Abstract
Background:
Which noninvasive brain stimulation (NIBS) treatment – transcranial direct current stimulation (tDCS) or transcranial magnetic stimulation (TMS) – is more beneficial for stroke patients’ cognitive rehabilitation is still up for debate.
Objectives:
Our goal is to provide an overview of the research on the effectiveness and safety of various NIBS protocols.
Design:
Systematic review and network meta-analysis (NMA) of randomized controlled trials (RCTs).
Methods:
This NMA compared any active NIBS versus sham stimulation in adult stroke survivors to enhance cognitive function, with a focus on global cognitive function (GCF), attention, memory, and executive function (EF) using the databases MEDLINE, Embase, Cochrane Library, Web of Science, and ClinicalTrials.gov. The NMA statistical approach was built on a frequency framework. The effect size was estimated by the standardized mean difference (SMD) and a 95% confidence interval (CI). We compiled a relative ranking of the competing interventions based on their surface under the cumulative ranking curve (SUCRA).
Results:
NMA showed that high-frequency repeated TMS (HF-rTMS) improved GCF compared with sham stimulation (SMD = 1.95; 95% CI: 0.47–3.43), while dual-tDCS improved memory performance versus sham stimulation significantly (SMD = 6.38; 95% CI: 3.51–9.25). However, various NIBS stimulation protocols revealed no significant impact on enhancing attention, executive function, or activities of daily living. There was no significant difference between the active stimulation protocols for TMS and tDCS and sham stimulation in terms of safety. Subgroup analysis demonstrated an effect favoring activation site of the left dorsolateral prefrontal cortex (DLPFC) (SUCRA = 89.1) for enhancing GCF and bilateral DLPFC (SUCRA = 99.9) stimulation for enhancing memory performance.
Conclusion:
The HF-rTMS over the left DLPFC appears to be the most promising NIBS therapeutic option for improving global cognitive performance after stroke, according to a comparison of numerous NIBS protocols. Furthermore, for patients with post-stroke memory impairment, dual-tDCS over bilateral DLPFC may be more advantageous than other NIBS protocols. Both tDCS and TMS are reasonably safe.
Registration:
PROSPERO ID: CRD42022304865
Keywords: cognition, memory, network meta-analysis, stroke, transcranial direct current stimulation, transcranial magnetic stimulation
Introduction
Over two-thirds of stroke survivors experience cognitive impairment or decline after their stroke,1,2 which makes it challenging for them to carry out their daily activities, 3 and which may be an indication of a poor prognosis with worse rehabilitation outcomes, 4 the onset of dementia, 5 and higher mortality. 6 Rehabilitation of stroke survivors has been a prominent research focus for academics and medical professionals as the occurrence of stroke has risen over the past 20 years.
As a system, attention, memory, executive function (EF), visuospatial ability, and language all act as part of cognition, which is not a single concept. 7 Many cognitive domains are affected by cognitive impairment following a stroke, although attention, 8 memory, 9 and EF 10 are some of the more prevalent ones. And these multidimensional cognitive deficits are likely to persist into the subacute or even chronic phase after stroke onset; 11%–31% of patients still have memory impairment 1 year after stroke,11,12 and 66% of stroke victims continue to experience EF decompensation in varied degrees. 13 On the other hand, roughly 30% of stroke survivors who experience attentional abnormalities end up developing dementia. 8 These cognitive problems also result in significant functional limitations, such as hampered rehabilitation efforts, a diminished ability to return to work, and a need for additional support. 14
Unfortunately, there are still very few cognitive therapies available for stroke patients, and the evidence is still insufficient. The effectiveness of several non-pharmacological rehabilitation techniques, such as cognitive training, 15 brain stimulation, 16 virtual reality training, 17 psychological interventions, 18 and many more, are still debatable but are thought to improve cognitive function in stroke survivors by promoting neuroplasticity.
Noninvasive brain stimulation (NIBS) technology, such as repeated transcranial magnetic stimulation (rTMS), theta-burst stimulation (TBS), and transcranial direct current stimulation (tDCS), which can induce change in excitability of the underlying brain cortex in a noninvasive fashion and potentially induce long-lasting neuroplastic changes by either magnetic or electric fields, has gradually become more prevalent in stroke rehabilitation in recent years.19–22 Briefly, TMS acts differently by generating a time-varying magnetic field perpendicular to the stimulation coil, which then induces a current in the superficial cerebral cortex nearly parallel to the coil, by affecting the excitability of neurons. Different stimulation frequencies have different effects on cortical activity, with high-frequency (> 1 Hz) stimulation (HF-rTMS) promoting local neuronal excitability, while low-frequency (⩽ 1 Hz) stimulation (LF-rTMS) shows inhibitory effects. 23 The TBS is a novel rTMS consisting of three pulse bursts at 50 Hz. It is divided into two forms, continuous TBS (cTBS) and intermittent TBS (iTBS), which play inhibitory and facilitatory roles in local cortical excitability, respectively. 24 On the other hand, tDCS is a weak, constant, low-intensity direct current (current intensity of 0.5–2 mA) applied to the cerebral cortex through two electrodes, anode and/or cathode, placed on the scalp, which, through subthreshold stimulation, changes the difference between the internal and external potential of the neuronal membrane of the brain and regulates the threshold of the action potential, thus affecting the excitability of neurons in the stimulated area. 5 According to the selection and placement of electrodes, they can be divided into anodal tDCS (a-tDCS), cathodal tDCS (c-tDCS), and dual tDCS (both anodal and cathodal tDCS).
In the present review, we will concentrate primarily on the aspect of cognition impairment from the perspective of the therapeutic efficacy of NIBS with various post-stroke dysfunctions. Some findings have been drawn from meta-analyses about the cognitive function of language25–28 and unilateral neglect,27–30 despite the lack of and conflicting evidence on attention, memory, and EF. The current meta-analysis for NIBS showed that most studies concluded that TMS had significant effects on attention, memory, working memory, global cognition,23,31 and activities of daily living 32 (ADL) in post-stroke patients, while the effect of different frequencies of treatment options did not differ significantly, 33 and HF-rTMS had significant improvements in global cognition on left dorsolateral prefrontal cortex (DLPFC). 32 The findings of tDCS research are disputed, with some advocating the treatment regardless of disease type for cognitive recovery in neurological disorders. 23 Some suggest an acute facilitative effect on repairing attentional impairment, but no improvement in general cognition or working memory. 27 Another report claims that tDCS improves attention and general cognition after stroke, but not memory. 34 But the effect of NIBS stimulation treatment on EF after stroke is limited.
Rationale
There is so far conflicting evidence from systematic reviews of randomized controlled trials (RCTs) on the effectiveness of different NIBS approaches for improving different domains of cognitive function after stroke. New RCTs on TMS and tDCS13,24,35,36,37 were not included in the prior meta-analysis. To acquire integrated results, it is required to expand these investigations. Besides, it is common that one study involves only electrical or magnetic stimulation, which has different costs and operational conveniences. And the same study frequently uses only one or two treatment protocols that may differ in terms of stimulation target, frequency or electrode selection, timing, intensity, and so on, while thus, combining TMS and tDCS therapies in one trial is often unnecessary. The lack of head-to-head comparative trials makes it difficult to compare the effectiveness of different NIBS protocols using conventional meta-analysis.
Network meta-analysis (NMA) combines head-to-head and indirect evidence from randomized trials.38–40 By utilizing the strength of indirect evidence, NMA guarantees all treatment comparisons, allowing the assessment and rating of comparative effects not expressly evaluated in randomized clinical trials.41,42
Objective
We aim to summarize the evidence network of RCTs of various NIBS protocols, including rTMS (HF-rTMS, LF-rTMS), TBS (iTBS, cTBS), and tDCS (a-tDCS, c-tDCS, dual-tDCS), and stimulation site, on cognitive rehabilitation, particularly for attention, memory, and EF in patients after stroke, as well as ADL and safety, to provide recommendations for clinical treatment decisions.
Methods
Protocol and registration
We prepared this protocol following the recommendations of Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidance 43 (Supplemental File 1), which has been registered in the PROSPERO database under the ID CRD42022304865.
Eligibility criteria
Participant
Studies on adults (over 18 years old) meeting the diagnostic criteria for stroke through head computed tomography or magnetic resonance imaging with cognitive impairment after a stroke were considered. Cognitive impairment includes attention, memory, and EF, tested by mini-mental status examination (MMSE), Montreal cognitive assessment (MoCA), or other domain-specific cognitive tests. There was no limitation on stroke type and stage. Studies with no more than five individuals were excluded.
Interventions and comparators
We chose groups comparing NIBS, including HF-rTMS, LF-rTMS, iTBS, cTBS, a-tDCS, c-tDCS, or dual-tDCS, with the control group eligible for inclusion. In the control group, sham stimulation with or without conventional/computerized cognitive treatment was acceptable. We excluded studies by no more than five sessions of NIBS for meta-analysis.
Outcomes
We set the primary outcome as cognitive function evaluated before and after neurostimulation therapy in the short term. Primary outcome indicators focused on scale scores assessing global cognitive function (GCF), attention, memory, and EF.
The ability to perform ADL was one of the secondary outcomes, measured by scale scores assessing ADL. Another secondary outcome was safety, measured by the number of dropouts and adverse events.
Studies
We included all genuine parallel or cross-over trials RCTs for review. Articles reporting protocols, in-progress trials, retrospective studies, or case reports were excluded. Studies reporting sufficient information to compute effect size statistics (i.e. mean and standard deviations or standard errors, or median and ranges or interquartile ranges, exact F-, p-, t-, or z-values], published in an international peer-reviewed journal until February 10, 2022, in only English language were considered.
Information sources
We searched the following databases for relevant English language literature: PubMed (MEDLINE), Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science from their inception to February 2022. For the ongoing trials register, we also searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.ClinicalTrials.gov/). We will also explore the reference lists of included trials, systematic reviews, and meta-analyses identified during the screening process to identify other eligible trials. Gray literature, such as conference proceedings, will also be searched.
Search
Supplemental File 2 contains the MEDLINE search technique. Other databases also can use this search approach.
