Abstract
Calotropis procera is a perennial flowering plant of the Apocynaceae family, traditionally used in medicine to treat various ailments. Recent investigations have revealed its potential therapeutic activities such as anti-inflammatory, gastroprotective, analgesic, anti-obesity, and anti-diabetic properties. RP-HPLC qualitatively and quantitatively evaluated the phenolic acids and flavonoids in the ethanolic extract at two different wavelengths, 280 and 330 nm. In addition, total phenolic and flavonoid contents were measured via spectrophotometric determination in addition to the antioxidant activity. The antiproliferative effects of C. procera were investigated on two cancer cell lines: human colon (HCT-116) and breast (MCF-7) cancer. Several methods were utilised to analyse the effectiveness of the plant extract on the cytotoxicity, apoptosis, cell cycle progression, genes involved in the cell cycle, and protein expression profiles of HCT-116 and MCF-7 cells. These included the MTT assay, Annexin V-FITC/PI, analysis of the cell cycle, and Western blot. Results indicated that ferulic and caffeic acids were the major compounds at λmax 280 nm (1.374% and 0.561%, respectively), while the major compounds at λmax 325 nm were kaempferol and luteolin (1.036% and 0.512%, respectively). The ethanolic extract had significantly higher antioxidant activity (80 ± 2.3%) compared to ascorbic acid (90 ± 3.1%).
C. procera extract exhibited dose-dependent cell growth inhibition, with an estimated IC50 of 50 μg/mL for MCF-7 and 55 μg/mL for HCT-116 cells at 24 h. Annexin V-FITC/PI confirmed the induction of apoptosis. Remarkably, cell cycle arrest occurred at the sub-G1 phase in MCF-7 cells, while in HCT-116 cells, it was observed at the G2-M phase. The sub-G1 arrest was associated with dysregulation of Akt, p-AKT, mTOR, and p-mTOR proteins, as confirmed by the Western blot analysis, while downregulation of CDK1, cyclin B1, and survivin caused G2-M arrest.
Keywords: Calotropis procera, Apoptosis, AKT, Breast cancer, Colon cancer
1. Introduction
The International Agency for Research on Cancer reports that nearly one in six deaths globally are caused by cancer, and in 2020 cancer led to 9.6 million deaths worldwide [1]. Despite the recent advances in the field of chemo-, immuno-, and targeted therapy, many inevitable side effects and complications are observed [2].
Plants have been used, for centuries, as a source of natural remedies owing to their medicinal and pharmacological properties. It is reported that approximately forty percent of medications are either directly extracted, derived from, or modelled after natural products found in plants [3]. Interestingly, less than twenty percent of plant species have been studied for their pharmacological properties, and only a few were analyzed for their antineoplastic activities [3]. As such, the vast repertoire of natural products is yet to be explored.
Calotropis procera (Al-ashkhar) is a xerophytic perennial flowering shrub of the Apocynaceae family, widely spread in arid and semiarid regions [4]. Colds, fevers, asthma, eczema, leprosy, dyspepsia, and dysentery are frequently treated with C. procera in traditional folk medicine [4]. The C. procera extracts are reported to have anticancer [5], anti-inflammatory [6], antidiabetic [7], gastroprotective [8], antioxidant [7], antifungal [9], anthelmintic [10], analgesic [6], and antibacterial properties [9,11]. In earlier research, two cell lines (HCT-116 and MCF-7) representing colon and breast cancers were used to assess the antitumor effects of C. procera extract. However, the underlying mechanism behind these effects was not investigated [12]. Earlier phytochemical studies on C. procera found cardenolides, flavonoids, steroids and saponins [13]. The authors previously researched the composition of its flowers' volatiles, lipoids, and flavonoids [14]. They investigated the polyphenolic components of the leaves, finding that C. procera leaf extract contains other abundant (such as rosmarinic acid and quercitrin) [14].
This study aimed to standardise the leaves extract and investigate the mechanistic antitumor pathway of the ethanolic extract of C. procera on the human colon (HCT-116) and breast (MCF-7) cancer cell lines.
2. Materials and methods
2.1. Chemicals
Absolute ethanol and aluminum chloride were obtained from Sigma-Aldrich, USA. 2,2-diphenyl-1-picrylhydrazyl (DPPH), and Folin-Ciocalteu reagent were acquired from Supelco, Germany. Sigma-Aldrich (Saint Louis, MO, USA) provided 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium (MTT) and dimethyl sulfoxide (DMSO), while Abcam (Cambridge, MA, USA) provided Annexin V-FITC and PI staining kits. Fisher Scientific (UK) provided all of the analytical grade solvents.
2.2. Plant material
C. procera leaves were collected in mid-November 2020 from the Muhaisnah desert, Dubai, UAE. Prof. Naglaa Shehab, a taxonomist, was responsible for identifying and authenticating the plant. The herbarium of Dubai Pharmacy College for Girls, UAE, houses the voucher specimen (#13-11-20).
2.3. Extraction of bound phytochemicals
The leaves of C. procera were thoroughly extracted using cold maceration and absolute ethanol (100 g × 1200 mL). A rotary evaporator was used to evaporate the solvent at 50 °C under reduced pressure. After that, the residue was weighed and used for the phytochemical analysis and biological assessment.
2.4. Standardisation of the plant extract
2.4.1. Determination of total phenolic and flavonoid content
The spectrophotometer (UV-1700 Pharma Spec, Shimadzu, Japan) was used to determine the total phenolic and flavonoid contents, and each experiment was performed three times.
The Folin-Ciocalteu reagent was used to determine the total phenolic content [15]. The calibration curve was obtained using gallic acid serial dilutions (10–50 mg/mL). Results were expressed as mean (mg/g of gallic acid equivalents per gram of dry weight of plant material, and the percentage was calculated). The absorbance was measured at 750 nm against the reagent blank.
While total flavonoid content was measured via spectrophotometer by the aluminium chloride method [16], and quercetin was used as standard. The mixture was allowed to sit for 5 min at room temperature after the plant extract (0.1 mL) was added to 0.3 mL of distilled water and 0.03 mL of 5% NaNO2. Following the addition of aluminium chloride (0.03 mL, 10%), the mixture was allowed to sit for a further 5 min before being treated with sodium hydroxide and then diluted with water.
