Abstract
Cyprinus carpio (Carp) is a fish of great economic importance in China. However, its population has declined considerably due to the construction of barrages. Thus, fishways need to be constructed at barrages to protect fish resources. It is essential for the fishway design to study the swimming performance of carp. By applying incremental flow velocities in a glass open-type flume, three indicators of swimming performance of the carp in China with the body length (BL) of 13–21 cm, including the induced flow velocity (IFV), the critical swimming speed (Ucrit) and the burst swimming speed (Uburst), are systematically assessed. The correlation between the swimming performance and the BL is also analyzed. The results indicate that the IFV of the carp is 15.56 ± 1.79 cm/s, which is not significantly influenced by the BL. The value of Ucrit varies from 60 to 82 cm/s and gradually increases with the increasing value of BL. The relative critical swimming speed (U'crit) is 4.23 ± 0.28 BL/s and gradually decreases with the increasing value of BL. The value of Uburst ranges from 77.2 to 105.1 cm/s, which is linearly positively correlated to BL. The relative burst swimming speed (U'burst) is 5.42 ± 0.39 BL/s. The value of Uburst is approximately 1.28 times of that of Ucrit for the carps with the same BL. These findings are meaningful to the further study of ecological behavior and to the fishway design and optimization of carps.
Keywords: Carp, Swimming performance, Induced flow velocity, Critical swimming speed, Burst swimming speed, Body length
1. Introduction
Fish migration is a characteristic that fish acquired through long-term adaptation to the external environmental changes, which is a periodic, orientated and clustered activity that occurs among their wintering habitats, spawning grounds and feeding grounds [1]. Nowadays, several barrages have been built globally for comprehensive utilization of water resources. However, the barrages have adversely affected river ecological systems. Specifically, they have segmented the previously continuous rivers and cut off fish migration routes. In this case, it is difficult for fish to migrate for spawning and feeding, which has further resulted in a gradual decline in fish resources in the rivers. It is a serious situation not only in China, but also worldwide [2,3]. The fishway is an ecological project that can maintain the continuity of rivers and meet the fish migration, which has been widely applied to protect the ecological environment during river exploitation. One of the key considerations for fishway design is to match the flow velocity in the fishway with the swimming performance of the passing fish to ensure that the fish can swim against the current and move upstream successfully. The swimming performance of fish varies among different species and body lengths. Therefore, it is particularly important to study the swimming performance of the target fish to guide the fishway design [[4], [6], [45]].
Cyprinus carpio (carp) is the most diverse and widely distributed commercial fish among freshwater fishes in China. Specifically, which is a valuable fish resource. Anthropogenic activities such as river management, construction of barrages and overfishing have adversely affected the wild carp resources in China. The number of wild carp in China has decreased drastically [7]. According to the existing literature, the management and exploitation of the mudflats and the construction of hydraulic projects, such as Longkou and
Wanjiazhai at the Hequ reach of the Yellow River, have reduced the proportion of the captured carp from 5.9% in the 1980s to less than 1% at present [8]. The original carp reserve in Qinghai Lake was 320,000 tons. However, activities such as overfishing and damming have severely damaged the local population structure of carp, and as a result, the carp reserve has declined to less than 0.26 million tons. Although the reserve has recovered slightly in recent years owing to the implementation of the carp stock enhancement policy and the fishing bans, the current reserve is still less than 10% of the highest historical reserve [9]. The carp reserve in the Shaanxi section of the Yellow River has decreased by 39.1% compared with that in the 1980s, and the original Yellow River carp with “golden scales and red tails” can now hardly be seen in Shanxi and Shandong section of Yellow River. In addition to the Yellow River basin, significant declines in the carp reserve have also been reported in the Poyang Lake area, Qiantang River and Xinyun Lake area in Yunnan province [10,11]. It is evident that numerous human activities, especially the construction of large-scale hydraulic projects, have had a serious impact on carp resources. Thus, the initiation of carp conservation projects has become an urgent requirement. Therefore, it is important to investigate the swimming performance of the carp in China to provide a foundation for hydraulic design of the fishway.
Swimming is the most basic life activity of fish that plays an essential role in escaping predators, hunting, migrating and avoiding disaster environments. As early as 1893, Regnaed et al. investigated the swimming performance of fish for the first time and introduced the concept of swimming performance in fish [12]. It has been a current consensus among researchers in this field that the main indicators of swimming performance of migratory fish related to fishway design are the induced flow velocity (IFV), the critical swimming speed (Ucrit), and the burst swimming speed (Uburst) [[13], [14], [15], [16]]. IFV is the flow velocity at which the rheotaxis in fish starts to occur. It is the minimum flow velocity at which the direction of flow can be discerned, providing a design guideline for the minimum flow velocity that needs to be achieved at both exit of fish passage facilities and the interior of fishway. Ucrit is the maximum sustained swimming speed of fish and is a measure of the maximum sustained aerobic capacity of fish, the swimming time usually exceeds 200 min. Uburst is the maximum swimming speed that fish can reach and maintain for a very short duration (usually less than 20 s). An excellent burst swimming performance can guarantee the fish to have the ability to pass areas with high flow velocities. Uburst is generally used as the design guideline for the upper limit of flow velocity in the high velocity area of the fishway. The relative critical swimming speed (U'crit) refers to the Ucrit of an individual fish normalized by its BL, while the relative burst swimming speed (U'burst) refers to the Uburst of an individual fish normalized by its BL. In the 1940s, Fry and Black et al. characterized fish swimming performance and suggested that fish have both strong aerobic and anaerobic metabolic capacities. In 1964, Brett first used the method of increasing velocity to determine the maximum sustained speed of fish and introduced the critical swimming speed (Ucrit) as an index for evaluating the maximum sustainable aerobic capacity of fish [17]. In 1995, Hammer proposed the burst swimming speed (Uburst) for assessing anaerobic swimming status of fish [18].
At present, studies on fish swimming performance for fishway design in China mainly focus on the four major Chinese carps including Mylopharyngodon piceus (black carp), Ctenopharyngodon idella (grass carp), Hypophthalmichthys molitrix (silver carp) and Hypophthalmichthys nobilis (bighead carp), and the endemic fish species, such as Schizothorax lissolabiatus, Percocypris retrodorslis, S. wangchiachii, Schizopygopsis malacanthus and Pelteobagrus fulvidraco (yellow catfish), in the river segment where the engineering projects were conducted [[19], [20], [21], [22], [23], [24], [25]]. Since many hydraulic projects in the main carp-producing area, such as Sanmenxia dam and Xiaolangdi hydrojunction on the Yellow River, have been constructed without fishways, there is a lack of swimming performance studies on carp. The conducted studies mainly focused on biochemical and breeding characteristics, such as how group breeding, rapid growth, and physical training would affect the swimming performance of carp [[26], [27], [28]]. Although some swimming performance studies have been conducted on European carp [29], the swimming performance of the same fish species varies largely with different geographical environments. The swimming capacity of fish is related to its own morphological parameters and the external environment. The morphological parameters affecting swimming capacity mainly include body length and caudal fin, and the external environmental factors are including velocity, water temperature, turbulent kinetic energy, etc[[30], [31], [32]]. For the same species of fish, the caudal fin is basically the same, and the body weight is proportional to the body length, so the body length is the main influencing factor. As for the external environment, the literature [33], points out that the flow velocity is the most important hydraulic factor affecting the swimming behavior of fish. Based on the above, in order to clarify the influence of single factor change, only the relationship between swimming capacity and body length under the change of flow velocity is researched in this study. Therefore, carps with the body length (BL) of 13–21 cm are studied in the present work to determine the correlation between the BL and three swimming performance indicators, including IFV, Ucrit and Uburst. The fitting equations are expected to deduce the swimming performance of carps with different BLs, which are useful for the design of fishways and to comprehend the ecological behavior of carps.