Study selection
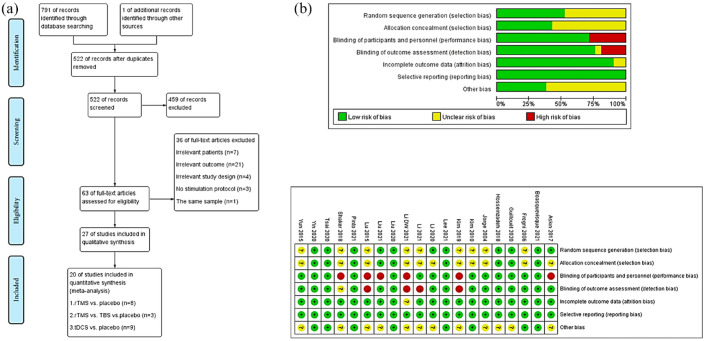
One reviewer (WL) screened titles and abstracts for irrelevant research. Two writers (YW and JR) examined all remaining full-text studies. Disagreements were discussed with the third author (JC) and resolved through consensus. Figure 1(a) depicts the flowchart structure.
Figure 1.
(a) Flow chart of literature screening. (b) Risk of bias graph.
Data collection process
YW and JR extracted trial and summary result data independently. If data were unavailable, we emailed the corresponding author to request the information; if the author did not reply, we contacted him again after 2 weeks and asked twice more.
Data items
We independently evaluated the following items utilizing checklists: (1) methods of random sequence generation, (2) methods of allocation concealment, (3) blinding of outcome assessors, participants and personnel, (4) use of an intention-to-treat analysis, (5) adverse effects and dropouts, (6) participants (number, sex, age, stroke type, days after stroke onset to study entry, intervention, protocol of stimulation, site of stimulation, etc.), (7) comparison (details of protocol in control groups, and (8) outcome data [MMSE, MoCA for GCF, Stroop test (ST), trail making test (TMT), digital span (DS), visual/auditory continuous performance test (VCPT/ACPT) for attention function, visual/verbal learning test-delayed recall (ViLT/VeLT) and Rivermead behavior memory test (RBMT) for memory function, digital symbol test (DST) and tower of London (TOL) for EF, and functional independence measure (FIM) or Barthel index (BI) for ADL].
We gathered data from ‘Results’ sections and tables or estimated them from figures and appendices using GetData Graph Digitizer 2.26 software (http://www.getdata-graph-digitizer.com/download.php). Mean and standard deviation (SD) changes revealed each neurostimulation therapy’s effectiveness. The mean change was calculated as the arithmetic difference between baseline and poststimulation values. When SD was unavailable for absolute baseline and poststimulation values, we used the correlation method. 44 We estimated the variance following what Hozo et al. 45 reported if the data were displayed by median and range. We contacted the corresponding author of the original text if the outcome of the study detail were unclear.
Geometry of the network
The network’s geometry defines direct comparisons’ relationship and precision. We compared rTMS (HF-rTMS, LF-TMS), TBS (iTBS, cTBS), and tDCS (a-tDCS, c-tDCS, dual-tDCS) with sham stimulation. The nodes were linked by a line when the treatments were directly comparable. The width of each line is proportional to the number of RCTs, and the size of each node is proportional to the number of patients (sample size). The colors of the edges indicate the risk of bias for this comparison (green = low, yellow = unclear, and red = high risk of bias).
Risk of bias within individual studies
The Cochrane Handbook 5.1.0 risk of bias table was used to evaluate the quality of each study, which included seven domains of bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. 46 The Cochrane Handbook 5.1.0 classifies each evaluation scenario as high, medium, or low risk. Figure 1(b) shows the study outcomes.
Summary measures
We calculated the standardized mean difference (SMD) and 95% confidence interval (CI) for studies that examined the same underlying construct employing various outcome measures. Odds ratio (OR) and 95% CIs were used to estimate dropouts and adverse events. We generated contrast-based forest plots for comparison. I2 more than 50% was considered significant heterogeneity. 46
We compiled a relative ranking of the competing interventions based on their surface under the cumulative ranking line (SUCRA). 47 Higher SUCRA means greater treatment results. RevMan 5.4.1 performed the conventional meta-analysis. We draw network evidence relationship diagrams, NMA forest diagrams, funnel diagrams, Egger test, sensitivity test, and the corresponding statistics 48 by STATA 14.2 (Stata Corporation, Lakeway, Texas, USA).
Planned method of analysis
The statistical method of an NMA is based on a frequency framework. All the outcome indicators use the random-effects model, 48 which follows the graph-theoretical methodology and permits multi-arm trials. 49
Assessment of inconsistency
There is no general inconsistency if p > 0.05 when testing global consistency. Local inconsistencies between direct and indirect evidence were checked via node splitting. 50 We evaluated local inconsistencies by calculating each network closed loop’s inconsistency factors (IFs) and 95% CIs. Direct and indirect evidence were consistent if the 95% CI’s bottom limit was 0 or near 0.
Risk of bias across studies
Publication bias was assessed with a funnel plot and Egger’s test. 51 We conducted a sensitivity analysis to exclude low-quality studies.
Quality of evidence
The overall quality of the results in this paper was assessed using a method that extends the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) system 52 to NMAs. We used directly comparable quality ratings to establish evidence certainty (confidence in the evidence/quality of the evidence). 53 Limitations in study design, inconsistency, indirectness, imprecision, and publication bias were scored. 52 Evidence can be high, moderate, low, or very low). 54 Evidence profiles were obtained using the GRADEpro GDT (https://gdt.gradepro.org/app/). 55
Additional analyses
We also included the stimulation site in our pre-specified subgroup analysis as a potential effect-influencing factor.
Results
Study selection
We identified 792 studies from electronic databases, and 522 articles were screened by title and abstract after removing duplicates. A total of 63 full-text articles were potentially eligible for inclusion in the review. We excluded 36 studies for the following reasons: irrelevant study design (case report, self-controlled study, and not placebo stimulation of intervention), the same sample, irrelevant patients or outcomes, and not NIBS for intervention over the brain – the remaining 27 studies with 1,041 participants in a qualitative analysis RCTs. Three studies56–58 with no more than five sessions of stimulation were removed. We also excluded three trials for data unextractable59–61 and one 62 without details about tDCS protocol, to which we contacted the corresponding authors without reply. The quantitative meta-analysis included 20 trials13,24,35–37,63–77 with 840 participants (see Figure 1(a), Table 1).
Table 1.
Characteristics of the included studies.
| Study | Study design | Sample size | Age (year), M ± SD or median (IQR) | Gender (male/female) | Days after onset M ± SD or median (IQR) | Stroke type | Group set | Protocol of stimulation | Site of stimulation | Outcomes | Adverse effect |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tdcs | |||||||||||
| Shaker 2018 63 | Parallel | I: 20/C: 20 | I: 54.45 ± 4.68 C: 53.05 ± 6.32 |
40/0 | I:14.05 ± 1.53 months C:16.55 ± 2.78 months |
IS | I: dual-tDCS ± RT/C: sham ± RT | 2 mA, 30 minutes/day, 3 days/week × 1 month, TS = 12 | Anode: right and left DLPFC/cathode: CSA | ②③④⑤ | NA |
| Pinto 2021 35 | Parallel | I: 31/C:29 | I: 45.6 ± 12.1 C:48.1 ± 9.4 |
I:25/6 C:19/10 | I:113.7 ± 109.7 days C:52.4 ± 60.8 days |
IS&HS | I: dual-tDCS ± RT/C: sham ± RT | 2–3 mA, 25 cm²,30 minutes/session, twice daily, 6 days/week×2 weeks, TS = 24 | Anode: Ip-M1/cathode: NI-M1 | ②③④⑤ | NA |
| Yun 2015 (three arms) 64 | Parallel | I1: 15/I2: 15/C: 15 | I1:60.9 ± 12.9 I2:58.9 ± 15.0 C:68.5 ± 14.6 |
I1:6/9 I2:7/8 C:7/8 | I1:42.2 ± 31.9 days I2:38.1 ± 27.0 days C:39.5 ± 29.6 days |
IS&HS | I1: Left a-tDCS/I2: right a-tDCS/C: sham-tDCS | 2 mA, 5 × 5 cm, 30 minutes/day, 5 days/week × 3 weeks, TS = 15 | I1 anode: the left FT/I2 anode: the right FT/cathode: NA | ①②③⑤ | NA |
| Boasquevisque 2021 36 | Parallel | I: 15 C: 15 | I:61.8 ± 15 C:61.9 ± 17.9 |
I: 8/7 C: 4/11 | I:37 (23.5; 45.5) days C:26.5 (20.8; 37.3) days |
IS | I: c-tDCS C: sham-tDCS | 1 mA, 7 × 5 cm, 20 minutes/day, 3 days/week × 2 weeks, TS = 6 | Anode: ISA/cathode: NI-M1 | ①⑤ | None |