2.4.2. RP-HPLC analysis for phenolic and flavonoid constituents
The reversed-phase high-performance liquid chromatography (RP-HPLC) (Agilent 1100) is composed of a quaternary pump, and a UV/V detector was used for the determination of phenolic acid constituents. The apparatus had an Alltima C18 column (particle size 5 mm, 150 × 4.6 mm) (Alltech, USA). The UV detector was adjusted at a wavelength of 280 nm to detect phenolic compounds. Flavonoids were separated on a Hypersil-ODS C18 column (particle size 5 m, 4.6250 mm) with the UV detector set to 325 nm. The column temperature was controlled at 25 °C in both experiments [17].
By using a gradient mobile phase of two solvents, solvent A (Methanol) and solvent B (Acetic acid in water) (1:25), phenolic acids were separated. The gradient program began with 100% B and was held at this concentration for the first 5 min. This was followed by 3 min of 50% eluent A. The concentration of A was then increased to 80% for the following 3 min and then decreased to 50% once more for the next 5 min. While the methanol/water (50:50 v/v) mixture was adjusted to pH 2.8 with phosphoric acid at an isocratic flow rate of 1.0 mL/min and was used to separate and identify the flavonoids. Individual components were identified based on the retention times compared to standards. The peak area was computed using the external standard approach for quantification. All experiments were performed in triplicate. The samples were examined at 280 and 330 nm [18,19].
2.4.3. DPPH radical scavenging assay for antioxidant activity
As previously reported, the capacity of hydrogen donation or radical scavenging was assessed using DPPH radical [20]. With the aid of a UV microplate reader, the absorbance was determined at 517 nm. The following equation (1) was used to calculate the percentage inhibition of the DPPH radical (antioxidant activity):
| (1) |
A0: absorbance of the control; A1: absorbance of the sample with DPPH; A2: absorbance of the sample without DPPH [21].
Ascorbic acid was used as a reference compound. The percentage of the inhibition was calculated, and the samples were analyzed in triplicate.
2.5. Cell lines
Cells for human breast (MCF-7) and colon cancer (HCT-116) purchased from the American Type Culture Collection were cultured with Dulbecco's Modified Eagle's Medium supplemented with 100 U/mL streptomycin, 100 U/mL penicillin, and 10% fetal bovine serum to maintain the cells. The containers were kept with 5% carbon dioxide at 37 °C. Culture bottles were subcultured every 48 h, and the cells were transferred into containers when they were almost fully grown, at around 80% confluence.
2.6. Cytotoxicity assay
The plant's extract, 10 mg, was dissolved in 1 mL of DMSO to create a stock solution. Concentrations between 3 and 200 μg/mL were prepared from this solution. The extract was then applied to the cells for 24 and 48 h in triplicates after they had been seeded into a 96-well plate with 5 × 103 cells per well. After treatment, MTT (3-[4,5-dimethylthiazol-2-yl] 2,5 diphenyl tetrazolium bromide) [5 mg/mL in phosphate-buffered saline (PBS)] was added to the medium and the cells were incubated (at 37 °C) for 2 h to allow for the reduction of MTT. DMSO (100 μL) was added to dissolve the MTT crystals and then incubated for 10 min. The absorbance at 570 nm was measured using a microtiter plate reader, and the absorbance of the treated cultures was contrasted with that of the control cultures that were left untreated to calculate the rate of cell proliferation [22].
2.7. Apoptosis assay (annexin V/PI)
Apoptosis was assessed using a standardised Annexin V/PI Detection kit based on manufacturer instructions. Briefly, After being exposed to the plant extract for 24 h, both cell lines were removed, followed by PBS washing, and stained for 20 min with annexin V/PI in the staining buffer. After that, the cells were examined for signs of apoptosis at 488 nm (for excitation) and 530/30 nm and 502 nm for fluorescein detection using a flow cytometer (BD FACS Aria III; Becton Dickinson). Early apoptotic cells are those that are positive for only annexin V, late apoptotic cells are those that are positive for both PI and annexin V, and necrotic cells are those that are positive for only PI. Watson's pragmatic algorithm in addition to the FlowJo program, were used to analyse the data and prepare the relevant figures [23].
2.8. Cell cycle analysis
Both cell lines were seeded and then treated with C. procera extract for 24 h. The cells were then harvested, fixed using 70% ethanol (1 mL) at −20 °C overnight, washed and stained for 30 min with PI and DNase-free RNase. The cell cycle phase progression was then analyzed using a flow cytometry platform [22].
2.9. Western blot analysis
The cells were lysed by a protease inhibitor cocktail containing NP40 lysis buffer, which was chilled on ice (Sigma-Aldrich). The Bradford method from Bio-Rad was used to calculate the protein levels. Thirty micrograms of protein were then separated by 12% SDS-PAGE, transferred to a nitrocellulose membrane from Bio-Rad, and blocked with 5% skim milk powder. The membrane was then washed with Tris-buffered saline with 0.1% Tween 20 and incubated overnight at 4 °C with primary antibodies against Cyclin B1 (AB32053), Cyclin D1 (AB134175), CDK4 (AB108357), CDK6 (ab124821), survivin (AB134170), AKT1 (A17909), Phospho-AKT-S473 (AP0637), mTOR (A2445), and Phospho-mTOR-S2448 (AP0115) were obtained from Abcam (Cambridge, MA, USA). The membrane was then treated for an hour with a 1:1000 dilution of the secondary antibodies (antirabbit and antimouse) purchased from Cell Signaling Technology. Chemiluminescence from Thermo Fisher Scientific was used to see the bands, and the band density was calculated using Bio-Rad's Image Lab software. β-Actin was used as a reference standard for normalisation [23].
2.10. Statistical analysis
Statistical analysis was performed using GraphPad Prism 8. Student's t-test was used to compare the means, and statistical significance was determined to be a p-value <0.05.
3. Results
3.1. Yield of the plant extract
Extraction of C. procera leaves with absolute ethanol rendered dark green residue in a yield of 50 g/100 g dry weight (50% w/w yield).
