Abstract
Background
Post-stroke care guidelines highlight continued rehabilitation as essential; however, many stroke survivors cannot participate in outpatient rehabilitation. Technological advances in wearable sensing, treatment algorithms, and care delivery interfaces have created new opportunities for high-efficacy rehabilitation interventions to be delivered autonomously in any setting (ie, clinic, community, or home).
Methods
We developed an autonomous rehabilitation system that combines the closed-loop control of music with real-time gait analysis to fully automate patient-tailored walking rehabilitation. Specifically, the mechanism-of-action of auditory-motor entrainment is applied to induce targeted changes in the post-stroke gait pattern by way of targeted changes in music. Using speed-controlled biomechanical and physiological assessments, we evaluate in 10 individuals with chronic post-stroke hemiparesis the effects of a fully-automated gait training session on gait asymmetry and the energetic cost of walking.
Results
Post-treatment reductions in step time (Δ: −12 ± 26%, P = .027), stance time (Δ: −22 ± 10%, P = .004), and swing time (Δ: −15 ± 10%, P = .006) asymmetries were observed together with a 9 ± 5% reduction (P = .027) in the energetic cost of walking. Changes in the energetic cost of walking were highly dependent on the degree of baseline energetic impairment (r =− .90, P < .001). Among the 7 individuals with a baseline energetic cost of walking larger than the normative value of healthy older adults, a 13 ± 4% reduction was observed after training.
Conclusions
The closed-loop control of music can fully automate walking rehabilitation that markedly improves walking after stroke. Autonomous rehabilitation delivery systems that can safely provide high-efficacy rehabilitation in any setting have the potential to alleviate access-related care gaps and improve long-term outcomes after stroke.
Keywords: digital therapeutic, music, rhythm, walking, symmetry, economy
Introduction
Post-stroke neuromotor deficits result in gait asymmetries and an increased energetic cost of walking.1-3 Such deficits in walking quality often persist beyond standard rehabilitation efforts and result in reduced physical activity.4,5 Care guidelines highlight continued rehabilitation as essential to improve mobility and function and mitigate costly future morbidities 6 ; however, most stroke survivors do not participate in outpatient rehabilitation, in part due to limited access to skilled care and disparities in transportation availability and time. 7
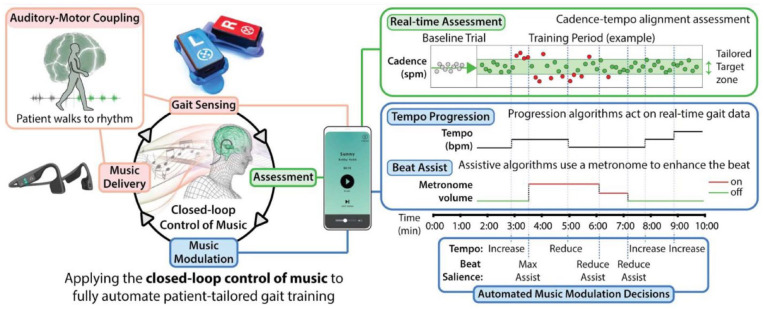
Technological developments in high fidelity wearable sensing, automated treatment algorithms, and novel care delivery interfaces8,9 have created new opportunities for high-efficacy interventions to be delivered autonomously in any setting (ie, clinic, home, or community). We present the closed-loop control of music (Figure 1) as a novel approach to leveraging such advancements to autonomously provide highly salient and targeted, patient-tailored. Here, “closed-loop” refers to the autonomous control of music, driven by input from foot sensors as the participant walks. More specifically, the autonomous rehabilitation system integrates real-time gait sensing with music-based treatment algorithms to enable the application of auditory-motor entrainment for enhancing post-stroke walking. 10
Figure 1.
Patient-tailored walking rehabilitation fully automated via the closed-loop control of music. Real-time gait sensing and assessment enable music modulation algorithms to apply the mechanism-of-action of auditory-motor entrainment to fully automate patient-tailored walking rehabilitation after stroke. Continuous gait assessments individualize the rehabilitation to the user’s baseline walking abilities and facilitate safe progression.
Plainly, auditory-motor entrainment is why people can effortlessly synchronize their movements (ie, entrain) to the beat of an external rhythm.11-15 Indeed, auditory-motor neural circuits cause rhythmic motor output to be attracted to, and eventually lock on to, the frequency of an external rhythmic auditory signal.16-19 Our autonomous rehabilitation system applies the neurobiological process of auditory-motor entrainment to induce targeted and personalized changes in the post-stroke gait pattern. More specifically, the rhythm of music is purposefully modulated based on continuous, real-time gait assessments, resulting in gait training autonomously tailored to each user’s gait.
The profound effect that rhythmic auditory stimuli have on neuromotor control12,15,20-23 has led to development and study of numerous clinical interventions centered on auditory-motor entrainment. The efficacy of these interventions has been extensively studied in people post-stroke, with their ability to improve walking well-established,24-36 resulting in their recommendation 37 and inclusion 38 in published clinical practice guidelines. However, as with any skilled intervention, clinical benefits can only be realized if the intervention is readily accessible. The objective of this foundational study is to determine if gait training autonomously delivered by way of the closed-loop control of music has potential to improve walking quality after stroke, as measured by improvements in the energetic cost of walking and gait asymmetry.
Methods
Overview
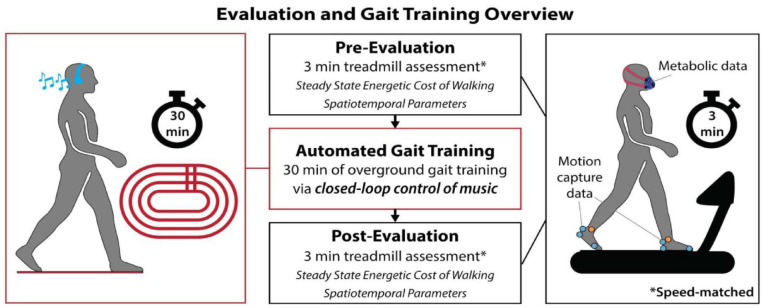
In brief, 10 individuals with chronic post-stroke hemiparesis (Table 1) completed 1 session of overground gait training fully automated by an autonomous rehabilitation system centered on the closed-loop control of music (see Supplemental Movie S1). Sessions were approximately 3 hours long with pre- and post-evaluations occurring immediately before and after the 30-minute overground training. These evaluations consisted of 4-minutes of quiet sitting to identify baseline oxygen consumption, overground walks to measure speed, and 3-minute speed-controlled treadmill trials to quantify biomechanical and physiological changes in walking quality (Figure 2). Participants used their usual device during all walking activities.