| Kim 2019 (three arms) 65 | Parallel | I1: 11/C: 10/I2: 9 | 63.5 ± 7.45 | 17/13 | 24.53 ± 11.56 months | IS&HS | I1: a-tDCS + RT/C: sham-tDCS + RT/I2: a-tDCS | 2.2 mA, 20 minutes/session, twice/week × 8 weeks, TS = 16 | Anode: Ip-M1/cathode: none | ② | NA |
| Liu 202113 | Parallel | I: 25/C: 25 | I: 65 (60.5: 70.5) C: 64 (55.0:70.0) |
I:15/10 C:12/13 | I:8 (7.0: 9.0) months C:8 (9.0: 10.5) months |
IS&HS | I: dual-tDCS + RT/C: sham-tDCS + RT | 2 mA, 5 × 5 cm, 20 minutes/day, 5 days/week × 4 weeks, TS = 20 | Anode: the left DLFPC/cathode: the right DLPFC | ①②④⑤ | Mild headaches and discomfort: I: 4/C: 2 |
| Lee 2021 66 | Parallel | I: 10/C: 10 | I: 67.5 ± 6.74 C: 65.00 ± 5.73 |
I:6/4 C:7/3 | I: 3.75 ± 1.48 months C:4.12 ± 1.55 months |
IS&HS | I: a-tDCS + RT/C: sham-tDCS + RT | 2 mA, 20 minutes/day, 5 days/week × 4 weeks, TS = 20 | Anode: Ip-M1/cathode: CSA | ①②④⑤ | NA |
| Guillouët 2020 67 | Crossover | 14 | 53.8 ± 13.1 | 10/4 | 18 ± 23.1 months | IS&HS | I: dual-tDCS + RT/C: sham-tDCS + RT | 2 mA, 7 × 5 cm, 20 minutes/session, 2–5 times per week × 3 weeks, washout: 1 week, TS = 21 | Anode: the IFG/cathode: the contralesional IFG | ② | None |
| Hosseinzadeh 2018 (four arms) 68 | Parallel | I1: 25/I2: 25 C1: 25/C2: 25 |
I1: 56 ± 8 I2: 60 ± 7 C1: 59 ± 7 C2: 59 ± 8 |
I1:13/12 I2:12/13 C1:12/13 C2:12/13 | 25–180 days | IS | I1: a-tDCS/I2: c-tDCS/C1: sham-tDCS/C2: RT | 2 mA, 7 × 5 cm, 30 minutes/session, 3 times/week × 1 month, TS = 12 | I1 anode: the left STG/cathode: the right STG I2 anode: CSA/cathode: the left gyrus | ② | None |
| Park 201359 | Parallel | I: 6/C: 5 | I: 65.3 ± 14.3 C: 66.0 ± 10.8 |
I:2/4 C:3/2 | I: 29.0 ± 18.7 days C:25.2 ± 17.5 days |
IS&HS | I: dual-tDCS + RT/C: sham-tDCS + RT | 2 mA, 5 × 5 cm, 30 minutes, TS = 18.5 | Anode: BI-M1/cathode: non-dominant arm | ①② | None |
| Kolskår 2020 60 | Parallel | I: 27/C: 27 | 69.13 ± 7.374 | I:23/4 C:17/10 | 25.74 ± 9.17 months | IS&HS | I: a-tDCS + RT/C: sham-tDCS + RT | 1 mA, 5 × 7 cm, 20 minutes/session, 2 times/week × 3 weeks, TS = 6 | Anode: the left DLPFC/cathode: O2 | Task-fMRI | NA |
| Kang 2009 57 | Crossover | P: 10/HC: 10 | 69.9 ± 13.0 | 6/4 | 544.1 ± 388.6 days | IS&HS | I: a-tDCS/C: sham-tDCS | 2 mA, 20 minutes/session, washout: 2 days, TS = 2 | Anode: the left DLPFC/cathode: CSA | ② | NA |
| Jo 2009 56 | Crossover | 10 | 47.9 ± 8.9 | 7/3 | 2.4 ± 1.0 months | IS&HS | I1: a-tDCS/I2: c-tDCS/C: sham-tDCS | 2 mA, 5 × 5 cm, 30 minutes/session, washout: 48 hours, TS = 2 | Anode: the left DLPFC/cathode: CSA | ② | Transient aching or burning sensations: 6 Transient skin redness at the electrode contact site: 3 |
| Au-yueng 2014 (three arms) 58 | Crossover | 10 | 62.6 ± 5.7 | 10/0 | 8.3 ± 3.2 years | NA | I: tDCS + RT/C: sham + RT | 2 mA, 35 cm2, 20 minutes/session, washout: at least 5 days, TS = 3 | I1 anode: Ip-M1/cathode: CSA I2 anode: CSA/cathode:NI-M1 | ② | NA |
| Szépfalusi 2017 62 | Parallel | I: 10/C: 7 | I: 53.6 ± 13.48 C: 58.29 ± 10.95 |
I:6/4 C:7/0 | NA | NA | I: tDCS + RT/C: sham + RT | NA | NA | ①② | NA |
| TMS | |||||||||||
| Li 2021 37 | Parallel | I: 33/C: 32 | I: 61.79 ± 5.51 C: 59.47 ± 6.75 |
I: 21/12 C: 19/13 | I: 28.64 ± 12.60 days C:27.78 ± 11.01 days |
IS&HS | I: 1 Hz-rTMS + RT / C: sham-rTMS + RT | 1 Hz, 90% MT, 1,000 pulse, 20 minutes/day, 5 days/week × 4 weeks, TS = 20 | Contralateral DLPFC | ①②③⑤ | NA |
| Li 2020 69 | Parallel | I:15/C:15 | I: 65.47 ± 3.68 C: 64.53 ± 4.72 | I: 7/8 C: 9/6 | I: 22.73 ± 8.05 days C:19.13 ± 7.95 days |
HS | I: 5 Hz rTMS/C: sham-rTMS | 5 Hz 100% MT, 2,000 pulse, 20 minutes/day, 5 days/week × 3 weeks, TS = 15 | The left DLPFC | ① | I: transient dizziness or headache: several C: light dizziness2 |
| Tsai 2020 (3 arms) 70 | Parallel | I1: 14/I2: 15/C: 15 | I1: 57.45 ± 12.3 I2: 60.13 ± 14.1 C: 56.23 ± 12 |
I1:9/2 I2:11/4 C:13/2 | I1:33.27 ± 26.4 months I2:18.47 ± 20.21 months C: 38 ± 7.9 months |
IS&HS | I1: 5 Hz-rTMS + RT/I2: iTBS + RT/C: sham-rTMS + RT | I1:5 Hz, 80% MT, 600 pulse, 20 minutes/day, 5 days/week × 2 weeks. I2: 3 pulses of 50 Hz bursts, repeated at 5 Hz, 600 pulses,80% MT, TS = 10 |
The left DLPFC | ①②③ | None |
| Liu 2020 71 | Parallel | I:29/C:29 | I: 58.55 ± 6.24 C: 57.69 ± 7.25 |
I: 10/19 C: 16/13 | I: 8.79 ± 1.84 months C:8.62 ± 1.84 months |
IS&HS | I: 10 Hz-rTMS + RT/C: sham-rTMS + RT | 70 mm figure-8 coil, 10 Hz, 90% MT, 700 pulse/day, 5 days/week × 4 weeks, TS = 20 | The left DLPFC | ①②④⑤ | None |
| Yin 2020 72 | Parallel | I: 16/C: 18 | I: 56.69 ± 12.92 C: 58.17 ± 11.27 |
I:14/2 C: 16/2 | I: 52 (38.25–98.75) days C:55 (39.75–94.75) days |
IS&HS | I: 10 Hz-rTMS + RT/C: sham-rTMS + RT | 70 mm figure-8 coil, 10 Hz, 80% MT, 2,000 pulse/day, 20 minutes/day, 5 days/week × 4 weeks, TS = 20 | The left DLPFC | ①②③⑤ | NA |
| Lu 2015 73 | Parallel | I: 19/C: 21 | I: 42.5 ± 12.3 C: 47.3 ± 11.8 |
I: 12/7 C: 13/8 | I: 67 (30, 365) days C: 56 (30, 296) days |
IS&HS | I: 1 Hz-rTMS/C: sham-rTMS | 1 Hz, 100% MT, 5 days/week × 4 weeks, TS = 20 | The right DLPFC | ①③ | Transient headache: I-1 C-1 Dizziness: I-1 |
| Jorge 2004 74 | Parallel | I: 10/C: 10 | I: 63.1 ± 8.1 C: 66.5 ± 12.2 |
I: 6/4 C: 5/5 | 17.8 ± 14.3 months | IS&HS | I: 10 Hz-rTMS/C: sham-rTMS | 70 mm figure-8 coil, 10 Hz, 110% MT, 1,000 pulse/day, 5 days/week × 2 weeks, TS = 10 | The left M1 | ① | Transient headaches: 6 Local discomfort at the site of the stimulation usually produced by tightness of the stimulation cap: 5 An exacerbation of initial insomnia: 1 |
| Kim 2010 (3 arms) 76 | Parallel | I1: 6/I2: 6/C: 6 | I1: 69.3 ± 7.4 I2: 53.5 ± 16.9 C: 66.8 ± 17.2 |
I1:2/4 I2:4/2 C:4/2 | I1: 404.4 ± 71.7 days I2: 241.2 ± 42.5 days C: 69.7 ± 39 days |
IS&HS | I1: 1 Hz-rTMS + RT/I2:10 Hz-rTMS + RT/C: sham-rTMS + RT | I1:1 Hz, 80% MT, 900 pulse/I2:10 Hz, 80% MT, 450 pulse, 20 minutes/day, 5 days/week × 2 weeks, TS = 10 | The left DLPFC | ②③④⑤ | None |
| Askın 2017 75 | Parallel | I: 20/C: 20 | I: 56.75 ± 11.46 C: 58.80 ± 12.02 |
I: 14/6 C: 15/5 | I: 28.35 ± 15.34 months C: 24.35 ± 15.39 months |
NA | I: 1 Hz-rTMS + RT/C: sham-rTMS + RT | 1 Hz, 90% MT, 1,200pulse/day, 20 minutes/session, 5 days/week × 2 weeks, TS = 10 | NI-M1 | ①⑤ | None |
| Fregni 2006 77 | Parallel | I: 10/C: 5 | I: 57.7 ± 11.27 C: 52.6 ± 12.56 |
I: 8/2 C: 3/2 | I: 3.52 ± 2.93 years C: 3.97 ± 2.64 years |
IS | I: 1 Hz-rTMS/C: sham-rTMS | 1 Hz, 100% MT, 1,200pulse/day, 20 minutes/session, TS = 5 | NI-M1 | ①② | I: A mild headache (contralateral to the side of TMS application):1 An increase in anxiety:1. C: An increase in the tiredness:1 C: A mild headache:1 |
| Li 2021 (three arms) 24 | Parallel | I1: 30/I2: 30/I3: 30 | I1: 56.77 ± 8.58 I2: 57.60 ± 7.40 I3: 55.13 ± 7.90 |
I1:20/10 I2:19/11 I3:18/12 | I1: 3.63 ± 1.85 months I2: 3.80 ± 1.71 months I3: 3.67 ± 1.84 months |
IS&HS | I1: 1 Hz-rTMS + cTBS/C1: 1 Hz-rTMS/C2: cTBS | I and C1:1 Hz, 80% MT, 1,000 pulse, 20 minutes/day, 6 days/week × 4 weeks C2: 80% of AMT, 20 seconds of stimulation time, 100 stimuli, 3 pulses, repeated 4 times, 1,200 pulse, TS = 24 |
I and C1: NI-M1 C2: right cerebellar hemisphere | ⑤ | NA |
| Georgiou 2022 61 | Parallel | I1: 3/I2: 3 | 56 ± 17.72 | 2/4 | NA | IS | I1: cTBS/ I2:1Hz-rTMS | 80% MT, system for guide coil position. I2:1,200pulses × 20 minutes | Right pars triangularis | ② | None |
AMT, active motor threshold; a-tDCS, anode-transcranial direct current stimulation; BI, bilateral; C, control group; CSA, contralateral supraorbital area; cTBS, continuous theta burst stimulation; c-tDCS, cathode-transcranial direct current stimulation; DLPFC, dorsolateral prefrontal cortex; FT, fronto-temporal; HC, healthy control; I, intervention; IFG, inferior frontal gyrus; Ip, ipsilesional; IQR, interquartile range; ISA, ipsilesional supraorbital area; iTBS, intermittent theta burst stimulation; M1, primary motor cortex; MT, motor threshold; NI, non-ipsilesional; RT, rehabilitation therapy; rTMS, repetitive transcranial magnetic stimulation; SD, standard deviation; STG, superior temporal gyrus; tDCS, transcranial direct current stimulation; TMS, transcranial magnetic stimulation; TS, total session of stimulation.