2. Materials and methods
2.1. Experimental apparatus
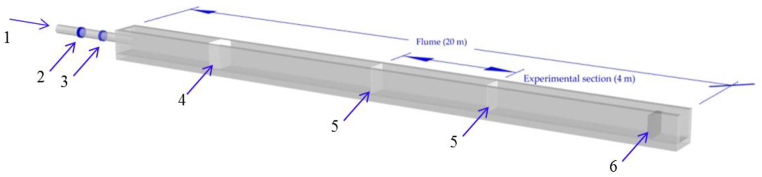
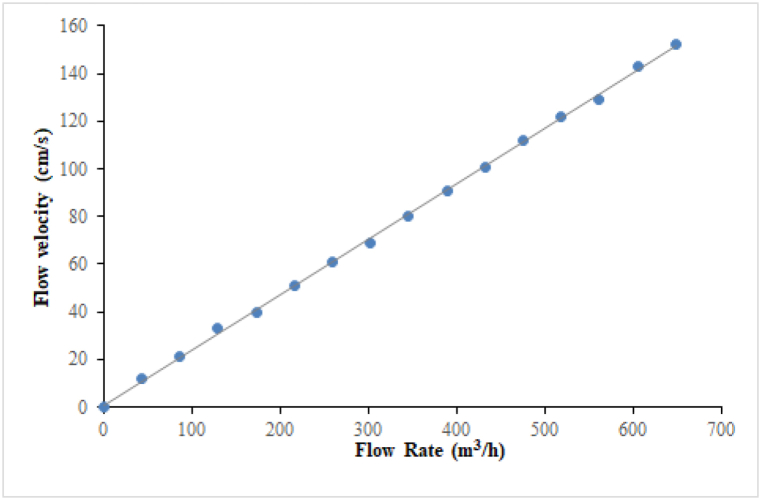
The performance test is conducted in a glass open-type flume (20 m L × 0.6 m W × 0.8 m D) with an electric butterfly valve (VKF42, Siemens) and an electromagnetic flow sensor (7ME6580-2DF14-2AA1, measurement accuracy ±0.2%, Siemens) at the flume inlet to control and measure the flow velocity, as illustrated in Fig. 1. The water level in the flume is regulated using an automatic gate developed by Tianjin Research Institute for Water Transport Engineering (M.O.T.), placed at the flume outlet. The flow velocity in the flume is controlled automatically by the opening of the inlet electric valve and the outlet gate. The flow velocity in experimental area varies from 0 to 1.5 m/s and was calibrated by inlet flow rate as shown in Fig. 2. A 4 m section at the middle of the flume is selected as the experimental section to ensure that all tests are conducted under a smooth flow while barrier meshes (hole size: 3 cm × 3 cm) are placed at both ends to keep the fish in the experimental area. The open-type flume is set in the comprehensive test hall of Tianjin Research Institute for Water Transport Engineering of the Ministry of Transport (Fig. 2). There is a complete water supply circulation system in the test hall. The maximum water flow of the supply system is 1 m3/s, which can meet the test requirements.
Fig. 1.
Illustration of the test flume (1. inlet pipe; 2. electromagnetic flow sensor; 3. electric butterfly valve; 4. wave elimination fence; 5. barrier meshes; 6. automatic outlet gate).
Fig. 2.
A photograph of the test flume.
2.2. Study object
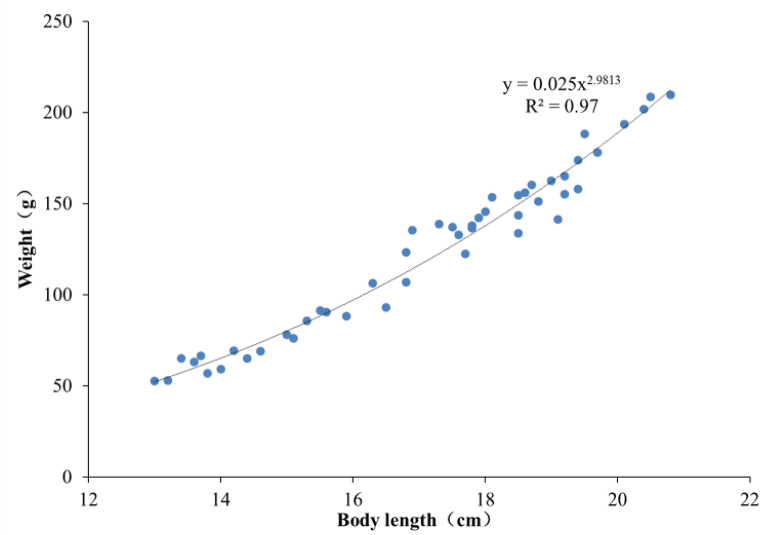
A total of 120 sample fish with the BL of 13–21 cm (length from snout to the last vertebrae of a fish) and the weight of 43.4–209.8 g were obtained from Tianjin Jinghai Aquaculture Fishery in mid-September 2021, as shown in Fig. 3. The correlation between the BL and the body weight of the sample fish W (Fig. 4) is fitted to the following equation:
| W = 0.025 BL2.9813 | (1) |
where the units of W and BL are g and cm, respectively. The coefficient of determination (R2) of Equation (1) reaches 0.97. This equation indicates that the growth of the sample fish is highly allometric [34].
Fig. 3.
Sample fish. (a) BL = 21 cm, (b) BL = 16.5 cm.
Fig. 4.
Length-weight relation of sample fish.
The fish are divided into four groups with 30 specimens in each group according to their BL, including 14.0 ± 1.0 cm, 16.0 ± 1.0 cm, 18.0 ± 1.0 cm and 20.0 ± 1.0 cm. For the first 2 weeks after the fish arrived from the fishery, they are placed in a rectangular water tank containing tap water that has been aerated for 5 days. The water temperature is 26 ± 1 °C, the dissolved oxygen concentration is maintained at 6.50 mg/L or more, and the ammonia nitrogen concentration is controlled below 0.01 mg/L. The lighting for the water tank is indoor natural light, and the fish are fasted for 2 days before the experiment. The experiment was conducted from September 26 to October 28, 2021, which is basically consistent with the migration season of Chinese carp generally from July to September.