3.2. Determination of total phenolic and flavonoid contents
The total phenolic and flavonoid contents of the ethanolic extract of the plant were investigated spectrophotometrically, yielding 2.5% and 2.8%, respectively. The calibration curves of the phenolics and flavonoids are represented in Figs. S1 and S2 in the supplementary material.
3.3. RP-HPLC analysis of the phenolic and flavonoid constituents
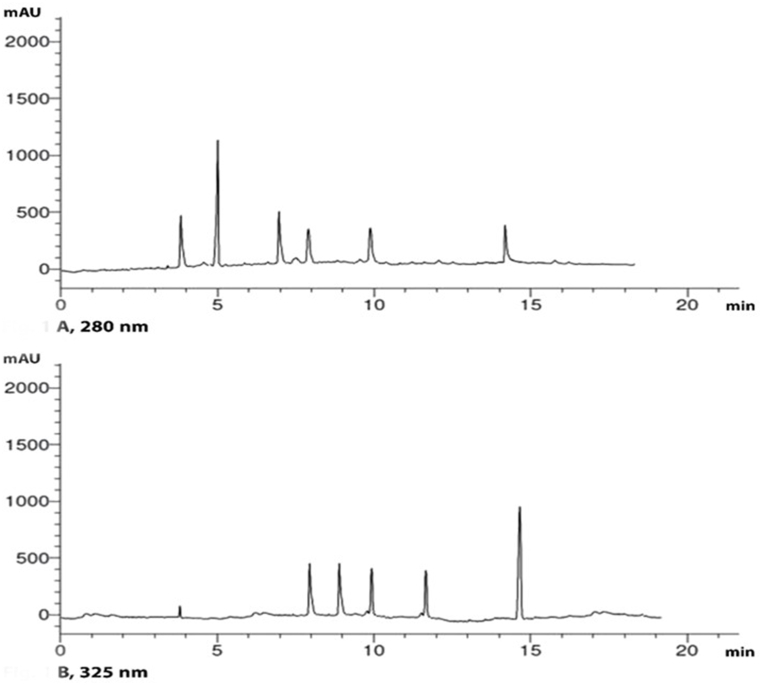
RP-HPLC evaluation of the ethanolic plant extract enabled the identification and quantitation of a variety of phenolic and flavonoid compounds (Table 1, Fig. 1 A and B). Eleven compounds were found in the ethanolic extract at both λmax 280 nm and 325 nm with total percentages of 3.746 and 2.439 w/w, respectively. Ferulic and caffeic acids were the major compounds at λmax 280 nm (1.374% and 0.561% w/w, respectively), while the major compounds at λmax 325 nm were kaempferol and luteolin (1.036% and 0.512% w/w, respectively).
Table 1.
RP-HPLC analysis of the phenolic and flavonoid constituents at different wavelengths.
| 280 nm | RT | (% w/w) | 325 nm | RT | (%w/w) |
|---|---|---|---|---|---|
| Catechol | 3.1 | – | Rutin | 4.1 | – |
| Caffeic acid | 4 | 0.561 | Naringin | 5.2 | – |
| Ferulic acid | 5 | 1.374 | Quercetin | 7 | – |
| O-Coumaric acid | 6.5 | – | Kaempferol | 8.1 | 1.036 |
| Gallic acid | 6.89 | 0.507 | Luteolin | 9 | 0.512 |
| Chlorogenic | 7.95 | 0.48 | Hesperidin | 10 | 0.478 |
| Syringic acid | 8.6 | – | Catechin | 12 | 0.266 |
| Cinnamic acid | 10 | 0.455 | Chrysoeriol | 14.9 | 0.147 |
| Salicylic acid | 11.2 | – | |||
| Ellagic acid | 12.3 | – | |||
| Protocatechuic acid | 14.3 | 0.369 | |||
| Total Identification | 6 | 5 | |||
| Total Percentages | 3.746 | 2.439 |
Fig. 1.
RP-HPLC analysis of the phenolic and flavonoid constituents at different wavelengths (A. 280 and B.325 nm).
3.4. Antioxidant activity
The stable free radical DPPH displays a distinct purple colour measured spectrophotometrically at λmax 517 nm. When a plant extract with antioxidant activity is added to the DPPH assay solution, it donates a hydrogen atom that scavenges the free radical, changing the colour of the solution to a yellowish blue and leading to a reduction in the absorbance. The DPPH free radical scavenging activity is believed to be an in vitro test for potential in vivo antioxidant and anticancer properties. The ethanolic extract was tested for its antioxidant activity compared to ascorbic acid as a reference standard, and the ethanolic extract was found to have significantly (p < 0.05) high antioxidant activity (80 ± 2.3%) compared to ascorbic acid (90 ± 3.1%).
3.5. Cytotoxic effect of C. Procera
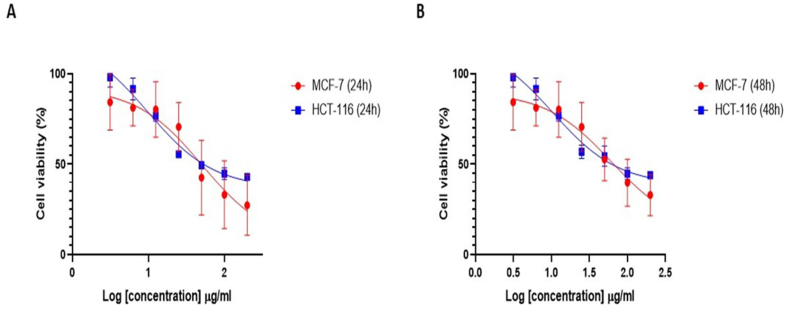
To investigate how the ethanolic extract of C. procera affects the proliferation of cancer cells, MCF-7 and HCT-116 cells were exposed to increasing concentrations of the extract (0, 3.125, 6.25, 12.5, 25, 50, 100 and 200 μg/mL) for different time points 24 and 48 h, followed by an MTT assay. MTT is known to be converted into formazan crystals by living cells determining mitochondrial activity, which we measure to determine the percentage of cell death. According to the findings, inhibition of C. procera cell growth was directly proportional to the dosage administered, with IC50 estimated to be 50 μg/mL for MCF-7 and 55 μg/mL for HCT-116 cells at 24 h (Fig. 2 A). However, at 48 h, the IC50 for MCF-7 and HCT-116 cells were 66 and 60 μg/mL, respectively (Fig. 2 B). For subsequent experiments, 30 μg/mL was used in both cell lines for a 24-h treatment period to prevent excessive cell death.