① Global cognitive function, ② attention, ③ memory, ④ executive function, ⑤ activities of daily living.
Study characteristics
Nineteen of 20 studies (95%) were RCTs, while one (5%) was a randomized cross-over. 67 Two tDCS,64,65 and three TMS24,70,76 had three arms, and one tDCS study 68 had four arms. The sample sizes of the included studies ranged from 14 to 100. Participants’ ages ranged from 42.5 to 70.3 years. Since stroke onset, the average time was 19.13 days to 3.97 years. Eighty-five patients treated with active anode-tDCS in four studies,64–66,68 90 patients with dual-tDCS in four studies,13,35,63,67 40 patients with cathode-tDCS in two studies,36,68 90 patients with HF-rTMS in six studies,69–72,74,76 118 patients with LF-rTMS in six studies,24,37,73,75–77 15 patients with iTBS in one study, 70 30 patients with cTBS, and 30 patients with LF-rTMS plus cTBS all in one study. 24 The median number of stimulation sessions was 15. Table 1 summarizes the trials’ features.
Network meta-analysis
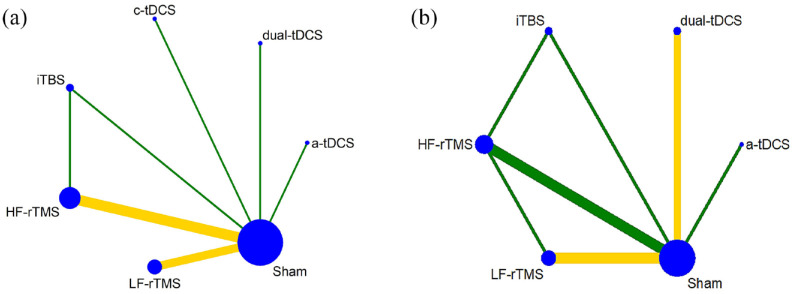
Figure 2 shows the network structure for GCF and memory evaluation. Supplemental Figure 1 shows the network structure for the rest of the main and secondary outcomes. League tables (Table 2 and Supplemental Table 1) exhibit the results of the NMA.
Figure 2.
Network diagrams of NIBS versus Sham stimulation in patients with stroke for global cognitive function (a) and memory (b).
The nodes were linked by a line when the treatments were directly comparable. The width of each line is proportional to the number of randomized controlled trials, and the size of each node is proportional to the number of patients (sample size). The colors of the edges indicate the risk of bias for this comparison (green = low, yellow = unclear, and red = high risk of bias).a-tDCS: anode transcranial direct current stimulation; c-tDCS: cathode transcranial direct current stimulation; dual-tDCS: dual transcranial direct current stimulation; HF-rTMS: high-frequency repetitive trancranial magnetic stimulation; iTBS: intermittent theta burst stimulation; LF-rTMS: low-frequency repetitive transcranial magnetic stimulation; NIBS: noninvasive brain stimulation.
Table 2.
The league table of the network and pairwise meta-analysis of cognition assessment after stroke: global cognitive function, and memory.
| Global cognitive function | ||||||
|---|---|---|---|---|---|---|
| Sham | 0.32 (−0.40,1.04) | 0.78 *(0.21, 1.36) | –1.20*(−1.99, –0.42) | 1.93* (0.93,2.93) | 1.47 (−0.26,3.20) | 1.03* (0.26,1.79) |
| −0.32 (−3.58, 2.95) | a-tDCS | NA | NA | NA | NA | NA |
| −0.78 (−4.02, 2.45) | −0.47 (−5.06, 4.12) | dual-tDCS | NA | NA | NA | NA |
| 1.20 (−2.07, 4.48) | 1.52 (−3.10, 6.14) | 1.99 (−2.62, 6.59) | c-tDCS | NA | NA | NA |
| −1.95*(−3.43, –0.47) | −1.63 (−5.21, 1.95) | −1.16 (−4.72, 2.39) | −3.15 (−6.74, 0.45) | HF-rTMS | NA | −0.24 (-0.97, 0.49) |
| −1.47 (−3.11, 0.17) | −1.15 (−4.81, 2.50) | −0.69 (−4.31, 2.94) | −2.67 (−6.34, 0.99) | 0.48 (−1.74, 2.69) | LF-rTMS | NA |
| −1.38 (−4.30, 1.54) | −1.06 (−5.44, 3.32) | −0.59 (−4.95, 3.76) | −2.58 (−6.97, 1.81) | 0.57 (−2.35, 3.49) | 0.09 (−3.26, 3.44) | iTBS |
| Memory | ||||||
| Sham | 0.20 (−0.52, 0.92) | 6.49* (2.46, 10.51) | 1.16* (0.13, 2.19) | 1.97 (−0.52, 4.47) | 1.06* (0.29, 1.84) | |
| −0.20 (−3.99, 3.59) | a-tDCS | NA | NA | NA | NA | |
| −6.38* (−9.25, –3.51) | −6.18* (−10.93, –1.43) | dual-tDCS | NA | NA | NA | |
| −1.47 (−3.61, 0.68) | −1.27 (−5.62, 3.09) | 4.91* (1.33, 8.50) | HF-rTMS | 0.08 (−1.05, 1.21) | −0.91* (−1.68, –0.14) | |
| −2.09 (−4.23, 0.06) | −1.89 (−6.24, 2.47) | 4.29* (0.71, 7.88) | −0.62 (−3.34, 2.10) | LF-rTMS | NA | |
| −0.86 (−4.32, 2.59) | −0.66 (−5.79, 4.46) | 5.52* (1.03, 10.01) | 0.60 (−2.85, 4.06) | 1.22 (−2.73, 5.18) | iTBS | |
a-tDCS, anode transcranial direct current stimulation; c-tDCS, cathode transcranial direct current stimulation; dual-tDCS, dual transcranial direct current stimulation; HF-rTMS, high-frequency repetitive transcranial magnetic stimulation; iTBS, intermittent theta burst stimulation; LF-rTMS, low-frequency repetitive transcranial magnetic stimulation; NA, not applicable.
Data were presented as SMDs with 95% CIs; the lower left part was the finding of network meta-analysis, and the upper right part was the finding of pairwise meta-analyses. The positive SMD reflects the better efficacy of the lower right intervention; the negative SMD favors the upper left intervention.
Statistically significant, p<0.05.
Summary of network geometry and Synthesis of results
Global cognitive function
Twelve studies13,24,36,64,69–75,77 (460 participants) involving seven treatment options were employed to evaluate GCF. Pairwise meta-analysis between NIBS options and sham stimulation revealed that dual-tDCS, HF-rTMS, and iTBS were significantly more effective than sham stimulation (SMD = 0.78; 95% CI: 0.21–1.36, N (number of studies) = 1, total sample size = 50, p = 0.008; SMD = 1.93; 95% CI: 0.93–2.93, N = 5, total sample size = 171, p = 0.0002, I2 = 85%; SMD = 1.03; 95% CI: 0.26–1.79, N = 1, total sample size = 30, p = 0.009, respectively. See Table 2 and Supplemental Figure 2A). Based on NMA, only HF-rTMS significantly enhanced the impact in comparison with sham stimulation (SMD = 1.95; 95% CI: 0.47–3.43, p < 0.05). None of the other treatment comparisons revealed a statistically significant difference (Table 2 and Supplemental Figure 3A).
Attention
Fourteen studies13,24,35,63–68,70–72,76,77 (558 patients) involving seven treatment options reported scales representing attention function were compared directly and indirectly. Direct evidence indicated that all tDCS and HF-rTMS were significantly more effective than sham (a-tDCS: SMD = 2.44; 95% CI: 0.14–4.73, N = 4, total sample size = 121, p = 0.04, I2 = 95%; dual-tDCS: SMD = 2.36; 95% CI: 0.65–4.07, N = 4, total sample size = 178, p = 0.007, I2 = 95%; c-tDCS: SMD = 2.56; 95% CI: 1.80–3.32, N = 1, total sample size = 50, p < 0.00001; HF-rTMS: SMD = 1.62; 95% CI: 0.19–3.05, N = 4, total sample size = 133, p = 0.03, I2 = 91%). And a-tDCS showed significantly more effective compared with c-tDCS (SMD = 1.59; 95% CI: 0.94–2.23, N = 1, total sample size = 50, p < 0.00001). HF-rTMS was significantly more effective than iTBS (SMD = 0.96; 95% CI: 0.19–1.74, N = 1, total sample size = 29, p = 0.01. See Supplemental Table 1A and Supplemental Figure 2B). However, we found no significant effect of NIBS alternatives on attention function measured by NMA (Supplemental Table 1A and Supplemental Figure 3B).
Memory
Eight RCTs35,37,63–65,70,72,73 (335 participants) on memory comprised six NIBS alternatives. The pairwise meta-analysis suggested a significant difference between dual-tDCS, HF-rTMS, iTBS, and sham stimulation (dual-tDCS: SMD = 6.49; 95% CI: 2.46–10.51, N = 2, total sample size = 100, p = 0.002, I2 = 92%; HF-rTMS: SMD = 1.16; 95% CI: 0.13–2.19, N = 3, total sample size = 75, p = 0.03, I2 = 74%; iTBS: SMD = 1.06; 95% CI: 0.29–1.84, N = 1, total sample size = 30, p = 0.007). And HF-rTMS was significantly more effective than iTBS (SMD = 0.91; 95% CI: 0.14–1.68, N = 1, total sample size = 29, p = 0.02. See Table 2 and Supplemental Figure 2C).