Table 1.
Participant Baseline Characteristics.
| ID | Side of paresis | Sex | Age (y) | Time since stroke (y) | Functional gait assessment (out of 30) | Usual device | Walking speed (m/s) | Energy cost of walking (ml O2/kg/m) | Spatiotemporal asymmetry | Cadence (steps/min) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Step time | Stance time | Swing time | Step length | ||||||||||
| 1 | Left | M | 46.5 | 12.6 | 20 | None | 1.43 | 0.24 | 0.15 | 0.04 | 0.08 | 0.04 | 121.9 |
| 2 | Right | M | 36.8 | 7.4 | 19 | AFO | 0.96 | 0.18 | 0.11 | 0.03 | 0.06 | 0.09 | 96.8 |
| 3 | Right | M | 55.5 | 3.1 | 12 | AFO | 0.64 | 0.24 | 0.03 | 0.05 | 0.12 | 0.02 | 80.2 |
| 4 | Right | M | 66.8 | 21.5 | 13 | None | 0.60 | 0.36 | 0.14 | 0.03 | 0.08 | 0.11 | 87.4 |
| 5 | Right | F | 57.0 | 9.6 | 24 | None | 1.00 | 0.20 | 0.17 | 0.02 | 0.04 | 0.11 | 105.5 |
| 6 | Left | M | 66.5 | 9.2 | 15 | AFO | 0.45 | 0.34 | 0.07 | 0.05 | 0.10 | 0.01 | 76.5 |
| 7 | Left | M | 77.2 | 7.1 | 18 | Bioness | 1.03 | 0.22 | 0.01 | 0.01 | 0.02 | 0.02 | 105.3 |
| 8 | Right | M | 35.0 | 5.2 | 28 | None | 1.30 | 0.18 | 0.10 | 0.04 | 0.08 | 0.02 | 109.6 |
| 9 | Left | F | 63.4 | 7.6 | 27 | None | 1.37 | 0.16 | 0.05 | 0.02 | 0.04 | 0.01 | 116.1 |
| 10 | Right | F | 64.0 | 7.4 | — | AFO and SBQC | 0.43 | 0.45 | 0.08 | 0.02 | 0.05 | 0.06 | 67.7 |
| Med | — | — | 60.2 | 7.50 | 19 | — | 0.98 | 0.23 | 0.09 | 0.03 | 0.07 | 0.03 | 101.0 |
| Semi-IQR | — | — | 8.6 | 1.16 | 4.50 | — | 0.31 | 0.07 | 0.04 | 0.01 | 0.02 | 0.03 | 13.3 |
Abbreviations: Med, median; y, year; m/s, meters/second; M, male; F, female; AFO, ankle-foot orthosis; SBQC, small base quad cane; IQR, interquartile range.
Figure 2.
Experimental procedures overview. Study participants completed 30 minutes of overground gait training automated via the closed-loop control of music, as described in Figure 1. Speed-controlled biomechanical and physiological evaluations were conducted immediately before and after the training to characterize changes in walking quality, as measured by the energetic cost of walking and gait asymmetry.
*Walking speed was experimentally controlled across the pre-training and post-training evaluations.
We expected that the autonomously-delivered gait intervention would induce improvements in both gait asymmetry and the energetic cost of walking. Gait asymmetry was quantified using inter-limb measurements of the spatiotemporal determinants of walking speed (ie, stance, swing, and step times, as well as step lengths). The energetic cost of walking was quantified using mass and speed-normalized oxygen consumption (ie, ml O2/kg/m), with each study participant’s energetic impairment measured as the difference between their energetic cost of walking and the average normative value for older adults (ie, 0.168 ml O2/kg/m). 39 For both the walking asymmetry and energetic impairment outcomes, values greater than zero represent a deviation from normal. Reductions in these metrics indicate more symmetrical and more economical walking, with a reduction to zero suggesting a full restoration of walking quality.
Study Participants
Eligible study participants were between 18 and 80 years old, greater than 6 months post-stroke, and able to walk independently with or without an assistive device. Exclusion criteria included severe aphasia impairing communication (score >1 on NIHSS question 1b and >0 on question 1c), pain that impaired walking, neglect, hemianopia, unexplained dizziness in the previous 6 months, resting heart rate outside of the range of 50 to 100 bpm, and resting blood pressure outside of the range of 90/60 to 200/110 mmHg. The study was approved by the Boston University Institutional Review Board. All study participants provided written informed consent.
Automated Gait Rehabilitation Delivered Via the Closed-Loop Control of Music
The safe and efficacious delivery of gait rehabilitation based on auditory-motor entrainment has historically required in-clinic care by therapists with advanced training in adapting standard treatment protocols to meet the needs of individual patients. Applying the principles of auditory-motor entrainment requires that the rhythmic stimuli be individualized and progressed according to an initial assessment of the patient’s baseline walking ability and continuous assessments of their ability to safely entrain to progressively more challenging rhythmic stimuli. Moreover, different parameters of the rhythmic stimuli—from the tempo and salience of the auditory cues to the familiarity of the music—are modified by the clinician to enhance entrainment and maximize treatment outcomes.40,41 Our autonomous rehabilitation system combines real-time gait sensing with adaptive music algorithms to fully automate gait training that is individualized to the immediate and evolving needs of each patient and can be delivered without direct input from a clinician.
Study participants completed one 30-minute overground gait rehabilitation session fully automated by the closed-loop control of music. Music during the session was delivered via bone conduction headphones (AfterShokz AS451OB Sportz Titanium Open Ear Wired, Austin, TX, USA) and was the same for all users. Songs were screened for consistent beat saliency and rhythmic stability, and the playlist spanned various tempos and musical genres. Gait data were collected by wireless inertial sensors (3D gyroscope, 2000°/second) clipped to each shoe. The automated treatment algorithms were run on a locked mobile device pre-loaded with the music (Figure 1, left). 42
More specifically, the system’s inertial sensors enable the real-time assessment of the user’s walking cadence (ie, steps per minute, or spm) and the prescribed tempo of the music (ie, beats per minute, or bpm), and thus the step-to-beat period alignment. These data are used as inputs in algorithms that modulate features of the music—that is, its tempo and beat salience (Figure 1, right). Within the algorithms, each stride is assigned an entrainment precision score, as previously described. 42 When a user has successfully entrained, the tempo of the song is automatically increased. When a user fails to entrain, the volume of the music and a synchronized metronome are adjusted to support enhanced audibility of the beat. More specifically, the algorithm automatically cycles through different levels of metronome audibility based on what the user needs to restore entrainment. When the user begins to entrain again, the algorithm returns to a music-only experience. Because this feature of the algorithm is triggered only when the user fails to entrain, the duration of metronome enhancement varies across participants and sessions. The goal of these automated changes is to induce auditory-motor entrainment to progressively faster tempos, and thus facilitate faster and more symmetrical stepping (see Supplemental Movie S1).