Fig. 2.
Effects of the C. procera ethanolic extract on cell viability in MCF-7 and HCT-116 cells after: (A) 24-h and (B) 48-h.
3.6. C. Procera promotes apoptosis
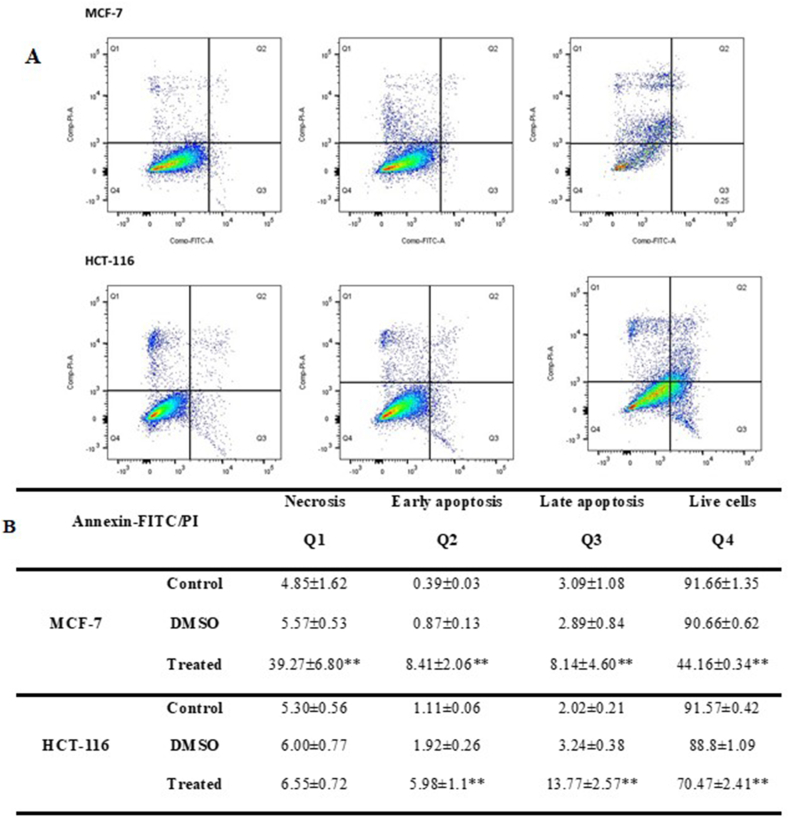
Membrane asymmetry is used as a marker for viable cells, where different phospholipids are asymmetrically distributed between the interior and the outer leaflet of the membrane. In apoptotic cells, membrane asymmetry is lost, and it is detected by annexin V through its interaction with phosphatidyl serine. Propidium iodide (PI) is used to detect late apoptotic and necrotic cells, as it strongly binds to DNA double helix due to its passage into the nucleus, hence indicating cell membrane loss. For this purpose, we have conducted an annexin V-FITC/PI double staining experiment to investigate C. procera action on HCT-116 and MCF-7 cells. Both cell lines were treated with C. procera ethanolic extract for 24 h. Our results show a significant increase in the apoptotic rate in HCT-116 cells upon treatment. The early and late apoptotic rates of treated cells were 5.98% and 13.76%, compared to 1.1% and 2.02% in untreated HCT-116 cells, respectively (Fig. 3 A and B). For MCF-7 cells, the ethanolic extract promoted cell death through both apoptosis and necrosis (Fig. 3 A and B). Cell death through necrosis is more dominant, with a rate of 39% compared to 4.85% in control cells, whereas early and late apoptosis showed a slight increase, 8.4% and 8.1%, respectively, in treated cells compared to 0.39% and 3.09% in untreated cells (Fig. 3 A and B).
Fig. 3.
C. procera ethanolic extract promotes cell death in both breast and colon cancer cells. (A) The percentage of apoptotic and necrotic cells was assessed by flow cytometry in MCF-7 cells (upper panel) and HCT-116 cells (lower panel) using Annexin V/PI staining. Control cells were cultured in media, whereas treated cells were treated with the extract for 24 h. DMSO was used as a negative control. Q1, Q2, Q3 and Q4 represent necrotic, late apoptotic, early apoptotic, and living cells, respectively. (B) Statistical analysis. The average of three independent experiments is represented.
3.7. Cell cycle analysis
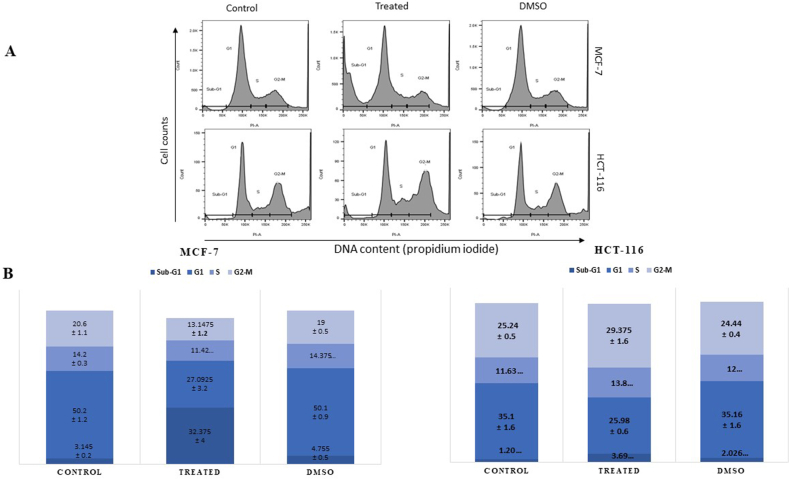
Both cell lines were seeded and treated for 24 h with 50 μg/mL and 55 μg/mL of the C. procera ethanolic extract for MCF-7 and HCT-116, respectively. Cells were then collected and subjected to cell cycle analysis. Interestingly, we found C. procera ethanolic extract causes a remarkable cell cycle arrest at the sub-G1 phase in MCF-7 cells: 32% of the cells arrested at the sub-G1 phase compared to 3.14% in the untreated cells and 4.7% in the DMSO condition. Whereas the extract had a minimal effect on HCT-116 cells, a small but significant arrest (29.4%) at the G2-M phase was observed compared to 25% and 24% in the untreated and DMSO-treated cells, respectively (Fig. 4 A and B).