In NMA, dual-tDCS is significantly more effective than sham (SMD = 6.38; 95% CI: 3.51–9.25, p < 0.05), a-tDCS (SMD = 6.18; 95% CI: 1.43–10.93, p < 0.05), HF-rTMS (SMD = 4.91; 95% CI: 1.33–8.50, p < 0.05), LF-rTMS (SMD = 4.29; 95% CI: 0.71–7.88, p < 0.05), and iTBS (SMD = 5.52; 95% CI: 1.03–10.01, p < 0.05), but not for other options compared directly and indirectly (Table 2 and Supplemental Figure 3C).
Executive Function
Five trials13,35,63,65,71 with 226 participants used NIBS (four stimulation choices) to improve EF. Dual-tDCS and LF-rTMS showed a substantial effect over sham (dual-tDCS: SMD = 3.97; 95% CI: 1.12–6.83, N = 3, total sample size = 150, p = 0.006, I2 = 97%; LF-rTMS: SMD = 1.34; 95% CI: 0.04–2.65, N = 1, total sample size = 12, p = 0.04. Supplemental Table 1B and Supplemental Figure 2D). NMA results showed no difference between NIBS treatment comparisons (see Supplemental Table 1B and Supplemental Figure 3D).
Secondary outcomes
Activities of daily living
Eleven RCTs13,24,35–37,63–65,71,72,75 with 515 participants and eight treatment alternatives were included for the secondary outcome of ADL. The pairwise meta-analysis suggested a significant difference between dual-tDCS and sham stimulation (SMD = 2.36; 95% CI: 0.05–4.68, N = 3, total sample size = 150, p = 0.05, I2 = 97%). cTBS showed a substantial effect over LF-rTMS (SMD = 1.74; 95% CI: 1.14–2.34, N = 1, total sample size = 60, p < 0.00001). LF-rTMS combined with cTBS options was more significant than either LF-rTMS or cTBS (versus LF-rTMS: SMD = 1.37; 95% CI: 0.81–1.94, N = 1, total sample size = 60, p < 0.00001; versus cTBS: SMD = 3.30; 95% CI: 2.51–4.10, N = 1, total sample size = 60, p < 0.00001. See Supplemental Table 1C and Supplemental Figure 2E). But NIBS options have no significant efficiency for improving ADL by NMA (Supplemental Table 1C and Supplemental Figure 3E).
Adverse effects
Six of 16 tDCS trials mentioned adverse or side effects, and in two studies13,56 mentioned mild side effects in active and sham groups. Liu et al. 13 reported that in the course of tDCS treatment, only six subjects experienced mild headaches and discomfort at a low discomfort rate. These side effects were relieved by relaxation. While in Jo’s 56 clinical trial, there were some adverse effects during tDCS that disappeared after a few seconds. Transient aching or burning sensations were reported in six cases, and transient skin redness at the electrode contact site was reported in three cases. Other four studies36,59,67,68 reported no adverse effects or dropout.
Nine of 13 TMS studies mentioned adverse or side effects; four69,73,74,77 reported mild to moderate side effects. There were seven patients in one of the four studies experienced transient dizziness or headache in the rTMS group, and two patients complained of light dizziness in the control group. 69 Another study reported one patient experienced a transient headache and another experienced dizziness in the rTMS group, and one patient experienced a headache in the sham group. 73 Jorge et al. 74 mentioned the mild level of side effects in the patients, including transient headaches (six patients) that were relieved with low doses of acetaminophen, local discomfort at the site of the stimulation usually produced by the tightness of the stimulation cap (five patients), and an exacerbation of initial insomnia observed in one patient. Finally, Fregni et al. 77 found that in the active group, only one patient reported a mild headache (contralateral to the side of TMS application) and one patient reported an increase in anxiety. In the sham rTMS group, one patient reported an increase in tiredness and one patient reported a mild headache. The rest of the five RCTs61,70, 71 ,75,76 reported no adverse effects. These articles did not observe severe side effect, such as seizes. The findings revealed that there was no discernible difference between tDCS and TMS in the frequency of adverse effects by NMA. (see Supplemental Table 1D and Supplemental Figure 2F).
SUCRA
According to the results of SUCRA (Table 3), HF-rTMS (SUCRA = 80.4) may be the best way to improve GCF. Table 3 showed that dual-tDCS may be the most effective intervention to improve attention (SUCRA = 70.9), memory (SUCRA = 99.5), EF (SUCRA = 75.0), and ADL scores (SUCRA = 73.4). Apart from sham stimulation (SUCRA = 78.3), tDCS (SUCRA = 37.2) may be slightly safer for patients than TMS (SUCRA = 34.5).
Table 3.
Rankings by SUCRA of different NIBS for improving cognitive function after stroke.
| Intervention | GCF | Attention | Memory | EF | ADL |
|---|---|---|---|---|---|
| Sham | 28.4 | 15.7 | 17.7 | 18.7 | 31.8 |
| a-tDCS | 42.3 | 65.9 b | 28.8 | – | 42.4 |
| dual-tDCS | 51.4 | 70.9 a | 99.5 a | 75.0 a | 73.4 a |
| c-tDCS | 15.6 | 53.0 | – | – | 27.6 |
| HF-rTMS | 80.4 a | 55.8 | 51.9 | 56.2 b | 61.7 |
| LF-rTMS | 68.6 b | 53.8 | 63.6 b | 50.1 | 57.6 |
| iTBS | 63.2 | 34.9 | 38.5 | – | – |
| cTBS | – | – | – | – | 33.5 |
| LF-rTMS + cTBS | – | – | – | 71.9 b |
ADL, ability of daily living; a-tDCS, anode transcranial direct current stimulation; cTBS, continuous theta burst stimulation; c-tDCS, cathode transcranial direct current stimulation; EF, executive function; GCF, global cognitive function; HF-rTMS, high-frequency repetitive transcranial magnetic stimulation; iTBS, intermittent theta burst stimulation; LF-rTMS, low-frequency repetitive transcranial magnetic stimulation; NIBS, noninvasive brain stimulation; rTMS, repetitive transcranial magnetic stimulation; SUCRA, surface under the cumulative ranking line; tDCS, transcranial direct current stimulation.
Presents the first-ranking.
Presents the second-ranking.
Risk of bias assessment
Figure 1(b) shows the bias summary and graph. Eleven RCTs13,35,36,66–72,75 were graded as having a low risk of bias in a random sequence using the random number table approach. Nine trials13,35,36,66–68,70–72 had a low risk of bias in allocation concealment. Three studies13,63,75 were rated the high risk of performance bias for a single-blinded design; two of them13,75 mentioned the assessor-blind method, and the other one did not describe the blinding object. 63 Four trials24,37,65,73 without mentioning the blinding way were high-risk bias, with one 24 describing the study hypothesis blinded to the patients in the ‘Methods’ section. The remaining 13 RCTs35,36,64,66–72,74,76,77 were double-blinded designs. Most studies with an adequate description for incomplete results were rated as low risk of attrition bias, except for one. 24 No RCTs had reporting bias.
Exploration for inconsistency
We did not observe any significant inconsistency between direct and indirect comparisons. Formal testing found no statistically significant design inconsistency in GCF (χ2 = 0.16; p = 0.6938), attention (χ2 = 0.87; p = 0.9287), memory (χ2 = 0.98; p = 0.8068), EF (χ2 = 0.23; p = 0.6352), or ADL (χ2 = 0.56; p = 0.7547) and no local inconsistency.
Risk of bias across studies
Egger’s test found publication bias in attention (p = 0.016) but not in GCF (p = 0.261), memory (p = 0.132), EF (p = 0.108), or ADL (p = 0.153). Supplemental Figures 4–8 show funnel plot analysis and Egger’s test of included studies. The network map’s line colors could indicate the bias across different NIBS (Figure 2 and Supplemental Figure 1). According to the GRADE system, GCF and memory outcomes were classified as moderate-quality evidence, while attention, EF, and ADL outcomes were low-quality evidence (Supplemental Table 2).
Results of additional analyses
We did sensitivity analysis by deleting one RCT each time. The results (Supplemental Figures 4–8) showed no significant change compared with those before the investigation, indicating that the meta-analysis results were relatively robust.
We detected subgroup network analysis of GCF and memory scores according to the stimulation site. The left DLPFC (L-DLPFC) showed more effectiveness than the sham site (SMD = 1.77; 95% CI: 0.88–2.66, p < 0.05) and non-ipsilesional primary motor cortex (NI-M1) (SMD = 2.06; 95% CI: 0.53–3.60, p < 0.05) for GCF recovery. In terms of memory restoration, bilateral DLPFC is significantly more effective than sham (SMD = 8.65; 95% CI: 6.15–11.15, p < 0.05), L-DLPFC (SMD = 7.71; 95% CI: 5.11–10.32, p < 0.05), right DLPFC (R-DLPFC) (SMD = 7.35; 95% CI: 4.42–10.27, p < 0.05), bilateral M1 (SMD = 4.11; 95% CI: 1.10–7.13, p < 0.05), left frontotemporal cortex (L-FT) (SMD = 8.44; 95% CI: 5.50–11.37, p < 0.05), and right FT (R-FT) (SMD = 8.53; 95% CI: 5.60–11.47, p < 0.05). Bilateral M1 is significantly superior to sham (SMD = 4.54; 95% CI: 2.85–6.22, p < 0.05), L-DLPFC (SMD = 3.60; 95% CI: 1.75–5.45, p < 0.05), R-DLPFC (SMD = 3.23; 95% CI: 0.96–5.50, p < 0.05), L-FT (SMD = 4.32; 95% CI: 2.03–6.61, p < 0.05), and R-FT (SMD = 4.42; 95% CI: 2.13–6.71, p < 0.05). A comparison of the ranking of treatment sites revealed that stimulation over left DLPFC (SUCRA = 89.1) contributed the most to the improvement of GCF. On the other hand, stimulation over bilateral DLPFC (SUCRA = 99.9) contributed the most to improving memory function. Supplemental Figure 9 and Supplemental Table 3 showed our subgroup analysis results.