Data Collection
Study participants completed speed-controlled, 3-minute evaluation trials on the treadmill immediately before and after the automated gait training. To isolate training-induced changes in gait asymmetry and the energetic cost of walking from training-induced changes in walking speed, the same treadmill speed was used for both the pre-training and post-training evaluation trials. This speed was set to each study participant’s comfortable walking speed measured during a 10-meter Walk Test prior to the training. A fall-prevention harness and heart rate monitor were worn during all walking activities as a safety precaution, and blood pressure was measured at baseline and monitored throughout the visit. Handrail use during treadmill walking was matched across pre- and post-training evaluation trials. Gait asymmetry and oxygen consumption data were collected concurrently during the evaluation trials (Figure 2).
Data Processing
Primary and Exploratory Gait Kinematics
Kinematic data were collected during the treadmill evaluation trials using an 18-camera motion analysis system (Qualisys, Göteborg, Sweden) at 200 Hz, except for 3 individuals whose data were collected at 100 Hz due to technical difficulties; collection frequencies were consistent within a participant. Marker data were low-pass filtered at 10 Hz with a fourth order Butterworth filter 43 using commercial biomechanics software (Visual3D, C-Motion, Inc. Germantown, MD, USA). Heelstrike and toe-off gait events were identified using markers placed on the heel and fifth metatarsal, respectively, and a velocity-based algorithm as described by Zeni et al. 44 In brief, heelstrike was identified when the velocity of the heel marker in the anterior-posterior direction transitioned from negative to positive, and toe-off was identified when the velocity of the fifth metatarsal marker transitioned from negative to positive in the anterior-posterior direction. Heelstrike and toe-off gait events were used to segment the walking data and compute pre- and post-training step length, step time, stance time, swing time, and cadence using custom Visual3D scripts. 45 These parameters were calculated as the average of 20 steps that were time-matched to the oxygen consumption data.
For our primary analyses, asymmetries for step length, step time, stance time, and swing time were calculated using the following equation, such that a value of 0 denotes perfect symmetry across limbs46-48:
A reduction in the asymmetry ratio indicates an improvement in gait symmetry.
To complete an exploratory post-hoc analysis of changes in gait variability, standard deviation and the coefficient of variation (ie, standard deviation/mean) were calculated for step length, step time, stance time, and swing time.
Energetic Cost of Walking
Oxygen consumption (VO2) data were collected on a breath-by-breath basis (Cosmed© K5, Rome, Italy) during each of the treadmill evaluation trials, as well as the 4 minutes of quiet sitting that preceded each trial. The steadiest 30-second window of VO2 data (based on standard deviation) from the final 90 seconds of each treadmill evaluation trial was selected for analysis. To ensure steady-state had been reached, the standard deviation of the data in the 30-second window had to be ≤2.0 ml O2/kg/minutes. 49 VO2 NET was calculated by subtracting the average VO2 from the last 2 minutes of quiet sitting (ie, VO2 REST) from the average VO2 of the selected 30-second steady-state window. VO2 NET was used in all analyses and normalized 49 by body weight (kg) and walking speed (m/minutes) to yield the energetic cost of walking, expressed as the net VO2 per meter walked (ml O2/kg/m).
Energetic Impairment
Because biological walking will always incur a metabolic cost, we consider reducing the energetic cost of walking to within the range of what is seen in healthy older adults as the target for intervention. To quantify each participant’s energetic impairment we computed the difference between their energetic cost of walking and the average value measured in healthy older adults (0.168 ml O2/kg/minutes, 95% confidence interval [0.156, 0.180] 39 ). An energetic impairment value of 0 indicates an energetic cost of walking equivalent to that of the average older adult.
Using their pre-training energetic impairment data, study participants were separated into 2 subgroups: those with and those without an energetic impairment. Individuals whose energetic cost of walking prior to training was within the 95% confidence interval of healthy older adults were classified as without an energetic impairment. In contrast, individuals whose energetic cost of walking was above the upper bound of the 95% confidence interval were classified as with an energetic impairment.
Statistical Analyses
Analyses were conducted using RStudio Version 1.3.1056. Data are presented as median ± semi-interquartile range, unless indicated. Alpha was set at 0.05.
Wilcoxon Signed-Rank Tests were used to evaluate differences between pre- and post-training measurements of gait asymmetry, gait variability, and the energetic cost of walking. Custom processing scripts (MATLAB, Mathworks, Natick, MA) were used to compute the change and percent change metrics. We report the percent change (%Δ), test statistic (W), P-value (P), and effect size r, where 0.10 to <0.3 is a small effect, 0.30 to 0.5 is a moderate effect and >0.5 is a large effect.
Spearman’s rank-order correlations were used to explore the relationship between (1) the percent change in each measure of gait asymmetry and baseline gait parameters, (2) the percent change in the energetic cost of walking and comfortable walking speed measured at baseline, and (3) the percent change in the energetic cost of walking and the energetic cost of walking measured at baseline. Energetic impairment subgroup analyses were also conducted to compare the post-training percent change in the energetic cost of walking between participants with and without a baseline impairment.
Results
Participant Baseline Characteristics
Study participants were 60.2 ± 8.6 years old and 7.5 ± 1.2 years post-stroke. A wide range of gait impairment was observed, with a median energetic impairment of 0.06 ml O2/kg/m (range: −0.01 to 0.29 ml O2/kg/m) above the normative value of 0.168 ml O2/kg/m and interlimb asymmetry ratios from 0.01 to 0.17 across the spatiotemporal parameters (see Table 1).