2.3. Experimental methodology
At present, there are two main methods for testing fish swimming capacity: the incremental flow velocity methodology and the fixed velocity test [5,35,36]. Both of the methods are widely used for measuring the critical swimming speed (Ucrit) and the burst swimming speed (Uburst), and the induced flow velocity (IFV) is mainly measured by the incremental flow velocity method. Since the two methods mentioned above are simplified methods for the convenience of measuring, they have certain limitations. For example, in the incremental flow velocity methodology, fish are forced to swim at a certain velocity increment. In this process, fish are in a certain degree of tension, which may deviate from the free swimming of fish in the natural state. But the method requires less time and less experimental samples to obtain statistically significant test results, and the flow velocity in the test is controllable [37].
The swimming state of fish in the fixed velocity test is closer to the natural state, but more samples are required, and there is no clear physiological basis for the measurement index of fish swimming time used in the test[38]. On this basis, combined with the focus of this study on the correlation between swimming capacity and body length of carp, the incremental flow velocity methodology was selected according to the related literature of similar swimming capacity experiments [[39], [40], [41], [42]].
The IFV, Ucrit and Uburst of the sample fish are measured separately for each fish using the incremental flow velocity methodology. Before the formal experiment, the sample fish are placed in the experimental flume section and allowed to acclimatize at a flow velocity of 1 BL/s for 1 h to eliminate the stress caused by transportation. Four groups of sample fish with different BLs (14.0 ± 1.0 cm, 16.0 ± 1.0 cm, 18.0 ± 1.0 cm and 20.0 ± 1.0 cm) are selected for the IFV test, and tests are repeated 8 times for each BL group. Sample fish used for Ucrit and Uburst tests are sorted in the same way as the fish for the IFV test. Fig. 5 shows a photograph of the experimental site.
Fig. 5.
A photograph of the experimental site.
Before the test, the flow velocity of test section is calibrated first (Fig. 6), and then the inlet flow and the tail gate height are adjusted to make the flow velocity of the test section uniform. The health of fish is also the focus of the experiment. The fish are basically in normal state during the test of the critical swimming speed and the burst swimming speed. According to the test method, when the fish is in a state of exhaustion, that is the end of the experiment. After the experiment is stopped, the experimental fish will be transferred to the temporary water tank to recover, without causing death. In addition, the experimental fish will not be reused.
-
(1)
IFV Tests
Fig. 6.
Relationship between flow velocity and flow rate.
In the test for IFV, the flow velocity in the flume is first set to zero. As the test started, the flow velocity is increased at an increment of 0.01 m/s with a preferable time interval of 5 s between each increment [22]. The swimming performance of the sample fish is observed and recorded. The IFV is recorded and the test is stopped as soon as the sample fish exhibits rheotaxis. After the test is stopped, the body length of the sample fish is measured, and the fish are transferred to the temporary flume to recover immediately. At the same time, the next sample fish are put into the test flume for adaptation, and the experiment is continued.
-
(2)
Ucrit Measurement
When the Ucrit test started, the flow velocity is increased directly in addition to the adaptive velocity at an increment of 0.5 BL/s and with a time interval of 20 min between increments [22]. The test is stopped when the sample fish is exhausted and swims against the barrier mesh for 20 s [17,19]. The duration of swimming at the highest flow velocity and the maximum swimming speed at which the sample fish succeeds in completing the entire increment interval are recorded. After the test is stopped, the body length of the sample fish is measured, and the fish are transferred to the temporary flume to recover immediately. At the same time, the next sample fish are put into the test flume for adaptation, and the experiment is continued. Ucrit is calculated according to equation (2):
| (2) |
where, is the maximum flow velocity corresponding to the exhaustion of the sample fish in the Ucrit test (cm/s), is the flow velocity increment in the Ucrit test (cm/s), t is the swimming duration at the highest flow velocity tested (min), and Δt is the time interval between flow velocity increments (min).
The relative critical swimming speed, U'crit, is calculated by equation (3):
| (3) |
-
(3)
Uburst Measurement
When the Uburst test started, the flow velocity is increased directly in addition to the adaptive velocity at an increment of 0.5 BL/s with a time interval of 20 s between each increment [22]. The test is stopped when the sample fish is exhausted and stops against the barrier mesh for 20 s [17,19]. The swimming duration at the highest flow velocity and the maximum swimming speed at which the sample fish succeeds in completing the entire increment interval are recorded. After the test is stopped, the body length of the sample fish is measured, and the fish are transferred to the temporary flume to recover immediately. At the same time, the next sample fish are put into the test flume for adaptation, and the experiment is continued. Uburst is calculated according to equation (4):
| (4) |
where, is the maximum flow velocity corresponding to the exhaustion of the sample fish in the Uburst test (cm/s), is the flow velocity increment in the Uburst test (cm/s). The units of t and Δt are second.
The relative burst swimming speed, U'burst, refers to the Uburst of an individual fish normalized by its BL, which is calculated by equation (5).
| (5) |
3. Results
3.1. IFVs
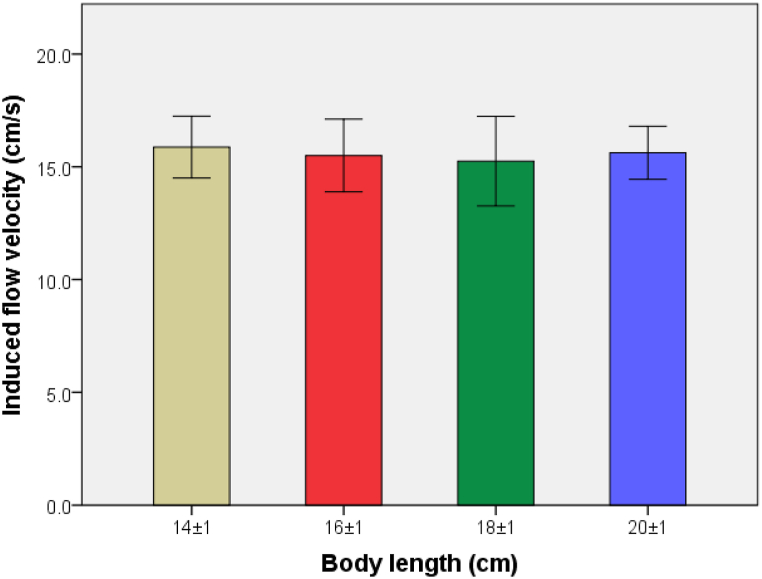
The experimental data are analyzed by SPSS23.0, the statistical values are described by mean ± standard deviation (mean ± SD), and the differences are analyzed by one-way ANOVA method. As shown in Fig. 7, the measured IFVs of carp in the four BL groups are 15.87 ± 1.64, 15.5 ± 1.93, 15.25 ± 2.38 and 15.62 ± 1.41 cm/s, respectively. The correlation analysis shows no significant difference (P > 0.05) in IFVs of carp among the four BL groups, indicating that the correlation between IFV and BL is not significant.