Fig. 4.
Cell cycle analysis in HCT116 and MCF-7 cells after treatment with C. procera ethanolic extract. (A) Treatment with C. procera leads to Sub-G1 accumulation in MCF-7 cells and G2-M cell cycle arrest in HCT-116 cells. (B) The average values of 5 independent experiments are presented as percentages of cells in Sub-G1, G1, S and G2-M.
3.8. Western blot
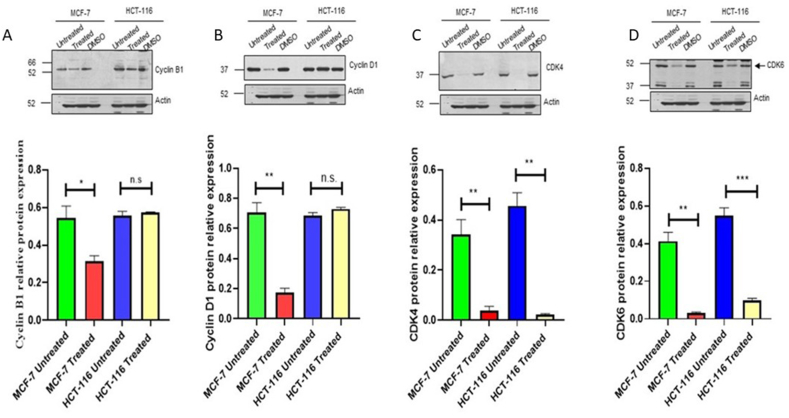
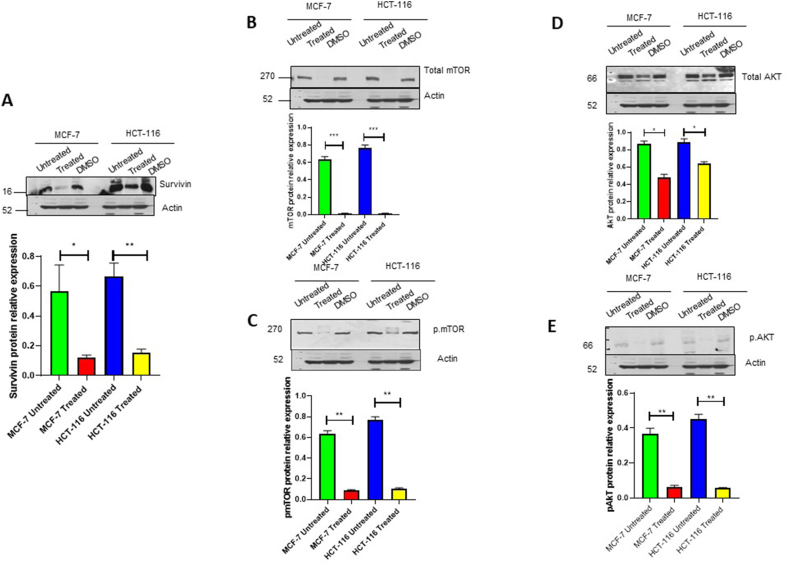
The expression of several cell cycle regulators, including Cyclin B1, Cyclin D1, CDK4, and CDK6, as well as significant cell death-regulating proteins, including survivin, mTOR, phospho-mTOR-S2448, AKT1, and phospho-AKT-S473, were assessed in order to investigate the molecular mechanisms underlying cell death in HCT-116 and MCF-7 cells. (Fig. 5, Fig. 6, respectively).
Fig. 5.
The effect of the C. procera extract on different cell cycle regulatory proteins. HCT-116 and MCF-7 cells were treated with C. procera extract for 24 h. DMSO was used as a negative control. (A) Cyclin B1, (B) Cyclin D1, (C) CDK4, (D) CDK6. The quantitative analysis of each band density after normalisation to the loading control (β-Actin) is represented in MCF-7 and HCT-116.
Fig. 6.
The expression of different cell death markers (A) survivin; (B) mTOR; (C) p-mTOR; (D) AKT; (E) p-AKT in HCT-116 and MCF-7 cells treated with C. procera extract for 24 h. DMSO was used as a negative control: The quantitative analysis of each band density after normalisation to the loading control (β-Actin).
The findings of our study revealed a significant reduction in protein levels of Cyclin B1 (Fig. 5 A), Cyclin D1 (Fig. 5 B), CDK4 (Fig. 5C), and CDK6 (Fig. 5 D), in MCF-7 cells. However, in HCT-116 cells, CDK4 and CDK6 levels experienced a significant decrease (Fig. 5C and D), while Cyclin B1 and D1 remained unchanged (Fig. 5 A and B). Furthermore, treating both cell lines with the plant extract for 24 h resulted in a considerable decrease in survivin expression levels (Fig. 6A). Additionally, the treatment caused a significant decrease in phosphorylation levels of mTOR (Fig. 6 B and C)and AKT (Fig. 6 D and E) in both cell lines, suggesting a dysregulation in the AKT/mTOR pathway (Fig. 6A–E).