Automated Training Via the Closed-Loop Control of Music Results in More Symmetric Walking
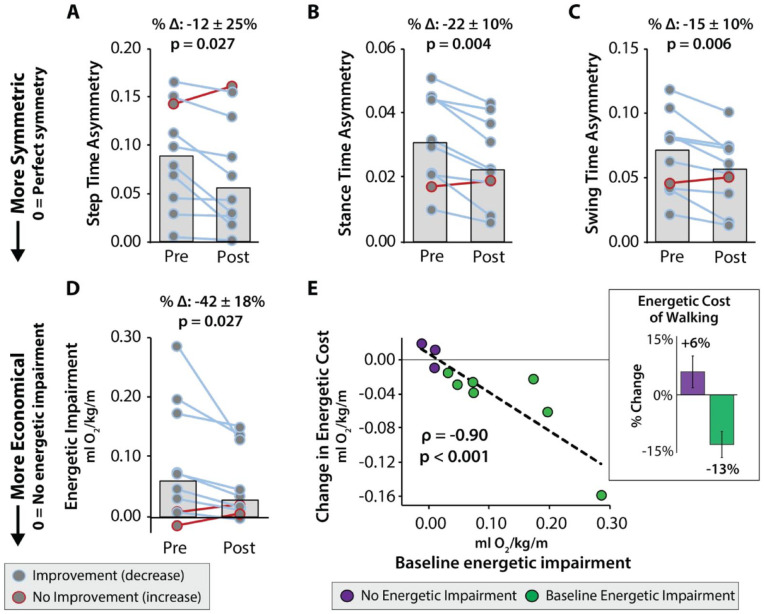
Study participants had a median baseline step time asymmetry of 0.09 ± 0.04, stance time asymmetry of 0.03 ± 0.01, swing time asymmetry of 0.07 ± 0.02, step length asymmetry of 0.03 ± 0.03, and cadence of 101.04 ± 13.3 steps per minute. At the group-level, significant large reductions in step time asymmetry (%Δ: 12.4 ± 25.7%, W = 6, P = .027, effect size r = .693), stance time asymmetry (%Δ: 21.8 ± 9.7%, W = 1, P = .004, effect size r = .854), and swing time asymmetry (%Δ: 15.4 ± 10.3%, W = 2, P = .006, effect size r = .822) were observed following training (Figure 3A-C). There were no observed significant changes in step length asymmetry (Δ: 22.4% ± 19.8%, W = 42, P = .16, effect size r = .467) and cadence (Δ: 0.4 ± 1.1%, W = 22, P = .625, effect size r = .177), nor in any of the individual limb spatiotemporal metrics (P > .05). There were also no observed significant changes in gait variability (P > .05). Changes in gait asymmetry were not related to study participants’ baseline characteristics (P > .05). At the individual-level, 9 out of 10 study participants improved in step, stance, and swing time asymmetries, 3 improved step length asymmetry, and 4 improved cadence. For each temporal asymmetry metric, the 1 study participant who did not improve differed: Participant 4 did not improve step time asymmetry and Participant 10 did not improve their stance or swing time asymmetries.
Figure 3.
Automated training via the closed-loop control of music results in more symmetric and economical walking. Gait rehabilitation fully automated by the closed-loop control of music significantly improves the walking quality of people post-stroke, measured as a reduction in gait asymmetry (A-C) and the energetic cost of walking (D). Gait asymmetry is calculated for step, stance, and swing times as the ratio of the difference between limbs divided by the sum of both limbs, with “0” indicative of no asymmetry. Energetic impairment is calculated as the difference from the normative energetic cost of walking of healthy older adults (ie, 0.168 ml/kg/m), with “0” indicative of no energetic impairment. (E) Changes in energetic impairment after training were highly related to baseline energetic impairment. All individuals with a baseline energetic impairment (green) reduced their energetic cost of walking after training. In contrast, individuals without an energetic cost impairment (purple) modestly increased their energetic cost of walking after training. Group and individual subject data are shown.
Note. All group data are reported as medians ± sIQR.
Automated Training Via the Closed-Loop Control of Music Results in Better Walking Economy
Study participants had a baseline energetic cost of walking of 0.229 ± 0.07 ml O2/kg/m that was reduced to 0.196 ± 0.05 ml O2/kg/m (P = .027) after the automated gait training. Compared to the normative value of healthy older adults (ie, 0.168 ml O2/kg/m 39 ) study participants had a median pre-training energetic impairment of 0.061 ml O2/kg/m (see Methods: Data Processing: Energetic Impairment) that reduced to only 0.028 ml O2/kg/m after training, indicating a substantial 42 ± 18% median reduction in energetic impairment (Figure 3D).
At the individual-level, 8 of the 10 study participants reduced their energetic impairment after training, with the magnitude of the change highly dependent on the degree of baseline impairment (ρ = −.90, P < .001; Figure 3E). All 7 of the 10 study participants with a baseline energetic impairment reduced their energetic impairment after training (Figure 3E, green). In contrast, 2 of the remaining 3 study participants modestly increased their energetic impairment after training (Figure 3E, purple). Examination of these energetic impairment subgroups in terms of changes in their energetic cost of walking reveals a median 13.4 ± 3.5% reduction in the 7 with a baseline energetic impairment, and a median 6.2 ± 4.3 % increase in the 3 without a baseline energetic impairment (Figure 3E, subpanel).
Discussion
Here we show that the closed-loop control of music can fully automate gait rehabilitation that improves the quality of post-stroke walking. Our findings add to the emerging field of closed-loop music interventions for movement retraining.42,50,51 Consistent with our hypotheses, the autonomously delivered gait rehabilitation improved the step time, stance time, and swing time gait asymmetries, and the energetic cost of walking of nearly all study participants. Inconsistent with our hypotheses, spatial asymmetries and cadence did not improve.
Automated Training Via the Closed-Loop Control of Music Results in More Symmetric Walking
The rhythmic beat produced by the closed-loop control of music provides a stable cue for the duration of each walking cycle that is personalized to the walking abilities of each user. 41 The neural processes governing auditory-motor entrainment allow these rhythmic cues to effectively induce changes in the temporal control of walking. Indeed, we observed post-training improvements in step time, stance time, and swing time asymmetries in 9 out of 10 study participants. Importantly, across these individuals, more symmetric walking was not the result of systematic changes in either paretic or non-paretic limb control, rather in improvements in their interlimb coordination. These findings show that fully automated gait training facilitated by the closed-loop control of music produces improvements in the temporal control of walking, as measured by treadmill evaluations conducted immediately after training and without the rhythmic auditory stimulus. These findings are consistent with prior studies of manually-delivered, entrainment-based gait training.25,28,30,33-35
Automated Training Via the Closed-Loop Control of Music Results in Better Walking Economy
Our finding of a substantial reduction in the energetic cost of walking following gait rehabilitation automated by the closed-loop control of music is a major contribution to the existing body of literature on entrainment-based gait rehabilitation.24-30,32-34,36 It is well established that stroke survivors expend more energy to walk than healthy individuals. 55 Because a high energetic cost of walking is associated with reduced community walking activity and worsened long-term health outcomes, 53 the development of gait interventions that can reduce the energetic cost of walking is a high priority. Taking the findings of this study together with the existing evidence on auditory-motor entrainment, we conclude that gait training automated by the closed-loop control of music has the potential to be an effective intervention for improving walking economy after stroke, warranting further clinical investigation into the effects of long-term training on retention of these benefits.