Fig. 7.
Comparison of induced flow velocities (IFVs) of carp in the four different body length (BL) groups.
3.2. Ucrit
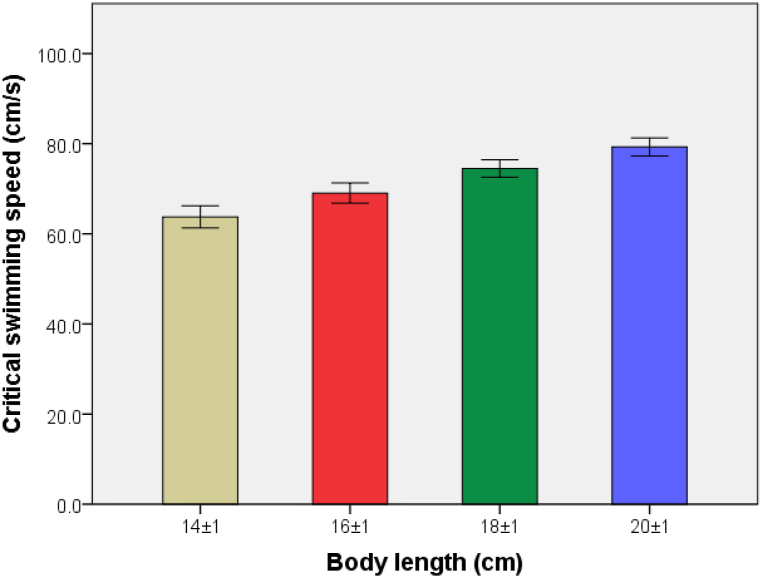
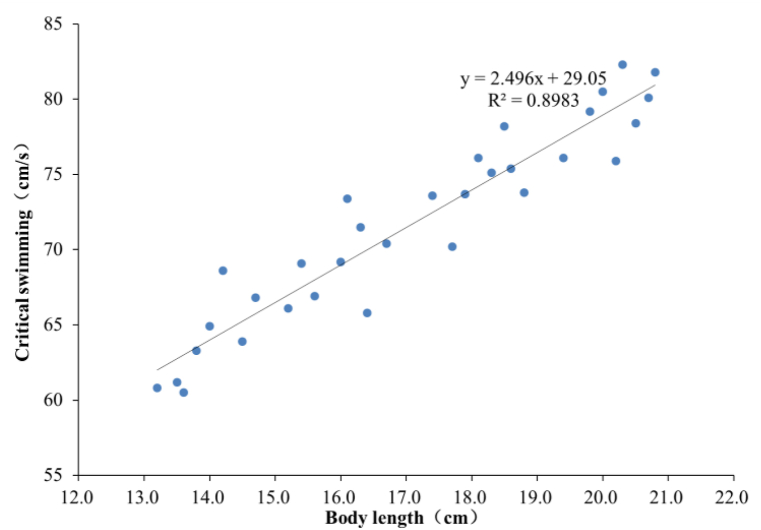
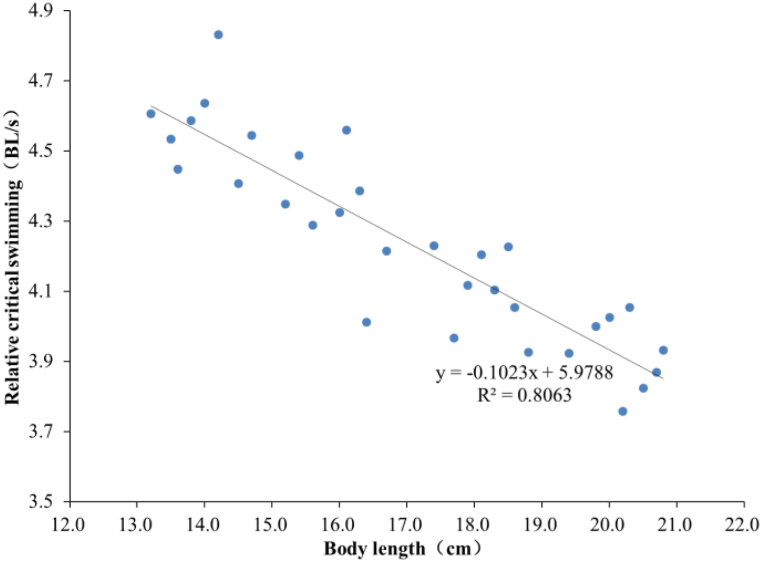
As shown in Fig. 8, Ucrit values of sample carp in the four BL groups are 63.75 ± 2.93, 69.05 ± 2.69, 74.51 ± 2.33 and 79.29 ± 2.39 cm/s, respectively. The correlation analysis shows that there are significant differences among the four groups (P < 0.05), suggesting a significant correlation between Ucirt and BL. According to the analysis, Ucrit increases linearly with increasing BL, as shown in Fig. 9, while the U'crit of carp decreases linearly with increasing BL, as shown in Fig. 10.
Fig. 8.
Comparison of Ucrit of carp in the four different body length (BL) groups.
Fig. 9.
Correlation between Ucrit and body length (BL).
Fig. 10.
Correlation between U'crit and body length (BL).
The regression equation for Ucrit and BL can be derived as
| (6) |
The regression equation for U'crit and BL can be derived as
| (7) |
Values of R2 for Equations (6), (7) are 0.8983 and 0.8063, respectively.
