Abstract
Background
Anticoagulant therapy has become a hallmark of treatment for critically ill COVID patients. Gastrointestinal and intracranial hemorrhage are known major complications of anticoagulation, but spontaneous hemothorax is a rare event, particularly in the absence of pre-existing structural lung disease, vascular malformations, or genetic bleeding diatheses. Herein is a case of spontaneous hemothorax following anticoagulation for microthrombi in a patient with acute hypoxic respiratory failure due to Covid pneumonia.
Case presentation
A 49 year old male with hypertension, asthma, and obesity was admitted for acute hypoxic respiratory failure due to Covid pneumonia. He was treated with dexamethasone, baricitinib, and therapeutic enoxaparin as empiric therapy for severe COVID disease. He subsequently developed a massive right hemothorax with associated hemorrhagic shock, which required initiation of massive transfusion protocol, vasopressor support and mechanical ventilation. No clear etiology for the hemothorax was determined upon investigations. The patient eventually improved and was discharged to a skilled nursing facility on chronic oxygen therapy.
Conclusions
Various mechanisms have been proposed for the development of non-traumatic hemothoraces, including tearing of adhesions and rupture of vascularized bullae. Such explanations find support in radiologic and pathologic studies of pleural changes in Covid pneumonia, and likely played a role in the hemorrhage experienced by our patient.
Keywords: Spontaneous hemothorax, Hemothorax, Covid, Anticoagulation complications
1. Background
Coronavirus 2019 disease (COVID) is the result of infection by the severe acute respiratory syndrome coronavirus 2. The disease states caused by COVID range from asymptomatic, to mild upper respiratory and gastrointestinal symptoms, to severe disease, which primarily manifests as pneumonia, but can also result in severe hematologic and neurologic dysfunction [1]. The most common form of hematologic dysfunction is venous thromboembolism (VTE), which is thought to occur due to endothelial damage in tandem with an uncontrolled immune response (“cytokine storm”) [2]. D-dimer levels have been shown to be associated with both disease severity and risk of VTE [3,4]. After several clinical trials, anticoagulant therapy has become a hallmark of treatment for critically ill COVID patients [5]. Previously described complications of anticoagulation include gastrointestinal and intracranial bleeding [6], muscular hematomas [7], in rare cases, spontaneous hemothorax [8].
Hemothorax is defined as a collection of blood in the pleural cavity with a pleural fluid hematocrit that is 50% or greater than the peripheral hematocrit, although the chronicity of the effusion can modulate this percentage [9]. Most instances of hemothorax are traumatic, with spontaneous etiologies accounting for only 1.7% [10] of hemothoraces. Previous studies have found that hemothorax occurred in 3–7% of pneumothoraces [11], and may be potentiated by anticoagulant use [9,12].
The present case report describes the occurrence of a spontaneous hemothorax (SH) in a patient who was treated with therapeutic anticoagulation for COVID pneumonia.
2. Case presentation
A 49 year old male with hypertension, asthma, morbid obesity (BMI 37), was admitted for acute hypoxic respiratory failure due to Covid pneumonia in February 2022. Physical exam did not reveal any abnormal skin lesions. Chest imaging demonstrated opacities, but no bullae or other evidence of chronic lung disease. He was treated with dexamethasone, baricitinib, and enoxaparin at 1mg/kg every 12 hours as empiric therapy for severe COVID disease.
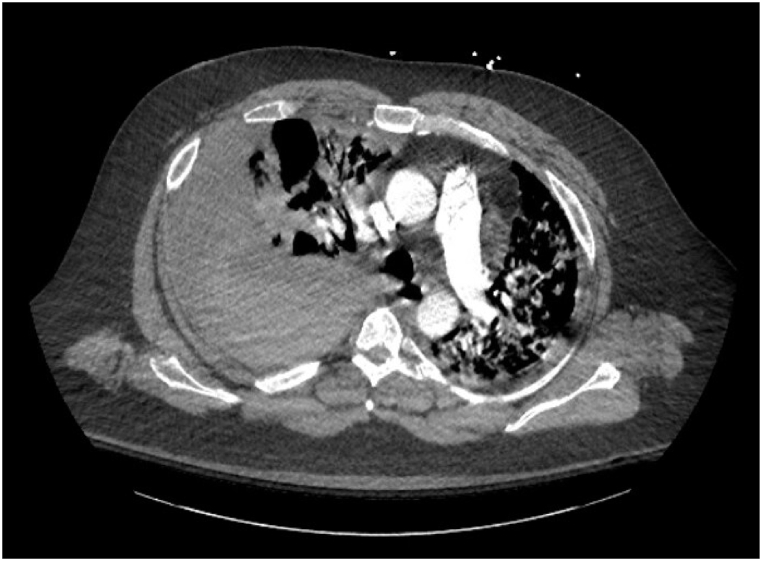
His hospital course was complicated by a mediastinal hematoma and massive right hemothorax with associated hemorrhagic shock, which required tube thoracostomy insertion, activation of a massive transfusion protocol, vasopressor support and transfer to the intensive care unit. CT angiography of the chest did not demonstrate any vascular abnormalities (Fig. 1). His respiratory status deteriorated while in the ICU, requiring orotracheal intubation and 3 days of mechanical ventilation. After being transferred back to the medical floor, he was found to have a left brachial vein deep vein thrombosis. He was placed on a heparin drip and, once it was clear that no further bleeding was occurring, transitioned to apixaban. He was discharged to a nursing facility for rehabilitation, with chronic oxygen supplementation. On 3-month follow up in Pulmonology clinic, the patient had been weaned from oxygen and was able to ambulate for ¼ mile without stopping.