4. Discussion
Calotropis is a plant genus frequently used to treat multiple conditions in South Asia, the Middle East, and North Africa such as colds, asthma, eczema, leprosy, indigestion, and dysentery [4]. A study comparing C. gigantea and C. procera found little variation and similar microscopic characteristics [24]. Previous studies employing RP-HPLC to determine and measure the phytochemicals present in the plant's alcoholic extract [25,26]. The extract was reported to have a substantial number of phenolic compounds along with non-phenolic compounds [25,26]. In the previous study, the leaves exhibited a high phenolic content (4 mg/g), with ellagic acid being prevalent and a low flavonoid content (0.3%), with quercitrin being prevalent [26]. The total phenolic and flavonoid levels of C. procera were found to be 2.5% and 2.8%, respectively, in the current study. The major phenolic compounds were ferulic and caffeic acids, while the major flavonoid compounds were kaempferol and luteolin in the plant's extract. The difference in the composition and the phenolic content may be attributed to many environmental factors in UAE. In the previous study, the leaves were collected in September (the weather is very hot and without rain), while in the current study, the leaves were collected at the end of November when the weather is moderate and sometimes with rain. Multiple studies showcased that phenolic compounds such as caffeic, cinnamic, and ferulic acids, and flavonoids such as kaempferol, luteolin, hesperidin, and catechin, all of which have anticancer properties against the studied cell lines [27,28].
The cytotoxic activity of the C. procera aqueous solution was also explored in vitro studies on hepatocellular carcinoma, non-small cell lung cancer, glioblastoma, and prostate cancer cell lines [29]. Additionally, in vivo studies using the plant's latex extract explored anticancer activity against hepatocellular carcinoma [30]. However, limited research has been conducted to investigate the reported findings' precise mechanism of action. Additionally, it has been demonstrated that reactive oxidative species play a role in the plant's aqueous extract's ability to inhibit the proliferation of colon cancer cell lines (HCT116 and HT-29) [30]. To examine the potential cytotoxic effects and molecular mechanism of C. procera on human colon (HCT-116) and breast (MCF-7) cell lines, ethanolic extract of the plant was utilised in this study.
In this study, C. procera ethanolic extract suppressed tumour cell proliferation in both breast and colon cancer. Controlling cancer cells’ cell cycle progression is an effective strategy in tumour growth control [31]. The present data indicated that treatment of MCF-7 and HCT-116 cells with C. procera resulted in a significant arrest in the sub-G1 phase in MCF-7 cells, whereas in HCT-116 cells, the findings revealed arresting of the cell cycle at the G2-M phase.
It is widely known and accepted that cells respond differently to different stimuli, and their mode of cell death might depend on their nature, the treatment concentration and the stimuli severity [32]. To this date, there are 15 different cell death classifications. The most common classification is based on cells morphology which classifies cell death into three different forms: (i) apoptosis, a well-defined programmed response, where dying cells exhibit morphologic and biochemical changes such as cytoplasmic shrinkage, chromatin condensation, nuclear fragmentation, plasma membrane blebbing; (ii) autophagy, characterised with an extensive cytoplasmic vacuolisation together with culminating phagocytic uptake and consequent lysosomal degradation; (iii) necrosis, which is very similar to apoptosis and autophagy [33,34]. According to our data, C. procera ethanolic leave extract promotes cell death in breast and colon cancer cells through different pathways. In HCT-116 cells, we noticed the presence of both apoptotic and necrotic cells; however, apoptosis appeared to be more prevalent. In contrast, necrosis was found to be dominant in MCF-7 cells. Interestingly, according to the literature, apoptosis and necrosis may coexist under pathologic conditions [35], which might explain the presence of both phenotypes in HCT-116 cells.
To further elucidate the cellular basis of the observed cytotoxicity and proapoptotic effects, a cell cycle analysis and molecular expression of the different Cyclins and their respective CDKs was carried out. Cell cycle progression is controlled by specific protein phosphorylation like CDKs, which are activated by binding to their partner cyclins which are expressed in a periodic manner [36]. Cyclin D/CDK4 and 6 are associated with the G1/S phase transition, whereas Cyclin B/CDK1 is associated with the M phase of the cell cycle [37]. We observed a significant decrease in Cyclin D, and both CDK4 and 6 in MCF-7 were detected, which might explain the observed cell cycle arrest in the sub-G1 phase. As for HCT-116, despite the presence of Cyclin D, a complete loss of CDK4 and a significant decrease in CDK6 levels was detected, which could be the result of the sub-G1 arrest observed upon treatment. Interestingly, a slight reduction in Cyclin B was also observed, which could explain the modest arrest at the G1-M phase of the cell cycle observed in HCT-116 cells. At a molecular level, the response towards the treatment was very similar in both cell lines. They both showed a decrease in CDK4 and 6, but the outcome was divergent. MCF-7 cells responded by arresting cells in the sub-G1 phase of the cell cycle and induced cell death through necrosis. HCT-116 cells, on the other hand, showed a higher tendency to induce cell death via apoptosis; G2-M arrest was also minimally detected. Multiple studies examining CDK4/6 inhibition on multiple cancer cell lines (i.e AML, prostate cancer) showed a significant increase in apoptotic cell percentage [38,39].
Survivin, a 142-amino acid protein, is a member of the apoptosis protein family inhibitor that plays a crucial part in controlling cell proliferation. Although differentiated tissues do not often express survivin [40], it is upregulated in most cancer cells, effectively reducing cell death and leading to the abnormal proliferation of various cancer cells. Therefore, a decrease in survivin expression upon treatment is a good indicator of cell death [[41], [42], [43], [44]]. In our study, the expression of survivin was completely abolished in MCF-7 cells and significantly decreased in HCT-116 cells. The AKT/mTOR pathway plays a crucial role in cell motility, growth, survival and metabolism [45] and is known to be one of the most commonly disrupted pathways in cancer. Following 24-h treatment with C. procera ethanolic extract, a decrease in mTOR and AKT phosphorylation was detected in both cell lines, which indicates inhibition of the AKT/mTOR pathway.
Together our data suggest that C. procera extract could be utilised in the fight against cancer. Our results showcase how unique each cell line is and how different the response could be from one cell line to another despite having the same outcome. This highlights the need for personalised medicine, especially in the field of cancer.
5. Conclusions
The ethanolic extract of C. procera has been found to induce cell cycle arrest and apoptosis in colon and breast cancer cells. Further investigation revealed that the extract's action targeted the sub-G1 and G2-M phases in colon and breast cancer cell lines, respectively. These findings suggest that C. procera extract may have potential as a complementary therapy for colon and breast cancer and warrant further in vivo studies on animal models to evaluate its efficacy in conjunction with contemporary cancer treatments.