3.3. Uburst
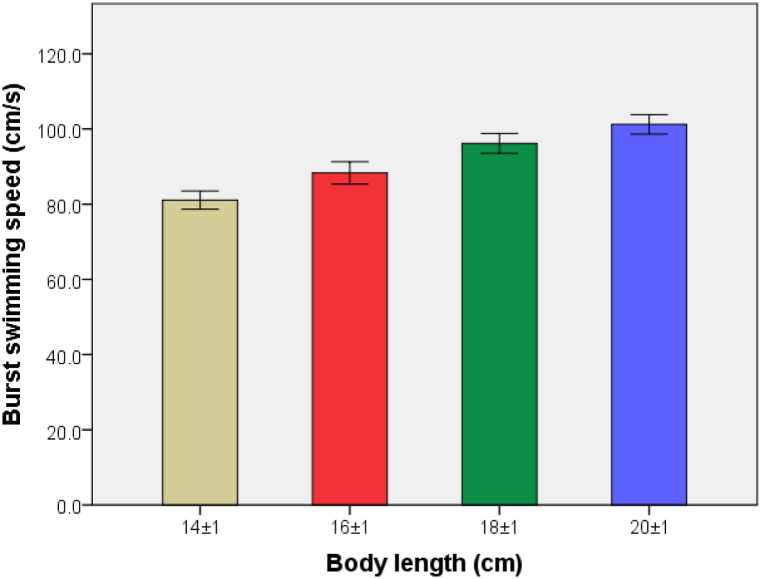
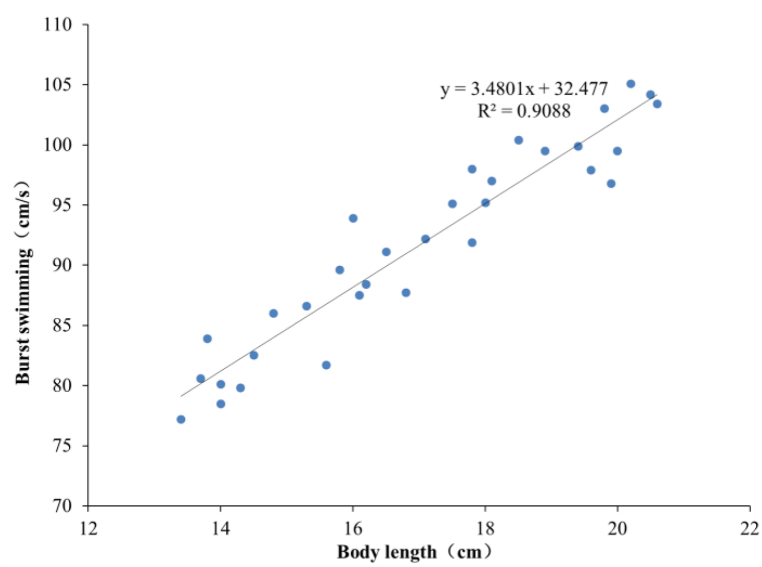
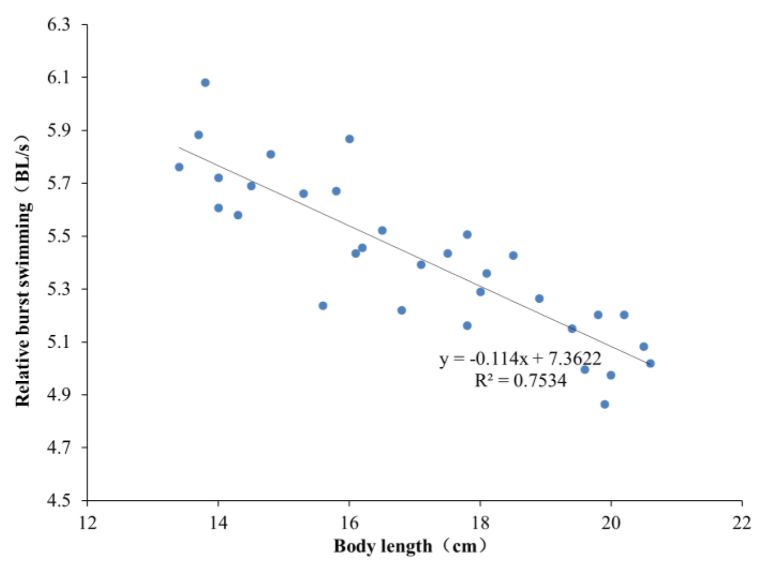
As shown in Fig. 11, Uburst values of the sample carp in the four BL groups are 81.08 ± 2.90, 88.31 ± 3.56, 96.16 ± 3.14 and 101.23 ± 3.09 cm/s, respectively. The correlation analysis shows that there are significant differences (P < 0.05) among the four groups and a significant linear correlation between Uburst and BL of carp (Fig. 12). Meanwhile, U'burst decreases linearly with increasing BL, as shown in Fig. 13. The regression equation for Uburst and BL is obtained as
| (8) |
Fig. 11.
Comparison of Uburst of carp in the four different BL groups.
Fig. 12.
Correlation between Uburst and BL.
Fig. 13.
Correlation between U'burst and BL.
The regression equation for U'burst and BL is obtained as
| (9) |
Values of R2 for Equations (8), (9) are 0.9088 and 0.7534, respectively.
4. Discussion
4.1. IFVs
IFV is an indicator of the fish's sensitivity to flow, which is closely associated with the developmental process, physiological features and habitat conditions of the fish. In this study, the IFV of the carp in China is 13–18 cm/s. Compared with the IFV of other fish species of Cyprinidae family, as shown in Table 1, the IFVs of carp observed in this study are almost equal to those of grass carp (10.72–15.55 cm/s), S. lissolabiatus (10–23 cm/s), S. lantsangensis (10–18 cm/s), and they are close to the IFVs of S. yunnanensis (9–13 cm/s), S. griseus (10–16 cm/s), P. Retrodorslis (7–12 cm/s) and S. wangchiachii (4–14 cm/s) [19,21,25]. However, IFVs of carp are significantly higher than those of silver carp (6.5–7.2 cm/s), Carassius auratus (0.3–7.23 cm/s) and bighead carp (0.47–4.26 cm/s) [22,43]. The variances in IFV of different fish species are mainly attributed to the diverse habitat environment and living habits of the fish. The flow velocity of each layer in the water body is different, owing to the decrease in flow velocity with the increase in depth of the water body. Thus, fish species such as silver carp and bighead carp that live in the upper layer of the water body are the most sensitive to the flow velocity and have the smallest IFV. Conversely, since carp and grass carp live in the lower layers of the water body, they are not very sensitive to the velocity of flow and have relatively higher IFVs.
Table 1.
Comparison of induced flow velocities (IFVs) of different fish species.
| Species | BL (cm) | IFV(cm/s) | Source |
|---|---|---|---|
| Cyprinus carpio | 13∼21 | 13∼18 | This study |
| Ctenopharyngodon idellus | 10.31–16.01 | 10.72–15.55 | [19] |
| Schizothorax lissolabiatus | 15.5–34.2 | 10∼23 | [22] |
| S. lantsangensis | 17.4–31.4 | 10∼18 | [22] |
| S. yunnanensis | 14.6–24.9 | 9∼13 | [22] |
| S.griseus | 13.9–24.4 | 10∼16 | [22] |
| Percocypris retrodorslis | 9.5–11.2 | 7∼12 | [22] |
| Schizothorax wangchiachii | 20∼25 | 4.0–14.0 | [25] |
| Hypophthalmichthys molitrix | 7.2–14.1 | 6.5–7.2 | [43] |
| Carassius auratus | 7.8–23 | 0.3–7.23 | [21] |
| Aristichthy snobilis | 27.4–37 | 0.47–4.26 | [21] |
The correlation between IFV and BL of carp is not significant in this study, which is consistent with the results of the studies conducted by Bai et al. [43] and Wang et al. [21]. However, Cai et al. [44] suggested that the body force per unit volume decreases as the size of fish increases, and as a result, IFV increases with increasing BL. In contrast, Zhong et al. [25] emphasized the role of BL played in decreasing IFV. Specifically, the fish with a larger BL is usually older with a more developed lateral line sensory system, which makes it more sensitive to water flow and has a lower IFV. The results in this study show that BL has no significant impact on IFV, which is the outcome of the combined effects of body force per unit volume and lateral line sensory system.