Discussion
Summary of evidence
This NMA demonstrated the possibility of obtaining evidence that HF-rTMS improves GCF in stroke patients compared with sham stimulation. In contrast to other NIBS protocols, dual-tDCS improved memory performance in stroke patients. However, various NIBS stimulation protocols show no significant impact on enhancing attention, memory, or executive function. Also, of the many NIBS protocols, HF-rTMS may be the most effective therapy for enhancing GCF; dual-tDCS may mostly increase attention, memory function, EF, and ADL. The NIBS examination of the safety of TMS and tDCS active stimulation protocols and sham stimulation revealed no significant difference. Moreover, tDCS may offer a higher level of patient safety. Our subgroup analysis demonstrated an effect favoring activation of the left DLPFC for enhancing GCF and bilateral DLPFC stimulation for enhancing memory performance.
As far as we know, there have only been a handful of papers comparing different TMS and tDCS protocols in stroke patients for cognition rehabilitation. A comparison of the efficacy of two NIBS protocols in patients with post-stroke dysphagia was observed.21,78 Following a stroke, patients with motor or speech impairments have been studied for various TMS protocols,79,80 and tDCS protocols.25,81
We believe our NMA offers fresh and significant insights into the relative efficacy of various NIBS therapy approaches. It provides evidence for selecting TMS or tDCS and comparing NIBS options for stroke survivors with cognitive dysfunction.
Our NMA results are partially compatible with a previous evaluation of the impact of NIBS on cognitive performance, especially on attention and memory deficits following stroke.27,31–33 For the main outcomes, rTMS improved GCF and memory in stroke patients,31–33 whereas tDCS does not.27,31 In the assessment of attention performance, all the meta-analyses of NIBS showed positive results,27,31–33 except the tDCS part in the review of Hara et al. 31 Due to the methodological restrictions of conventional meta-analysis, the authors of these reviews could only conduct pairwise comparisons. Furthermore, the authors analyzed a single group consisting of several rTMS or tDCS stimulation options. While low-frequency (1 Hz) transcranial magnetic stimulation seemed to exhibit a similar effect with a high-frequency (10 Hz) treatment for improving GCF and memory. 33 This might explain the uniformity of the combined results of different rTMS protocols. However, no researchers have performed a subgroup analysis of different tDCS options, so that, the combined effect of tDCS is more disagreement and needs further study and analysis. In addition, few papers were included in some of these meta-analyses, particularly for tDCS, for which only two literature mergers were conducted. 31 We discovered that one of the literatures 59 contained data that could not be retrieved directly: pre- and post-treatment ratios. Although it did not cause significant heterogeneity, it might still have a specific influence on the results.
In the secondary outcome evaluation, only the pairwise meta-analysis suggested a significant improvement for ADL by the dual-tDCS option, with no favorable effect by NMA. One NMA of tDCS 81 on motor function in stroke patients indicated that cathodal-tDCS enhanced ADL relative to sham stimulation and were superior to other treatments. Nonetheless, we discovered that all tDCS stimulation sites in the reviewed literature were affected, the non-affected, or the bilateral primary motor cortex, which may account for the discrepancy between our findings. Another two meta-analyses showed improvement by TMS treatment on ADL for stroke patients.32,33 However, the main outcomes reflecting ADL in these two reviews were both modified Barthel index (MBI), while our study set a combination of MBI and FIM scores as the target outcomes. Another reason for the different results from our study may lie in the different included literature. Xu et al. 33 included only three Chinese studies, but we did not take Chinese literature into account due to the consideration of the overall quality of the literature.
In terms of safety studies, we were in general agreement with the results of previous studies. The authors found no major adverse effects in an NMA 78 investigating noninvasive treatments for post-stroke dysphagia; Another NMA 21 reported that one rTMS study 82 and one tDCS study 83 said dizziness, headache, or nosebleed after the intervention. However, neither of these reviews compared rTMS and tDCS, probably due to the small number of articles. In our study, 12.5% (2/16) of tDCS articles reported side effects in both real and sham groups, with 25% (4/16) mentioning no adverse effect after tDCS intervention; 30.8% (4/13) of TMS studies reported adverse reactions in both the true and sham stimulation groups, while 38.5% (5/13) found no side effects. NMA ranked tDCS higher than TMS, although neither treatment was significantly different from sham stimulation. Mild to moderate adverse effects were reported and resolved quickly. Therefore, we have grounds to believe that these two NIBS approaches are reasonably safe.
Another challenge for therapists is selecting the stimulation site. The majority of research focused on the area of the DLPFC: 8/15 of tDCS studies selected excitatory stimulation for the DLPFC (four on L-DLPFC, one on R-DLPFC, and three on bilateral DLPFC). Eight of 12 TMS studies chose the DLPFC for stimulation site (seven on L-DLPFC and one on R-DLPFC). Although our study did identify a benefit of the left DLPFC region over other regions, functional imaging confirmation is still required to enhance the study’s objectivity. When just one target could be selected, all TMS studies used single-head stimulation; hence, the left DLPFC may be the preferred alternative. Dual-tDCS protocols with the anode over the left DLPFC and the cathode over the right DLPFC are favored and may be the first choice for enhancing memory function.
It was indicated that DLPFC was an important site in cognitive function. Some studies have shown that DLPFC is related to cognitive function mainly for processing speed, selective attention, working memory, and episodic memory.69–72,84–87 Besides, DLPFC plays an important part in the central executive network (CEN), which is responsible for high-level cognitive functions, notably the control of attention and working memory. 88 A meta-analysis has shown that HF-rTMS stimulation of DLPFC could improve cognitive function for healthy people, patients with Alzheimer’s disease, depression, executive dysfunction, memory complaints, and Parkinson’s disease. 89 The mechanism that produces these effects may be based on the theory of interhemispheric inhibition, where excitation of the left DLPFC (and/or inhibition of the right DLPFC) serves to modulate the function of the cognitively relevant cortex, and important brain networks, which consequently promotes the recovery of cognitive function. 90
However, it is currently unclear if DLPFC should be used as a stimulation location for all patients, regardless of stroke type, stroke origin, or damage site, despite functional neuroimaging studies suggesting DLPFC plays a crucial role in the neural circuitry of cognitive function and it has been shown that therapeutic interventions, which aim to interfere with improper cortical activity, may address pathological connections not only at the DLPFC but also among distant brain regions.91,92 In our findings, the DLPFC is not the only beneficial site. A treatment plan delivered bilaterally to the M1 region is effective in improving memory function in stroke patients. Inadequate studies included in our analysis, notably the lack of studies using stimulation sites other than the DLPFC, also contributed to the outcome. Furthermore, the original study did not perform a stratified analysis of the different stroke locations or stroke types, which suggests that future studies might be able to concentrate on these areas.
Our results for primary and secondary indicators imply a high degree of heterogeneity, presumably because we aggregated the impacts of different scales reflecting similar functions, intervention parameters (number of stimuli, current or magnetic stimulation intensity, etc.), and populations. A large number of outcome variables and a small sample size might cause publication bias in attention outcomes. More than half of EF and ADL studies did not address random sequence generation or allocation concealment; the research was less rigorous in design and less descriptive in the report.
Limitations
Our study has several limitations. First, NIBS (particularly c-tDCS and cTBS) to improve cognitive function after stroke lacked high-quality RCTs, and even fewer trials examined stroke patients’ memory and EF, thus the certainty of the evidence is reduced. Our study on the subgroup of protocols and stimulation sites is limited by the absence of related RCTs in comparison to published meta-analyses. However, this constraint points the way for future research. And due to the small number of included studies and the several ways of cognitive function evaluation, the method we pooled the data may have resulted in a large degree of heterogeneity, which compromised the robustness of the conclusions. Some of the included literature incorporated various rehabilitation training, such as visual reaction (VR), computer-assisted cognitive training (CCT), and so on, and the kind and duration of stroke varied, leading to considerable heterogeneity. A lack of relevant gray research may prejudice study selection. Half of the trials had questionable allocation concealment bias, reducing RCT quality.
Second, although we did not detect inconsistencies in the primary and secondary outcomes, this does not mean any inconsistencies occurred. 41 This is partly because most of the network diagrams are not closed loop, which leads to the fact that our analyses are not strictly NMA or multiple treatment comparisons (MTCs), but belongs partly to the NMA genus of adjusted indirect treatment comparison (ITC). 93
Finally, we only evaluated the immediate effects after NIBS treatment, and some articles followed up on the long-term effects, which deserve further concentration.
Conclusion
Our NMA of RCTs indicates that NIBS positively affects GCF and memory performance after stroke, with no apparent side effects. When NIBS is utilized to enhance GCF, HF-rTMS, particularly over the left DLPFC, maybe the appropriate therapeutic option. Dual-tDCS, over bilateral DLPFC, may improve memory function after stroke, which is superior to other NIBS options. No difference regarding safety (in terms of dropouts and adverse events) was seen between different types of NIBS.
However, the effect of NIBS on attention, EF, and ADL performance was insignificant, while the impact of c-tDCS and cTBS protocols was even weaker. New RCTs of interventions of high quality are required to increase the exploration of the efficacy of different NIBS intervention modalities, particularly tDCS and TBS protocols, for different dimensions of cognitive function to establish their evidence of efficacy with greater reliability. In the future, we advise clinical therapists to use left DLPFC site HF-rTMS and bilateral DLPFC dual-tDCS as supplementary treatments for cognitive impairment and memory deficits in stroke rehabilitation.
Supplemental Material
Supplemental material, sj-docx-19-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-20-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-21-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-22-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-23-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-1-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-10-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-11-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-12-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-13-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-14-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-2-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-3-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-4-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-5-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-6-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-7-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-8-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-9-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-png-15-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-png-16-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-png-17-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-png-18-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-png-24-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors would like to thank the School of Public Health, Nanjing Medical University, for assistance in data analysis for this article.
Footnotes
ORCID iD: Yao Wang  https://orcid.org/0000-0002-5940-4988
https://orcid.org/0000-0002-5940-4988
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yao Wang, Department of Rehabilitation Medicine, Affiliated Nanjing Brain Hospital, Nanjing Medical University, Nanjing, China.
Wan Liu, Department of Rehabilitation Medicine, Affiliated Nanjing Brain Hospital, Nanjing Medical University, Nanjing, China.