Walking Quality Improvements in Context
Autonomous gait rehabilitation systems like the music-based system presented in this paper have the potential to improve the reach and accessibility of skilled gait interventions beyond specialized clinical centers and reduce care gaps that hinder long-term outcomes in the millions of people with chronic mobility impairments. Indeed, the magnitude of walking quality improvements observed in this study are comparable with other rehabilitation paradigms including wearable robotics, functional electrical stimulation, and treadmill training. Improvements following these rehabilitation approaches range from 0% to 50% reduction in temporal asymmetries47,54-58 and 0% to 32% reduction in the energetic cost of walking.59-63 Our study is especially notable given that we report changes resulting from a single session of training, whereas most other intervention studies report improvements after several days or weeks of training. Though additional training visits are likely required to facilitate the durable behavioral changes desired of rehabilitation interventions, interventions that facilitate substantial walking improvements after a single training visit have great potential to increase the likelihood of behavioral change by catalyzing a positive cycle of improvements in self-efficacy, activity, and habit formation. 64
The walking quality improvements observed in this study build on our recent work showing that the autonomously delivered gait rehabilitation has the potential to be safe, enjoyable, and meaningfully improve the overground walking speed of people with post-stroke hemiparesis. 42 Because post-stroke gait interventions are thought to commonly increase walking speed through compensatory strategies linked to a highly inefficient and unstable walking pattern, 65 the substantial, speed-independent improvements in walking quality observed in this study highlight the potential therapeutic value of this autonomous neurorehabilitation technology.
Reducing the Energetic Cost of Walking: The Moderating Role of Baseline Impairment
To the best of our knowledge, this study is the first to show a reduction in the energetic cost of walking following gait training centered on auditory-motor entrainment. The presence of a baseline energetic impairment played an important role in determining the magnitude and direction of energetic changes following training. Though we hypothesized a reduction in the energetic cost of walking, it was not clear if this would be universal. Indeed, it is often suggested that the asymmetric walking patterns of individuals post-stroke may be their most economical walking patterns.66-68 In partial support of this hypothesis, we found a subset of individuals who walked more symmetrically after training but not more economically, with 2 of these individuals modestly increasing their energetic cost of walking. However, it is important to note that these 3 individuals did not present with an energetic impairment prior to training (see Table 1).
It is widely believed that human locomotion can be so well-tuned that a “barrier of energy cost” exists such that any changes in the walking pattern would increase energy expenditure. 69 Like the “normal barrier of energy cost” posited to exist in healthy individuals, despite their walking deficits, an energy cost barrier may exist for individuals post-stroke who have a more typical energetic cost of walking. That is to say, biological walking will always incur an energetic cost, and some post-stroke gait patterns may be just as economical as the bipedal gait pattern of individuals without neuromotor deficits. Though the findings of this study suggest that an energy cost barrier may exist for the small subset of people post-stroke with near normal energy expenditure during walking, individuals with a substantial baseline energetic impairment—which may be attributable to some combination of a slow walking speed and asymmetric power generation across limbs48,68,70—do appear to benefit substantially from the gait training automated by the closed-loop control of music (Figure 3E, Green).
Methodological Considerations
It is important to note that we experimentally controlled walking speed across the study’s pre- and post-training treadmill evaluations. Our decision to match speeds across evaluation timepoints enabled the analysis of changes in post-stroke gait asymmetry and the energetic cost of walking without the confound of changes in walking speed—which is known to be a major determinant of these biomechanical and physiological variables. When coupled with the serendipitous absence of a change in walking cadence, our results indicate that the observed improvements in gait symmetry and the energetic cost of walking should not be attributed to changes in speed or cadence, rather are likely fundamental changes in walking quality likely arising from changes in the neural control of walking. Indeed, recent work suggests the presence of music-induced neuromodulation of spinal reflexes. 71
Though warranted given our research question, the experimental control of walking speed across timepoints likely explains why we did not observe improvements in walking cadence, individual limb step lengths, or step length symmetry following training. Indeed, the speed-controlled biomechanical assessment used in our study is a major methodological difference from prior studies that showed improvements in stride length24,27-29,32,34 and cadence25-28,32,33,35 after entrainment-based gait training; these other studies conducted evaluations overground and allowed study participants to walk at their new self-selected speeds after training.
Limitations
Though the training was conducted overground, evaluations were conducted on the treadmill. Speed-controlled treadmill trials were used to isolate changes in the primary outcomes of gait quality from the accompanying changes in walking speed reported in prior work 42 ; however, there are known differences between treadmill and overground walking patterns72,73 that may have affected the findings of the study. Indeed, the treadmill evaluations may have been unable to detect actual gait changes induced by the overground gait training. For example, surprisingly, we failed to detect an effect of the gait training on measures of gait variability; however, actual effects may have been masked by the treadmill’s known effects on gait variability.74,75 Additionally, though we observed improvements in both gait asymmetry and the energetic cost of walking, examining the direct relationship between these outcomes was beyond the scope of this study and should be evaluated in future studies with a larger sample size. Likewise, future work is necessary to evaluate the effects of the intervention on other important metrics, like balance during walking, to provide a more complete understanding of the effects on post-stroke gait. Moreover, participants were not screened for amusia or anhedonia which may impact an individual’s response to gait training automated by the closed-loop control of music.
The potential effects of a larger treatment dose—that is, a longer treatment session or multiple treatment sessions—on the gait asymmetry and energetic outcomes is not known, warranting follow-on studies with longer treatment periods. Although this novel gait rehabilitation delivery system is meant for unsupervised use in the community and home, the gait training provided in this study, though fully automated by the technology, was performed in a supervised laboratory environment. More work is necessary to understand the feasibility of unsupervised gait training in the community or home.
Although this study achieved the objective of determining if the closed-loop control of music can autonomously deliver gait training that meaningfully improves walking after stroke, determining the specific treatment factors contributing to the observed improvements requires a control group—which was not included in this translational study of the closed-loop music technology. The largely homogenous positive response in several gait quality metrics that are often targeted by post-stroke walking therapies, but are difficult to modify, demonstrates the technology’s rehabilitative promise and supports progression to a randomized controlled trial that can evaluate clinical efficacy.