4.2. Ucrit
The calculated U'crit of the carp in China with the BL of 13–21 cm is 4.23 ± 0.28 BL/s, which is comparable to that of European carp (4.84 ± 0.31 BL/s) [29]. But it is lower than the four major Chinese carps (black, grass, silver, and bighead carps) of the Cyprinidae family, as shown in Table 2. For example, Xian et al. [40] reported that the U'crit of black carp was 5.25 ± 0.18 BL/s, and Gong et al. [39] reported that U'crit of grass carp was 7.71 ± 0.18 BL/s. Moreover, Liu et al. [41] stated that U'crit of silver carp and bighead carp was 5.87 ± 0.25 BL/s and 4.60 ± 0.56 BL/s, respectively. The swimming performance of different fish species is closely related to the body shape and living habits. From the morphological and hydrodynamical aspects, crescent-shaped caudal fins, narrower caudal peduncles, stiffer bodies, streamlined body shapes and proper aspect ratios are more conducive to continuous moving. Compared to the four major Chinese carps, the carp has a plump body and a larger height to length ratio and lives mainly in water bodies with slower currents [20], which are the main reasons for its weaker swimming performance.
Table 2.
Comparison of U'crit among carp, European carp and four major Chinese carps.
| Species | BL (cm) | U'crit (BL/s) | Source |
|---|---|---|---|
| Cyprinus carpio | 13∼21 | 4.23 ± 0.28 | This study |
| European Cyprinus carpio | 10.7–22.8 | 4.84 ± 0.31 | [29] |
| M.piceus | 17.8–18.2 | 5.25 ± 0.18 | [40] |
| Ctenopharyngodon idellus | 5.0–15.0 | 7.71 ± 0.18 | [39] |
| Hypophthalmichthys molitrix | 17.9–18.8 | 5.87 ± 0.25 | [41] |
| Aristichthy snobilis | 18.2–21.5 | 4.60 ± 0.26 | [41] |
In this study, Ucrit of carp increases with increasing BL, while U'crit decreases with increasing BL. These are consistent with the findings of previous studies on other Cyprinidae species. In addition, Ucrit of carp measured in this study is linearly correlated with BL.
4.3. Relationship between Uburst and Ucrit
Burst swimming is a behavior of fish in response to environmental stress. It is an important characteristic of fish, which enables it to cross high flow velocity areas of the rivers or fish facilities and to escape from predators. Thus it plays an important role in the survival of fish. In this study, the measured U'burst of carp is 5.42 ± 0.39 BL/s, which is comparable to that of European carp (5.50 ± 0.32 BL/s), but lower than that of the four major Chinese carps (black, grass, silver and bighead carps, as shown in Table 3.
Table 3.
Comparison of U'burst among carp, European carp and four major Chinese carps.
| Species | BL (cm) | U'burst (BL/s) | Source |
|---|---|---|---|
| Cyprinus carpio | 13∼21 | 5.42 ± 0.39 | This sduty |
| European Cyprinus carpio | 10.7–22.8 | 5.50 ± 0.32 | [29] |
| M.piceus | 17.8–18.2 | 5.67 ± 1.22 | [40] |
| Ctenopharyngodon idellus | 5.0–15.0 | 8.86 ± 0.49 | [40] |
| Hypophthalmichthys molitrix | 17.9–18.8 | 6.60 ± 1.62 | [42] |
| Aristichthy snobilis | 18.2–21.5 | 5.75 ± 0.94 | [42] |
It is observed that the correlation between Uburst and BL for the carp in China is similar to that between Ucrit and BL, because both Uburst and Ucrit increase with increasing BL. Uburst is linearly correlated with BL. For carp with the same BL, Uburst is approximately 1.28 times of Ucrit. Due to the limitation of laboratory and experimental apparatus, little research has been reported on the swimming performance of fish with larger BL. The swimming performance of fish with different BLs is mainly deduced by fitting correlation equations based on the juvenile fish. Therefore, the results presented in this study can provide guidelines for the design of various fish passage facilities and habitat protection projects that target carp. Meanwhile, due to the small population of rare fish, it is difficult to obtain a large amount of experimental data and determine the correlation equation between BL and swimming performance. Thus, the swimming performance equations proposed in this study can also be used to estimate the swimming performance of rare fish with similar morphology.
5. Conclusions
In this study, various swimming performance indicators of carp with the BL of 13–21 cm, including IFV, Ucrit and Uburst, are systematically investigated using an open-type glass flume by applying the incremental flow velocity methodology. The correlations between the swimming performance and the BL of carp are analyzed. The main conclusions are as follows:
-
(1)
The value of IFV of carp ranges from 13 to 18 cm/s, and the difference of BLs has no significant effect on IFV. As a benthic fish, the carp lives on or near the river bottom all year round and shows lower sensitivity to currents and a higher IFV compared with other Cyprinidae species.
-
(2)
The value of Ucrit of carp with the BL of 13–21 cm varies from 60.2 to 82.3 cm/s, which is positively correlated with BL (). In contrast, the value of U'crit of carp is negatively correlated with BL, i.e., .
-
(3)
The value of Uburst of carp with the BL of 13–21 cm ranges from 77.2 to 105.1 cm/s, which is linearly and positively correlated with BL, i.e., . Conversely, the value of U'burst of carp is negatively correlated with BL, i.e., .
-
(4)
Because of its plump body and living habits in water bodies with slower currents, carp has a weaker swimming performance in both Ucrit and Uburst when compared to the four major Chinese carps (black, grass, silver, and bighead carps).
The above results can provide fundamental data and reference materials for the further study of the ecological behavior of the carp in China and the design of the carp fishway engineering.
Ethics statement
This study was conducted in strict accordance with the laws governing animal experimentation in China. The protocol was approved by the China Tianjin University. All efforts were made to minimize suffering.
Funding
This research was jointly funded by the National Key Research and Development Program of China (No. 2022YFC3204202; No. 2021YFB2600200), the Special Program of Tianjin Research Institute for Water Transport Engi-neering of Ministry of Transport (TKS20210208) and the National Natural Science Foundation of China (No. 51879187; No. 51890915).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
J. Li, H. Liu, and Z. Xiao conceived and designed the experiments, performed the experiments. X. Wei and Z. liu analyzed and interpreted the data; Z. Zhang contributed reagents, materials and analysis tools. J. Li drafted the article and critically revised its important intellectual content. All authors read and final approval of the version submitted.
Contributor Information
Haixiao Liu, Email: liuhx@tju.edu.cn.