Jiu Chen, Department of Radiology, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China; Institute of Medical Imaging and Artificial Intelligence, Nanjing University, Nanjing, China.
Jianling Bai, Department of Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, China.
Hao Yu, Department of Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, China.
Hongxia Ma, Department of Epidemiology, Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China.
Jiang Rao, Department of Rehabilitation Medicine, Affiliated Nanjing Brain Hospital, Nanjing Medical University, No. 264 Guangzhou Road, Jiangsu Province, Nanjing 210029, China.
Guangxu Xu, Rehabilitation Medicine Center, The First Affiliated Hospital of Nanjing Medical University, No. 300 Guangzhou Road, Jiangsu Province, Nanjing 210029, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Yao Wang: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Software; Visualization; Writing – original draft; Writing – review & editing.
Wan Liu: Data curation; Formal analysis; Investigation.
Jiu Chen: Data curation; Investigation; Project administration.
Jianling Bai: Formal analysis; Methodology; Software; Visualization.
Hao Yu: Methodology; Visualization.
Hongxia Ma: Validation; Writing – review & editing.
Jiang Rao: Data curation; Funding acquisition; Investigation; Project administration; Supervision; Writing – review & editing.
Guangxu Xu: Conceptualization; Funding acquisition; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Nanjing Municipal Science and Technology Bureau (grant no. 2019060002), Nanjing Municipal Special Fund Key Project for Health Science and Technology Development (grant no. ZKX22042), and Nanjing Municipal Special Fund General Project for Health Science and Technology Development (grant no. YKK22137).
The authors declare that there is no conflict of interest.
Availability of data and materials: By e-mail to the corresponding author
References
- 1.Qu Y, Zhuo L, Li N, et al. Prevalence of post-stroke cognitive impairment in china: a community-based, cross-sectional study. PLoS ONE 2015; 10: e0122864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aben HP, Reijmer YD, Visser-Meily JM, et al. A role for new brain magnetic resonance imaging modalities in daily clinical practice: protocol of the Prediction of Cognitive Recovery After Stroke (PROCRAS) Study. JMIR Res Protoc 2018; 7: e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toglia J, Askin G, Gerber LM, et al. Association between 2 measures of cognitive instrumental activities of daily living and their relation to the montreal cognitive assessment in persons with stroke. Arch Phys Med Rehabil 2017; 98: 2280–2287. [DOI] [PubMed] [Google Scholar]
- 4.Nys GM, van Zandvoort MJ, van der Worp HB, et al. Early cognitive impairment predicts long-term depressive symptoms and quality of life after stroke. J Neurol Sci 2006; 247: 149–156. [DOI] [PubMed] [Google Scholar]
- 5.Mijajlovic MD, Pavlovic A, Brainin M, et al. Post-stroke dementia – a comprehensive review. BMC Med 2017; 15: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leys D, Henon H, Mackowiak-Cordoliani MA, et al. Poststroke dementia. Lancet Neurol 2005; 4: 752–759. [DOI] [PubMed] [Google Scholar]
- 7.Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke 2013; 8: 38–45. [DOI] [PubMed] [Google Scholar]
- 8.Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: e98–e169. [DOI] [PubMed] [Google Scholar]
- 9.Stephens S, Kenny RA, Rowan E, et al. Neuropsychological characteristics of mild vascular cognitive impairment and dementia after stroke. Int J Geriatr Psychiatry 2004; 19: 1053–1057. [DOI] [PubMed] [Google Scholar]
- 10.van Rooij FG, Plaizier NO, Vermeer SE, et al. Executive function declines in the first 6 months after a transient ischemic attack or transient neurological attack. Stroke 2017; 48: 3323–3328. [DOI] [PubMed] [Google Scholar]
- 11.Nair RD, Lincoln NB. Cognitive rehabilitation for memory deficits following stroke. Cochrane Database Syst Rev 2007; 3: D2293. [DOI] [PubMed] [Google Scholar]
- 12.Cappa SF, Benke T, Clarke S, et al. Cognitive rehabilitation. In: Hughes R, Brainin M, Gilhus NE. (eds) European handbook of neurological management. New York: John Wiley & Sons, 2011, pp. 545–567. [Google Scholar]
- 13.Liu YW, Chen ZH, Luo J, et al. Explore combined use of transcranial direct current stimulation and cognitive training on executive function after stroke. J Rehabil Med 2021; 53: m162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown AW, Moessner AM, Mandrekar J, et al. A survey of very-long-term outcomes after traumatic brain injury among members of a population-based incident cohort. J Neurotrauma 2011; 28: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y, Xing Y, Zhu Z, et al. The effects of 7-week cognitive training in patients with vascular cognitive impairment, no dementia (the Cog-VACCINE Study): a randomized controlled trial. Alzheimers Dement 2019; 15: 605–614. [DOI] [PubMed] [Google Scholar]
- 16.Draaisma LR, Wessel MJ, Hummel FC. Non-invasive brain stimulation to enhance cognitive rehabilitation after stroke. Neurosci Lett 2020; 719: 133678. [DOI] [PubMed] [Google Scholar]
- 17.Manuli A, Maggio MG, Latella D, et al. Can robotic gait rehabilitation plus Virtual Reality affect cognitive and behavioural outcomes in patients with chronic stroke? A randomized controlled trial involving three different protocols. J Stroke Cerebrovasc Dis 2020; 29: 104994. [DOI] [PubMed] [Google Scholar]
- 18.Merriman NA, Sexton E, McCabe G, et al. Addressing cognitive impairment following stroke: systematic review and meta-analysis of non-randomised controlled studies of psychological interventions. BMJ Open 2019; 9: e24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldema J, Gharabaghi A. Non-invasive brain stimulation for improving gait, balance, and lower limbs motor function in stroke. J Neuroeng Rehabil 2022; 19: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding X, Zhang S, Huang W, et al. Comparative efficacy of non-invasive brain stimulation for post-stroke aphasia: a network meta-analysis and meta-regression of moderators. Neurosci Biobehav Rev 2022; 140: 104804. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Huang H, Jia Y, et al. Systematic review and network meta-analysis of noninvasive brain stimulation on dysphagia after stroke. Neural Plast 2021; 2021: 3831472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allida S, Cox KL, Hsieh CF, et al. Pharmacological, psychological, and non-invasive brain stimulation interventions for treating depression after stroke. Cochrane Database Syst Rev 2020; 1: D3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begemann MJ, Brand BA, Ćurcˇicć-Blake B, et al. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med 2020; 50: 2465–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D, Cheng A, Zhang Z, et al. Effects of low-frequency repetitive transcranial magnetic stimulation combined with cerebellar continuous theta burst stimulation on spasticity and limb dyskinesia in patients with stroke. BMC Neurol 2021; 21: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elsner B, Kugler J, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving aphasia after stroke: a systematic review with network meta-analysis of randomized controlled trials. J Neuroeng Rehabil 2020; 17: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norise C, Hamilton RH. Non-invasive brain stimulation in the treatment of post-stroke and neurodegenerative aphasia: parallels, differences, and lessons learned. Front Hum Neurosci 2016; 10: 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan A, Yuan K, Bao SC, et al. Can transcranial electrical stimulation facilitate post-stroke cognitive rehabilitation? A systematic review and meta-analysis. Front Rehabil Sci 2022; 3: 795737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitti E, Hillis AE. Treatment of post-stroke aphasia: a narrative review for stroke neurologists. Int J Stroke 2021; 16: 1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashiwagi FT, El Dib R, Gomaa H, et al. Noninvasive brain stimulations for unilateral spatial neglect after stroke: a systematic review and meta-analysis of randomized and nonrandomized controlled trials. Neural Plast 2018; 2018: 1638763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houben M, Chettouf S, Van Der Werf YD, et al. Theta-burst transcranial magnetic stimulation for the treatment of unilateral neglect in stroke patients: a systematic review and best evidence synthesis. Restor Neurol Neurosci 2021; 39: 447–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hara T, Shanmugalingam A, McIntyre A, et al. The effect of non-invasive brain stimulation (NIBS) on attention and memory function in stroke rehabilitation patients: a systematic review and meta-analysis. Diagnostics 2021; 11: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li KP, Sun J, Wu CQ, et al. Effects of repetitive transcranial magnetic stimulation on post-stroke patients with cognitive impairment: a systematic review and meta-analysis. Behav Brain Res 2023; 439: 114229. [DOI] [PubMed] [Google Scholar]
- 33.Xu WW, Liao QH, Zhu DW. The effect of transcranial magnetic stimulation on the recovery of attention and memory impairment following stroke: a systematic review and meta-analysis. Expert Rev Neurother 2022; 22: 1031–1041. [DOI] [PubMed] [Google Scholar]
- 34.Yan RB, Zhang XL, Li YH, et al. Effect of transcranial direct-current stimulation on cognitive function in stroke patients: a systematic review and meta-analysis. PLoS ONE 2020; 15: e0233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto EF, Gupta A, Kulkarni GB, et al. A randomized, double-blind, sham-controlled study of transcranial direct current stimulation as an augmentation intervention for the attenuation of motor deficits in patients with stroke. J ECT 2021; 37: 281–290. [DOI] [PubMed] [Google Scholar]
- 36.Boasquevisque DS, Servinsckins L, de Paiva J, et al. Contralesional cathodal transcranial direct current stimulation does not enhance upper limb function in subacute stroke: a pilot randomized clinical trial. Neural Plast 2021; 2021: 8858394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Ma J, Zhang J, et al. Repetitive transcranial magnetic stimulation (rTMS) modulates thyroid hormones level and cognition in the recovery stage of stroke patients with cognitive dysfunction. Med Sci Monit 2021; 27: e931914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bafeta A, Trinquart L, Seror R, et al. Reporting of results from network meta-analyses: methodological systematic review. BMJ 2014; 348: g1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ioannidis JP, Karassa FB. The need to consider the wider agenda in systematic reviews and meta-analyses: breadth, timing, and depth of the evidence. BMJ 2010; 341: c4875. [DOI] [PubMed] [Google Scholar]
- 40.Mills EJ, Bansback N, Ghement I, et al. Multiple treatment comparison meta-analyses: a step forward into complexity. Clin Epidemiol 2011; 3: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ 2013; 346: f2914. [DOI] [PubMed] [Google Scholar]
- 42.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005; 331: 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 44.Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat Med 2005; 24: 3823–3844. [DOI] [PubMed] [Google Scholar]
- 45.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins JPGS. Cochrane handbook for systematic reviews of interventions (Version 5.1.0), http://handbook.cochrane.org/ (accessed 30 October 2018).