Author contribution statement
Waseem El-Huneidi, Eman Abu-Gharbieh: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Lara J. Bou Malhab: Performed the experiments; Analyzed and interpreted the data; Wrot the paper.
Khuloud Bajbouj: Performed the experiments; Analyzed and interpreted the data.
Naglaa G. Shehab: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrot the paper.
Jalal Taneera, Mohamed Saleh, Wael M. Abdel-Rahman, Mohammad H. Semreen, Karem H Alzoubi, Yasser Bustanji: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrot the paper.
Salma. M. Elayoty, Jithna Sinoj, Saryia Adra: Performed the experiments.
Data availability statement
Data included in article/supplementary material/referenced in article.
Funding
The research was supported by a grants from the College of Research and Graduate Studies (Grants No. 2001090185 and 2001090271), University of Sharjah, United Arab Emirates.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16706.
Contributor Information
Waseem El-Huneidi, Email: welhuneidi@sharjah.ac.ae.
Eman Abu-Gharbieh, Email: eabugharbieh@sharjah.ac.ae.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.E.M. Ferlay J, Lam F, Colombet M, Mery L, Piñeros M, et al., Cancer Today, Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer.
- 2.Basak D., Arrighi S., Darwiche Y., Deb S. Comparison of anticancer drug toxicities: paradigm shift in adverse effect profile. Life. 2021;12 doi: 10.3390/life12010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen T., Samanta S.K. Medicinal plants, human health and biodiversity: a broad review. Adv. Biochem. Eng./Biotechnol. 2015;147:59–110. doi: 10.1007/10_2014_273. [DOI] [PubMed] [Google Scholar]
- 4.Al-Rowaily S.L., Abd-ElGawad A.M., Assaeed A.M., Elgamal A.M., Gendy A.E.N.G.E., Mohamed T.A., Dar B.A., Mohamed T.K., Elshamy A.I. Essential oil of Calotropis procera: comparative chemical profiles, antimicrobial activity, and allelopathic potential on weeds. Molecules. 2020;25 doi: 10.3390/molecules25215203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Qahtani M.M., Farah M., Abou-Tarboush F., Al-Anazi K., Al-Harbi N., Ali M., Hailan W.Q. Anticancer effects of Calotropis procera latex extract in mcf-7 breast cancer cells. Phcog. Mag. 2020;16:550–556. [Google Scholar]
- 6.Basu A., Chaudhuri A.K.N. Preliminary studies on the antiinflammatory and analgesic activities of Calotropis procera root extract. J. Ethnopharmacol. 1991;31:319–324. doi: 10.1016/0378-8741(91)90017-8. [DOI] [PubMed] [Google Scholar]
- 7.Nadeem M., Mumtaz M.W., Danish M., Rashid U., Mukhtar H., Anwar F., Raza S.A. Calotropis procera: UHPLC-QTOF-MS/MS based profiling of bioactives, antioxidant and anti-diabetic potential of leaf extracts and an insight into molecular docking. J. Food Meas. Char. 2019;13:3206–3220. [Google Scholar]
- 8.Tour N., Talele G. Anti-inflammatory and gastromucosal protective effects of Calotropis procera (Asclepiadaceae) stem bark. J. Nat. Med. 2011;65:598–605. doi: 10.1007/s11418-011-0522-1. [DOI] [PubMed] [Google Scholar]
- 9.Falana M.B., Nurudeen Q.O. Evaluation of phytochemical constituents and in vitro antimicrobial activities of leaves extracts of Calotropis procera against certain human pathogens. Not. Sci. Biol. 2020;12:208–221. [Google Scholar]
- 10.Cavalcante G.S., de Morais S.M., André W.P.P., de Araújo-Filho J.V., Muniz C.R., da Rocha L.O., Ribeiro W.L.C., Rodrigues A.L.M., de Oliveira L.M.B., Bevilaqua C.M.L., Ramos M.V. Chemical constituents of Calotropis procera latex and ultrastructural effects on Haemonchus contortus. Rev. Bras. Parasitol. Vet. 2020;29:1–12. [Google Scholar]
- 11.Rani R., Sharma D., Chaturvedi M., Parkash Yadav J. 2017. Antibacterial Activity of Twenty Different Endophytic Fungi Isolated from Calotropis Procera and Time Kill Assay. [Google Scholar]
- 12.Al-Taweel A.M., Perveen S., Fawzy G.A., Rehman A.U., Khan A., Mehmood R., Fadda L.M. Evaluation of antiulcer and cytotoxic potential of the leaf, flower, and fruit extracts of Calotropis procera and isolation of a new lignan glycoside. Evid. base Compl. Alternative Med. 2017;2017 doi: 10.1155/2017/8086791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moustafa A.M.Y., Ahmed S.H., Nabil Z.I., Hussein A.A., Omran M.A. Extraction and phytochemical investigation of Calotropis procera: effect of plant extracts on the activity of diverse muscles. Pharmaceut. Biol. 2010;48(10):1080–1190. doi: 10.3109/13880200903490513. [DOI] [PubMed] [Google Scholar]
- 14.Shehab N.G., Abu-Gharbieh E., Bayoumi F.A. Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Compl. Alternative Med. 2015;15(1):1–12. doi: 10.1186/s12906-015-0919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shehab N.G., Abu-Gharbieh E., Bayoumi F.A. Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Compl. Alternative Med. 2015;15 doi: 10.1186/s12906-015-0919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewanto V., Xianzhong W., Adom K.K., Liu R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X., Wang J., Wu Q., Li L., Wang Y., Yang H. Determination of kanamycin by high performance liquid chromatography. Molecules. 2019;24(10):1902. doi: 10.3390/molecules24101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goupy P., Hugues M., Boivin P., Amiot M.J. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J. Sci. Food Agric. 1999;79(12):1625–1634. [Google Scholar]
- 19.Mattila P., Astola J., Kumpulainen J. Determination of flavonoids in plant material by HPLC with diode-array and electro-array detections. J. Agric. Food Chem. 2000;48(12):5834–5841. doi: 10.1021/jf000661f. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Z., Moore J., Yu L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 2006;54:7429–7436. doi: 10.1021/jf0611668. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Z., Moore J., Yu L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 2006;54(20):7429–7436. doi: 10.1021/jf0611668. [DOI] [PubMed] [Google Scholar]