Zhong Xiao, Email: tjuzhongxiao@tju.edu.cn.
References
- 1.Cooke S.J., Hinch S.G., Donaldson M.R., Clark T.D., Eliason E.J., Crossin G.T., Raby G.D., Jeffries K.M., Lapointe M., Miller K. Conservation physiology in practice: how physiological knowledge has improved our ability to sustainably manage Pacific salmon during up-river migration. J. Phil. Trans. R. Soc. B. 2012;367:1757–1769. doi: 10.1098/rstb.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X.Y., Guo J. Brief review on research and construction of fishways at home and abroad. J. China Inst. Water Resour. Hydropower Res. 2002;3:222–228. doi: 10.3969/j.issn.1672-3031. 2005.03.012. [DOI] [Google Scholar]
- 3.Nestler J., Goodwin R., Smith D., Anderson J., Li S. Optimum fish passage and guidance designs are based in the hydrogeomorphology of natural rivers. J. River Res. Appl. 2008;24:148–168. doi: 10.1002/rra.1056. [DOI] [Google Scholar]
- 4.Castro-Santos T. Modeling the effect of varying swim speeds on fish passage through velocity barriers. Trans. Am. Fish. Soc. 2006;135:1230–1237. doi: 10.1577/T05-262.1. [DOI] [Google Scholar]
- 5.Castro M.A., Santos H.A., Sampaio F.A.C., Pompeu P.S. Swimming performance of the small characin Bryconamericus stramine-us (Characiformes: characidae) Zool. 2010;27(6):939–944. doi: 10.1590/S1984-46702010000600015. [DOI] [Google Scholar]
- 6.Li G.N., Sun S.K., Liu H.T., Zheng T.G. Schizothorax prenanti swimming behavior in response to different flow patterns in vertical slot fishways with different slot positions. Sci. Total Environ. 2021;754:1–14. doi: 10.1016/j.scitotenv.2020.142142. [DOI] [PubMed] [Google Scholar]
- 7.Ding Y.T., Pan B.Z. The aquatic organisms resources in the Yellow River basin: assessment and problem diagnosis. Environ. Monitor. China. 2022;38:1–13. doi: 10.19316/j.issn.1002-6002. 2022.01.01. [DOI] [Google Scholar]
- 8.Han J.F. Investigation on fish resources in Hequ reach of the Yellow River and research on their breeding protection. Shanxi Hydrotech. 2014;194:67–71. doi: 10.3969/j.issn.1006-8139.2014.04.025. [DOI] [Google Scholar]
- 9.Tan L.F., Xu D.P., Qi H.F., Fang D.A., Ding L.Q., Li Y.D., Wu Y.H. The spatial and temporal distribution in early life history stages of gymnocypris przewalskii in shaliu river. Acta Hydrobiol. Sin. 2022;46:265–272. doi: 10.7541/2021.2020.126. [DOI] [Google Scholar]
- 10.Wang Y.C. Investigation and analysis of fish resources at the confluence of the weihe river and the yellow river. Acta Ecologiae Animale Domactici. 2019;40:41–45. doi: 10.3969/j.issn.1673-1182. 2019.01.008. [DOI] [Google Scholar]
- 11.Hao Y.B., Liu J.D., Zhang A.J., Guo A.H., Lian Q.P., Yuan J.L., Gu z.m. Current status of fishery resources in lanxi section of Qiantang River drainage. Fish. Sci. 2019;38:555–562. doi: 10.16378/j.cnki.1003-1111. 2019.04.018. [DOI] [Google Scholar]
- 12.Stobutzki I.C., Bellwood D.R. An analysis of the sustained swimming abilities of pre- and post-settlement coral reef fishes. J. Exp. Mar. Biol. Ecol. 1994;175:275–286. doi: 10.1016/0022-0981(94)90031-0. [DOI] [Google Scholar]
- 13.Meixler M.S., Bain M.B., Todd Walter M. Predicting barrier passage and habitat suitability for migratory fish species. Ecol. Model. 2009;220(20):2782–2791. doi: 10.1016/j.ecolmodel.2009.07.014. [DOI] [Google Scholar]
- 14.Yanase K., Eayrs S., Arimoto T. Influence of water temperature and fish length on the maximum swimming speed of sand flathead, Platycephalus bassensis: implications for trawl selectivity. Fish. Res. 2007;84(2):180–188. doi: 10.1016/j.fishres.2006.10.015. [DOI] [Google Scholar]
- 15.Cai L., Fang M., Tu Z.Y., Liu G.Y., Shi X.T., Huang Y.P. Research progress on the fish swimming performance related to migration. J. Wuhan Univer. Natur. Sci. Ed. 2013;4:363–368. [Google Scholar]
- 16.Mu X.P., Cao P., Gong L., Baiyin B.L.G., Li X. A classification method for fish swimming behaviors under incremental water velocity for fishway hydraulic design. Water. 2019;11(10):1–14. https://doi:10.3390/w11102131 [Google Scholar]
- 17.Brett J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish. Res. Board Can. 1964;21:1183–1226. doi: 10.1139/f64-103. [DOI] [Google Scholar]
- 18.Hammer C. Fatigue and exercise tests with fish. Comp. Biochem. Physiol. Physiol. 1995;112(1):1–20. doi: 10.1016/0300-9629(95)00060-k. [DOI] [Google Scholar]
- 19.Cao P., Mu X.P., Li X., Baiyin B.L.G., Wang X.Y., Chen Y.F. Study on swimming behavior of juvenile grass carp for the fish channel hydraulic design. J. Hydraul. Eng. 2017;48:1456–1464. doi: 10.13243/j.cnki.slxb.20170758. [DOI] [Google Scholar]
- 20.Wang X.C., Xing J.J. Comparison of induction velocities for five fish species. J. Hydroecol. 2018;39:77–81. doi: 10.15928/j.1674-3075. 2018.02.011. [DOI] [Google Scholar]
- 21.Wang X.C., Lu B.B., Xing J.J., Li D., Wang C.N., Ren S.J. Induced flow speed and its relationship to body length of two Schizothoracinae fishes in upper reaches of Yellow River. South China Fisher. Sci. 2020;16:47–53. doi: 10.12131/20190249. [DOI] [Google Scholar]
- 22.Cai L., Wang W.Y., Wang H.L., Hu W.B., Zhao P., Zhao N., Hou Y.Q., Chen S.L., Chen H., Zhang P. Response of induced flow speed to fish body length and its application in flow design of fish passage facilities. Trans. Chin. Soc. Agric. Eng. 2018;34:176–181. doi: 10.11975/j.issn.1002-6819. 2018.02.024. [DOI] [Google Scholar]