Fig. 1.
Thoracic CT angiogram obtained immediately upon diagnosis of hemothorax.
3. Discussion and conclusions
The current NIH Guidelines’ recommendation for empiric anticoagulation (with preference for enoxaparin) in hospitalized, non-critically ill patients with COVID pneumonia [13] renders careful consideration of hemorrhage risks vitally important. While SH is a rare clinical entity, it can confer significant morbidity and mortality [9].
Previous case reports and case series of SH have noted risk factors of genetic abnormalities, such as Hereditary Hemorrhagic Telangiectasia or von Recklinghausen disease [14,15]; arterial aneurysms or other malformations [12,16]; and vessel wall weakening due to acquired states, such as amyloid angiopathy or angiosarcoma [12,17]. The few cases of SH described in patients with COVID have noted contributory factors such as ECMO-induced coagulopathy and pulmonary aneurysms [18], barotrauma [19], and intercostal artery bleeding [20,21]. However, Jung et al. described a protracted case of COVID-induced critical illness in which SH could only be attributed to necrotizing pneumonia [22]. The patient in the present case did not demonstrate any of these features.
Hsu et al., in a historical analysis of SH case series, proposed three mechanisms for hemorrhage in cases of pneumothorax [1]: tearing of adhesions between parietal and visceral pleurae [2], rupture of vascularized bullae or parenchyma, and [3] tearing of congenitally present aberrant vessels [11]. Although forensic pathology investigations into SH have been sparse, a 2014 review considered these mechanisms plausible [23]. Indeed, given the severe parenchymal inflammation as well as pleural edema and adhesions observed in both radiologic [24,25] and pathologic [[26], [27], [28]] studies of COVID pathophysiology, adhesional traction seems to be reasonable explanation for our patient's SH.
Additional potential contributory elements in this patient's SH include the less predictable pharmacokinetics of enoxaparin in obese patients [29] as well as the effect of concomitant administration of baricitinib.
COVID presents clinicians with the challenge of a multi-system disease that probes the extent of medical knowledge. The above case illustrates the need for further research into the interactions between different organ systems in order to elucidate the optimal treatment plan for individual patients.
Ethics approval and consent to participate
According to the RUHS IRB, no need for consent or IRB approval was warranted for this case report.
Consent for publication
Written informed consent for publication was obtained from the patient discussed in this article.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
AF analyzed and interpreted the clinical data, performed a review of the literature, and was a major contributor in writing the manuscript. MU analyzed and interpreted the clinical data and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Authors’ information
AF and MU are a hospital based internists (hospitalists) at Riverside University Health Systems Medical Center in Riverside, California, USA. Both hold appointments as Assistant Professors, at the University of California at Riverside and Loma Linda University Medical Center.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Alexander Friedman, Email: DrAlexFriedman12@gmail.com.
Michael Ulrich, Email: m.ulrich.md@gmail.com.
References
- 1.Stawicki S.P., Jeanmonod R., Miller A.C., et al. The 2019-2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: a joint American college of academic international medicine-world academic council of emergency medicine multidisciplinary COVID-19 working group consensus paper. J. Global Infect. Dis. 2020;12(2):47–93. doi: 10.4103/jgid.jgid_86_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bösmüller H., Matter M., Fend F., Tzankov A. The pulmonary pathology of COVID-19. Virchows Arch. 2021;478(1):137–150. doi: 10.1007/s00428-021-03053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu, Qin, Chen, Wang, Tian D-dimer level is associated with the severity of COVID-19. Thromb Res. 2020 Nov;195:219–225. doi: 10.1016/j.thromres.2020.07.047. https://www.sciencedirect.com/science/article/pii/S0049384820303388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nauka P.C., Baron S.W., Assa A., et al. Utility of D-dimer in predicting venous thromboembolism in non-mechanically ventilated COVID-19 survivors. Thromb. Res. 2021;199:82–84. doi: 10.1016/j.thromres.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N. Engl. J. Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piran S., Schulman S. Treatment of bleeding complications in patients on anticoagulant therapy. Blood. 2019;133(5):425–435. doi: 10.1182/blood-2018-06-820746. [DOI] [PubMed] [Google Scholar]
- 7.Rogani S., Calsolaro V., Franchi R., Calabrese A.M., Okoye C., Monzani F. Spontaneous muscle hematoma in older patients with COVID-19: two case reports and literature review. BMC Geriatr. 2020;20(1):539. doi: 10.1186/s12877-020-01963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azfar Ali H., Lippmann M., Mundathaje U., Khaleeq G. Spontaneous hemothorax: a comprehensive review. Chest. 2008;134(5):1056–1065. doi: 10.1378/chest.08-0725. [DOI] [PubMed] [Google Scholar]
- 9.Zeiler J., Idell S., Norwood S., Cook A. Hemothorax: a review of the literature. Clin. Pulm. Med. 2020;27(1):1–12. doi: 10.1097/CPM.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schweigert M., Beron M., Dubecz A., Stadlhuber R., Stein H. Video-assisted thoracoscopic surgery for posttraumatic hemothorax in the very elderly. Thorac. Cardiovasc. Surg. 2012;60(7):474–479. doi: 10.1055/s-0031-1298069. [DOI] [PubMed] [Google Scholar]
- 11.Hsu N.Y., Shih C.S., Hsu C.P., Chen P.R. Spontaneous hemopneumothorax revisited: clinical approach and systemic review of the literature. Ann. Thorac. Surg. 2005;80(5):1859–1863. doi: 10.1016/j.athoracsur.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 12.Patrini D., Panagiotopoulos N., Pararajasingham J., Gvinianidze L., Iqbal Y., Lawrence D.R. Etiology and management of spontaneous haemothorax. J. Thorac. Dis. 2015;7(3):520–526. doi: 10.3978/j.issn.2072-1439.2014.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/clinical-data. Published February 24, 2022. Accessed August 21, 2022.