- 22.El-Huneidi W., Shehab N.G., Bajbouj K., Vinod A., El-Serafi A., Shafarin J., Bou Malhab L.J., Abdel-Rahman W.M., Abu-Gharbieh E. Micromeria fruticosa induces cell cycle arrest and apoptosis in breast and colorectal cancer cells. Pharmaceuticals. 2020;13(6):115. doi: 10.3390/ph13060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Huneidi W., Bajbouj K., Muhammad J.S., Vinod A., Shafarin J., Khoder G., Saleh M.A., Taneera J., Abu-Gharbieh E. Carnosic acid induces apoptosis and inhibits Akt/mTOR signaling in human gastric cancer cell lines. Pharmaceuticals. 2021;14(3):230. doi: 10.3390/ph14030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava S., Singh A.P., Rawat A.K.S. Comparative botanical and phytochemical evaluation of Calotropis procera Linn. and Calotropis gigantea Linn. Root. J. Appl. Pharmaceut. Sci. 2015;5:41–47. [Google Scholar]
- 25.Parihar G., Balekar N. Calotropis procera: a phytochemical and pharmacological review. TJPS. 2016:115–131. [Google Scholar]
- 26.Ayemele A.G., Ma L., Li X., Yang P., Xu J., Yu Z., Bu D. Identification of bioactive phytochemicals from six plants: mechanistic insights into the inhibition of rumen protozoa, ammoniagenesis, and α-glucosidase. Biology. 2021;10:1055. doi: 10.3390/biology10101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopustinskiene D.M., Jakstas V., Savickas A., Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020;12(2) doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez L., Trostchansky A., Vogel H., Wood I., Palomo I., Wehinger S., Fuentes E. A comprehensive literature review on cardioprotective effects of bioactive compounds present in fruits of aristotelia chilensis stuntz (maqui) Molecules. 2022;27(19) doi: 10.3390/molecules27196147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim S.R.M., Mohamed G.A., Shaala L.A., Moreno L., Banuls Y., Kiss R., Youssef D.T.A., Proceraside A. A new cardiac glycoside from the root barks of Calotropis procera with in vitro anticancer effects. Nat. Prod. Res. 2014;28:1322–1327. doi: 10.1080/14786419.2014.901323. [DOI] [PubMed] [Google Scholar]
- 30.Choedon T., Mathan G., Arya S., Kumar V.L., Kumar V. Anticancer and cytotoxic properties of the latex of Calotropis procera in a transgenic mouse model of hepatocellular carcinoma. World J. Gastroenterol. 2006;12:2517–2522. doi: 10.3748/wjg.v12.i16.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grana X., Reddy E.P. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11(2):211–219. [PubMed] [Google Scholar]
- 32.Thornberry N.A., Lazebnik Y. Caspases: enemies within. Science. 1998;281(5381):1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 33.Schweichel J.U., Merker H.J. The morphology of various types of cell death in prenatal tissues. Teratology. 1973;7(3):253–266. doi: 10.1002/tera.1420070306. [DOI] [PubMed] [Google Scholar]
- 34.Galluzzi L., Maiuri M.C., Vitale I., Zischka H., Castedo M., Zitvogel L., Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14(7):1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 35.Yu S.F., Chen T.M., Chen Y.H. Apoptosis and necrosis are involved in the toxicity of Sauropus androgynus in an in vitro study. J. Formos. Med. Assoc. 2007;106(7):537–547. doi: 10.1016/S0929-6646(07)60004-7. [DOI] [PubMed] [Google Scholar]
- 36.Bai J., Li Y., Zhang G. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017;14(4):348–362. doi: 10.20892/j.issn.2095-3941.2017.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding L., Cao J., Lin W., Chen H., Xiong X., Ao H., Yu M., Lin J., Cui Q. The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. Int. J. Mol. Sci. 2020;21(6) doi: 10.3390/ijms21061960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakatani K., Matsuo H., Harata Y., Higashitani M., Koyama A., Noura M., Nishinaka-Arai Y., Kamikubo Y., Adachi S. Inhibition of CDK4/6 and autophagy synergistically induces apoptosis in t (8; 21) acute myeloid leukemia cells. Int. J. Hematol. 2021;113:243–253. doi: 10.1007/s12185-020-03015-4. [DOI] [PubMed] [Google Scholar]
- 39.Wu C., Peng S., Pilié P.G., Geng C., Park S., Manyam G.C., Lu Y., Yang G., Tang Z., Kondraganti S. PARP and CDK4/6 inhibitor combination therapy induces apoptosis and suppresses neuroendocrine differentiation in prostate cancer. Mol. Cancer Therapeut. 2021;20(9):1680. doi: 10.1158/1535-7163.MCT-20-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garg H., Suri P., Gupta J.C., Talwar G.P., Dubey S. Survivin: a unique target for tumor therapy. Cancer Cell Int. 2016;16 doi: 10.1186/s12935-016-0326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tazo Y., Hara A., Onda T., Saegusa M. Bifunctional roles of survivin-DeltaEx3 and survivin-2B for susceptibility to apoptosis in endometrial carcinomas. J. Cancer Res. Clin. Oncol. 2014;140 doi: 10.1007/s00432-014-1762-8. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y.B., Gao X., Deeb D., Brigolin C., Zhang Y., Shaw J., Pindolia K., Gautam S.C. Ubiquitin-proteasomal degradation of antiapoptotic survivin facilitates induction of apoptosis in prostate cancer cells by pristimerin. Int. J. Oncol. 2014;45(4):1735–1741. doi: 10.3892/ijo.2014.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dohi T., Beltrami E., Wall N.R., Plescia J., Altieri D.C. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J. Clin. Invest. 2004;114(8):1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altieri D.C. Survivin and IAP proteins in cell-death mechanisms. Biochem. J. 2010;430(2):199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng Y., Wang Y., Zhou C., Mei W., Zeng C. PI3K/Akt/mTOR pathway and its role in cancer therapeutics: are we making headway? Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.819128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.