Supplemental Material
Supplemental material, sj-jpg-1-nnr-10.1177_15459683231174223 for Autonomous Control of Music to Retrain Walking After Stroke by Ashley N. Collimore, BSE, Anna V. Roto Cataldo, PhD, Ashlyn J. Aiello, MS, Regina Sloutsky, DPT, Karen J. Hutchinson, DPT, PhD, Brian Harris, MA, Terry Ellis, PhD and Louis N. Awad, DPT, PhD in Neurorehabilitation and Neural Repair
Supplemental material, sj-xlsx-2-nnr-10.1177_15459683231174223 for Autonomous Control of Music to Retrain Walking After Stroke by Ashley N. Collimore, BSE, Anna V. Roto Cataldo, PhD, Ashlyn J. Aiello, MS, Regina Sloutsky, DPT, Karen J. Hutchinson, DPT, PhD, Brian Harris, MA, Terry Ellis, PhD and Louis N. Awad, DPT, PhD in Neurorehabilitation and Neural Repair
Supplemental material, sj-xlsx-3-nnr-10.1177_15459683231174223 for Autonomous Control of Music to Retrain Walking After Stroke by Ashley N. Collimore, BSE, Anna V. Roto Cataldo, PhD, Ashlyn J. Aiello, MS, Regina Sloutsky, DPT, Karen J. Hutchinson, DPT, PhD, Brian Harris, MA, Terry Ellis, PhD and Louis N. Awad, DPT, PhD in Neurorehabilitation and Neural Repair
Acknowledgments
We thank S. Hanson, J. Paskewitz, C. Ledwick, K. Rezowalli, A. Alvarez, M. O’Connor, B. Adams, and G. Chen for their assistance with data collection. We thank E. Richardson, K. Smayda, and the entire MedRhythms team for their contributions. We thank our study participants who generously gave their time for this research. This device feasibility study does not meet the criteria for an applicable device clinical trial as described in 42 CFR 11.10 of the Final Rule for Clinical Trials Registration and Results Information Submission and was not prospectively registered.
Footnotes
Author Contributions: Conceptualization: BH, TE, and LNA. Methodology: RS, KJH, BH, TE, and LNA. Investigation: ANC, AVRC, AJA, and RS. Formal Analysis: ANC, AVRC, and LNA. Visualization: ANC, AVRC, and LNA. Funding acquisition: BH and LNA. Project administration: ANC, RS, and LNA. Supervision: BH and LNA. Writing—original draft: ANC, AVRC, and LNA. Writing—review & editing: ANC, AVRC, AJA, RS, KJH, BH, TE, and LNA.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: LNA is a paid advisor to MedRhythms Inc. BH is cofounder and CEO of MedRhythms Inc. This study was completed under a master management plan instituted by the Boston University Financial Conflicts of Interest Committee.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by MedRhythms, Inc. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressly or implied, of MedRhythms, Inc.
Data and Materials Availability: The data supporting the main conclusions of this manuscript are located within the main text or supplemental materials. Additional data are available upon request.
ORCID iDs: Ashlyn J. Aiello  https://orcid.org/0000-0002-9472-7336
https://orcid.org/0000-0002-9472-7336
Louis N. Awad  https://orcid.org/0000-0002-0159-8011
https://orcid.org/0000-0002-0159-8011
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website along with the online version of this article.
References
- 1.Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22:51-56. [DOI] [PubMed] [Google Scholar]
- 2.Balaban B, Tok F. Gait disturbances in patients with stroke. Phys Med Rehabil. 2014;6(7):635-642. [DOI] [PubMed] [Google Scholar]
- 3.Zamparo P, Francescato MP, De Luca G, Lovati L, di Prampera PE. The energy cost of level walking in patients with hemiplegia. Scand J Med Sci Sports. 1995;5:348-352. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro JAM, Oliveira SG, Di Thommazo-Luporini L, et al. Energy cost during the 6-minute walk test and its relationship to real-world walking after stroke: a correlational, cross-sectional pilot study. Phys Ther. 2019;99(12):1656-1666. [DOI] [PubMed] [Google Scholar]
- 5.Franceschini M, Rampello A, Agosti M, Massucci M, Bovolenta F, Sale P. Walking performance: correlation between energy cost of walking and walking participation. New statistical approach concerning outcome measurement. PLoS One. 2013;8(2):e56669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98-e169. [DOI] [PubMed] [Google Scholar]
- 7.Ayala C, Fang J, Luncheon C, et al. Use of outpatient rehabilitation among adult stroke survivors—20 states and the District of Columbia, 2013, and four states, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(20):575-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porciuncula F, Roto AV, Kumar D, et al. Wearable movement sensors for rehabilitation: a focused review of technological and clinical advances. PM R. 2018;10(9suppl 2):S220-S232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis TD, Earhart GM. Digital therapeutics in Parkinson’s disease: practical applications and future potential. J Parkinsons Dis. 2021;11(s1):S95-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun Janzen T, Koshimori Y, Richard NM, Thaut MH. Rhythm and music-based interventions in motor rehabilitation: current evidence and future perspectives. Front Hum Neurosci. 2022;15:789467. doi: 10.3389/fnhum.2021.789467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaut MH. The Discovery of Human Auditory-Motor Entrainment and Its Role in the Development of Neurologic Music Therapy. 1st ed. Elsevier B.V.; 2015. [DOI] [PubMed] [Google Scholar]
- 12.Chauvigné LAS, Gitau KM, Brown S. The neural basis of audiomotor entrainment: an ALE meta-analysis. Front Hum Neurosci. 2014;8:1-18. doi: 10.3389/fnhum.2014.00776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Large EW. On synchronizing movements to music. Hum Mov Sci. 2000;19(4):527-566. [Google Scholar]
- 14.Large EW, Herrera JA, Velasco MJ. Neural networks for beat perception in musical rhythm. Front Syst Neurosci. 2015;9:159. doi: 10.3389/fnsys.2015.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forner-Cordero A, Pinho JP, Umemura G, et al. Effects of supraspinal feedback on human gait: rhythmic auditory distortion. J Neuroeng Rehabil. 2019;16(159):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaut MH, Kenyon GP, Schauer ML, McIntosh GC. The connection between rhythmicity and brain function. IEEE Eng Med Biol Mag. 1999;18(2):101-108. [DOI] [PubMed] [Google Scholar]