- 14.Parrilla Quinones F., Marmorato Rivera R., Calderon R.E., Vargas Otero P., Hidalgo Rios A. Spontaneous hemothorax: first manifestation of pulmonary arteriovenous malformation in a patient with hereditary hemorrhagic telangiectasia. Bol. Asoc. Med. P. R. 2011;103(4):41–44. [PubMed] [Google Scholar]
- 15.Garg S.K., Malik A. Spontaneous hemothorax and implication of diagnostic evaluation: a case report. Respir Med Case Rep. 2022;36 doi: 10.1016/j.rmcr.2022.101604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haridas K.K., Neeraakal G.M., Moorthy S., Prabhu N.K., Kumar V. Ruptured idiopathic pulmonary artery aneurysm: unusual case of hemothorax treated by selective embolization. Indian Heart J. 2001;53(6):769–772. [PubMed] [Google Scholar]
- 17.Liu J., Closser D., Satoskar A., Stasek J. Spontaneous hemothorax: an unusual presentation of a rare disease. Chest. 2013;144(4):495A. [Google Scholar]
- 18.Desnos C., Boussouar S., Hekimian G., Redheuil A., Combes A. Spontaneous hemothorax in 4 COVID-19 ARDS patients on VV-ECMO revealing pulmonary artery aneurysms. Crit. Care. 2020;24(1):638. doi: 10.1186/s13054-020-03359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guven B.B., Erturk T., Kompe Ö., Ersoy A. Serious complications in COVID-19 ARDS cases: pneumothorax, pneumomediastinum, subcutaneous emphysema and haemothorax. Epidemiol. Infect. 2021;149:e137. doi: 10.1017/S0950268821001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan S.A., Fadzaily Z.S., Abdullah Hashim S.H. Spontaneous haemothorax in a patient with COVID-19. Case Rep. Med. 2022:2022. doi: 10.1155/2022/8275326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long A., Grimaldo F. Spontaneous hemopneumothorax in a patient with COVID-19. Am. J. Emerg. Med. 2021;40 doi: 10.1016/j.ajem.2020.07.065. 228.e1-e228.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung C., Gillmann H.J., Stueber T., Hinken L. Spontaneous massive hemothorax as a complication of necrotizing pneumonia in a patient with severe acute respiratory syndrome coronavirus 2 induced acute respiratory distress syndrome: a case report. J. Med. Case Rep. 2021;15(1):444. doi: 10.1186/s13256-021-03032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janik M., Straka L., Krajcovic J., Hejna P., Hamzik J., Novomesky F. Non-traumatic and spontaneous hemothorax in the setting of forensic medical examination: a systematic literature survey. Forensic Sci. Int. 2014;236:22–29. doi: 10.1016/j.forsciint.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Saha B.K., Chong W.H., Austin A., et al. Pleural abnormalities in COVID-19: a narrative review. J. Thorac. Dis. 2021;13(7):4484–4499. doi: 10.21037/jtd-21-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichter Y., Topilsky Y., Taieb P., et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020;46(10):1873–1883. doi: 10.1007/s00134-020-06212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C., Xie J., Zhao L., et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arslan M.N., Büyük Y., Ziyade N., et al. COVID-19 autopsies of Istanbul. Ir. J. Med. Sci. 2022;191(2):529–541. doi: 10.1007/s11845-021-02602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curry M.A., LaFollette J.A., Alexander B.R., Evans K.S., Tran R.H., Kempton C.L. Evaluation of treatment-dose enoxaparin in acutely ill morbidly obese patients at an academic medical center: a randomized clinical trial. Ann. Pharmacother. 2019;53(6):567–573. doi: 10.1177/1060028018821149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.