- 47.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011; 64: 163–171. [DOI] [PubMed] [Google Scholar]
- 48.Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS ONE 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rucker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Stat Med 2014; 33: 4353–4369. [DOI] [PubMed] [Google Scholar]
- 50.van Valkenhoef G, Dias S, Ades AE, et al. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods 2016; 7: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res 1993; 2: 121–145. [DOI] [PubMed] [Google Scholar]
- 52.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brignardello-Petersen R, Bonner A, Alexander PE, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018; 93: 36–44. [DOI] [PubMed] [Google Scholar]
- 54.Puhan MA, Schunemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014; 349: g5630. [DOI] [PubMed] [Google Scholar]
- 55.Brignardello-Petersen R, Mustafa RA, Siemieniuk RAC, et al. GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. J Clin Epidemiol 2019; 108: 77–85. [DOI] [PubMed] [Google Scholar]
- 56.Jo JM, Kim YH, Ko MH, et al. Enhancing the working memory of stroke patients using tDCS. Am J Phys Med Rehabil 2009; 88: 404–409. [DOI] [PubMed] [Google Scholar]
- 57.Kang EK, Baek MJ, Kim S, et al. Non-invasive cortical stimulation improves post-stroke attention decline. Restor Neurol Neurosci 2009; 27: 645–650. [DOI] [PubMed] [Google Scholar]
- 58.Au-Yeung SS, Wang J, Chen Y, et al. Transcranial direct current stimulation to primary motor area improves hand dexterity and selective attention in chronic stroke. Am J Phys Med Rehabil 2014; 93: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 59.Park SH, Koh EJ, Choi HY, et al. A double-blind, sham-controlled, pilot study to assess the effects of the concomitant use of transcranial direct current stimulation with the computer assisted cognitive rehabilitation to the prefrontal cortex on cognitive functions in patients with stroke. J Korean Neurosurg Soc 2013; 54: 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolskår KK, Richard G, Alnaes D, et al. Reliability, sensitivity, and predictive value of fMRI during multiple object tracking as a marker of cognitive training gain in combination with tDCS in stroke survivors. Hum Brain Mapp 2021; 42: 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Georgiou AM, Kambanaros M. The effectiveness of transcranial magnetic stimulation (TMS) paradigms as treatment options for recovery of language deficits in chronic poststroke aphasia. Behav Neurol 2022; 2022: 7274115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szépfalusi N, Németh VL, Vékony T, et al. The use of transcranial direct current stimulation (tDCS) combined with cognitive training in stroke rehabilitation-a sham-controlled pilot study. Cerebrovasc Dis 2017; 43: 61. [Google Scholar]
- 63.Shaker HA, Sawan S, Fahmy EM, et al. Effect of transcranial direct current stimulation on cognitive function in stroke patients. Egypt J Neurol Psychiatr Neurosurg 2018; 54: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yun GJ, Chun MH, Kim BR. The effects of transcranial direct-current stimulation on cognition in stroke patients. J Stroke 2015; 17: 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Lee M, Yim J. A new approach to transcranial direct current stimulation in improving cognitive motor learning and hand function with the nintendo switch in stroke survivors. Med Sci Monit 2019; 25: 9555–9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee S, Cha H. The effect of clinical application of transcranial direct current stimulation combined with non-immersive virtual reality rehabilitation in stroke patients. Technol Health Care 2021; 30: 117–127. [DOI] [PubMed] [Google Scholar]
- 67.Guillouët E, Cogné M, Saverot E, et al. Impact of combined transcranial direct current stimulation and speech-language therapy on spontaneous speech in aphasia: a randomized controlled double-blind study. J Int Neuropsychol Soc 2020; 26: 7–18. [DOI] [PubMed] [Google Scholar]
- 68.Hosseinzadeh SA, Mazhari S, Najafi K, et al. Anodal transcranial direct current stimulation enhances positive changes in movement functions, visual attention and depression of patients with chronic ischemic stroke: a clinical trial. Biomed Res Ther 2018; 5: 2841–2849. [Google Scholar]
- 69.Li Y, Luo H, Yu Q, et al. Cerebral functional manipulation of repetitive transcranial magnetic stimulation in cognitive impairment patients after stroke: an fMRI Study. Front Neurol 2020; 11: 977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsai PY, Lin WS, Tsai KT, et al. High-frequency versus theta burst transcranial magnetic stimulation for the treatment of poststroke cognitive impairment in humans. J Psychiatry Neurosci 2020; 45: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Yin M, Luo J, et al. Effects of transcranial magnetic stimulation on the performance of the activities of daily living and attention function after stroke: a randomized controlled trial. Clin Rehabil 2020; 34: 1465–1473. [DOI] [PubMed] [Google Scholar]
- 72.Yin M, Liu Y, Zhang L, et al. Effects of rTMS treatment on cognitive impairment and resting-state brain activity in stroke patients: a randomized clinical trial. Front Neural Circuits 2020; 14: 563777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu H, Zhang T, Wen M, et al. Impact of repetitive transcranial magnetic stimulation on post-stroke dysmnesia and the role of BDNF Val66Met SNP. Med Sci Monit 2015; 21: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jorge RE, Robinson RG, Tateno A, et al. Repetitive transcranial magnetic stimulation as treatment of poststroke depression: a preliminary study. Biol Psychiatry 2004; 55: 398–405. [DOI] [PubMed] [Google Scholar]
- 75.Aşkın A, Tosun A, Demirdal ÜS. Effects of low-frequency repetitive transcranial magnetic stimulation on upper extremity motor recovery and functional outcomes in chronic stroke patients: a randomized controlled trial. Somatosens Mot Res 2017; 34: 102–107. [DOI] [PubMed] [Google Scholar]
- 76.Kim BR, Kim DY, Chun MH, et al. Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am J Phys Med Rehabil 2010; 89: 362–368. [DOI] [PubMed] [Google Scholar]
- 77.Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke 2006; 37: 2115–2122. [DOI] [PubMed] [Google Scholar]
- 78.Chiang CF, Lin MT, Hsiao MY, et al. Comparative efficacy of noninvasive neurostimulation therapies for acute and subacute poststroke dysphagia: a systematic review and network meta-analysis. Arch Phys Med Rehabil 2019; 100: 739–750. [DOI] [PubMed] [Google Scholar]
- 79.Xie YJ, Chen Y, Tan HX, et al. Repetitive transcranial magnetic stimulation for lower extremity motor function in patients with stroke: a systematic review and network meta-analysis. Neural Regen Res 2021; 16: 1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong Z, Zheng H, Luo J, et al. Effects of low-frequency repetitive transcranial magnetic stimulation on language recovery in poststroke survivors with aphasia: an updated meta-analysis. Neurorehabil Neural Repair 2021; 35: 680–691. [DOI] [PubMed] [Google Scholar]
- 81.Elsner B, Kwakkel G, Kugler J, et al. Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: a network meta-analysis of randomised controlled trials. J Neuroeng Rehabil 2017; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ünlüer NÖ, Temuçin ÇM, Demir N, et al. Effects of low-frequency repetitive transcranial magnetic stimulation on swallowing function and quality of life of post-stroke patients. Dysphagia 2019; 34: 360–371. [DOI] [PubMed] [Google Scholar]
- 83.Zhong L, Rao J, Wang J, et al. Repetitive transcranial magnetic stimulation at different sites for dysphagia after stroke: a randomized, observer-blind clinical trial. Front Neurol 2021; 12: 625683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaudeau-Bosma C, Moulier V, Allard AC, et al. Effect of two weeks of rTMS on brain activity in healthy subjects during an n-back task: a randomized double blind study. Brain Stimul 2013; 6: 569–575. [DOI] [PubMed] [Google Scholar]
- 85.Nejati V, Salehinejad MA, Nitsche MA, et al. Transcranial direct current stimulation improves executive dysfunctions in ADHD: implications for inhibitory control, interference control, working memory, and cognitive flexibility. J Atten Disord 2020; 24: 1928–1943. [DOI] [PubMed] [Google Scholar]
- 86.Alcalá-Lozano R, Morelos-Santana E, Cortés-Sotres JF, et al. Similar clinical improvement and maintenance after rTMS at 5 Hz using a simple vs. complex protocol in Alzheimer’s disease. Brain Stimul 2018; 11: 625–627. [DOI] [PubMed] [Google Scholar]
- 87.Parkin BL, Ekhtiari H, Walsh VF. Non-invasive human brain stimulation in cognitive neuroscience: a primer. Neuron 2015; 87: 932–945. [DOI] [PubMed] [Google Scholar]
- 88.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 2010; 14: 277–290. [DOI] [PubMed] [Google Scholar]
- 89.Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm 2010; 117: 105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 2014; 10: 597–608. [DOI] [PubMed] [Google Scholar]
- 91.Cheng CPW, Wong CSM, Lee KK, et al. Effects of repetitive transcranial magnetic stimulation on improvement of cognition in elderly patients with cognitive impairment: a systematic review and meta-analysis. Int J Geriatr Psychiatry 2018; 33: e1–e13. [DOI] [PubMed] [Google Scholar]
- 92.Grefkes C, Fink GR. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain 2011; 134(Pt. 5): 1264–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tonin FS, Rotta I, Mendes AM, et al. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract (Granada) 2017; 15: 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-19-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-20-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-21-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-22-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-23-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-1-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-10-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-11-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-12-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-13-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-14-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-2-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-3-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-4-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-5-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-6-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-7-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-8-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-jpg-9-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-png-15-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-png-16-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-png-17-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-png-18-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease
Supplemental material, sj-png-24-taj-10.1177_20406223231168754 for Comparative efficacy of different noninvasive brain stimulation therapies for recovery of global cognitive function, attention, memory, and executive function after stroke: a network meta-analysis of randomized controlled trials by Yao Wang, Wan Liu, Jiu Chen, Jianling Bai, Hao Yu, Hongxia Ma, Jiang Rao and Guangxu Xu in Therapeutic Advances in Chronic Disease