- 17.Nozaradan S. Exploring how musical rhythm entrains brain activity with electroencephalogram frequency-tagging. Philos Trans R Soc Lond B Biol Sci. 2014;369(1658):20130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nozaradan S, Peretz I, Keller PE. Individual differences in rhythmic cortical entrainment correlate with predictive behavior in sensorimotor synchronization. Sci Rep. 2016;6:20612. doi: 10.1038/srep20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comstock DC, Hove MJ, Balasubramaniam R. Sensorimotor synchronization with auditory and visual modalities: behavioral and neural differences. Front Comput Neurosci. 2018;12:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damm L, Varoqui D, De Cock VC, Dalla Bella S, Bardy B. Why do we move to the beat? A multi-scale approach, from physical principles to brain dynamics. Neurosci Biobehav Rev. 2020;112:553-584. [DOI] [PubMed] [Google Scholar]
- 21.Proksch S, Comstock DC, Médé B, Pabst A, Balasubramaniam R. Motor and predictive processes in auditory beat and rhythm perception. Front Hum Neurosci. 2020;14:578546. doi: 10.3389/fnhum.2020.578546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt N, McGrath D, Stergiou N. The influence of auditory-motor coupling on fractal dynamics in human gait. Sci Rep. 2014;4:5879. doi: 10.1038/srep05879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoles-Frenkel M, Avron M, Prut Y. Impact of auditory context on executed motor actions. Front Integr Neurosci. 2016;10(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobinata N, Ueno M, Imanishi Y, Yoshikawa H. Immediate effects of rhythmic auditory stimulation on gait in stroke patients in relation to the lesion site. J Phys Ther Sci. 2016;28(9):2441-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SH, Lee KJ, Song CH. Effects of rhythmic auditory stimulation (RAS) on gait ability and symmetry after stroke. J Phys Ther Sci. 2012;24(4):311-314. [Google Scholar]
- 26.Mainka S, Wissel J, Völler H, Evers S. The use of rhythmic auditory stimulation to optimize treadmill training for stroke patients: a randomized controlled trial. Front Neurol. 2018;9:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh JH, Han SJ, Jeon SY, et al. Effect of rhythmic auditory stimulation on gait and balance in hemiplegic stroke patients. NeuroRehabilitation. 2014;34(1):193-199. [DOI] [PubMed] [Google Scholar]
- 28.Thaut MH, Leins AK, Rice RR, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single-blind, randomized trial. Neurorehabil Neural Repair. 2007;21(5):455-459. [DOI] [PubMed] [Google Scholar]
- 29.Thaut MH, Mcintosh GC, Rice RR. Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J Neurol Sci. 1997;151(2):207-212. [DOI] [PubMed] [Google Scholar]
- 30.Crosby LD, Wong JS, Chen JL, Grahn J, Patterson KK. An initial investigation of the responsiveness of temporal gait asymmetry to rhythmic auditory stimulation and the relationship to rhythm ability following stroke. Front Neurol. 2020;11:517028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moumdjian L, Buhmann J, Willems I, Feys P, Leman M. Entrainment and synchronization to auditory stimuli during walking in healthy and neurological populations: a methodological systematic review. Front Hum Neurosci. 2018;12:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha Y, Kim Y, Hwang S, Chung Y. Intensive gait training with rhythmic auditory stimulation in individuals with chronic hemiparetic stroke: a pilot randomized controlled study. NeuroRehabilitation. 2014;35(4):681-688. [DOI] [PubMed] [Google Scholar]
- 33.Cha Y, Kim Y, Chung Y. Immediate effects of rhythmic auditory stimulation with tempo changes on gait in stroke patients. J Phys Ther Sci. 2014;26(4):479-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Oh D. Home-based auditory stimulation training for gait rehabilitation of chronic stroke patients. J Phys Ther Sci. 2012;24:775-777. [Google Scholar]
- 35.Lee S, Lee K, Song C. Gait training with bilateral rhythmic auditory stimulation in stroke patients: a randomized controlled trial. Brain Sci. 2018;8(164):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thaut MH, Abiru M. Rhythmic auditory stimulation in rehabilitation of movement disorders: a review of current research. Music Percept. 2010;27(4):263-269. [Google Scholar]
- 37.Sall J, Eapen BC, Elizabeth J, Bowles AO, Bursaw A, Rodgers ME. The management of stroke rehabilitation: a synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann Intern Med. 2019;171(12):916-924. [DOI] [PubMed] [Google Scholar]
- 38.Hornby TG, Reisman DS, Ward IG, et al. Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. J Neurol Phys Ther. 2020;44:49-100. [DOI] [PubMed] [Google Scholar]
- 39.Berryman N, Gayda M, Nigam A, Juneau M, Bherer L, Bosquet L. Comparison of the metabolic energy cost of overground and treadmill walking in older adults. Eur J Appl Physiol. 2012;112(5):1613-1620. [DOI] [PubMed] [Google Scholar]
- 40.Thaut MH. Rhythm, Music, and the Brain: Scientific Foundations and Clinical Applications. Routledge; 2005. [Google Scholar]
- 41.Thaut M, Hodges DA. The Oxford Handbook of Music and the Brain. 1st ed. Oxford University Press; 2019. [Google Scholar]
- 42.Hutchinson K, Sloutsky R, Collimore A, et al. A music-based digital therapeutic: proof-of-concept automation of a progressive and individualized rhythm-based walking training program after stroke. Neurorehabil Neural Repair. 2020;34(11):986-996. [DOI] [PubMed] [Google Scholar]
- 43.Nuckols RW, Lee S, Swaminathan K, Orzel D, Howe RD, Walsh CJ. Individualization of exosuit assistance based on measured muscle dynamics during versatile walking. Sci Robot. 2021;6(60):eabj1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeni JA, Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture. 2008;27(4):710-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anon. Temporal distance calculations for gait. C-Motion WIKI Documentation. 2021. Accessed December 15, 2021. https://c-motion.com/v3dwiki/index.php/Temporal_Distance_Calculations_for_Gait [Google Scholar]
- 46.Lauzière S, Betschart M, Aissaoui R, Nadeau S. Understanding spatial and temporal gait asymmetries in individuals post stroke. Int J Phys Med Rehabil. 2014;2(201):1-11. [Google Scholar]
- 47.Lewek MD, Braun CH, Wutzke C, Giuliani C. The role of movement errors in modifying spatiotemporal gait asymmetry post-stroke: a randomized controlled trial HHS public access. Clin Rehabil. 2018;32(2):161-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padmanabhan P, Rao KS, Gulhar S, Cherry-Allen KM, Leech KA, Roemmich RT. Persons post-stroke improve step length symmetry by walking asymmetrically. J Neuroeng Rehabil. 2020;17(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. J Appl Physiol. 1955;8(1):73-80. [DOI] [PubMed] [Google Scholar]
- 50.van den Berghe P, Derie R, Bauwens P, et al. Reducing the peak tibial acceleration of running by music-based biofeedback: a quasi-randomized controlled trial. Scand J Med Sci Sports. 2022;32(4):698-709. [DOI] [PubMed] [Google Scholar]