- 23.Hou Y.Q., Newbold L., Cai L., Wang X., Hu W.B., Qiao Y. Swimming performance of juvenile Aristichthys nobilis under fixed velocity swimming tests. Chin. J. Ecol. 2016;35:1583–1588. doi: 10.13292/j.1000-4890.201606.013. [DOI] [Google Scholar]
- 24.Li Y.X., Zhu Z.Q., Hou Y.Q., Wang Y.M., Ke F.S., Shi S.S., Shi X.T., Zhang D.Y. A study on swimming ability of Schizopygopsis malacanthus. J. Hydroecol. 2021;7 doi: 10.15928/j.1674-3075.202012030335. [DOI] [Google Scholar]
- 25.Zhong Z.Y., Shi X.T., Tan J.J., Ke S.F., Li Z.M., Lei Q.S., Cheng B.X., Shi X.L. Fishway velocity design analysis based on fish swimming ability. J. Hydroecol. 2021;42:92–99. doi: 10.15928/j.1674-3075.201910080249. [DOI] [Google Scholar]
- 26.Lin S.C., Bai Y.L., Xiong J.L. Effects of dietary selenium on growth performance and blood parameters of juvenile Yellow River carp. Hubei Agric. Sci. 2022;61:121–126. doi: 10.14088/j.cnki.issn0439-8114. 2022.04.024. [DOI] [Google Scholar]
- 27.Fu B.R., Liu M.Q., Zhang R.J., Zhang N., Ren J. Effects of Brevibacillus brevis on aquiculture water quality and carps growth characteristics. Ecolog. Sci. 2018;37:146–151. doi: 10.14108/j.cnki.1008-8873. 2018.03.019. [DOI] [Google Scholar]
- 28.Xia D.Y., Shen C., Li Q., Zhu X.L., Luo Y.P. Resting Metabolism,Swimming ability and growth performance of juvenile Cyprinus carpioin individual-fed and group-fed bonditons. J. Chongqing Normal Univ. (Nat. Sci. Ed.) 2019;36:36–40. doi: 10.11721/cqnuj20190605. [DOI] [Google Scholar]
- 29.Tudorache C., Viaene P., Blust R., Vereecken H., De Boeck G. A comparison of swimming capacity and energy use in seven European freshwater fish species. Ecol. Freshw. Fish. 2008;17(2):284–291. doi: 10.1111/j.1600-0633.2007.00280.x. [DOI] [Google Scholar]
- 30.Jin Z.J., Ma W.Z., Shi X.T. Assessing the swimming ability and performance of Schizothorax oconnori to cross velocity barriers in fishway. J. Hydraul. Eng. 2018;49(4):512–522. doi: 10.13243/j.cnki.slxb.20171067. [DOI] [Google Scholar]
- 31.Li Z.M., Chen M.X., Jin Z.J., Shi X.T. Swimming ability of Schizothorax irregularis ddy in yarkand river. Chin. J. Ecol. 2018;37(6):1897–1902. doi: 10.13292/j.1000-4890.201806.005. [DOI] [Google Scholar]
- 32.Tan J.J., Gao Z., Dai H.C., Shi X.T. The correlation analysis between hydraulic characteristics of vertical slot fishway and fish movement characteristics. J. Hydraul. Eng. 2017;48(8):924–932. doi: 10.13243/j.cnki.slxb.20160822. [DOI] [Google Scholar]
- 33.Qi L., Yang Y., Wang Y. Fish behavior characteristics in response to change of hydrodynamic environment. J. Hohai Univer.(Natural Sciences) 2012;40(4):438–445. doi: 10.3876/j.issn.1000-1980. 2012.04.014. [DOI] [Google Scholar]
- 34.Huang Z.L., Chang J.B. Fractal characteristics of length-weight relationship in fish. Acta Hydrobiol. Sin. 1999;23:330–336. doi: 10.3321/j.issn:1000-3207.1999.04.006. [DOI] [Google Scholar]
- 35.Sanz-Ronda F.J., Ruiz-Legazpi J., Bravo-Córdoba F.J., Makrakis S., Castro-Santos T. Sprinting performance of two Iberian fish: luciobarbus bocagei and Pseudochondrostoma duriense in an open channel flume. Ecol. Eng. 2015;83:61–70. doi: 10.1016/j.ecoleng.2015.05.033. [DOI] [Google Scholar]
- 36.Castro-Santos T., Sanz-Ronda F.J., Ruiz-Legazpi J. Breaking the speed limit — comparative sprinting performance of brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta) Can. J. Fish. Aquat. Sci. 2013;70(2):280–293. doi: 10.1139/cjfas-2012-0186. [DOI] [Google Scholar]
- 37.Peake S.J. Gait transition speed as an alternate measure of maximum aerobic capacity in fishes. J. Fish. Biol. 2008;72(3):645–655. doi: 10.1111/j.1095-8649.2007.01753.x. [DOI] [Google Scholar]
- 38.Zhang S., Chen Y. Preliminary study on the rheotaxis of juvenile Sebastodes fuscescens. J. Shanghai Fish. Univ. 2005;(3):3282–3287. doi: 10.3969/j.issn.1004-7271.2005.03.011. [DOI] [Google Scholar]
- 39.Gong L., Wu Y.H., Baiyin B.L.G., Mu X.P. Experimental study on swimming capability and swimming behavior of juvenile grass carp. J. China Inst. Water Resour. Hydropower Res. 2015;13:211–216. doi: 10.13244/j.cnki.jiwhr. 2015.03.010. [DOI] [Google Scholar]
- 40.Xian X.M., Cao Z.D., Fu S.J. The comparison of critical swimming speed and endurance at high speed of four species of juvenile fish. J. Chong. Nor. Univer.(Natural Science Edition) 2010;27:16–20. doi: 10.3969/J.ISSN.1672-6693. 2010.04.005. [DOI] [Google Scholar]
- 41.Liu H.J., Wang C.F., Zhu L.K., Chen M.M. Comparative study of critical swimming speeds for juvenile silver and bighead carp. J. Hydroecol. 2016;37:63–69. doi: 10.15928/j.1674-3075. 2016.04.010. [DOI] [Google Scholar]
- 42.Xiong F., Wang C.F., Liu D.F. Comparative study of burst swimming speed of black carp,grass carp,silver carp and bighead carp from Songhua River. Ecolog. Sci. 2014;33:339–343. doi: 10.3969/j.issn.1008-8873. 2014.02.022. [DOI] [Google Scholar]
- 43.Bai Y.Q., Lu B., Luo J. Induction velocity of juvenile grass carp, silver carp, and darkbarbel catfish. Chin. J. Ecol. 2013;32:2085–2089. [Google Scholar]
- 44.Cai L., Tu Z.Y., Yuan X., Liu G.Y., Liu D.F., Shi X.T., Huang Y.P. Swimming capability and swimming behaviour of juvenile aristichthys nobilis. Resour. Environ. Yangtze Basin. 2012;21:89–95. [Google Scholar]
- 45.Castro-Santos T., Haro A. Science Publishers; Enfield, UK: 2010. Fish Guidance and Passage at Barriers. [DOI] [Google Scholar]