and its metabolites in food samples. Food Chem. 2021;356 doi: 10.1016/j.foodchem.2021.129710. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z., Zhang H., Ni H., Zhang S., Shen J. Development of a highly sensitive and specific immunoassay for enrofloxacin based on heterologous coating haptens. Anal. Chim. Acta. 2014;820:152–158. doi: 10.1016/j.aca.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Shen W., Wu S., Zhu Y., Hou R., Li L., Dai M., Peng D. Preparation of monoclonal antibody and development of an enzyme-linked immunosorbent assay for the detection of ceftiofur in animal-derived foods. J. Pharmaceut. Biomed. Anal. 2021;206 doi: 10.1016/j.jpba.2021.114378. [DOI] [PubMed] [Google Scholar]
- 23.Ye Y., Liu A., Wang X., Chen F. Spectra analysis of coating antigen: a possible explanation for difference in anti-AFB1 polyclonal antibody sensitivity. J. Mol. Struct. 2016;1121:74–79. [Google Scholar]
- 24.Zhao C., Liu W., Ling H., Lu S., Zhang Y., Liu J., Xi R. Preparation of anti-gatifloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for the detection of gatifloxacin residue in milk. J. Agric. Food Chem. 2007;55(17):6879–6884. doi: 10.1021/jf070978g. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z., Lu S., Zhao C., Ding K., Cao Z., Zhan J., Ma C., Liu J., Xi R. Preparation of anti-danofloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of danofloxacin residue in chicken liver. J. Sci. Food Agric. 2009;89(7):1115–1121. [Google Scholar]
- 26.Liu J., Xu X., Wu A., Wang Z., Song S., Kuang H., Liu L., Xu C. Development of a gold nanoparticle-based lateral flow immunoassay for the detection of pyridaben. Microchem. J. 2021;170 [Google Scholar]
- 27.Ni T., Peng D., Wang Y., Pan Y., Xie S., Chen D., Wang Y., Tao Y., Yuan Z. Development of a broad-spectrum monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for the multi-residue detection of avermectins in edible animal tissues and milk. Food Chem. 2019;286:234–240. doi: 10.1016/j.foodchem.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt D.J., Clarkson C.E., Swanson T.A., Egger M.L., Carlson R.E., Van Emon J.M., Karu A.E. Monoclonal antibodies for immunoassay of avermectins. J. Agric. Food Chem. 1990;38(8):1763–1770. [Google Scholar]
- 29.Samdal I.A., Løvberg K.E., Briggs L.R., Kilcoyne J., Xu J., Forsyth C.J., Miles C.O. Development of an ELISA for the detection of azaspiracids. J. Agric. Food Chem. 2015;63(35):7855–7861. doi: 10.1021/acs.jafc.5b02513. [DOI] [PubMed] [Google Scholar]
- 30.Wang S., Zhang C., Wang J., Zhang Y. Development of colloidal gold-based flow-through and lateral-flow immunoassays for the rapid detection of the insecticide carbaryl. Anal. Chim. Acta. 2005;546(2):161–166. [Google Scholar]
- 31.Suryoprabowo S., Liu L., Peng J., Kuang H., Xu C. Development of a broad specific monoclonal antibody for fluoroquinolone analysis. Food Anal. Methods. 2014;7(10):2163–2168. [Google Scholar]
- 32.Li Q., Zhang Y., Wu Z., Huang J., Yue N., Huang L., Zhang X. Tyrosine–EDC conjugation, an undesirable side effect of the EDC-catalyzed carboxyl labeling approach. Anal. Chem. 2021;93(2):697–703. doi: 10.1021/acs.analchem.0c03487. [DOI] [PubMed] [Google Scholar]
- 33.Yan T.T., Wu L.P., Zhang Q., Chen Y.Q., Tang X.Q., Zhang W., University H. Effect of hapten coupling ratio on aflatoxin antigen molecule in immunoassay sensitivity. Chin. J. Oil Crop Sci. 2018;40(3):426–431. [Google Scholar]
- 34.Luo S.J., Zhong H.Y., Liu C.M., Chen T.T., Chen F.R., Yan J.L. Preparation of chloramphenicol immunogen and coating antigen. Food Sci. (N. Y.) 2010;31(10):91–94. [Google Scholar]
- 35.Xu L., Yang F., Dias A.C.P., Zhang X. Development of quantum dot-linked immunosorbent assay (QLISA) and ELISA for the detection of sunset yellow in foods and beverages. Food Chem. 2022;385 doi: 10.1016/j.foodchem.2022.132648. [DOI] [PubMed] [Google Scholar]
- 36.Pang Y., Zhao S., Liu Z., Chen J., Yang Z., He Z., Shen X., Lei H., Li X. An enhanced immunochromatography assay based on colloidal gold-decorated polydopamine for rapid and sensitive determination of gentamicin in animal-derived food. Food Chem. 2022;387 doi: 10.1016/j.foodchem.2022.132916. [DOI] [PubMed] [Google Scholar]
- 37.Mei X., Sun M., Zhang Y., Shen J., Li J., Xue C., Chang Y. Establishment of a carbohydrate binding module-based lateral flow immunoassay method for identifying hyaluronic acid. Int. J. Biol. Macromol. 2022;223:1180–1185. doi: 10.1016/j.ijbiomac.2022.11.122. [DOI] [PubMed] [Google Scholar]
- 38.Cao B., Yang H., Song J., Chang H., Li S., Deng A. Sensitivity and specificity enhanced enzyme-linked immunosorbent assay by rational hapten modification and heterogeneous antibody/coating antigen combinations for the detection of melamine in milk, milk powder and feed samples. Talanta. 2013;116:173–180. doi: 10.1016/j.talanta.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Lei H., Su R., Haughey S.A., Wang Q., Xu Z., Yang J., Shen Y., Wang H., Jiang Y., Sun Y. Development of a specifically enhanced enzyme-linked immunosorbent assay for the detection of melamine in milk. Molecules. 2011;16(7) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.