- 51.van den Berghe P, Derie R, Gerlo J, et al. Influence of music-based feedback training on peak tibial acceleration during running outside of the biomechanics laboratory. Footwear Sci. 2021;13(S1):S39-S41. [Google Scholar]
- 52.Kramer S, Johnson L, Bernhardt J, Cumming T. Energy expenditure and cost during walking after stroke: a systematic review. Arch Phys Med Rehabil. 2016;97(4):619-632.e1. [DOI] [PubMed] [Google Scholar]
- 53.Saunders DH, Greig CA, Mead GE. Physical activity and exercise after stroke: review of multiple meaningful benefits. Stroke. 2014;45(12):3742-3747. [DOI] [PubMed] [Google Scholar]
- 54.Karniel N, Raveh E, Schwartz I, Portnoy S. Functional electrical stimulation compared with ankle-foot orthosis in subacute post stroke patients with foot drop: a pilot study. Assist Technol. 2021;33(1):9-16. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka H, Nankaku M, Nishikawa T, et al. Spatiotemporal gait characteristic changes with gait training using the hybrid assistive limb for chronic stroke patients. Gait Posture. 2019;71:205-210. [DOI] [PubMed] [Google Scholar]
- 56.Cleland B, Madhavan S. Changes in walking speed after high-intensity treadmill training are independent of changes in spatiotemporal symmetry after stroke. Front Neurol. 2021;12:647338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen G, Patten C, Kothari DH, Zajac FE. Gait deviations associated with post-stroke hemiparesis: improvement during treadmill walking using weight support, speed, support stiffness, and handrail hold. Gait Posture. 2005;22:57-62. [DOI] [PubMed] [Google Scholar]
- 58.Meder KG, Lojacono CT, Rhea CK. A systematic review of non-pharmacological interventions to improve gait asymmetries in neurological populations. Symmetry. 2022;14:281. [Google Scholar]
- 59.Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA. Reducing the cost of transport and increasing walking distance after stroke: a randomized controlled trial on fast locomotor training combined with functional electrical stimulation. Neurorehabil Neural Repair. 2016;30(7):661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Awad LN, Bae J, O’Donnell K, et al. A soft robotic exosuit improves walking in patients after stroke. Sci Transl Med. 2017;9:1-12. [DOI] [PubMed] [Google Scholar]
- 61.Lee HJ, Lee SH, Seo K, et al. Training for walking efficiency with a wearable hip-assist robot in patients with stroke a pilot randomized controlled trial. Stroke. 2019;50(12):3545-3552. [DOI] [PubMed] [Google Scholar]
- 62.Munari D, Pedrinolla A, Smania N, et al. High-intensity treadmill training improves gait ability, VO2peak and cost of walking in stroke survivors: preliminary results of a pilot randomized controlled trial. Eur J Phys Rehabil Med. 2018;54(3):408-418. [DOI] [PubMed] [Google Scholar]
- 63.Awad LN, Kudzia P, Revi DA, Ellis TD, Walsh CJ. Walking faster and farther with a soft robotic exosuit: implications for post-stroke gait assistance and rehabilitation. IEEE Open J Eng Med Biol. 2020;1:108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anon. National Institutes of Health Science of Behavior Change Common Fund Program. National Institutes of Health. Accessed January 20, 2022. https://commonfund.nih.gov/behaviorchange [Google Scholar]
- 65.Awad LN, Lewek MD, Kesar TM, Franz JR, Bowden MG. These legs were made for propulsion: advancing the diagnosis and treatment of post-stroke propulsion deficits. J Neuroeng Rehabil. 2020;17:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roemmich RT, Leech KA, Gonzalez AJ, Bastian AJ. Trading symmetry for energy cost during walking in healthy adults and persons poststroke. Neurorehabil Neural Repair. 2019;33(8):602-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen TM, Jackson RW, Aucie Y, de Kam D, Collins SH, Torres-Oviedo G. Self-selected step length asymmetry is not explained by energy cost minimization in individuals with chronic stroke. J Neuroeng Rehabil. 2020;17(119):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reisman DS, Rudolph KS, Farquhar WB. Influence of speed on walking economy poststroke. Neurorehabil Neural Repair. 2009;23(6):529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collins SH, Wiggin MB, Sawicki GS. Reducing the energy cost of human walking using an unpowered exoskeleton. Nature. 2015;522(7555):212-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabil Neural Repair. 2015;29(5):416-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peyre I, Hanna-Boutros B, Lackmy-Vallee A, et al. Music restores propriospinal excitation during stroke locomotion. Front Syst Neurosci. 2020;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brouwer B, Parvataneni K, Olney SJ. A comparison of gait biomechanics and metabolic requirements of overground and treadmill walking in people with stroke. Clin Biomech. 2009;24(9):729-734. [DOI] [PubMed] [Google Scholar]
- 73.Kautz SA, Bowden MG, Clark DJ, Neptune RR. Comparison of motor control deficits during treadmill and overground walking poststroke. Neurorehabil Neural Repair. 2011;25(8):756-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hollman JH, Watkins MK, Imhoff AC, Braun CE, Akervik KA, Ness DK. A comparison of variability in spatiotemporal gait parameters between treadmill and overground walking conditions. Gait Posture. 2016;43:204-209. [DOI] [PubMed] [Google Scholar]
- 75.Harris-Love ML, Forrester LW, Macko RF, Silver KHC, Smith GV. Hemiparetic gait parameters in overground versus treadmill walking. Neurorehabil Neural Repair. 2001;15(2):105-112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-nnr-10.1177_15459683231174223 for Autonomous Control of Music to Retrain Walking After Stroke by Ashley N. Collimore, BSE, Anna V. Roto Cataldo, PhD, Ashlyn J. Aiello, MS, Regina Sloutsky, DPT, Karen J. Hutchinson, DPT, PhD, Brian Harris, MA, Terry Ellis, PhD and Louis N. Awad, DPT, PhD in Neurorehabilitation and Neural Repair
Supplemental material, sj-xlsx-2-nnr-10.1177_15459683231174223 for Autonomous Control of Music to Retrain Walking After Stroke by Ashley N. Collimore, BSE, Anna V. Roto Cataldo, PhD, Ashlyn J. Aiello, MS, Regina Sloutsky, DPT, Karen J. Hutchinson, DPT, PhD, Brian Harris, MA, Terry Ellis, PhD and Louis N. Awad, DPT, PhD in Neurorehabilitation and Neural Repair
Supplemental material, sj-xlsx-3-nnr-10.1177_15459683231174223 for Autonomous Control of Music to Retrain Walking After Stroke by Ashley N. Collimore, BSE, Anna V. Roto Cataldo, PhD, Ashlyn J. Aiello, MS, Regina Sloutsky, DPT, Karen J. Hutchinson, DPT, PhD, Brian Harris, MA, Terry Ellis, PhD and Louis N. Awad, DPT, PhD in Neurorehabilitation and Neural Repair